
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 27 June 2023
Sec. Cardio-Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1168651
This article is part of the Research TopicCancer Treatment-Related Cardiovascular Disease - Real World Data in Cardio-OncologyView all 10 articles
 Kai Yi Wu1
Kai Yi Wu1 Sarah Parent2
Sarah Parent2 Lingyu Xu3
Lingyu Xu3 Maryam Yaqoob1
Maryam Yaqoob1 W. Allan Black4
W. Allan Black4 Andrea Shysh1
Andrea Shysh1 John R. Mackey5
John R. Mackey5 Karen King5
Karen King5 Harald Becher1
Harald Becher1 Edith Pituskin4,5
Edith Pituskin4,5 D. Ian Paterson6*
D. Ian Paterson6*Background: Many patients with breast cancer receive therapies with the potential to cause cardiotoxicity. Echocardiography and multiple-gated acquisition (MUGA) scans are the most used modalities to assess cardiac function during treatment in high-risk patients; however, the optimal imaging strategy and the impact on outcome are unknown.
Methods: Consecutive patients with stage 0-3 breast cancer undergoing pre-treatment echocardiography or MUGA were identified from a tertiary care cancer center from 2010-2019. Demographics, medical history, imaging data and clinical events were collected from hospital charts and administrative databases. The primary outcome is a composite of all-cause death or heart failure event. Clinical and imaging predictors of outcome were evaluated on univariable and multivariable analyses.
Results: 1028 patients underwent pre-treatment MUGA and 1032 underwent echocardiography. The groups were well matched for most clinical characteristics except patients undergoing MUGA were younger, had more stage 3 breast cancer and more HER2 over-expressing and triple negative cases. Routine follow-up cardiac imaging scan was obtained in 39.3% of patients with MUGA and 38.0% with echocardiography. During a median follow-up of 2448 (1489, 3160) days, there were 194 deaths, including 7 cardiovascular deaths, and 28 heart failure events with no difference in events between the MUGA and echocardiography groups. There were no imaging predictors of the primary composite outcome or cardiac events. Patients without follow-up imaging had similar adjusted risk for the composite outcome compared to those with imaging follow-up, hazard ratio 0.8 (95% confidence interval 0.5,1.3), p=0.457.
Conclusion: The selection of pretreatment echocardiography or MUGA did not influence the risk of death or heart failure in patients with early breast cancer. Many patients did not have any follow-up cardiac imaging and did not suffer worse outcomes. Cardiovascular deaths and heart failure event rates were low and the value of long-term cardiac imaging surveillance should be further evaluated.
Cardiotoxicity is a recognized complication arising from anticancer therapy and may lead to cardiac and cancer morbidity and premature death (1). Cancer treatment regimens containing anthracyclines and/or human epidermal growth factor-2 (HER2) targeted therapies have significant cardiotoxic potential, therefore, patients with breast cancer are particularly vulnerable to adverse cardiac outcomes (2). Anthracyclines can cause direct myocardial damage in dose-dependent fashion and may result in cardiac dysfunction, irreversible cardiomyopathy, and heart failure (1). Trastuzumab, a monoclonal antibody directed against HER2 receptors, has been associated with a two-fold risk of worsening cardiac function and a five-to-seven-fold risk of overt heart failure (3, 4). Monitoring of left ventricular ejection fraction (LVEF) is recommended for the first 12 months in all patients receiving HER2-targeted therapy and/or anthracyclines with a cumulative dose of 250 mg/m2 of doxorubicin or equivalent (5). Furthermore, long-term (5+ years) imaging surveillance of cardiac function is recommended for cancer survivors at high-risk for heart failure (4). Cancer treatment related cardiac dysfunction may be prevented and treated with beta blockers and/or renin-angiotensin inhibitors (5–7).
