
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 October 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1170464
 Xinrui Liang1†
Xinrui Liang1† Yu Wang2†
Yu Wang2† Guanghua Fu3
Guanghua Fu3 Pingmig Fan2
Pingmig Fan2 Ke Ma4
Ke Ma4 Xu-Chen Cao4
Xu-Chen Cao4 Guang-Xun Lin5*‡
Guang-Xun Lin5*‡ Wu-ping Zheng3*‡
Wu-ping Zheng3*‡ Peng-fei Lyu2*‡
Peng-fei Lyu2*‡Parts of this article's content have been modified or rectified in:
Erratum: Top 100 cited classical articles in sentinel lymph nodes biopsy for breast cancer
Background: The sentinel lymph node biopsy (SLNB) takes on a critical significance in breast cancer surgery since it is the gold standard for assessing axillary lymph node (ALN) metastasis and determining whether to perform axillary lymph node dissection (ALND). A bibliometric analysis is beneficial to visualize characteristics and hotspots in the field of sentinel lymph nodes (SLNs), and it is conducive to summarizing the important themes in the field to provide more insights into SLNs and facilitate the management of SLNs.
Materials and methods: Search terms relating to SLNs were aggregated and searched in the Web of Science core collection database to identify the top 100 most cited articles. Bibliometric tools were employed to identify and analyze publications for annual article volume, authors, countries, institutions, keywords, as well as hotspot topics.
Results: The period was from 1998 to 2018. The total number of citations ranged from 160 to 1925. LANCET ONCOLOGY and JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION were the top two journals in which the above articles were published. Giuliano, AE was the author with the highest number of articles in this field with 15. EUROPEAN INST ONCOL is the institution with the highest number of publications, with 35 articles. Hotspots include the following 4 topics, false-negative SLNs after neoadjuvant chemotherapy; prediction of metastatic SLNs; quality of life and postoperative complications; and lymphography of SLNs.
Conclusion: This study applies bibliometric tools to analyze the most influential literature, the top 100 cited articles in the field of SLNB, to provide researchers and physicians with research priorities and hotspots.
Breast cancer has become the disease with the highest morbidity and mortality among women in most countries (1). The axillary lymph node (ALN) status is one of the most important indicators for evaluating the prognosis of breast cancer (2), and axillary lymph node dissection (ALND) has always been considered the gold standard for assessing the metastasis of ALNs, and it has been commonly employed in the surgical treatment of breast cancer. However, as a result of the obstruction of the axillary lymphatic system easily by axillary surgery, which can result in a series of complications (e.g., lymphedema of the affected limb, limited shoulder joint movement, and decreased muscle strength (3–5)), ALND has resulted in great inconvenience to the patients in postoperative life.
With the improvement of early diagnosis and comprehensive treatment of breast cancer, de-escalation has been more generally recommended as the surgical treatment of breast cancer. A considerable number of researches have confirmed that the sentinel lymph node (SLN) of breast cancer is the first-stop lymph node draining the primary tumor, as well as the first-stop lymph node draining the entire breast organ (6, 7). Since this concept has been progressively recognized, sentinel lymph node biopsy (SLNB) has been extensively employed in clinical trials. SLNB can accurately stage the ALN status of breast cancer (8–12) while effectively reducing the incidence of postoperative complications (13–17). It has replaced conventional axillary surgery and become the standard surgical treatment for early breast cancer (18, 19).
Bibliometric analysis is effective in investigating the development of a field and identifying vital research hotspots (20). It is imperative to use the bibliometric analysis that can provide various quantitative indicators of the number of publications, scientific achievements, and the effect of authors (21, 22) to identify the trends and hotspots of SLNB and then help scholars explore new directions for future researches in the academic realm.
The publication on sentinel lymph nodes of breast cancer retrieved 7076 articles in the Web of Science core collection database (WoSCC) from 1998 to August 2022. The search strategy is presented as follows: “sentinel node*” or “sentinel lymph node*” or “sentinel lymphadenectomy” or “SLNs” AND “breast cancer” or “breast carcinoma” or “breast tumor*” or “breast neoplasm”. 3614 English articles were included. Lastly, the 100 most cited publications were collected. Two authors (Pengfei Lyu and Pingming Fan) assessed the retrieved data respectively to identify the literature relating to SLNB in breast cancer. If there are any opinions, another author (Ke Ma) will be consulted, and a consensus will be reached through discussion.
Data were input into the tools of bibliometrics. The analysis was conducted using RStudio (version 4.1.3), gCLUTO (version 1.0), and VOSviewer (version 1.6.14), which were adopted to build networks of bibliographic couplings based on keywords and co-occurrence analysis, among others for the visual analysis of the network.
The period was from 1998 to 2018; the sources (journals) were 29; the average number of citations per document was 365.6; the average number of citations per document per year was 24.8. Article type: article 75; proceedings paper 18; editorial material 1; review 6.
The total number of citations ranged from 160 to 1925. The most cited articles have been written by Giuliano, AE, and so forth published in the JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION in 2011 on a comparative study of ALND and non-ALND in breast cancer with sentinel lymph node metastasis. The top 100 most cited articles in the field of SLNB for breast cancer are listed in Table 1. Table 2 lists the annual average citation (citation rate) of the top 10 articles relating to SLNB. Articles published by Giuliano, AE, and so forth occupied four of them, and the period was from 1994 to 2017. LANCET ONCOLOGY and JAMA-JOURNAL OF THE AMERICAN MEDICAL ASSOCIATION were the top two journals of the above articles published.
Figure 1 shows annual scientific production and annual average article citations. The scientific output of the top 100 most cited articles reached the top in 1999(n=10, Figure 1A). The average yearly number of article citations reached 80.8 in 2017(Figure 1B).
