
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol., 22 May 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1168942
This article is part of the Research TopicKey Proteins of Tumor Angiogenesis: Potential Therapeutic Targets for Gastrointestinal TumorsView all 5 articles
 Makan Cheraghpour1†
Makan Cheraghpour1† Masomeh Askari1
Masomeh Askari1 Sascha Tierling2
Sascha Tierling2 Sajad Shojaee1
Sajad Shojaee1 Amir Sadeghi3
Amir Sadeghi3 Pardis Ketabi Moghadam3
Pardis Ketabi Moghadam3 Maryam Khazdouz4
Maryam Khazdouz4 Hamid Asadzadeh Aghdaei1
Hamid Asadzadeh Aghdaei1 Moein Piroozkhah1
Moein Piroozkhah1 Ehsan Nazemalhosseini-Mojarad3
Ehsan Nazemalhosseini-Mojarad3 Nayeralsadat Fatemi1*†
Nayeralsadat Fatemi1*†Background: The receptors, ligands, and associated proteins of the insulin-like growth factor (IGF) family are involved in cancer development. The IGF1 receptor and its accompanying signaling cascade are a crucial growth-regulatory mechanism that plays an important role in colorectal cancer (CRC) proliferation and differentiation. IRS1 (Insulin receptor substrate-1), a major substrate for the IGF1R, is involved in cell growth and promotes tumorigenesis. There are shreds of evidence from prior research suggesting that IGF system polymorphisms may influence susceptibility to CRC. However, the findings in this area were contradictory. Accordingly, we carried out a systematic literature search to identify all case-control, cross-sectional, and cohort studies on the association between various polymorphisms across four IGF1 pathway genes (IGF1, IGF1R, IRS1, and IRS2) and the risk of CRC.
Methods: We performed a comprehensive search strategy in PubMed, Scopus, and Web of Science databases for articles available until Aug 30, 2022. A total of 26 eligible studies with IGF1/IGF1R, IRS1 and IRS2 polymorphisms; met the inclusion criteria. All case-control studies for IGF1 rs6214C>T, IRS1 rs1801278G>A, and IRS2 rs1805097G>A comprising 22,084 cases and 29,212 controls were included in the current meta-analysis. The pooled odds ratios (ORs) with 95% confidence intervals (CIs) were used to evaluate relationships between the polymorphisms and CRC susceptibility. All statistical analyses were performed using STATA software version 14.0.
Results: The meta-analysis of available data for rs6214C>T, rs1801278G>A, and rs1805097G>A showed a significant association between these polymorphisms and an increased CRC risk in some of the comparisons studied (rs6214C>T, pooled OR for CC = 0.43, 95% CI 0.21- 0.87, P = 0.019; rs1801278G>A, OR for GA = 0.74, 95% CI 0.58-0.94, P = 0.016; rs1805097G>A, OR for GA = 0.83, 95% CI 0.71-0.96, P = 0.013). Nevertheless, the meta-analysis did not include other genetic variations in IGF1, IGF1R, IRS1, and IRS2 due to heterogeneity and limited sample size.
Conclusions: This systematic review and meta-analysis provide evidence that genetic variants in IGF1 rs6214C>T, IRS1 rs1801278G>A, and IRS2 rs1805097G>A are associated with an increased risk of CRC. These findings may contribute to a better understanding of the complex genetic mechanisms involved in CRC development and could inform future research on prevention and treatment strategies for this disease.
The Insulin Growth Factor (IGF) protein family plays a key role in cell proliferation, apoptosis, and cell transformation through regulatory proteins synthesis (1). The Insulin Growth Factor-1 (IGF)-/Insulin-like Growth Factor-Receptor1 (IGF1R) pathway plays critical roles in the regulation of tumor cell metabolism, proliferation, survival, and angiogenesis (2, 3). IGF signaling pathway is activated when cell surface receptors like IGF1R bind to Insulin-like growth factors 1 and 2 (IGF1,2), and stimulate the phosphatidylinositol-3 kinase (PI3k)/Akt signaling pathway (4, 5). Any genetic alteration in IGF/IGF1R pathway members may result in insulin sensitivity (6).
This signaling pathway as a critical determinant has been linked to the development of colorectal cancer (CRC) (7, 8) and much evidence displayed hyperinsulinemia as a determinant of CRC risk, especially in those with younger onset (9–12). Consequently, evidence implicated the IGF1R and its ligands, IGF1, and IGF2, in tumor development and progression including CRC (13, 14).
Insulin receptor substrate (IRS) proteins including IRS1 and IRS2 are the major cytoplasmic molecules regulating the downstream signaling of IGF/IGF1R (15). They can interact with IGF receptors, leptin, vascular endothelial growth factors, growth hormone, prolactin, integrin, cytokine, and interferon receptors. These interactions display the critical role of IRS proteins in cancer development (16). In addition, these proteins activate and regulate intracellular signaling cascades including phosphatidylinositol 3-kinase/Akt (PI3K/Akt) and extracellular signal-regulated kinase (ERK) pathways that are involved in metabolism and protein synthesis, cell proliferation, and key regulators of CRC development and progression (17, 18).
Genetic variation in the insulin‐like growth factor (IGF) pathway would prove a role in IGF-related factors in colorectal tumorigenesis. Indeed, several studies revealed a significant association with CRC risk for genetic variants in genes encoding IGF-related factors (19–24). However, most single SNPs confer a small increase in the risk and the gene-gene and gene-environment interactions (25) and functional genetic compensation between genes may exist (26).
As mentioned above, the IGF pathway has been shown to play a critical role in the development and progression of CRC, and genetic variations in the pathway may contribute to CRC risk. However, there is limited integration of IGF pathway-linked genetic data in previous studies. This review and meta-analysis aim to address this gap in knowledge by systematically analyzing the available evidence on the associations between four IGF1 pathway gene (IGF1, IGF1R, IRS1, and IRS2) polymorphisms and CRC risk. The novelty of this study lies in the comprehensive analysis of multiple genetic variants across four genes in the IGF1 pathway, which may provide insights into the complex genetic mechanisms underlying CRC development and inform future research on the prevention and treatment of CRC.
This systematic review and meta-analysis were performed based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (27). Searches were conducted in PubMed, Scopus, and Web of Science databases until Aug 30, 2022, using keywords shown in Supplementary Text. In the search, only English language and human studies were considered. Two reviewers independently searched the literature, screened titles, abstracts, and full texts, and consulted the third author whenever disagreements occurred.
Inclusion criteria were: 1) Case-control, cross-sectional, and cohort studies in CRC populations; 2) Quantitative analysis of interplay between Genetic variants and CRC risk were reported; 3) Full text in the English language was available. Randomized controlled trials (RCT), reviews, letters, comments, editorials, case reports, conference abstracts, and personal communications, and studies focused solely on hereditary nonpolyposis colorectal cancer (HNPCC) were excluded.
According to the inclusion criteria, two reviewers extracted the following data from the included studies: the name of the first author, year of publication, participant’s race and ethnicity, type of study, number of cases and control, family history of cancer, body mass index, age, KRAS status, gene and variation genotype, clinical outcome, type of drug, and CRC stages. The quality of each study was assessed using Critical Appraisal Skills Programme (CASP) checklist by three reviewers (Supplementary Table 1). As part of the screening process and quality assessment, conflicts were resolved with the fourth author through discussions or consultation.
Meta-analyses were performed on the extracted data for dichotomous outcome variables of colorectal cancer. We calculated a pooled odds ratio (OR) and 95% confidence intervals (CI) for several genetic variations including IGF1 (969(CA), rs6214C>T, rs35767C>T), IRS1 (rs1801278G>A), and IRS2 (rs1805097G>A). We applied fixed or random effects of meta-analyses due to heterogeneity with the inverse variance (IV) weighting in overall analysis by Forest plots. If heterogeneity was rejected, a random model was used to calculate pooled estimates. Heterogeneity of variances was assessed using Cochran’s Q test and I2 measure and was plotted with a radial diagram (28). We also assessed publication bias by a funnel plot and calculating Egger’s test (29). All statistical analysis was conducted using STATA, version 14.0 (Stata Corp, College Station, TX).
A total of 2620 articles were retrieved through electronic and manual searches. Title and abstract reviews led to the removal of duplicates (n=110) and the exclusion of articles that were not in English or did not meet our inclusion criteria (n=2510) (Figure 1). Baseline characteristics of the included manuscripts are presented in Table 1. A quality appraisal determined that all inquiries were high-quality studies.
There were 23 studies that analyzed the association between IGF1 and IGF1R polymorphisms and CRC, 20 of which were case-control studies and three were cohort studies. A total of eight studies reported associations between IRS1 and IRS2 polymorphisms and CRC, but only one of them was a cohort study.