Echocardiography (echo) and multiple-gated acquisition (MUGA) radionuclide ventriculography scans are commonly used modalities to assess cardiac function in patients at high-risk for cancer therapy related cardiotoxicity. Current European Society of Cardiology guidelines recommend echo before MUGA as the first-line modality to assess cardiac function due to a superior safety profile and a more comprehensive cardiac assessment (5). However, in many centers, MUGA is more available and accessible than echo. Previous studies found that MUGA is more sensitive for detecting changes in LVEF than echo (8–10). However, the main disadvantage of MUGA is radiation exposure (~5 to 10 mSv per scan) which is significant in patients undergoing long-term surveillance with repeated exams (1). Furthermore, it has been suggested that patients with cancer are more likely to receive beta-blocker therapy and be referred to a cardiologist for reduced cardiac function detected on echo compared to MUGA (11).
Therefore, we sought to determine if the cardiac imaging modality (echo or MUGA) used for cardiotoxicity surveillance in patients with early breast cancer influences short and long-term cardiovascular outcomes. Furthermore, we sought to understand the pattern of use and duration of cardiac imaging monitoring in these patients.
Consecutive adult patients with stage 0-3 breast cancer were identified from a prospective cardio-oncology echocardiography registry at a tertiary cardiac hospital (Mazankowski Alberta Heart Institute, Edmonton, Alberta, Canada) from January 1, 2010, to December 31, 2019. Similar patients undergoing pre-treatment MUGA during the same timeframe were also identified from an affiliated tertiary cancer center (Cross Cancer Institute, Edmonton, Alberta, Canada).
Individual patient charts and electronic health records were reviewed to determine baseline characteristics including prior medical history, cancer type and staging, cancer treatments, cardiac imaging surveillance, and clinical outcomes. Patients with Eastern Cooperative Oncology Group performance status 3 or 4 were excluded. Breast cancer staging was determined according to the TNM classification proposed by the American Joint Committee on Cancer (12). The European Society of Cardiology (ESC) 2022 Heart Failure Association–International Cardio-Oncology Society (HFA-ICOS) risk classification was used to assess the baseline cardiovascular toxicity risk (5). However, previous chemotherapy data and baseline serum biomarkers were unavailable for most patients and were therefore not included in the risk calculation.
The treating medical oncology team ordered cardiac imaging prior to initiating cancer therapy and the modality, echo or MUGA, was usually selected according to availability. As MUGA was scheduled and performed at the cancer center, patients requiring rapid access to cardiac imaging (e.g. stage 3 or neoadjuvant) more commonly underwent this exam. Echocardiograms were acquired at the cardio-oncology clinic using an ultrasonographic system (EPIQ 7C, Philips Medical Systems, N.A., Bothell, USA) equipped with a X5-1 transducer. All patients received echocardiographic contrast (Definity, Lantheus Medical Imaging, North Billerica, USA) bolus regardless of non-contrast image quality in order to minimize variability of LVEF measurements. LVEF was measured from the contrast recordings using the biplane Simpson’s method on commercially available software (13). MUGA scans were performed with technetium 99 m-labeled red blood cells with an activity of approximately 11 to 13 MBq/kg. Images were acquired with a dual-head gamma camera (Siemens Healthineers, Erlangen, Germany and Philips Medical Systems, N.A., Bothell, USA). Scintigrams were smoothed off-line using standard algorithms, and background correction was performed. LVEF was calculated from left ventricular time-activity curves according to the current recommendations (14). For this analysis, patients were assigned either to a MUGA, or echo cohort based on the imaging modality used at baseline. Patients with both imaging modalities at baseline were assigned to the imaging group with the date closest to their cancer diagnosis. Follow-up imaging surveillance strategies were recorded and classified as all echo, all MUGA or mixed modality (both MUGA and echo were performed). Only cardiac imaging performed within 15 months from the baseline scan was included to reflect the standard duration of follow-up at our cancer center. Left ventricular ejection fraction was recorded for each cardiac imaging encounter.
The primary outcome is a composite of all-cause death and/or new or worsening heart failure diagnosis, and secondary outcomes are (i) cardiovasular death or heart failure event, and (ii) new or worsening heart failure. Events were collected from Jan 11, 2010, to December 31, 2020.
Clinical outcomes data were obtained from medical charts and an independent review of health administrative databases (Alberta Strategy for Patient Oriented Research Support Unit). Information obtained included (i) all admissions to acute care facilities; (ii) all ambulatory encounters, including emergency department visits and (iii) vital statistics including the date and cause of death. Diagnoses were classified using the International Classification of Diseases, Canadian Enhancement; ICD-10. Heart failure events included any new heart failure or cardiomyopathy related encounters during the follow-up period.