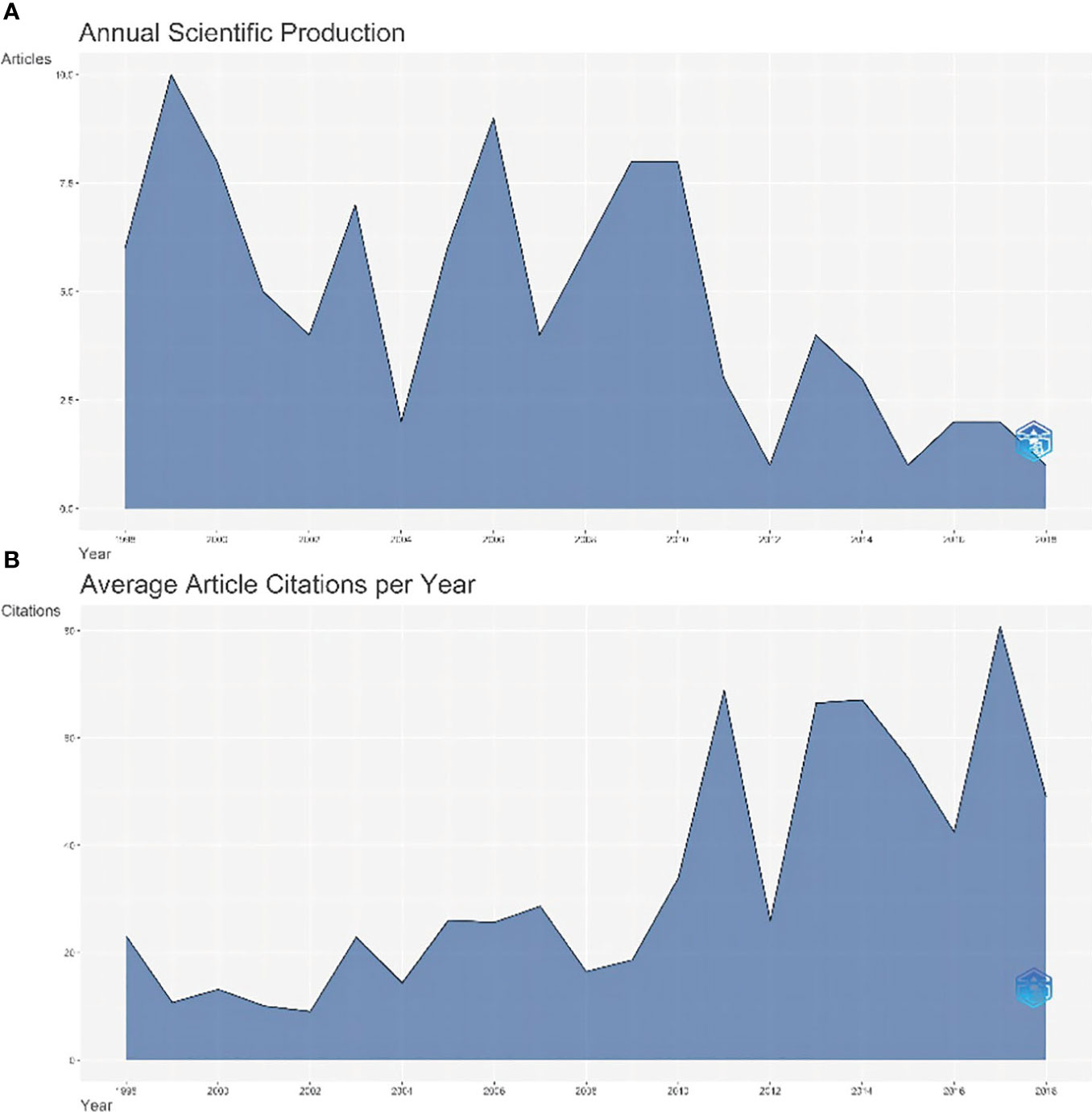
Figure 1 The map of annual scientific production and annual average article citations. (A) The annual scientific output of the top 100 most cited articles, (B) The average yearly number of article citations.
57 of the 100 articles originated from the US, accounting for 57%. ITALY is the second-largest country with 12 pieces, far less than the US. Figure 2A illustrates the cooperation network of countries. As depicted in the figure, there are more than half of the articles from the US, as well as the most relationships with the US. Thus, the distribution of highly cited articles centered on the US has taken shape.
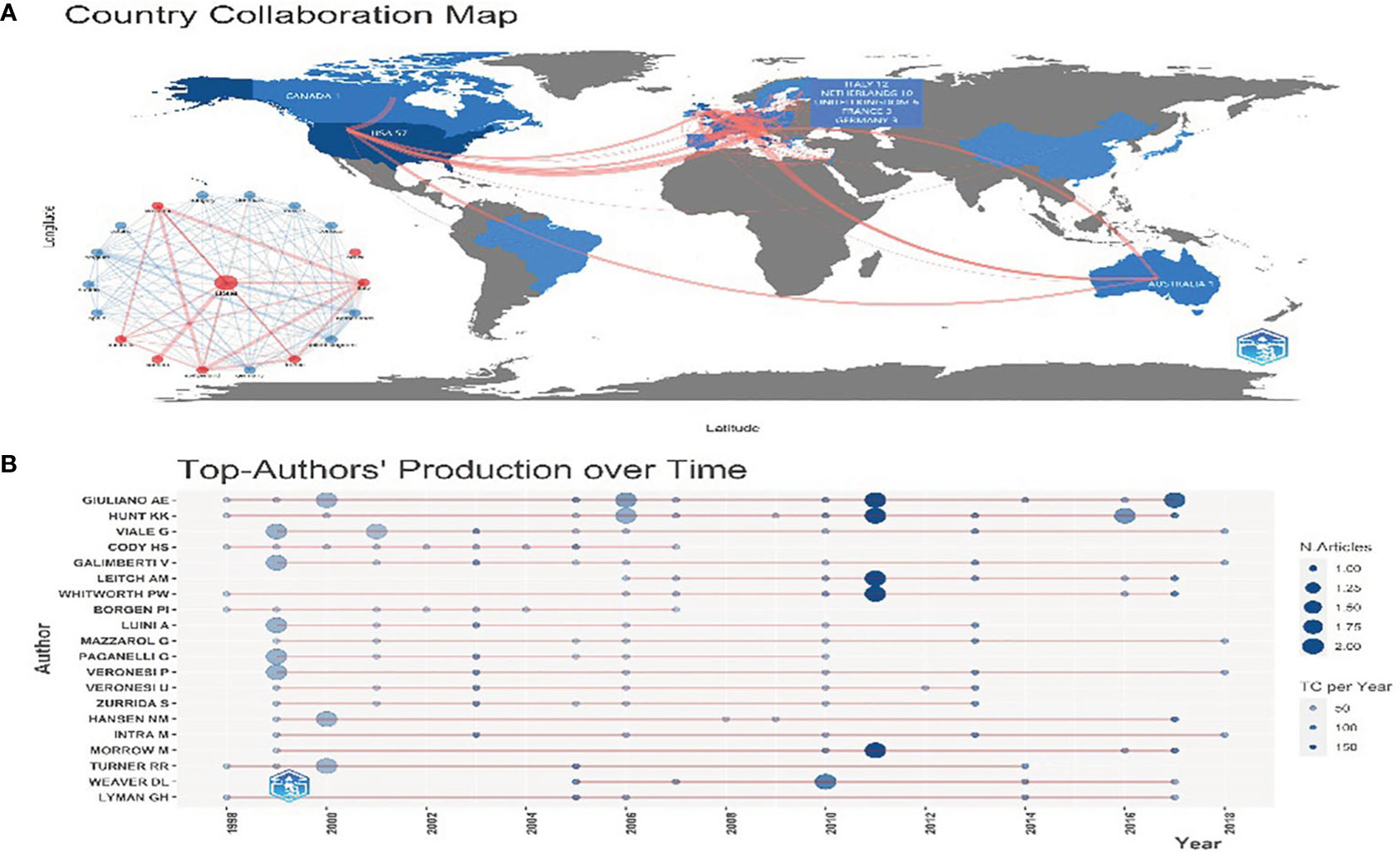
Figure 2 (A) Country Cooperation Map. The color depth represents the number of documents sent. The red line represents cooperation between countries. The thicker the line, the more the connections will be. (B) The top 20 authors’ production over time. The light blue circle represents the total citation (TC) per year, and the dark blue represents the number of articles.