Three IGF1 polymorphisms (-969(CA) repeat, rs35767C>T, and rs6214C>T) have been most intensely investigated (Table 2). The CA repeat polymorphism, which is located 969 bp upstream from the transcription start site, has been investigated in six studies. Wong et al. found no association between risks of CRC and IGF1 genotypes [-969(CA)19, -969(CA)18] in the 290 case and 873 control from Chinese population. However, they found that CA(21) was associated with CRC risk (P=0.02) (30).
It was demonstrated that every genotype of CA repeat, except homozygous CA(19), had increased risks of CRC in 782 American patients (p<0.05) (33). Also, these genotypes modulated the association of BMI, physical activity, and consumption of hormones in postmenopausal women with CRC risk in women with the 19/19 genotype (33). In a study on 5047 subjects, 1346 with colon cancer, 952 with rectal cancer, and 2217 healthy controls, the correlation of CA(19) repeat with risk of CRC was analyzed. No significant association in either colon or rectal cases was found (32). Samowitz et al. conducted a multicenter study to assess the impact of CA(19) repeats on the risk of CRC. According to these results, there was no significant association between CA(19) repeats and 1788 cases of CRC (34). In line with this evidence, Pechlivanis et al. also observed no association between CA(19) genotype and CRC risk in the 661 case and 667 control from German population (35). A case-control study including 528 case and 836 control by Keku et al. examined the distribution of CA(19) repeat genotypes among two races. It was found that homozygous CA(19) genotype and colon cancer risk was associated in whites (p-value not reported), but not in African Americans (19). In cohort studies including 3440 CRC cases, it was proven that females carrying less than 38 repeats of the CA (19) allele had lower CRC risks at all subsites (p<0.001), except the rectum compared to those carrying more than 38 repeats (21). Conflicting results have been reported by Chao et al. who found CA(19) genotype with less than 38 repeats was associated with reduced CRC risk in 219 males (p-value not reported), but no interaction between CA(19) and CRC stage appeared to exist (51).
Six studies evaluated the correlation of rs35767C>T located in the 5’UTR of IGF1 and CRC risk (Table 2). Four studies conducted on different populations, including German, Caucasians, Americans, and Egyptians, found no significant association of rs35767C>T with CRC risk (35, 38, 39, 46). However, two recent studies have reported the opposite results. Li et al. found an increased risk of CRC for CT genotype carrier in 367 Chines cases (CT vs. CC, OR=1.399, 95% CI 1.029-1.901 P = 0.032), although no significant differences between genotype and stage were shown (23). A possible role for rs35767C>T in the risk of CRC has been suggested by Li et al. reported an association of TT genotype with CRC risk in 208 Chines cases (TT vs. CC: OR = 2.26, 95% CI = 1.35–3.80, P = 0.003). Furthermore, it was also found that in advanced tumor stages the incidence of TT or TC genotype is significantly higher (50).
For rs6214C>T that located in the 3’UTR of exon 4 in IGF1, five studies reported its relationship with CRC risk in different populations. Feik et al. found an increased risk of CRC for the carriers of TT genotype (OR = 1.79, 95% CI 1.04–1.90) in a cohort of 178 Caucasian cases (39), a finding confirmed in two other studies. Patients with TT or TC genotype had an increased risk of CRC in the Egyptian (OR 17.68, 95% CI; 2.27 - 137.99) (38) and Arab (p < 0.001) population (40). However, the association between rs6214C>T and CRC risk was not confirmed by two other studies (43, 49).
A study, on 178 patients found no significant association between rs6220A>G and risk of CRC (39). These results were confirmed by another case-control study conducted by Yosry et al. included a total of 66 patients to evaluate an association between rs6220A>G and risk of CRC. However, the distribution of the rs6220A>G genotype was not different between cases and controls (38).
As for other polymorphisms located in the promoter region of IGF1, some studies have investigated the role of rs5742612T>C and rs12579108C>A. In 290 Chines patients, CC and CT genotypes of rs5742612T>C were associated with a decreased risk of CRC (P-value not reported), and a protective effect of the C allele was observed among participants younger than 60 years (30). However, Mahmoudi et al. found no association of rs5742612T>C with risk of CRC in 261 Iranian patients (41). The rs12579108C>A has been investigated by one group, which found genotypes AA and CA considerably associated with reduced colorectal cancer risk (P-value not reported). The association was also significantly more substantial in the subgroup of patients with colon cancer compared to patients with rectal cancer (heterogeneity p<0.001) (47). 950 CRC patients enrolled in a cohort study and genotype distribution of rs12579108C>A were investigated between old and young patients. It was demonstrated that the frequency of the AA genotype of rs12579108C>A was 12.7%, which was significantly higher than young patients (31).A further SNP that was investigated only in one case-control study was rs7136446C>A. The results showed that rs7136446C>A was not associated with CRC risk in the German population (35). Four SNPs, including rs1520220C>G, rs5742678C>G, rs10735380G>A, and rs5742694G>T have been investigated in a large cohort study. Simons et al. found that rs5742694G>T (P=0.02) and rs1520220C>G (P=0.04) were associated with an increased risk of CRC in men (21).
The association between IGF1 genotype and circulating level of IGF1 was assessed in five studies. A case–control study from a multiethnic Cohort showed that the rs35767C>T was associated with circulating IGF1 levels (P= 0.001) (46). A similar observation was made by Li et al. when they found higher IGF1 levels for three genotypes in the CRC group than for the control group (P<0.05) (23).
A study of 1364 subjects found that plasma concentrations of IGF1 were not significantly affected by the genotype of the CA(19) polymorphism (19). Supporting results have also been reported that CA(19) repeat polymorphism did not have any significant association with circulating IGF1 levels (51).
The results of a study involving 80 colorectal cancer patients and 80 matched controls revealed that TT and CT genotypes of rs6214C>T had the highest serum IGF1 levels (P= < 0.001) (40).
A study carried out by Stanilov et al. examined the relationship between the rs2229765G>A polymorphism in the IGF1R gene and the risk of CRC as well as the activity of the disease (Table 3). The genotypes AA or AG were more prevalent among 110 advanced CRC Caucasian cases as compared to controls (AA/AG vs. GG: OR= 3.06, P= 0.004) (48).
The rs1801278G>A polymorphism (Gly972Arg) located in exon 1 of IRS1 has been assessed in six different studies, two of which have found an association of this polymorphism with CRC risk (Table 4). One of the significant reported results was in the case-control study of Slattery et al., who demonstrated that having at least one minor allele (GA or AA) was associated with an increased risk of colon cancer (OR 1.4, 95% CI 1.1-1.9). Furthermore, individuals without a family history of colon cancer were found to have an increased risk of colon cancer if they carried the GA/AA genotype (32). In line with this evidence, the multicenter study of 1788 American cases and 1981 healthy control found a significantly increased risk of CRC in the GA/AA genotypes carrier (OR 1.3, 95% CI 1.0-1.5) (48). However, the results of four studies including, three case-control and one cohort study, did not support an association between rs1801278G>A and CRC risk (21, 32, 41, 44).
Among IRS2 polymorphisms, rs1805097G>A has been evaluated in six studies (Table 5). Three of them have found that rs1805097G>A was not associated with CRC in Caucasian, Turkish and Iranian populations (36, 41, 45). The only significant result reported was in the study conducted by Slattery et al. who found an association of GA genotype with risk of CRC in 1346 colon cancer patients (OR 0.8, 95% CI 0.6-0.9) (32). The other study of the American population indicated that rs1805097G>A was not associated with CRC (34). Furthermore, a cohort study was performed on a total of 3440 CRC patients from the Netherlands, confirming that not only rs1805097G>A but also rs2289046A>G, rs754204C>T, and rs4773082T>C were not associated with risk of CRC (21). Karimi et al. assessed the genotype distribution of rs2289046A>G within 167 Iranian CRC patients. Although the genetic association of rs2289046A>G with CRC risk was excluded, GG genotype was associated with reduced risk of CRC in subjects in the normal range weight (p=0.035, OR=0.259, 95%CI=0.074-0.907) (43).
