- 1Department of Medicine V–Hematology, Oncology and Rheumatology, University Hospital Heidelberg, Heidelberg, Germany
- 2Department of Hematology and Stem-Cell Transplantation, University Hospital Essen, Essen, Germany
- 3Clinic for Cardiology and General Internal Medicine, Städtisches Klinikum Solingen gemeinnützige GmbH, Solingen, Germany
- 4Department of Medicine III–Cardiology, Angiology and Intensive Care, University Hospital Heidelberg, Heidelberg, Germany
- 5Institute of Medical Biometry (IMBI), University Hospital Heidelberg, Heidelberg, Germany
- 6Klinikum Ernst von Bergmann–Department for Hematology, Oncology and Palliative Care, Potsdam, Germany
Background: In patients with cardiovascular (CV) comorbidities that necessitate antiplatelet therapy (APT), its optimal management during chemotherapy-induced thrombocytopenia remains elusive, as the risk of bleeding has to be balanced against the risk of CV events. The purpose of this study was to assess the risk for bleeding with APT during thrombocytopenia in patients with multiple myeloma undergoing high-dose chemotherapy and subsequent autologous stem-cell transplantation (ASCT) with and without acetylsalicylic acid (ASA) as comedication.
Methods: We assessed patients who underwent ASCT at the Heidelberg University Hospital between 2011 and 2020 for bleeding events, management strategies for ASA intake during thrombocytopenia, transfusion requirements, and the occurrence of CV events.
Results: There were 57/1,113 patients who continued ASA until at least 1 day after ASCT; thus, a continuous platelet inhibition during thrombocytopenia was assumed. Most of the patients (41/57) continued ASA until they had a platelet count of 20–50/nl. This range reflects the kinetics of thrombocytopenia and nondaily measurements of platelets during ASCT. A tendency toward a higher risk for bleeding events in the ASA group was demonstrated (1.9% (control group) vs. 5.3% (ASA), p = 0.082). The risk factors for bleeding in multivariate analysis were the duration of thrombocytopenia < 50/nl, a history of gastrointestinal bleeding, and diarrhea. The factors predicting the duration of thrombocytopenia were age >60 years, a hematopoietic stem-cell transplantation comorbidity index ≥3, and an impaired bone marrow reserve at admission. CV events occurred in three patients; none of them took ASA or had an indication for APT.
Conclusions: The intake of ASA until thrombocytopenia with a platelet count of 20–50/nl appears safe, although an elevated risk cannot be excluded. If ASA is indicated for the secondary prevention of CV events, the evaluation of risk factors for bleeding and a prolonged time of thrombocytopenia before conditioning is crucial to adapt the strategy for ASA intake during thrombocytopenia.
Introduction
Cardiovascular (CV) disease is common in patients with cancer (1, 2). Due to impaired bone marrow function and more often therapy related, cancer patients frequently present thrombocytopenia with a platelet count (PLC) ≤50 × 109/L which does not preclude from CV events (3, 4). Acetylsalicylic acid (ASA) has an outstanding role for the prevention of major CV events (MACE) (5), including nonfatal stroke, myocardial infarction, and CV death. Many strategies to handle ASA during thrombocytopenia exist without appropriate evidence as data are lacking to what extent ASA increases the bleeding risk in these scenarios.
In daily practice, ASA is frequently withheld during thrombocytopenia National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) III–IV° (PLC < 25–50 × 109/L). However, the discontinuation of ASA leads to the rebound reactivity of platelets caused by increasing thromboxane A2 (TXA2) levels (6). MACE after the discontinuation of ASA are as frequent as one additional event in every 36 patients (7) in studies outside an oncological setting (7–13).
Bleeding events (BLEDs) during thrombocytopenia (14) or antiplatelet therapy (APT) (15) can be life-threatening. The risk for serious BLEDs is not necessarily related to the severity of thrombocytopenia (16, 17), although a critical PLC of 5–7 × 109/L was suggested as required to maintain endothelial integrity to prevent bleeding (18, 19). Although life-threatening bleeding during autologous hematopoietic stem-cell transplantation (ASCT) is rare (14, 20), less severe BLEDs can potentially lead to patient discomfort, prolonged in-patient stay, increased healthcare costs, or further complications. In a recent trial on different platelet transfusion (PLTX) strategies, 19% of ASCT patients developed mild to moderate BLEDs (20). A recent large prospective randomized trial assessed a prophylactic PLTX approach with a morning PLC of < 10 × 109/L in comparison to a therapeutic approach in case of apparent bleeding signs in patients undergoing ASCT or intensive chemotherapy for acute leukemia. The therapeutic approach led to a significant reduction of provided PLTX without increasing the risk for major BLEDs in patients undergoing ASCT (21). Thus, a therapeutic approach appears to be safe. Moreover, trials and retrospective data analysis that assessed the effect of PLTXs in patients with intracranial or gastrointestinal (GI) bleeding upon APT and a mostly normal PLC found even a negative impact on death and/or disability (22–24). Thus, there is no evidence that fosters a liberal transfusion approach so far.
To evaluate the risk of bleeding during thrombocytopenia and concomitant APT, we reviewed BLEDs in a large cohort of multiple myeloma patients with and without APT undergoing ASCT.
Materials and methods
Ethical considerations
The study design and data acquisition were approved by the institutional review board of the University Heidelberg, Germany, No S-721/2018, and the study was performed in accordance with the Declaration of Helsinki.
Study population
Patients undergoing ASCT for multiple myeloma at the in-patient service of the University Hospital Heidelberg between 2011 and February 2020 were included. Patients undergoing their ASCT as outpatients, being on dual APT or therapeutic anticoagulation, were excluded. Patient characteristics and the course of ASCT were captured by the review of electronical medical records (EMRs).
Study definitions and outcomes
The primary outcome was the occurrence of BLEDs during hospital stay. These were staged according to CTCAE, version 5.0 (National Cancer Institute, Bethesda, MD), the Bleeding Severity Measurement Scale (BSMS) (25), and the World Health Organization (WHO) bleeding scale. In-depth discussion and rationale for the use of these bleeding scales are summarized and discussed in the Supplementary Material (1.1 Supplementary Methods, 1.1.1 Grading of bleeding events, their definitions, and rationale).
Secondary outcomes included the number of PLTXs, the time to the engraftment of platelets, and MACE. For the purpose of this study, MACE was defined as nonfatal stroke, nonfatal myocardial infarction, and CV death.
Management of antiplatelet therapy and patient allocation
ASA therapy at a dose of 75–100 mg during ASCT was managed at individual physicians’ discretion. ASA was regarded as having an efficient antiplatelet function during thrombocytopenia if at least one dose of ASA after the reinfusion of stem cells (day 0) was provided as the autologous stem-cell preparation contained platelets and plasma potentially reverting the ASA effect. Furthermore, it was assumed that all platelets at this point were sufficiently inhibited and no turnover took place until hematopoietic recovery. ASA was usually recommenced with the recovery of PLC >20–50 × 109/L. Patients who stopped ASA intake between hospital admission and earlier than 1 day after stem-cell reinfusion (day +1) were excluded from main analyses because neither ASA efficacy could not be assumed nor excluded. BLEDs and the patient characteristics of this group were separately assessed and analyzed to exclude a selection bias.
Center-specific transfusion management during autologous stem-cell transplantation
During the study period, two major transfusion strategies existed at our center (1): prophylactic PLTX if morning PLC < 10 × 109/L and (2) PLTX restricted to signs of bleeding as recently described (21), based on the treating physician’s discretion. Because minor bleeding signs were not always documented in the EMR, a prophylactic versus therapeutic approach could not assigned to every provided PLTX and was therefore not further integrated into analyses.
There were 1 to 2 units of packed red blood cell (PRBC) transfusions that were usually provided in case of hemoglobin ≤ 70 or 80 g/L with symptomatic anemia.
Statistical analysis
Categorical variables were summarized by number and percentage, and continuous variables were summarized by mean, standard deviation, and range (minimum/maximum). Continuous variables were compared between ASA and the control group (or as stated otherwise) using Welch’s t-test for unequal variances and categorial variables by the chi-square test. Univariate logistic regression models were built to test the association between BLEDs or PLTX requirements, respectively, and all relevant patient characteristics, clinical, and laboratory variables to determine the potential predictors of BLEDs or PLTX requirements. For PLTX requirements, patients with BLEDs were excluded from the analysis to avoid bias due to increased PLTX requirements during bleeding. Multivariable logistic regression models using stepwise estimation by backward and forward selection were then generated from variables with p ≤ 0.05 from the univariate models and known/expected factors to be associated with BLEDs or PLTX requirements. Firth’s logistic regression was used to confirm results for BLEDs as logistic regression could be biased by unbalanced predictors.
