- 1Department of Oncology, Thoraxklinik and National Center for Tumor Diseases at Heidelberg University Hospital, Heidelberg, Germany
- 2Translational Lung Research Center at Heidelberg University Hospital, Member of the German Center for Lung Research (DZL), Heidelberg, Germany
- 3Translational Radiobiology, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany
- 4Comprehensive Cancer Center Erlangen-European Metropolitan Area Nürnberg (CCC ER-EMN), Erlangen, Germany
Editorial on the Research Topic
Systemic immune dysregulation in malignant disease: Insights, monitoring and therapeutic exploitation
Each tumor does not only trigger immune responses aiming to control its growth, but also causes profound immune dysregulation in the host: chronic antigen stimulation, contact-dependent effects through invariant receptors, and paracrine or systemic release of mediators induce alterations on virtually every immune cell type in the body. Precise characterization of these changes has key importance for the development of novel immunomodulatory strategies alone or in combination with standard modalities, such as radio- and chemotherapy (1, 2). Aim of this Research Topic was to provide a broad and comprehensive overview about recent discoveries in the field of cancer-associated systemic immune dysregulation, explore pathophysiologic links between the genetic contexture and immunologic tumor microenvironment (TME), analyze the potential prognostic and predictive value of immunologic biomarkers, and highlight opportunities for direct therapeutic exploitation using novel drugs or in the context of cell therapies.
A main subject was the potential clinical utility of blood-based and other biomarkers to guide patient management under immunotherapy (IO, Table 1). Using cytometric bead arrays for multiplex quantification of serum proteins, Schindler et al. demonstrated that blood cytokines can serve as predictors of efficacy and toxicity for PD-(L)1-treated non-small-cell lung cancer patients (NSCLC), but are not suitable for disease monitoring, since serial measurements cannot capture disease progression. This resembles the properties of simple blood-based biomarkers, like the neutrophil-to-lymphocyte ratio and advanced lung inflammation index (ALI) (17, 18), but is in contrast to circulating tumor DNA (ctDNA) assays, which have recently demonstrated superiority compared to radiologic imaging for the longitudinal monitoring of lung and other cancers under immunotherapy or targeted drugs (19, 20). Besides, Zhou et al. (4) demonstrated how machine learning methods can be leveraged in order to improve the predictive power of single blood-based biomarkers, like the C-reactive protein (CRP) and platelet-to-lymphocyte ratio (PLR), in order create complex, more accurate predictors for the immune-related adverse events (irAE) of cancer immunotherapy. Complementary immune cell-based matrices obtained from the peripheral blood of cancer patients might succeed to predict therapy responses more easily and accurately in the future (21).
Derangement of T-cell immunity also plays an essential role in the pathogenesis of cancer and treatment-related complications (Figure 1). One prominent example is cerebral pseudoprogression, which is caused by immune cell influx rather than tumor growth, may affect approximately 5% of patients receiving PD-(L)1 inhibitors, and requires meticulous radiologic criteria for accurate diagnosis in order to avoid therapeutic mistakes, as Urban et al. demonstrated. Alterations of cellular immunity are particularly prominent in case of tumors caused by chronic infection. This was well illustrated by the study of Meng et al. who analyzed head-and-neck squamous cell carcinoma (HNSCC) and revealed a lower degree of cancer cell stemness, heavier CD8+ cell infiltration, stronger expression of immune checkpoint molecules of T-cells, and elevated CCL4 in HPV-positive compared to HPV-negative tumor cells, as assessed by bulk and single-cell RNAseq data from various patient cohorts and cell lines. These data provide a mechanistic basis for the higher IO-sensitivity and more favorable prognosis of HPV-positive compared to HPV-negative HNSCC. Similar observations were reported by Huang et al. in soft-tissue sarcoma (STS), where a lower degree of stemness, more pronounced immune cell infiltrates, better sensitivity to chemotherapy and immunotherapy, as well as longer survival were observed in tumors with less N6-methyladenosine (m6A) RNA methylation. Besides, γδ T cells are important for the control of another virally induced pathology, i.e. HTLV-1-associated adult T-cell leukemia/lymphoma (ATLL) and tropical spastic paraparesis (TSP), as highlighted by Ruggieri et al.: cytotoxic Vγ9δ2 lymphocytes can eliminate eukaryotic cells expressing the HTLV-1 proteins HBZ or Tax, while progression from asymptomatic HTLV-1 infection to clinically overt ATLL/TSP is accompanied by depletion of the protective effectors in vivo. Instrumental for these insights was fine-granular analysis of T-cell receptor (TCR) repertoire in HTLV-1 infected patients and healthy donors using a custom spectratyping protocol that could differentiate between very similar transcripts belonging to various human Vγ and Vδ families (22). This is proof-of-principle for the potential clinical utility of comprehensive TCR profiling to elucidate pathogenesis and refine patient stratification in various cancers, as also suggested by earlier pivotal studies in melanoma, large-cell neuroendocrine lung carcinoma and indolent B-cell lymphoma (B-NHL) (23–26).