A cohort study by Winder et al. investigated the association between polymorphisms of the IGF1 and IGF1R genes with clinical outcome of 130 metastatic CRC (mCRC) patients treated with cetuximab monotherapy. A significant correlation was found between three IGF1 polymorphisms, rs6214C>T (P=0.048), rs2946834G>A (p<0.001), and rs7136446C>A (P=0.034) with PFS (1.3 months, 95% CI, 1.3-1.5). In addition, PFS for mCRC with wt KRAS was independently predicted by two polymorphisms of IGF1, including rs2946834G>A (P=0.001) and rs713664 (P=0.022). As a result of the OS analysis, IGF1 (rs7136446C>T), IGF1R (rs2272037T>C and rs2016347G>T) were associated with shorter OS in all patients (P=0.026, P=0.039, P=0.038 respectively), while in patients with wt KRAS only IGF1R (rs2016347G>T) significantly predicted shorter OS (P=0.004). Additionally, it was found that IGF1 rs6214C>T, and rs2946834G>A, and IGF1R rs2016347G>T were negative predictors of cetuximab efficacy in mCRC patients (52). In another cohort study conducted by Cho et al., 440 Korean CRC patients were subjected to an analysis of the association of rs2288378T>C, rs6220A>G, rs5742612T>C, rs5742714C>G, and rs12579108C>A with OS and PFS. However, no correlation was observed between the distribution of genotypes of polymorphisms and OS and PFS in these patients (42). The role of IGF1 and IGFR1 polymorphism on OS and PFS was also studied in 132 patients treated with first-line bevacizumab (BV) and FOLFOX or XELOX. Gerger et al. provide evidence that patients carrying the AG or GG genotype of rs6220A>G showed a median OS of 32.4 months, while those carrying AA genotype had a median OS of 22 months (HR 0.51; 95%CI 0.32–0.83) (37).
The results of the meta-analysis on the association of IGF1 rs6214C>T, IRS1 rs1801278G>A, and IRS2 rs1805097G>A polymorphisms with CRC risk are shown in Table 6.
Overall, pooled results from 5 studies (comprising 1,035 cases and 2,726 controls) for IGF1 rs6214C>T, revealed a significant association between the polymorphism and an increased CRC risk in some of the comparisons studied (CC, OR = 0.43, 95% CI 0.21- 0.87, P = 0.019; CT, OR = 0.97, 95% CI 0.64–1.47, P = 0.879; TT, OR = 1.37, 95% CI 0.83– 2.26, P = 0.216) (Figure 2).
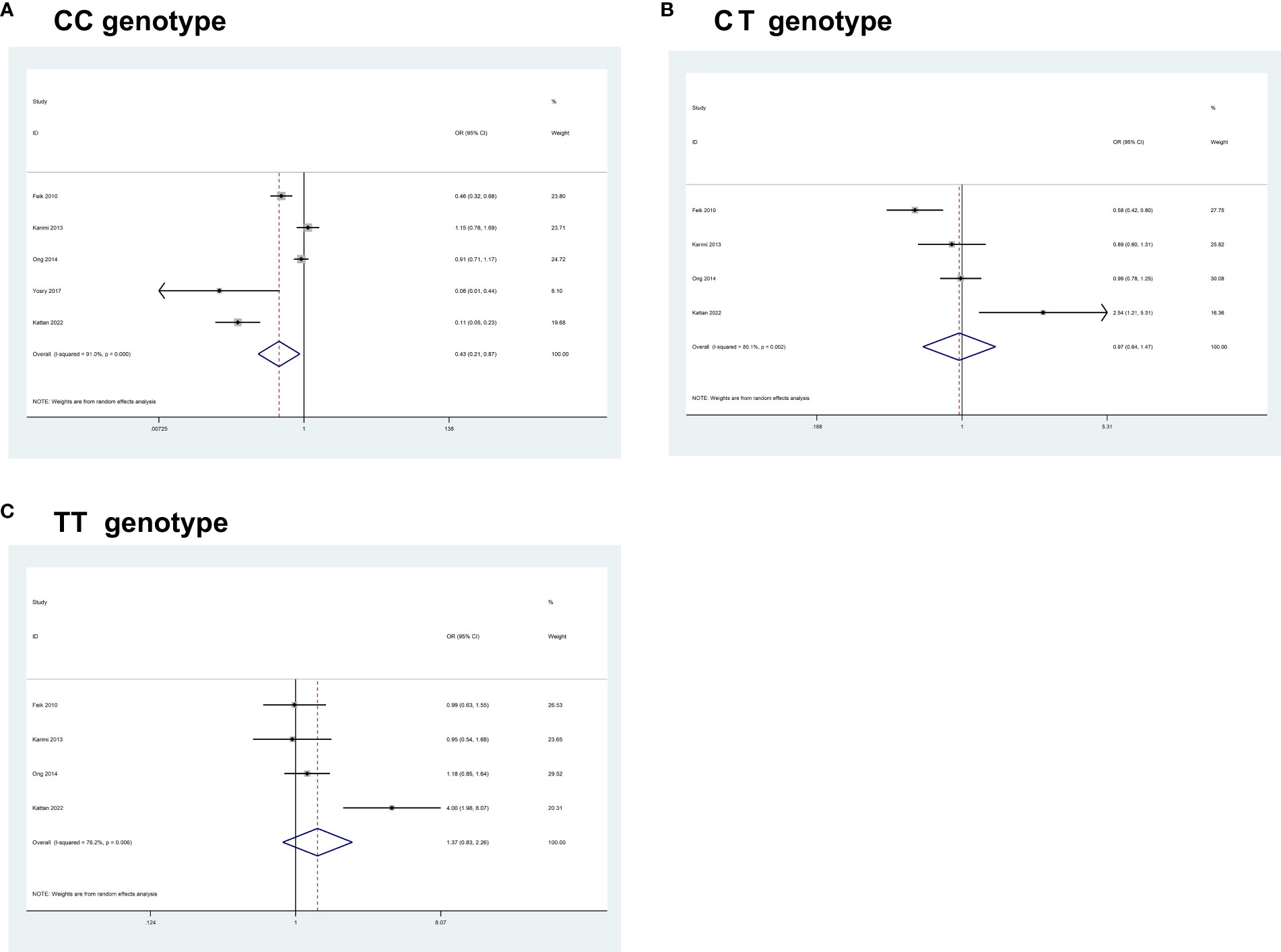
Figure 2 Forest plots of the association between IGF1 rs6214C>T polymorphism and the risk of colorectal cancer. (A) CC genotype; (B) CT genotype; (C) TT genotype.
Heterogeneity between study designs was obtained in the radial plot for IGF1 rs6214C>T (CC, I2 = 91%, P=0.000; CT, I2 = 80.1%, P=0.002; TT, I2 = 76.2%, P=0.006) (The results are not shown here). The pooled results based on 7 included studies for -969(CA) repeat (comprising 7,024 cases and 8,519 controls) and 6 eligible studies for IGF1 rs35767C>T (comprising 3,434 cases and 5,698 controls) indicated that no significant association between these polymorphisms and CRC risk was found in any of the comparisons studied (Table 6).
For IRS1 rs1801278G>A, pooled results based on 5 studies (comprising 5,371 cases and 6,255 controls) revealed a significant association between the polymorphism and an increased CRC risk in some of the comparisons studied (AA, OR = 0.75, 95% CI 0.17-3.33, P = 0.712; GA, OR = 0.74, 95% CI 0.58-0.94, P = 0.016; GG, OR = 1.06, 95% CI 0.82-1.37, P = 0.644) (Figure 3).