A linear regression model to assess risk factors for a prolonged duration of thrombocytopenia in patients without BLEDs to avoid a bias for increased transfusions during bleeding was built. Additionally, two outlier cases with an unusual requirement were excluded to avoid a bias for this analysis.
The rationale and sample size calculation for a randomized controlled trial to evaluate different ASA discontinuation strategies are described in the Supplement Material (1.1 Supplementary Methods, 1.1.2 Design of a randomized controlled trial to assess different APT strategies, and Supplementary Figure S1).
All statistical analyses were performed in SPSS version 28.0.0.0 and SAS version 9.4. Figures were created with R-studio version 4.1.2, Excel, and Biorender.
Results
Study population and patient characteristics
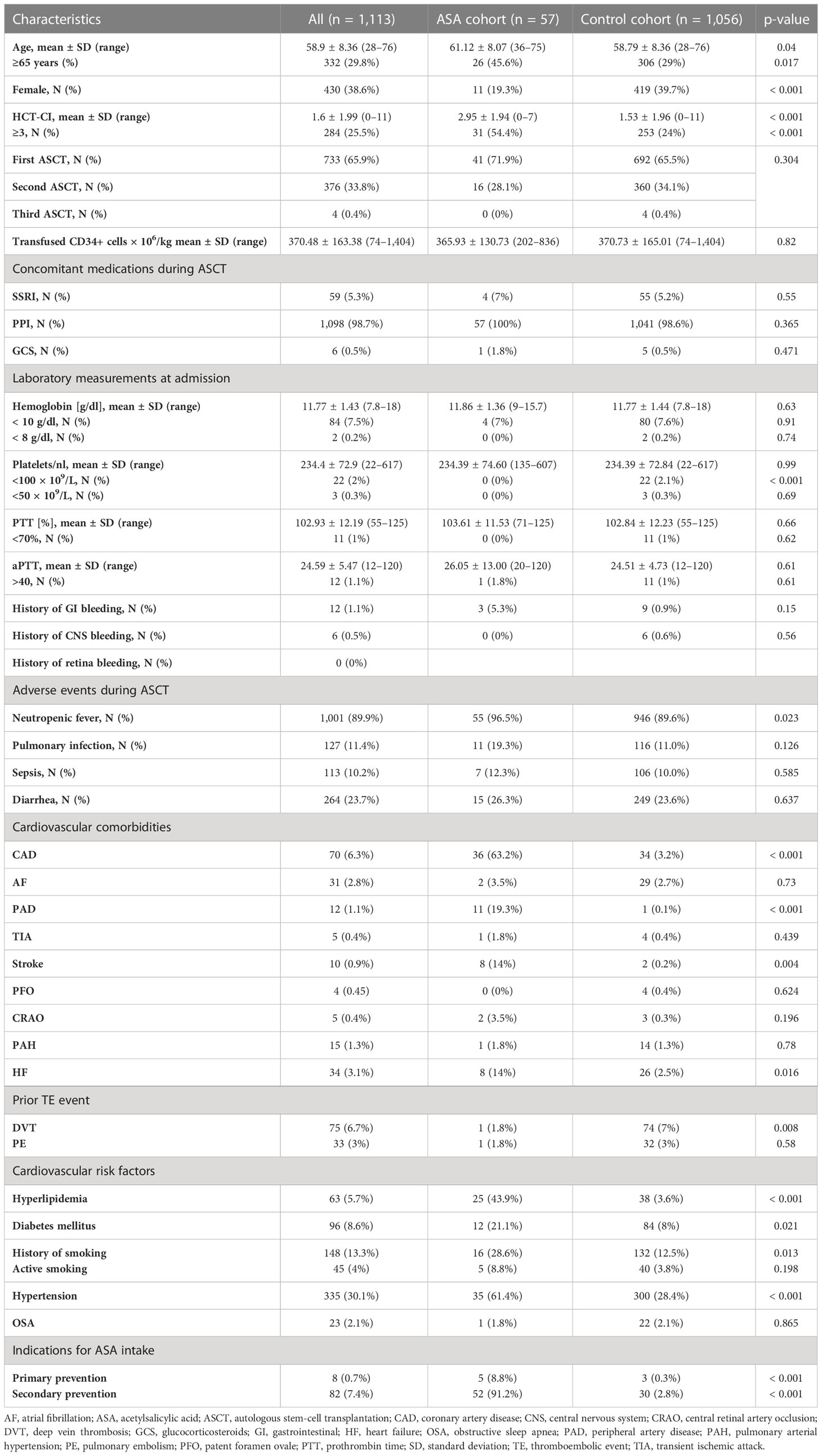
There were 13 patients who were excluded as they paused ASA intake earlier than 1 day after stem-cell reinfusion (day +1); therefore, neither ASA efficacy could be expected nor excluded (the patient characteristics of this group are depicted in Supplementary Table S1). Thus, 1,113 patients were included into analysis. Baseline patient characteristics are summarized in Table 1. The patient characteristics of those who took ASA in comparison to those without (control group) revealed significant differences in age, gender, and the hematopoietic stem-cell transplantation comorbidity index (HCT-CI). Furthermore, patients in the ASA group had more CV comorbidities as expected. All other characteristics were similar between groups.
Management of acetylsalicylic acid intake during thrombocytopenia
For 57 patients, continuous ASA efficacy during thrombocytopenia was assumed as ASA was continued until at least 1 day after ASCT. Of those, the majority (41/57) continued ASA until PLC 20–50 × 109/L (11/57 patients stopped with PLC < 70–80 × 109/L, 28/57 with PLC < 50 × 109/L, 13/57 with PLC < 20–30 × 109/L, and 2 with PLC < 10 × 109/L. Only one patient continued ASA throughout thrombocytopenia, and for 2/57 patients, no clear strategy could be defined). The mean day of last ASA intake after ASCT was day +4.89 ± 1.37 (range: day 2–7), the mean days of ASA discontinuation were 9.39 ± 6.02 (range: 0–42 days; 95% confidence interval (CI) [8, 11.19]).
Bleeding events
A clinically meaningful, documented BLED occurred in 23 patients (2.1%). Of these 23 patients, three patients were in the ASA group. Two of them discontinued ASA before the onset of a BLED, although a continued efficacy of APT was suspected. The third patient took ASA on the day of bleeding, but PLC was still normal. The clinical course of these three patients is depicted in Figure 1. No significant difference in bleeding incidence was demonstrated for patients receiving ASA, although a higher tendency was observed [1.9% (control group) vs. 5.3% (ASA), p = 0.082].
