- 1Department of Student Affairs, Affiliated Cancer Hospital of Xinjiang Medical University, Urumqi, China
- 2Department of Cancer Epidemiology, Affiliated Cancer Hospital of Zhengzhou University & Henan Cancer Hospital, Henan Engineering Research Center of Cancer Prevention and Control, Henan International Joint Laboratory of Cancer Prevention, Zhengzhou, China
- 3Department of Clinical Research, The First Affiliated Hospital of Jinan University, Guangzhou, Guangdong, China
- 4The Clinical Epidemiology of Research Center, Department of Public Health and Preventive Medicine, Baotou Medical College, Baotou, China
- 5Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Beijing Office for Cancer Prevention and Control, Peking University Cancer Hospital & Institute, Beijing, China
- 6Department of Public Health, Gansu Provincial Cancer Hospital, Lanzhou, China
- 7School of Nursing, Jining Medical University, Jining, China
- 8Department of Cancer Prevention, The Cancer Hospital of the University of Chinese Academy of Sciences, Zhejiang Cancer Hospital, Hangzhou, China
- 9Department of Preventive Health, Xinxiang Central Hospital, Xinxiang, China
- 10Center for Cancer Prevention Research, Sichuan Cancer Hospital & Institute, Sichuan Cancer Center, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
- 11Liaoning Office for Cancer Control and Research, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang, China
- 12Department of Gastrodiges, Wuzhou Red Cross Hospital, Wuzhou, China
- 13Department of Cancer Prevention and Control Office, the First Affiliated Hospital of Guangxi Medical University, Nanning, China
- 14State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-Sen University Cancer Center, Guangzhou, China
- 15Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital, Chongqing, China
- 16School of Public Health and Management, Chongqing Medical University, Chongqing, China
- 17School of Public Health, Chengdu Medical College, Chengdu, China
- 18Public Health School, Dalian Medical University, Dalian, China
- 19School of Population Medicine and Public Health, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Introduction: This cross-sectional study evaluated the involvement of patients with advanced colorectal cancer (CRC) in treatment decision-making, assessed the treatment efficacy according to their self-reports, and investigated the influencing factors.
Methods: Patients with advanced CRC were recruited from 19 hospitals from March 2020 to March 2021 by a multi-stage multi-level sampling method. A self-designed questionnaire was used to collect demographic and clinical characteristics, involvement of CRC patients in treatment decision-making, treatment methods, and self-reported efficacy. Univariate and unordered multinomial logistic regression analyses were used to evaluate the factors affecting the involvement in treatment decision-making and self-reported efficacy.
Results: We enrolled 4533 patients with advanced CRC. The average age at diagnosis was 58.7 ± 11.8 years. For the treatment method, 32.4% of patients received surgery combined with chemotherapy, 13.1% of patients underwent surgery combined with chemotherapy and targeted therapy, and 9.7% of patients were treated with surgery alone. For treatment decision-making, 7.0% of patients were solely responsible for decision-making, 47.0% of patients shared treatment decision-making with family members, 19.0% of patients had family members solely responsible for treatment decision-making, and 27.0% of patients had their physicians solely responsible for treatment decision-making. Gender, age, education level, family income, marital status, treatment cost, hospital type, and treatment method were significantly associated with the involvement of patients in treatment decision-making. A total of 3824 patients submitted self-reported efficacy evaluations during treatment. The percentage of patients with good self-reported efficacy was 76.5% (for patients treated for the first time), 61.7% (for patients treated for the second time), and 43.2% (for patients treated after recurrence and metastasis), respectively. Occupation, education level, average annual family income, place of residence, time since cancer diagnosis, hospital type, clinical stage, targeted therapy, and involvement in treatment decision-making were the main influencing factors of self-reported efficacy of treatment.
Discussion: Conclusively, CRC patients are not highly dominant in treatment decision-making and more likely to make treatment decisions with their family and doctors. Timely and effective communication between doctors and patients can bolster patient involvement in treatment decision-making.