For the author, Giuliano AE has published 15 articles, followed by Hunt KK published 14 articles and Viale G published 10 articles. The top 20 authors’ production over time is shown in Figure 2B. High-yielding authors such as Giuliano AE and Hunt KK are also highly cited.
The most relevant sources (Top 20 journals) are presented in Supplementary Figure 1. To be specific, the JOURNAL OF CLINICAL ONCOLOGY and ANNALS OF SURGERY both published 16 articles, followed by the Journal of ANNALS OF SURGICAL ONCOLOGY published 13 articles.
The top 20 publishing institutions are displayed in Supplementary Figure 2. Since EUROPEAN INST ONCOL published the largest number of 35 articles, it was the institution that contributed the most to the research of the sentinel lymph nodes. MEM SLOAN KETTERING CANC CTR and UNIV TEXAS published 28 and 15 articles, respectively. The three-domain diagram of authors, institutions, and countries is depicted in Supplementary Figure 3. Most of the highly prolific authors and institutions are from the US and Italy.
Figure 3A presents the co-occurrence analysis profile of the keywords. The top ten frequency keywords are presented as follows: lymphadenectomy, biopsy, carcinoma, axillary dissection, surgery, breast cancer, validation trial, metastases or micro-metastases, multicenter. Hence, the above high-frequency keywords suggest that the research focus of the above 100 articles was on biopsies relating to sentinel lymph nodes, axillary lymph node dissection, multicenter clinical validation trials, and management of metastases or micro-metastases in axillary lymph nodes.
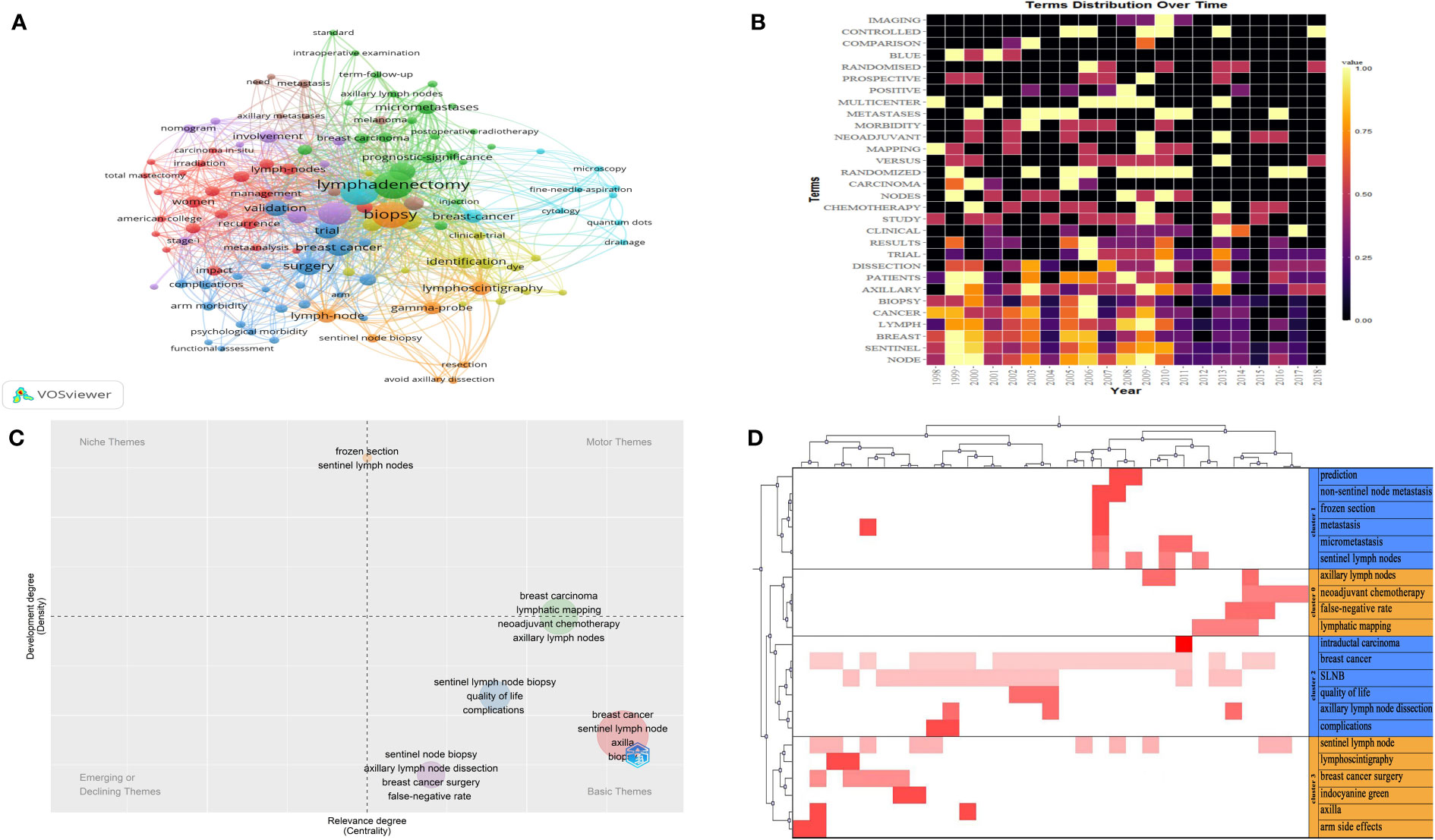
Figure 3 (A) The co-occurrence analysis profile of the keywords. The size of the node represents the frequency of occurrence. The same color represents the same theme. Connecting lines represent co-occurrence. (B) The distribution of high-frequency subject terms over time. Lighter colors represent higher frequency of occurrence, darker colors represent lower frequency of occurrence, and black colors represent a frequency of 0. (C) A thematic map of the SLNB. The horizontal coordinate represents the degree of relevance and the vertical coordinate represents the degree of development. (D) A heat map of the visualization of four clusters. The red color represents values in the original data, the dark color represents larger corresponding values and the white color represents corresponding values of 0.
