- 1Department of Medical Oncology, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 2State Key Laboratory of Respiratory Disease, Center for Infection and Immunity, Guangzhou Institutes of Biomedicine and Health, Chinese Academy of Sciences (CAS), Guangzhou, Guangdong, China
- 3CAS-Lamvac (Guangzhou) Biomedical Technology Co., Ltd., Guangzhou, Guangdong, China
- 4Department of Hepatobiliary Surgery, First Affiliated Hospital of Kunming Medical University, Kunming, Yunnan, China
- 5The Ministry of Education Key Laboratory of Laboratory Medical Diagnostics, College of Laboratory Medicine, Chongqing Medical University, Chongqing, China
- 6Department of Applied Biosciences and Biotechnology, Faculty of Science and Technology, Midlands State University, Gweru, Zimbabwe
- 7Department of Pathogen Biology and Immunology, Kunming Medical University, Kunming, Yunnan, China
Objective: Our previous studies have demonstrated that Plasmodium immunotherapy (infection) has antitumor effects in mice. However, as a new form of immunotherapy, this therapy has a weakness: its specific killing effect on tumor cells is relatively weak. Therefore, we tested whether Plasmodium immunotherapy combined with gemcitabine (Gem), a representative chemotherapy drug, has synergistic antitumor effects.
Methods: We designed subcutaneously and intravenously implanted murine Lewis lung cancer (LLC) models to test the antitumor effect of Plasmodium chabaudi ASS (Pc) infection in combination with Gem treatment and explored its underlying mechanisms.
Results: We found that both Pc infection alone and Gem treatment alone significantly inhibited tumor growth in the subcutaneous model, and combination therapy was more effective than either monotherapy. Monotherapy only tended to prolong the survival of tumor-bearing mice, while the combination therapy significantly extended the survival of mice, indicating a significant synergistic effect of the combination. In the mechanistic experiments, we found that the combination therapy significantly upregulated E-cadherin and downregulated Snail protein expression levels, thus inhibiting epithelial-mesenchymal transition (EMT) of tumor cells, which may be due to the blockade of CXCR2/TGF-β-mediated PI3K/Akt/GSK-3β signaling pathway.
Conclusion: The combination of Pc and Gem plays a synergistic role in inhibiting tumor growth and metastasis, and prolonging mice survival in murine lung cancer models. These effects are partially attributed to the inhibition of EMT of tumor cells, which is potentially due to the blockade of CXCR2/TGF-β-mediated PI3K/Akt/GSK-3β/Snail signaling pathway. The clinical transformation of Plasmodium immunotherapy combined with Gem for lung cancer is worthy of expectation.
Introduction
Lung cancer is the most common cancer in the world, with the highest mortality and the second morbidity among all types of malignant tumors (1). Even though the traditional methods of surgery and chemoradiotherapy have improved, more than 40% of patients with non-small cell lung cancer (NSCLC) treated in early stage still have tumor recurrence and metastasis (2). In addition, about 65.33% of patients diagnosed with NSCLC are in the late stage (3), and the effect of conventional chemotherapy or radiotherapy is limited. Although some patients respond to targeted therapy, the development of drug resistance leads to tumor progression in a certain period (4). Since 2013, cancer immunotherapy represented by immune checkpoint inhibitors has been crowned as one of the breakthroughs of science and technology (5). However, some challenges remain to be addressed, including immune-related toxicities (6, 7), and primary/acquired resistance to therapy (8). There are no ideal and effective treatment models with little toxicity and the ability to inhibit tumor metastasis to improve the therapeutic effect of lung cancer patients so far.
Malaria is a disease caused by the infection of Plasmodium parasite, which is the most common parasitic infection in humans and animals. Studies have indicated that Plasmodium infection activates the immune system (9–11), which may help to repress tumor growth. Our previous studies have demonstrated that Plasmodium infection inhibits Lewis lung cancer (LLC) growth and metastasis by activating antitumor immune response (12), inhibiting angiogenesis (13, 14) and counteracting the tumor immunosuppressive microenvironment in mice (15). However, as a new form of immunotherapy, namely, Plasmodium immunotherapy, we still need to further enhance the specific killing effect of this therapy to increase its efficacy. In principle, chemotherapeutic drugs just have this effect, and gemcitabine (Gem) as a typical representative of these drugs is an effective and commonly used cytotoxic agent for the treatment of various tumors including NSCLC (16). Therefore, we designed murine NSCLC (LLC) models to examine the effects of Plasmodium infection in combination with Gem treatment on the inhibition of tumor growth and metastasis.
Tumor metastasis is a complex process that involves numerous factors and multiple steps, of which the epithelial-mesenchymal transition (EMT) is considered the initial and critical event in many types of carcinoma (17, 18). Chemokines, which are small chemoattractants in regulating cell positioning and cell recruitment into tissues, have been found to play an important role in cancer metastasis and EMT (19). Multiple signaling pathways participate in the progression of EMT, of which the chemokine-mediated PI3K/Akt/GSK−3β/Snail signaling is an essential process (20, 21). Therefore, in our current study, we tested whether Plasmodium infection in combination with Gem inhibited tumor metastasis and EMT, and whether these inhibitions were associated with the blocking of the above-mentioned pathways. Our results indicated that the combination of Plasmodium infection and Gem treatment significantly suppressed tumor metastasis and EMT, and these suppressions were potentially associated with the blockade of CXCR2/TGF-β-mediated PI3K/Akt/GSK-3β/Snail signaling pathway.
Materials and methods
Ethics statement
Our animal experiment facilities were approved by the Guangdong Provincial Department of Science and Technology. The animal experiments were approved by the Welfare Committee of the Center of Experimental Animals, Guangzhou Institutes of Biomedicine and Health (GIBH), Chinese Academy of Sciences (approval no. N2019014; Guangzhou, China), and strictly followed the standard guidelines for the care of animals. Animal suffering was minimized during the experiments.
Animals, cells, and parasites
Six- to eight-week-old female C57BL/6 mice were purchased from Vital River Experiment Animal Limited Company (Beijing, China) and kept in a specific pathogen-free (SPF) animal facility of the GIBH. Plasmodium yoelii 17XNL (MRA-593, Py) and Plasmodium chabaudi ASS (MRA-429, Pc) strains were both donated by Malaria Research and Reference Reagent Resource Center (MR4). The murine LLC cells were obtained from ATCC and cultured with the medium containing Dulbecco’s modified Eagle’s medium (DMEM, Gibco), penicillin (80 U/ml), streptomycin (100 U/ml) and 10% fetal bovine serum (FBS, Gibco) in a humidified atmosphere of 5% CO2 at 37°C. Py and Pc were both intraperitoneally injected and propagated in C57BL/6 mice, respectively. Peripheral blood parasitemia was measured in thin blood smear by Giemsa staining (Sigma-Aldrich). Parasitemia (%) was calculated by counting the parasitized erythrocytes in at least 1000 erythrocytes.
Subcutaneous tumor model and treatment
For the subcutaneous tumor model, 5×105 LLC cells were subcutaneously (s.c.) inoculated into the right flank of C57BL/6 mice. Mice were randomly divided into four groups of 15 mice each according to body weight stratification: Control group (Con); Pc infection group (Pc); Gem treatment group (Gem); Pc infection combined with Gem treatment group (Pc+Gem). Mice were then intraperitoneally (i.p.) injected with 5×105 Pc infected red blood cells on day 7 in the Pc group and Pc+Gem group. Mice were treated with Gem (50 mg/kg; 0.2 mL per time) i.p. for twice on day 6 and day 13 in the Gem group and Pc+Gem group. The tumor volume was recorded every two days after the tumor became palpable. The formula for calculating the volume is V = (ab2)/2, where “a” represents the long diameter, and “b” represents the short diameter of the tumor (12). Five mice randomly selected from each group were sacrificed on day 17 for calculating their body weight and tumor weight, and for analyzing their tumor tissues. The remaining mice were continually observed for their survival.
Intravenous tumor model and treatment
For intravenous tumor model, 5×105 LLC cells were intravenously (i.v.) injected into the C57BL/6 mice. Mice were randomly divided into four groups of 15 mice each: Con group, Pc group, Gem group, and Pc+Gem group. Each mouse of the Pc group and Pc+Gem group was i.p. injected with 5×105 (Pc) parasitized erythrocytes on day 2. Each mouse in the Gem group and Pc+Gem group was (i.p.) given Gem (50 mg/kg, 0.2 ml per time) for twice on day 12 and 19. Five mice randomly selected from each group were sacrificed for counting individual metastatic nodules on the surface of lung under a microscope on day 32. The remaining mice continued to be observed for their survival.
