- 1Department of Oncology, Hebei General Hospital, Shijiazhuang, Hebei, China
- 2Department of Radiotherapy, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, China
Background: The treatment of local–regional recurrent breast cancer (BC) after external beam radiotherapy is challenging. We aim to evaluate the effectiveness and safety of computed tomography (CT)–guided percutaneous iodine-125 brachytherapy for local recurrent BC.
Methods: We retrospectively analyzed 15 patients with local recurrent BC treated with CT-guided interstitial implantation of iodine-125 seeds. Regular contrast-enhanced CT was conducted to evaluate the tumor response. Follow-up survival, quality of life, and adverse events were analyzed.
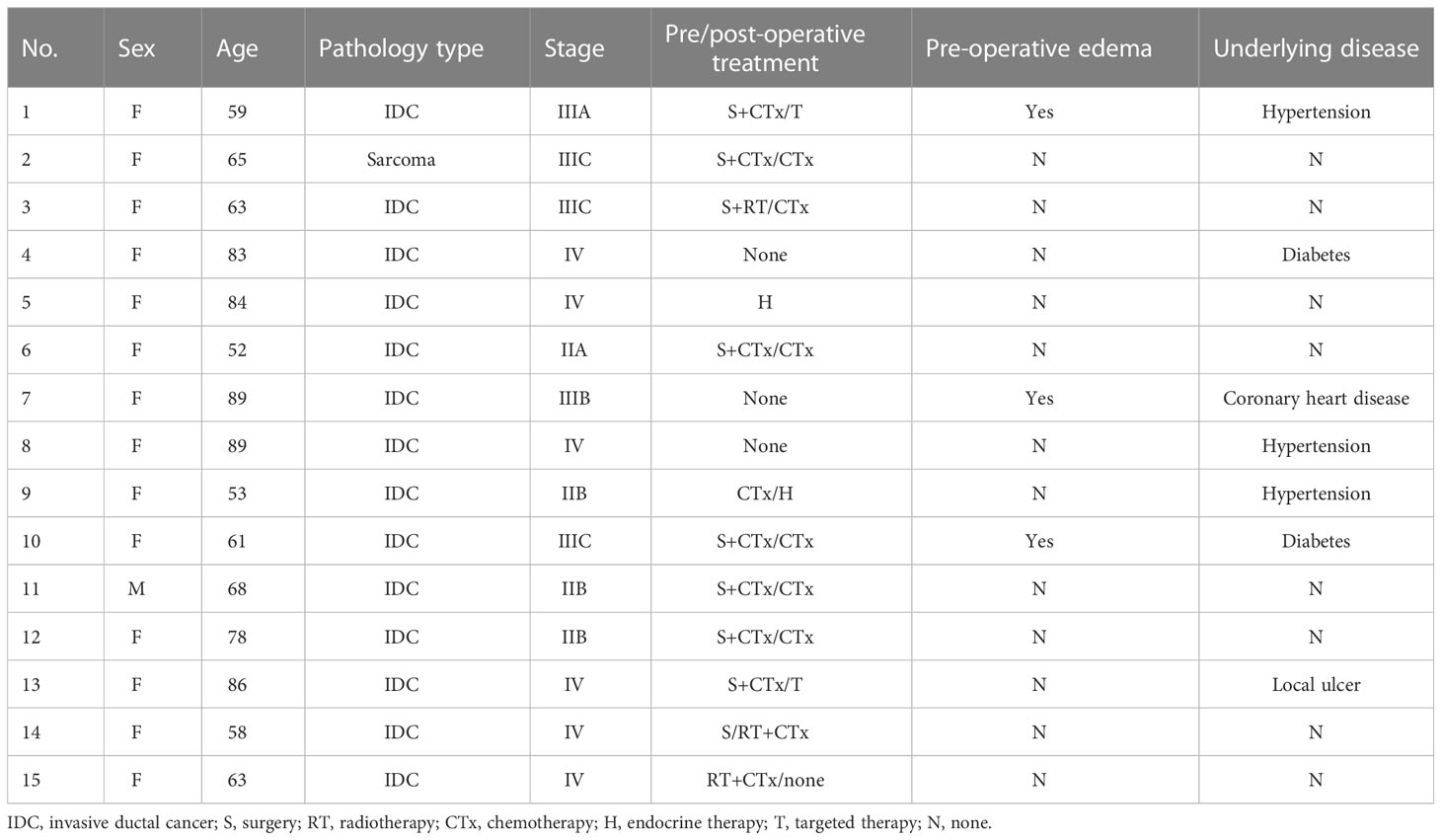
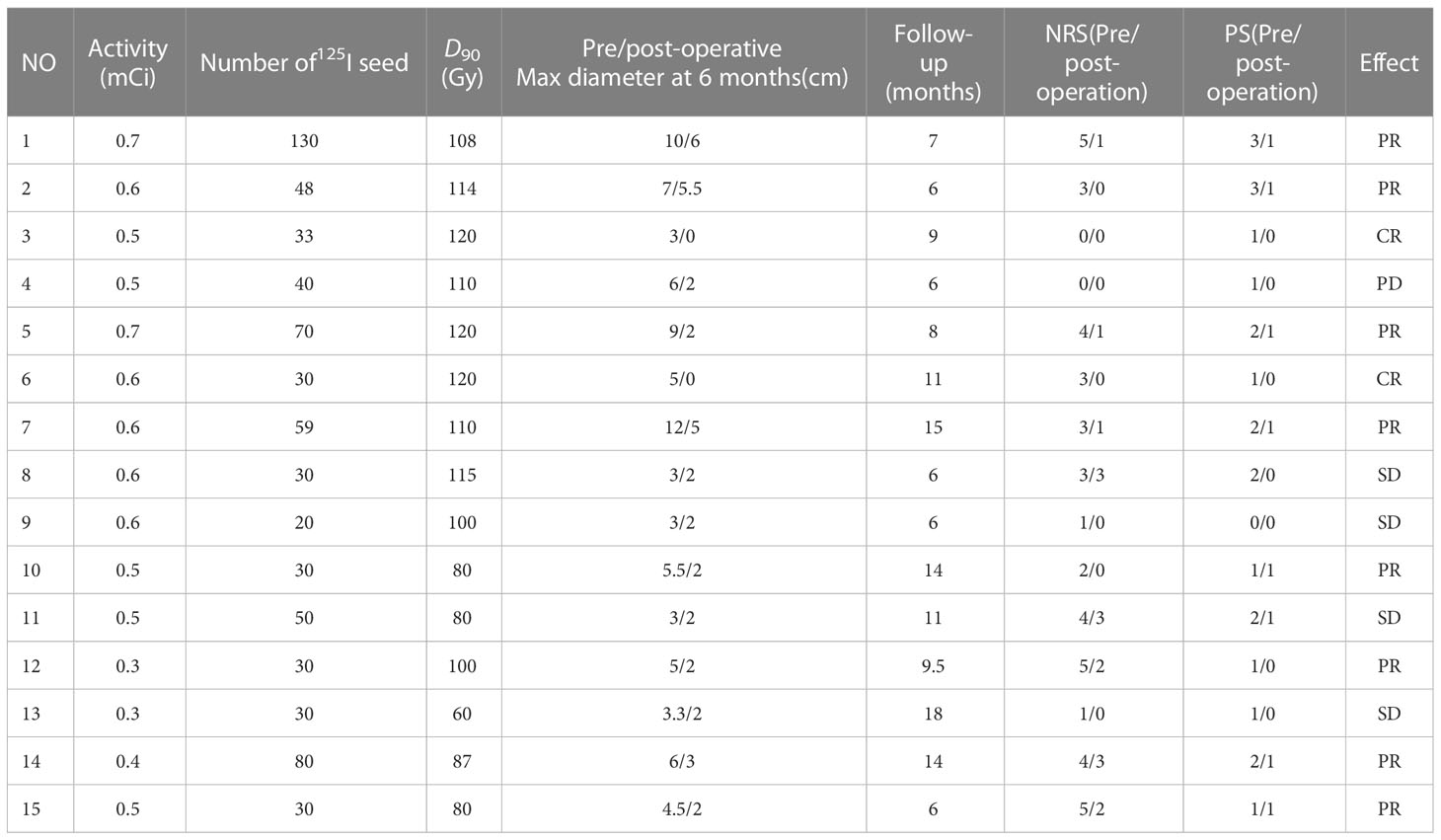
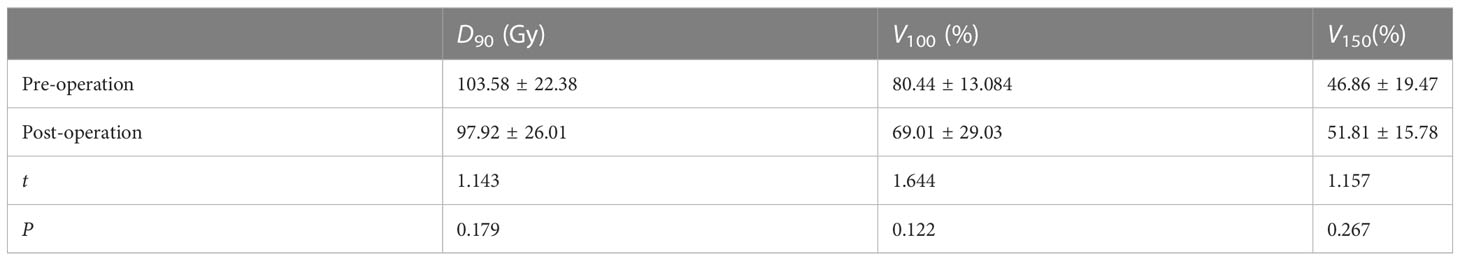
Results: Among the 15 patients, five were elderly patients (older than 80 years) and six were complicated with chronic underlying diseases. The median number of 125I seeds implantation was 33 (range: 20–130) with median dose 90 (D90, the minimum dose covering 90% of the target volume) of 108 Gy (range: 60–120 Gy). There was no significant difference in D90, V100 (the volume of the target receiving 100% of the prescription dose), and V150 (the volume of the target receiving 150% of the prescription dose) before and after operation (p > 0.05). The median follow-up was 14 months (range: 6–18 months). Six months after operation, the ORR was 66.7% (10/15) and the LCR was 93.3% (14/15). The 6- and 12-month survival rates were 100 and 41.6%, respectively, and the median survival time was 12.5 months. PS score decreased from 1.53 ± 0.81 to 0.53 ± 0.49. The pain score decreased from 2.87 ± 1.67 before operation to 1.07 ± 1.18 after operation, and the differences were statistically significant (p< 0.05). No severe complications occurred.