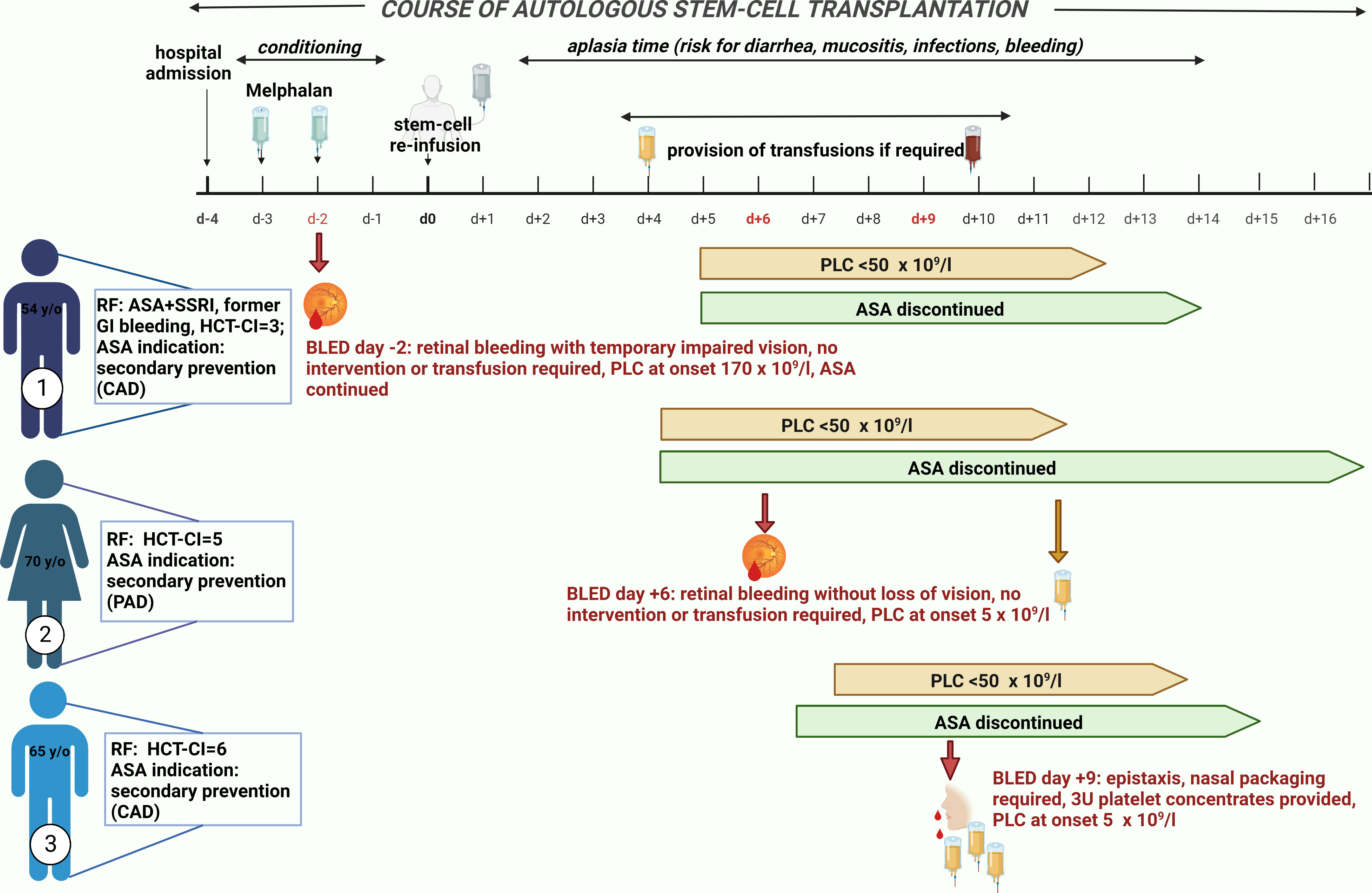
Figure 1 Bleeding events in patients taking acetylsalicylic acid (ASA). Three patients who took ASA developed a bleeding event. Patient 1 suffered from a retinal hemorrhage on day −2. He took ASA on the day of bleeding onset but still had a normal platelet count of 170 × 109/L. As risk factors for bleeding, he took a selective serotonin reuptake inhibitor (SSRI) in addition to ASA and had a former gastrointestinal (GI) bleeding and a hematopoietic stem-cell transplantation comorbidity index (HCT-CI) of 6. The indication for ASA was secondary preventive due to coronary heart disease. During bleeding, he had a temporarily impaired vision but without any need of intervention or transfusion. He reported no sequelae. Patient 2 suffered also from a retinal hemorrhage on day +6 after autologous stem-cell reinfusion during a thrombocytopenia of 5 × 109/L. ASA was discontinued 2 days before due to onset of thrombocytopenia < 50 × 109/L. She did not receive any platelet transfusion (PLTX) prior to bleeding onset; thus, continuous full platelet inhibition by ASA could be expected. She developed only very mild symptoms; thus, no intervention or transfusion was required and no sequelae were reported. Her indication for ASA intake was peripheral artery disease and, as risk factors for bleeding, only an HCT-CI of 5 can be described. Patient 3 developed epistaxis on day +9 after stem-cell reinfusion during a thrombocytopenia of 5 × 109/L. Nasal packaging by an ear–nose–throat specialist was performed, and he received 3 units of PLTXs. ASA was discontinued 3 days prior to bleeding onset without any PLTX in the meantime; thus, continued platelet inhibition could be assumed. As risk factors for bleeding, an HCT-CI of 6 existed and the indication for ASA intake was secondary preventive due to coronary heart disease. All three bleeding events were single events without ongoing bleeding after first onset. ASA, acetylsalicylic acid; BLED, bleeding events; CAD, coronary artery disease; GI, gastrointestinal; HCT-CI, hematopoietic stem-cell transplantation comorbidity index; PAD, peripheral artery disease; PLC, platelet count; RF, risk factor; SSRI, selective serotonin reuptake inhibitor.
The mean PLC at bleeding onset was 30.87 × 109/L ± 46.48 (range: 4–179 × 109/L). There were 52.2% (N = 12) of BLEDs that occurred during PLC ≤ 10 × 109/L. In total, 82.6% of BLEDs were documented during PLC ≤ 50 × 109/L (Figure 2C). The median day of bleeding onset was day +6 after ASCT (range: day −2 to +40). There were 16/23 BLEDs (69.6%) that occurred during a documented infection. The majority constituted lower GI bleedings (34.8%) (Figure 2A).
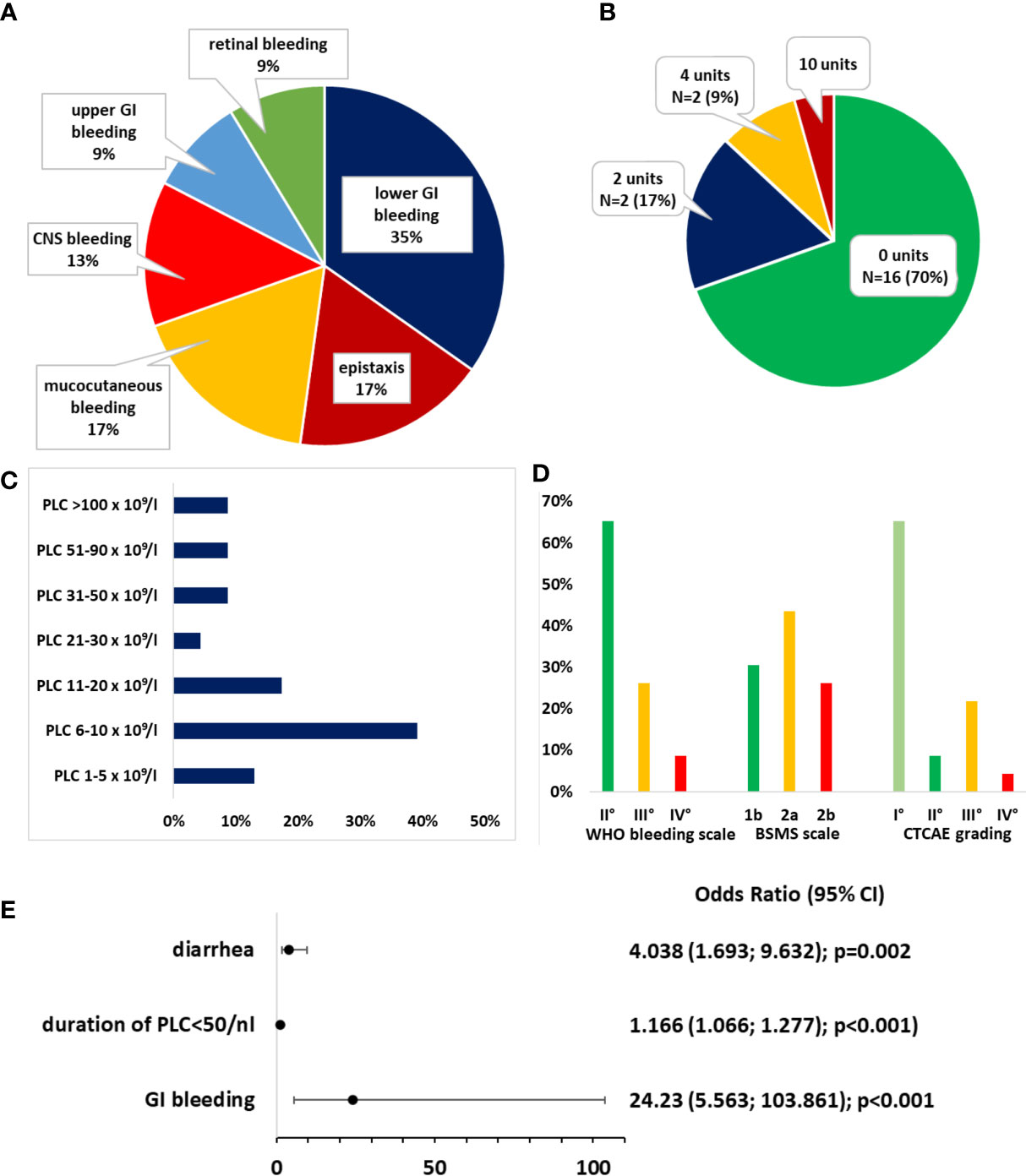
Figure 2 Bleeding events. (A) Distribution of bleeding sites. (B) Distribution of transfusion requirements for packed red blood cells. (C) Distribution of platelet count (PLC) at bleeding onset. (D) Distribution of bleeding severity according to the World Health Organization (WHO) bleeding scale, Bleeding Severity Measurement Scale (BSMS), and NCI Common Terminology Criteria for Adverse Events (CTCAE). (E) Odds ratios (ORs) for the risk of bleeding. A binomial logistic regression model was generated after forward selection.
