- 1Department of Pediatrics and Pediatric Hematology/Oncology, University Children’s Hospital, Carl von Ossietzky University Oldenburg, Klinikum Oldenburg AöR, Oldenburg, Germany
- 2Department of Particle Therapy, University Hospital Essen, West German Proton Therapy Centre Essen (WPE), West German Cancer Center (WTZ), Essen, Germany
- 3Department of Pediatrics, Faculty of Medicine, Srinakharinwirot University, Bangkok, Thailand
- 4Department of Pediatric Hematology/Oncology, University of Bonn Medical Center, Bonn, Germany
- 5Institute of Biostatistics and Clinical Research, University of Münster, Münster, Germany
- 6Department of Radiotherapy and Radiation Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, Technische Universität Dresden, Dresden, Germany
- 7Diagnostic and Interventional Neuroradiology, Faculty of Medicine, University of Augsburg, Augsburg, Germany
- 8Department of Radiation Oncology, Heidelberg University Hospital, Heidelberg, Germany
- 9National Center for Tumor Diseases (NCT) Dresden with German Cancer Research Center (DKFZ), University Hospital and Faculty of Medicine Dresden, Helmholtz-Zentrum Dresden – Rossendorf, Dresden, Germany
- 10German Cancer Consortium (DKTK), Dresden and German Cancer Research Center (DKFZ) Heidelberg, Dresden, Germany
- 11Department of Particle Therapy, University Hospital Essen, West German Proton Therapy Centre Essen (WPE), West German Cancer Centre (WTZ) and German Cancer Consortium (DKTK), Essen, Germany
Background: Proton beam therapy (PBT) is being increas16ingly used to treat residual craniopharyngioma (CP) after hypothalamus-sparing surgery. Compared to photon-based radiation therapy (XRT) with PBT, less irradiation in the penumbra reduces the scattered dose to critical organs neighboring but outside the area of treatment, minimizing the risk of sequelae.
Patients and methods: Between 2007 and 2019, 99 of 290 (34%) childhood-onset CP patients recruited in KRANIOPHARYNGEOM 2007 received external radiation therapy (RT) (65% PBT, 35% XRT). Outcome was analyzed in terms of survival, endocrinological and anthropometric parameters (BMI and height SDS), quality of life (QoL using PEDQOL), and functional capacity (FMH) with special regard to irradiation technique.
Results: PBT became predominant (used in 43% and 72% of all irradiated patients registered within the first and second halves of the recruitment period, between 2008 and 2013 and 2013 and 2018, respectively). Five-year event-free survival rates after PBT or XRT were comparable (92% ± 4% vs. 91% ± 4%, p = 0.42) and higher than for the whole cohort since diagnosis, including non-RT patients (37% ± 4%). Radiation doses to the hypothalamus and pituitary did not differ between PBT and XRT. Endocrine deficits due to disturbances of the hypothalamic-pituitary axis (HPA) were already common before irradiation. During the first 5 years after CP diagnosis/RT, no differences between PBT, XRT, and non-RT CP patients concerning functional capacity and anthropometric parameters have been obtained. Only for the PEDQOL domain “physical function”, parental-assessed QoL was lower 12 months after PBT versus XRT or non-RT patients.
Conclusion: QoL, functional capacity, degree of obesity, and endocrinopathy varied over time from diagnosis, but by 5 years, there was no significant difference between PBT and XRT upfront or delayed, nor was there any compromise in historic survival rates, which remained high >90%. RT of any type is extremely effective at stabilizing disease after hypothalamic-sparing surgery. The purported specific benefits of PBT-reducing sequelae are not proven in this study where the organ of critical interest is itself diseased, increasing an urgent need to better address and treat the tumor-induced endocrine harm from diagnosis in dedicated pituitary services. Other hypothesized benefits of PBT versus XRT on vascular events and secondary cancers await longer comparison.
Clinical trial registration number: https://clinicaltrials.gov/study/, identifier NCT01272622.
Introduction
Although craniopharyngiomas (CP) are slow-growing WHO °I tumors of the sellar and parasellar region (1), they may infiltrate adjacent structures, causing relevant morbidity and mortality (2). Previous studies show that attempts at gross-total resection (GTR) in patients with tumors invading the hypothalamus can result in significant morbidity in terms of hypothalamic dysfunction and altered neurophysiological and neuropsychological profiles (3–7). KRANIOPHARYNGEOM 2007 is a prospective multicenter trial in children and adolescents diagnosed with CP at an age younger than 18 years and treated with a risk-adapted multimodality strategy using a preoperative radiological grading system to avoid significant morbidity (8). In hypothalamic involvement grade 0/1 (no involvement of anterior hypothalamic structures in front of mammillary bodies on MRI), GTR is recommended, whereas in hypothalamic involvement grade 2 (involvement of posterior hypothalamic nuclei including mammillary bodies and structures beyond these), a subtotal tumor resection (STR) is aimed to keep the integrity of hypothalamic structures (9–12).
Incomplete surgery is associated with CP progression in 50%–91%, leading to their eligibility of these patients for postoperative radiation therapy (RT) if repeated surgery should be avoided (13). The optimal timing for RT after STR with a significant residual tumor remains an open question. In KRANIOPHARYNGEOM 2007, a subgroup of patients aged >5 years at diagnosis with postoperative residual tumors were randomized for immediate RT or RT at progression, showing no meaningful differences in quality of life (QoL) (8). We reported that for the whole cohort, RT was very useful in preventing further progression after STR, and the hypothalamus-sparing treatment strategy was associated with a higher long-term QoL (8).
Different RT techniques were used for the adjuvant treatment of CP in the KRANIOPHARYNGEOM 2007 trial. The advantage of proton beam therapy (PBT) is the lower dose to critical organs, as shown by treatment planning studies, which may decrease the risk of neurocognitive, vascular, and optic nerve complications and second cancers (14–16). However, studies comparing PBT and XRT in one large patient cohort are scarce.
Herein, we analyzed the use of different RT techniques within the KRANIOPHARYNGEOM 2007 cohort and whether PBT compared to XRT or no adjuvant RT influenced outcome parameters for survival and hypothalamic syndrome.
Materials and methods
Patients
Between 2007 and 2019, 290 patients diagnosed with childhood-onset adamantinomatous CP at an age ≤18 years have been enrolled in the trial KRANIOPHARYNGEOM 2007 (NCT01272622). The surgical approach was not part of the KRANIOPHARYNGEOM 2007 trial, but a hypothalamic sparing technique according to the preoperative radiological grading was recommended (8). Confirmation of the histological diagnosis adamantinomatous CP by pathological reference assessment was mandatory. Data on histological and molecular tumor characteristics, treatment, early and late toxicities, and tumor control was prospectively collected. For this cross-sectional study, trial data on physical condition, anthropometric parameters, clinical manifestations, imaging results, resection status, and RT (technique, dosage, age at initiation) has been analyzed. Endocrine deficiencies were assessed preoperative, 6 months postoperative, and then once yearly and interpreted by the local endocrinologist using local center reference values.
Neuroimaging
Cranial MRIs were performed at the time of CP diagnosis and after initial diagnosis/progression prospectively every 3 months in the first year and then every 6 to 12 months for at least 10 years of follow-up using the guidelines of the KRANIOPHARYNGEOM 2007 protocol (8). A central imaging review by an experienced neuroradiologist (B.B.) blinded for clinical and surgical information included assessment of CP location, preoperative hypothalamic involvement (HI), surgical hypothalamic lesions (HL), and postoperative residual tumor on pre- and postoperative axial, coronal, and sagittal planes of magnetic resonance imaging (MRI). CP location was classified according to the degree of HI: grade 0, no HI; HI grade 1, involving anterior hypothalamic structures and not mammillary bodies and hypothalamic structures dorsal of those; HI grade 2, involving both anterior + posterior hypothalamic areas, i.e., mammillary bodies and areas dorsal of those (8, 10, 17). Surgical HLs were graded according to the same criteria in three categories: HL grade 0, no HL; HL grade 1, anterior HL; and HL grade 2, anterior + posterior HL. The tumor size calculation of CP was performed using the formula “½ (A × B × C)” (aligned to the ellipsoid model: 4/3 π [A/2 × B/2 × C/2]), with A, B, and C being the maximum dimensions in the standard planes: axial (transverse, A), coronal (cranio-caudal, B), and sagittal (antero-posterior, C). A complete resection needed to be confirmed by centrally reviewed MRI.