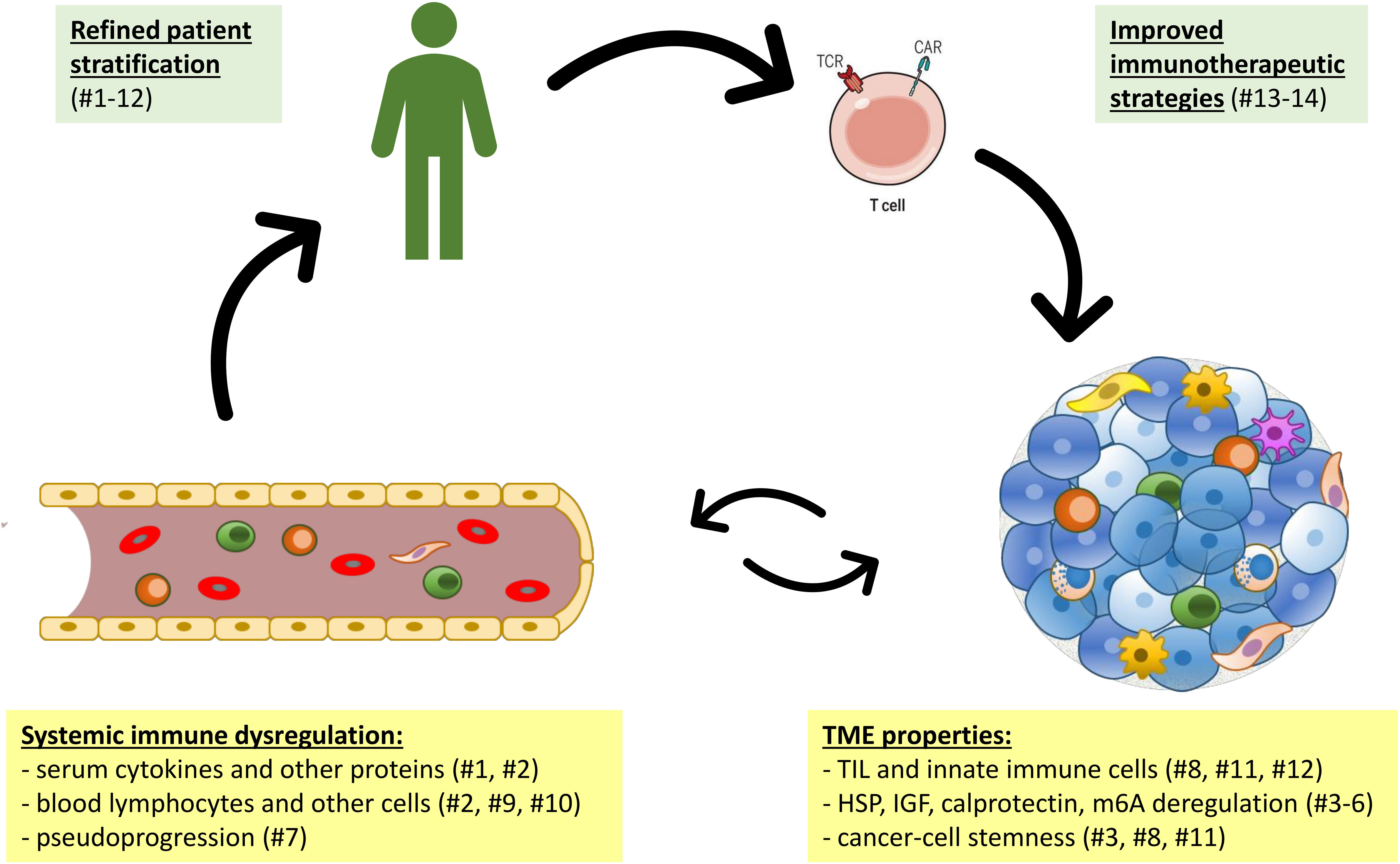
Figure 1 The interplay of systemic immune dysregulation and the tumor microenvironment (TME) of cancer as basis for clinical exploitation. Articles of the current research topic are shown in parentheses numbered according to Table 1.