Cancer therapy–related cardiac dysfunction (CTRCD) was defined as a reduction in LVEF of ≥10% to a value <50% (5, 15). Cardiac biomarkers and left ventricular strain measurements were not available for many patients and were therefore not used for determining CTRCD events.
The Shapiro-Wilk normality test was used to test the normal distribution of continuous variables and continuous variables were expressed as mean ± standard deviation or median (25th, 75th percentile), as appropriate. Categorical variables were expressed as frequency and percentage. Chi-square testing or Fisher’s exact test was used to compare categorical variables between two groups undergoing echo or MUGA at baseline. Two sample t-test or Mann-Whitney U test was used to compare continuous variables among two groups of patients, as appropriate. Univariable Cox proportional regression of outcome was performed in all clinical (cancer-related and cardiovascular disease-related) and imaging metrics at baseline and stepwise forward selection of parameters with p-value<0.2 was used to identify the best predictors of outcome. In the multivariable Cox proportional hazard analysis, all non-collinear 1-year parameters of interest with univariable p-value<0.2 were independently tested for their association with adverse outcomes after adjustment for baseline risk. The Kaplan-Meier method was used to plot time to clinical events for significant parameters from multivariable analysis. A p value less than 0.05 was considered significant for all tests. Statistical analyses were performed using STATA version 17.0 software (StataCorp LP, College Station, Texas).
This study complies with the Declaration of Helsinki and was approved by the University of Alberta Research Ethics Board (HREBA.CC-16-0511). Informed patient consent was not required due to the minimal risk to the patients involved.
During the study period, we identified 1028 patients with early-stage breast cancer undergoing pre-treatment MUGA and 1032 patients undergoing pre-treatment echo who fulfilled study entry criteria. Significant baseline differences included older age and prior cancer diagnosis in the echo group and more advanced cancer stage, more aggressive cancer receptor types and more anthracycline and trastuzumab use in the MUGA group (Table 1). Patients were well balanced for their baseline cardiovascular risk factors and cardiovascular medications except for more angiotensin-converting enzyme inhibitor use in the MUGA group, and more angiotensin receptor blocker use in the echo group. Baseline HFA-ICOS cardiovascular toxicity risk was similar between cohorts, with 54.1% of MUGA patients and 57.3% of echo patients classified as low risk (Table 1).
Baseline LVEF was slightly lower in the MUGA group compared to echo, median LVEF 64% vs. 65%, p = 0.0064 (Table 2). At least one follow-up cardiac imaging scan was obtained in 39.3% of patients undergoing pre-treatment MUGA and in 38% with pre-treatment echo within 15 months from the baseline scan. No follow-up imaging was found in 90.4% of patients receiving anthracycline-based treatment compared to 5.1% of patients receiving trastuzumab. Patients who had a baseline MUGA were more likely to be scanned with another imaging modality and had more cardiac imaging tests compared to patients with echo (Table 2). The incidence of CTRCD was similar in both groups, 12.2% for MUGA and 12.1% for echo (Table 2). For patients with high or very high HFA-ICOS risk, CTRCD occurred in 36% compared to 10.8% and 9.8% for moderate and low risk patients respectively, odds ratio 2.2 (95% confidence interval (1.9, 2.7), p < 0.001.
During a median follow-up of 2448 (1489, 3160) days, there were 194 deaths and 28 heart failure events with no difference in events between the MUGA and echo cohorts. The cause of death was cancer related in 171 (88%) cases and cardiovascular related in 7 (3.6%). The 7 cardiovascular related deaths included 4 from coronary artery disease, 1 from arrhythmia, 1 from stroke and 1 from complications of diabetes mellitus. For the 12 patients with late heart failure events after 24 months, 3 were low HFA-ICOS risk at baseline, 5 were moderate risk, 1 was high risk and 3 were very high risk. The timing of cardiac events was also not significantly different between the MUGA and echo groups (Table 3).