A specialist consultation (e.g., neurology and urology) was initiated in 8/23 patients, an endoscopy without further intervention was performed in two, and three patients underwent nasal packing to control epistaxis, which was the only intervention that was performed. The transfusion requirements and gradings of bleedings according to the WHO bleeding scale, CTCAE grading, and the BSMS are depicted in Figures 2B, D. In 16 patients, the BLED was deemed clinically significant according to the BSMS (comprising grade 2a/b). No relevant sequelae after hospital discharge were documented.
Of note, among patients who were excluded from analysis as they paused ASA intake earlier than 1 day after stem-cell reinfusion (day +1), therefore, no ASA efficacy was expected, one more patient developed a retinal hemorrhage. Thus, all three documented retinal hemorrhages occurred in patients with ASA. Further details on BLEDs are summarized in Supplementary Table S2.
Risk factors for bleeding
We further assessed the risk factors for BLEDs. Hypertension, the duration of PLC ≤ 50 × 109/L, diarrhea, a history of GI bleeding, and the HCT-CI score were significantly (but weakly) associated with BLEDs (data not shown). The odds ratio (OR) for ASA intake was 2.78 (95% CI, [0.83, 9.98], p = 0.096). In multivariate binomial logistic regression, ASA intake as a risk factor for bleeding was also not significant (Supplementary Figure S2A). After multivariate binomial logistic regression by backward selection, only the duration of PLC ≤ 50 × 109/L (OR = 1.166, 95% CI, [1.066, 1.277]; p < 0.001), diarrhea (OR = 4.038, 95% CI, [1.693, 9.632], p = 0.002), and a history of GI bleeding (OR = 24.230, 95% CI, [5.653, 103.861], p < 0.001) remained significantly associated with bleeding risk (Figure 1E). This binomial logistic regression model was statistically significant, χ² (3) = 31.500, p < 0.001. Goodness-of-fit as assessed using Hosmer–Lemeshow test indicated a good model fit, χ²(3) = 8.690, p > 0.050. The correlations between predictor variables were low (r < 0.70), indicating that multicollinearity was not a confounding factor in the analysis. As only few BLEDs occurred, results were confirmed with Firth regression to avoid biased results by disproportions among the groups. The complete model and Firth regression are depicted in Supplementary Figure S2A/B. The duration of PLC ≤ 10 × 109/L was not associated with an increased bleeding risk (Supplementary Figure S2C).
Platelet count and transfusion strategies
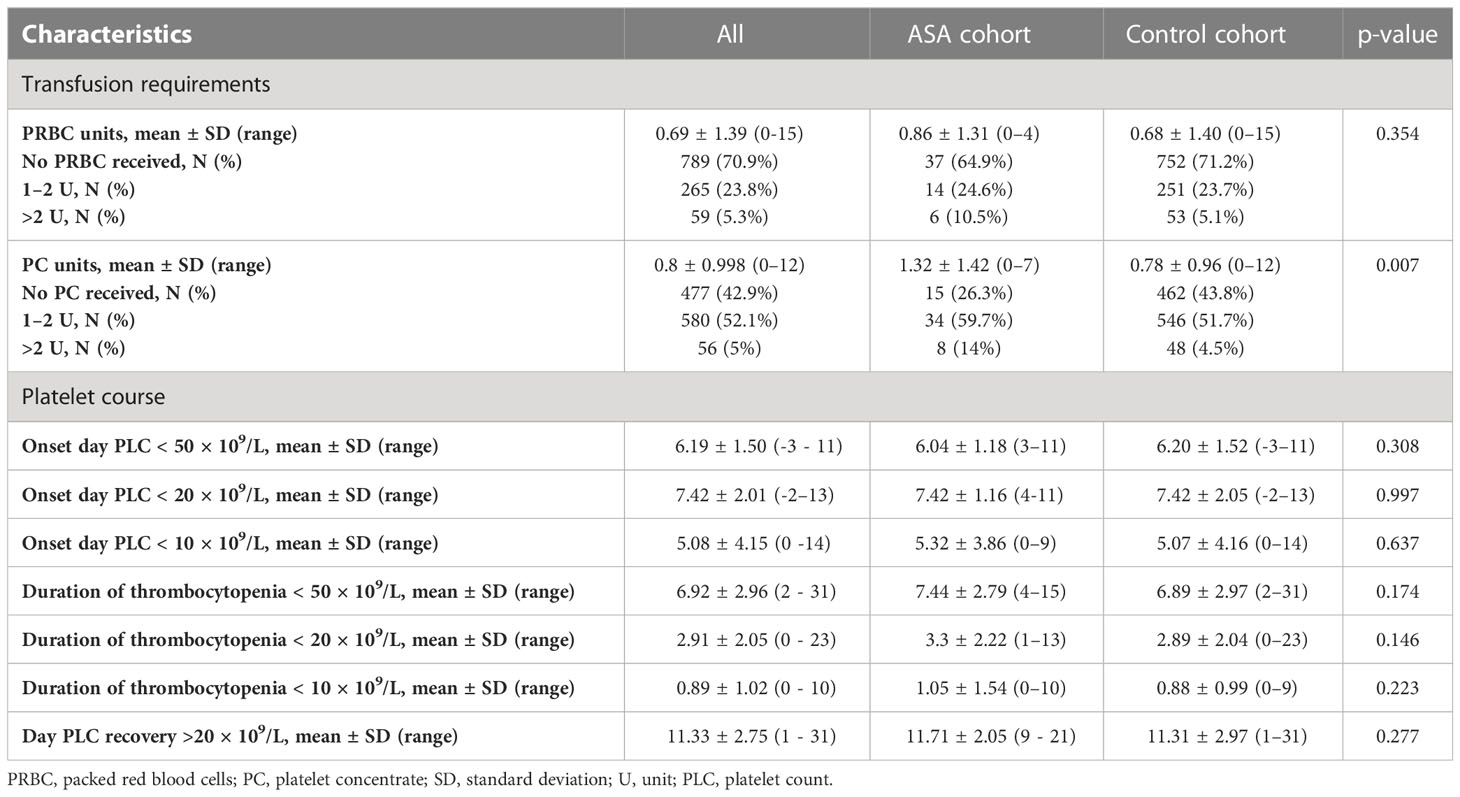
Basic transfusion requirements and PLCs are summarized in Table 2. A total of 477 (42.9%) patients received no PLTX at all; 481 (43.2%) received only one unit. Patients with ASA intake received significantly more units of PLTX (1.32 ± 1.42 vs. 0.78 ± 0.96, p < 0.001). No significant differences regarding the units of PRBCs or the duration of thrombocytopenia was demonstrated. The correlation between PLTX requirements and clinical factors is shown in Figure 3A. In binomial logistic regression analysis for PLTX requirements above the average of 0–1 unit of platelets, smoking, PRBCs, and the duration of PLC < 50 × 109/L remained significant. ORs are depicted in Figure 3B. The binomial logistic regression model was statistically significant, χ²(15) = 198.747, p < 0.001. Goodness-of-fit as assessed by the Hosmer–Lemeshow test indicated a good model fit, χ²(15) = 7.326, p > 0.050. The correlations between predictor variables were low (r < 0.70). Since the duration of PLC < 50 × 109/L showed a strong and clinical meaningful impact on PLTX requirements and BLEDs, risk factors for prolonged duration were further explored. The duration of PLC < 50 × 109/L correlated significantly with age, PLC, hemoglobin, and prothrombin time (PTT) at admission, HCT-CI, hypertension, diarrhea, smoking, diabetes, hyperlipidemia, and infections (Figure 3A). The same factors were significant in univariant linear regression analysis. Based on that, a multiple linear regression model was built including all of these factors known before the start of conditioning. Smoking, diabetes, hypertension, and hyperlipidemia did not remain significant; thus, the strongest model was built including PLC at admission < 100 × 109/L (ß = 0.241, p < 0.001), hemoglobin at admission < 100 g/L (ß = 0.073, p = 0.013), PTT at admission < 70% (ß = 0.103, p > 0.001), age > 60 years (ß = 0.111, p < 0.001), and HCT-CI ≥3 (ß = 0.084, p = 0.004). The R² for the overall model was 0.113 (adjusted R² = 0.109), indicating a weak goodness-of-fit according to Cohen. The model showed no autocorrelation as the value of the Durbin–Watson statistic was 1.698. An increased risk for BLEDs by a combination of these factors including PLC at admission < 100 × 109/L, hemoglobin at admission < 100 g/L, PTT at admission < 70%, age > 60 years, and HCT-CI ≥3 was excluded by another binomial regression analysis using a sum score of these factors. The OR for this sum score was 1.194 (95% CI, [0.731, 19.52], p = 0.479).
