
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 17 May 2023
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1179431
This article is part of the Research Topic Transarterial Chemoembolization for Hepatocellular Carcinoma Patients View all 13 articles
Objective: At present, several molecular targeted agents(MTAs) combined with transarterial chemoembolization (TACE) have been employed to treat unresectable hepatocellular carcinoma (HCC). In this meta-analysis, we compared the efficacy and safety of different MTAs combined with TACE to enable effective decision-making for the clinical treatment of unresectable HCC.
Methods: Pubmed, Web of Science, EMBASE, and Cochrane Library were retrieved to evaluate the efficacy and safety of different MTAs combined with TACE in cohort studies and randomized controlled trials. The hazard ratios and 95% confidence intervals (CIs) were calculated to investigate the impact of various therapies on overall survival (OS) and progression-free survival. However, the objective response rate (ORR), disease control rate (DCR), adverse events (AEs), and ≥grade-3 adverse events (≥G3-AEs) were calculated using odd ratios and 95% CIs. The node-splitting approach was used to test the heterogeneity. The funnel plot was utilized to analyze the publication bias. Additionally, according to the ranking plots, we ranked various treatments.
Results: A total of 45 studies involving 10,774 patients with 8 treatment strategies were included in our network meta-analysis. Our network meta-analysis showed that apatinib+TACE provided the highest OS (62.2%), ORR (44.7%), and DCR (45.6%), while and lenvatinib+TACE offered the best PFS (78.9%). Besides, there was no statistically significant difference in AEs and ≥G3-AEs among treatment options.
Conclusion: Apatinib+TACE demonstrated the best OS, ORR, and DCR with no additional AEs and ≥G3-AEs. Therefore, for the treatment scheme of MTAs combined with TACE, apatinib+TACE may be the best option for patients with unresectable HCC.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42023388609.
As one of the most prevalent kinds of cancer, primary liver cancer (PLC) incidence rate and mortality rank sixth and third globally, respectively (1). Hepatocellular carcinoma (HCC) accounts for 75% to 95% of PLC cases (1). Features of HCC include insidious onset, lengthy latency, and swift progression. Patients are frequently diagnosed at an advanced stage, making them miss out on the best opportunity for surgery (2). The diagnosis of advanced HCC is found in patients who do not follow the recommended monitoring plan according to the guidelines. According to the guidelines, they should better accept different treatment strategies based on the number and size of HCC. Meanwhile, the median survival of advanced HCC is under one year, making it a significant global health problem (3).
For unresectable HCC (uHCC), the available treatment options mainly include transarterial chemoembolization (TACE), transarterial radioembolization (TARE), liver transplantation, stereotactic body radiation therapy (SBRT), targeted therapy, and immunotherapy (4). Due to its safety, efficacy, minimally invasive nature, and repeatability, TACE has been included as a first-line treatment in the non-radical treatment of HCC which cannot be surgically resected (5). TACE is mainly used to achieve the therapeutic purpose by injecting chemotherapy drugs into the tumor supply arteries and then blocking the above arteries with embolic materials. However, after TACE, the local hypoxia of the tumor blood supply artery will disturb the tumor microenvironment, leading to the upward regulation of hypoxia-inducible factor-1 (HIF-1), which upregulates vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR), further increasing tumor angiogenesis (6). Tumor neovascularization forms collateral circulation with other intrahepatic vessels, causing local recurrence and metastasis (7).
Over the past decades, the molecular mechanism of the onset and development of liver cancer has gradually become known through the continued exploration of molecular cell biology. The above progress provides a theoretical basis for the emergence of more molecular targeted agents (MTAs) which inhibit anomalous molecular targets (8). Tumor angiogenesis produced by ascending regulation of VEGFR and PDGFR is the primary cause of tumor spread and relapse following TACE. MTAs can inhibit the PFGF and VEGFR pathways, preventing tumor neovascularization. Meanwhile, molecular targeted therapy reduces tumor growth and differentiation by disrupting tumor signal transduction pathways, resulting in apoptosis and destruction of tumor cells. The Wnt/β-Catenin pathway, Ras/Raf/MAPK pathway, PI3/AKT/mTOR pathway, JAK/STAT pathway, Ubiquitin Proteasome pathway, and IGF1/IGF1R pathway are the principal targeted pathways for the therapy of HCC (8). Growth factors, signaling molecules, cyclins, apoptotic regulators, and chemicals that encourage angiogenesis in the route are among the compounds that targeted medications target (9). Different MTAs act on various transduction pathways, depending on the targets they are meant to affect. By obstructing signals that encourage cancer cell development, disrupting the control of the cell cycle, or inducing cell death, MTAs destroy cancer cells (10). Both TACE and MTAs have anti-tumor properties. At the same time, MTAs can reverse the tumor recurrence and metastasis caused by TACE treatment, which promotes tumor angiogenesis. Consequently, there is an increasing trend in clinical practice to combine TACE and MTAs to treat uHCC.
As more and more MTAs arrive on the market, so does the number of MTAs that TACE can jointly choose. However, due to the lack of head-to-head comparison of TACE in combination with MTAs, the ideal strategy for TACE in combination with MTA still needs to be discovered. Consequently, a network meta-analysis (NMA) was performed to compare the efficacy and safety of various MTAs combined with TACE.
This NMA was registered in PROSPERO (CRD42023388609). Additionally, the study was conducted in strict adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
We systematically searched Pubmed, Web of Science, EMBASE, and Cochrane Library from the date of establishment to January 4, 2023. The paramount search terms were “liver neoplasms”, “chemoembolization, therapeutic”, “sorafenib”, “sunitinib”, “brivanib”, “anlotinib”, “apatinib”, “orantinib”, “lenvatinib” along with their synonyms. The detailed search strategy is outlined in Supplementary Table 1.
Studies were included in this NMA if they met the following inclusion criteria: (a) Patients: adults who were at least 18 years old diagnosed with uHCC; uHCC patients did not receive systematic treatment prior to receiving MTA combined with TACE or TACE alone; no additional treatment was administered during the studies, including radiofrequency ablation, percutaneous ethanol injection or iodine-125 seed implantation. (b) Intervention: TACE as monotherapy therapy or in combination with several MTAs. (c) Comparison: studies that compared the outcomes of various interventions in treating uHCC. (d) Outcomes: efficacy indicators included overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR); safety indicators included the incidence of adverse events (AEs) and ≥grade-3 adverse events (≥G3-AEs). (e) Study design: randomized controlled trials (RCTs) and cohort studies.
The following studies were eliminated from this NMA: case reports, reviews, case-control studies, editorials, and studies with insufficient data.
After determining the RCTs and cohort studies to be included in this study, two researchers (BC and ZL) independently extracted data. Any differences were resolved by a third researcher (JL). The following data were extracted: first author, publication year, region, treatment measures, sample size, gender, age, and disease characteristics.