1 Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide, with morbidity ranking third and mortality ranking second. More than half of new cases and CRC-related deaths are from China, Europe, and North America (1). It is estimated that approximately 1.9 million newly diagnosed CRC cases and 935,000 CRC-related deaths in 2020, accounting for approximately one-tenth of new cancer cases and deaths (2). In recent years, the morbidity and mortality of CRC have decreased in some countries in Europe and the United States. However, China is still suffering a remarkable CRC burden, which accounts for 28.11% of global deaths. In China, both men and women have higher crude mortality rates for CRC than the global average. Moreover, the prevalence of CRC is on the rise in China (3, 4).
In 2015, there were about 387,600 new CRC cases and 187,100 CRC-related deaths, accounting for 9.87% and 8.01% of all malignant tumors, respectively, in China (5, 6). In recent years, with the implementation of screening, early diagnosis, and treatment in China, the age-standardized mortality of CRC has decreased from 10.01/100,000 in 2005 to 9.68/100,000 in 2020, and the 5-year survival rate has increased from 47.2% in 2003-2005 to 56.9% in 2012-2015. However, it is worth noting that more than half of the patients have advanced CRC at initial diagnosis (7), with a 5-year survival rate of approximately 20% (8). It has been shown that there are significant differences in the prognosis of CRC between different treatment methods, countries, or regions (9, 10). Moreover, satisfaction and compliance with treatment may be improved by the involvement of patients in treatment decision-making, thereby indirectly prompting outcomes (11). Therefore, the involvement of patients in treatment decisions making and further analysis of factors associated with the prognosis of advanced CRC is important for making individual diagnoses and treatment plans and for improving compliance and treatment efficacy, further reducing disease burden.
Previously, most of the studies on CRC were designed as single-centered (12) or focused on specific subjects in a certain clinical stage (13), the results of which could not be generalized. Generally, patients have high expectations of participating in treatment decision-making, but the actual involvement is low (14). Moreover, there are rare studies on the involvement of CRC patients in diagnosis and treatment decision-making. Additionally, several prognostic factors of CRC have been reported, such as distant metastasis of CRC, the location of the primary tumor, molecular markers, age, and radical surgery (15–17). However, there are few studies on the self-reported prognosis of patients.
Therefore, in this study, we conducted a nationwide multi-center cross-sectional study. The involvement of patients with advanced CRC in treatment decision-making and the influencing factors were evaluated. Moreover, the prognosis of advanced CRC patients was comprehensively assessed by using patient self-report efficacy. Additionally, the influencing factors of patient self-reported efficacy were also analyzed. Our findings may provide evidence for further improvement of treatment in patients with advanced CRC.
2 Materials and methods
2.1 Study design
This is a nationwide multicenter cross-sectional study and the details of the study design have been published (18). In brief, a multi-stage sampling method was used to identify the 19 tertiary hospitals (10 tertiary cancer hospitals and 9 tertiary general hospitals) in China from March 2020 to March 2021. Firstly, two cities were randomly selected from seven administrative regions in East China, North China, Central China, South China, Northeast China, Southwest China, and Northwest China; subsequently, one tertiary cancer hospital or tertiary general hospital in each city was selected as the research center. This study was approved by the Medical Ethics Committee of Henan Cancer Hospital (No. 2019273) and also by the Ethics Committee of all other participating hospitals subsequently. Informed consent was obtained from each participant.
2.2 Patients
As previously described (18), it was estimated that more than 4445 advanced CRC patients would be enrolled. The total sample size was proportionally allocated to each region according to its population size and based on the estimated sample size, patients should be recruited from each region. A total of 4,589 inpatients with advanced CRC who had stage III or IV CRC across seven geographic regions of China’s mainland were included in this study from March 2020 to March 2021. Fifty-three cases were excluded due to a lack of essential information for the current analysis. Ultimately, 4,533 cases were included.