Radiotherapy
RT was performed between 3 and 12 weeks postoperatively, or as soon as possible after the diagnosis of recurrence if no repeat surgery was performed. All patients were treated once a day, five times a week, with a fraction of 1.8 Gy per session. The total dose was 54.0 Gy. In general, CT-based therapy planning was recommended, allowing the evaluation of the integral dose within the target volumes and organs at risk. The clinical target volume corresponded to the areas of preoperative tumor contact adjusted for current anatomy, including the residual tumor extent shown on postoperative imaging, with a safety margin of 0.5 cm. The planning target volume captured the clinical target volume depending on the geometric precision of the technique used, typically 0.3–0.5 mm. The following organs at risk should have been contoured to create the integral dose distribution: brainstem, chiasm, pituitary gland, optic nerve, bulbus, lens, thalamus, hypothalamus, and inner ear. The planning target volume and the organs at risk were defined by image fusion with a diagnostic MRI not older than 21 days before the CT-based planning. The three-dimensional conformation technique with standing fields was commonly applied. A convergence technique was also used if the clinical target volume was spherical in configuration. In this situation, the dose specification was based on the specifications for stereotactic one-time therapy. The dose specifications were based on the ICRU 50/62 report. If possible, the target volume should be irradiated within the tolerance ranges of 95%–107%. The dose maximum and minimum within the target volume as well as possible dose peaks “hot spots” (dose maximum outside the target volume) were specified.
Anthropometric parameters
Standing height was measured in triplicate, of which the median was calculated as height SDS according to the Prader et al. references (18). Patients were weighed on calibrated electronic step-scales. The degree of obesity was derived from the body mass index (BMI) SDS (w/h2; w = weight (kg), h = height (m)) according to the reference of Rolland-Cachera et al. (19).
Quality-of-life questionnaire
In CP patients diagnosed at an age ≥5 years, health-related QoL was analyzed using the Pediatric Quality of Life (PEDQOL) (8, 20) questionnaire at time points 1, 3, and 5 years after CP diagnosis/RT. Furthermore, parental estimation of their child’s QoL was obtained by PEDQOL if patients where ≤18 years old at the time of study. Health-related QoL is obtained through the PEDQOL questionnaire by seven domains (autonomy, emotional stability, body image, cognition, physical function, and social functionality in family and among friends). An assessment yielding a higher score (self or by proxy) is equivalent to a worse health-related QoL (20).
Functional capacity questionnaire
With the German daily life ability scale (Fertigkeitenskala Münster-Heidelberg (FMH)), the functional capacity as a measure of QoL was analyzed 1, 3, and 5 years after CP diagnosis/RT (21). FMH uses 56 items, representing the capacity for routine actions in daily life. The test was normalized with 971 persons (45% female participants, aged 0–102 years), resulting in age-dependent percentiles. It takes an average of 4.5 min to answer the FMH questionnaire for first-time users (21).
Statistical methods
Statistical analysis was performed using SPSS, version 27 (SPSS, Inc.). Data are displayed as median (range) or frequency (percent). Event-free survival (EFS) (OS) rates were assessed using the Kaplan–Meier method. For comparison of continuous variables between two independent groups, the Mann–Whitney U test was used. For comparison of different groups for categorical variables, the Fisher’s exact test was used. The Kruskal–Wallis test was used for comparisons of multiple independent samples. EFS was defined as the date of irradiation (PBT or XRT group), diagnosis to the occurrence of an event, or last contact specified for censoring. An event was defined as a >25% increase of residual tumor volume, tumor recurrence after complete surgical resection on central imaging review, or death. OS was defined as date of diagnosis to death of any cause or last contact specified by censoring.
Results
Between 2007 and 2019, 290 patients with childhood-onset CP were recruited in the trial KRANIOPHARYNGEOM 2007. Of 290 patients (34%), 99 received external RT after diagnosis of adamantinomatous CP, which was confirmed by histological reference assessment in all cases. Of these 99 patients, 64 were treated by PBT (65%), and 35 received XRT (35%). PBT became the predominant irradiation technique during the study period (used in 23% and 77% of all irradiated patients registered within the first and second halves of the recruitment period, respectively) (Figure 1). PBT was applied in six different centers treating a median of 6 (range, 2–32) patients with CP, while XRT was applied in 27 different centers treating a median of 1 (range, 1–3) patient with CP. The radiation techniques applied reflect the spectrum commonly used within the follow-up period of the KRANIOPHARYNGEOM 2007 trial. For PBT pencil beam scanning (75%), and for XRT, IMRT (51%) was predominant (Table 1).

Figure 1 Number of patients treated with proton beam therapy (PBT, white column) or photon-based radiation therapy (XRT, black column) in relation to the year of enrollment in KRANIOPHARYNGEOM 2007.
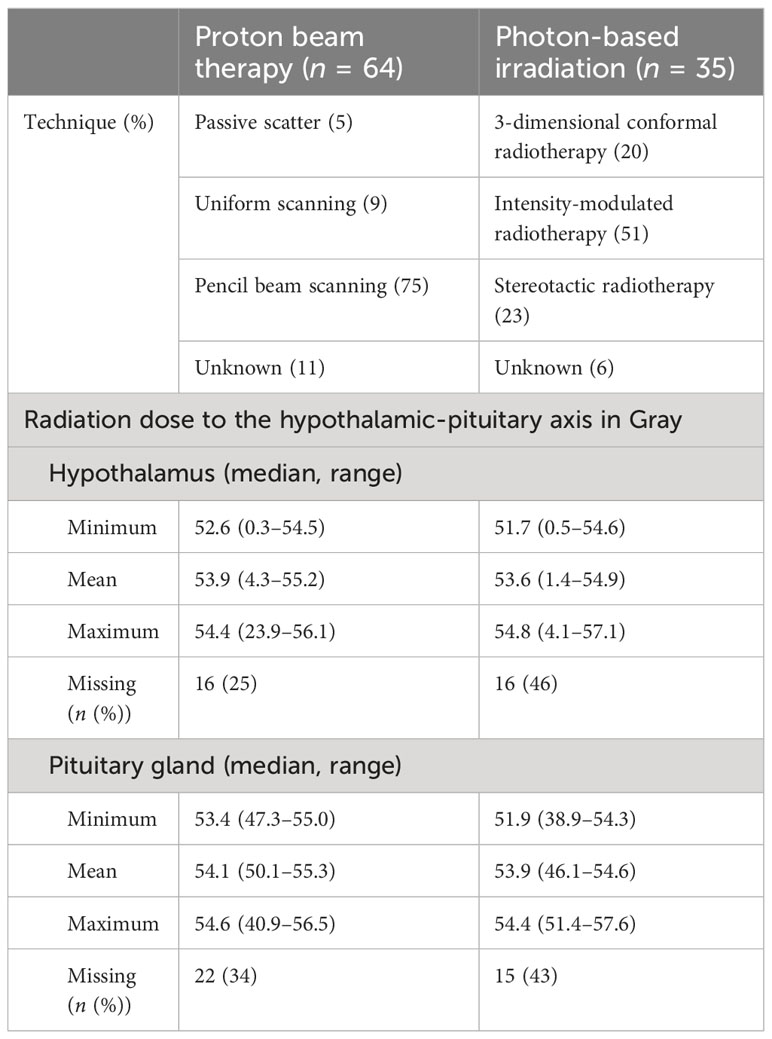
Table 1 Irradiation techniques and integral dose to organs at risk: comparing proton beam therapy with photon-based radiation therapy.
Irradiated patients presented with larger (p < 0.001) initial tumor volume (PBT: 22.1 cm3, range: 0.1–286.3 cm3; XRT: 23.0 cm3, range: 0.9–107.2 cm3) when compared with nonirradiated patients (12.6 cm3, range: 0.1–168.7 cm3) and a higher rate (p = 0.004) of initial hydrocephalus (PBT: 51%; XRT: 52%; non-RT: 32%). However, differences in terms of presurgical HI and HL as potential risk factors for the development of a hypothalamic syndrome were nondetectable between patients with PBT, patients with XRT, and patients without RT (Table 2). Complete surgical resections were more frequently (p = 0.001) achieved in nonirradiated patients (30%) when compared with patients treated with PBT (17%) and XRT-treated patients (3%).