To avoid the bias of keywords representing the topic of the article, I have researched the subject terms (title keywords and abstract keywords). Figure 3B presents the distribution of high-frequency subject terms over time. The main subject terms were distributed year by year. We found that the main subject words occurred with high frequency from 1998 to 2013.
A thematic map of the SLNs is shown in Figure 3C. Basic themes are located in the lower right quadrant, including quality of life and complications after surgery; false-negative sentinel lymph node dissection; the impact of neoadjuvant chemotherapy on lymphatic imaging; and prediction of positive sentinel lymph nodes. The above small clustered themes are well-centered and poorly developed, suggesting that the above issues are extensively studied in the above 100 highly cited articles.
After the extraction of the subject terms and cluster analysis from 3 to 10, the result indicated that four clusters worked best, having the greatest in-group similarity and the smallest out-group similarity. The parameters related to four clusters are shown in the Supplementary Table 1. A heat map of the visualization of clusters is shown in Figure 3D. Using the semantics of the articles corresponding to the keywords we identified four major hotspots in SLNB research.
Cluster 0: False-negative sentinel lymph nodes after neoadjuvant chemotherapy
Cluster 1: Prediction of metastatic sentinel lymph nodes
Cluster 2: Quality of life and postoperative complications in sentinel lymph node biopsy versus axillary lymph node dissection
Cluster 3: Lymphography of the sentinel lymph nodes
With a series of large samples of prospective clinical research on SLNB over the past few years, this operation has more crucial means for patients to assess lymph nodes status, staging, treatment plan formulation, and prognosis judgment of breast cancer and represents the development level of breast surgery to a certain extent (23, 24). There are considerable articles on sentinel lymph nodes from 1998 to 2022. In the above thousands of documents, many researchers have made outstanding contributions to the treatment of breast cancer, solved many clinical problems, and benefited most patients with breast cancer. In this study, bibliometrics was adopted to summarize the 100 most cited articles relating to SLNB. Moreover, characteristics, current hotspots, and possible trends can be assessed through the study of authors, countries, institutions, magazines, keywords, and so forth. Although most articles still use total citations to sort articles, there is a certain deviation in the early cited articles because they are time-dependent (25–27). Some literature is published in the recent period, whereas the number of citations is not enough to reflect the importance, and the articles with high citation rates are always worth exploring. Accordingly, we also analyzed the citation rate of articles (the average citations per year).
There is frequent cooperation between the US and European countries. The US not only accounts for a large proportion of the top 100 most cited articles. Most of the authors and institutions with great achievement come from the US, indicating that the US has made significant contributions to the research relating to SLNB in breast cancer and significantly affects the whole world. In terms of the research on reasons, the US government has given great financial support to sentinel lymph node researchers. The top 10 funding agencies are listed in Supplementary Table 2. Analysis of the keywords in the 100 most cited articles resulted in four major clusters, as follows.
Cluster 0: False-negative sentinel lymph nodes after neoadjuvant chemotherapy
Neoadjuvant chemotherapy (NAC) has been increasingly applied to locally advanced or early breast cancer to achieve the goal of radical or breast-conserving surgery at the lower stage. For patients without clinical metastasis of ALNs after NAC, the detection rate of SLNs accounted for 96%, the accuracy rate reached 99%, and the false negative rate (FNR) was 6%, similar to the FNR of SLNB in patients with early breast cancer who were not eligible for NAC (28). In other words, SLNB is adequately safe and feasible after NAC in this part of patients. For patients with clinical metastasis of ALNs, NAC may cause lymphatic vascular obstruction or fibrosis that changes the lymphatic drainage pathway, thus increasing the FNR of SLNB and affecting the judgment of tumor staging (29). Accordingly, ALND continues to be the standard treatment of patients whose metastatic ALNs disappeared by palpation and imaging assessment after NAC in routine clinical practice. Following the clinical guidelines, the FNR of SLNB should be lower than 10% (30), and on the premise of meeting this requirement, false-negative events may not affect the prognosis of patients after surgery (31). A meta-analysis enrolling 1521 patients who underwent SLNB or ALND after NAC in 23 articles showed that the accuracy rate of SLNB in evaluating the status of ALNs status was 89%, and the FNR was 13% which was higher than the threshold of 10% (32). As revealed by the results of relevant clinical trials, the FNR can be reduced to a certain extent by placing marker clips in lymph nodes before NAC, using ultrasound and other auxiliary examinations to assist in assessment before the operation, using the dye and nuclide dual tracer technology to map SLNs and increasing the number of SLNs during operation, as well as using immunohistochemical detection and defining the concept of SLNs strictly (33–38). However, the number of SLNs detected cannot be accurately predicted in clinical practice, the application of nuclide is limited, and even the clips placed by some patients may not be identified during the operation and others. As a result, other technologies (e.g., the application of targeted axillary lymph node dissection combining SLNB with marked lymph node biopsy (39)) and novel tracers (e.g., superparamagnetic iron oxide (40) and carbon nanoparticle suspension (41)) should be developed to provide the possibility for the safe use of SLNB in patients with clinically positive ALNs turning into negativity after NAC.