We applied two quality evaluation tools to evaluate two types of studies. For cohort studies, we applied the Newcastle-Ottawa scale, which evaluated cohort studies through eight items. The eight items mentioned above consisted of the representativeness of the exposure cohort, the selection of the non-exposed cohort, the determination of the exposure, the absence of the disease to be studied at the beginning of the study, the comparability of the exposure cohort and the non-exposed cohort, the measurement method of the results, whether the follow-up time was long enough and the integrity of the follow-up. Apart from the item of comparability between exposed and non-exposed cohorts, which could be rated up to two stars, other items could be rated up to one star, with a total score of nine stars. For RCTs, the Cochrane’s Risk of Bias Tool, recommended by the Cochrane Handbook, was used to investigate sources of bias from seven dimensions. The seven dimensions were described in terms of six aspects, namely, selection bias, implementation bias, measurement bias, follow-up bias, reporting bias, and other biases. Each dimension was judged and divided by low, high, and unclear risk of bias.
R version 3.6.1 and StataMP 14.0 were used to analyze relevant data. We conducted a Bayesian NMA employing a random effect model to compare directly or indirectly the efficacy and safety of each treatment included in the study. To obtain the posterior distribution, we established three independent Markov chains for each outcome measure. The number of iterations per chain was set at 50,000, with the first 5,000 being considered burn-in samples. The model’s convergence was assessed employing Brooks-Gelman-Rubin plots and trace plots.
For OS and PFS, the pooled hazard ratio (HR) and 95% confidence intervals (CIs) were used for comparison. For ORR, DCR, AEs, and ≥G3-AEs, the pooled odds ratio (OR) and 95% CIs were used for comparison. We extracted data from Kaplan-Meier plots for those studies that did not offer HR values utilizing Engauge Digitizer version 11.3 software. The ranking probability was used to evaluate the ranking of each treatment measure. The node-splitting approach was used to determine if direct or indirect comparisons were coherent. Funnel plots were used to assess whether the included study had publication bias. If the funnel plot was symmetrical, it indicated no publication bias. Otherwise, there may be publication bias. Two-tailed P<0.05 was deemed statistically significant.
In our selected database, 9,370 studies were initially identified, and another study was obtained through other means. After removing 2,966 duplicate articles, 6,305 articles were abstracted and screened. Following the preliminary screening, 777 articles met the evaluation criteria. Subsequently, after excluding 607 systematic reviews or case reports, 121 non-human trials, 1 article with incomplete data, and 3 articles receiving other treatments, a total of 10 RCTs (11–20) and 35 cohort studies (21–55) were included for NMA. The literature screening process is illustrated in Figure 1.
In our NMA, a total of 7 MTAs+TACE treatment schemes were included, namely: sorafenib+TACE (Sora+TACE), lenvatinib+TACE (Lenv+TACE), sunitinib+TACE (Suni+TACE), brivanib+TACE (Briv+TACE), anlotinib+TACE (Anlo+TACE), apatinib+TACE (Apat+TACE) and orantinib+TACE (Oran+TACE). There were 10,774 HCC patients in our 45 included studies. Among them, 19 studies (11–14, 21–35) were about the comparison of Sora+TACE and TACE monotherapy, 3 studies (36–38) were about the comparison of Lenv+TACE and TACE monotherapy, 11 studies (15, 39–48) were about the comparison of Apat+TACE and TACE monotherapy, 1 studies (49) was about the comparison of Anlo+TACE and TACE monotherapy, 1 study (16) was about the comparison of Briv+TACE and TACE monotherapy, and 2 studies (17, 50) were about the comparison of Suni+TACE and TACE monotherapy, and 3 studies (18–20) were about the comparison of Oran+TACE and TACE monotherapy. Moreover, there were 2 studies (51, 52) on the comparison of Lenv+TACE and Sora+TACE, 2 studies (53, 54) on the comparison of Sora+TACE and Apat+TACE, and 1 study (55) on the comparison of Suni+TACE and Sora+TACE. In the included studies, the patient count was between 42 and 1,719. The age of patients varied between 18 and 87 years. The characteristics of the included study are shown in Supplementary Table 2. The quality evaluation of the included literature is shown in Supplementary Table 3.
For OS, 8 treatment strategies were documented altogether (Figure 2) and in comparison with TACE monotherapy and Oran+TACE, Apat+TACE, Lenv+TACE, and Sora+TACE demonstrated significant OS benefits (HR 0.62, 95% CI 0.50-0.75; HR 0.60, 95% CI 0.44-0.77; HR 0.68, 95% CI 0.49-0.88; HR 0.66, 95% CI 0.44-0.90; HR 0.78, 95% CI 0.68-0.86; HR 0.75, 95% CI 0.58-0.93) (Figure 3). Moreover, Apat+TACE supplied better OS than Sora+TACE and Suni+TACE (HR 0.80, 95% CI 0.67-0.95; HR 0.69, 95% CI 0.49-0.94). In the light of the ranking plot, Apat+TACE had the highest probability (62.2%) of delivering a better OS, followed by Lenv+TACE (40.6%), Sora+TACE (40.4%) and Anlo+TACE (18.3%) (Figure 4; Supplementary Table 4).
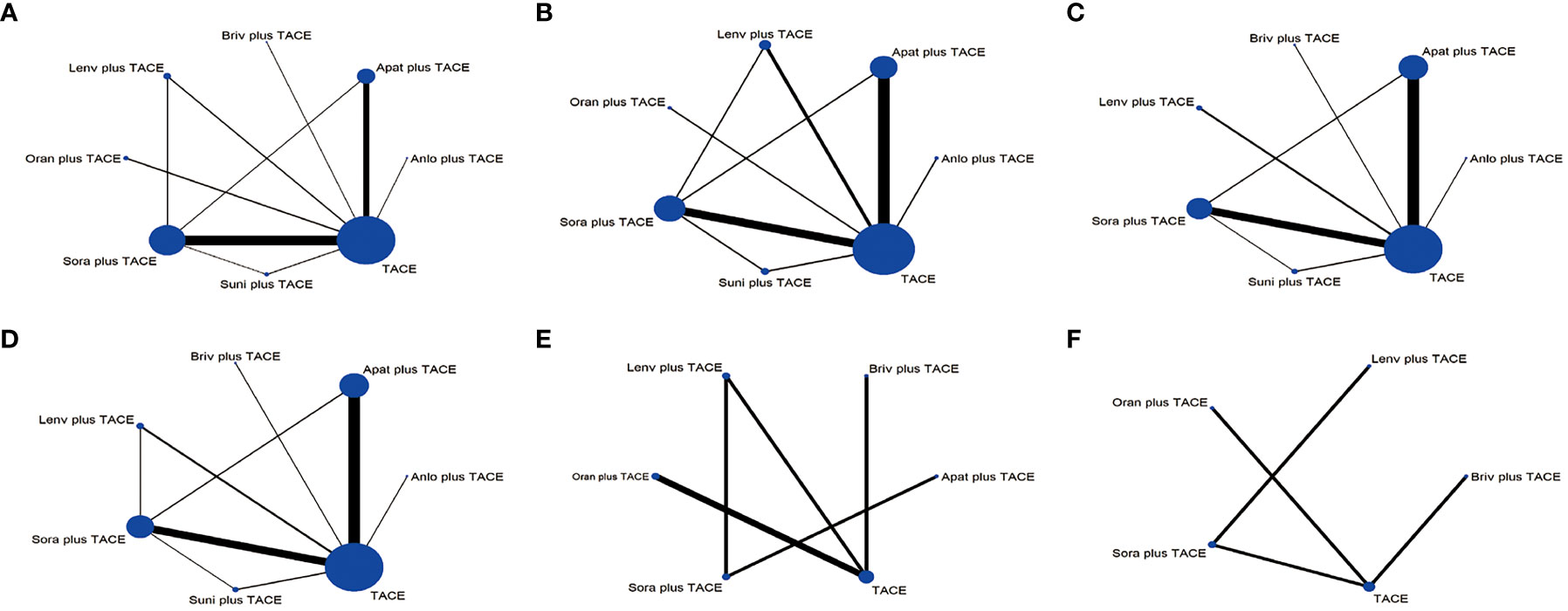
Figure 2 Network plots of the comparisons for the network meta-analysis. (A) Overall survival. (B) Progression-free survival. (C) Objective response rate. (D) Disease control rate. (E) ≥Grade-3 adverse events. (F) Adverse events. The size of the circle is proportional to the number of studies. The width of the line is proportional to the study of direct comparison. Lenv plus TACE, lenvatinib+TACE; Briv plus TACE, brivanib+TACE; Apat plus TACE, apatinib+TACE; Anlo plus TACE, anlotinib+TACE; Suni plus TACE, sunitinib+TACE; Sora plus TACE, sorafenib+TACE; Oran plus TACE, Orantinib+TACE.