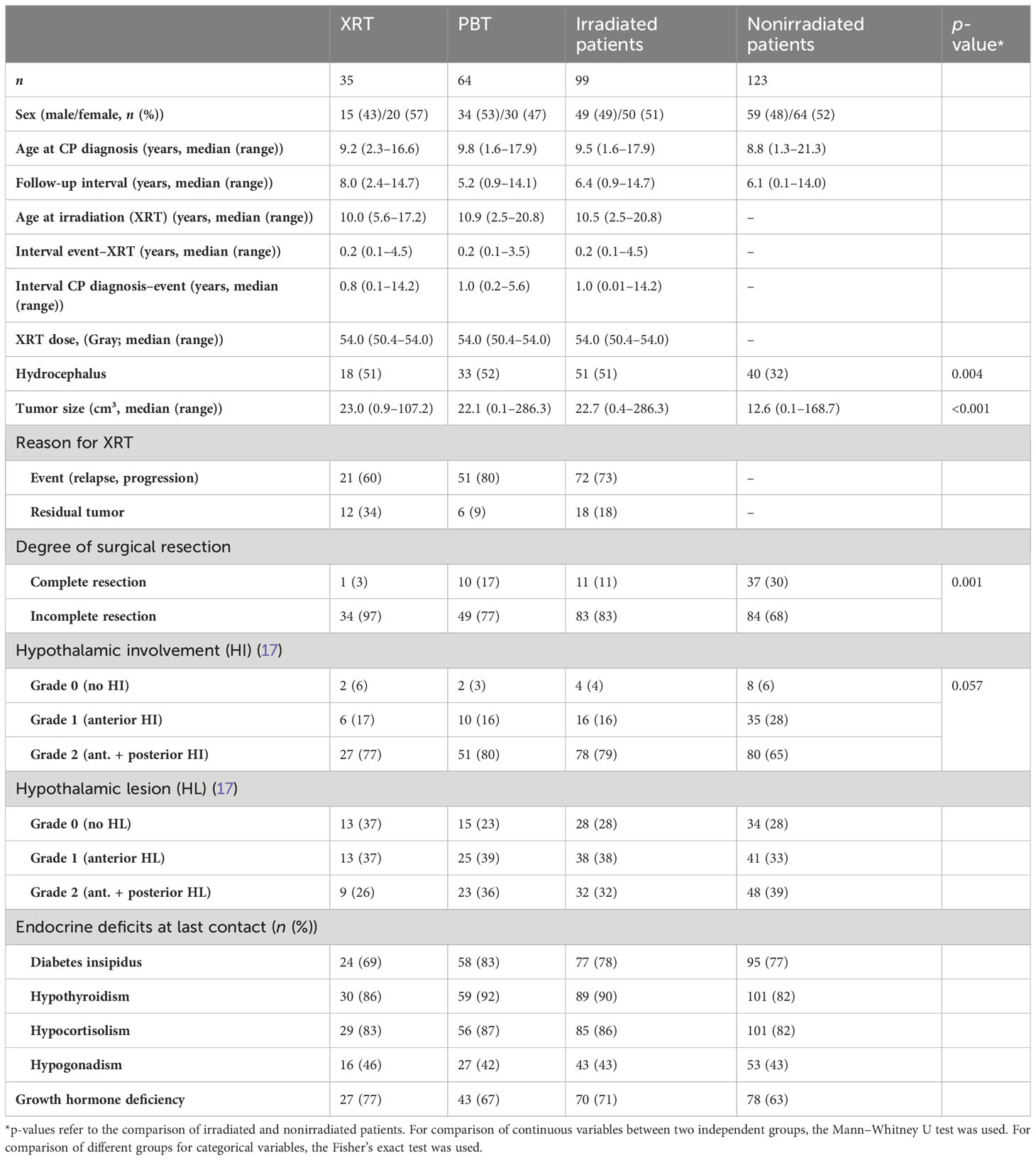
Table 2 Characteristics of the study population showing data for patients with childhood-onset adamantinomatous craniopharyngioma (CP) recruited in KRANIOPHARYNGEOM 2007 treated with photon-based radiation therapy (XRT), proton beam therapy (PBT), and patients without external RT.
External RT was started at a median interval of 11 months (range: 1–70 months) after CP diagnosis, whereas PBT was started at a median interval of 13 months (range: 1–70 months) and XRT at a median interval of 7 months (range: 3–63 months) after CP diagnosis. Reasons for RT (PBT/XRT) were preceding events in terms of relapses after complete resection (14%/3%) and progression of residual tumor after incomplete resection (59%/57%), respectively. Residual CP after incomplete surgical resection without signs of progression was the reason for RT in 18 patients (9% PBT; 34% XRT). The doses (Gray (Gy)) of radiation therapy were similar for PBT (median: 54.0 Gy; range: 50.4–54.0 Gy) and XRT (median: 54.0 Gy; range: 50.4–54.0 Gy). Dosismetric evaluations of organs at risk were available in 52 of 64 (81%) patients treated with PBT and 30 of 35 (86%) patients treated with XRT. Within the patients with dosimetric evaluation, hypothalamus and pituitary were contoured in the XRT group at 69% and 72%, respectively, and in the PBT group at 92% and 81%, respectively. The doses to the hypothalamic-pituitary axis were very similar when comparing PBT and XRT (Table 1).
The overall follow-up interval of surviving patients was 6.4 years (range: 0.9–14.6 years) in irradiated patients and 6.1 years (0.1–14.0 years) in nonirradiated patients. The 5-year EFS rate for the whole group of CP patients (n = 222) was 38% ± 4% (Figure 2A). The EFS after irradiation was higher, while there was no clear difference between PBT- (n = 64) and XRT-treated (n = 35) patients (PBT: 5-year EFS rate: 92% ± 4% vs. XRT: 91% ± 5%, p = 0.42) (Figure 2B). Whether prior to radiation an event (n = 20) or no event (n = 72) occurred did not influence EFS (5-year EFS rate 100% ± 0% vs. 88% ± 4%, p = 0.64) (Figure 2C). OS rates were similar in all analyzed subgroups. Only one fatal event occurred in the PBT subgroup. The patient had a long course of disease with a lot of complications, including cerebral infarction, but the reason of death was not declared.
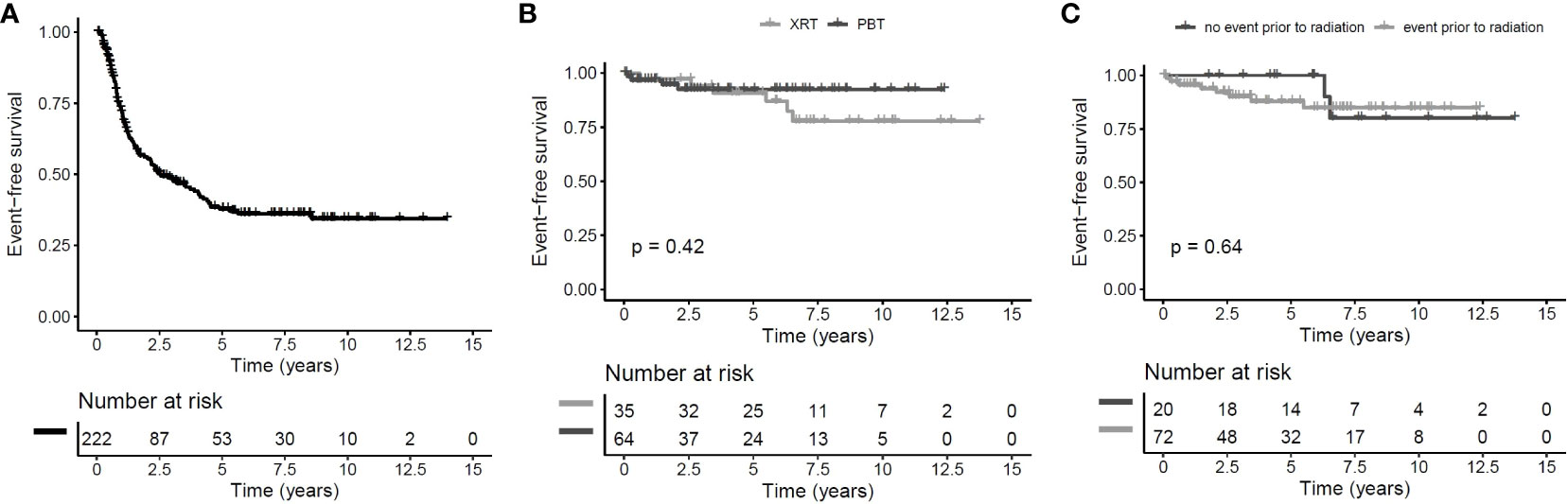
Figure 2 Event-free survival (EFS) of childhood-onset craniopharyngioma (CP) patients recruited between 2007 and 2019 in KRANIOPHARYNGEOM 2007. Cohort of all patients with EFS defined as time from diagnosis to first event and censoring at end of follow-up or in case of irradiation without prior event (A) patients receiving proton beam therapy (PBT) compared to patients receiving photon-based irradiation since the start of radiotherapy with log-rank test with EFS defined as time from irradiation to first event after start of irradiation (B) and patients receiving irradiation after a prior event compared to patients receiving irradiation without an event since the start of radiotherapy with log-rank test with EFS defined as time from irradiation to first event after start of irradiation (C).