Protein extraction and Western blotting
Total protein was extracted from the tumor tissue using lysis buffer (RIPA, Beyotime) with protease inhibitor (Biotool) and phosphatase inhibitor cocktail (CST). Protein concentration was determined by BCA method. Total protein (50-100 μg) was separated by 10%-12% SDS-PAGE electrophoresis and then transferred to a PVDF membrane (Millipore). The membrane was blocked with 3% skim milk powder in tris-buffered saline with 0.1% tween 20 (TBST, Thermo Fisher Scientific, Inc) for 90 minutes at room temperature, and then it was incubated overnight with a solution containing the primary antibody. After washing with TBST for three times, the membrane was incubated at room temperature for 1 hour with horseradish peroxidase–conjugated secondary antibody diluted in TBST. Protein bands were observed by enhanced chemiluminescence (Pierce) and detected by BioImaging Systems (BIO-RAD ChemiDoc™ MP Imaging System, USA). GAPDH expression was used as a normalized protein.
Reagents list used in the study
Antibodies: GAPDH (cat. no. ab9385; Abcam), E-cadherin (cat. no. BS1098; Bioworld Technology, Inc.), Snail (cat. no. 3879S; Cell Signaling Technology, Inc.), PI3K (cat. no. ab151549; Abcam), phosphorylated (p)PI3K p85 (Tyr458)/p55 (Tyr199) (cat. no. 4228S; Cell Signaling Technology, Inc.), Akt (cat. no. 9272S; Cell Signaling Technology, Inc.), pAkt (Ser473) (cat. no. 4060S; Cell Signaling Technology, Inc.), GSK 3β (cat. no. 12456S; Cell Signaling Technology, Inc.), pGSK 3β Ser9 (cat. no. 5558S; Cell Signaling Technology, Inc.), CXCR2 (cat. no. ab217314; Abcam) and TGF-β (cat. no. ab25121; Abcam).
Statistical analysis
The survival was analyzed by Kaplan-Meier and compared by the Log-rank test. Data between groups were analyzed by Student’s t-test. p values less than 0.05, 0.01, and 0.001 were considered statistically significant and indicated by *, ** and ***, respectively, ns means “no significance” in each. All statistical analysis was performed using GraphPad Prism8.0 (GraphPad Software, Inc. https://www.graphpad.com/scientific-software/prism/).
Results
The combination of Pc with Gem significantly inhibited LLC subcutaneous tumor growth in mice
To investigate the antitumor effect of the combination of Pc and Gem, subcutaneously implanted tumor-bearing mice were treated with Pc in combination with Gem (Figure 1A). The subcutaneous tumors of mice in Pc+Gem group had less blood supply, and looked like benign tumors (Figure 1B). Both Pc infection alone (p = 0.005) and Gem treatment alone (p = 0.002) significantly inhibited tumor growth in the subcutaneous model. Furthermore, tumor growth was significantly slower in the combination (Pc+Gem) group than in the control group(p < 0.001), and Pc alone (p = 0.006) or Gem alone (p = 0.03) group respectively (Figure 1C). The tumor weights and the ratios of tumor weight to the body weight in the combination group were also significantly lower than those in any other group (all p < 0.05) (Figures 1D, S1). The median survival time was 27.5 days, 34 days, 35 days and 41 days in the control group, Pc group, Gem group and Pc+Gem group, respectively (Figure 1E). Compared with the control, single treatment (Pc alone or Gem alone) only tended to prolong the life span of tumor-bearing mice without statistically significant differences in survival time between groups. Nevertheless, the life span of the combined treatment group was significantly longer than that of the control group (p = 0.007), suggesting a synergistic effect of the combination (Figure 1E).
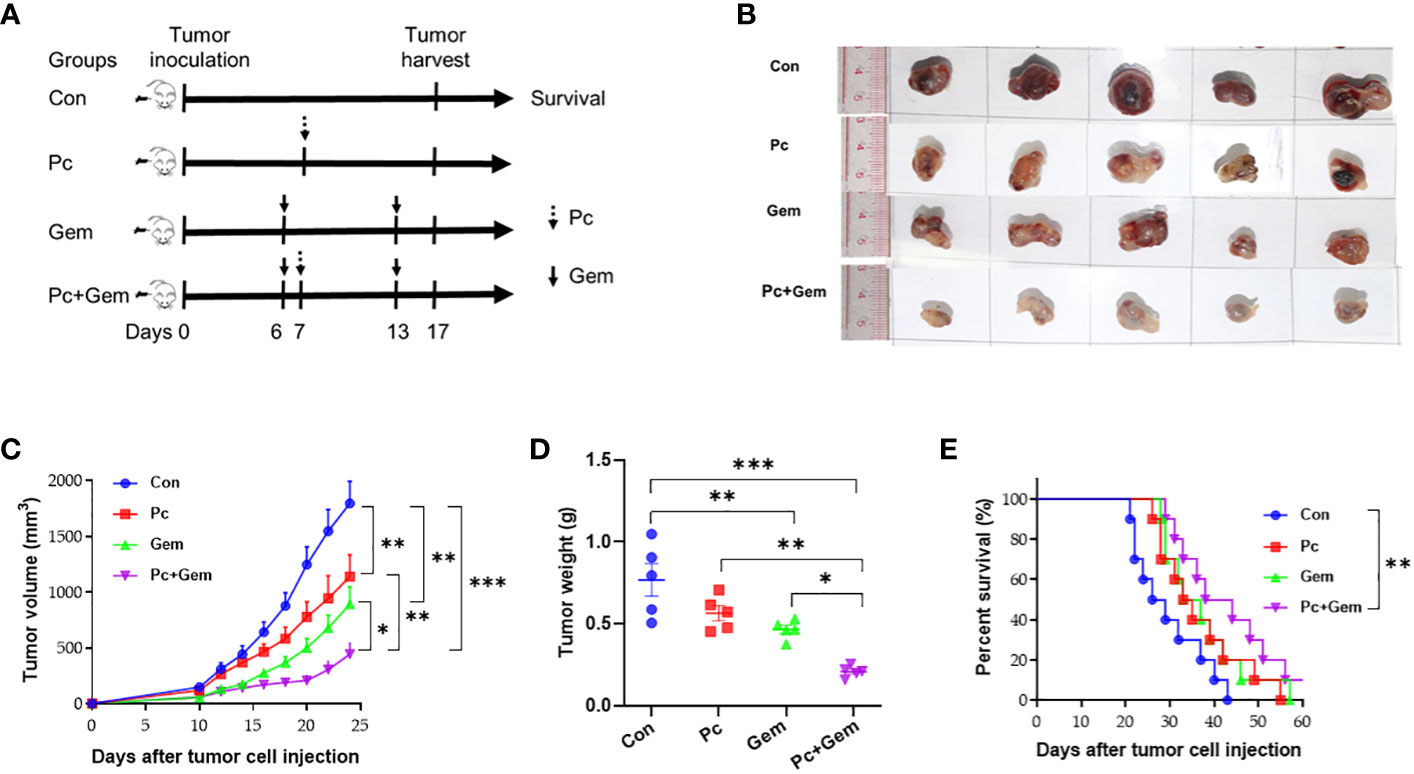
Figure 1 Pc+Gem combination treated lung cancer in the s.c. implanted murine LLC model. (A) Simplified experimental flow chart of s.c. implanted murine LLC model. (B) The image of tumor size on day 17 after tumor cells injection (n=5). (C) Tumor growth curves (n = 10 each group). (D) Tumor weight on day 17 after tumor cells injection (n = 5). The statistical differences were analyzed with an unpaired two-tailed Student’s t-test. (E) Kaplan-Meier survival curves (n = 10). Survival curves were analyzed by a log-rank test. p values less than 0.05, 0.01, and 0.001 were considered statistically significant and indicated by *, ** and ***, respectively.
The combination of Pc with Gem significantly inhibited LLC metastasis in mice
To determine the anti-metastatic effect of Pc+Gem, we established a mouse tumor model with intravenous injection of LLC cells (Figure 2A). The number of lung tumor nodules on day 32 in the Pc alone group or combination group was significantly less than that in the control group (p = 0.005 and p = 0.003, respectively) and Gem alone group (p = 0.02 and p = 0.02, respectively) (Figure 2B). These phenomena suggested that the role of Pc on the inhibition of metastasis was greater than that of Gem. In the control group, large transparent gelatinoid metastastic nodules were observed on the lung surface, some of which were accompanied by local bleeding. In the treatment groups, the metastases were smaller, and most of the metastases were only observed under magnifying glass after lung tissue dissection (Figure 2C). The median survival time was 44.5 days, 57 days, 59.5 days and 69.5 days in the control group, Pc group, Gem group and Pc+Gem group, respectively (Figure 2D). Accordingly, we also found that the mice in the Pc group, Gem group and Pc+Gem group survived significantly longer than the mice in the control group (p = 0.02, p = 0.006 and p < 0.001, respectively), the mice in the combination treatment group appeared to live longer than those in any monotherapy group, but the differences between the groups were not statistically significant (Figure 2D).