Cluster 1: Prediction of metastatic sentinel lymph nodes
Due to a considerable number of factors for the FNR of SLNB (42), many scholars have also tried to establish models for predicting metastatic SLNs, to select patients who are necessary to perform ALND after SLNB. The bibliometric analysis indicates that the nomogram is the main form of model construction, and the analysis of the above articles indicates that the size of the primary tumor, the number of SLN macrometastases and positive SLN resections, and peripheral vascular invasion are critical factors for additional metastasis of ALNs (43–49). The role of SLNB at the time that the risk of invasive disease on final pathology in patients with an initial diagnosis of ductal carcinoma in situ (ductal carcinoma in situ, DCIS) was sufficiently high and has not been well defined. The result of the study in the bibliometric analysis indicated that 55 years of age or younger, diagnosed by needle core biopsy, mammography with a size of at least 4 cm and high-grade DCIS were more likely to develop into invasive cancer. The presence of comedonecrosis and larger tumor sizes were the independent predictors of patients receiving SLNB, whereas the accessibility of the tumor was the only independent predictor of positive SLNs (50). Therefore, SLNB should not be performed routinely in all patients initially diagnosed with DCIS.
Cluster 2: Quality of life and postoperative complications in sentinel lymph node biopsy versus axillary lymph node dissection
ALND is the most accurate method to assess the status of ALNs in breast cancer, whereas it is also the main cause of postoperative complications (e.g., edema of the upper limb, pain, sensory and motor dysfunction). Since the screening methods for breast cancer are progressively enriched, the detection rate of early breast cancer increases year by year, and the proportion of new cases of breast cancer without the metastasis of ALNs also rises. If ALND is performed on all patients, most patients are excessive diagnosis and treatment, thus significantly affecting their quality of life and causing greater psychological stress.
Among the top 100 most cited articles, numerous articles have compared the complications and the quality of life of SLNB and ALND. The most cited and representative clinical trial called the ALMANAC trial showed that the postoperative situation of patients performing SLNB was superior to that of patients performing ALND in lymphedema, sensory disturbance, wound drainage, length of hospital stays, upper limb functional index, postoperative motor function recovery and mental illness (51, 52). A recent meta-analysis including 67 articles showed that SLNB was significantly lower than ALND in the prevalence of lymphedema and pain, and was also better than ALND in the range and strength of the affected limbs (53). In addition, a small-scale prospective clinical trial in the bibliometric statistics only reported the complications of SLNB which could not also be ignored. The article highlighted that older age and more SLNs resection are capable of increasing the incidence rate of axillary seroma, whereas this cannot affect the choice of axillary surgery for elderly patients with breast cancer and increase the number of SLNs dissection to reduce the false negative rate for surgeons (54).
Complications (e.g., lymphedema) can be currently treated by further surgery and should be consistent with the principle of prevention first and treatment as a supplement due to the difficulty of implementation and relatively high cost and long duration (55, 56). In brief, clinicians have sufficient evidence to consider that SLNB should be employed as the optimal operation for breast cancer patients with clinically negative ALNs.
Cluster 3: Lymphography of the sentinel lymph nodes
With the continuous development of lymphography technology of SLNB in breast cancer, people can easily understand the drainage pattern of SLNs and increase the detection rate of SLNB through this way. In the bibliometric analysis, more articles have focused on lymphography combining different imaging systems with an injection of indocyanine green and confirmed this method was feasible and safe for intraoperative SLNB and could be observed in real time without long-term training (57–61). Furthermore, novel tracers and relevant imaging systems (e.g., axillary ultrasound, and computed tomography lymphography) to locate and visualize positive lymph nodes in different ways can be adopted to guide the implementation of SLNB in clinical practice (62–64).
The lymphography of the SLNs is still in the experimental stage and needed to assess and confirm by more and further clinical articles. Although this technology enhances the ability of lymph node drainage, it may complicate subsequent surgery and radiation therapy. According to the results of the research by Jung et al., the use of indocyanine green fluorescence plus radioisotope dual imaging can also improve the detection rate of SLNs after NAC (65). Consequently, the lymphography of the SLNs may not be necessary and the FNR can still be effectively reduced by using the double-tracer method of different tracers.
Firstly, only a single database was included in the bibliometric analysis which resulted in incomplete search results. Secondly, the retrieved articles only included English language literature. Thirdly, articles from the last few years have low citation rates due to their recent publication, but this does not mean that they are not important. Despite the above limitations, the analysis of this study provides insights into current controversies and future research directions for SLNB.
In this study, the top 100 most cited articles on SLNB for breast cancer were analyzed through bibliometrics in combination with network visualization analysis. Notably, the US was the leading country of most cited articles, and the research hotspots focused on the quality of life and complications after surgery, the impact of neoadjuvant chemotherapy on the false-negative rate of SLNB, lymphography, and prediction of metastatic SLNs. Future research directions may include decreasing the false-negative rate and increasing the accuracy of SLNB to expand its indications through the continuous development of advanced lymphatic imaging and mapping techniques and the establishment of reasonably predictive models of lymph node metastasis.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
P-FL and PZ designed the study. KM and X-CC conducted a literature search. P-FL, PZ, KM, and X-CC analyzed data and wrote this thesis. All authors contributed to the article and approved the submitted version.
The study was supported by the Hainan Provincial Key Research and Development Program Project Fund (No. ZDYF2021SHFZ248).
Thanks to these authors: Xinrui Liang, Yu Wang and Guanghua Fu for their contributions to this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1170464/full#supplementary-material
Supplementary Figure 1 | Most relevant sources (top 20 journals).
Supplementary Figure 2 | The top 20 publishing institutions.