The Tumor Lymph Node Metastasis (TNM) staging system by the American Joint Committee on Cancer (AJCC) was used to identify the eligible subjects. The inclusion criteria were as follows: 1) CRC patients with TNM stage III or IV; 2) patients aged more than 18 years; 3) patients with normal cognitive ability; 4) patients willing to participate in the research and signed the informed consent. Exclusion criteria: patients with severe physical, cognitive, and/or verbal limitations were excluded.
2.3 Data collection
A self-designed questionnaire was used to collect the demographic and clinical characteristics, patient awareness of CRC risk factors, involvement in treatment decision-making, medical experience, treatment methods, treatment efficacy, etc. Before the formal survey, a preliminary survey was conducted among 50 CRC patients at the Henan Cancer Hospital and the First Affiliated Hospital of Baotou Medical College to evaluate the validity and reliability of the questionnaire. Then we revised the questionnaire based on the preliminary results. The final questionnaire consisted of four parts in 9 pages. To ensure the study quality, all investigators received standard training. Questionnaires were filled out face-to-face by the investigators, with a mean survey time of 20 min.
Involvement in treatment decision-making was classified into “full treatment decision-making by patients, joint treatment decision-making by patients and their family members, treatment decision-making by family members, and treatment decision-making by doctors”. The evaluation of the efficacy was mainly based on the patient’s self-report, and was designed as “poor, good, or stable (unchanged)”. The awareness of CRC risk factors was evaluated by multiple-choice questions, namely, “What do you think are the risk factors for CRC before diagnosis?”, “What do you think is the appropriate CRC screening method before diagnosis?”, and “Which method do you use to acquire knowledge about CRC?”, which contain 10, 6, and 10 options, respectively. Any selected option was scored as 1 point, while “don’t know” or “seldom see” options were scored as 0 points. Therefore, the total score of each question was 9 points, 5 points, and 9 points, respectively.
The clinical characteristics and treatment methods in the questionnaire were provided by doctors according to patient medical records of diagnosis and treatment, mainly including clinical stage, metastasis, and the treatment and surgical methods during the treatment process.
2.4 Quality control and data processing
The questionnaire was designed in standard Chinese. To avoid possible biases, the survey was conducted face-to-face by trained local investigators who were fluent in standard Chinese and the local language to ensure an adequate understanding of the questions by the study participants. To ensure the consistency and the quality of the questionnaire distribution process, in addition to standard training, each investigator had an implementation manual for timely review and to ensure that all processes were carried out following the standard steps and procedures specified in the manual. After the completion of the questionnaire by the investigators, members of the project team would review the questionnaire, and if any missing information or obvious logical errors were found, verification with the patients was required. After data collection, double data entry and validation were performed by two investigators using Epidata software V.3.1.
2.5 Statistical analysis
All statistical analyses were performed by using SASV.9.4 software. Continuous variables were expressed as mean and standard deviations and categorical variables were expressed as absolute frequencies and percentages. Univariate analysis was performed by using the t-test, analysis of variance, and chi-square test. The variables with P<0.1 in the univariate analysis were included in the multinomial logistic regression analysis. For treatment decision-making, full decision-making by patients served as the reference. For efficacy, the poor treatment effect was used as the reference. Unordered multinomial logistic regression was used to evaluate influencing factors. All statistical analyses were two-sided with a significance level of 0.05.