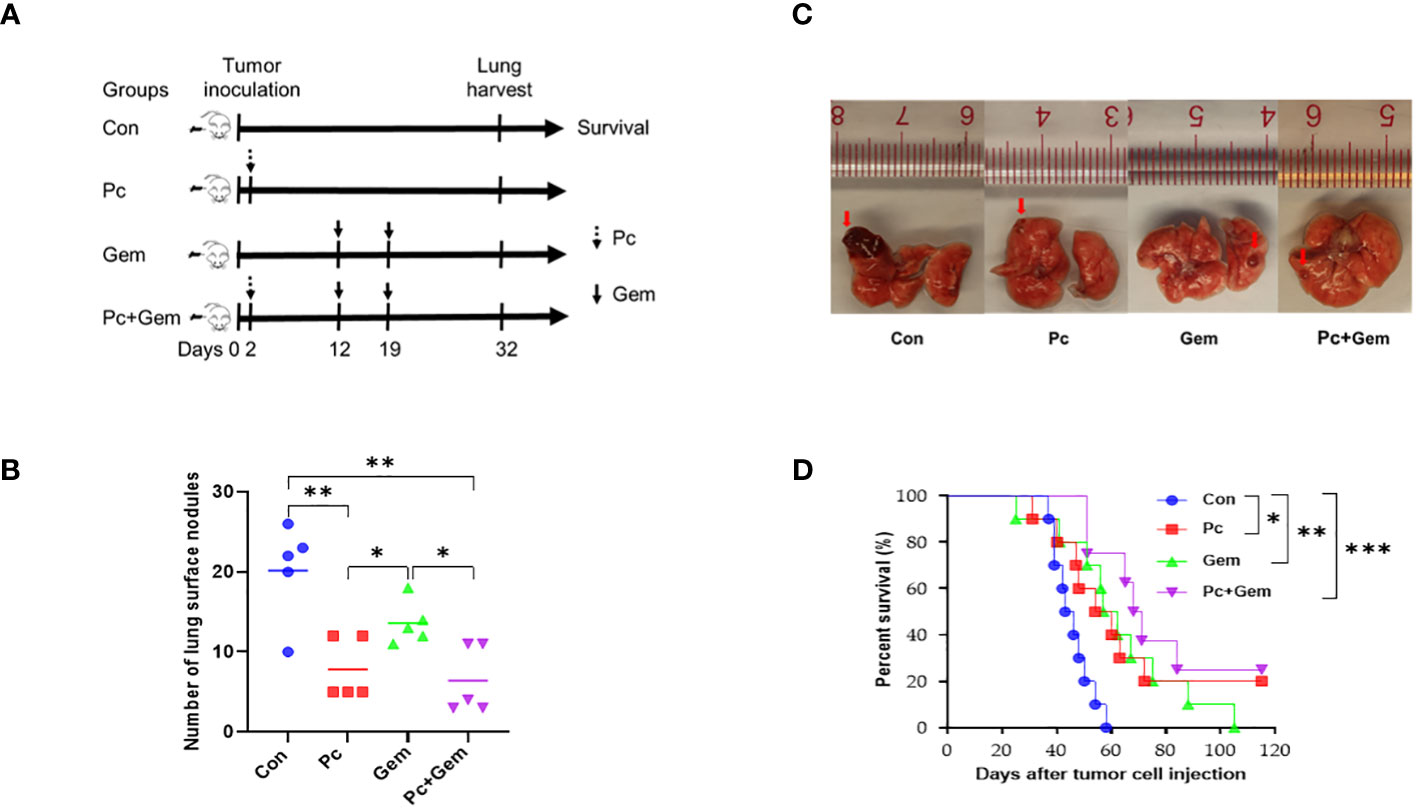
Figure 2 Pc+Gem treated lung cancer in the i.v. inoculated murine LLC model. (A) Simplified experimental flow chart of i.v. inoculated murine LLC model. (B) Number of tumor nodules in the lung tissues on day 32 after intravenous tumor inoculation (n = 5). The statistical differences were analyzed with an unpaired two-tailed Student’s t-test. (C) The representative image of tumor nodules in the lung tissues on day 32 after intravenous tumor inoculation. (D) Kaplan-Meier survival curve (n = 10). Survival curves were analyzed by a log-rank test. p values less than 0.05, 0.01, and 0.001 were considered statistically significant and indicated by *, ** and ***, respectively.
The combination of Pc and Gem significantly inhibited the EMT of tumor cells
Tumor cells obtain an invasive phenotype for metastatic progression through EMT, which allows them to dissociate from the primary tumor into the blood circulation (22). The loss of E-cadherin expression has been considered a landmark for EMT (23). Our experimental results showed that there were no significant differences in the protein expression level of E-cadherin between each monotherapy group and the control group, but the level of this molecule in the combination treatment group was significantly higher than that in the control group or that in Pc group and Gem group (p < 0.001, p = 0.002 and p < 0.001, respectively) (Figures 3A, B), suggesting a significantly synergistic effect of the combined treatment. The Snail superfamily of Zinc-finger transcription factors is a crucial transcription inhibitor for EMT, which can directly lead to inhibition of E-cadherin (24). Our results indicated that the expression level of Snail protein in the Pc group and Pc+Gem group was significantly lower than that in the control group (p = 0.02 and p = 0.002, respectively). Although there were no significant differences between the treatment groups, the level of this molecule in Gem group was not statistically significant than that in the control group, which suggested that combination treatment group has a synergistic effect on the inhibition of Snail protein expression and the role of Pc on the inhibition of Snail protein expression was greater than that of Gem (Figures 3A, C).
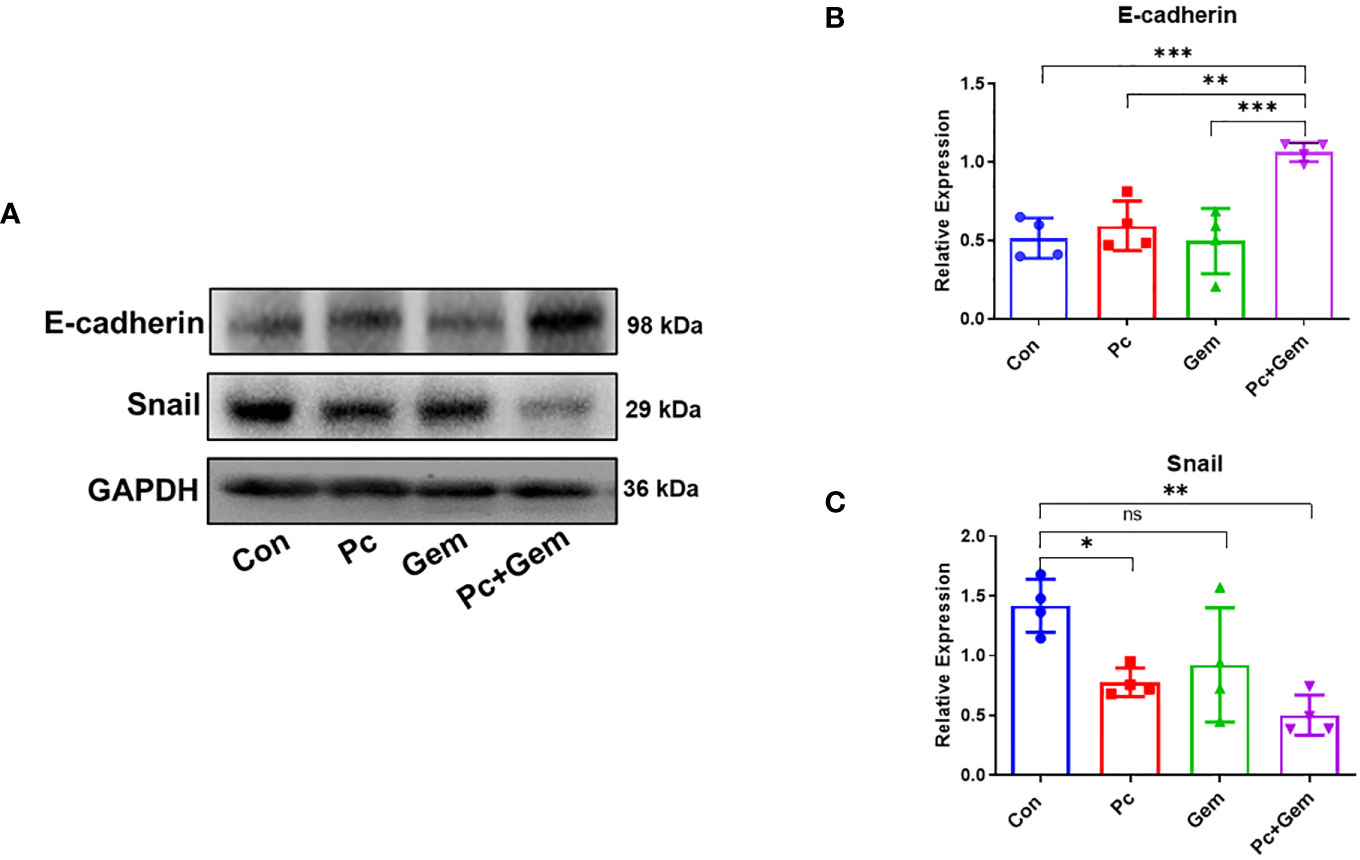
Figure 3 Pc+Gem significantly up-regulated E-cadherin and down-regulated Snail protein expression levels. The experimental scheme was shown in Figure 1A. (A) The results of Western blotting analysis of E-cadherin and Snail in LLC subcutaneous tumors on day 17 after tumor cells injection. (B) The relative expression of E-cadherin/GAPDH (n = 4). (C) The relative expression of Snail/GAPDH (n = 4). The statistical differences were analyzed with an unpaired two-tailed Student’s t-test. p values less than 0.05, 0.01, and 0.001 were considered statistically significant and indicated by *, ** and ***, respectively. p values more than 0.05 were considered statistically non-significantly and indicated by ns.