Figure 3 Pooled efficacy indicators estimates of network meta-analysis. (A) Pooled hazard ratios (95% confidence intervals) of overall survival. (B) Pooled hazard ratios (95% confidence intervals) of progression-free survival. (C) Pooled odds ratios (95% confidence intervals) for objective response rate. (D) Pooled odds ratios (95% confidence intervals) for disease control rate.

Figure 4 Relative rank plots based on probabilities of treatment strategies. (A) Overall survival. (B) Progression-free survival. (C) Objective response rate. (D) Disease control rate. (E) Adverse events. (F) ≥Grade-3 adverse events. Dark to light colors in the bar chart correspond to the ranking from top to bottom. LenvplusTACE, lenvatinib+TACE; BrivplusTACE, brivanib+TACE; ApatplusTACE, apatinib+TACE; AnloplusTACE, anlotinib+TACE; SuniplusTACE, sunitinib+TACE; SoraplusTACE, sorafenib+TACE; OranplusTACE, Orantinib+TACE.
For PFS, 7 treatment strategies were documented altogether (Figure 2). Lenv+TACE and Apat+TACE were significantly ahead of Sora+TACE, Suni+TACE, and TACE monotherapy. Lenv+TACE offered better PFS than Sora+TACE (HR 0.53, 95%CI 0.32-0.88), Suni+TACE (HR 0.50, 95% CI 0.26-0.92), and TACE monotherapy (HR 0.46, 95% CI 0.28-0.73) (Figure 3). Similarly, Apat+TACE provided a better PFS than Sora+TACE (HR 0.69, 95% CI 0.53-0.93), Suni+TACE(HR 0.64, 95% CI 0.40-1.02), and TACE monotherapy(HR 0.60, 95% CI 0.46-0.75). In the light of the ranking plot, Lenv+TACE had the highest probability (78.9%) of providing a superior PFS, followed by Apat+TACE (58.1%), Oran+TACE (34.4%) and Anlo+TACE(32.7%) (Figure 4; Supplementary Table 4).
For ORR, 7 treatment strategies were documented altogether (Figure 2). Compared with TACE monotherapy, Suni+TACE, and Anlo+TACE, Apat+TACE showed a significantly better ORR rate (OR 1.97, 95% CI 1.50-2.66; OR 2.05, 95% CI 1.01-4.04; OR 2.79 95% CI 1.00-8.32) (Figure 3). Sora+TACE was also demonstrated to have a significantly higher ORR rate than TACE monotherapy (OR 1.79, 95% CI 1.34-2.52). Additionally, no significant differences were found among the other treatments. In the light of the ranking plot, Apat+TACE had the highest probability of yielding a higher ORR rate (44.7%), followed by Sora+TACE (36.6%), Lenv+TACE (30.7%) and Briv+TACE (32.7%) (Figure 4; Supplementary Table 4).
For DCR, 7 treatment strategies were documented altogether (Figure 2). Compared to the remaining six treatment measures, TACE monotherapy showed a lower DCR rate (OR 0.70, 95% CI 0.59-0.82; OR 0.75, 95% CI 0.63-0.88; OR 0.79, 95% CI 0.59-1.06; OR 0.88, 95% CI 0.49-1.57; OR 1.00, 95% CI 0.57-1.75; OR 0.98, 95% CI 0.69-1.45), but most differences were not statistically significant (Figure 3). Whereas there was no significant difference in the DCR of the six treatment strategies when compared with each other, Apat+TACE showed a higher DCR than Sora+TACE (OR 1.07, 95% CI 0.87-1.34), Lenv+TACE (OR 1.13, 95% CI 0.81-1.59), Anlo+TACE (OR 1.25, 95% CI 0.69-2.32), Briv+TACE (OR 1.42, 95% CI 0.80-2.58) and Suni+TACE(OR 1.40, 95% CI 0.96-2.17). In the light of the ranking plot, Apat+TACE had the highest probability of delivering a maximum DCR (45.6%), followed by Sora+TACE (33.7%), Lenv+TACE (26.1%) and Anlo+TACE (16.6%) (Figure 4; Supplementary Table 4).
For ≥G3-AEs, 6 treatment strategies were documented altogether (Figure 2). The various combined therapies did not significantly differ from one another (Figure 5). TACE demonstrated no statistically significant advantage in the low incidence of ≥G3-AEs while being a relatively safe treatment compared to other combined regimens (OR 0.95, 95% CI 0.27-3.28; OR 0.69, 95% CI 0.29-1.65; OR 0.64, 95% CI 0.35-1.12; OR 0.62, 95% CI 0.27-1.4; OR 0.41, 95% CI 0.09-1.82). In the light of the ranking plot, Apat+TACE was most likely to deliver the highest incidence of ≥G3-AEs (68.8%), followed by Briv+TACE (30.0%). Besides, TACE had the highest probability of delivering the safest treatment (48.2%), followed by Sora+TACE (27.3%) (Figure 4; Supplementary Table 4).