No irradiation-related secondary malignancies were observed in any of the three subgroups.
The outcome parameters—endocrine status, anthropometric measurements, functional capacity, and QoL—were assessed at defined timepoints (1, 3, and 5 years after CP diagnosis/RT). Of all tested patients at their last visit before RT, 84% (n = 63) had diabetes insipidus, 87% (n = 68) hypothyroidism, 61% (n = 61) had growth hormone deficiency (GHD), 84% (n = 61) had hypocortisolism, and 35% (n = 15) had hypogonadism. Analyzing all patients at their last visit after irradiation with regard to endocrine status revealed no significant difference between PBT- and XRT-treated or irradiated and nonirradiated patients (Table 2). Patients with PBT, XRT, and without RT presented with similar BMI SDS and height SDS during follow-up (Figure 3). Differences in terms of functional capacity were not observed between patients after PBT or XRT and patients without RT during follow-up (Figure 4). In terms of QoL, only for the PEDQOL domain physical function, parental-assessed QoL was lower 12 months after PBT compared with CP patients after XRT and patients without RT (Figure 5).
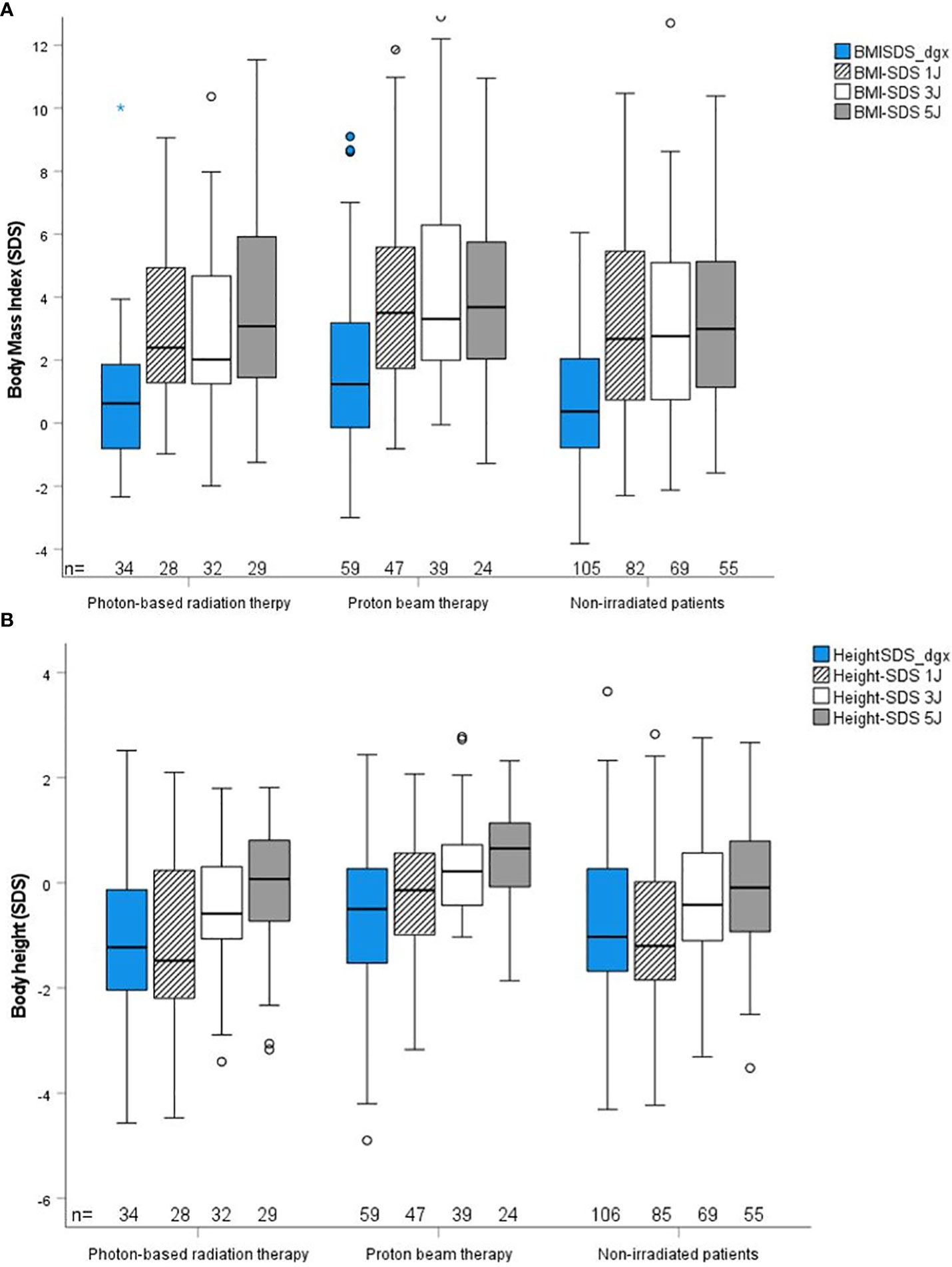
Figure 3 Anthropometric parameter body mass index (BMI) SDS (19) (A) and body height SDS (18) (B) during follow-up in childhood-onset craniopharyngioma (CP) patients (recruited in KRANIOPHARYNGEOM 2007) treated with photon-based radiation therapy (XRT), with proton beam therapy (PBT), and in CP patients without external irradiation at defined time points. Blue boxes: time point at diagnosis; crossed lines boxes: time point 1 year after diagnosis/RT; white boxes: time point 3 years after diagnosis/RT; grey boxes: time point 5 years after diagnosis;RT. The horizontal line in the middle of the box depicts the median. The top and bottom edges of the box, respectively, mark the 25th and 75th percentiles. Whiskers indicate the range of values that fall within 1.5 box lengths. The Kruskal–Wallis test was used for comparisons of multiple independent samples.
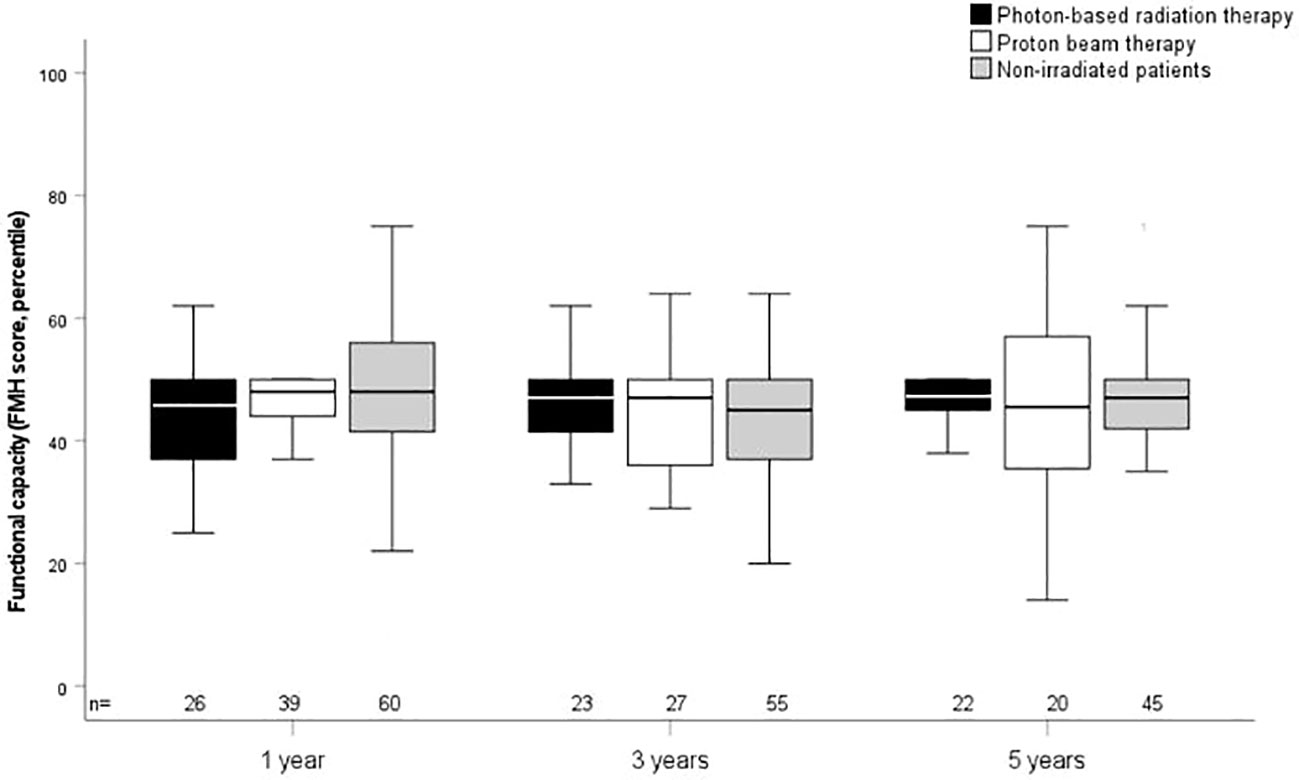
Figure 4 Functional capacity as measured by the capability scale Fertigkeitenskala Münster Heidelberg (FMH) in childhood-onset craniopharyngioma (CP) patients (recruited in KRANIOPHARYNGEOM 2007) treated with photon-based radiation therapy (XRT), with proton beam therapy (PBT), and in CP patients without external irradiation at the defined time points. White boxes: PBT; black boxes: XRT; grey boxes: no external irradiation. FMH scores are shown for the time points 1 year, 3 years, and 5 years after irradiation/CP diagnosis. The horizontal line in the middle of the box depicts the median. The top and bottom edges of the box, respectively, mark the 25th and 75th percentiles. Whiskers indicate the range of values that fall within 1.5 box lengths.