The combination of Pc and Gem significantly suppressed the activation of PI3K/Akt/GSK-3β signaling pathway
Some studies have indicated that the PI3K/Akt/GSK-3β signaling pathway plays an important role by affecting the activity of the transcription factor, Snail that is related to EMT in many types of tumors (20, 21). Therefore, we examined PI3K/Akt/GSK-3β protein expression levels in the tumors of these mice. The results of Western blotting analysis indicated that the phosphorylation level of PI3K in any treatment group (Pc, Gem, or Pc+Gem) was lower than that in the control group (p = 0.007, p = 0.04 and p = 0.02, respectively), but there were no significant differences between the treatment groups, suggesting that the combination of the two treatments had neither synergistic nor antagonistic effect on this molecule (Figures 4A, B). Similarly, the phosphorylation level of Akt in any treatment group (Pc, Gem, or Pc+Gem) was also lower than that in the control group (p = 0.02, p = 0.03 and p < 0.001, respectively). Furthermore, the phosphorylation level of Akt in Pc+Gem group was significantly lower than the Gem group (p = 0.02). At the same time, the expression level of phosphorylated Akt in the Pc+Gem group also appeared to be lower than the Pc group, but the difference between the two groups was not statistically significant. These results suggested that the combination of the two treatments had a synergistic effect on the expression of this molecule (Figures 4A, C). The phosphorylation level of GSK-3β in any of the treatment groups (Pc, Gem, or Pc+Gem) was lower than that in the control group (p = 0.02, p = 0.03 and p = 0.005, respectively), but there were no significant differences between the treatment groups, suggesting that the combination of the two treatments did not have a synergistic effect on the expression of this molecule, neither did it have an antagonistic effect (Figures 4A, D).
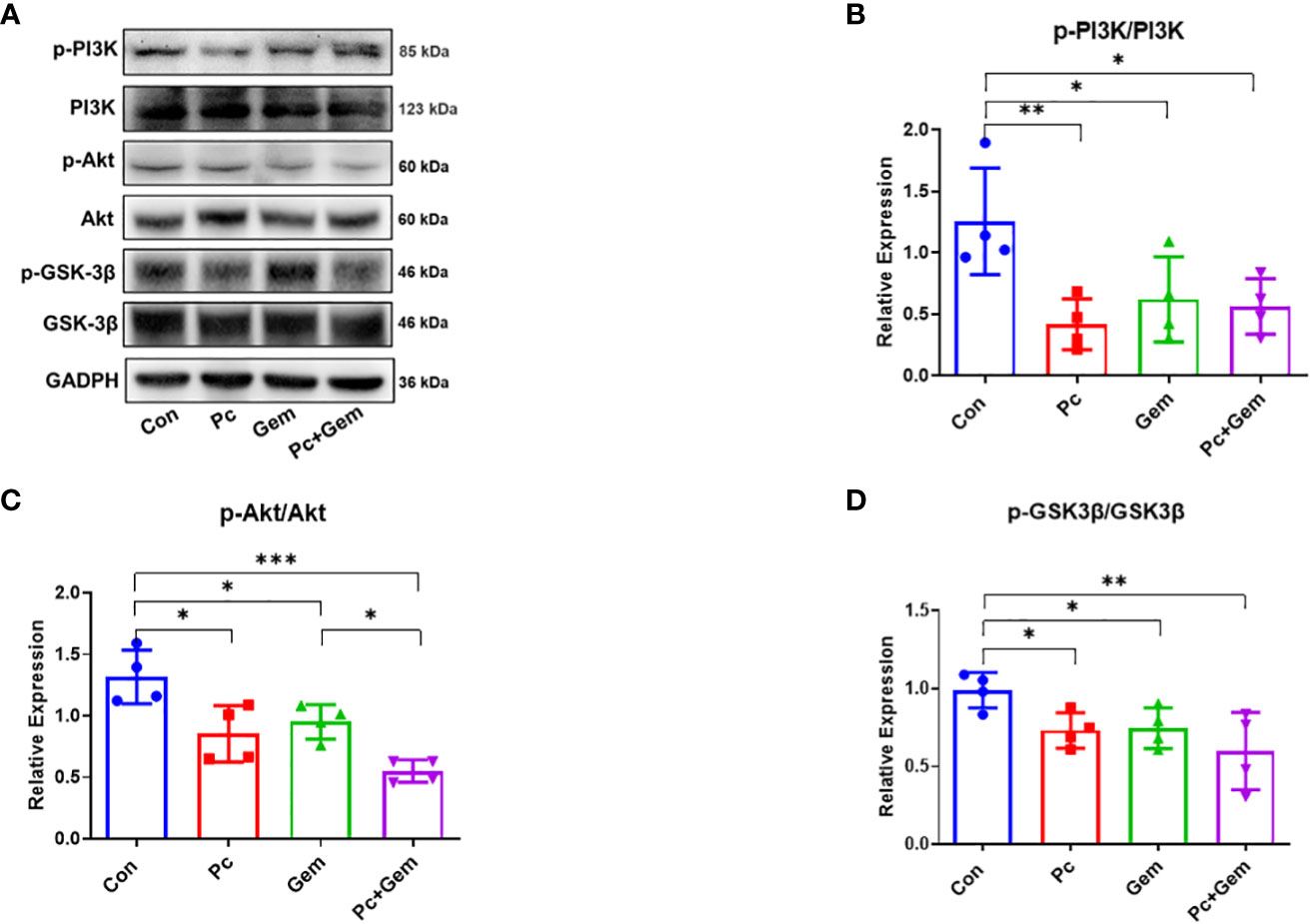
Figure 4 Pc+Gem significantly inhibited the activation of the PI3K/Akt/GSK-3β signaling pathway. The experimental scheme was shown in Figure 1A. (A) The results of Western blotting analysis of PI3K/Akt/GSK-3β in LLC subcutaneous tumors on day 17 after tumor cells injection. (B) The relative expression of p-PI3K/PI3K (n = 4). (C) The relative expression of p-Akt/Akt (n = 4). (D) The relative expression of p-GSK-3β/GSK-3β (n = 4). The statistical differences were analyzed with an unpaired two-tailed Student’s t-test. p values less than 0.05, 0.01, and 0.001 were considered statistically significant and indicated by *, ** and ***, respectively.
The combination of Pc and Gem significantly downregulated the expression of CXCR2/TGF-β
CXC-chemokine receptor 2 (CXCR2) has been demonstrated to have the ability to activate PI3K/Akt/GSK-3β/Snail signaling pathways and promote cell migration (25, 26). Therefore, we examined the expression of these molecules in tumor tissues of mice. The Western blotting results showed that there were no significant differences in CXCR2 protein expression level between any single treatment (Pc or Gem) group and the control group, but the level of this molecule in the Pc+Gem group was significantly lower than that in the control group (p = 0.03). In addition, the expression level of this molecule in the Pc+Gem group was also significantly lower than that in the Pc group (p = 0.02). These phenomena suggested that the combination of the two treatments had a synergistic effect on this molecule (Figures 5A, B). We further examined the expression level of TGF-β which has also been shown to promote EMT through PI3K/Akt/GSK-3β/Snail signaling (27). Our results indicated that the expression level of TGF-β protein in any treatment (Pc, Gem or Pc+Gem) group was significantly lower than that in the control group (p < 0.001, p = 0.03 and p = 0.003, respectively), but there were no significant differences between the treatment groups, suggesting that the combined treatment had no synergistic effect, neither did it show any antagonistic effect on the expression of this molecule (Figures 5A, C).