Multivariable analysis identified prior heart failure, chronic kidney disease, chronic obstructive pulmonary disease, aldosterone antagonist therapy, stage 3 breast cancer, triple negative receptor status and absence of cardiac imaging surveillance as predictive of the primary outcome (Table 4). The selection of MUGA or echo at baseline was not predictive of clinical events (Tables 4–6, Figure 1). Baseline LVEF and the type of cardiac imaging were not predictive of outcomes (Tables 4–6). Lack of imaging follow-up was not predictive of adverse outcomes, even after excluding patients receiving non-anthracycline, non-trastuzumab treatments (Supplemental Tables 1-3) and after excluding low HFA-ICOS risk patients (Supplemental Tables 4-6). In the overall cohort, risk of cardiac death or heart failure was similar in the 1264 patients without follow-up imaging compared to the 796 patients with follow-up imaging, hazard ratio 0.9 (95% confidence interval 0.5,1.9), p=0.813 (Figure 2). However, the HFA-ICOS risk was predictive cardiac death or heart failure on multivariable and adjusted survival analyses (Table 5 and Figure 3).
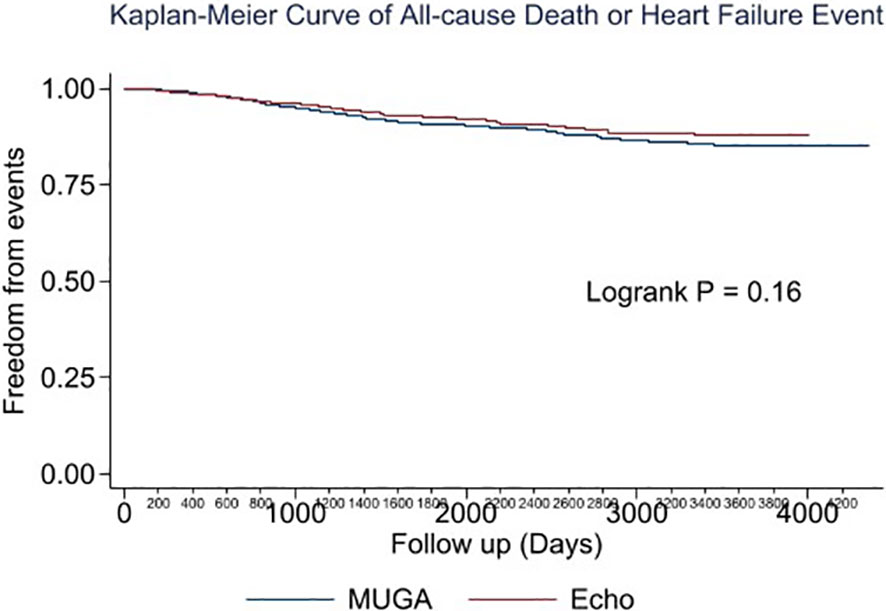
Figure 1 Plot of unadjusted Kaplan-Meier event-free survival for patients with breast cancer assigned to pretreatment multiple-gated acquisition (MUGA) scan (Blue) or echocardiography (Red). The composite event included all-cause death or new heart failure.