Conclusions: CT-guided iodine-125 brachytherapy provided a safe and effective choice for recurrent BC with significant local therapeutic effects and minor complications, especially for elderly patients with chronic underlying disease and those who were not eligible for surgical resection and had failed to benefit from systemic therapy.
Introduction
Breast cancer (BC) is the most common malignancy in women, and the latest data showed that BC had been the most commonly diagnosed cancer worldwide and the leading cause of cancer death worldwide in women in 2020 (1). In China, the latest data from the National Cancer Center demonstrate that its morbidity ranks the fifth (2). To date, external beam radiotherapy (EBRT), which could reduce the local recurrence rate and prolong the progression-free survival (PFS) of BC patients, has become the standard treatment after modified radical mastectomy and Breast-Sparing Surgery (3, 4). Unfortunately, approximately 5–30% of patients with BC who underwent surgical resection, chemoradiotherapy, endocrine, and/or target therapy presented with local recurrence (5, 6). Recurrence is considered the major prognostic factor for BC patients; to data, the treatment is challenging, given their specific location and the modality of previous multiple therapies, such as surgery, chemotherapy, and radiation. Currently, there is no standard treatment approach for recurrent BC. Iodine-125 (125I) seed implantation with low-dose rate (LDR), is a type of brachytherapy radiation technique that is characterized by extremely a sharp drop-fall off dose achieving an accurate dose delivery and sparing the normal tissues that EBRT could never achieve within treated lesions, has been considered as a standard therapy