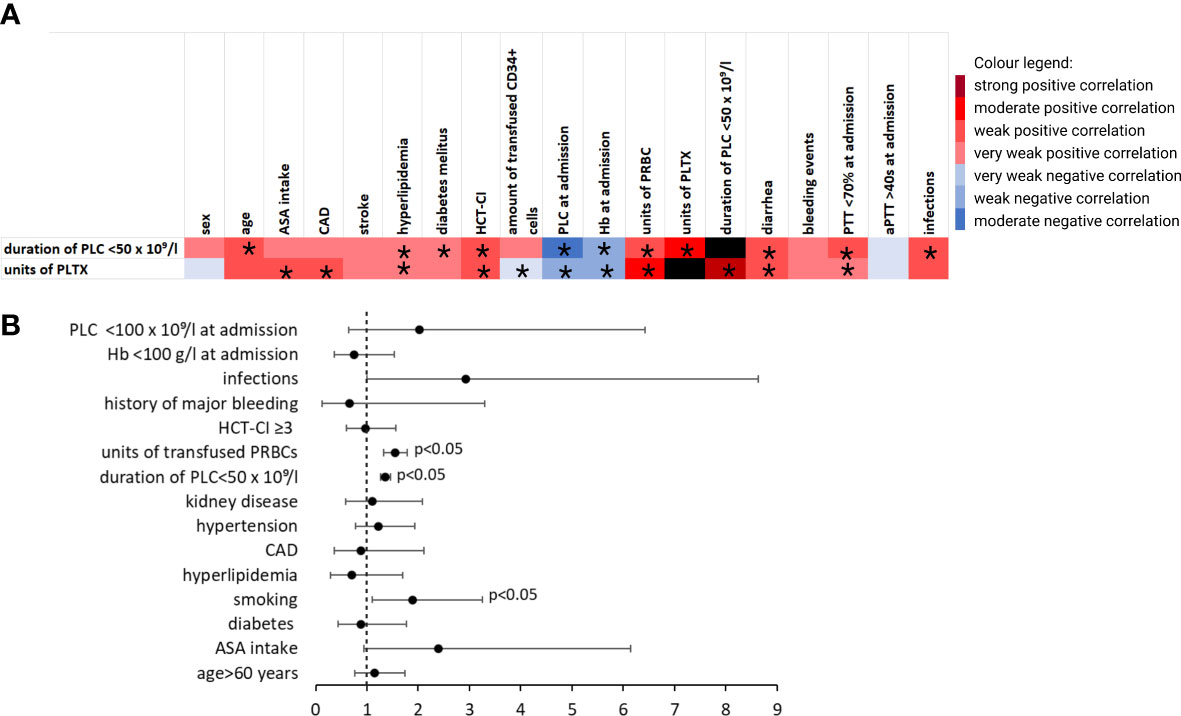
Figure 3 Transfusion requirements and duration of thrombocytopenia. (A) Heatmap showing Pearson’s correlation coefficient (phi coefficient for binary variables, respectively) visualizing factors that correlate with requirement of PLTX and duration of PLC < 50 × 109/L. *indicates p < 0.05. (B) ORs for the requirement of >1 unit of platelets in patients without a bleeding event from multivariate logistic regression analysis after using stepwise estimation by backward and forward selection. aPTT, activated partial thromboplastin time; ASA, acetylsalicylic acid; CAD, coronary artery disease; Hb, hemoglobin; HCT-CI, hematopoietic stem-cell transplantation comorbidity index; PLC, platelet count; PLTX, platelet transfusion; PRBC, packed red blood cells (in units); PTT, prothrombin time..
Description of major cardiovascular event
Given the high frequency of the CV risk factor within our cohort, we assessed the incidence of MACE during the hospital stay. Three patients suffered a MACE. Two MACEs were likely associated with the transfusion of stem cells or a serious infection and are therefore discussed in the Supplementary Material (1.2 Supplementary Results and Discussion, 1.2.1 Case discussions on patients presenting with MACE, and Supplementary Table S3. Cardiac comorbidities and risk factors in patients below versus above 60 years of age). Nonetheless, one patient was found unresponsive due to ventricular fibrillation and was successfully resuscitated. Subsequent coronary angiography revealed a significant occlusion of the left anterior descending artery (LAD), global hypokinesia, and reduced left ventricular ejection fraction. A drug-eluting stent and a single-chamber implantable cardiac defibrillator were implanted. The patient recovered with no obvious deficit. As CV risk factors, type II diabetes and hypertension were known prior to this event.
Discussion
No evidence-based approach on how to handle APT during chemotherapy-induced thrombocytopenia exists, although a recent expert consensus recommended to withhold ASA with thrombocytopenia CTCAE III° unless severe CV comorbidities are present (26). In our analysis, we approached this question by assessing BLEDs and ASA discontinuation strategies in a large single-center cohort of patients with multiple myeloma undergoing ASCT. To our knowledge, this is the first study with a systematic evaluation of APT during thrombocytopenia.
The PLADO trial (14), which is the largest (prospective) trial assessing bleeding risk, included only 378 patients undergoing ASCT. Thus, we assessed the largest and most homogenous patient sample for BLEDs during ASCT so far and present true real-world data.
Strategies for preventive acetylsalicylic acid use during autologous stem-cell transplantation
ASA represents the gold standard for preventing MACE (5). Despite its net benefit, major BLEDs were increased in both primary and secondary prevention trials (RR 2.69 [99% CI 1.25–5, p = 0.01) (5). Several retrospective analyses of cancer patients with thrombocytopenia and acute coronary syndrome suggested ASA and even dual APT to be safe and advantageous in regard to cardiac outcomes with a little risk of major bleeding (4, 27–30).
In our cohort, ASA was discontinued during thrombocytopenia of various degrees after high-dose chemotherapy. During aplasia, no or only a small percentage of new platelets are generated. Therefore, the remaining platelets were assumed to be continuously inhibited by ASA. The in vitro reversal of platelet inhibition in patients taking ASA was shown to be achieved by mixing inhibited platelets with 30% untreated donor platelets (31). The restoration of platelet function is mediated by TXA2 from (donor) platelets, which activates the thromboxane receptor of ASA-inhibited (host) platelets (31). This concept was supported by small case series and proof-of-principal studies in vivo (32–34). Despite, clinical outcome data on PLTX in patients taking ASA are scarce and the data from thrombocytopenic patients are not available at all. In our cohort, >70% of cases with ASA intake received only 0–1 unit PLTX after ASA pause during aplasia; thus, a relevant impact on continued ASA efficacy seems unlikely. Of note, if PLTX is provided during continued ASA intake, the strongest platelet inhibition by ASA 60 min after oral intake (35) should be considered, depending on the pursued effect.
We observed no MACE during the discontinuation of ASA in our cohort. Large analyses that assessed the discontinuation of ASA without surgery or bleeding after long-term use for secondary prevention demonstrated a 46% higher rate of MACE (7). This translates into one additional event in 1 of every 36 patients (7). Of note, the time from discontinuation to the thrombotic event was as early as 7–30 days in most reports (8–12, 36). In our cohort, patients discontinued ASA on average for ~9 days during thrombocytopenia. Although infections are related to a prothrombotic state, this time frame might be short enough to avoid a clinically relevant increased risk of MACE. In addition, the protective impact of thrombocytopenia for MACE is difficult to estimate or measure. This is supported by the fact that neither patients who discontinued ASA before day +1 nor patients who had an indication for secondary ASA intake but did not receive it experienced an additional MACE during ASCT. The latter group included notably 2.8% (N = 30) of control cases who had an indication for ASA as secondary prophylaxis and 0.3% (N = 3) for primary prophylaxis but did not receive it. Whether these patients developed an event after discharge could not be assessed as many of those received their follow-up visits outside our center. The only patient who developed a severe MACE during ASCT had no indication for preventive ASA to our knowledge based on his chart review.
Association of bleeding with acetylsalicylic acid intake
We found no significant increase of BLEDs in patients taking ASA but observed a tendency toward a higher risk of bleeding. In addition, patients on ASA received significantly more PLTX although >70% received only 0–1 unit. Above this amount, the requirements for PLTX were not related to ASA intake. Despite the increased PLTX requirements, it is unlikely that BLEDs were abrogated by the more generous transfusion approach. Patients on ASA required 1.32 units of PLTX, which is still below the reported mean requirement of 2 units (37) during ASCT and underlines our strict transfusion policy. We cannot completely exclude that ASA intake leads to a higher risk of bleeding, but in none of our analysis did it reach statistical significance.
To exclude a selection bias for analyzing BLEDs, we compared the group taking ASA until at least 1 day after ASCT with those who were excluded due to ASA discontinuation prior to day +1 (Supplementary Table S1). Patients differed significantly with regard to chronic kidney failure. The only documented BLED in this group was a retinal hemorrhage.