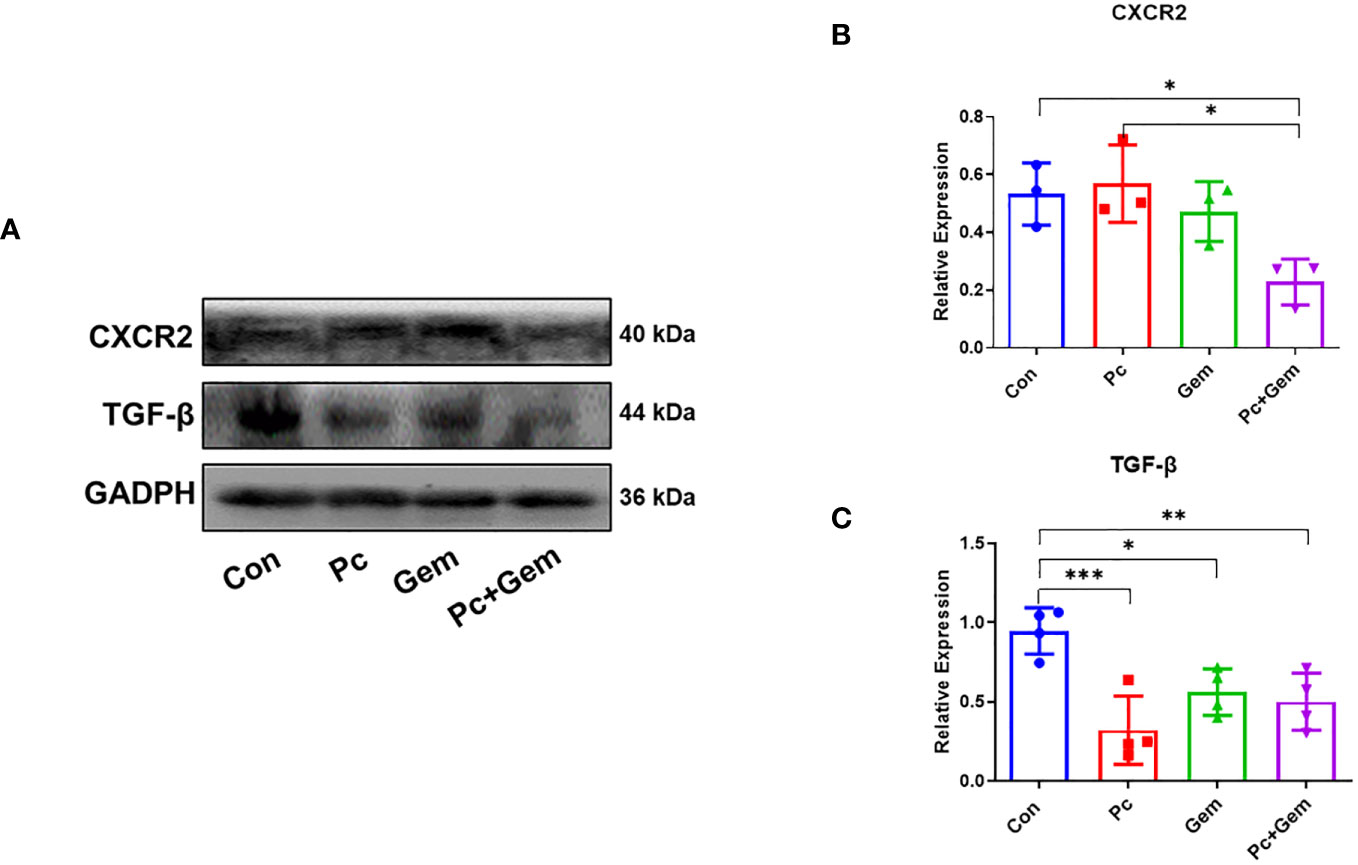
Figure 5 Pc+Gem significantly down-regulated the protein expression levels of CXCR2/TGF-β. The experimental scheme was shown in Figure 1A. (A) The results of Western blotting analysis of CXCR2/TGF-β in LLC subcutaneous tumors on day 17 after tumor cells injection. (B) The relative expression of CXCR2/GAPDH (n = 3). (C) The relative expression of TGF-β/GAPDH (n = 4). The statistical differences were analyzed with an unpaired two-tailed Student’s t-test. p values less than 0.05, 0.01, and 0.001 were considered statistically significant and indicated by *, ** and ***, respectively.
Discussion
Our current study indicates that the combination of Plasmodium (Pc) infection with Gem treatment has a synergistic effect on the inhibition of tumor growth and prolongation of survival in a subcutaneously inoculated mouse lung cancer model (Figure 1) and a synergistic effect on the inhibition of tumor metastasis and prolongation of survival in an intravenously inoculated mouse tumor model (Figure 2). Pc infection may contribute more than the Gem treatment in the inhibition of tumor metastasis (Figure 2B). These results are consistent with those of our previous studies that Plasmodium yoelii 17XNL (Py) infection significantly reduces tumor metastasis and recurrence after the original tumor is removed through operation in a murine liver cancer model (21), and that the combination of Py infection with DNA cancer vaccine treatment has a synergistic antitumor effect in a murine lung cancer model (12).
Studies have shown that tumor metastasis is highly related to EMT of tumor cells and the apparent characteristics of EMT are a downregulation of E-cadherin and an upregulation of Snail in tumor cells (17–19). Our current results have demonstrated that Pc infection in combination with Gem treatment significantly up-regulates the protein expression level of E-cadherin and down-regulates the protein expression level of Snail in tumor tissues (Figure 3), which indicates a significant inhibition of EMT. Even though Gem alone may induce EMT in patient-derived pancreatic ductal adenocarcinoma xenografts (28), we did not observe a similar effect represented by the expression levels of E-cadherin and Snail in our current murine lung cancer model (Figure 3).
Our previous study has suggested that Py infection inhibits PI3K/Akt/GSK-3β/Snail signaling pathway and therefore inhibits EMT in a murine liver cancer model (21). In our current study, we tested whether the combination of Pc and Gem or Pc infection alone or Gem treatment alone inhibited this pathway in a murine lung cancer model. The results show that the combination or Pc infection alone or Gem treatment alone indeed inhibits this pathway (Figure 4). Then we asked whether CCR10 mediated this pathway as demonstrated in our previous study of a liver cancer model infected with Py (21). The result suggested that the CCR10 protein level in lung cancer tissue was not affected by Pc infection, or Gem treatment or the combination of both (Figure S2). Then we tested the protein expression levels of CXCR2 and TGF-β, because some studies have indicated that CXCR2 can also mediate PI3K/Akt/GSK-3β/Snail signaling pathway (25, 26), and many researches have revealed that CXCR2 is also significant in the recruitment of different cells (tumor-associated macrophages, tumor-associated neutrophils, myeloid-derived suppressor cells, regulatory T cells) which constitute the tumor microenvironment (29–32). These components can cause EMT by regulating TGF-β secretion which can be involved in PI3K/Akt/GSK-3β pathway (27). The results show that the combination of Pc and Gem treatment significantly downregulates the protein level of CXCR2 compared with the control group, but Pc alone or Gem alone does not affect its level (Figures 5A, B). Finally, our current results show that Pc infection alone or Gem treatment alone or the combination of both significantly inhibits the protein expression level of TGF-β (Figures 5A, C). Based on the results mentioned above, we summarize the possible mechanism of action of Pc infection in combination with Gem treatment on EMT of tumor cells in murine lung cancer models as shown in Figure 6.