for localized prostate carcinoma (7, 8). To date, an increasing number of studies showed adequate efficacy of 125I brachytherapy in many solid tumors (9–11). However, there are a few reports regarding 125I brachytherapy for recurrent BC. Therefore, we conducted this study to evaluate the effectiveness and safety of 125I brachytherapy for the treatment of recurrent BC patients to provide an alternative therapy for these difficult cases.
Material and methods
Patients
A total of 15 patients diagnosed with chest wall recurrence BC (total of 15 lesions) who underwent interstitial 125I brachytherapy as a salvage treatment with a CT-guided coplanar template-assisted technique between December 2011 and May 2022 in our hospital were retrospectively collected. Among these patients, one was man, 14 were women, while five of these 15 patients were elderly patients (older than 80 years), six of these 15 patients were complicated with chronic underlying diseases (such as hypertension, diabetes, coronary heart disease), and one of these patients with local huge ulcer at the chest wall recurrent lesion. All of these patients had previously received surgical resection, chemotherapy, and external beam radiation. Baseline clinical characteristics of these patients were shown in Table 1. This study was approved by the institutional review board of our hospital, and written informed consent was obtained from all of the patients. The inclusion criteria in our study were all follows: All cases were pathologically diagnosed, and inability to tolerate or refuse surgical resection and external radiotherapy; all patients agreed to receive 125I brachytherapy as a salvage treatment; Karnofsky performance status (KPS) ≥ 70; blood routine examination and coagulation function were normal; and expected survival ≥ 3 months.