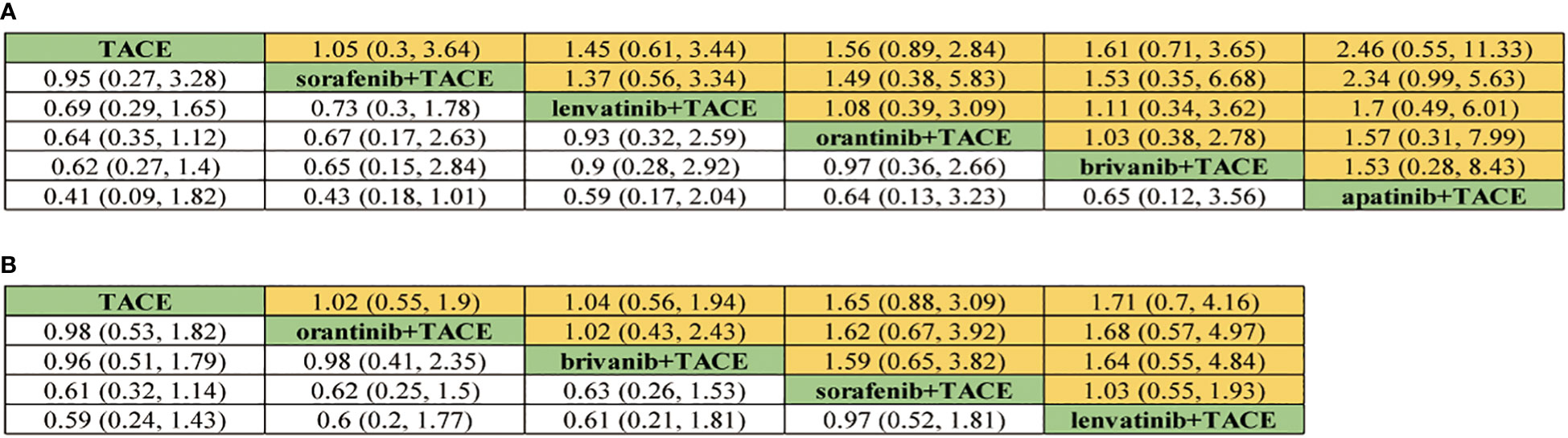
Figure 5 Pooled safety indicators estimates of network meta-analysis. (A) Pooled odds ratios (95% confidence intervals) of ≥Grade-3 adverse events. (B) Pooled odds ratios (95% confidence intervals) of adverse events.
For AEs, 5 treatment strategies were documented altogether (Figure 2). There was no significant difference between the various treatment measures (Figure 5). Given the ranking plot, Oran+TACE had the highest probability of providing the safest treatment (34.3%), followed by TACE (44.2%). Besides, Sora+TACE was most likely to deliver the highest incidence of AEs (53.4%), followed by Lenv+TACE (53.4%) (Figure 4, Supplementary Table 4).
The funnel plots of all indicators in the included study were nearly symmetrical, indicating no publication bias (Figure 6). Utilizing the node-splitting method, we found no inconsistency between direct and indirect comparison (Supplementary Table 5).
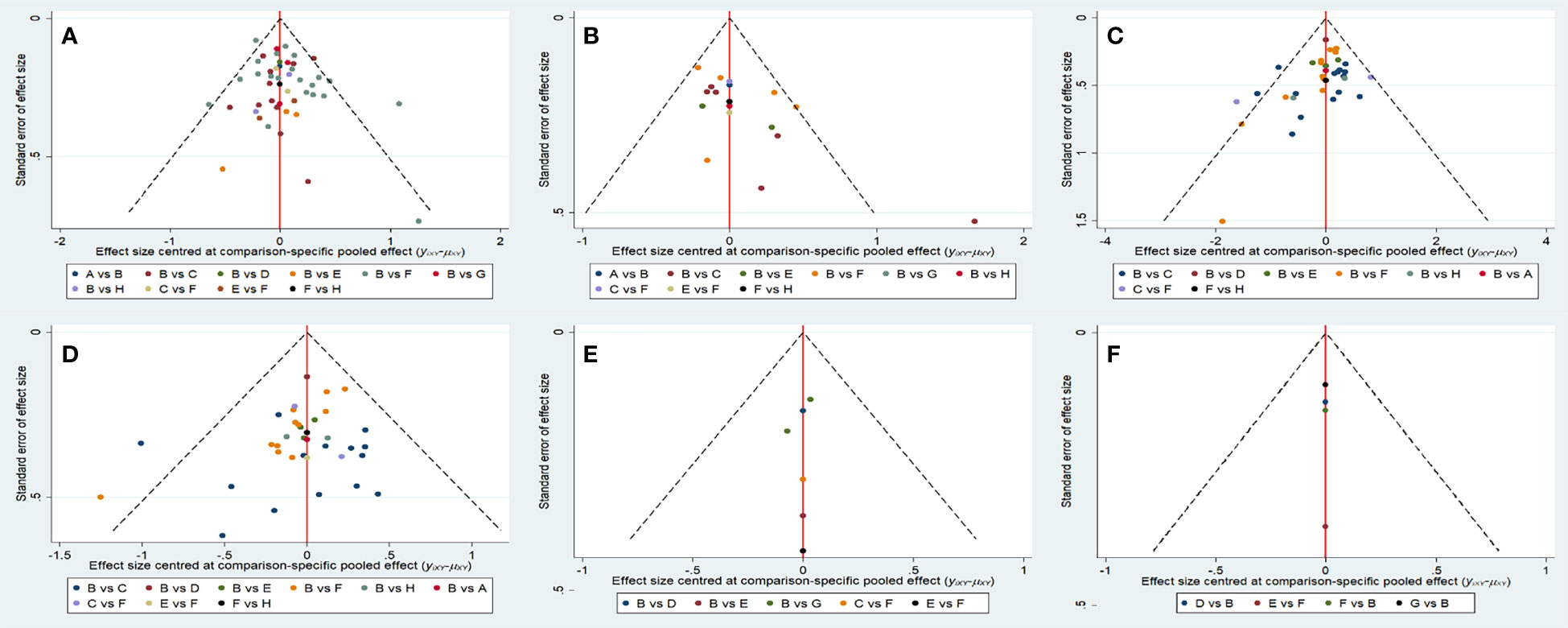
Figure 6 Funnel plots of each evaluation index. (A) Overall survival. (B) Progression-free survival. (C) Objective response rate. (D) Disease control rate. (F) ≥Grade-3 adverse events. (E) Adverse events. A, anlotinib+TACE; B, TACE; C, apatinib+TACE; D, brivanib+TACE; E, lenvatinib+TACE; F, sorafenib+TACE; G, orantinib+TACE; H, sunitinib+TACE.
So far, TACE combined with MTAs has become an essential approach for treating uHCC. Increasing combined therapies are applied in clinical practice. However, it is not easy to compare these therapies directly.
In this NMA, we primarily concentrated on comparing the efficacy and safety of TACE combined with MTAs. The results manifested that Apat+TACE had the best OS outcomes; Lenv+TACE and Sora+TACE placed second and third, respectively. The first three treatment strategies related to higher PFS outcomes were Lenv+TACE, Apat+TACE, and Oran+TACE. The first three treatment strategies related to higher ORR were Apat+TACE, Sora+TACE, and Lenv+TACE, while DCR was ranked similarly. In addition, there was no statistically significant difference among all treatment regimens with respect to the incidence of AEs and ≥G3-AEs. As a result, it can conclude that Apat+TACE was related to the best OS, ORR, and DCR, whereas Lenv+TACE was linked with the greatest PFS, with no extra AEs or ≥G3-AEs.
Apat, as an anti-angiogenic drug, preferentially inhibits VEGFR-2 tyrosine kinase, as well as slightly inhibits c-kit, c-src and, RET tyrosine kinases (56). It selectively binds to the intracellular ATP binding domain, inhibiting vascular endothelial cell proliferation and migration, reducing tumor angiogenesis, and inhibiting tumor formation (57). Furthermore, it can reverse the multidrug resistance caused by ABC protein and enhance the effectiveness of conventional anticancer drugs (58, 59). By inducing traditional chemotherapy medications and stimulating cell apoptosis, it can also have an anticancer effect (57). The above characteristics of apatinib are highly compatible with TACE treatment, which may be the important reason why Apat+TACE can stand out in many combined therapies.