Another important principle nicely demonstrated by the study of Ruggieri et al. is that systemic immune dysregulation and lymphocyte aberrations worsen with further disease progression in advanced cancer. Similar findings have also been described in many other hematologic and solid tumors, for example B-NHL and NSCLC Hao et al. (27, 28). Therefore, studies focusing on early–stage disease are of key importance to deconvolute the complex pathogenetic chains. Using multiparametric flow cytometry to analyze longitudinal blood samples from NSCLC patients, Hao et al. could demonstrate that significant depletion of multiple T–cell subsets alongside systemic immune activation are present already in patients with localized disease and aggravated after radical surgery, for example CD4+ cell counts and the CD4/CD8–cell ratio significantly decreased, but NKT increased in many patients after tumor resection. Thus, quantification of peripheral immune cell subsets could be used alongside TCR–based and ctDNA–based liquid biopsies in the future to improve monitoring of lung and other cancers (29, 30), as well as guide application of consolidative immunotherapies (31, 32). These tools could pave the way for personalized strategies of perioperative immunotherapy, which will become a main objective of translational research in the next few years, after the recent approval of the first adjuvant and neoadjuvant PD–(L)1 inhibitor treatments in thoracic oncology based on the Impower010 and Checkmate–816 trials, respectively (33, 34).
Accumulating evidence suggests that alterations of blood lymphocytes in lung and other cancers originate in the TME, which is hijacked by the growing tumor and transformed in an immunosuppressive niche (1). For example, using multispectral flow cytometry with hierarchical clustering to analyze samples from diverse murine lung cancer models, Olesch et al. could demonstrate alterations of CD324+ epithelial cells, alveolar macrophages, dendritic cells (DCs) and endothelial cells in animals with primary tumors, while fibroblasts, vascular smooth muscle cells, monocytes (Ly6C+ and Ly6C–) and neutrophils were elevated in metastatic models. On the other hand, Bazzi et al. could identify alterations of mast cells and DCs as predominant prognostic markers in early colorectal cancer (CRC) by comparative CIBERSORT analysis vs. the adjacent normal tissue. These changes are facilitated by deregulation of several important biological axes in human cancers, whose precise characterization could reveal specific therapeutic vulnerabilities. For example, expression of the calprotectin–receptor CD147 on penile cancer cells was associated with increased counts of S100A8+/S100A9+ neutrophil–derived suppressor cells in the TME and an elevated risk of metastasis (Mohr et al.). Furthermore, lower expression of IGF regulators in renal cell cancer (RCC) was linked to reduced infiltration by CD8+, Th1, and plasmacytoid DCs, activation of multiple metabolic pathways that fuel cancer progression, and lower sensitivity to antiangiogenics and immunotherapy, despite a higher tumor mutational burden (Jiang et al.). A third example are heat–shock proteins (HSP), which support tumor development by both intracellular and paracrine effects by regulating a wide array of biological processes, including unfolded protein responses, mitochondrial bioenergetics, apoptosis, autophagy, necroptosis, lipid metabolism, angiogenesis, cancer cell stemness, epithelial–mesenchymal transition and tumor immunity (Albakova and Mangasarova). At the same time, they can also serve as danger signal, for example by delivering antigens to DCs, or by direct activation of NK cells (35). The predictive value of serum Hsp70 is currently under investigation (36). Many recent insights into TME processes have relied on global transcriptomic profiling of tumor biopsies to dissect the TME at the functional level, but this method is still too resource–intensive for routine application. An attractive alternative is Nanostring–based targeted RNA profiling, a hybridization method suitable for formalin–fixed paraffin–embedded (FFPE) tissue specimens that requires very small amounts of input material, can be incorporated into the routine molecular workup, and may provide useful prognostic information for newly diagnosed with lung and other cancers (37–40). On the other hand, purely genetic markers, like the tumor mutational burden (TMB), pose technical challenges and have failed to meet expectations (41). Considering the significant variability among patients and cancer entities, wide adoption of practicable TME analysis methods will be crucial for personalized insights and tailored immunotherapeutic approaches. That being said, there are also important similarities across tumors, like the blood lymphopenia, which is a common feature and key adverse prognostic factor of carcinomas, sarcomas, and lymphomas (42). The sole exception to this rule appear to be thymic epithelial tumors, which uniquely cause an accumulation of hyporesponsive CD247–deficient naive T–cells in the periphery due to the unique thymic role in T–cell maturation (43, 44).