The exclusion criteria were all follows: severe organ dysfunction; coagulation dysfunction, anticoagulant therapy should be stopped at least 5–7 days before implantation; poor general condition or cachexia; the interval from last radiotherapy was less than 3 months; no CT and other imaging data after 125I seed implantation.
Instrument and equipment
The 18-G implantation needles and seed implant applicator were provided from Mick Radio-Nuclear company, USA. The 125I seeds (ZHIBO Bio-Medical Tech Ltd. Beijing, China) used in this study were cylindrical and 4.5-mm long with a diameter of 0.8 mm. The seed activity was 0.3–0.7 mCi and had a half-life of 59.4 days. The γ-ray energy was 27–35 KeV. The tissue half-value layer was 1.7 cm. About 93–97% of the energy of the seed was delivered into the tumor after 8–10 months.
A treatment planning system (TPS) (Panther Brachy version 5.0 TPS, Prowess Inc., Concord, CA, USA) was used to develop a treatment plan. The RM-905a radioactivity meter was provided by the Chinese Institute of Metrology. PET-CT (Discovery CT750 HD, GE, USA) was used to scan images.
Methods of 125I seeds implantation
Preoperative preparation: Enhanced CT scan (slice thickness 5 mm) was performed 1 week before surgery, and the images were transmitted to TPS to make preoperative plan. The dose of 125I seed implantation was prescribed as D90, V100, and V150, which encompassed the planning treatment volume (PTV). PTV included the entire gross tumor volume (GTV) and 0.5- to 1.0-cm margins.
Intraoperative operation: The position was consistent with the preoperative design, local anesthesia with 1% lidocaine, CT, and ultrasound localization scan to determine the puncture point, angle, and depth of the body surface. According to the preoperative plan, the center was sparsely implanted with particles spaced between 0.5–1.0 cm. CT scan was performed immediately after operation to observe the spatial distribution of particles, and the existing dosimetric cold area was re-implanted.
Postoperative treatment: The quality of postoperative CT scan images was verified by transferring them into TPS. The actual dose to the tumor was obtained according to the dose-volume histogram (DVH).
Treatment of post 125I seed implantation
After 125I seed implantation, seven of these 15 patients received four to six cycles of chemotherapy, two patients just received endocrine therapy, two patients received target therapy, and the other four patients received no antitumor drug therapy. Chemotherapy and endocrine therapy were performed according to the latest guidelines of the National Comprehensive Cancer Network (NCCN).
Follow-up and clinical efficacy evaluation
Follow-up CT was conducted at 1 month after the procedure, 3 months after the procedure, and then every 3 months. The primary end points were the objective response rate (ORR) and tumor local control rate (LCR). The secondary end points included the clinical benefit response (CBR), overall survival (OS), and incidence of adverse events. The ORR was defined as the proportion of patients achieving complete response (CR) and partial response (PR). LCR = (CR + PR + SD/total cases)%. The clinical efficacy evaluation of 125I brachytherapy was assessed by the Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1). The CBR was evaluated according to the PS score and pain score. The pain was evaluated by digital assessment (NRS) and positive CBR indicating an improvement in the patient’s functional impairment. PS score according to eastern cooperative oncology group (ECOG): 0-5. Upper limb edema was measured by measuring the circumcubitus 10 cm. OS was defined as the time between the date of 125I brachytherapy and the last follow-up or death. Adverse reactions were assessed according to the new classification of the Society of Interventional Radiology.
Complications
The patients were observed for fever, bleeding, bone marrow suppression, liver and kidney dysfunction, radioactive skin and mucosa reaction, particle displacement, and other symptoms after seed implantation. The skin-mucosal response was assessed according to the Radiological Collaboration in Oncology/European Research and Treatment in Oncology (RTOC/EORTC) radiological injury grading criteria.
Statistical analysis
The data analysis was performed using SPSS (version 24.0). Continuous variables were expressed as the mean ± SD. Paired t-test was used to analyze the scores of D90, V100, and V150 and pain and physical status before and after surgery. Comparisons of PS before and after the procedure were performed using the Mann–Whitney U test. Survival analysis was assessed by Kaplan–Meier methods. p< 0.05 was considered as statistical significance.