As all documented retinal hemorrhages occurred in patients taking ASA, retinal hemorrhages bear the potential of long-term vision impairments. Hypertension is one potential risk factor for retinal hemorrhages and was more prevalent in the ASA group. Thus, it is important to be aware of a possible causality between ASA and this specific BLED.
Risk factors for bleeding
Over 80% of BLEDs occurred during PLC < 50 × 109/L and majority of these with a PLC < 10 × 109/L. The severity of thrombocytopenia was repetitively shown not to correlate with BLEDs (38), although a PLC < 5 × 109/L was demonstrated as a risk factor for BLEDs during ASCT (14). In our cohort, multivariate analysis validated the duration of PLC < 50 × 109/L, not below < 10 × 109/L, as a risk factor for bleeding. Although the duration of PLC < 10 × 109/L might be skewed by PLTX, these results are in concordance with previous reports.
Three risk factors for bleeding remained statistically significant in multivariate analysis: a history of GI bleeding, diarrhea, and the duration of PLC < 50 × 109/L. The number of patients with former GI bleeding was very low; thus, its predictive value might be overestimated. We therefore suggest to rather focus on factors that predict a prolonged duration of PLC < 50 × 109/L than the direct risk factors for bleeding. Herein, a decreased bone marrow reserve (hemoglobin at admission < 100 g/L, PLC < 100 × 109/L), impaired coagulation (PTT < 70% at admission), age >60 years, or HCT-CI ≥3 points are known prior to conditioning and can help to further guide physicians in assessing the indirect risk for bleeding. None of them were significantly associated with BLEDs itself in multivariate analysis (Supplementary Figure S2A), neither alone or as a sum score.
The recently reported risk factors for BLEDs comprised fever (HR: 1.7, 95% CI [1.3, 2.4]) (39, 40). Neither fever nor sepsis remained an independent risk factor for bleeding in the multivariate analysis of our cohort. This might be explained by the high number of infections during ASCT: 89.9% of cases developed at least one documented episode of fever/infection, and 69.6% of BLEDs occurred during an infection. Whether this constitutes a co-occurrence or a causality remains unclear, but a statistical association could not be demonstrated.
Limitations
The incidence of BLEDs in our cohort was lower than recently reported (20, 21). In these trials, a daily structured bleeding assessment was mandatory and was documented as patient days with bleeding to account for the course of the bleeding with regard to PLTX. We documented every BLED only once; thereby, the bleeding incidence is hardly comparable. In addition, our data are based on EMRs without these structured assessments and the underreporting of mild BLEDs (e.g., mild epistaxis) is possible.
Furthermore, the number of patients on ASA was limited, although this is a large real-world cohort and the largest sample size assessing BLEDs during ASCT so far. Therefore, our results are hypothesis-generating and this question can only be answered in a randomized controlled trial. Such a trial would require a large international cooperative initiative that does not appear to be feasible (for the design and sample size calculation of such a trial, please refer to Supplementary Material, 1.1 Supplementary Methods, 1.1.2 Design of a randomized controlled trial to assess different APT strategies, and Supplementary Figure S1).
Recommendations on management of antiplatelet therapy during autologous stem-cell transplantation
As an evidence-based strategy guided by high-quality randomized trial data will most likely never be available, the following rationale-based approach can be considered: if ASA is indicated based on a primary preventive approach, the discontinuation of ASA before conditioning can be considered based on the high number needed to treat (NNT) to prevent MACE and the relevant risk for bleeding even with normal PLC. If ASA is indicated by a secondary preventive approach, it appears safe to continue ASA until a PLC of 20–50 × 109/L (CTCAE III°). We consider this PLC range rather than a fixed cut-off to pause ASA, as PLC was not measured daily in our routine clinical practice. Furthermore, a PLC range of 20–50 × 109/L reflects the real-world scenario better than a fixed cut-off given the standard kinetics of thrombocytopenia during ASCT. Whether the application of ASA even during PLC < 20 × 109/L with a lower transfusion trigger (e.g., 20–30 × 109/L) is also safe cannot be concluded from our data as only one patient was guided with this strategy. The rationale behind such an approach is a potentially increased prothrombotic state due to a rebound effect after the discontinuation of ASA. When the ASA discontinuation strategy for the individual patient is discussed, we recommend to consider age >60 years, HCT-CI ≥3, PLC < 100 × 109/L, hemoglobin < 100 g/L, and PTT < 70% at admission as predictors for a prolonged duration of PLC < 50 × 109/L, and therefore, an indirect bleeding risk together with a clinical bleeding history (especially GI bleedings). Recommendations are summarized in Figure 4; general results are shown in Figure 5.
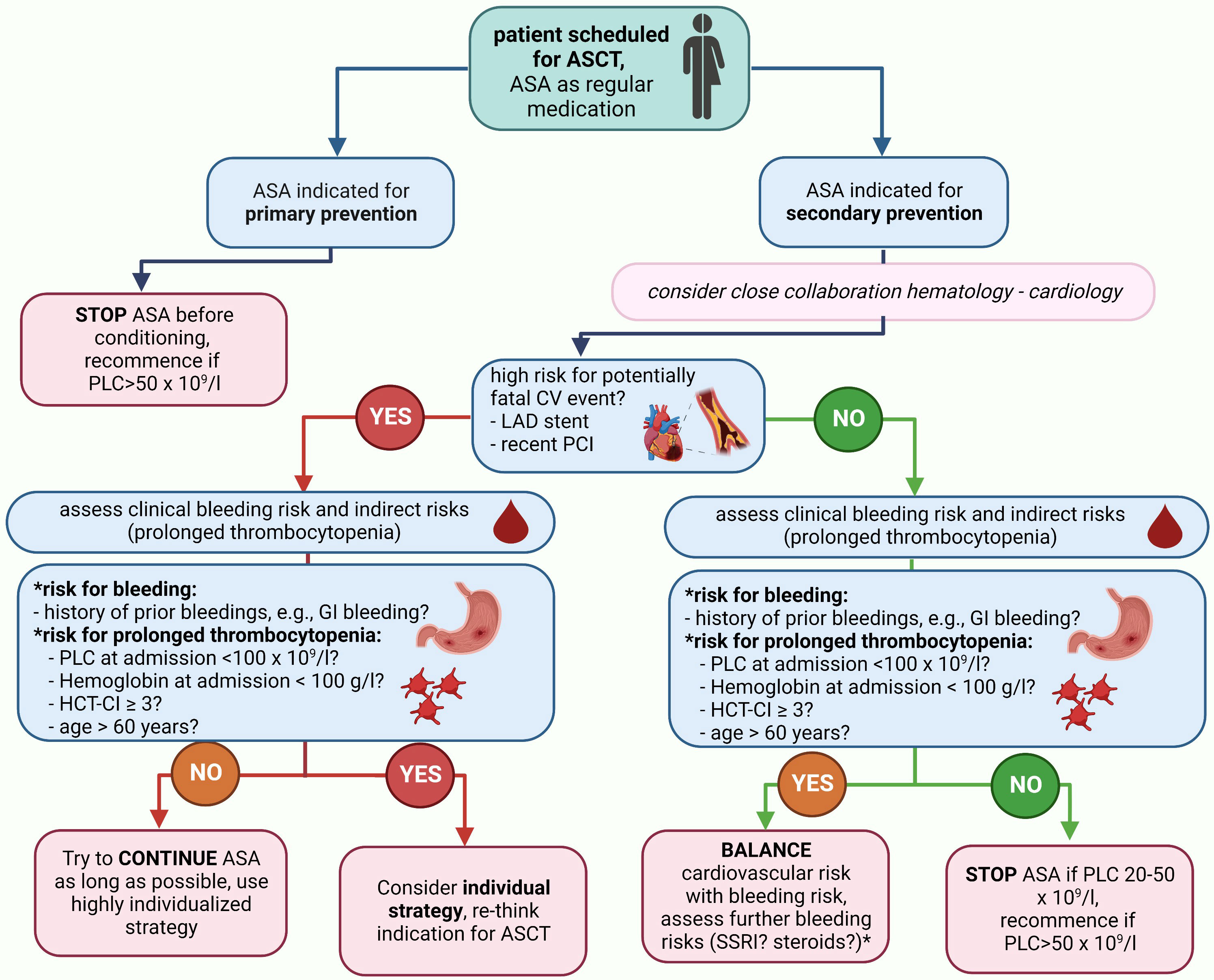
Figure 4 Summary of recommendations for management of APT during ASCT. If a patient is scheduled for ASCT and has an indication for APT, we recommend the depicted management strategy. *Although the use of steroids or selective serotonin reuptake inhibitors were not demonstrated to carry an additional bleeding risk in our cohort, they carry well-described bleeding risks and we would recommend to integrate considerations on their use into the individual patient management plan. ASA, acetylsalicylic acid; CV, cardiovascular; HCT-CI, hematopoietic stem-cell transplantation comorbidity index; LAD, left-anterior descending; PCI, percutaneous coronary intervention; PLC, platelet count..