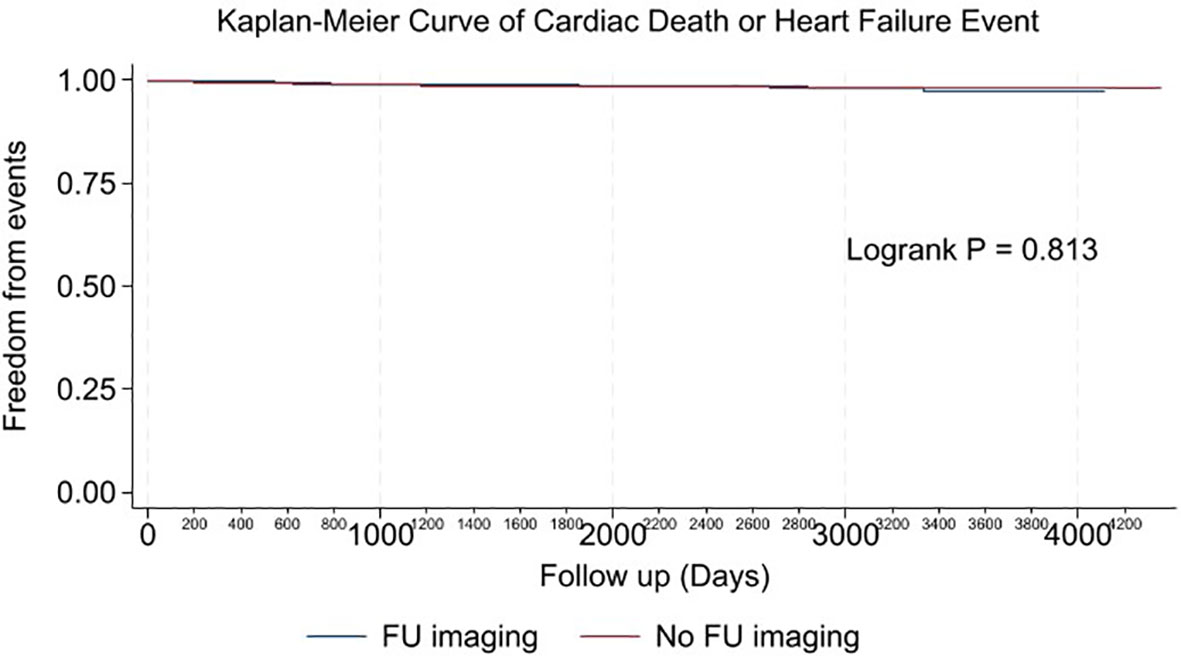
Figure 2 Plot of unadjusted Kaplan-Meier event-free survival curve for cardiac death or new heart failure in patients with imaging follow-up (Blue), and no imaging follow-up (Red).
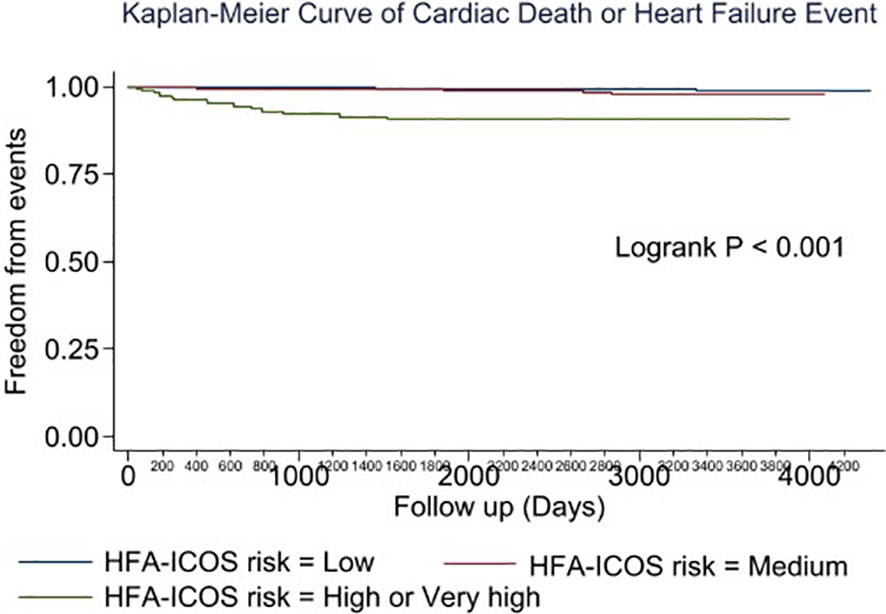
Figure 3 Plot of the adjusted survival curve for cardiac death or new heart failure in patients with breast cancer assigned to low (Blue), moderate (Red), high or very high (Green) European Society of Cardiology Heart Failure Association-International Cardio-Oncology Society risk.
In this large, real-world cohort study of patients with early-stage breast cancer, we found that the pretreatment cardiac imaging modality (MUGA or echo) was not associated with all-cause death or new heart failure during extended follow-up. Over 60% of patients had no follow-up cardiac imaging after the baseline scan and lack of imaging surveillance was not associated with adverse cardiac outcomes. HFA-ICOS risk was consistently associated with death, cardiac death and heart failure on multivariable analysis.