Results
125I implantation and dosimetry description
A total of 15 patients received 125I seed implantation to meet the TPS criteria and followed by a postoperative dose evaluation. The seed activity was 0.3–0.7 mCi, and the median number of 125I seeds implanted was 33 (range: 20–130) with a median dose 90 (D90) of 108 Gy (range: 60–120 Gy) (Table 2). There was no significant difference in D90, V100, and V150 before and after operation (p > 0.05), as shown in Table 3.
Clinical efficacy evaluation and overall survival
The patients were followed for 6–18 months (median: 9 months). Six months after operation, among the 15 patients, CT images showed that there were two cases of CR, eight cases of PR, four cases of SD, and one case of PD (Figure 1, CT images of 2 patients showed CR after 125I seed implantation therapy). The ORR was 66.7% (10/15), and the LCR was 93.3% (14/15) at 6 months. The maximum diameter of the target lesion decreased from (5.69 ± 2.69) cm before operation to (2.50 ± 1.74) cm after operation, and the difference was statistically significant (p< 0.05). At last of follow-up, five of these patients died of systemic metastasis and three of 15 patients died of organ failure. The 6- and 12-month OS were 100 and 41.6%, respectively, and the median survival time was 12.5 months.

Figure 1 (A) An 89-year-old woman with the chest wall lesion recurred from right BC was accurately inserted into the tumor to implant 125I seeds. (a) Pre-brachytherapy; (b) 3 months after brachytherapy, tumor shrinked; (c) 5 months after brachytherapy, tumor disappeared; (d) 9 months after brachytherapy, no recurrence. (B) An 82-year-old woman with the chest wall lesion recurred from right BC. (e) Pre-brachytherapy; (f) 1 month after brachytherapy, tumor shrinked; (g) 11 months after brachytherapy, tumor disappeared; (h) 15 months after brachytherapy, no recurrence.
After operation, 11 patients received medicine therapy, including chemotherapy, endocrine and target, two cases showed CR, six cases with PR, three cases with SD, the ORR was 72.7%, LCR was 100%, the 6- and 12- month OS rate were 100 and 27.3%, respectively. While four patients just received single-radioactive seed implantation therapy, two cases showed PR, one with SD, one with PD, no case showed CR, ORR was 50%, LCR was 75%, and the 6- and 12-month OS were 75 and 25%, respectively. Survival analysis was shown in Figure 2. It seems that patients who received drug therapy after 125I seed implantation therapy has better local control and 6-OS.
Clinical benefit response
We used PS and pain score to evaluate the CBR. PS score of these patients decreased from 1.53 ± 0.81 to 0.53 ± 0.49. The pain score decreased from 2.87 ± 1.67 before operation to 1.07 ± 1.18 after operation, and the differences were statistically significant (p< 0.05). The swelling of three cases of upper limb edema was gradually reduced within 1–14 days after operation, as shown in Table 1.
Adverse events
Among these patients, five of 15 patients were elderly patients (older than 80 years), and six of 15 patients were complicated with chronic underlying diseases. There were no complications such as fever, hemorrhage, bone marrow suppression, liver and kidney dysfunction, radioactive skin and mucous membrane reaction, and particle displacement during follow-up period in all of these 15 patients. Furthermore, one patient with local huge ulcer at the recurrent lesion of the chest wall also has no radioactive skin injury after operation.
Discussion
BC has been the most commonly diagnosed cancer worldwide and the leading cause of cancer death worldwide in women. For patients with local recurrence and metastasis, the overall prognosis is poor. Study showed that the majority of BC recurrences occur during the first decade after initial diagnosis with a peak incidence 2–5 years (12). However, the 10-year OS of patients with postoperative recurrence of BC was 39% and the distant disease-free survival rates was 36%, even after successful salvage mastectomy (13). The treatment for loco-regionally recurrent BC is challenging, especially the cases previously treated with multi-modality therapy. The most common therapies such as surgery, EBRT, chemotherapy, or hormonal interventions have limitations. Some large local-regional recurrent tumors cannot be completely removed. Chemotherapy and hormonal interventions failed to achieve therapeutic benefits; the second EBRT has certain curative effect, but with increasing toxicities (14). Therefore, another palliative therapy should be considered.