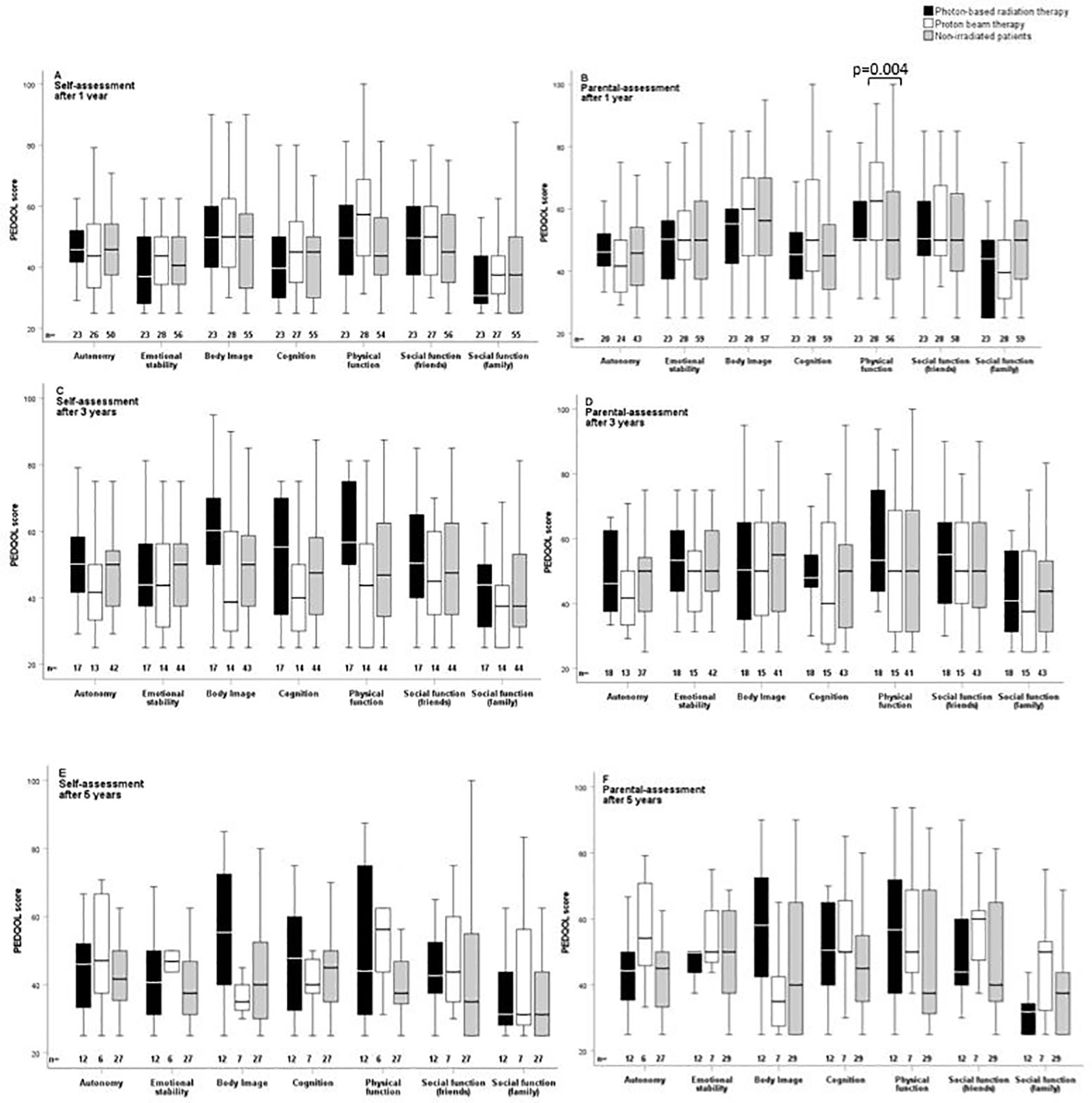
Figure 5 Self-assessment (A, C, E) and parental assessment (B, D, F) of quality of life by the Pediatric Quality of Life questionnaire (PEDQOL) in childhood-onset craniopharyngioma (CP) patients recruited in KRANIOPHARYNGEOM 2007 with regard to external irradiation. White boxes: proton beam therapy (PBT); black boxes: photon-based radiation therapy (XRT); grey boxes: no external irradiation. PEDQOL scores are shown as negative ratings at the time points 1 year (A, B), 3 years (C, D), and 5 years (E, F) after RT/CP diagnosis. The horizontal line in the middle of the box depicts the median. The top and bottom edges of the box respectively mark the 25th and 75th percentiles. Whiskers indicate the range of values that fall within 1.5 box lengths.
Discussion
In our study, we compared the outcome between 99 irradiated CP patients and 123 nonirradiated CP patients, proving that hypothalamic sparing surgery and irradiation, irrespective of the technique used, are efficient treatments with 5-year EFS rates over 90%. This approach has been at least equivalent in maintaining “historical cure rates” by more aggressive surgery while not increasing morbidity. A surgical cure is only advisable in smaller tumors. In our cohort, baseline tumor differences in terms of hydrocephalus and tumor volumes already existed in those receiving any form of irradiation. They predispose these patients a priori before radiation, and not as a result of it, to sequelae (10, 22, 23).
Risk-appropriate treatment of CP is a challenging multidisciplinary task (8, 11, 24). Based on recent reports, hypothalamus-sparing surgical strategies are preferred in childhood-onset CP patients presenting initially with a sellar mass involving hypothalamic structures (8, 10, 25). Accordingly, radiooncological approaches for residual tumor treatment after incomplete hypothalamus-sparing surgical interventions play a major role in the current consensus on appropriate treatment for CP patients (11, 26). The occurrence of disease progression or not before irradiation was not associated with a difference in EFS. Therefore, it seems to be better to postpone irradiation of CP patients with residual tumor until the first progression.
Modern, highly conformal, intensity-modulated RT techniques are especially advantageous in treating pediatric CP patients. The tumor in general is well demarcated toward healthy tissue and does much less infiltrate it compared to other brain tumors. In patients irradiated according to the KRANIOPHARYNGEOM 2007 trial, the target volume included all relevant tissues in contact with previous tumor (tumor bed of the primary tumor, tumor bed of relapse/progressive tumors, and residual tumor), regardless of the intervening time interval. In cases of very extensive disease, compromises to reduce the target volume were performed, depending on an individual risk assessment. To reduce the irradiation volume and associated increased probabilities of toxicities, in more recent guidelines like that for the KRANIOPHARYNGEOM Registry 2019, areas with an expected low risk of relapse (e.g., areas of cysts with resected cyst walls, tumor beds of primary tumors in cases of late relapses) could be excluded, although we herein show that most endocrinopathies were not caused by irradiation.