ESC cardio-oncology guidelines recommend that asymptomatic patients receiving trastuzumab have follow-up cardiac imaging every 3 months for the first 12 months and repeat testing at 24 months (5). Similarly, patients receiving anthracycline-based chemotherapy should have follow-up cardiac imaging at 12 months and higher risk individuals should undergo 4 intervening scans during and after treatment (5). Additionally, asymptomatic patients at high or very high HFA-ICOS risk are recommended to have follow-up imaging at years 1, 3 and 5 and possibly every 5 years thereafter (5). In this real-world study of patients with early breast cancer receiving cardiotoxic therapy, no follow-up imaging was seen in 61.4%, including 90.4% of patients treated with anthracyclines. Patients with no cardiac imaging follow-up had similar outcomes compared to patients with echo and/or MUGA surveillance, although, heart failure event rates were low. Our findings build on existing knowledge regarding the utility of surveillance cardiac imaging in patients with breast cancer (16, 17). While cardiology involvement in the care of breast cancer patients can lead to adherence to guideline recommended cardiac surveillance during cancer treatment (18), the impact of cardiac monitoring on hard endpoints is unclear. For example, Yu et al. found a lack of association between adherence to routine echocardiogram monitoring and clinical heart failure, suggesting that routine LVEF assessments may be insufficient to decrease the risk of heart failure (17).
In our cohort, we found that patients undergoing echo were more likely to have same modality compared to MUGA (Table 2). Although current guidelines recommend using the same imaging method given observed differences in LVEF measurements between different modalities (1, 8, 19), our study did not find that the type of follow-up imaging (same modality or mixed modality) to be a significant predictor of clinical outcomes.
Among patients with follow-up imaging, 12.2% in the MUGA group and 12.1% in the echo group developed CTRCD. Furthermore, cardiac death or heart failure occurred in only 32 patients (1.6%) during a median follow-up of 6.7 years. The incidence of CTRCD in our cohort is similar to that found in other studies. In the CARDIOTOX registry, which included 865 patients receiving high-risk cancer treatment regimens (84.5% anthracyclines), López-Sendón et al. found the overall incidence of CTRCD was 37.5% (20). However, the majority of the CTRCD were mild (31.6%), defined as asymptomatic patients with LVEF ≥50% with elevated biomarkers or at least one additional abnormal echo parameter, whereas 5.9% of patients had LVEF <50% (20). In another study of 373 patients with breast cancer followed for a mean of 2.4 ± 1 years, there were no cases of LV dysfunction in the anthracycline cohort (0/202), and 16/171 (9%) in the anti-HER2 therapy group (21). In a study by Battisti et al. which included patients with early breast cancer treated with trastuzumab, 5.91% of the patients experienced a LVEF decline ≥ 10% to below 50% but only 5% developed symptomatic heart failure (4.5% with New York Heart Association class II and 0.5% with class III-IV) (22).
ESC guidelines recommend using HFA-ICOS risk to guide strategies preventing cancer therapy associated cardiotoxicity (5). In our study, patients with high or very high HFA-ICOS risk (7.5% of the overall cohort) were at increased risk for CTRCD as well as cardiac death or heart failure compared to patients with moderate or low risk. This finding is consistent with recent studies that examined the utility of HFA-ICOS proforma in predicting LV dysfunction and heart failure events in patients with breast cancer (21). In a smaller breast cancer cohort, Tini et al. found a similar distribution of HFA-ICOS baseline risk and that patients with increased risk receiving anti-HER2 therapy experienced a greater incidence of LV dysfunction (21). While HFA-ICOS risk predicts cardiac events in patients with breast cancer receiving anthracycline and/or anti-HER2 therapies, future studies should evaluate whether imaging surveillance in higher risk individuals mitigates this risk.
This was a single-center, retrospective study of the relationship between cardiac imaging and clinical outcomes. This design introduces the potential for bias and these results should therefore be confirmed in multicenter, prospective studies. Nevertheless, this is one of the largest studies of cardiotoxicity with a long period of follow-up (median 6.7 years). Heart failure event rates were relatively low compared to other studies of cardiotoxicity in patients with breast cancer. However, it is unlikely that heart failure events were missed given that data was extracted multiple sources including manual chart review and provincial health administrative databases. Referrals to cardiology and the initiation of cardiac medications were not systematically collected in our study and we are therefore not able to determine the potential impact of cardiac imaging on these outcomes. Cardiac biomarkers and left ventricular strain measurements were unavailable for most patients. Therefore, CTRCD was defined using only the LVEF criteria. The role of echo strain to guide management is unclear (23, 24).