Supplementary Figure 3 | The three-domain diagram of authors, institutions, and countries. The size of the squares represents the number of articles published, and the linking lines to each other represent attribution.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE Jr., et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of national surgical adjuvant breast and bowel project B-18 and B-27. J Clin Oncol (2012) 30(32):3960–6. doi: 10.1200/JCO.2011.40.8369
3. Sakorafas GH, Peros G, Cataliotti L. Sequelae following axillary lymph node dissection for breast cancer. Expert Rev Anticancer Ther (2006) 6(11):1629–38. doi: 10.1586/14737140.6.11.1629
4. Crane-Okada R, Wascher RA, Elashoff D, Giuliano AE. Long-term morbidity of sentinel node biopsy versus complete axillary dissection for unilateral breast cancer. Ann Surg Oncol (2008) 15(7):1996–2005. doi: 10.1245/s10434-008-9909-y
5. Voogd AC, Ververs JMMA, Vingerhoets AJJM, Roumen RMH, Coebergh JWW, Crommelin MA. Lymphoedema and reduced shoulder function as indicators of quality of life after axillary lymph node dissection for invasive breast cancer. Br J Surg (2003) 90(1):76–81. doi: 10.1002/bjs.4010
6. Turner RR, Ollila DW, Krasne DL, Giuliano AE. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg (1997) 226(3):271–6. doi: 10.1097/00000658-199709000-00006
7. Weaver DL, Krag DN, Ashikaga T, Harlow SP, O'Connell M. Pathologic analysis of sentinel and nonsentinel lymph nodes in breast carcinoma: A multicenter study. Cancer (2000) 88(5):1099–107. doi: 10.1002/(SICI)1097-0142(20000301)88:5<1099::AID-CNCR22>3.0.CO;2-7
8. Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, et al. The sentinel node in breast cancer–a multicenter validation study. N Engl J Med (1998) 339(14):941–6. doi: 10.1056/NEJM199810013391401
9. Schulze T., Mucke J, Markwardt J, Schlag P. M., Bembenek A. Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J Surg Oncol, (2006) 93(2):109–19. doi: 10.1002/jso.20406
10. Hunt KK, Yi M, Mittendorf EA, Guerrero C, Babiera GV, Bedrosian I, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg (2009) 250(4):558–66. doi: 10.1097/SLA.0b013e3181b8fd5e
11. Giuliano AE, Haigh PI, Brennan MB, Hansen NM, Kelley MC, Ye W, et al. Prospective observational study of sentinel lymphadenectomy without further axillary dissection in patients with sentinel node-negative breast cancer. J Clin Oncol (2000) 18(13):2553–9. doi: 10.1200/JCO.2000.18.13.2553
12. Stearns V, Ewing CA, Slack R, Penannen MF, Hayes DF, Tsangaris TN. Sentinel Lymphadenectomy after Neoadjuvant Chemotherapy for Breast Cancer May Reliably Represent the Axilla except for Inflammatory Breast Cancer. Ann Surg Oncol (2002) 9(3):235–42. doi: 10.1007/BF02573060
13. Schrenk P, Rieger R, Shamiyeh A, Wayand W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer (2000) 88(3):608–14. doi: 10.1002/(sici)1097-0142(20000201)88:3<608::aid-cncr17>3.0.co;2-k
14. Purushotham AD, Upponi S, Klevesath MB, Bobrow L, Millar K, Myles JP, et al. Morbidity after sentinel lymph node biopsy in primary breast cancer: results from a randomized controlled trial. J Clin Oncol (2005) 23(19):4312–21. doi: 10.1200/JCO.2005.03.228
15. Ashikaga T, Krag DN, Land SR, Julian TB, Anderson SJ, Brown AM, et al. Morbidity results from the nsabp B-32 trial comparing sentinel lymph node dissection versus axillary dissection. J Surg Oncol (2010) 102(2):111–8. doi: 10.1002/jso.21535
16. Langer I, Guller U, Berclaz G, Koechli OR, Schaer G, Fehr MK, et al. Morbidity of sentinel lymph node biopsy (Sln) alone versus sln and completion axillary lymph node dissection after breast cancer surgery: A prospective swiss multicenter study on 659 patients. Ann Surg (2007) 245(3):452–61. doi: 10.1097/01.sla.0000245472.47748.ec
17. Schijven MP, Vingerhoets AJJM, Rutten HJT, Nieuwenhuijzen GAP, Roumen RMH, van Bussel ME, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol (2003) 29(4):341–50. doi: 10.1053/ejso.2002.1385
18. Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol (2014) 32(13):1365–83. doi: 10.1200/JCO.2013.54.1177
19. Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: american society of clinical oncology clinical practice guideline update. J Clin Oncol (2017) 35(5):561–4. doi: 10.1200/JCO.2016.71.0947
20. Lyu P-F, Li J-T, Deng T, Lin G-X, Fan P-M, Cao X-C. Research trends and hotspots of breast cancer management during the covid-19 pandemic: A bibliometric analysis. Front Oncol (2022) 12:918349. doi: 10.3389/fonc.2022.918349
21. Chen X, Xie H, Wang FL, Liu Z, Xu J, Hao T. A bibliometric analysis of natural language processing in medical research. BMC Med Inform Decis Mak (2018) 18(Suppl 1):14. doi: 10.1186/s12911-018-0594-x
22. Wang J, Deng H, Liu B, Hu A, Liang J, Fan L, et al. Systematic evaluation of research progress on natural language processing in medicine over the past 20 years: bibliometric study on pubmed. J Med Internet Res (2020) 22(1):e16816. doi: 10.2196/16816
23. Giuliano AE, Ballman KV, McCall L, Beitsch PD, Brennan MB, Kelemen PR, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the acosog Z0011 (Alliance) randomized clinical trial. JAMA (2017) 318(10):918–26. doi: 10.1001/jama.2017.11470
24. Krag DN, Anderson SJ, Julian TB, Brown AM, Harlow SP, Costantino JP, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the nsabp B-32 randomised phase 3 trial. Lancet Oncol (2010) 11(10):927–33. doi: 10.1016/S1470-2045(10)70207-2