Brachytherapy demonstrates definite curative effect and few complications, which has been a safe, effective, and extremely versatile radiation technique (15). Meanwhile, existing studies have preliminarily confirmed that brachytherapy is also an ideal technique for residual disease and recurrences in BC patients based on its efficacy, appropriate toxicity, and cosmetic outcomes (15). 125I seed implantation is LDR brachytherapy for the release of low-energy γ-rays to kill tumor cells continuously; it is so safe with a sharp drop-fall off dose, and only 1.7-cm soft tissue could shield half the radiation dosage (16). To date, 125I brachytherapy is emerging as a salvage method for the treatment of many tumors, such as recurrent gliomas, retroperitoneal recurrent carcinoma, the head and neck, and recurrent ovarian cancer (17–20). In our center, 125I seed implantation is confirmed as a effective and safe therapy in most solid tumors, such as lung cancer (21), skin squamous cell carcinoma (22), malignant solitary fibrous tumor (23), and also as an effective salvage treatment for lymph metastases or loco-regional recurrence (24, 25). Furthermore, 125I seed implantation has become a promising new strategy for the treatment of cancer pain because of bone metastasis (26). However, there are few reports on local recurrence and metastasis BC patients, especially elderly patients with complications.
The current study preliminarily analyzed the clinical efficacy and safety of 125I seed implantation in the treatment of 15 cases of local recurrent metastatic BC patients. Results showed that the ORR and LCR of 6 months after 125I seed implantation therapy were 66.7 and 93.3%, respectively; the 6- and 12-month OS were 100 and 41.6%, respectively; and the median survival time was 12.5 months, no complications related to radioactive seed implantation occurred. These results were similar to Yu YH’study (27), who retrospectively analyzed 36 locoregionally recurrent and unresectable BCs to assess the efficacy of 125I seed implantation brachytherapy as a palliative management, results showed that 6-month local control was 97.2%, pain relief response rate was 88.9%, no serious complications were detected during the follow-up period, but 1-year OS was 97.2%, higher than that in our study (41.6%), and the reasons were analyzed that most of the BC patients in our study were elderly patients with underlying disease including heart disease, diabetes, and so forth. Furthermore, the diameter of tumor lesions were relatively large, the maximum diameter of tumors in this study was 12 cm, no tumors were less than 3cm, 60% of tumors were more than 5 cm, and last but not least, most patients had multiple systemic metastases; the systemic condition was poor. Some cases showed local tumor rupture accompanied by infection, so the prognosis was poor and the survival rate was relatively low. It was worth noting that we found that the pain score of BC patients decreased from 2.87 ± 1.67 before operation to 1.07 ± 1.18 after operation; PS score of these patients decreased from 1.53 ± 0.81 to 0.53 ± 0.49. It suggests that 125I brachytherapy is an effective method for the treatment of cancer pain. Furthermore, we also found that patients who received drug therapy after 125I seed implantation therapy seems has better local control and short survival, but it need larger cohort to confirm our findings.
To date, the dose and the activity choice of 125I seed implantation are controversial, no standard guidelines. Our previous study showed that 125I seed brachytherapy with a dose (EQD2) of 85-123.25Gy was safe for previously treated patients (23). In this study, the activity of125I seed implanted was 0.3–0.7 mCi, the median dose of 125I seeds implanted was 130 Gy (range: 110–160 Gy), and median dose of D90 was 97.92 ± 26.01 Gy (range: 80–120 Gy) after operation; there was no significant complications. Yu YH et al (26)reported the median activity of 125I seed was 0.6 mCi (range: 0.4–0.7mCi), which was similar to our study, but MPD was 110Gy (range: 90–140 Gy), lower than that in our center. The experience of our center was based on the location, volume, and distance between the target area and the surrounding organs: First, some patients had received external radiotherapy and their overall condition was poor, the prescription dose was reduced, and the irradiation in the high-dose area was reduced. For example, the D90 of ulcerative cancer focus of case no. 13 was 60 Gy. On the one hand, due to the patient’s previous external radiotherapy, the tumor was large and the surface skin was ruptured and infected. The target area of this case was zero distance from the skin of the organ at risk, and we selected the particle activity with 0.3 mCi. The ulcer was reduced and stable after surgery, and no complications occurred. Second, based on the pathological type, BC is moderately sensitive to radiation, and prescription dose should be reduced.
This study has several limitations. First, it is a retrospective study; prospective multi-center study is needed in the future to avoid the bias. Second, the number of patients is relatively small; a larger cohort should be conducted in the future to confirm our findings. Finally, this is a single-arm study, no parallel controls, and only external historical data can be compared with evaluate the safety and efficacy of this method. It is difficult to obtain historical research data that are completely consistent with the current study design, resulting in a bias in evaluating the results.