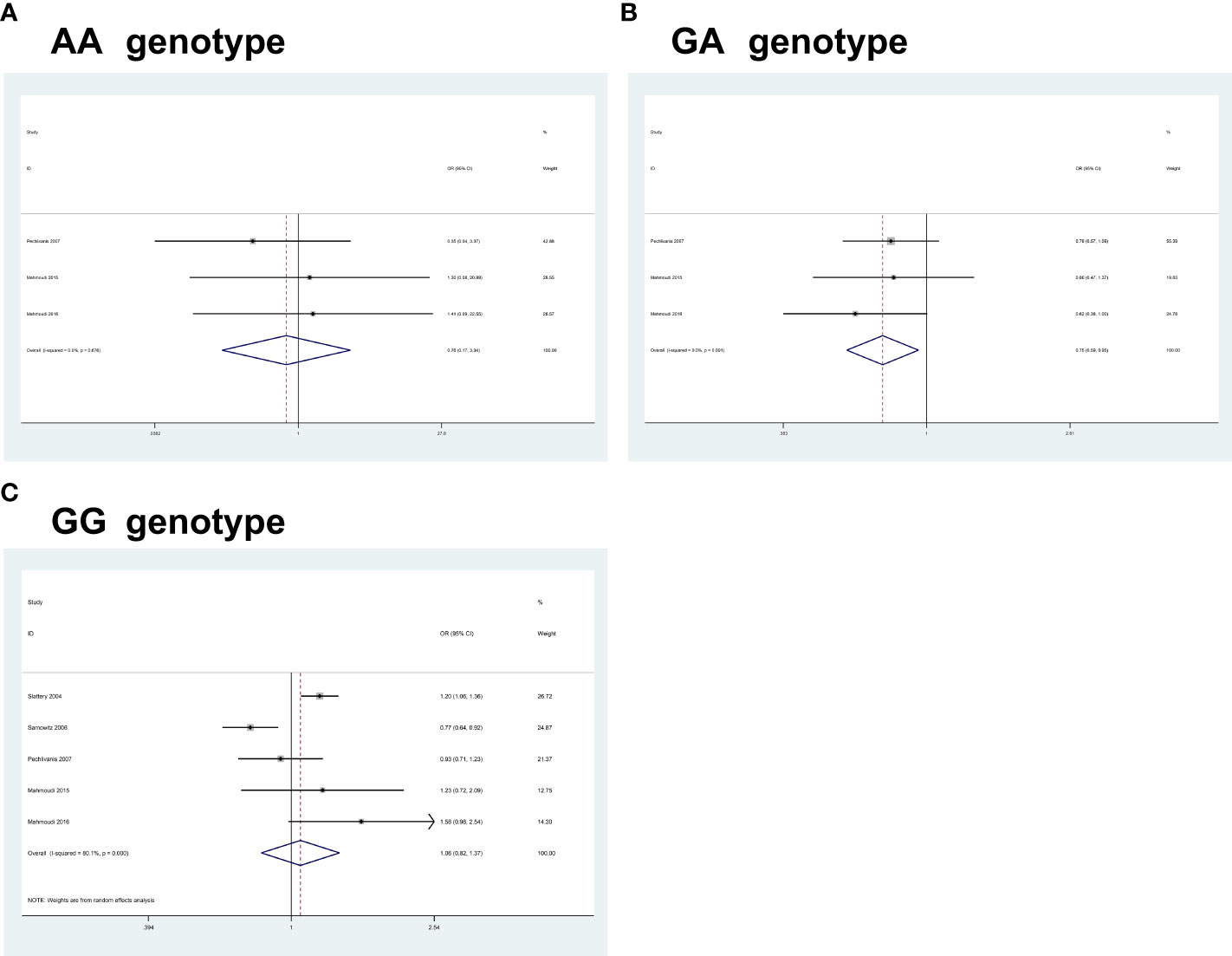
Figure 3 Forest plots of the association between IRS1 rs1801278G>A polymorphism and the risk of colorectal cancer. (A) AA genotype; (B) GA genotype; (C) GG genotype.
Heterogeneity between study designs was obtained in the radial plot for IRS1 rs1801278G>A (AA, I2 = 0.0%, P=0.676; GA, I2 = 0.0%, P=0.691; GG, I2 = 80.1%, P=0.000) (The results are not shown here).
For IRS2 rs1805097G>A, pooled results from 5 studies (comprising 5,220 cases and 6,014 controls) revealed a significant association between the polymorphism and an increased CRC risk in some of the comparisons studied (AA, OR = 0.94, 95% CI 0.84-1.06, P = 0.358; GA, OR = 0.83, 95% CI 0.71-0.96, P = 0.013; GG, OR = 0.96, 95% CI 0.84-1.10, P = 0.627) (Figure 4). Heterogeneity between study designs was obtained in the radial plot for IRS2 rs1805097G>A (AA, I2 = 0.0%, P=0.620; GA, I2 = 63.5%, P=0.027; GG, I2 = 57.7%, P=0.051) (The results are not shown here).
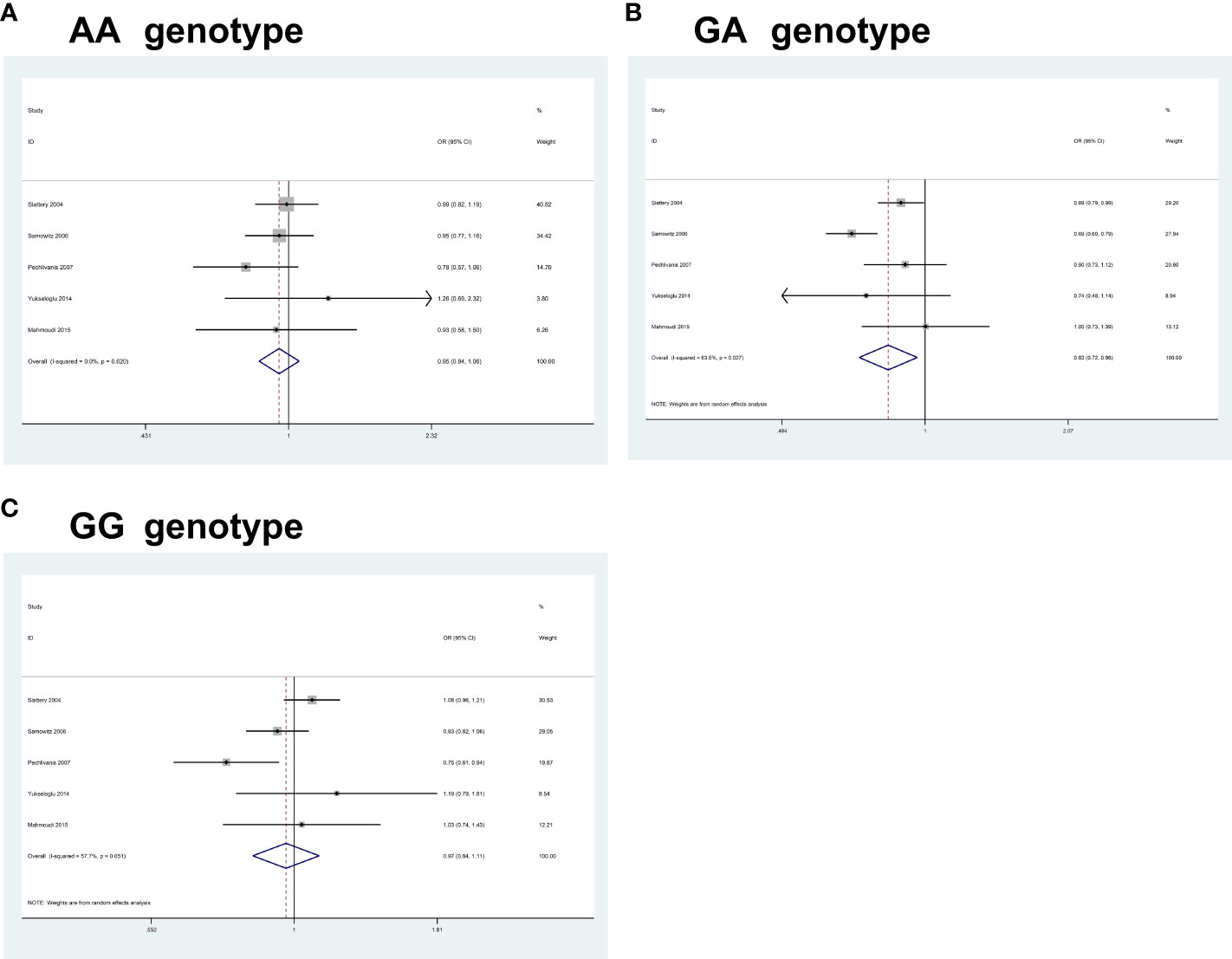
Figure 4 Forest plots of the association between IRS2 rs1805097G>A polymorphism and the risk of colorectal cancer. (A) AA genotype; (B) GA genotype; (C) GG genotype.
The presence of publication bias was examined using Egger’s tests and visually verified using funnel plots. For rs6214C>T, no significant publication bias was detected according to the Egger’s test (CC, P=0.146; CT, P= 0.532; TT, P= 0.442). Similarly, no publication bias was detected for rs1801278G>A (AA, P= 0.029; GA, P= 0.729; GG, P= 0.980) and rs1805097G>A (AA, P= 0.902; GA, P= 0.733; GG, P= 0.866) (The results are not shown here). It is also noted that tests for funnel plot asymmetry should be used only when there are at least 10 studies included in the meta-analysis, because when there are fewer studies the power of the tests is too low to distinguish chance from real asymmetry. This was a limitation of the present study.
Colorectal cancer pathogenesis involves both genetic and environmental factors. As the effects of genetic mutations on CRC continued to be revealed, many authors have focused on the associations between SNPs and CRC susceptibility. There is a huge body of evidence on the implications of the IGF/insulin signaling pathway in the progression and development of cancers, which indicates an important prognostic factor for patients (53–55). This pathway activates the transformation-facilitating pathways of colorectal epithelial cells including proapoptotic and mitogenic signaling pathways (53). In particular, previous studies have brought strong evidence of the potential role of the IGF axis in the initiation and progression of cancer (56, 57). In other words, impairment in the regulation of the IGF axis considerably contributes to the malignancy phenotype by modifying cell behavior and boosting survival and invasion of cancer cells (58).