25. Huang Y, Chen P, Peng B, Liao R, Huang H, Huang M, et al. The top 100 most cited articles on triple-negative breast cancer: A bibliometric analysis. Clin Exp Med (2022) 175–201. doi: 10.1007/s10238-022-00800-9
26. Brandt JS, Hadaya O, Schuster M, Rosen T, Sauer MV, Ananth CV. A bibliometric analysis of top-cited journal articles in obstetrics and gynecology. JAMA Netw Open (2019) 2(12):e1918007. doi: 10.1001/jamanetworkopen.2019.18007
27. Ahmad P, Asif JA, Alam MK, Slots J. A bibliometric analysis of periodontology 2000. Periodontol 2000 (2020) 82(1):286–97. doi: 10.1111/prd.12328
28. Geng C, Chen X, Pan X, Li J. The feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemotherapy: A systematic review and meta-analysis. PloS One (2016) 11(9):e0162605. doi: 10.1371/journal.pone.0162605
29. Soebhi T, Yarso KY, Sobri F, Budhi IB. Methylene blue absorption in sentinel lymph node biopsy for early breast cancer after neoadjuvant chemotherapy. Asian Pac J Cancer Prev (2020) 21(6):1767–71. doi: 10.31557/APJCP.2020.21.6.1767
30. Goetz MP, Gradishar WJ, Anderson BO, Abraham J, Aft R, Allison KH, et al. Nccn guidelines insights: breast cancer, version 3.2018. J Natl Compr Canc Netw (2019) 17(2):118–26. doi: 10.6004/jnccn.2019.0009
31. Roos MM, van Steenhoven JEC, Aalders KC, Schreuder K, Burgmans JPJ, Siesling S, et al. Regional recurrence risk following a negative sentinel node procedure does not approximate the false-negative rate of the sentinel node procedure in breast cancer patients not receiving radiotherapy or systemic treatment. Ann Surg Oncol (2019) 26(2):372–8. doi: 10.1245/s10434-018-6940-5
32. Shirzadi A, Mahmoodzadeh H, Qorbani M. Assessment of sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer in two subgroups: initially node negative and node positive converted to node negative - a systemic review and meta-analysis. J Res Med Sci (2019) 24:18. doi: 10.4103/jrms.JRMS_127_18
33. Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-Lymph-Node Biopsy in Patients with Breast Cancer before and after Neoadjuvant Chemotherapy (Sentina): A Prospective, Multicentre Cohort Study. Lancet Oncol (2013) 14(7):609–18. doi: 10.1016/S1470-2045(13)70166-9
34. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the acosog Z1071 (Alliance) clinical trial. JAMA (2013) 310(14):1455–61. doi: 10.1001/jama.2013.278932
35. Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Factors affecting sentinel lymph node identification rate after neoadjuvant chemotherapy for breast cancer patients enrolled in acosog Z1071 (Alliance). Ann Surg (2015) 261(3):547–52. doi: 10.1097/SLA.0000000000000551
36. Boughey JC, Ballman KV, Le-Petross HT, McCall LM, Mittendorf EA, Ahrendt GM, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from acosog Z1071 (Alliance). Ann Surg (2016) 263(4):802–7. doi: 10.1097/SLA.0000000000001375
37. Boughey JC, Ballman KV, Hunt KK, McCall LM, Mittendorf EA, Ahrendt GM, et al. Axillary ultrasound after neoadjuvant chemotherapy and its impact on sentinel lymph node surgery: results from the american college of surgeons oncology group Z1071 trial (Alliance). J Clin Oncol (2015) 33(30):3386–93. doi: 10.1200/JCO.2014.57.8401
38. Boileau J-F, Poirier B, Basik M, Holloway CMB, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the sn fnac study. J Clin Oncol (2015) 33(3):258–64. doi: 10.1200/JCO.2014.55.7827
39. Swarnkar PK, Tayeh S, Michell MJ, Mokbel K. The evolving role of marked lymph node biopsy (Mlnb) and targeted axillary dissection (Tad) after neoadjuvant chemotherapy (Nact) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel) (2021) 13(7) 1539. doi: 10.3390/cancers13071539
40. Douek M, Klaase J, Monypenny I, Kothari A, Zechmeister K, Brown D, et al. Sentinel node biopsy using a magnetic tracer versus standard technique: the sentimag multicentre trial. Ann Surg Oncol (2014) 21(4):1237–45. doi: 10.1245/s10434-013-3379-6
41. Wei N, Hou J, Chen J, Dai M, Du K, Wang S, et al. Sentinel lymph node biopsy with carbon nanoparticle suspension after neoadjuvant chemotherapy for breast cancer patients. Ann R Coll Surg Engl (2021) 103(10):752–6. doi: 10.1308/rcsann.2021.0084
42. Goyal A, Newcombe RG, Chhabra A, Mansel RE. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer–results of the almanac validation phase. Breast Cancer Res Treat (2006) 99(2):203–8. doi: 10.1007/s10549-006-9192-1
43. Nadeem R. M., Gudur L. D., Saidan A. Z. An independent assessment of the 7 nomograms for predicting the probability of additional axillary nodal metastases after positive sentinel lymph node biopsy in a cohort of British patients with breast cancer. Clin Breast Cancer (2014) 14(4):272–9. doi: 10.1016/j.clbc.2014.02.006
44. Viale G, Maiorano E, Pruneri G, Mastropasqua MG, Valentini S, Galimberti V, et al. Predicting the risk for additional axillary metastases in patients with breast carcinoma and positive sentinel lymph node biopsy. Ann Surg (2005) 241(2):319–25. doi: 10.1097/01.sla.0000150255.30665.52
45. Barranger E, Coutant C, Flahault A, Delpech Y, Darai E, Uzan S. An axilla scoring system to predict non-sentinel lymph node status in breast cancer patients with sentinel lymph node involvement. Breast Cancer Res Treat (2005) 91(2):113–9. doi: 10.1007/s10549-004-5781-z
46. Kohrt HE, Olshen RA, Bermas HR, Goodson WH, Wood DJ, Henry S, et al. New models and online calculator for predicting non-sentinel lymph node status in sentinel lymph node positive breast cancer patients. BMC Cancer (2008) 8:66. doi: 10.1186/1471-2407-8-66