PBT is increasingly applied in pediatric central nervous tumors, and there is a consensus among treating physicians that it is the treatment of choice in CP patients (27, 28). PBT patterns of care studies often do not record the utilization of both PBT and XRT) (29). In our study, we compared the PBT with XRT patterns of care in a prospective trial with population-based recruitment in place. Over the inclusion period of 12 years, PBT became the predominant irradiation technique. PBT was performed centralized in only a few facilities, while XRT was applied in many different facilities, treating in median only one patient per recruitment period of 12 years. However, whether a more centralized RT increases treatment quality needs to be determined in the future.
We further compared unpaired data from 64 PBT versus 34 XRT patients, testing the hypothesis that one RT technique (PBT) causes less hypothalamic obesity, endocrinopathy, and functional morbidity than the other by reducing scatter to critical organs at risk, which include the HPA and optic pathway, without compromising cure.
The conventional cumulative target dose (total volume dose) applied in CP is 54.0 Gray (13, 30–36). After XRT, we observed a 5-year EFS rate of 94% that is comparable to 10- and 20-year PFS rates of 95% and 54%, respectively, reported before in the literature (37–43). Using PBT, the 5-year EFS rate was 92% in our cohort and in line with earlier reports of 5- and 10-year local control rates of 93% and 85%, respectively, and a 10-year OS rate of 72% (44). Our results demonstrate that PBT is not compromising the cure compared with XRT.
The HPA includes critical structures that are often disturbed in CP patients (11). Our study demonstrates that most HPA deficits occur before RT due to CP or the surgery performed before the irradiation, with diabetes insipidus, hypothyroidism, hypocortisolism being more common than GHD, and hypogonadism. As shown by height SDS decrements, GHD is often already clinically existent before diagnosis of CP, and laboratory testing is suspectable to faults (6). This can also be seen in Figure 3B, but a longitudinal intergroup comparison of BMI or height SDS was not within the scope of this study. Because of the already preexistent damage, a clear benefit of PBT compared to XRT with regard to HPA deficits could not be expected. Studies on patients with suprasellar optic gliomas confirm that endocrinopathies are more tumor- or surgery-induced than radiation-induced (45, 46).
For multicenter trials, it is a challenge to diagnose endocrinopathies uniformly and receive robust data. However, this challenge needs to be overcome in future trials or registries in order to establish endocrine event-free survival (EEFS) and endocrine morbidity score (EMS) as important outcome parameter together with QoL and functional capacity (45, 46). In the PEDQOL domain “physical function”, parental-assessed QoL was lower 12 months after PBT versus XRT or non-RT patients but not again at the other time points, so the clinical relevance remains inconclusive. In addition, differences in our CP cohort in terms of anthropometric parameters, functional capacity, and health-related QoL as outcome parameters were not observed between different RT techniques. These results confirm previous reports and trials performed in smaller cohorts with shorter follow-up intervals (3, 5, 6, 24, 47, 48).
It has been shown before that the outcome parameters depend on the degree of HL, especially those involving posterior hypothalamic structures (8, 11). For that reason, radical surgery in this region should be avoided, and RT should be applied. A dosimetric comparison of treatment plans in our cohort demonstrated that there were no relevant differences in radiation doses to hypothalamus or pituitary gland between PBT and XRT. The hypothalamus or HPA, respectively, are so close to residual CP and within or near the clinical target volume that both structures likely receive a full dose irrespective of whether XRT or PBT was applied (49). Of note, not all patients with dosimetric evaluations received a contouring of the hypothalamus and pituitary gland, probably due to a displacement by the CP or surgery.
A dosimetric comparison between PBT and XRT treatment plans for CP demonstrates that PBT is associated with a lower dose to important structures like the hippocampus, subventricular zone, and the vascular system (15). Initial publications of first-generation PBT suggested that, in comparison to XRT, the rate and pattern of treatment failure and occurrence rates of vasculopathy, necrosis, and severe neurological and neuroendocrine sequelae were equivalent (27). Due to the anatomical location of CP close to the Circle of Willis, the rate of vasculopathy after RT was 5%–32% (34, 47, 50–53). Many of these vasculopathies have only been detected on MR angiography and are subclinical. It has been shown that, besides RT, the tumor or surgical manipulation contributes predominately to the development of vasculopathies (54). In line with these results, we have shown within the cohort of KRANIOPHARYNGEOM 2007 patients that all cerebral infarctions (11%, 28 of 244 patients) were detected on MRI before RT and mainly after surgery (55). However, the use of MRI angiography was not established for follow-up in the KRANIOPHARYNGEOM 2007 cohort, so the incidence of subclinical vasculopathies may be underreported and especially no comparison between PBT and XRT can be drawn yet.
It has been shown in a direct comparison with a correction for the distribution of RT dose to a normal brain that patients treated with PBT had no change in academic (i.e., reading and math) achievement scores while those receiving XRT demonstrated a significant decline (56). While these preliminary reports are promising, we did not observe a difference in functional capacity and health-related QoL in the KRANIOPHARYNGEOM 2007 cohort that would be in accordance with these results.
However, in future studies it is important to analyze the locoregional distribution of radiation doses to normal brain tissue beyond the HPA, comparing PBT and XRT in a large cohort like that of the KRANIOPHARYNGEOM 2007 trial (15). The results need to be correlated to neurocognitive function in order to receive an idea of the brain damage caused by different irradiation techniques (57).
The results of our study are limited, and some observations are speculative at this point. Due to the rareness of the disease, the cohort size of our study remains improvable. Nevertheless, we are reporting on the outcome of the—to our knowledge—largest group of childhood-onset CP patients treated with different radiooncological techniques. With that focus, no comparisons of the outcome parameters to healthy controls were obtained. Furthermore, our investigation was done retrospectively and not in a randomized fashion. The utilization of both radiation techniques was different over time, with PBT being more predominant to the end of the inclusion period, so that a bias through other evolving techniques in XRT cannot be excluded. The follow-up period after PBT is still short in our study. However, major clinical manifestations of severe side effects such as hypothalamic syndrome or consecutive severe hypothalamic obesity have already been observed after short-term follow-up, i.e., during the first year after surgery (5, 6, 8). Increasing patient numbers and prolonging follow-up for assessment of long-term outcomes are goals of future studies on CP survivors.
We conclude that PBT is similarly efficient in preventing relapses and progressions in childhood-onset CP patients when compared with patients treated with XRT. During follow-up, clinically relevant differences between PBT and XRT in terms of QoL, functional capacity, and the degree of obesity as a marker of hypothalamic syndrome were not observed. Studies on larger CP cohorts with longer follow-up after RT are warranted in order to analyze whether PBT has the advantage of preventing long-term sequelae such as second malignant neoplasms, neurocognitive deficits, and vasculopathies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by local standing-committee on ethical practice of the Medizinische Fakultät, Julius-Maximilians-Universität Würzburg, Germany (140/99; 94/06, respectively). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
CF researched the data and wrote the manuscript. SB collected data and prepared statistical analyses, contributed to the analytical plan and discussion and reviewed/edited the manuscript. MB collected data and reviewed/edited the manuscript. JB contributed to the analytical plan and discussion and reviewed/edited the manuscript. PS contributed to the discussion and reviewed/edited the manuscript. GC contributed to the discussion and reviewed/edited the manuscript. ME performed statistical analyses, contributed to the analytical plan and discussion and reviewed/edited the manuscript. CV collected data and reviewed/edited the manuscript. BB did neuroradiological assessment of all imaging. BB is the neuroradiologist, who performs reference-assessment of imaging in all patients recruited in KRANIOPHARYNGEOM 2007. She prepared the imaging data and their presentation and reviewed/edited the manuscript. SH collected data and reviewed/edited the manuscript. BT did a radiooncological assessment in all cases. BT is the radiooncologist who performs reference assessment of all patients recruited in KRANIOPHARYNGEOM 2007. HM initiated and conducted the multicenter trial KRANIOPHARYNGEOM 2007, contributed to the analytical plan and discussion, and reviewed/edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by grants (HM, DKS2014.13; BB, DKS2018.02) of the German Childhood Cancer Foundation, Bonn, Germany.
Acknowledgments
The authors would like to thank colleagues, especially in the radiotherapy centers, patients and their families for participating in trials on craniopharyngioma.
Conflict of interest
HM has received reimbursement of participation fees for scientific meetings and continuing medical education events from the following companies: Ferring, Lilly, Pfizer, Sandoz/Hexal, Novo Nordisk, IPSEN, and Merck Serono. He has received reimbursement of travel expenses from IPSEN and lecture honoraria from Pfizer. BB has received reimbursement for continuing medical education events from Merck Serono.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Santagata S K-DB, Komori T, Müller HL, Pietsch T. Adamantinomatous Craniopharyngioma. In: Brat DJGA, Wesseling P, editors. Central Nervous System Tumours Who Classification of Tumours, 6. 5 ed. Lyon (France: International Agency for Research on Cancer (2021). p. 393–6. Tumours of the sellar region.