From a clinical standpoint, of utmost importance is the therapeutic exploitation of tumor–related immunologic changes. Up until a few years ago, the most effective immunotherapy for malignant disease has been allogeneic hematopoietic stem cell transplantation (HSCT), which represented the only curative option for patients with refractory hematologic cancers, like relapsed acute myeloid leukemia (AML) (45). However, this therapy was notoriously toxic, as the preceding conditioning regimens and subsequent graft–versus–host disease (GvHD) caused significant morbidity and mortality. One rare and exceptionally mild complication is secondary sarcoidosis, which occurs with a very low frequency <1% following HSCT, particularly in the presence of specific HLA–haplotypes, and usually resolves under standard glucocorticoid treatment without long–term sequelae, as observed by Wurm-Kuczera et al.. A newer cell–based cancer therapeutic are chimeric antigen receptor (CAR)–T cells, which have revolutionized the treatment of hematologic malignancies, but face two major challenges in application against lung and other solid tumors: the paucity of suitable dispensable extracellular target antigens, and an immunosuppressive TME that hinders penetration and activation of effector cells, as described by Kandra et al.. Very promising in this regard is the development of transgenic TCR–T cell therapies, which can be directed against the much larger pool of intracellular antigens and further augmented by genetic engineering in order to “heat–up” cold tumors for increased efficacy (46). Another innovative strategy with huge momentum currently are multi–specific antibodies, which can recruit endogenous T– or NK–cells against tumor cells bearing specific surface or even intracellular antigens (47). Important advantages of antibody– vs. cell–based strategies are immediate, off–the–shelf availability and better tolerability, since no previous conditioning is needed, so that these therapies can be started faster and combined with any other modality, like radiotherapy, chemotherapy or other immunotherapies for synergistic effects (48–50).
Since the advent of PD–(L)1 inhibitors, modern cancer medicine has been increasingly focused on the better unraveling, monitoring and reversal of the cancer–associated immune dysregulation for further therapeutic progress. Recent developments in these fields offer a justified hope for cure of several cancers in the near future, a snapshot of which the current Research Topic aspired to capture.
Author contributions
Both authors made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gaissmaier L, Christopoulos P. Immune modulation in lung cancer: Current concepts and future strategies. Respiration (2020), 1–27. doi: 10.1159/000510385
2. Rückert M, Flohr AS, Hecht M, Gaipl US. Radiotherapy and the immune system: More than just immune suppression. Stem Cells (2021) 39:1155–65. doi: 10.1002/stem.3391
3. Schindler H, Lusky F, Daniello L, Elshiaty M, Gaissmaier L, Benesova K, et al. Serum cytokines predict efficacy and toxicity, but are not useful for disease monitoring in lung cancer treated with PD–(L)1 inhibitors. Front Oncol (2022) 12:1010660. doi: 10.3389/fonc.2022.1010660
4. Zhou J–G, Wong AH–H, Wang H, Tan F, Chen X, Jin S–H, et al. Elucidation of the application of blood test biomarkers to predict immune–related adverse events in atezolizumab–treated NSCLC patients using machine learning methods. Front Immunol (2022) 13:862752. doi: 10.3389/fimmu.2022.862752
5. Mohr T, Zwick A, Hans MC, Bley IA, Braun FL, Khalmurzaev O, et al. The prominent role of the S100A8/S100A9–CD147 axis in the progression of penile cancer. Front Oncol (2022) 12:891511. doi: 10.3389/fonc.2022.891511