47. Pal A, Provenzano E, Duffy SW, Pinder SE, Purushotham AD. A model for predicting non-sentinel lymph node metastatic disease when the sentinel lymph node is positive. Br J Surg (2008) 95(3):302–9. doi: 10.1002/bjs.5943
48. Coutant C, Olivier C, Lambaudie E, Fondrinier E, Marchal F, Guillemin F, et al. Comparison of models to predict nonsentinel lymph node status in breast cancer patients with metastatic sentinel lymph nodes: A prospective multicenter study. J Clin Oncol (2009) 27(17):2800–8. doi: 10.1200/JCO.2008.19.7418
49. McMasters KM, Wong SL, Chao C, Woo C, Tuttle TM, Noyes RD, et al. Defining the optimal surgeon experience for breast cancer sentinel lymph node biopsy: A model for implementation of new surgical techniques. Ann Surg (2001) 234(3) 292–300. doi: 10.1097/00000658-200109000-00003
50. Yen TWF, Hunt KK, Ross MI, Mirza NQ, Babiera GV, Meric-Bernstam F, et al. Predictors of invasive breast cancer in patients with an initial diagnosis of ductal carcinoma in situ: A guide to selective use of sentinel lymph node biopsy in management of ductal carcinoma in situ. J Am Coll Surg (2005) 200(4):516–26. doi: 10.1016/j.jamcollsurg.2004.11.012
51. Mansel RE, Fallowfield L, Kissin M, Goyal A, Newcombe RG, Dixon JM, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the almanac trial. J Natl Cancer Inst (2006) 98(9):599–609. doi: 10.1093/jnci/djj158
52. Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post-operative arm morbidity and quality of life. Results of the almanac randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Res Treat (2006) 95(3):279–93. doi: 10.1007/s10549-005-9025-7
53. Che Bakri NA, Kwasnicki RM, Khan N, Ghandour O, Lee A, Grant Y, et al. Impact of axillary lymph node dissection and sentinel lymph node biopsy on upper limb morbidity in breast cancer patients: A systematic review and meta-analysis. Ann Surg (2022) 572–580. doi: 10.1097/SLA.0000000000005671
54. Wilke LG, McCall LM, Posther KE, Whitworth PW, Reintgen DS, Leitch AM, et al. Surgical complications associated with sentinel lymph node biopsy: results from a prospective international cooperative group trial. Ann Surg Oncol (2006) 13(4):491–500. doi: 10.1245/ASO.2006.05.013
55. McLaughlin SA, Wright MJ, Morris KT, Sampson MR, Brockway JP, Hurley KE, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: patient perceptions and precautionary behaviors. J Clin Oncol (2008) 26(32):5220–6. doi: 10.1200/JCO.2008.16.3766
56. McLaughlin SA, Wright MJ, Morris KT, Giron GL, Sampson MR, Brockway JP, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol (2008) 26(32):5213–9. doi: 10.1200/JCO.2008.16.3725
57. Troyan SL, Kianzad V, Gibbs-Strauss SL, Gioux S, Matsui A, Oketokoun R, et al. The flare intraoperative near-infrared fluorescence imaging system: A first-in-human clinical trial in breast cancer sentinel lymph node mapping. Ann Surg Oncol (2009) 16(10):2943–52. doi: 10.1245/s10434-009-0594-2
58. Kim C, Song KH, Gao F, Wang LV. Sentinel lymph nodes and lymphatic vessels: noninvasive dual-modality in vivo mapping by using indocyanine green in rats–volumetric spectroscopic photoacoustic imaging and planar fluorescence imaging. Radiology (2010) 255(2):442–50. doi: 10.1148/radiol.10090281
59. Tagaya N, Yamazaki R, Nakagawa A, Abe A, Hamada K, Kubota K, et al. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am J Surg (2008) 195(6):850–3. doi: 10.1016/j.amjsurg.2007.02.032
60. Hirche C, Murawa D, Mohr Z, Kneif S, Hünerbein M. Icg fluorescence-guided sentinel node biopsy for axillary nodal staging in breast cancer. Breast Cancer Res Treat (2010) 121(2):373–8. doi: 10.1007/s10549-010-0760-z
61. Murawa D, Hirche C, Dresel S, Hünerbein M. Sentinel lymph node biopsy in breast cancer guided by indocyanine green fluorescence. Br J Surg (2009) 96(11):1289–94. doi: 10.1002/bjs.6721
62. Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: A systematic review. Lancet Oncol (2014) 15(8):e351–e62. doi: 10.1016/S1470-2045(13)70590-4
63. Erpelding TN, Kim C, Pramanik M, Jankovic L, Maslov K, Guo Z, et al. Sentinel lymph nodes in the rat: noninvasive photoacoustic and us imaging with a clinical us system. Radiology (2010) 256(1):102–10. doi: 10.1148/radiol.10091772
64. Suga K, Ogasawara N, Okada M, Matsunaga N. Interstitial ct lymphography-guided localization of breast sentinel lymph node: preliminary results. Surgery (2003) 133(2):170–9. doi: 10.1067/msy.2003.17
65. Jung S-Y, Han JH, Park SJ, Lee E-G, Kwak J, Kim SH, et al. The sentinel lymph node biopsy using indocyanine green fluorescence plus radioisotope method compared with the radioisotope-only method for breast cancer patients after neoadjuvant chemotherapy: A prospective, randomized, open-label, single-center phase 2 trial. Ann Surg Oncol (2019) 26(8):2409–16. doi: 10.1245/s10434-019-07400-0
Keywords: breast cancer, sentinel lymph node, biopsy, trends, bibliometric
Citation: Liang X, Wang Y, Fu G, Fan P, Ma K, Cao X-C, Lin G-X, Zheng W-p and Lyu P-f (2023) Top 100 cited classical articles in sentinel lymph nodes biopsy for breast cancer. Front. Oncol. 13:1170464. doi: 10.3389/fonc.2023.1170464
Received: 20 February 2023; Accepted: 03 August 2023;
Published: 09 October 2023.
Edited by:
Efe Sezgin, Izmir Institute of Technology, TürkiyeReviewed by:
Isabella Castellano, University of Turin, ItalyCopyright © 2023 Liang, Wang, Fu, Fan, Ma, Cao, Lin, Zheng and Lyu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guang-Xun Lin, linguangxun@xmu.edu.cn; linguangxun@hotmail.com; Wu-ping Zheng, hnzwp2000@163.com; Peng-fei Lyu, sky125585117@163.com
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.