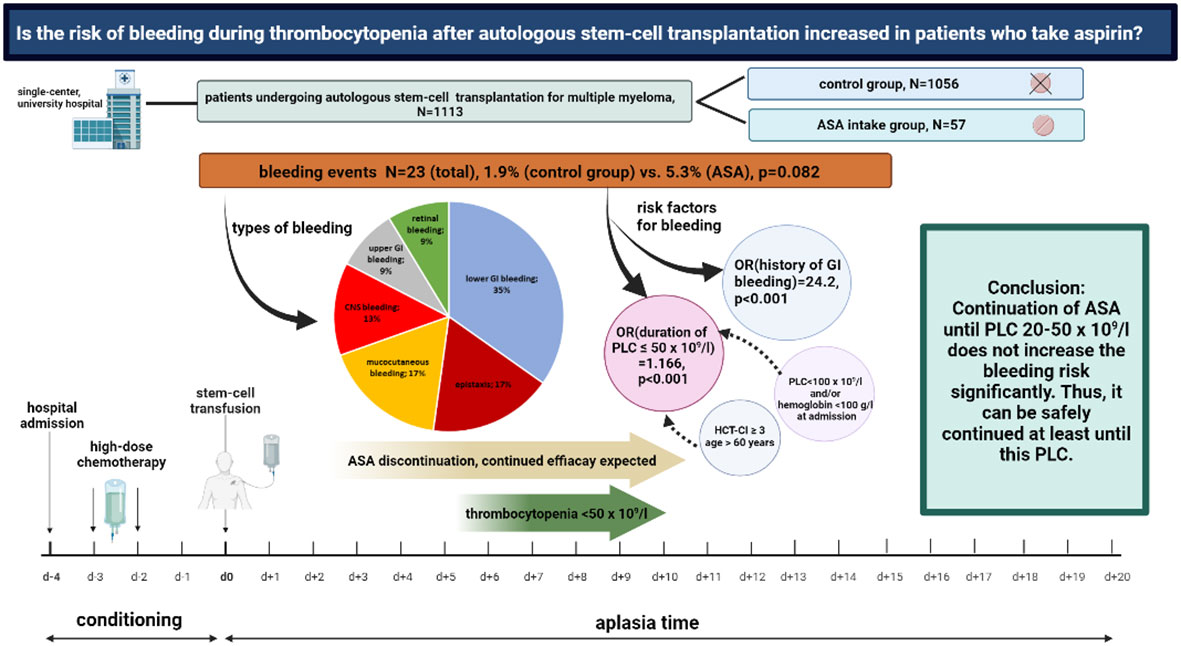
Figure 5 Aspirin use and bleeding events during thrombocytopenia following autologous stem-cell transplantation for multiple myeloma. Overview on the study design and major findings.
Conclusions
Severe BLEDs are rare during ASCT even if ASA is continued until thrombocytopenia CTCAE III°. The APT in such a situation will remain an “one-size-does-not-fit-all” situation and requires a thorough balance between the individual risks for MACE and bleeding, ideally achieved by a close collaboration between hematologists and cardiologists. Especially high-risk situations for a fatal MACE (e.g., recent PCI and the involvement of LAD) might drive ASA continuation until there are very low PLCs.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional review board of the University Heidelberg, Germany, No S-721/2018.
Author contributions
NN participated in research design, data acquisition and analysis, and writing of the paper. BB participated in data acquisition and writing of the paper. LF participated in research design and the final editing of the paper. MK participated in data analysis and the final editing of the paper. CR participated in data analysis and the final editing of the paper. HG participated in research design and the final editing of the paper. NF participated in the final editing of the paper. CM-T participated in the final editing of the paper. KJ participated in research design and the final editing of the paper. SS participated in the final editing of the paper. MJ participated in research design, data acquisition and analysis, and the writing of the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Deutsche Forschungsgemeinschaft (DFG) in the framework of the DFG University Medicine Essen Clinician Scientist Academy (UMEA), FU 356/12-2 to NN.
Acknowledgments
We are grateful to Miriam Freund, MD, for her insightful comments on our work and Ulrike Buttkereit, MD, for her important input on questions regarding transfusion medicine.
Conflict of interest
NF reports speaker honoraria from AstraZeneca, Blyer, and Daiichi Sankyo. KJ reports personal fees as an invited speaker from Amgen, art tempi, Helsinn, Hexal, med update GmbH, MSD, Mundipharma, Onkowissen, Riemser, Roche, Shire Takeda, and Vifor; personal fees for advisory board membership from Amgen, AstraZeneca, BD Solutions, Hexal, Karyopharm, and Voluntis; and royalties from Elsevier and Wolters Kluwer; NRN reports honoraria and travel support from Janssen-Cilag, Medac, Novartis, Abbvie, and Jazz Pharmaceutical. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1168120/full#supplementary-material
References
1. Battisti NML, Welch CA, Sweeting M, de Belder M, Deanfield J, Weston C, et al. Prevalence of cardiovascular disease in patients with potentially curable malignancies: a national registry dataset analysis. JACC CardioOncol (2022) 4(2):238–53. doi: 10.1016/j.jaccao.2022.03.004
2. Backs D, Saglam I, Loffler C, Ihne S, Morbach C, Brenner S, et al. Prevalence of cardiovascular risk factors and diseases in patients with multiple myeloma undergoing autologous peripheral blood stem cell transplantation. Oncotarget (2019) 10(34):3154–65. doi: 10.18632/oncotarget.26872
3. Libourel EJ, Sonneveld P, van der Holt B, de Maat MP, Leebeek FW. High incidence of arterial thrombosis in young patients treated for multiple myeloma: results of a prospective cohort study. Blood (2010) 116(1):22–6. doi: 10.1182/blood-2009-12-257519
4. Iliescu C, Balanescu DV, Donisan T, Giza DE, Munoz Gonzalez ED, Cilingiroglu M, et al. Safety of diagnostic and therapeutic cardiac catheterization in cancer patients with acute coronary syndrome and chronic thrombocytopenia. Am J Cardiol (2018) 122(9):1465–70. doi: 10.1016/j.amjcard.2018.07.033
5. Antithrombotic Trialists C, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet (2009) 373(9678):1849–60. doi: 10.1016/S0140-6736(09)60503-1
6. Aguejouf O, Eizayaga F, Desplat V, Belon P, Doutremepuich C. Prothrombotic and hemorrhagic effects of aspirin. Clin Appl Thromb Hemost (2009) 15(5):523–8. doi: 10.1177/1076029608319945
7. Sundstrom J, Hedberg J, Thuresson M, Aarskog P, Johannesen KM, Oldgren J. Low-dose aspirin discontinuation and risk of cardiovascular events: a Swedish nationwide, population-based cohort study. Circulation (2017) 136(13):1183–92. doi: 10.1161/CIRCULATIONAHA.117.028321
8. Collet JP, Montalescot G, Blanchet B, Tanguy ML, Golmard JL, Choussat R, et al. Impact of prior use or recent withdrawal of oral antiplatelet agents on acute coronary syndromes. Circulation (2004) 110(16):2361–7. doi: 10.1161/01.CIR.0000145171.89690.B4
9. Burger W, Chemnitius JM, Kneissl GD, Rucker G. Low-dose aspirin for secondary cardiovascular prevention - cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation - review and meta-analysis. J Intern Med (2005) 257(5):399–414. doi: 10.1111/j.1365-2796.2005.01477.x
10. Fischer LM, Schlienger RG, Matter CM, Jick H, Meier CR. Discontinuation of nonsteroidal anti-inflammatory drug therapy and risk of acute myocardial infarction. Arch Intern Med (2004) 164(22):2472–6. doi: 10.1001/archinte.164.22.2472
11. Ferrari E, Benhamou M, Cerboni P, Marcel B. Coronary syndromes following aspirin withdrawal: a special risk for late stent thrombosis. J Am Coll Cardiol (2005) 45(3):456–9. doi: 10.1016/j.jacc.2004.11.041
12. Eisenberg MJ, Richard PR, Libersan D, Filion KB. Safety of short-term discontinuation of antiplatelet therapy in patients with drug-eluting stents. Circulation (2009) 119(12):1634–42. doi: 10.1161/CIRCULATIONAHA.108.813667
13. García Rodríguez LA, Cea Soriano L, Hill C, Johansson S. Increased risk of stroke after discontinuation of acetylsalicylic acid: a UK primary care study. Neurology (2011) 76(8):740–6. doi: 10.1212/WNL.0b013e31820d62b5
14. Uhl L, Assmann SF, Hamza TH, Harrison RW, Gernsheimer T, Slichter SJ. Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood (2017) 130(10):1247–58. doi: 10.1182/blood-2017-01-757930
15. Uchiyama S, Goto S, Origasa H, Uemura N, Sugano K, Hiraishi H, et al. Major cardiovascular and bleeding events with long-term use of aspirin in patients with prior cardiovascular diseases: 1-year follow-up results from the management of aspirin-induced gastrointestinal complications (MAGIC) study. Heart Vessels (2020) 35(2):170–6. doi: 10.1007/s00380-019-01484-0
16. Triulzi DJ. How well do platelets prevent bleeding? Hematol Am Soc Hematol Educ Program (2020) 2020(1):518–22. doi: 10.1182/hematology.2020000136