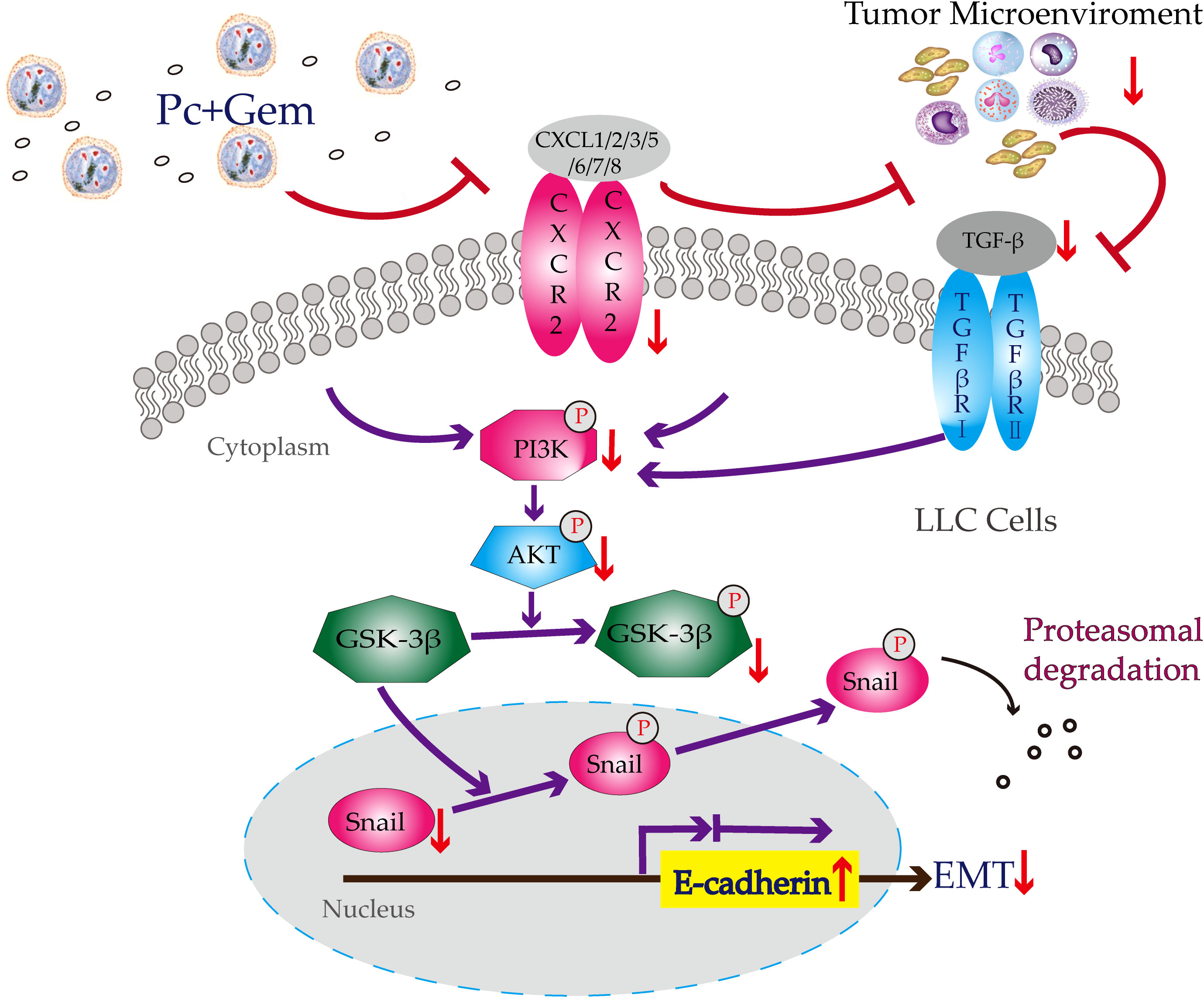
Figure 6 Potential mechanism of action of Pc+Gem in the inhibition of tumor cell EMT in murine Lewis lung cancer models. Pc+Gem induces: 1) down-regulation of CXCR2/TGF-β; 2) down-regulation of p-PI3K, but not PI3K, suggesting the inhibition of the phosphorylase of PI3K; 3) downregulation of p-Akt, but not Akt, suggesting the inhibition of the phosphorylase of Akt; 4) down-regulation of p-GSK-3β, but not GSK-3β, suggesting the inhibition of the phosphorylase of GSK-3β; 5) down-regulation of Snail; 6) up-regulation of E-cadherin. The down-regulation of Snail and up-regulation of E-cadherin are the main characteristics of suppression of the EMT in murine LLC models.
Studies have suggested that high EMT score of tumor cells promotes immune evasion, while low EMT score promotes antitumor immune response, and vice versa, an effective antitumor immune response can inhibit EMT and kill tumor cells at the same time (33–35). Other studies have pointed out that STAT3 signaling plays an important role in the occurrence and development of EMT, and the interaction between STAT3 and TGF-β not only induces EMT, but also serves as the central regulatory signal molecules for the formation of immunosuppressive network in the tumor microenvironment (36–39). Our previous study in mouse tumor models shows that Plasmodium infection significantly downregulates the phosphorylated level of STAT3 (pSTAT3) within MDSCs in tumor tissues and the expression level of TGF-β in tumor tissues, thus releasing the tumor immunosuppressive microenvironment (15). Combined with the findings of current study, namely, Plasmodium infection blocks TGF-β-induced EMT, we believe that the activation of the immune system of the tumor-bearing host, the relief of the immunosuppressive state, and the inhibition of EMT by Plasmodium infection, are highly unified because they share certain signaling pathways and key targets. By inhibiting these targets and pathways, Plasmodium infection can promote the body’s anti-tumor immune response, inhibit tumor growth and metastasis, and thus prolong the life of tumor-bearing hosts.
However, there have been many conflicting results and controversies regarding whether Plasmodium infection suppresses or activates the immune system (40–42). Urban BC et al. proposed a hypothesis to reconcile these conflicting results, where they suggested that high density of parasitemia or high concentration of malaria pigment (hemozoin) inhibited dendritic cells (DC) and downstream T and B cells, while low density of parasitemia or low concentration of hemozoin activated DC and its downstream immune cells (43). Nevertheless, what we observed in Plasmodium-infected tumor-bearing mice is inconsistent with this hypothesis. First, we compared the parasitemia induced by Py and Pc. In both tumor-free mice and lung cancer-bearing mice, the parasite density (red blood cell infection rate) induced by Py was significantly higher than that induced by Pc, and the duration of high parasitemia was significantly longer in Py than in Pc (Figure S3). Then, we compared the inhibitory effect of Py and Pc on lung cancer in mice and found that the effect of Py was significantly better than that of Pc (Figure S4). Finally, we observed a positive correlation of parasite density with spleen size (represents the degree of immune response to the infection) and a negative correlation of parasite density with tumor size (Figure S5), suggesting that Plasmodium infection could induce antitumor immune response in a parasitemia-dependent manner. In fact, one of the best ways to test whether Plasmodium infection activates or suppresses the immune system is to observe it in a tumor-bearing host, because the immune system of the tumor-bearing host is inhibited by tumor cells, if it suppresses the immune system, it would promote tumor growth, if it activates the immune system, it would inhibit tumor growth. In brief, what we observed in the tumor-bearing hosts is that Plasmodium infection generally activates rather than suppresses the immune system, when the immune system has already been suppressed by the tumor cells. This has also been preliminarily demonstrated in our clinical trial of Plasmodium immunotherapy for advanced solid tumors. We used a relatively benign form of human parasite, namely, Plasmodium vivax (Pv), in clinical trials, and the parasitemia level of natural Pv infection in humans is fairly low compared to Py or Pc in mice (44). Furthermore, to ensure the safety of clinical trials, we used artesunate to control the parasitemia to very low levels (0.1% or less), and the low parasitemia has been shown to activate the immune system of patients with advanced cancer (45) and therefore has effect on the treatment of clinical tumors (unpublished data). Our clinical data preliminarily suggest that high parasitemia not only increases toxicity, but is also positively associated with poor prognosis in cancer patients (unpublished data), so we must strictly control the infection rate of the parasite. It is very important that the parasite that we have selected is a strain of Plasmodium vivax that is sensitive to all current antimalarial drugs, especially artesunate, and a single low dose of artesunate administered intravenously in a very short period of time can control the parasite density to less than 0.1%, so the side effects of Plasmodium immunotherapy are limited and manageable.
It is worthy of note that in our previous study, Py infection inhibits the CCR10-mediated PI3K/Akt/GSK-3β/Snail signaling pathway in a murine liver cancer model, but in our current study, Pc infection inhibits the CXCR2/TGF-β-mediated PI3K/Akt/GSK-3β/Snail signaling pathway in murine lung cancer models. There are two possible reasons for these differences. (1) Different murine cancer models: liver cancer versus lung cancer; (2) different murine Plasmodium parasites: Py versus Pc. This merits further study.