Over the decades, studies have been focused on identifying important genetic variations and SNPs of this pathway, which potentially influence the function of these genes. Despite being widely investigated, the relationship between the genes of the IGF axis and the risk of CRC is still poorly understood, and studies indicate conflicting findings. The contradicting results could attribute to the sample size of studies, ethnicity of participants, changes in the place of residence, lifestyle factors, and dietary patterns (59). For determining these heterogeneities, we designed a systematic study and meta-analysis according to the previous results, in order to achieve a more precise estimation of the correlation between the genes involved in the IGF pathway (IGF1, IGF1R, IRS1, and IRS2) and the risk of CRC for the first time.
One of the most important genes of this axis is IGF1, which may increase the risk of CRC through the regulation of cell proliferation, and apoptosis (23, 60). Epidemiological findings have indicated that elevation of serum concentration of IGF1 is related to increased risk of CRC because it can considerably increase the growth of cancer cells, suggesting evidence of the role of the IGF pathway in the risk and progression of carcinogenesis (7, 32, 61–63). Indeed, IGF1 is expressed locally in various tissues such as skeletal muscle and controls tissue growth via local paracrine and autocrine effects (64). Furthermore, it arouses cell proliferation and suppresses apoptosis (65). Therefore, it can be proposed that irregular expression of the IGF1 gene leads to the progression of CRC (65, 66). There is evidence claiming that polymorphisms of the IGF1 gene may be associated with the risk of CRC by affecting its serum level (40). Importantly, we showed that some polymorphisms especially IGF1 rs35767C>T, and rs6214C>T CT and TT are associated with the serum IGF1 level (23, 40), so it makes sense to classify these polymorphisms as regulatory SNPs.
Our current findings showed that there was a significant correlation between polymorphism of IGF1 rs6214C>T and the risk to develop CRC. However, there was a conflicting result regarding the relationship between IGF genotypes and the risk to develop CRC in previous studies. The significant association between rs6214C>T polymorphisms and risk of cancer development has been reported in various tumors including pancreatic, acute lymphocytic leukemia (ALL), esophageal, head and neck, and colorectal (38, 49, 67). Our result was in line with a previous study that revealed an association with cancer susceptibility in a population of Saudi Arabia, Egypt which clarified the correlation of rs6214C>T polymorphism with CRC susceptibility (38, 40). However, a meta-analysis study detect no significant relationship between rs6214C>T with overall cancer risk, but it had a significant correlation with breast and pancreatic cancer (68). Interestingly, we also observed that rs6214C>T polymorphism had a significant correlation with PFS.
Previous studies evaluated the association of IGF1 rs35767C>T with the risk of different cancers which revealed contradicting results (23, 69, 70). Our meta-analysis proposed that IGF1 rs35767C>T didn’t influence CRC risk. In contrast with our finding, a meta-analysis of 10 studies that included 9,415 CRC cases and 14,179 controls in Caucasians and Asian population showed that the IGF1 rs35767C>T polymorphism was associated with decreased susceptibility to CRC in Caucasians, and has a protective effect against cancer (65). In a previous investigation, it was demonstrated that the IGF1 CA-repeat polymorphism exhibited an association with an elevated susceptibility to develop CRC within the context of HNPCC (71). Nevertheless, Chen et al. conducted a meta-analysis and did not find a statistically significant correlation between IGF1 (CA)n and the overall risk of cancer (72). Although, Qin et al. in a meta-analysis study declared that IGF1 rs35767C>T polymorphism hadn’t a significant association with cancer risk (73). However, this result should be interpreted cautiously because studies on the IGF1 rs35767C>T polymorphism exhibit high heterogeneity in terms of ethnicity and population. Additionally, this meta-analysis evaluated the association of IGF1 rs35767C>T with several types of cancer in eight studies with 11,257 CRC patients and 16,213 healthy controls which only three of studies were about CRC (73).
IGF1R is predominately expressed in the digestive system and is involved in the proliferation of colon crypts (74). Considering the function of IGF1/IGF1R in cell differentiation, proliferation, and apoptosis, its activation is correlated with initiation, progression of cancer, and poor survival (53, 75). Thus, it can be supposed to be an attractive therapeutic target. Previous studies identified that various polymorphisms of the IGF1R gene could modify the susceptibility of cancer. Stanilov found that the IGF1R rs2229765G>A polymorphism was related to CRC progression; the allele A-carrying patients had higher levels of circulating IGF1 and were in higher stages of CRC compared to the GG genotype (48). Another study showed that serum concentration of IGF1 was higher in allele-A carrying IGF1R rs7166348 polymorphism and associated with a higher risk of colorectal neoplasm (76). To the contrary, in the current meta-analysis, we did not observe a significant correlation between IGF1R rs2229765G>A and the risk of developing CRC. On the other hand, the expression of IGF1R is regulated by P53 and P73. Thus, epigenetic modifications, deletion, and silencing of these proteins can impair the activation of IGF1R and lead to progression and metastasis in CRC (53).
IRSs are scaffold proteins, which mediate the IGF/insulin signaling pathway (77). They have critical roles in cell growth, proliferation, and cellular metabolism (77). Evidence has shown that these proteins play a significant role in the regulation of tumor development and progression of solid tumors (78). Several epidemiological studies have examined the relationship between IRSs polymorphisms and CRC risk (32, 79, 80). Based on our meta-analysis, a significant association was found between IRS1 rs1801278G>A with the risk to develop CRC. Previous studies showed contradictory results for this polymorphism. In agreement with our findings, Su Yon Jung et al. demonstrated that the polymorphism of IRS1 rs1801278G>A increases the risk of developing CRC by 30% in women who are inactive and consume exogenous estrogen (81). Similarly, Slattery reported a significant association between the IRS1 rs1801278G>A (Gly972Arg) polymorphism and CRC risk in individuals using aspirin and NSAIDs. The G972R IRS1 polymorphism has been linked to a 50% reduction in insulin sensitivity, suggesting that the GG genotype would reduce insulin resistance and the risk of CRC (81). However, a meta-analysis study conducted by Li et al. revealed that the IRS1 rs1801278G>A polymorphism did not have a significant correlation with increased susceptibility of individuals to CRC (82) (Table 4).
Additionally, we found that IRS2 rs1805097G>A significantly increased the risk of CRC. In line with our study, a meta-analysis found that IRS2 rs1805097G>A polymorphism, leading to an aspartate replaced by glycine in the codon 1057 of its gene, changed the structure and function of the IRS2 protein and lowered the risk of overall CRC (78). Yin et al. conducted a meta-analysis comprising 6 case-control studies with a total of 4,333 cases and 5,333 controls, which demonstrated that the IRS2 gene rs1805097G>A polymorphism plays a crucial role in the pathogenesis of CRC. This polymorphism was associated with a reduced risk of CRC, particularly colon cancer. Additionally, an ethnicity-based stratification analysis revealed that the rs1805097G>A polymorphism decreased the risk of CRC among Americans (83). In other words, the influence of this polymorphism on the manifestation of CRC depends upon the genetic background of individuals and the location of cancer within different populations. However, there were studies that reported no significant association between rs1805097G>A and CRC risk (21, 36, 41).
In our methodological systematic review, we managed to include all research related to our research questions and provided a quality assessment of each paper. To the best of our knowledge, this study represents a significant contribution to the current understanding of the relationship between IGF/insulin pathway polymorphisms and CRC risk. While previous investigations have explored the link between IGF1 gene polymorphisms and CRC risk, this study is among the few that have evaluated multiple major SNPs of this pathway in assessing CRC susceptibility. The findings of this study add to the existing literature on the subject matter and provide novel insights into the influence of the IGF/insulin pathway on individual susceptibility to CRC. Furthermore, we demonstrate links between different clinical outcomes such as PFS, OS, and drug response with multiple genetic variations in IGF axis pathway.
There are several limitations related to this study. First, our study was based on the studies published in indexed journals, which may increase bias related to time lag and publication bias. In the time-lag bias, literature with negative results compared to enthusiastic results is published over a long period of time (84). Concerning publication bias, studies with small sample sizes and negative results would not appear in the literature, while studies with small sample sizes and positive results are quickly published (85). Second, our search was restricted to English literature, and hence there was also an English language bias. Third, non-differential misclassification may exist because it is possible that the study’s control groups would develop cancer in the future. Finally, our data has been obtained according to the non-adjusted data. Thus, it seems that a more accurate adjusted analysis based on confounding factors such as age, tobacco, alcohol, and other environmental factors, can provide a precise estimate.
In addition, we were able to conduct a meta-analysis to quantify the contribution of genetic polymorphisms of IGF1 (rs6214C>T), IRS1 (rs1801278G>A), and IRS2 (rs1805097G>A) to CRC risk. Of note, other genetic variations in IGF1, IGFR1, IRS1, and IRS2 were not included in meta-analysis due to heterogeneity and small sample size.