In conclusion, CT-guided 125I seed implantation brachytherapy is a safe and effective radiation technique for recurrent BC with definite curative local therapeutic effect and safety. This therapy may become a promising new strategy for local-regional recurrent BC patients, especially for elderly patients with complications who are not eligible for surgical resection and/or fail to benefit from EBRT and systemic therapy.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the institutional review board of Hebei General Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HZ was responsible for the overall design of this study. JW and XC was responsible for manuscript writing as well as data analysis. KX, YL JZ and ZL were responsible for data collection. All the authors reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135(5):584–590. doi: 10.1097/CM9.0000000000002108
3. Speers C, Pierce LJ. Postoperative radiotherapy after breast-conserving surgery for early-stage breast cancer: A review. JAMA Oncol (2016) 2(8):1075–82. doi: 10.1001/jamaoncol.2015.5805
4. Liu B, Huang H, Pan L, Ma Y. Value analysis of neoadjuvant radiotherapy for breast cancer after modified radical mastectomy based on data mining. Comput Intell Neurosci (2022) 2022:6257536. doi: 10.1155/2022/6257536
5. Ragaz J, Olivotto IA, Spinelli JJ, Phillips N, Jackson SM, Wilson KS, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst (2005) 97(2):116–26. doi: 10.1093/jnci/djh297
6. Zhao XR, Xuan L, Yin J, Tang Y, Sun HR, Jing H, et al. Prognosis and prophylactic regional nodal irradiation in breast cancer patients with the first isolated chest wall recurrence after mastectomy. Front Oncol (2021) 10:600525. doi: 10.3389/fonc.2020.600525
7. Wei S, Li C, Li M, Xiong Y, Jiang Y, Sun H, et al. Radioactive iodine-125 in tumor therapy: advances and future directions. Front Oncol (2021) 11:717180. doi: 10.3389/fonc.2021.717180
8. Langley SE, Laing RW. Iodine seed prostate brachytherapy: an alternative first-line choice for early prostate cancer. Prostate Cancer Prostatic Dis (2004) 7(3):201–7. doi: 10.1038/sj.pcan.4500727
9. Shin HS. Comments on: Safety and efficacy of 3D-printed templates assisted CT-guided radioactive iodine-125 seed implantation for the treatment of recurrent cervical carcinoma after external beam radiotherapy. J Gynecol Oncol (2021) 32(2):e50. doi: 10.3802/jgo.2021.32.e5
10. Xu T, Peng WD, Gu X, Yu WJ. Endobronchial ultrasound-guided iodine-125 radioactive seed implantation as a novel therapy for mediastinal tumors. Cancer Biother Radiopharm (2019) 34(9):547–50. doi: 10.1089/cbr.2019.2818
11. Zhang F, Zheng L, Li D, Yang H. To explore the curative effect of CT-guided Iodine-125 radioactive seed implantation in the treatment of stage IVprimary hepatocellular carcinoma. J Interv Med (2021) 4(2):82–6. doi: 10.1016/j.jimed.2021.02.009
12. Retsky M, Demicheli R. Multimodal hazard rate for relapse in breast cancer: quality of data and calibration of computer simulation. Cancers (Basel) (2014) 6(4):2343–55. doi: 10.3390/cancers6042343
13. Voogd AC, van Oost FJ, Rutgers EJ, Elkhuizen PH, van Geel AN, Scheijmans LJ, et al. Dutch Study Group on Local Recurrence after Breast Conservation (BORST Group). Long-term prognosis of patients with local recurrence after conservative surgery and radiotherapy for early breast cancer. Eur J Cancer (2005) 41(17):2637–44. doi: 10.1245/s10434-013-3032-4
14. Weksberg DC, Allen PK, Hoffman KE, Litton JK, Strom EA, Shah RR, et al. Outcomes and predictive factors for salvage therapy after local–regional recurrence following neoadjuvant chemotherapy and breast conserving therapy. Ann Surg Oncol (2013) 20(11):3430–7. doi: 10.3390/cancers14102564