For a long time, the comparison of survival time of HCC patients between Sora+TACE and Apat+TACE has been controversial. Qiu et al. (54) found that the PFS of the Apat+TACE group was shorter than that of the Sora+TACE group, while the OS of the two groups was not significantly different. Besides, Cao et al. (53) argued that in a retrospective study, the prognosis for both treatments was equivalent in patients with portal vein tumor thrombosis. It is worth mentioning that Apatinib has a tenfold higher affinity for VEGFR-2 tyrosine kinase than sorafenib (60). In our NMA, the OS and PFS of Apat+TACE were significantly better than those of Sora+TACE.
Regarding ORR, Apat+TACE had a higher ORR, suggesting a more significant proportion of patients with a 30% tumor decrease and maintenance for more than 4 weeks after Apat+TACE treatment. Because doctors or patients can clearly see the comparison of tumor bodies before and after therapy from the imaging, this sign is more intuitive for effect after treatment. However, ORR has a disadvantage in that it can only assess the efficacy of individual treatments and cannot reflect the overall advantages of the patient’s whole course of therapy. Apat+TACE fared better in terms of DCR, suggesting that it helped increase patient compliance and provided opportunities for more follow-up treatment in the future.
Hand-foot syndrome, hypertension, fatigue, and diarrhea were some of the most frequent adverse reactions to using TACE combined with MTAs. Compared to other combination therapy, Sora+TACE had more significant instances of erythema multiforme, rash, liver dysfunction, and alopecia. Thrombocytopenia and neutropenia were the side effects of Suni+TACE that occurred more frequently than those of other combination therapies. Pyrexia was the side effect that occurred more frequently with Oran+TACE than with other combination therapies. Liu et al. (43) reported in their study that two patients in the Apat+TACE group had to stop the trial for antihypertensive treatment due to severe hypertension but could continue the trial after treatment. Lu et al. (15) found that patients with severe hand-foot syndrome need to stop taking medication for two weeks and resume treatment after symptoms subside. Two other patients withdrew from treatment due to severe hand-foot syndrome. Lencioni et al. (13) reported that 4 deaths in the Sora+TACE group might be related to Sora. Zhu et al. (32) reported that patients with ≥G3-AEs in the Sora+TACE group needed to reduce the dosage of Sora or interrupt treatment. In their study, Kudo et al. (14) reported a case of death within 30 days after receiving Sora+TACE treatment. Chen et al. (36) reported in their study that 6 patients were forced to stop taking Lenv+TACE due to uncontrolled hypertension. However, in other studies, adverse reactions can be controlled by reducing drug dosage or providing symptomatic therapy without the occurrence of drug-related deaths. Therefore, it can be considered that combination therapy is within an acceptable range and is tolerable. In our NMA, there was no statistically significant difference in the incidence of AEs and ≥G3-AEs among various treatment strategies.
In our NMA, the OS, ORR, and DCR of Apat+TACE were better than that of Lenv+TACE apart from PFS, but there was no statistical difference between them. Similarly, Zhang et al. (61) compared the efficacy of TACE combined with four different tyrosine kinase inhibitors (TKIs) in their study. They deemed that the OS, PFS, ORR, and DCR of Lenv+TACE were better than those of Apat+TACE, but the difference was not statistically significant. We considered the reasons as follows: First, for OS and PFS indicators, since most of them are extracted from the Kaplan-Meier curve, different extractors had different subjective feelings, resulting in different extracted data. Second, we did not include those studies that had received systemic HCC treatment before the experiment or received other therapies at the same time during the experiment, which was different from the study of Zhang et al. (61). As a result, it is necessary to carry out a sizeable multi-center RCT to compare the efficacy between Apat+TACE and Lenv+TACE.
As an oral multikinase inhibitor, Sora directly suppresses tumor growth by regulating RAF/MEK/ERK pathway (62). It can also indirectly inhibit tumor growth and proliferation by inhibiting VEGFR and PDGFR to inhibit tumor neovascularization (63). There are already many meta-analyses comparing Sora+TACE with TACE. Zhang et al. (64) reported that Sora+TACE significantly outperformed TACE in terms of 1-year OS, 2 years OS, 3 years OS, 5 years OS, ORR, and DCR. Patients tolerated combination treatment well, despite the possibility of side effects related to Sora. Li et al. (65) mainly focused on the efficacy comparison between Sora+TACE and TACE. They thought that OS and time to progression (TTP) of combined treatment were significantly better than monotherapy. Chen et al. (66) believed that compared to the monotherapy group, the combined treatment group showed a significant increase in OS, TTP, and ORR. Also, there was no statistically significant difference in the incidence of AEs between the two treatment groups.
In our NMA, the Apat+TACE group had significantly better OS, PFS, ORR, and DCR than the TACE group, while there was no statistically significant difference in AEs and ≥G3-AEs between the two groups. Many meta-analyses currently exist comparing Apat+TACE with TACE. Wei et al. (67) indicated that compared with the TACE group, the Apat+TACE group had significant benefits in 6 months OS, 1 year OS, and 2 years OS. Exception for the incidence of hand-foot syndrome, proteinuria, hypertension, and diarrhea, the Apat+TACE group was significantly higher than the TACE group. There was no statistical difference in the incidence of other adverse reactions. At the same time, Gong et al. (68) also concluded similarly to the foregoing.
Lenv, as an oral multi-kinase inhibitor, inhibits VEGFR-1/2/3, fibroblast growth factor receptor (FGFR) 1-4, and PDGFR-α, RET, and KIT targets, thereby inhibiting tumor cell growth and tumor angiogenesis (69). Liu et al. (70) conducted a meta-analysis comparing Lenv+TACE with Sora+TACE and found that the OS and PFS of the Lenv+TACE group were significantly better than those of Sora+TACE. In terms of safety, the incidence of hypertension and proteinuria was significantly higher in the Lenv+TACE group than in the Sora+TACE group, while the opposite was true for the hand-foot syndrome. The PFS of the Lenv+TACE group was significantly better than that of the Sora+TACE group in our NMA. In terms of OS, AEs, and ≥G3-AEs, there was no statistically significant difference between the two groups. As a result, there is disagreement on the efficacy of the comparison between Lenv+TACE and Sora+TACE, and a large, multicenter RCT is required to validate it.
Suni, as a tyrosine kinase inhibitor (TKI), targets PDGF-α/β, VEGFR-1/2/3, KIT, FLT-3, CSF-1, and RET. Briv, as a TKI, selectively inhibits VEGFR and FGFR. Anlo, as a TKI, targets VEGFR, FGFR, PDGFR, and c-kit (71). Oran, as a TKI, targets VEGFR-2 and PDGFR-β. Through the above mechanisms, it exerts its anti-tumor proliferation and anti-tumor angiogenesis effects (72). Due to the limited number of studies on the combination of the three MTAs combined with TACE for the treatment of HCC, there is no meta-analysis on the combination of the MTAs and TACE for the treatment of HCC.