In summary, our findings indicated that genotypes of CC in IGF1 rs6214C>T, and GA in IRS1 rs1801278G>A, and IRS2 rs1805097G>A are associated with an increased risk of CRC which can serve as diagnostic biomarkers in CRC. Indeed, the identification of specific genetic variants associated with an increased risk of CRC could inform future research on prevention and treatment strategies for this disease. These findings may also facilitate the development of personalized medicine approaches, allowing for targeted interventions in at-risk populations. -
Thus, given the limitation points of this study and practical reasons, epidemiological studies with a larger sample scale evaluating various populations as well as incorporating more comprehensive and accurate assessments are required to validate the findings of this study. However, there is still limited information regarding early detection of CRC, and gene-environment and gene-gene interactions must also be considered in succeeding research to apply early detection of CRC.
The data analyzed in this study is subject to the following licenses/restrictions: <b>No additional data available.</b>. Requests to access these datasets should be directed to <b>n_fatemi_1363@yahoo.com</b>.
MC and NF conceived and designed the study. MC, MA, AS and NF conducted systematic search, screened articles, and selected eligible articles. PM, MK, EN-M and MP extracted the result from included study and performed eligible evaluation. MC, NF, MA, HA and SS performed analysis and interpreted the findings. MC and NF wrote the first version of the manuscript. ST, AS, HA and EN-M revised the manuscript. All authors read and approved the final version of the manuscript.
The authors declare that they have no conflicts of interest. All authors have completed the ICMJE uniform disclosure form.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1168942/full#supplementary-material
1. Kasprzak A, Kwasniewski W, Adamek A, Gozdzicka-Jozefiak A. Insulin-like growth factor (IGF) axis in cancerogenesis. Mutat Research/Reviews Mutat Res (2017) 772:78–104. doi: 10.1016/j.mrrev.2016.08.007
2. Bowers LW, Rossi EL, O’Flanagan CH, deGraffenried LA, Hursting SD. The role of the insulin/IGF system in cancer: lessons learned from clinical trials and the energy balance-cancer link. Front Endocrinol (2015) 6:77. doi: 10.3389/fendo.2015.00077
3. Brahmkhatri VP, Prasanna C, Atreya HS. Insulin-like growth factor system in cancer: novel targeted therapies. BioMed Res Int (2015) 2015:1–24. doi: 10.1155/2015/538019
4. Tao Y, Pinzi V, Bourhis J, Deutsch E. Mechanisms of disease: signaling of the insulin-like growth factor 1 receptor pathway–therapeutic perspectives in cancer. Nat Clin Pract Oncol (2007) 4(10):591–602. doi: 10.1038/ncponc0934
5. Tognon CE, Sorensen PH. Targeting the insulin-like growth factor 1 receptor (IGF1R) signaling pathway for cancer therapy. Expert Opin Ther Targets (2012) 16(1):33–48. doi: 10.1517/14728222.2011.638626
6. Yakar S, Liu J-L, Fernandez AM, Wu Y, Schally AV, Frystyk J, et al. Liver-specific igf-1 gene deletion leads to muscle insulin insensitivity. Diabetes (2001) 50(5):1110–8. doi: 10.2337/diabetes.50.5.1110
7. Knuppel A, Fensom GK, Watts EL, Gunter MJ, Murphy N, Papier K, et al. Circulating insulin-like growth factor-I concentrations and risk of 30 cancers: prospective analyses in UK BiobankIGF-I concentrations and cancer risk in UK biobank. Cancer Res (2020) 80(18):4014–21. doi: 10.1158/0008-5472.CAN-20-1281
8. Murphy N, Carreras-Torres R, Song M, Chan AT, Martin RM, Papadimitriou N, et al. Circulating levels of insulin-like growth factor 1 and insulin-like growth factor binding protein 3 associate with risk of colorectal cancer based on serologic and mendelian randomization analyses. Gastroenterology (2020) 158(5):1300–1312.e1320. doi: 10.1053/j.gastro.2019.12.020
9. Hua F, Yu J-J, Hu Z-W. Diabetes and cancer, common threads and missing links. Cancer Lett (2016) 374(1):54–61. doi: 10.1016/j.canlet.2016.02.006
10. Tabung FK, Wang W, Fung TT, Smith-Warner SA, Keum N, Wu K, et al. Association of dietary insulinemic potential and colorectal cancer risk in men and women. Am J Clin Nutr (2018) 108(2):363–70. doi: 10.1093/ajcn/nqy093
11. Ali Khan U, Fallah M, Sundquist K, Sundquist J, Brenner H, Kharazmi E. Risk of colorectal cancer in patients with diabetes mellitus: a Swedish nationwide cohort study. PloS Med (2020) 17(11):e1003431. doi: 10.1371/journal.pmed.1003431
12. Khan UA, Fallah M, Tian Y, Sundquist K, Sundquist J, Brenner H, et al. Personal history of diabetes as important as family history of colorectal cancer for risk of colorectal cancer: a nationwide cohort study. Off J Am Coll Gastroenterology| ACG (2020) 115(7):1103–9. doi: 10.14309/ajg.0000000000000669
13. Fürstenberger G, Senn H-J. Insulin-like growth factors and cancer. Lancet Oncol (2002) 3(5):298–302. doi: 10.1016/S1470-2045(02)00731-3
14. Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer (2003) 107(6):873–7. doi: 10.1002/ijc.11487
15. Gorgisen G, Gulacar I, Ozes O. The role of insulin receptor substrate (IRS) proteins in oncogenic transformation. Cell Mol Biol (2017) 63(1):1–5. doi: 10.14715/cmb/2017.63.1.1
16. Gibson SL, Ma Z, Shaw LM. Divergent roles for IRS-1 and IRS-2 in breast cancer metastasis. Cell Cycle (2007) 6(6):631–7. doi: 10.4161/cc.6.6.3987
17. Coppola D, Ferber A, Miura M, Sell C, D'Ambrosio C, Rubin R, et al. A functional insulin-like growth factor I receptor is required for the mitogenic and transforming activities of the epidermal growth factor receptor. Mol Cell Biol (1994) 14(7):4588–95. doi: 10.1128/mcb.14.7.4588-4595.1994
18. Hu YP, Patil SB, Panasiewicz M, Li W, Hauser J, Humphrey LE, et al. Heterogeneity of receptor function in colon carcinoma cells determined by cross-talk between type I insulin-like growth factor receptor and epidermal growth factor receptor. Cancer Res (2008) 68(19):8004–13. doi: 10.1158/0008-5472.CAN-08-0280
19. Keku TO, Vidal A, Oliver S, Hoyo C, Hall IJ, Omofoye O, et al. Genetic variants in IGF-I, IGF-II, IGFBP-3, and adiponectin genes and colon cancer risk in African americans and whites. Cancer Causes Control (2012) 23(7):1127–38. doi: 10.1007/s10552-012-9981-2
20. Quan H, Tang H, Fang L, Bi J, Liu Y, Li H. IGF1 (CA) 19 and IGFBP-3-202A/C gene polymorphism and cancer risk: a meta-analysis. Cell Biochem Biophys (2014) 69(1):169–78. doi: 10.1007/s12013-013-9784-4
21. Simons CC, Schouten LJ, Godschalk RW, van Engeland M, van den Brandt PA, van Schooten FJ, et al. Genetic variants in the insulin-like growth factor pathway and colorectal cancer risk in the Netherlands cohort study. Sci Rep (2015) 5(1):1–14. doi: 10.1038/srep14126
22. Simons CC, Schouten LJ, Godschalk RW, van Engeland M, van den Brandt PA, van Schooten FJ, et al. Energy restriction at young age, genetic variants in the insulin-like growth factor pathway and colorectal cancer risk in the Netherlands cohort study. Int J Cancer (2017) 140(2):272–84. doi: 10.1002/ijc.30439
23. Li Y, Zhou X, Liu R, Cao Y, Wang L, Chao X, et al. Relationship of SNP rs35767 in IGF-1 promoter region with susceptibility to colorectal cancer. Int J Clin Exp Pathol (2018) 11(10):5110–6.