6. Jiang A, Wu X, Wang D, Wang A, Dong K, Liu B, et al. A new thinking: Deciphering the aberrance and clinical implication of IGF axis regulation pattern in clear cell renal cell carcinoma. Front Immunol (2022) 13:935595. doi: 10.3389/fimmu.2022.935595
7. Albakova Z, Mangasarova Y. The HSP immune network in cancer. Front Immunol (2021) 12:796493. doi: 10.3389/fimmu.2021.796493
8. Huang Z–D, Lin L–L, Liu Z–Z, Hu C, Gu H–Y, Wei R–X. m6A modification patterns with distinct immunity, metabolism, and stemness characteristics in soft tissue sarcoma. Front Immunol (2021) 12:765723. doi: 10.3389/fimmu.2021.765723
9. Urban H, Steidl E, Hattingen E, Filipski K, Meissner M, Sebastian M, et al. Immune checkpoint inhibitor–induced cerebral pseudoprogression: Patterns and categorization. Front Immunol (2021) 12:798811. doi: 10.3389/fimmu.2021.798811
10. Meng L, Lu H, Li Y, Zhao J, He S, Wang Z, et al. Human papillomavirus infection can alter the level of tumour stemness and T cell infiltration in patients with head and neck squamous cell carcinoma. Front Immunol (2022) 13:1013542. doi: 10.3389/fimmu.2022.1013542
11. Ruggieri M, Ducasa N, Juraske C, Polo VG, Berini C, Quiroga MF, et al. Phenotypic and functional analysis of gd T cells in the pathogenesis of human T–cell lymphotropic virus type 1 infection. Front Immunol (2022) 13:920888. doi: 10.3389/fimmu.2022.920888
12. Hao Z, Lin M, Du F, Xin Z, Wu D, Yu Q, et al. Systemic immune dysregulation correlates with clinical features of early non–small cell lung cancer. Front Immunol (2021) 12:754138. doi: 10.3389/fimmu.2021.754138
13. Olesch C, Brunn D, Aktay–Cetin Ö, Sirait–Fischer E, Pullamsetti SS, Grimminger F, et al. Picturing of the lung tumor cellular composition by multispectral flow cytometry. Front Immunol (2022) 13:827719. doi: 10.3389/fimmu.2022.827719
14. Bazzi ZA, Sneddon S, Zhang PG, Tai IT. Characterization of the immune cell landscape in CRC: Clinical implications of tumour–infiltrating leukocytes in early– and late–stage CRC. Front Immunol (2022) 13:978862. doi: 10.3389/fimmu.2022.978862
15. Wurm–Kuczera RI, Buentzel J, Koenig JF, Legler T, Valk J–J, Hasenkamp J, et al. Sarcoidosis following hematopoietic stem cell transplantation: Clinical characteristics and HLA associations. Front Immunol (2021) 12:746996. doi: 10.3389/fimmu.2021.746996
16. Kandra P, Nandigama R, Eul B, Huber M, Kobold S, Seeger W, et al. Utility and drawbacks of chimeric antigen receptor T cell (CAR–T) therapy in lung cancer. Front Immunol (2022) 13:903562. doi: 10.3389/fimmu.2022.903562
17. Mountzios G, Samantas E, Senghas K, Zervas E, Krisam J, Samitas K, et al. Association of the advanced lung cancer inflammation index (ALI) with immune checkpoint inhibitor efficacy in patients with advanced non–small–cell lung cancer. ESMO Open (2021) 6:100254. doi: 10.1016/j.esmoop.2021.100254
18. Daniello L, Elshiaty M, Bozorgmehr F, Kuon J, Kazdal D, Schindler H, et al. Therapeutic and prognostic implications of immune–related adverse events in advanced non–Small–Cell lung cancer. Front Oncol (2021) 11:703893. doi: 10.3389/fonc.2021.703893
19. Bratman SV, Yang SY, Iafolla MA, Liu Z, Hansen AR, Bedard PL, et al. Personalized circulating tumor DNA analysis as a predictive biomarker in solid tumor patients treated with pembrolizumab. Nat Cancer (2020) 1:873–81. doi: 10.1038/s43018–020–0096–5
20. Dietz S, Christopoulos P, Yuan Z, Angeles AK, Gu L, Volckmar A–L, et al. Longitudinal therapy monitoring of ALK–positive lung cancer by combined copy number and targeted mutation profiling of cell–free DNA. EBioMedicine (2020) 62:103103. doi: 10.1016/j.ebiom.2020.103103