A series of previous studies conducted by us have shown that Plasmodium infection plays anticancer roles through multiple targets and multiple pathways, for example, it activates the innate and adaptive antitumor immune responses (12, 46); systematically removes the tumor immunosuppressive microenvironment through Plasmodium-associated exosomes that inhibit tumor cell secretion of cytokines and chemokines which have the ability to recruit the precursors of immune suppressor cells including myeloid-derived suppressor cells (MDSC) and regulatory T cells (Treg) into tumor tissue, thereby significantly down-regulating the number of these cells and inhibiting their function through undefined mechanisms (15); inhibits tumor angiogenesis through micro-RNA (miRNA) 16/322/497/17 within exosomes and long noncoding RNA (lncRNA F66), both of which target VEGF/VEGFR2 pathway via different mechanisms of action or through changing the functional phenotype of tumor-associated macrophages (TAM) via engulfing hemozoin that blocks IGF-1/MMP9 signal pathways (13, 14, 47), and inhibits EMT of tumor cells (21). By comparing Plasmodium immunotherapy with a single-target anticancer immunotherapy known as immune checkpoint blockade, we propose the notion that Plasmodium immunotherapy is an ecological therapy that systemically targets cancer as an ecological disease (45). However, Plasmodium immunotherapy also has an obvious shortage, that is, the specific killing of tumor cells is relatively weak, therefore, needs to be combined with other therapies to further improve its efficacy. Our current study has preliminarily confirmed that Plasmodium (Pc) infection combined with Gem treatment has a synergistic effect on the inhibition of EMT and tumor metastasis, and the prolongation of survival in lung cancer-bearing mice, without significantly enhancing their toxicity (Figure S6). Since Gem treatment induces immunogenic death of cancer cells (48) and inhibits MDSC, Treg, and TGF-β (49), Pc infection in combination with Gem may also have synergistic effects on immune killing of tumor cells. This merits further study. Nevertheless, since both Plasmodium infection and Gem treatment exert anticancer effects through multiple targets and multiple pathways, their synergistic effects on the overall anticancer effect, that is, inhibiting tumor growth and metastasis and prolonging the life span of tumor-bearing mice, do not mean that they must have synergistic effects on every signaling pathway and every target. Importantly, antagonism between them was not observed throughout the study.
There are some shortcomings in this study: we were unable to use some specific gene knockout mice for experiments. For example, IFN-γ, an inflammatory cytokine, is important for both anti-tumor immunity (50, 51) and anti-Plasmodium immunity, especially for its antagonistic effect on anti-inflammatory cytokine TGF-β (52). However, IFN-γ knockout mice would die when infected with Plasmodium parasites (53). Therefore, we are currently designing single-cell transcriptome studies to look for important target genes for Plasmodium immunotherapy.
In summary, we report for the first time the antitumor results of Pc alone and the synergistic effects of Pc combined with Gem in murine lung cancer models in our present study. These results indicate that Pc infection in combination with Gem treatment significantly inhibits tumor growth and metastasis, and prolongs the survival of lung cancer-bearing mice partially through inhibiting EMT of tumor cells that is possibly related to the blockade of CXCR2/TGF-β-mediated PI3K/Akt/GSK-3β/Snail signaling pathway. Based on the results of our series of preclinical studies in murine tumor models and our epidemiological data analysis showing a significant negative correlation between global malaria incidence and tumor mortality (54), clinical trials of Plasmodium immunotherapy for advanced cancer have been approved and are ongoing in China (NCT02786589, NCT03474822 and NCT04165590). Our current study provides a candidate for future clinical trials of Plasmodium immunotherapy in combination with other therapies for the treatment of cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was approved by Guangzhou Institutes of Biomedicine and Health. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
XC and ZT conceived, planned and carried out this work. YL, MM, DA, LC, WD, XL, LD, SF, and SZ helped in the animal experiments. WH, DW, ZD, and FZ performed consultation and analyzed the results. LQ, XPC, and ZY supervised the research project. XPC and ZY revised the manuscript. All authors discussed the results and contributed to this work. All authors read and approved the final manuscript.
Funding
This work was supported by grants from the Open Project of the State Key Laboratory Respiratory Disease (grant number: SKLRD-OP-201802), Program Grant of Guangzhou Innovation Leading Team in Sciences and Technologies (grant number: 201909010007) and the Applied Basic Research Projects of Yunnan Province (grant number: 202101AY070001-084).
Conflict of interest
Authors ZT, WD, SZ, WH, FZ, and XPC were employed by CAS-Lamvac Guangzhou Biomedical Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1181176/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Zheng YW, Li RM, Zhang XW, Ren XB. Current adoptive immunotherapy in non-small cell lung cancer and potential influence of therapy outcome. Cancer Invest (2013) 31(3):197–205. doi: 10.3109/07357907.2013.775294
3. Chen VW, Ruiz BA, Hsieh MC, Wu XC, Ries LA, Lewis DR. Analysis of stage and clinical/prognostic factors for lung cancer from SEER registries: AJCC staging and collaborative stage data collection system. Cancer (2014) 120 Suppl 23:3781–92. doi: 10.1002/cncr.29045
4. Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev (2018) 65:1–10. doi: 10.1016/j.ctrv.2018.02.006
5. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science (2013) 342(6165):1432–3. doi: 10.1126/science.342.6165.1432
6. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med (2016) 375(18):1749–55. doi: 10.1056/NEJMoa1609214
7. Kanai O, Nakatani K, Fujita K, Okamura M, Mio T. Concurrence of nivolumab-induced interstitial lung disease and cancer invasion. Respirol Case Rep (2017) 5(6):e00257. doi: 10.1002/rcr2.257
8. Marin-Acevedo JA, Dholaria B, Soyano AE, Knutson KL, Chumsri S, Lou Y. Next generation of immune checkpoint therapy in cancer: new developments and challenges. J Hematol Oncol (2018) 11(1):39. doi: 10.1186/s13045-018-0582-8
9. Stevenson MM, Riley EM. Innate immunity to malaria. Nat Rev Immunol (2004) 4(3):169–80. doi: 10.1038/nri1311
10. Dobbs KR, Crabtree JN, Dent AE. Innate immunity to malaria-The role of monocytes. Immunol Rev (2020) 293(1):8–24. doi: 10.1111/imr.12830
11. Kurup SP, Butler NS, Harty JT. T cell-mediated immunity to malaria. Nat Rev Immunol (2019) 19(7):457–71. doi: 10.1038/s41577-019-0158-z
12. Chen L, He Z, Qin L, Li Q, Shi X, Zhao S, et al. Antitumor effect of malaria parasite infection in a murine Lewis lung cancer model through induction of innate and adaptive immunity. PloS One (2011) 6(9):e24407. doi: 10.1371/journal.pone.0024407
13. Yang Y, Liu Q, Lu J, Adah D, Yu S, Zhao S, et al. Exosomes from Plasmodium-infected hosts inhibit tumor angiogenesis in a murine Lewis lung cancer model. Oncogenesis (2017) 6(6):e351. doi: 10.1038/oncsis.2017.52
14. Qin L, Zhong M, Adah D, Qin L, Chen X, Ma C, et al. A novel tumour suppressor lncRNA F630028O10Rik inhibits lung cancer angiogenesis by regulating miR-223-3p. J Cell Mol Med (2020) 24(6):3549–59. doi: 10.1111/jcmm.15044
15. Adah D, Yang Y, Liu Q, Gadidasu K, Tao Z, Yu S, et al. Plasmodium infection inhibits the expansion and activation of MDSCs and Tregs in the tumor microenvironment in a murine Lewis lung cancer model. Cell Commun Signal (2019) 17(1):32. doi: 10.1186/s12964-019-0342-6
16. Suzuki E, Sun J, Kapoor V, Jassar AS, Albelda SM. Gemcitabine has significant immunomodulatory activity in murine tumor models independent of its cytotoxic effects. Cancer Biol Ther (2007) 6(6):880–5. doi: 10.4161/cbt.6.6.4090
17. Brabletz T, Kalluri R, Nieto MA, Weinberg RA. EMT in cancer. Nat Rev Cancer (2018) 18(2):128–34. doi: 10.1038/nrc.2017.118
18. Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, et al. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature (2015) 525(7568):256–60. doi: 10.1038/nature14897
19. Alizadeh AM, Shiri S, Farsinejad S. Metastasis review: from bench to bedside. Tumour Biol (2014) 35(9):8483–523. doi: 10.1007/s13277-014-2421-z
20. Zhang B, Yin C, Li H, Shi L, Liu N, Sun Y, et al. Nir1 promotes invasion of breast cancer cells by binding to chemokine (C-C motif) ligand 18 through the PI3K/Akt/GSK3β/Snail signalling pathway. Eur J Cancer (2013) 49(18):3900–13. doi: 10.1016/j.ejca.2013.07.146
21. Liang Y, Chen X, Tao Z, Ma M, Adah D, Li X, et al. Plasmodium infection prevents recurrence and metastasis of hepatocellular carcinoma possibly via inhibition of the epithelial−mesenchymal transition. Mol Med Rep (2021) 23(6). doi: 10.3892/mmr.2021.12057
22. Nieto MA, Huang RY, Jackson RA, Thiery JP. EMT: 2016. Cell (2016) 166(1):21–45. doi: 10.1016/j.cell.2016.06.028
23. Campbell K, Casanova J. A role for E-cadherin in ensuring cohesive migration of a heterogeneous population of non-epithelial cells. Nat Commun (2015) 6:7998. doi: 10.1038/ncomms8998
24. Dong C, Wu Y, Wang Y, Wang C, Kang T, Rychahou PG, et al. Interaction with Suv39H1 is critical for Snail-mediated E-cadherin repression in breast cancer. Oncogene (2013) 32(11):1351–62. doi: 10.1038/onc.2012.169
25. Zhou SL, Zhou ZJ, Hu ZQ, Li X, Huang XW, Wang Z, et al. CXCR2/CXCL5 axis contributes to epithelial-mesenchymal transition of HCC cells through activating PI3K/Akt/GSK-3β/Snail signaling. Cancer Lett (2015) 358(2):124–35. doi: 10.1016/j.canlet.2014.11.044
26. Zhou Y, Shurin GV, Zhong H, Bunimovich YL, Han B, Shurin MR. Schwann cells augment cell spreading and metastasis of lung cancer. Cancer Res (2018) 78(20):5927–39. doi: 10.1158/0008-5472.CAN-18-1702
27. Gonzalez DM, Medici D. Signaling mechanisms of the epithelial-mesenchymal transition. Sci Signal (2014) 7(344):re8. doi: 10.1126/scisignal.2005189
28. Bulle A, Dekervel J, Libbrecht L, Nittner D, Deschuttere L, Lambrecht D, et al. Gemcitabine induces Epithelial-to-Mesenchymal Transition in patient-derived pancreatic ductal adenocarcinoma xenografts. Am J Transl Res (2019) 11(2):765–79.