2. Apps JR, Muller HL, Hankinson TC, Yock TI, Martinez-Barbera JP. Contemporary biological insights and clinical management of craniopharyngioma. Endocr Rev (2022) 44(3):518–38. doi: 10.1210/endrev/bnac035
3. Bogusz A, Boekhoff S, Warmuth-Metz M, Calaminus G, Eveslage M, Muller HL. Posterior hypothalamus-sparing surgery improves outcome after childhood craniopharyngioma. Endocr Connect (2019) 8(5):481–92. doi: 10.1530/EC-19-0074
4. De Vile CJ, Grant DB, Kendall BE, Neville BG, Stanhope R, Watkins KE, et al. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J Neurosurg (1996) 85(1):73–81. doi: 10.3171/jns.1996.85.1.0073
5. Muller HL, Bueb K, Bartels U, Roth C, Harz K, Graf N, et al. Obesity after childhood craniopharyngioma–german multicenter study on pre-operative risk factors and quality of life. Klin Padiatr (2001) 213(4):244–9. doi: 10.1055/s-2001-16855
6. Muller HL, Emser A, Faldum A, Bruhnken G, Etavard-Gorris N, Gebhardt U, et al. Longitudinal Study on Growth and Body Mass Index before and after Diagnosis of Childhood Craniopharyngioma. J Clin Endocrinol Metab (2004) 89(7):3298–305. doi: 10.1210/jc.2003-031751
7. Beckhaus J, Friedrich C, Boekhoff S, Calaminus G, Bison B, Eveslage M, et al. Outcome after Pediatric Craniopharyngioma - the Role of Age at Diagnosis and Hypothalamic Damage. Eur J Endocrinol (2023) 188(3):lvad027. doi: 10.1093/ejendo/lvad027
8. Eveslage M, Calaminus G, Warmuth-Metz M, Kortmann RD, Pohl F, Timmermann B, et al. The postopera tive quality of life in children and adolescents with craniopharyngioma. Dtsch Arztebl Int (2019) 116(18):321–8. doi: 10.3238/arztebl.2019.0321
9. Mallucci C, Pizer B, Blair J, Didi M, Doss A, Upadrasta S, et al. Management of craniopharyngioma: the liverpool experience following the introduction of the cclg guidelines. Introducing a new risk assessment grading system. Childs Nerv Syst (2012) 28(8):1181–92. doi: 10.1007/s00381-012-1787-8
10. Muller HL, Gebhardt U, Faldum A, Warmuth-Metz M, Pietsch T, Pohl F, et al. Xanthogranuloma, rathke's cyst, and childhood craniopharyngioma: results of prospective multinational studies of children and adolescents with rare sellar malformations. J Clin Endocrinol Metab (2012) 97(11):3935–43. doi: 10.1210/jc.2012-2069
11. Muller HL, Merchant TE, Warmuth-Metz M, Martinez-Barbera JP, Puget S. Craniopharyngioma. Nat Rev Dis Primers (2019) 5(1):75. doi: 10.1038/s41572-019-0125-9
12. Puget S, Garnett M, Wray A, Grill J, Habrand JL, Bodaert N, et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J Neurosurg (2007) 106(1 Suppl):3–12. doi: 10.3171/ped.2007.106.1.3
13. Becker G, Kortmann RD, Skalej M, Bamberg M. The role of radiotherapy in the treatment of craniopharyngioma–indications, results, side effects. Front Radiat Ther Oncol (1999) 33:100–13. doi: 10.1159/000061227
14. Beltran C, Roca M, Merchant TE. On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int J Radiat Oncol Biol Phys (2012) 82(2):e281–7. doi: 10.1016/j.ijrobp.2011.01.005
15. Boehling NS, Grosshans DR, Bluett JB, Palmer MT, Song X, Amos RA, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys (2012) 82(2):643–52. doi: 10.1016/j.ijrobp.2010.11.027
16. Conroy R, Gomes L, Owen C, Buchsbaum J, Ahern V. Clinical equipoise: protons and the child with craniopharyngioma. J Med Imaging Radiat Oncol (2015) 59(3):379–85. doi: 10.1111/1754-9485.12264
17. Muller HL, Gebhardt U, Teske C, Faldum A, Zwiener I, Warmuth-Metz M, et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial kraniopharyngeom 2000 after 3-year follow-up. Eur J Endocrinol (2011) 165(1):17–24. doi: 10.1530/EJE-11-0158
18. Prader A, Largo RH, Molinari L, Issler C. Physical growth of swiss children from birth to 20 years of age. First zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl (1989) 52:1–125.
19. Rolland-Cachera MF, Cole TJ, Sempe M, Tichet J, Rossignol C, Charraud A. Body mass index variations: centiles from birth to 87 years. Eur J Clin Nutr (1991) 45(1):13–21.
20. Calaminus G, Weinspach S, Teske C, Gobel U. Quality of life in children and adolescents with cancer. First results of an evaluation of 49 patients with the pedqol questionnaire. Klin Padiatr (2000) 212(4):211–5. doi: 10.1055/s-2000-9679
21. Muller HL, Gebhardt U, Faldum A, Emser A, Etavard-Gorris N, Kolb R, et al. Functional capacity and body mass index in patients with sellar masses–cross-sectional study on 403 patients diagnosed during childhood and adolescence. Childs Nerv Syst (2005) 21(7):539–45. doi: 10.1007/s00381-005-1166-9
22. Daubenbuchel AM, Hoffmann A, Gebhardt U, Warmuth-Metz M, Sterkenburg AS, Muller HL. Hydrocephalus and hypothalamic involvement in pediatric patients with craniopharyngioma or cysts of rathke's pouch: impact on long-term prognosis. Eur J Endocrinol (2015) 172(5):561–9. doi: 10.1530/EJE-14-1029
23. Muller HL. Consequences of craniopharyngioma surgery in children. J Clin Endocrinol Metab (2011) 96(7):1981–91. doi: 10.1210/jc.2011-0174
24. Ajithkumar T, Mazhari AL, Stickan-Verfurth M, Kramer PH, Fuentes CS, Lambert J, et al. Proton therapy for craniopharyngioma - an early report from a single european centre. Clin Oncol (R Coll Radiol) (2018) 30(5):307–16. doi: 10.1016/j.clon.2018.01.012
25. Elowe-Gruau E, Beltrand J, Brauner R, Pinto G, Samara-Boustani D, Thalassinos C, et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab (2013) 98(6):2376–82. doi: 10.1210/jc.2012-3928
26. Gan HW, Morillon P, Albanese A, Aquilina K, Chandler C, Chang YC, et al. National uk guidelines for the management of paediatric craniopharyngioma. Lancet Diabetes Endocrinol (2023) 11(9):694–706. doi: 10.1016/S2213-8587(23)00162-6
27. Indelicato DJ, Merchant T, Laperriere N, Lassen Y, Vennarini S, Wolden S, et al. Consensus report from the stockholm pediatric proton therapy conference. Int J Radiat Oncol Biol Phys (2016) 96(2):387–92. doi: 10.1016/j.ijrobp.2016.06.2446
28. Odei B, Frandsen JE, Boothe D, Ermoian RP, Poppe MM. Patterns of care in proton radiation therapy for pediatric central nervous system Malignancies. Int J Radiat Oncol Biol Phys (2017) 97(1):60–3. doi: 10.1016/j.ijrobp.2016.09.011
29. Tseng YD, Hartsell W, Tsai H, Badiyan S, Wang CJ, Rossi C, et al. Proton therapy patterns of care among pediatric and adult patients with cns tumors. Neuro Oncol (2018) 20(11):1556–7. doi: 10.1093/neuonc/noy116
30. Aggarwal A, Fersht N, Brada M. Radiotherapy for craniopharyngioma. Pituitary (2013) 16(1):26–33. doi: 10.1007/s11102-012-0429-1
31. Beltran C, Naik M, Merchant TE. Dosimetric effect of target expansion and setup uncertainty during radiation therapy in pediatric craniopharyngioma. Radiother Oncol (2010) 97(3):399–403. doi: 10.1016/j.radonc.2010.10.017
32. Iannalfi A, Fragkandrea I, Brock J, Saran F. Radiotherapy in craniopharyngiomas. Clin Oncol (R Coll Radiol) (2013) 25(11):654–67. doi: 10.1016/j.clon.2013.07.005
33. Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, et al. Phase ii trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg (2006) 104(2 Suppl):94–102. doi: 10.3171/ped.2006.104.2.5