In this contemporary study of patients with early breast cancer undergoing cardiotoxic cancer therapy, the selection of baseline cardiac imaging (MUGA or echo) did not influence the risk of death or heart failure. Many patients did not have any follow-up cardiac imaging and did not suffer worse outcomes. Cardiac death and heart failure event rates were low and the value of long-term cardiac imaging surveillance should be further evaluated.
The datasets presented in this article are not readily available because we would require approval from our health research ethics board and possibly a legal agreement to share this data with individuals from outside institutions. Requests to access the datasets should be directed to ZHBhdGVyc29uQG90dGF3YWhlYXJ0LmNh.
The studies involving human participants were reviewed and approved by University of Alberta Health Research Ethics Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Study conception: KW, SP, JRM, KK, HB, EP, DIP. Data acquisition: KW, SP, MY, WB, AS, HB, DIP. Data analysis: KW, SP, LX, DIP. Manuscript writing and critical review: All. All authors contributed to the article and approved the submitted version.
EP is supported by a Tier 2 Canada Research Chair.
DIP reports funding from Pfizer and AstraZeneca. HB receives funding from Lantheus and Bracco and is on the advisory board for Lantheus.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1168651/full#supplementary-material
1. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging (2014) 15(10):1063–93. doi: 10.1093/ehjci/jeu192
2. Posch F, Niedrist T, Glantschnig T, Moik F, Kolesnik E, Wallner M, et al. Left-ventricular ejection fraction and cardiac biomarkers for dynamic prediction of cardiotoxicity in early breast cancer. Front Cardiovasc Med (2022) 2100. doi: 10.3389/fcvm.2022.933428
3. Dahabreh IJ, Linardou H, Siannis F, Fountzilas G, Murray S. Trastuzumab in the adjuvant treatment of early-stage breast cancer: a systematic review and meta-analysis of randomized controlled trials. Oncologist (2008) 13(6):620–30. doi: 10.1634/theoncologist.2008-0001
4. Moja L, Tagliabue L, Balduzzi S, Parmelli E, Pistotti V, Guarneri V, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Systematic Rev (2012) 2021(2). doi: 10.1002/14651858.CD006243.pub2
5. Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, et al. 2022 ESC guidelines on cardio-oncology developed in collaboration with the European hematology association (EHA), the European society for therapeutic radiology and oncology (ESTRO) and the international cardio-oncology society (IC-OS): developed by the task force on cardio-oncology of the European society of cardiology (ESC). Eur Heart J (2022) 43(41):4229–361. doi: 10.1093/eurheartj/ehac244
6. Lewinter C, Nielsen TH, Edfors LR, Linde C, Bland JM, Lewinter M, et al. A systematic review and meta-analysis of beta-blockers and renin–angiotensin system inhibitors for preventing left ventricular dysfunction due to anthracyclines or trastuzumab in patients with breast cancer. Eur Heart J (2022) 43(27):2562–9. doi: 10.1093/eurheartj/ehab843
7. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. (2015) 131(22):1981–8. doi: 10.1161/CIRCULATIONAHA.114.013777
8. Bellenger NG, Burgess MI, Ray SG, Lahiri A, Coats AJS, Cleland JGF, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. are they interchangeable? Eur Heart J (2000) 21(16):1387–96. doi: 10.1053/euhj.2000.2011
9. Walker J, Bhullar N, Fallah-Rad N, Lytwyn M, Golian M, Fang T, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol (2010) 28(21):3429–36. doi: 10.1200/JCO.2009.26.7294
10. van Royen N, Jaffe CC, Krumholz HM, Johnson KM, Lynch PJ, Natale D, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol (1996) 77(10):843–50. doi: 10.1016/S0002-9149(97)89179-5
11. Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies: are clinicians responding optimally? J Am Coll Cardiol (2010) 56(20):1644–50. doi: 10.1016/j.jacc.2010.07.023
12. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
13. Lang RM, Badano LP, Victor MA, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr (2015) 28(1):1–39.e14. doi: 10.1016/j.echo.2014.10.003
14. Corbett JR, Akinboboye OO, Bacharach SL, Borer JS, Botvinick EH, DePuey EG, et al. Equilibrium radionuclide angiocardiography. J Nucl Cardiol (2006) 13(6):e56–79. doi: 10.1016/j.nuclcard.2006.08.007
15. Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, et al. Anthracycline-induced cardiotoxicity: a multicenter randomised trial comparing two strategies for guiding prevention with enalapril: the international CardioOncology society-one trial. Eur J Canc (2018) 94:126–37. doi: 10.1016/j.ejca.2018.02.005
16. Tini G, Ameri P, Buzzatti G, Sarocchi M, Murialdo R, Guglielmi G, et al. Diversity of cardiologic issues in a contemporary cohort of women with breast cancer. Front Cardiovasc Med (2021) 8:1266. doi: 10.3389/fcvm.2021.654728
17. Yu AF, Moskowitz CS, Lee Chuy K, Yang J, Dang CT, Liu JE, et al. Cardiotoxicity surveillance and risk of heart failure during HER2 targeted therapy. JACC CardioOncol (2020) 2(2):166–75. doi: 10.1016/j.jaccao.2020.03.002
18. Demissei BG, Adusumalli S, Hubbard RA, Denduluri S, Narayan V, Clark AS, et al. Cardiology involvement in patients with breast cancer treated with trastuzumab. JACC CardioOncol (2020) 2(2):179–89. doi: 10.1016/j.jaccao.2020.04.010
19. Møgelvang J, Stokholm KH, Saunämaki K, Reimer A, Stubgaard M, Thomsen C, et al. Assessment of left ventricular volumes by magnetic resonance in comparison with radionuclide angiography, contrast angiography and echocardiography. Eur Heart J (1992) 13(12):1677–83. doi: 10.1093/oxfordjournals.eurheartj.a060124
20. López-Sendón J, Álvarez-Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, et al. Classification, prevalence, and outcomes of anticancer therapy-induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J (2020) 41(18):1720–9. doi: 10.1093/eurheartj/ehaa006
21. Tini G, Cuomo A, Battistoni A, Sarocchi M, Mercurio V, Ameri P, et al. Baseline cardio-oncologic risk assessment in breast cancer women and occurrence of cardiovascular events: the HFA/ICOS risk tool in real-world practice. Int J Cardiol (2022) 349:134–7. doi: 10.1016/j.ijcard.2021.11.059
22. Battisti NML, Andres MS, Lee KA, Ramalingam S, Nash T, Mappouridou S, et al. Incidence of cardiotoxicity and validation of the heart failure association-international cardio-oncology society risk stratification tool in patients treated with trastuzumab for HER2-positive early breast cancer. Breast Cancer Res Treat (2021) 188(1):149–63. doi: 10.1007/s10549-021-06192-w
23. Moslehi JJ, Witteles RM. Global longitudinal strain in cardio-oncology. J Am Coll Cardiol (2021) 77(4):402–4. doi: 10.1016/j.jacc.2020.12.014
Keywords: cardiac imaging, breast cancer, cardiotoxicity, surveillance, survival
Citation: Wu KY, Parent S, Xu L, Yaqoob M, Black WA, Shysh A, Mackey JR, King K, Becher H, Pituskin E and Paterson DI (2023) Does cardiac imaging surveillance strategy influence outcomes in patients with early breast cancer? Front. Oncol. 13:1168651. doi: 10.3389/fonc.2023.1168651
Received: 17 February 2023; Accepted: 12 June 2023;
Published: 27 June 2023.
Edited by:
Susan Dent, Duke University, United StatesReviewed by:
Eman Hamad, Temple University, United StatesCopyright © 2023 Wu, Parent, Xu, Yaqoob, Black, Shysh, Mackey, King, Becher, Pituskin and Paterson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: D. Ian Paterson, ZHBhdGVyc29uQG90dGF3YWhlYXJ0LmNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.