24. de Kort S, Simons CC, van den Brandt PA, Janssen-Heijnen ML, Sanduleanu S, Masclee AA, et al. Diabetes mellitus, genetic variants in the insulin-like growth factor pathway and colorectal cancer risk. Int J Cancer (2019) 145(7):1774–81. doi: 10.1002/ijc.32365
25. Maher B. Personal genomes: the case of the missing heritability. Nature (2008) 456(7218):18–21. doi: 10.1038/456018a
26. Zhang J. Genetic redundancies and their evolutionary maintenance. Evol (2012) 751:279–300. doi: 10.1007/978-1-4614-3567-9_13
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
28. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. Bmj (2011) 343:1–9. doi: 10.1136/bmj.d5928
29. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
30. Wong H-L, DeLellis K, Probst-Hensch N, Koh W-P, Van Den Berg D, Lee H-P, et al. A new single nucleotide polymorphism in the insulin-like growth factor I regulatory region associates with colorectal cancer risk in Singapore Chinese. Cancer Epidemiol Biomarkers Prev (2005) 14(1):144–51. doi: 10.1158/1055-9965.144.14.1
31. Lin JK, Shen MY, Lin TC, Lan YT, Wang HS, Yang SH, et al. Distribution of a single nucleotide polymorphism of insulin-like growth factor-1 in colorectal cancer patients and its association with mucinous adenocarcinoma. Int J Biol Markers (2010) 25(4):195–9. doi: 10.5301/JBM.2010.6119
32. Slattery ML, Samowitz W, Curtin K, Ma KN, Hoffman M, Caan B, et al. Associations among IRS1, IRS2, IGF1, and IGFBP3 genetic polymorphisms and colorectal cancer. Cancer Epidemiol Biomarkers Prev (2004) 13(7):1206–14. doi: 10.1158/1055-9965.1206.13.7
33. Morimoto LM, Newcomb PA, White E, Bigler J, Potter JD. Insulin-like growth factor polymorphisms and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev (2005) 14(5):1204–11. doi: 10.1158/1055-9965.EPI-04-0695
34. Samowitz WS, Wolff RK, Ma KN, Andersen K, Caan B, Slattery ML. Polymorphisms in insulin-related genes predispose to specific KRAS2 and TP53 mutations in colon cancer. Mutat Research/Fundamental Mol Mech Mutagenesis (2006) 595(1-2):117–24. doi: 10.1016/j.mrfmmm.2005.10.014
35. Pechlivanis S, Wagner K, Chang-Claude J, Hoffmeister M, Brenner H, Försti A. Polymorphisms in the insulin like growth factor 1 and IGF binding protein 3 genes and risk of colorectal cancer. Cancer Detect Prev (2007) 31(5):408–16. doi: 10.1016/j.cdp.2007.10.001
36. Pechlivanis S, Pardini B, Bermejo JL, Wagner K, Naccarati A, Vodickova L, et al. Insulin pathway related genes and risk of colorectal cancer: INSR promoter polymorphism shows a protective effect. Endocrine-related Cancer (2007) 14(3):733–40. doi: 10.1677/ERC-07-0107
37. Gerger A, El-Khoueiry A, Zhang W, Yang D, Singh H, Bohanes P, et al. Pharmacogenetic angiogenesis profiling for first-line bevacizumab plus oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Clin Cancer Res (2011) 17(17):5783–92. doi: 10.1158/1078-0432.CCR-11-1115
38. Yosry A, Omran D, Yousef M, Salah M, Omar H, Hamdy S, et al. SNPs in the insulin-like growth factor gene and obesity impact on colorectal cancer in egyptians. Asian Pacific J Cancer prevention: APJCP (2017) 18(11):2959. doi: 10.22034/APJCP.2017.18.11.2959
39. Feik E, Baierl A, Hieger B, Führlinger G, Pentz A, Stättner S, et al. Association of IGF1 and IGFBP3 polymorphisms with colorectal polyps and colorectal cancer risk. Cancer Causes Control (2010) 21(1):91–7. doi: 10.1007/s10552-009-9438-4
40. Kattan SW, Allah AMKA, Mohamed KI, Alruwetei AM, Hegazy AH, El Gayed EMA. Linking insulin like growth factor-1 (IGF-1) rs6214 gene polymorphism and its serum level with risk of colorectal cancer. Beni-Suef Univ J Basic Appl Sci (2022) 11(1):1–8. doi: 10.1186/s43088-022-00254-8
41. Mahmoudi T, Majidzadeh-a K, Karimi K, Karimi N, Farahani H, Dabiri R, et al. An exon variant in insulin receptor gene is associated with susceptibility to colorectal cancer in women. Tumor Biol (2015) 36(5):3709–15. doi: 10.1007/s13277-014-3010-x
42. Cho YY, Kim JG, Chae YS, Sohn SK, Kang BW, Moon JH, et al. No association of insulin-like growth factor gene polymorphisms with survival in patients with colorectal cancer. Cancer Res Treatment: Off J Korean Cancer Assoc (2011) 43(3):189–94. doi: 10.4143/crt.2011.43.3.189
43. Karimi K, Mahmoudi T, Karimi N, Dolatmoradi H, Arkani M, Farahani H, et al. Is there an association between variants in candidate insulin pathway genes IGF-I, IGFBP-3, INSR, and IRS2 and risk of colorectal cancer in the Iranian population? Asian Pacific J Cancer Prev (2013) 14(9):5011–6. doi: 10.7314/APJCP.2013.14.9.5011
44. Mahmoudi T, Majidzadeh-A K, Karimi K, Farahani H, Dabiri R, Nobakht H, et al. Gly972Arg variant of insulin receptor substrate 1 gene and colorectal cancer risk in overweight/obese subjects. Int J Biol Markers (2016) 31(1):68–72. doi: 10.5301/jbm.5000159
45. Yukseloglu EH, Celik SK, Kucuk MU, Yalin E, Ozkal SS, Ates C, et al. IRS-2 G1057D polymorphism in Turkish patients with colorectal cancer. Gastroenterol Review/Przegląd Gastroenterologiczny (2014) 9(2):88–92. doi: 10.5114/pg.2014.42503
46. Ollberding NJ, Cheng I, Wilkens LR, Henderson BE, Pollak MN, Kolonel LN, et al. Genetic variants, prediagnostic circulating levels of insulin-like growth factors, insulin, and glucose and the risk of colorectal cancer: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev (2012) 21(5):810–20. doi: 10.1158/1055-9965.EPI-11-1105
47. Wong H-L, Koh W-P, Probst-Hensch NM, Van Den Berg D, Yu MC, Ingles SA. Insulin-like growth factor-1 promoter polymorphisms and colorectal cancer: a functional genomics approach. Gut (2008) 57(8):1090–6. doi: 10.1136/gut.2007.140855
48. Stanilov NS, Karakolev IA, Deliysky TS, Jovchev JP, Stanilova SA. Association of insulin-like growth factor-I receptor polymorphism with colorectal cancer development. Mol Biol Rep (2014) 41(12):8099–106. doi: 10.1007/s11033-014-3708-2
49. Ong J, Salomon J, te Morsche RH, Roelofs HM, Witteman BJ, Dura P, et al. Polymorphisms in the insulin-like growth factor axis are associated with gastrointestinal cancer. PloS One (2014) 9(3):e90916. doi: 10.1371/journal.pone.0090916
50. Li X, Yang J, Wang X, Gao X. The association of IGF1 rs35767 polymorphism with colorectal cancer risk in the Chinese han population. Nucleosides Nucleotides Nucleic Acids (2022) 41(9):815–23. doi: 10.1080/15257770.2022.2081703
51. Chao X, Wang L, Liu R, Li Y, Zhou X. Association between CA repeat polymorphism in IGF1 gene promoter and colorectal cancer risk in a native Chinese population. Neoplasma (2019) 66(6):1002–8. doi: 10.4149/neo_2019_190117N51
52. Winder T, Zhang W, Yang D, Ning Y, Bohanes P, Gerger A, et al. Germline polymorphisms in genes involved in the IGF1 pathway predict efficacy of cetuximab in wild-type KRAS mCRC PatientsIGF1 and IGF1R polymorphisms predict cetuximab efficacy. Clin Cancer Res (2010) 16(22):5591–602. doi: 10.1158/1078-0432.CCR-10-2092
53. Vigneri PG, Tirrò E, Pennisi MS, Massimino M, Stella S, Romano C, et al. The insulin/IGF system in colorectal cancer development and resistance to therapy. Front Oncol (2015) 5:230. doi: 10.3389/fonc.2015.00230
54. Osher E, Macaulay VM. Therapeutic targeting of the IGF axis. Cells (2019) 8(8):895. doi: 10.3390/cells8080895
55. Mancarella C, Morrione A, Scotlandi K. Novel regulators of the IGF system in cancer. Biomolecules (2021) 11(2):273. doi: 10.3390/biom11020273