Our NMA had the following advantages. Firstly, studies that used other systemic therapies before or during MTAs+TACE therapy or TACE monotherapy were excluded to reflect the efficacy of MTAs+TACE more accurately. Secondly, it summarized the current research on MTAs combined with TACE to compare the efficacy and safety of various MTAs+TACE.
At the same time, however, there were also some limitations in the NMA. Firstly, among the 45 studies we included, only 5 were related to the comparison between MTAs and TACE, while the rest was compared between MTAs+TACE and TACE. Therefore, merging and comparing the comparisons of MTAs+TACE may undermine the credibility of the research. Secondly, since the HR and 95% CIs of the OS and PFS were rarely directly provided by the original study, we needed to extract data from the curve. Due to the subjective nature of extracting data, the accuracy of HR and its CIs related to OS and PFS may be affected. Thirdly, out of the 45 studies we included, only 10 were RCTs, which may bring confounding factors to our research and lead to the risk of selective bias.
The network meta-analysis showed that apatinib+TACE displayed the best OS, ORR, and DCR with no additional AEs and ≥G3-AEs. Therefore, for the treatment scheme of MTAs combined with TACE, apatinib+TACE may be the most effective treatment.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
JL was responsible for writing the manuscript. BC and ZL analyzed the data. BC was responsible for research and design. JL, BC, and ZL searched the literature together. BC participated in the revision of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1179431/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Liu CY, Chen KF, Chen PJ. Treatment of liver cancer. Cold Spring Harb Perspect Med (2015) 5(9):a021535. doi: 10.1101/cshperspect.a021535
3. Sayiner M, Golabi P, Younossi ZM. Disease burden of hepatocellular carcinoma: a global perspective. Dig Dis Sci (2019) 64(4):910–7. doi: 10.1007/s10620-019-05537-2
4. Lee VHF, Seong J, Yoon SM, Wong TCL, Wang B, Zhang JL, et al. Contrasting some differences in managing advanced unresectable hepatocellular carcinoma between the East and the West. Clin Oncol (2019) 31(8):560–9. doi: 10.1016/j.clon.2019.06.002
5. Torimura T, Iwamoto H. Optimizing the management of intermediate-stage hepatocellular carcinoma: current trends and prospects. Clin Mol Hepatol (2021) 27(2):236–45. doi: 10.3350/cmh.2020.0204
6. Ranieri G, Marech I, Lorusso V, Goffredo V, Paradiso A, Ribatti D, et al. Molecular targeting agents associated with transarterial chemoembolization or radiofrequency ablation in hepatocarcinoma treatment. World J Gastroenterol (2014) 20(2):486–97. doi: 10.3748/wjg.v20.i2.486
7. Liu J, Yi J. Relationship between the changes of VEGF level and dendritic cells in peripheral blood of patients with hepatocellular carcinoma after transcatheter arterial chemoembolization. J Huazhong Univ Sci Technolog Med Sci (2007) 27(1):58–60. doi: 10.1007/s11596-007-0117-y
8. Alqahtani A, Khan Z, Alloghbi A, Said Ahmed TS, Ashraf M, Hammouda DM. Hepatocellular carcinoma: molecular mechanisms and targeted therapies. Medicina (Kaunas) (2019) 55(9):526. doi: 10.3390/medicina55090526
9. Akula SM, Abrams SL, Steelman LS, Emma MR, Augello G, Cusimano A, et al. RAS/RAF/MEK/ERK, PI3K/PTEN/AKT/mTORC1 and TP53 pathways and regulatory miRs as therapeutic targets in hepatocellular carcinoma. Expert Opin Ther Targets (2019) 23(11):915–29. doi: 10.1080/14728222.2019.1685501
10. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol (2018) 15(10):599–616. doi: 10.1038/s41571-018-0073-4
11. Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, et al. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut (2019) 69(8):1492–501. doi: 10.1136/gutjnl-2019-318934
12. Meyer T, Fox R, Ma YT, Ross PJ, James MW, Sturgess R, et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): a randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol Hepatol (2017) 2(8):565–75. doi: 10.1016/S2468-1253(17)30156-5
13. Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol (2016) 64(5):1090–8. doi: 10.1016/j.jhep.2016.01.012
14. Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, Takayama T, et al. Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer (2011) 47(14):2117–27. doi: 10.1016/j.ejca.2011.05.007
15. Lu W, Jin X-L, Yang C, Du P, Jiang FQ, Ma JP, et al. Comparison of efficacy between TACE combined with apatinib and TACE alone in the treatment of intermediate and advanced hepatocellular carcinoma: a single-center randomized controlled trial. Cancer Biol Ther (2017) 18(6):433–8. doi: 10.1080/15384047.2017.1323589
16. Kudo M, Han G, Finn RS, Poon RTP, Blanc JF, Yan L, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: a randomized phase III trial. Hepatology (2014) 60(5):1697–707. doi: 10.1002/hep.27290
17. Turpin A, de Baere T, Heurgué A, Le Malicot K, Ollivier-Hourmand I, Lecomte T, et al. Liver transarterial chemoembolization and sunitinib for unresectable hepatocellular carcinoma: results of the PRODIGE 16 study. Clin Res Hepatol Gastroenterol (2021) 45(2):101464. doi: 10.1016/j.clinre.2020.05.012
18. Hidaka H, Izumi N, Aramaki T, Ikeda M, Inaba Y, Imanaka K, et al. Subgroup analysis of efficacy and safety of orantinib in combination with TACE in Japanese HCC patients in a randomized phase III trial (ORIENTAL). Med Oncol (2019) 36(6):52. doi: 10.1007/s12032-019-1272-2
19. Kudo M, Cheng A-L, Park J-W, Park JH, Liang P-C, Hidaka H, et al. Orantinib versus placebo combined with transcatheter arterial chemoembolisation in patients with unresectable hepatocellular carcinoma (ORIENTAL): a randomised, double-blind, placebo-controlled, multicentre, phase 3 study. Lancet Gastroenterol Hepatol (2018) 3(1):37–46. doi: 10.1016/S2468-1253(17)30290-X
20. Inaba Y, Kanai F, Aramaki T, Yamamoto T, Tanaka T, Yamakado K, et al. A randomised phase II study of TSU-68 in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Eur J Cancer (2013) 49(13):2832–40. doi: 10.1016/j.ejca.2013.05.011
21. Kuang J, Wan D, Wan P, Wu D. Efficacy of sorafenib combined with transcatheter hepatic arterial chemoembolization in treating intermediate-advanced hepatocellular carcinoma. J BUON (2021) 26(3):868–74.
22. Koch C, Göller M, Schott E, Waidmann O, den Winkel M, Paprottka P, et al. Combination of sorafenib and transarterial chemoembolization in selected patients with advanced-stage hepatocellular carcinoma: a retrospective cohort study at three German liver centers. Cancers (2021) 13(9):2121. doi: 10.3390/cancers13092121
23. Zou X, Fan W, Xue M, Li J. Evaluation of the benefits of TACE combined with sorafenib for hepatocellular carcinoma based on untreatable TACE (unTACEable) progression. Cancer Manag Res (2021) 13:4013–29. doi: 10.2147/CMAR.S304591
24. Cao J, Zhang D, Zhang Y, Yue Y, Cai H, Zhang J, et al. Survival analysis of sorafenib combined with TACE in hepatocellular carcinoma patients. Int J Clin Exp Med (2020) 13(9):6823–8.