29. Zhang M, Huang L, Ding G, Huang H, Cao G, Sun X, et al. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J Immunother Cancer (2020) 8(1). doi: 10.1136/jitc-2019-000308
30. Chao T, Furth EE, Vonderheide RH. CXCR2-dependent accumulation of tumor-associated neutrophils regulates T-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol Res (2016) 4(11):968–82. doi: 10.1158/2326-6066.CIR-16-0188
31. Lv M, Xu Y, Tang R, Ren J, Shen S, Chen Y, et al. miR141-CXCL1-CXCR2 signaling-induced Treg recruitment regulates metastases and survival of non-small cell lung cancer. Mol Cancer Ther (2014) 13(12):3152–62. doi: 10.1158/1535-7163.MCT-14-0448
32. Li BH, Garstka MA, Li ZF. Chemokines and their receptors promoting the recruitment of myeloid-derived suppressor cells into the tumor. Mol Immunol (2020) 117:201–15. doi: 10.1016/j.molimm.2019.11.014
33. Terry S, Savagner P, Ortiz-Cuaran S, Mahjoubi L, Saintigny P, Thiery JP, et al. New insights into the role of EMT in tumor immune escape. Mol Oncol (2017) 11(7):824–46. doi: 10.1002/1878-0261.12093
34. Chockley PJ, Keshamouni VG. Immunological consequences of epithelial-mesenchymal transition in tumor progression. J Immunol (2016) 197(3):691–8. doi: 10.4049/jimmunol.1600458
35. Chou MY, Yang MH. Interplay of immunometabolism and epithelial-mesenchymal transition in the tumor microenvironment. Int J Mol Sci (2021) 22(18). doi: 10.3390/ijms22189878
36. Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol (2014) 44(5):1643–51. doi: 10.3892/ijo.2014.2310
37. Hu R, Han Q, Zhang J. STAT3: A key signaling molecule for converting cold to hot tumors. Cancer Lett (2020) 489:29–40. doi: 10.1016/j.canlet.2020.05.035
38. Sadrkhanloo M, Entezari M, Orouei S, Ghollasi M, Fathi N, Rezaei S, et al. STAT3-EMT axis in tumors: Modulation of cancer metastasis, stemness and therapy response. Pharmacol Res (2022) 182:106311. doi: 10.1016/j.phrs.2022.106311
39. Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer (2009) 9(11):798–809. doi: 10.1038/nrc2734
40. Millington OR, Di Lorenzo C, Phillips RS, Garside P, Brewer JM. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. J Biol (2006) 5(2):5. doi: 10.1186/jbiol34
41. Amorim KN, Chagas DC, Sulczewski FB, Boscardin SB, et al. Dendritic cells and their multiple roles during malaria infection. J Immunol Res (2016) 2016:2926436. doi: 10.1155/2016/2926436
42. Osii RS, Otto TD, Garside P, Ndungu FM, Brewer JM. The impact of malaria parasites on dendritic cell-T cell interaction. Front Immunol (2020) 11:1597. doi: 10.3389/fimmu.2020.01597
43. Urban BC, Todryk S. Malaria pigment paralyzes dendritic cells. J Biol (2006) 5(2):4. doi: 10.1186/jbiol37
44. Fink J, Jones PD. High prevalence of low-level parasitemia with plasmodium vivax in makira-ulawa province presents a challenge for the diagnosis and eradication of malaria in Solomon Islands. Ochsner J (2021) 21(1):76–80. doi: 10.31486/toj.20.0023
45. Chen X, Qin L, Hu W, Adah D. The mechanisms of action of Plasmodium infection against cancer. Cell Commun Signal (2021) 19(1):74. doi: 10.1186/s12964-021-00748-5
46. Pan J, Ma M, Qin L, Kang Z, Adah D, Tao Z, et al. Plasmodium infection inhibits triple negative 4T1 breast cancer potentially through induction of CD8(+) T cell-mediated antitumor responses in mice. BioMed Pharmacother (2021) 138:111406. doi: 10.1016/j.biopha.2021.111406
47. Wang B, Li Q, Wang J, Zhao S, Nashun B, Qin L, et al. Plasmodium infection inhibits tumor angiogenesis through effects on tumor-associated macrophages in a murine implanted hepatoma model. Cell Commun Signal (2020) 18(1):157. doi: 10.1186/s12964-020-00570-5
48. Zhang X, Wang D, Li Z, Jiao D, Jin L, Cong J, et al. Low-dose gemcitabine treatment enhances immunogenicity and natural killer cell-driven tumor immunity in lung cancer. Front Immunol (2020) 11:331. doi: 10.3389/fimmu.2020.00331
49. Eriksson E, Wenthe J, Irenaeus S, Loskog A, Ullenhag G. Gemcitabine reduces MDSCs, tregs and TGFβ-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Transl Med (2016) 14(1):282. doi: 10.1186/s12967-016-1037-z.
50. Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U.S.A. (1998) 95(13):7556–61. doi: 10.1073/pnas.95.13.7556
51. Takeda K, Nakayama M, Hayakawa Y, Kojima Y, Ikeda H, Imai N, et al. IFN-γ is required for cytotoxic T cell-dependent cancer genome immunoediting. Nat Commun (2017) 8:14607. doi: 10.1038/ncomms14607
52. Omer FM, de Souza JB, Riley EM. Differential induction of TGF-beta regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. J Immunol (2003) 171(10):5430–6. doi: 10.4049/jimmunol.171.10.5430
53. Su Z, Stevenson MM. Central role of endogenous gamma interferon in protective immunity against blood-stage Plasmodium chabaudi AS infection. Infect Immun (2000) 68(8):4399–406. doi: 10.1128/IAI.68.8.4399-4406.2000
Keywords: Plasmodium immunotherapy, Plasmodium chabaudi ASS, gemcitabine, anticancer effect, synergism, mouse lung cancer model
Citation: Chen X, Tao Z, Liang Y, Ma M, Adah D, Ding W, Chen L, Li X, Dai L, Fanuel S, Zhao S, Hu W, Wu D, Duan Z, Zhou F, Qin L, Chen X and Yang Z (2023) Plasmodium immunotherapy combined with gemcitabine has a synergistic inhibitory effect on tumor growth and metastasis in murine Lewis lung cancer models. Front. Oncol. 13:1181176. doi: 10.3389/fonc.2023.1181176
Received: 07 March 2023; Accepted: 18 September 2023;
Published: 17 October 2023.
Edited by:
Qun Xue, Affiliated Hospital of Nantong University, ChinaCopyright © 2023 Chen, Tao, Liang, Ma, Adah, Ding, Chen, Li, Dai, Fanuel, Zhao, Hu, Wu, Duan, Zhou, Qin, Chen and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaoqing Yang, eWFuZ3poYW9xaW5nMUBrbW11LmVkdS5jbg==; Xiaoping Chen, Y2hlbl94aWFvcGluZ0BnaWJoLmFjLmNu; Li Qin, cWluX2xpQGNhcy1sYW12YWMuY29t
†These authors have contributed equally to this work
 Xiao Chen
Xiao Chen Zhu Tao
Zhu Tao Yun Liang2,4
Yun Liang2,4 Meng Ma
Meng Ma Lili Chen
Lili Chen Ziyuan Duan
Ziyuan Duan Fang Zhou
Fang Zhou Xiaoping Chen
Xiaoping Chen