15. Cozzi S, Augugliaro M, Ciammella P, Botti A, Trojani V, Najafi M, et al. The role of interstitial brachytherapy for breast cancer treatment: an overview of indications, applications, and technical notes. Cancers (Basel) (2022) 14(10):2564. doi: 10.3390/cancers14102564
16. Li Q, Tian Y, Yang D, Liang Y, Cheng X, Gai B. Permanent iodine-125 seed implantation for the treatment of nonresectable retroperitoneal malignant tumors. Technol Cancer Res Treat (2019) 18:1533033819825845. doi: 10.1177/1533033819825845
17. Wang C, Liu S, Peng L, Zhang K, Li W, Zhang H, et al. Permanent iodine-125 brachytherapy for patients with progressive or recurrent high-grade gliomas. BMC Cancer (2020) 20(1):591. doi: 10.1186/s12885-020-07086-8
18. Jiang W, Jiang P, Wei S, Jiang Y, Ji Z, Sun H, et al. The accuracy and safety of CT-guided iodine-125 seed implantation assisted by 3D non-coplanar template for retroperitoneal recurrent carcinoma. World J Surg Oncol (2020) 18(1):307. doi: 10.1186/s12957-020-02087-0
19. Jiang Y, Ji Z, Guo F, Peng R, Sun H, Fan J, et al. Side effects of CT-guided implantation of 125I seeds for recurrent malignant tumors of the head and neck assisted by 3D printing non co-planar template. Radiat Oncol (2018) 13(1):18. doi: 10.1186/s13014-018-0959-4
20. Liu P, Tong L, Huo B, Dai D, Liu W, Wang K, et al. CT-guided 125I brachytherapy for recurrent ovarian cancer. Oncotarget (2017) 8(35):59766–76. doi: 10.18632/oncotarget.15905
21. Chen E, Wang J, Zhang H, Zhang Y, Jia C, Min X, et al. Analysis of the efficacy and safety of iodine-125 seeds implantation in the treatment of patients with inoperable early-stage non-small cell lung cancer. J Contemp Brachyther (2021) 13(3):347–57. doi: 10.5114/jcb.2021.106241
22. Liang Y, Di X, Liu Z, Zhao J, Wang Z, Zhao J, et al. 125I seeds implantation for an elderly patient of skin squamous cell carcinomas with ulcer guided by ultrasound. J Cancer Res Ther (2018) 14(7):1660–4. doi: 10.4103/jcrt.JCRT_1032_17
23. Gao Z, Yu H, Di X, Zhao J, Liang Y, Liu Z, et al. Case report: 125I seed implantation for rare malignant solitary fibrous tumor in the pelvic cavity: a case report. Front Oncol (2022) 12:884491. doi: 10.3389/fonc.2022.884491
24. Zhang Y, Liu Z, Liang Y, Chen E, Zhang H, Gao Z, et al. The effectiveness and prognostic factors of radioactive iodine-125 seed implantation for the treatment of cervical lymph node recurrence of esophageal squamous cell carcinoma after external beam radiation therapy. J Contemp Brachyther (2020) 12(6):579–85. doi: 10.5114/jcb.2020.101691
25. Yu H, Zhang H, Gao Z, Liu X, Zhang L, Di X, et al. 125I seed brachytherapy for refractory loco-regional recurrence of non-anaplastic thyroid cancer. Front Oncol (2022) 12:773708. doi: 10.3389/fonc.2022.773708
26. Yang Z, Chen G, Cui Y, Su T, Yu J, Xiao G, et al. Iodine-125 seed implantation combined with arterial chemoembolization therapy for pain palliation in metastatic bone cancer: a retrospective study. Cancer Biol Ther (2019) 20(2):212–8. doi: 10.1080/15384047.2018.1523847
Keywords: iodine-125 seed, brachytherapy, computed tomography guidance, breast cancer, salvage therapy, recurrence
Citation: Wang J, Chang X, Xu K, Liang Y, Zhao J, Liu Z and Zhang H (2023) CT-guided iodine-125 brachytherapy as salvage therapy for local-regional recurrent breast cancer. Front. Oncol. 13:1171813. doi: 10.3389/fonc.2023.1171813
Received: 03 April 2023; Accepted: 06 July 2023;
Published: 18 August 2023.
Edited by:
Georgios S. Limouris, National and Kapodistrian University of Athens, GreeceReviewed by:
Athanasios G. Zafeirakis, Army Share Fund Hospital (NIMTS), GreeceZhe Ji, Peking University Third Hospital, China
Copyright © 2023 Wang, Chang, Xu, Liang, Zhao, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongtao Zhang, aG9uZ3Rhb3poYW5nbWRAMTYzLmNvbQ==
 Juan Wang1
Juan Wang1 Xiaojing Chang
Xiaojing Chang Yansong Liang
Yansong Liang Hongtao Zhang
Hongtao Zhang