25. Liu Q, Dai Y. Sorafenib combined with transarterial chemoembolization prolongs survival of patients with advanced hepatocellular carcinoma. J BUON (2020) 25(2):945–51.
26. Liu KC, Hao YH, Lv WF, Jia WD, Ji CS, Zhou CZ, et al. Transarterial chemoembolization combined with sorafenib in patients with BCLC stage c hepatocellular carcinoma. Drug Des Devel Ther (2020) 14:3461–8. doi: 10.2147/DDDT.S248850
27. Wang Z, Wang E, Bai W, Xia D, Ding R, Li J, et al. Exploratory analysis to identify candidates benefitting from combination therapy of transarterial chemoembolization and sorafenib for first-line treatment of unresectable hepatocellular carcinoma: a multicenter retrospective observational study. Liver Cancer (2020) 9(3):308–25. doi: 10.1159/000505692
28. Ren B, Wang W, Shen J, Li W, Ni C, Zhu X. Transarterial chemoembolization (TACE) combined with sorafenib versus TACE alone for unresectable hepatocellular carcinoma: a propensity score matching study. J Cancer (2019) 10(5):1189–96. doi: 10.7150/jca.28994
29. Lei XF, Ke Y, Bao TH, Tang HR, Wu XS, Shi ZT, et al. Effect and safety of sorafenib in patients with intermediate hepatocellular carcinoma who received transarterial chemoembolization: a retrospective comparative study. World J Clin cases (2018) 6(5):74–83. doi: 10.12998/wjcc.v6.i5.74
30. Wan X, Zhai X, Yan Z, Yang P, Li J, Wu D, et al. Retrospective analysis of transarterial chemoembolization and sorafenib in Chinese patients with unresectable and recurrent hepatocellular carcinoma. Oncotarget (2016) 7(50):83806–16. doi: 10.18632/oncotarget.11514
31. Hu H, Duan Z, Long X, Hertzanu Y, Shi H, Liu S, et al. Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PloS One (2014) 9(5):e96620. doi: 10.1371/journal.pone.0096620
32. Zhu K, Chen J, Lai L, Meng X, Zhou B, Huang W, et al. Hepatocellular carcinoma with portal vein tumor thrombus: treatment with transarterial chemoembolization combined with sorafenib–a retrospective controlled study. Radiology (2014) 272(1):284–93. doi: 10.1148/radiol.14131946
33. Bai W, Wang YJ, Zhao Y, Qi XS, Yin ZX, He CY, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis (2013) 14(4):181–90. doi: 10.1111/1751-2980.12038
34. Muhammad A, Dhamija M, Vidyarthi G, Amodeo D, Boyd W, Miladinovic B, et al. Comparative effectiveness of traditional chemoembolization with or without sorafenib for hepatocellular carcinoma. World J Hepatol (2013) 5(7):364–71. doi: 10.4254/wjh.v5.i7.364
35. Qu XD, Chen CS, Wang JH, Yan Z, Chen J, Gong G, et al. The efficacy of TACE combined sorafenib in advanced stages hepatocellullar carcinoma. BMC Cancer (2012) 12:263. doi: 10.1186/1471-2407-12-263
36. Chen YX, Zhang JX, Zhou CG, Liu J, Liu S, Shi HB, et al. Comparison of the efficacy and safety of transarterial chemoembolization with or without lenvatinib for unresectable hepatocellular carcinoma: a retrospective propensity score–matched analysis. J Hepatocell Carcinoma (2022) 9:685–94. doi: 10.2147/JHC.S373250
37. Xie QY, Huang LP, Gao FW, Liu DQ, Wang X, Jiang KY, et al. Efficacy of lenvatinib combined with sequential transarterial chemoembolization for primary hepatocellular carcinoma and the effects on serum basic fibroblast growth factor and vascular endothelial growth factor. Front Pharmacol (2022) 13:965770. doi: 10.3389/fphar.2022.965770
38. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int (2021) 15(3):663–75. doi: 10.1007/s12072-021-10184-9
39. Li N, Chen J. Efficacy and safety of drug-eluting bead transarterial chemoembolization (DEB-TACE) plus apatinib versus DEB-TACE alone in treating huge hepatocellular carcinoma patients. Ir J Med Sci (2022) 191(6):2611–7. doi: 10.1007/s11845-021-02884-w
40. Li N, Yang P, Fang J. Transarterial chemoembolization (TACE) plus apatinib vs. TACE alone for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol (2022) 46(9):102022. doi: 10.1016/j.clinre.2022.102022
41. Wang H, Liu D, Wang C, Yu S, Jin G, Wang C, et al. Transarterial chemoembolization (TACE) plus apatinib-combined therapy versus TACE alone in the treatment of intermediate to advanced hepatocellular carcinoma patients: a real-world study. Clin Res Hepatol Gastroenterol (2022) 46(6):101869. doi: 10.1016/j.clinre.2022.101869
42. Kan X, Liang B, Zhou G, Xiong B, Pan F, Ren Y, et al. Transarterial chemoembolization combined with apatinib for advanced hepatocellular carcinoma: a propensity score matching analysis. Front Oncol (2020) 10:970. doi: 10.3389/fonc.2020.00970
43. Liu S, Zhao G, Yu G, Guo N, Zhang Y, Li Q, et al. Transcatheter arterial chemoembolization combined with low-dose apatinib in the treatment of unresectable hepatocellular carcinoma in elderly patients: efficacy and safety. J Cancer Res Ther (2020) 16(5):1165–70. doi: 10.4103/jcrt.JCRT_1030_19
44. Shen L, Chen S, Qiu Z, Qi H, Yuan H, Cao H, et al. Transarterial chemoembolization combined with apatinib versus transarterial chemoembolization alone for hepatocellular carcinoma with macroscopic vascular invasion: a propensity score matching analysis. J Cancer Res Ther (2020) 16(5):1063–8. doi: 10.4103/jcrt.JCRT_801_19
45. Liu J, Xie S, Duan X, Chen J, Zhou X, Li Y, et al. Assessment of efficacy and safety of the transcatheter arterial chemoembolization with or without apatinib in the treatment of large hepatocellular carcinoma. Cancer Chemother Pharmacol (2020) 85(1):69–76. doi: 10.1007/s00280-019-04004-z
46. Fan W, Yuan G, Fan H, Li F, Wu Y, Zhao Y, et al. Apatinib combined with transarterial chemoembolization in patients with hepatocellular carcinoma and portal vein tumor thrombus: a multicenter retrospective study. Clin Ther (2019) 41(8):1463–76. doi: 10.1016/j.clinthera.2019.04.036
47. Chen S, Yu W, Zhang K, Liu W. Comparison of the efficacy and safety of transarterial chemoembolization with and without apatinib for the treatment of BCLC stage c hepatocellular carcinoma. BMC Cancer (2018) 18(1):1131. doi: 10.1186/s12885-018-5081-3
48. Yang Z, Chen G, Cui Y, Xiao G, Su T, Yu J, et al. The safety and efficacy of TACE combined with apatinib on patients with advanced hepatocellular carcinoma: a retrospective study. Cancer Biol Ther (2019) 20(3):321–7. doi: 10.1080/15384047.2018.1529099