56. LeRoith D, Roberts JCT. The insulin-like growth factor system and cancer. Cancer Lett (2003) 195(2):127–37. doi: 10.1016/S0304-3835(03)00159-9
57. Samani AA, Yakar S, LeRoith D, Brodt P. The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocrine Rev (2007) 28(1):20–47. doi: 10.1210/er.2006-0001
58. Pohlman AW, Moudgalya H, Jordano L, Lobato GC, Gerard D, Liptay MJ, et al. The role of IGF-pathway biomarkers in determining risks, screening, and prognosis in lung cancer. Oncotarget (2022) 13:393. doi: 10.18632/oncotarget.28202
59. Tan SC. Low penetrance genetic polymorphisms as potential biomarkers for colorectal cancer predisposition. J Gene Med (2018) 20(4):e3010. doi: 10.1002/jgm.3010
60. Hosseini SA, Zand H, Cheraghpour M. The influence of curcumin on the downregulation of MYC, insulin and IGF-1 receptors: a possible mechanism underlying the anti-growth and anti-migration in chemoresistant colorectal cancer cells. Medicina (2019) 55(4):90. doi: 10.3390/medicina55040090
61. Gao Y, Katki H, Graubard B, Pollak M, Martin M, Tao Y, et al. Serum IGF1, IGF2 and IGFBP3 and risk of advanced colorectal adenoma. Int J Cancer (2012) 131(2):E105–13. doi: 10.1002/ijc.26438
62. Larsson SC, Carter P, Vithayathil M, Kar S, Mason AM, Burgess S. Insulin-like growth factor-1 and site-specific cancers: a mendelian randomization study. Cancer Med (2020) 9(18):6836–42. doi: 10.1002/cam4.3345
63. Watling CZ, Schmidt JA, Dunneram Y, Tong TY, Kelly RK, Knuppel A, et al. Risk of cancer in regular and low meat-eaters, fish-eaters, and vegetarians: a prospective analysis of UK biobank participants. BMC Med (2022) 20(1):1–13. doi: 10.1186/s12916-022-02256-w
64. Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skeletal Muscle (2011) 1(1):1–14. doi: 10.1186/2044-5040-1-4
65. Wang W, Wu B, Chen G, Zhou Y, Li Z, Zhang J, et al. Meta-analysis of the association of IGFBP3 and IGF1 polymorphisms with susceptibility to colorectal cancer. Neoplasma (2018) 65(6):855–64. doi: 10.4149/neo_2018_170720N491
66. Ge W, Li Y, Xiang H, Li H. Lack of association of IGFBP-3 gene polymorphisms with colorectal cancer: evidence from 17,380 subjects. Mol Biol Rep (2014) 41(4):2609–15. doi: 10.1007/s11033-014-3119-4
67. Lu L, Wang F, He L, Xue Y, Wang Y, Zhang H, et al. Interaction between IGF1 polymorphisms and the risk of acute lymphoblastic leukemia in Chinese children. Cell Physiol Biochem (2015) 36(4):1346–58. doi: 10.1159/000430301
68. Xu G-P, Chen W-X, Xie W-Y, Wu L-F. The association between IGF1 gene 3’-UTR polymorphisms and cancer risk: a meta-analysis. Medicine (2018) 97(51):1–9. doi: 10.1097/MD.0000000000013829
69. Canzian F, McKay J, Cleveland R, Dossus L, Biessy C, Rinaldi S, et al. Polymorphisms of genes coding for insulin-like growth factor 1 and its major binding proteins, circulating levels of IGF-I and IGFBP-3 and breast cancer risk: results from the EPIC study. Br J Cancer (2006) 94(2):299–307. doi: 10.1038/sj.bjc.6602936
70. Mao J, Zhuang G, Chen Z. Genetic polymorphisms of insulin-like growth factor 1 are associated with osteosarcoma risk and prognosis. Med Sci Monitor: Int Med J Exp Clin Res (2017) 23:5892. doi: 10.12659/MSM.908004
71. Zecevic M, Amos CI, Gu X, Campos IM, Jones JS, Lynch PM, et al. IGF1 gene polymorphism and risk for hereditary nonpolyposis colorectal cancer. J Natl Cancer Institute (2006) 98(2):139–43. doi: 10.1093/jnci/djj016
72. Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y, et al. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. Eur J Hum Genet (2009) 17(12):1668–75. doi: 10.1038/ejhg.2009.86
73. Qin L, Zhao J, Wu Y, Zhao Y, Chen C, Xu M, et al. Association between insulin-like growth factor 1 gene rs35767 polymorphisms and cancer risk: a meta-analysis. Medicine (2019) 98(46):1–10. doi: 10.1097/MD.0000000000018017
74. Davies M, Gupta S, Goldspink G, Winslet M. The insulin-like growth factor system and colorectal cancer: clinical and experimental evidence. Int J Colorectal Dis (2006) 21(3):201–8. doi: 10.1007/s00384-005-0776-8
75. Heckl SM, Pellinghaus M, Behrens H-M, Krüger S, Schreiber S, Röcken C. Questioning the IGF1 receptor’s assigned role in CRC–a case for rehabilitation? BMC Cancer (2020) 20(1):1–12. doi: 10.1186/s12885-020-07173-w
76. LeRoy EC, Moore JH, Hu C, Martínez ME, Lance P, Duggan D, et al. Genes in the insulin and insulin-like growth factor pathway and odds of metachronous colorectal neoplasia. Hum Genet (2011) 129(5):503–12. doi: 10.1007/s00439-010-0942-0
77. Schirripa M, Zhang W, Heinemann V, Cao S, Okazaki S, Yang D, et al. Single nucleotide polymorphisms in the IGF-IRS pathway are associated with outcome in mCRC patients enrolled in the FIRE-3 trial. Int J Cancer (2017) 141(2):383–92. doi: 10.1002/ijc.30715
78. Lin J, Wang Y, Tang W, Jiang H, Liu C, Guo Z, et al. Insulin receptor substrate-2 (IRS-2) rs1805097 g> a polymorphism is associated with colorectal cancer susceptibility: a meta-analysis involving 11,234 subjects. Int J Clin Exp Med (2016) 9(7):12639–48.
79. Almind K, Bjørbaek C, Vestergaard H, Hansen T, Echwald S, Pedersen O. Aminoacid polymorphisms of insulin receptor substrate-1 in non-insulin-dependent diabetes mellitus. Lancet (1993) 342(8875):828–32. doi: 10.1016/0140-6736(93)92694-O
80. Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr (2001) 131(11):3109S–20S. doi: 10.1093/jn/131.11.3109S
81. Jung SY, Rohan T, Strickler H, Bea J, Zhang Z-F, Ho G, et al. Genetic variants and traits related to insulin-like growth factor-I and insulin resistance and their interaction with lifestyles on postmenopausal colorectal cancer risk. PloS One (2017) 12(10):e0186296. doi: 10.1371/journal.pone.0186296
82. Li P, Wang L, Liu L, Jiang H, Ma C, Hao T. Association between IRS-1 Gly972Arg polymorphism and colorectal cancer risk. Tumor Biol (2014) 35:6581–5. doi: 10.1007/s13277-014-1900-6
83. Yin J, Zhang Z, Zheng H, Xu L. IRS-2 rs1805097 polymorphism is associated with the decreased risk of colorectal cancer. Oncotarget (2017) 8(15):25107. doi: 10.18632/oncotarget.15342
84. Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. Jama (1998) 279(4):281–6. doi: 10.1001/jama.279.4.281
Keywords: insulin-like growth factor family, colorectal cancer, polymorphism, meta-analysis, insulin receptor substrate
Citation: Cheraghpour M, Askari M, Tierling S, Shojaee S, Sadeghi A, Ketabi Moghadam P, Khazdouz M, Asadzadeh Aghdaei H, Piroozkhah M, Nazemalhosseini-Mojarad E and Fatemi N (2023) A systematic review and meta-analysis for the association of the insulin-like growth factor1 pathway genetic polymorphisms with colorectal cancer susceptibility. Front. Oncol. 13:1168942. doi: 10.3389/fonc.2023.1168942
Received: 30 March 2023; Accepted: 04 May 2023;
Published: 22 May 2023.
Edited by:
Shuang Chen, Tianjin International Joint Academy of Biomedicine, ChinaReviewed by:
Zorana Dobrijevic, Institute for the Application of Nuclear Energy (INEP), SerbiaCopyright © 2023 Cheraghpour, Askari, Tierling, Shojaee, Sadeghi, Ketabi Moghadam, Khazdouz, Asadzadeh Aghdaei, Piroozkhah, Nazemalhosseini-Mojarad and Fatemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nayeralsadat Fatemi, bl9mYXRlbWlfMTM2M0B5YWhvby5jb20=
†ORCID: Makan Cheraghpour, https://orcid.org/0000-0003-4459-4528
Nayeralsadat Fatemi, https://orcid.org/0000-0001-7906-2260
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.