21. Zhou JG, Donaubauer AJ, Frey B, Becker I, Rutzner S, Eckstein M, et al. Prospective development and validation of a liquid immune profile–based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors. J Immunother Cancer (2021) 9. doi: 10.1136/jitc–2020–001845
22. Christopoulos P, Bukatz D, Kock S, Malkovsky M, Finke J, Fisch P. Improved analysis of TCRγδ variable region expression in humans. J Immunol Methods (2016) 434:66–72. doi: 10.1016/j.jim.2016.04.009
23. Kirsch I, Vignali M. Robins h. T–cell receptor profiling cancer Mol Oncol (2015) 9:2063–70. doi: 10.1016/j.molonc.2015.09.003
24. Christopoulos P, Schneider MA, Bozorgmehr F, Kuon J, Engel–Riedel W, Kollmeier J, et al. Large Cell neuroendocrine lung carcinoma induces peripheral T–cell repertoire alterations with predictive and prognostic significance. Lung Cancer (2018) 119:48–55. doi: 10.1016/j.lungcan.2018.03.002
25. Poran A, Scherer J, Bushway ME, Besada R, Balogh KN, Wanamaker A, et al. Combined TCR repertoire profiles and blood cell phenotypes predict melanoma patient response to personalized neoantigen therapy plus anti–PD–1. Cell Rep Med (2020) 1:100141. doi: 10.1016/j.xcrm.2020.100141
26. Christopoulos P, Follo M, Fisch P, Veelken H. The peripheral helper T–cell repertoire in untreated indolent b–cell lymphomas: Evidence for antigen–driven lymphomagenesis. Leukemia (2008) 22:1952–4. doi: 10.1038/leu.2008.82
27. Christopoulos P, Pfeifer D, Bartholomé K, Follo M, Timmer J, Fisch P, et al. Definition and characterization of the systemic T–cell dysregulation in untreated indolent b–cell lymphoma and very early CLL. Blood (2011) 117:3836–46. doi: 10.1182/blood–2010–07–299321
28. Thommen DS, Schreiner J, Müller P, Herzig P, Roller A, Belousov A, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res (2015) 3:1344–55. doi: 10.1158/2326–6066.CIR–15–0097
29. Christopoulos P. Liquid biopsies come of age in lung cancer. Transl Lung Canc Res (2022) 11:706–10. doi: 10.21037/tlcr–22–268
30. Hiam–Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer (2021) 21:345–59. doi: 10.1038/s41568–021–00347–z
31. Hecht M, Gostian AO, Eckstein M, Rutzner S, von der GrünJ, Illmer T, et al. Safety and efficacy of single cycle induction treatment with cisplatin/docetaxel/ durvalumab/tremelimumab in locally advanced HNSCC: First results of CheckRad–CD8. J Immunother Cancer (2020) 8. doi: 10.1136/jitc–2020–001378
32. Faehling M, Schumann C, Christopoulos P, Hoffknecht P, Alt J, Horn M, et al. Durvalumab after definitive chemoradiotherapy in locally advanced unresectable non–small cell lung cancer (NSCLC): Real–world data on survival and safety from the German expanded–access program (EAP). Lung Cancer (2020) 150:114–22. doi: 10.1016/j.lungcan.2020.10.006
33. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386:1973–85. doi: 10.1056/NEJMoa2202170
34. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non–small–cell lung cancer (IMpower010): A randomised, multicentre, open–label, phase 3 trial. Lancet (2021) 398:1344–57. doi: 10.1016/S0140–6736(21)02098–5
35. Gaipl US, Multhoff G, Scheithauer H, Lauber K, Hehlgans S, Frey B, et al. Kill and spread the word: Stimulation of antitumor immune responses in the context of radiotherapy. Immunotherapy (2014) 6:597–610. doi: 10.2217/imt.14.38
36. Seier S, Bashiri Dezfouli A, Lennartz P, Pockley AG, Klein H, Multhoff G. Elevated levels of circulating Hsp70 and an increased prevalence of CD94+/CD69+ NK cells is predictive for advanced stage non–small cell lung cancer. Cancers (Basel) (2022) 14. doi: 10.3390/cancers14225701