49. Guo W, Chen S, Wu Z, Zhuang W, Yang J. Efficacy and safety of transarterial chemoembolization combined with anlotinib for unresectable hepatocellular carcinoma: a retrospective study. Technol Cancer Res Treat (2020) 19:1533033820965587. doi: 10.1177/1533033820965587
50. Chen J, Zhou C, Long Y, Yin X. Sunitinib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. Tumour Biol (2015) 36(1):183–91. doi: 10.1007/s13277-014-2608-3
51. Ding X, Sun W, Li W, Shen Y, Guo X, Teng Y, et al. Transarterial chemoembolization plus lenvatinib versus transarterial chemoembolization plus sorafenib as first-line treatment for hepatocellular carcinoma with portal vein tumor thrombus: a prospective randomized study. Cancer (2021) 127(20):3782–93. doi: 10.1002/cncr.33677
52. Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T, et al. TACE plus lenvatinib versus TACE plus sorafenib for unresectable hepatocellular carcinoma with portal vein tumor thrombus: a prospective cohort study. Front Oncol (2021) 11:821599. doi: 10.3389/fonc.2021.821599
53. Cao Y, Sun T, Guo X, Ouyang T, Kan X, Chen L, et al. Sorafenib versus apatinib both combined transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a comparative retrospective study. Front Oncol (2021) 11:673378. doi: 10.3389/fonc.2021.673378
54. Qiu Z, Shen L, Jiang Y, Qiu J, Xu Z, Shi M, et al. Transarterial chemoembolization (TACE) combined with apatinib versus TACE combined with sorafenib in advanced hepatocellular carcinoma patients: a multicenter retrospective study. Ann Transl Med (2021) 9(4):283. doi: 10.21037/atm-20-5360
55. Xu Q, Huang Y, Shi H, Song Q, Xu Y. Sunitinib versus sorafenib plus transarterial chemoembolization for inoperable hepatocellular carcinoma patients. J BUON (2018) 23(1):193–9.
56. Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther (2015) 9:6075–81. doi: 10.2147/DDDT.S97235
57. Fathi Maroufi N, Rashidi MR, Vahedian V, Akbarzadeh M, Fattahi A, Nouri M. Therapeutic potentials of apatinib in cancer treatment: possible mechanisms and clinical relevance. Life Sci (2020) 241:117106. doi: 10.1016/j.lfs.2019.117106
58. Scott LJ. Apatinib: a review in advanced gastric cancer and other advanced cancers. Drugs (2018) 78(7):747–58. doi: 10.1007/s40265-018-0903-9
59. Mi YJ, Liang YJ, Huang HB, Zhao HY, Wu CP, Wang F, et al. Apatinib (YN968D1) reverses multidrug resistance by inhibiting the efflux function of multiple ATP-binding cassette transporters. Cancer Res (2010) 70(20):7981–91. doi: 10.1158/0008-5472.CAN-10-0111
60. Peng S, Zhang Y, Peng H, Ke Z, Xu L, Su T, et al. Intracellular autocrine VEGF signaling promotes EBDC cell proliferation, which can be inhibited by apatinib. Cancer Lett (2016) 373(2):193–202. doi: 10.1016/j.canlet.2016.01.015
61. Zhang Z, Wu Y, Zheng T, Chen X, Chen G, Chen H, et al. Efficacy of transarterial chemoembolization combined with molecular targeted agents for unresectable hepatocellular carcinoma: a network meta-analysis. Cancers (2022) 14(15):3710. doi: 10.3390/cancers14153710
62. Kong FH, Ye QF, Miao XY, Liu X, Huang SQ, Xiong L, et al. Current status of sorafenib nanoparticle delivery systems in the treatment of hepatocellular carcinoma. Theranostics (2021) 11(11):5464–90. doi: 10.7150/thno.54822
63. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res (2004) 64(19):7099–109. doi: 10.1158/0008-5472.CAN-04-1443
64. Zhang T, Huang W, Dong H, Chen Y. Trans-catheter arterial chemoembolization plus sorafenib, an unsuccessful therapy in the treatment of hepatocellular carcinoma?: a systematic review and meta-analysis. Med (Baltimore) (2020) 99(29):e20962. doi: 10.1097/MD.0000000000020962
65. Li H, Li S, Geng J, Zhao S, Tan K, Yang Z, et al. Efficacy evaluation of the combination therapy of sorafenib and transarterial chemoembolization for unresectable HCC: a systematic review and meta-analysis of comparative studies. Ann Transl Med (2020) 8(8):540. doi: 10.21037/atm.2020.02.115
66. Chen A, Li S, Yao Z, Hu J, Cao J, Topatana W, et al. Adjuvant transarterial chemoembolization to sorafenib in unresectable hepatocellular carcinoma: a meta-analysis. J Gastroenterol Hepatol (2021) 36(2):302–10. doi: 10.1111/jgh.15180
67. Wei Y, Liu J, Yan M, Zhao S, Long Y, Zhang W. Effectiveness and safety of combination therapy of transarterial chemoembolization and apatinib for unresectable hepatocellular carcinoma in the Chinese population: a meta-analysis. Chemotherapy (2019) 64(2):94–104. doi: 10.1159/000502510
68. Gong A, Li X. The efficacy and safety of apatinib combined with TACE in the treatment of hepatocellular carcinoma: a meta-analysis. World J Surg Oncol (2022) 20(1):69. doi: 10.1186/s12957-021-02451-8
69. Koyama N, Saito K, Nishioka Y, Yusa W, Yamamoto N, Yamada Y, et al. Pharmacodynamic change in plasma angiogenic proteins: a dose-escalation phase 1 study of the multi-kinase inhibitor lenvatinib. BMC Cancer (2014) 14:530. doi: 10.1186/1471-2407-14-530
70. Liu JN, Li JJ, Yan S, Zhang GN, Yi PS. Transarterial chemoembolization combined with lenvatinib versus transarterial chemoembolization combined with sorafenib for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Front Oncol (2023) 13:1074793. doi: 10.3389/fonc.2023.1074793
71. Shen G, Zheng F, Ren D, Du F, Dong Q, Wang Z, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol (2018) 11(1):120. doi: 10.1186/s13045-018-0664-7
Keywords: transarterial chemoembolization, molecular targeted agents, hepatocellular carcinoma, network meta-analysis, systematic review
Citation: Long J, Chen B and Liu Z (2023) Comparative efficacy and safety of molecular targeted agents combined with transarterial chemoembolization in the treatment of unresectable hepatocellular carcinoma: a network meta-analysis. Front. Oncol. 13:1179431. doi: 10.3389/fonc.2023.1179431
Received: 04 March 2023; Accepted: 09 May 2023;
Published: 17 May 2023.
Edited by:
Tommaso Maria Manzia, University of Rome Tor Vergata, ItalyCopyright © 2023 Long, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoxiang Chen, MjgwNDk2MzQwQHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.