34. Merchant TE, Kiehna EN, Sanford RA, Mulhern RK, Thompson SJ, Wilson MW, et al. Craniopharyngioma: the St. Jude children's research hospital experience 1984-2001. Int J Radiat Oncol Biol Phys (2002) 53(3):533–42. doi: 10.1016/s0360-3016(02)02799-2
35. Merchant TE, Kun LE, Hua CH, Wu S, Xiong X, Sanford RA, et al. Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int J Radiat Oncol Biol Phys (2013) 85(4):e187–92. doi: 10.1016/j.ijrobp.2012.10.030
36. Minniti G, Esposito V, Amichetti M, Enrici RM. The role of fractionated radiotherapy and radiosurgery in the management of patients with craniopharyngioma. Neurosurg Rev (2009) 32(2):125–32. doi: 10.1007/s10143-009-0186-4
37. Carmel PW, Antunes JL, Chang CH. Craniopharyngiomas in children. Neurosurgery (1982) 11(3):382–9. doi: 10.1227/00006123-198209000-00008
38. Flickinger JC, Lunsford LD, Singer J, Cano ER, Deutsch M. Megavoltage external beam irradiation of craniopharyngiomas: analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys (1990) 19(1):117–22. doi: 10.1016/0360-3016(90)90143-8
39. Habrand JL, Ganry O, Couanet D, Rouxel V, Levy-Piedbois C, Pierre-Kahn A, et al. The role of radiation therapy in the management of craniopharyngioma: A 25-year experience and review of the literature. Int J Radiat Oncol Biol Phys (1999) 44(2):255–63. doi: 10.1016/s0360-3016(99)00030-9
40. Hetelekidis S, Barnes PD, Tao ML, Fischer EG, Schneider L, Scott RM, et al. 20-year experience in childhood craniopharyngioma. Int J Radiat Oncol Biol Phys (1993) 27(2):189–95. doi: 10.1016/0360-3016(93)90227-m
41. Pemberton LS, Dougal M, Magee B, Gattamaneni HR. Experience of external beam radiotherapy given adjuvantly or at relapse following surgery for craniopharyngioma. Radiother Oncol (2005) 77(1):99–104. doi: 10.1016/j.radonc.2005.04.015
42. Rajan B, Ashley S, Gorman C, Jose CC, Horwich A, Bloom HJ, et al. Craniopharyngioma–a long-term results following limited surgery and radiotherapy. Radiother Oncol (1993) 26(1):1–10. doi: 10.1016/0167-8140(93)90019-5
43. Varlotto JM, Flickinger JC, Kondziolka D, Lunsford LD, Deutsch M. External beam irradiation of craniopharyngiomas: long-term analysis of tumor control and morbidity. Int J Radiat Oncol Biol Phys (2002) 54(2):492–9. doi: 10.1016/s0360-3016(02)02965-6
44. Fitzek MM, Linggood RM, Adams J, Munzenrider JE. Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at harvard cyclotron laboratory and massachusetts general hospital. Int J Radiat Oncol Biol Phys (2006) 64(5):1348–54. doi: 10.1016/j.ijrobp.2005.09.034
45. Gan HW, Phipps K, Aquilina K, Gaze MN, Hayward R, Spoudeas HA. Neuroendocrine Morbidity after Pediatric Optic Gliomas: A Longitudinal Analysis of 166 Children over 30 Years. J Clin Endocrinol Metab (2015) 100(10):3787–99. doi: 10.1210/jc.2015-2028
46. Picariello S, Cerbone M, D'Arco F, Gan HW, O'Hare P, Aquilina K, et al. A 40-year cohort study of evolving hypothalamic dysfunction in infants and young children (<3 years) with optic pathway gliomas. Cancers (Basel) (2022) 14(3):747. doi: 10.3390/cancers14030747
47. Bishop AJ, Greenfield B, Mahajan A, Paulino AC, Okcu MF, Allen PK, et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int J Radiat Oncol Biol Phys (2014) 90(2):354–61. doi: 10.1016/j.ijrobp.2014.05.051
48. Luu QT, Loredo LN, Archambeau JO, Yonemoto LT, Slater JM, Slater JD. Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J (2006) 12(2):155–9.
49. Greenberger BA, Yock TI. The role of proton therapy in pediatric Malignancies: recent advances and future directions. Semin Oncol (2020) 47(1):8–22. doi: 10.1053/j.seminoncol.2020.02.002
50. Bavle A, Srinivasan A, Choudhry F, Anderson M, Confer M, Simpson H, et al. Systematic review of the incidence and risk factors for cerebral vasculopathy and stroke after cranial proton and photon radiation for childhood brain tumors. Neurooncol Pract (2021) 8(1):31–9. doi: 10.1093/nop/npaa061
51. Fouda MA, Scott RM, Marcus KJ, Ullrich N, Manley PE, Kieran MW, et al. Sixty years single institutional experience with pediatric craniopharyngioma: between the past and the future. Childs Nerv Syst (2020) 36(2):291–6. doi: 10.1007/s00381-019-04294-x
52. Lo AC, Howard AF, Nichol A, Hasan H, Martin M, Heran M, et al. A cross-sectional cohort study of cerebrovascular disease and late effects after radiation therapy for craniopharyngioma. Pediatr Blood Cancer (2016) 63(5):786–93. doi: 10.1002/pbc.25889
53. Winkfield KM, Tsai HK, Yao X, Larson E, Neuberg D, Pomeroy SL, et al. Long-term clinical outcomes following treatment of childhood craniopharyngioma. Pediatr Blood Cancer (2011) 56(7):1120–6. doi: 10.1002/pbc.22884
54. Lucas JT Jr., Faught AM, Hsu CY, Wilson LJ, Guo Y, Li Y, et al. Pre- and posttherapy risk factors for vasculopathy in pediatric patients with craniopharyngioma treated with surgery and proton radiation therapy. Int J Radiat Oncol Biol Phys (2022) 113(1):152–60. doi: 10.1016/j.ijrobp.2021.12.172
55. Boekhoff S, Bison B, Genzel D, Eveslage M, Otte A, Friedrich C, et al. Cerebral infarction in childhood-onset craniopharyngioma patients: results of kraniopharyngeom 2007. Front Oncol (2021) 11:698150. doi: 10.3389/fonc.2021.698150
56. Merchant T, Indelicato D, Hua C, Wu S, Conklin H. Comparison of Academic Achievement Scores after Proton and Photon Therapy in Children and Young Adults with Craniopharyngioma. In: Pediatric blood & cancer. NJ USA: WILEY 111 RIVER ST, HOBOKEN 07030-5774 (2017).
Keywords: craniopharyngioma, proton beam therapy, irradiation, quality of life, hypothalamus, obesity, photon-based radiation therapy, survival
Citation: Friedrich C, Boekhoff S, Bischoff M, Beckhaus J, Sowithayasakul P, Calaminus G, Eveslage M, Valentini C, Bison B, Harrabi SB, Krause M, Timmermann B and Müller HL (2023) Outcome after proton beam therapy versus photon-based radiation therapy in childhood-onset craniopharyngioma patients—results of KRANIOPHARYNGEOM 2007. Front. Oncol. 13:1180993. doi: 10.3389/fonc.2023.1180993
Received: 06 March 2023; Accepted: 09 October 2023;
Published: 27 October 2023.
Edited by:
David Walker, University of Nottingham, United KingdomReviewed by:
Thomas Edward Merchant, St. Jude Children’s Research Hospital, United StatesGianluca Ingrosso, University of Perugia, Italy
Helen Spoudeas, University College London, United Kingdom
Copyright © 2023 Friedrich, Boekhoff, Bischoff, Beckhaus, Sowithayasakul, Calaminus, Eveslage, Valentini, Bison, Harrabi, Krause, Timmermann and Müller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hermann L. Müller, bXVlbGxlci5oZXJtYW5uQGtsaW5pa3VtLW9sZGVuYnVyZy5kZQ==
†ORCID: Hermann L. Müller, orcid.org/0000-0003-4929-9966
 Carsten Friedrich
Carsten Friedrich Svenja Boekhoff
Svenja Boekhoff Martin Bischoff2
Martin Bischoff2 Julia Beckhaus
Julia Beckhaus Chiara Valentini
Chiara Valentini Brigitte Bison
Brigitte Bison Semi B. Harrabi
Semi B. Harrabi Mechthild Krause
Mechthild Krause Beate Timmermann
Beate Timmermann Hermann L. Müller
Hermann L. Müller