37. Budczies J, Kirchner M, Kluck K, Kazdal D, Glade J, Allgäuer M, et al. A gene expression signature associated with b cells predicts benefit from immune checkpoint blockade in lung adenocarcinoma. Oncoimmunology (2021) 10:1860586. doi: 10.1080/2162402X.2020.1860586
38. Stepp WH. High–throughput NanoString analysis of oncogenic human papillomavirus and tumor microenvironment transcription in head and neck squamous cell carcinoma. Curr Protoc (2021) 1:e146. doi: 10.1002/cpz1.146
39. Budczies J, Kirchner M, Kluck K, Kazdal D, Glade J, Allgäuer M, et al. Deciphering the immunosuppressive tumor microenvironment in ALK– and EGFR–positive lung adenocarcinoma. Cancer Immunol Immunother (2022) 71(2):251-265. doi: 10.1007/s00262–021–02981–w
40. Kirchner M, Kluck K, Brandt R, Volckmar A–L, Penzel R, Kazdal D, et al. The immune microenvironment in EGFR– and ERBB2–mutated lung adenocarcinoma. ESMO Open (2021) 6:100253. doi: 10.1016/j.esmoop.2021.100253
41. Budczies J, Kazdal D, Allgäuer M, Christopoulos P, Rempel E, Pfarr N, et al. Quantifying potential confounders of panel–based tumor mutational burden (TMB) measurement. Lung Cancer (2020) 142:114–9. doi: 10.1016/j.lungcan.2020.01.019
42. Ray–Coquard I, Cropet C, van Glabbeke M, Sebban C, Le Cesne A, Judson I, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res (2009) 69:5383–91. doi: 10.1158/0008–5472.CAN–08–3845
43. Christopoulos P, Dopfer EP, Malkovsky M, Esser PR, Schaefer H–E, Marx A, et al. A novel thymoma–associated immunodeficiency with increased naive T cells and reduced CD247 expression. J Immunol (2015) 194:3045–53. doi: 10.4049/jimmunol.1402805
44. Christopoulos P, Fisch P. Acquired T–cell immunodeficiency in thymoma patients. Crit Rev Immunol (2016) 36:315–27. doi: 10.1615/CritRevImmunol.2017018916
45. Christopoulos P, Schmoor C, Waterhouse M, Marks R, Wäsch R, Bertz H, et al. Reduced–intensity conditioning with fludarabine and thiotepa for second allogeneic transplantation of relapsed patients with AML. Bone Marrow Transplant (2013) 48:901–7. doi: 10.1038/bmt.2012.267
46. Gaissmaier L, Elshiaty M, Christopoulos P. Breaking bottlenecks for the TCR therapy of cancer. Cells (2020) 9. doi: 10.3390/cells9092095
47. Elshiaty M, Schindler H, Christopoulos P. Principles and current clinical landscape of multispecific antibodies against cancer. Int J Mol Sci (2021) 22. doi: 10.3390/ijms22115632
48. Frey B, Rückert M, Deloch L, Rühle PF, Derer A, Fietkau R, et al. Immunomodulation by ionizing radiation–impact for design of radio–immunotherapies and for treatment of inflammatory diseases. Immunol Rev (2017) 280:231–48. doi: 10.1111/imr.12572
49. Saalfeld FC, Wenzel C, Christopoulos P, Merkelbach–Bruse S, Reissig TM, Laßmann S, et al. Efficacy of immune checkpoint inhibitors alone or in combination with chemotherapy in NSCLC harboring ERBB2 mutations. J Thor Oncol (2021) 16:1952–8. doi: 10.1016/j.jtho.2021.06.025
Keywords: immune checkpoint inhibitors, immune microenviroment, immune dysregulation, cell therapies, biomarkers
Citation: Christopoulos P and Gaipl US (2023) Editorial: Systemic immune dysregulation in malignant disease: Insights, monitoring and therapeutic exploitation. Front. Oncol. 13:1182081. doi: 10.3389/fonc.2023.1182081
Received: 08 March 2023; Accepted: 10 March 2023;
Published: 03 April 2023.
Edited and Reviewed by:
Catherine Sautes-Fridman, INSERM U1138 Centre de Recherche des Cordeliers (CRC), FranceCopyright © 2023 Christopoulos and Gaipl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Petros Christopoulos, cGV0cm9zLmNocmlzdG9wb3Vsb3NAbWVkLnVuaS1oZWlkZWxiZXJnLmRl
 Petros Christopoulos
Petros Christopoulos Udo S. Gaipl
Udo S. Gaipl