17. Friedmann AM, Sengul H, Lehmann H, Schwartz C, Goodman S. Do basic laboratory tests or clinical observations predict bleeding in thrombocytopenic oncology patients? a reevaluation of prophylactic platelet transfusions. Transfus Med Rev (2002) 16(1):34–45. doi: 10.1053/tmrv.2002.29403
18. Slichter SJ, Kaufman RM, Assmann SF, McCullough J, Triulzi DJ, Strauss RG, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med (2010) 362(7):600–13. doi: 10.1056/NEJMoa0904084
19. Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood (1985) 66(5):1105–9. doi: 10.1182/blood.V66.5.1105.1105
20. Wandt H, Schaefer-Eckart K, Frank M, Birkmann J, Wilhelm M. A therapeutic platelet transfusion strategy is safe and feasible in patients after autologous peripheral blood stem cell transplantation. Bone Marrow Transplant (2006) 37(4):387–92. doi: 10.1038/sj.bmt.1705246
21. Wandt H, Schaefer-Eckart K, Wendelin K, Pilz B, Wilhelm M, Thalheimer M, et al. Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open-label, multicentre, randomised study. Lancet (2012) 380(9850):1309–16. doi: 10.1016/S0140-6736(12)60689-8
22. Baharoglu MI, Cordonnier C, Al-Shahi Salman R, de Gans K, Koopman MM, Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): a randomised, open-label, phase 3 trial. Lancet (2016) 387(10038):2605–13. doi: 10.1016/S0140-6736(16)30392-0
23. Zakko L, Rustagi T, Douglas M, Laine L. No benefit from platelet transfusion for gastrointestinal bleeding in patients taking antiplatelet agents. Clin Gastroenterol Hepatol (2017) 15(1):46–52. doi: 10.1016/j.cgh.2016.07.017
24. Sung JJ, Lau JY, Ching JY, Wu JC, Lee YT, Chiu PW, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: a randomized trial. Ann Intern Med (2010) 152(1):1–9. doi: 10.7326/0003-4819-152-1-201001050-00179
25. Webert KE, Arnold DM, Lui Y, Carruthers J, Arnold E, Heddle NM. A new tool to assess bleeding severity in patients with chemotherapy-induced thrombocytopenia. Transfusion (2012) 52(11):2466–74. doi: 10.1111/j.1537-2995.2012.03634.x
26. Falanga A, Leader A, Ambaglio C, Bagoly Z, Castaman G, Elalamy I, et al. EHA guidelines on management of antithrombotic treatments in thrombocytopenic patients with cancer. HemaSphere (2022) 6(8):e750. doi: 10.1097/HS9.0000000000000750
27. Raphael CE, Spoon DB, Bell MR, Psaltis PJ, Kidd S, Loh SX, et al. Effect of preprocedural thrombocytopenia on prognosis after percutaneous coronary intervention. Mayo Clin Proc (2016) 91(8):1035–44. doi: 10.1016/j.mayocp.2016.05.008
28. Shiraishi J, Koshi N, Matsubara Y, Nishimura T, Ito D, Kimura M, et al. Effects of baseline thrombocytopenia on in-hospital outcomes in patients undergoing elective percutaneous coronary intervention. Intern Med (2019) 58(12):1681–8. doi: 10.2169/internalmedicine.2063-18
29. Sarkiss MG, Yusuf SW, Warneke CL, Botz G, Lakkis N, Hirch-Ginsburg C, et al. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer (2007) 109(3):621–7. doi: 10.1002/cncr.22434
30. Yusuf SW, Iliescu C, Bathina JD, Daher IN, Durand JB. Antiplatelet therapy and percutaneous coronary intervention in patients with acute coronary syndrome and thrombocytopenia. Tex Heart Inst J (2010) 37(3):336–40.
31. Li C, Hirsh J, Xie C, Johnston MA, Eikelboom JW. Reversal of the anti-platelet effects of aspirin and clopidogrel. J Thromb Haemost (2012) 10(4):521–8. doi: 10.1111/j.1538-7836.2012.04641.x
32. Thiele T, Sumnig A, Hron G, Muller C, Althaus K, Schroeder HW, et al. Platelet transfusion for reversal of dual antiplatelet therapy in patients requiring urgent surgery: a pilot study. J Thromb Haemost (2012) 10(5):968–71. doi: 10.1111/j.1538-7836.2012.04699.x
33. Baschin M, Selleng S, Hummel A, Diedrich S, Schroeder HW, Kohlmann T, et al. Preoperative platelet transfusions to reverse antiplatelet therapy for urgent non-cardiac surgery: an observational cohort study. J Thromb Haemost (2018) 16(4):709–17. doi: 10.1111/jth.13962
34. Pruller F, Drexler C, Archan S, Macher S, Raggam RB, Mahla E. Low platelet reactivity is recovered by transfusion of stored platelets: a healthy volunteer in vivo study. J Thromb Haemost (2011) 9(8):1670–3. doi: 10.1111/j.1538-7836.2011.04392.x
35. Nagalla S, Sarode R. Role of platelet transfusion in the reversal of anti-platelet therapy. Transfus Med Rev (2019) 33(2):92–7. doi: 10.1016/j.tmrv.2019.01.002
36. Sibon I, Orgogozo JM. Antiplatelet drug discontinuation is a risk factor for ischemic stroke. Neurology (2004) 62(7):1187–9. doi: 10.1212/01.WNL.0000118288.04483.02
37. Khan R, Mott SL, Schultz A, Jethava YS, Tricot G. Bloodless tandem autologous transplant in jehovah's witness patients. Bone Marrow Transplant (2018) 53(11):1428–33. doi: 10.1038/s41409-018-0132-6
38. Kaur D, Ashrani AA, Pruthi R, Khan SP, Bailey K, Rodriguez V. Thrombotic and hemorrhagic complications in children and young adult recipients of hematopoietic stem cell transplant (HSCT). Thromb Res (2018) 167:44–9. doi: 10.1016/j.thromres.2018.04.023
39. Stanworth SJ, Hudson CL, Estcourt LJ, Johnson RJ, Wood EM. Risk of bleeding and use of platelet transfusions in patients with hematologic malignancies: recurrent event analysis. Haematologica (2015) 100(6):740–7. doi: 10.3324/haematol.2014.118075
Keywords: multiple myeloma, autologous stem-cell transplantation, antiplatelet therapy, aspirin, bleeding, cardio-vascular events
Citation: Neuendorff NR, Boshikova B, Frankenstein L, Kirchner M, Rohde C, Goldschmidt H, Frey N, Müller-Tidow C, Jordan K, Sauer S and Janssen M (2023) Aspirin use and bleeding events during thrombocytopenia after autologous stem-cell transplantation for multiple myeloma. Front. Oncol. 13:1168120. doi: 10.3389/fonc.2023.1168120
Received: 17 February 2023; Accepted: 06 April 2023;
Published: 27 April 2023.
Edited by:
Chun Ka Wong, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
Stephen S. Y. Lam, The University of Hong Kong, Hong Kong SAR, ChinaOmer Iqbal, Loyola University Chicago, United States
Copyright © 2023 Neuendorff, Boshikova, Frankenstein, Kirchner, Rohde, Goldschmidt, Frey, Müller-Tidow, Jordan, Sauer and Janssen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maike Janssen, bWFpa2UuamFuc3NlbkBtZWQudW5pLWhlaWRlbGJlcmcuZGU=
 Nina Rosa Neuendorff
Nina Rosa Neuendorff Boryana Boshikova1,3
Boryana Boshikova1,3 Marietta Kirchner
Marietta Kirchner Hartmut Goldschmidt
Hartmut Goldschmidt Norbert Frey
Norbert Frey Maike Janssen
Maike Janssen