3 Results
3.1 Demographic characteristics
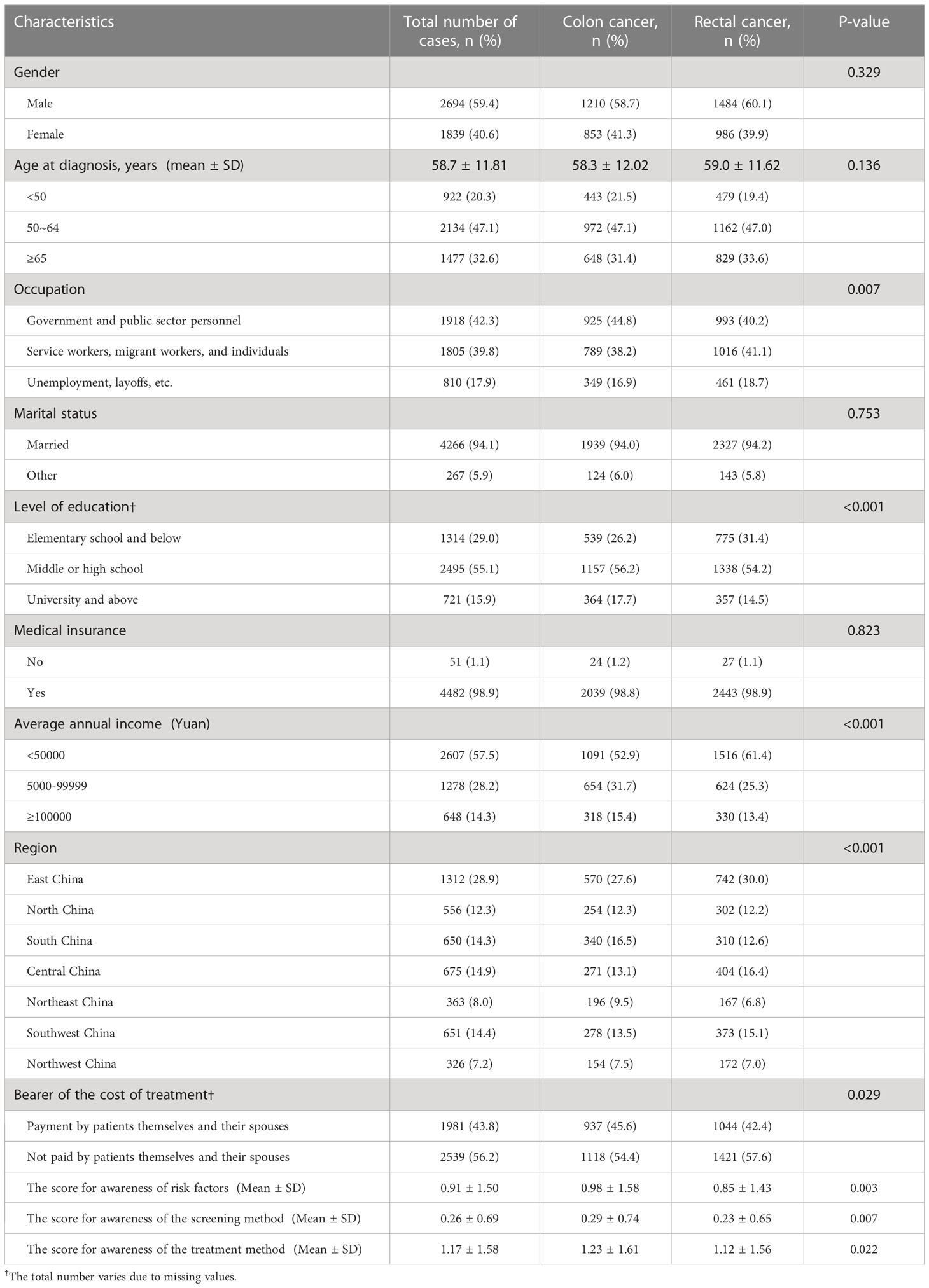
A total of 4533 patients with advanced CRC were enrolled. Their demographic characteristics are shown in Table 1. The average age at diagnosis of enrolled patients was 58.7 ± 11.81 years old, and there were 2694 males (59.4%) and 1839 females (40.6%). A total of 2063 (45.5%) patients had colon cancer, while 2470 (54.5%) cases were with rectal cancer. About 17.9% of patients were unemployed, 29% had an education level of primary school and below, 98.9% had medical insurance, 57.5% had an average annual family income of less than 50,000 Yuan, and, 56.2% had medical costs not covered by themselves or their spouses.
3.2 Clinical characteristics
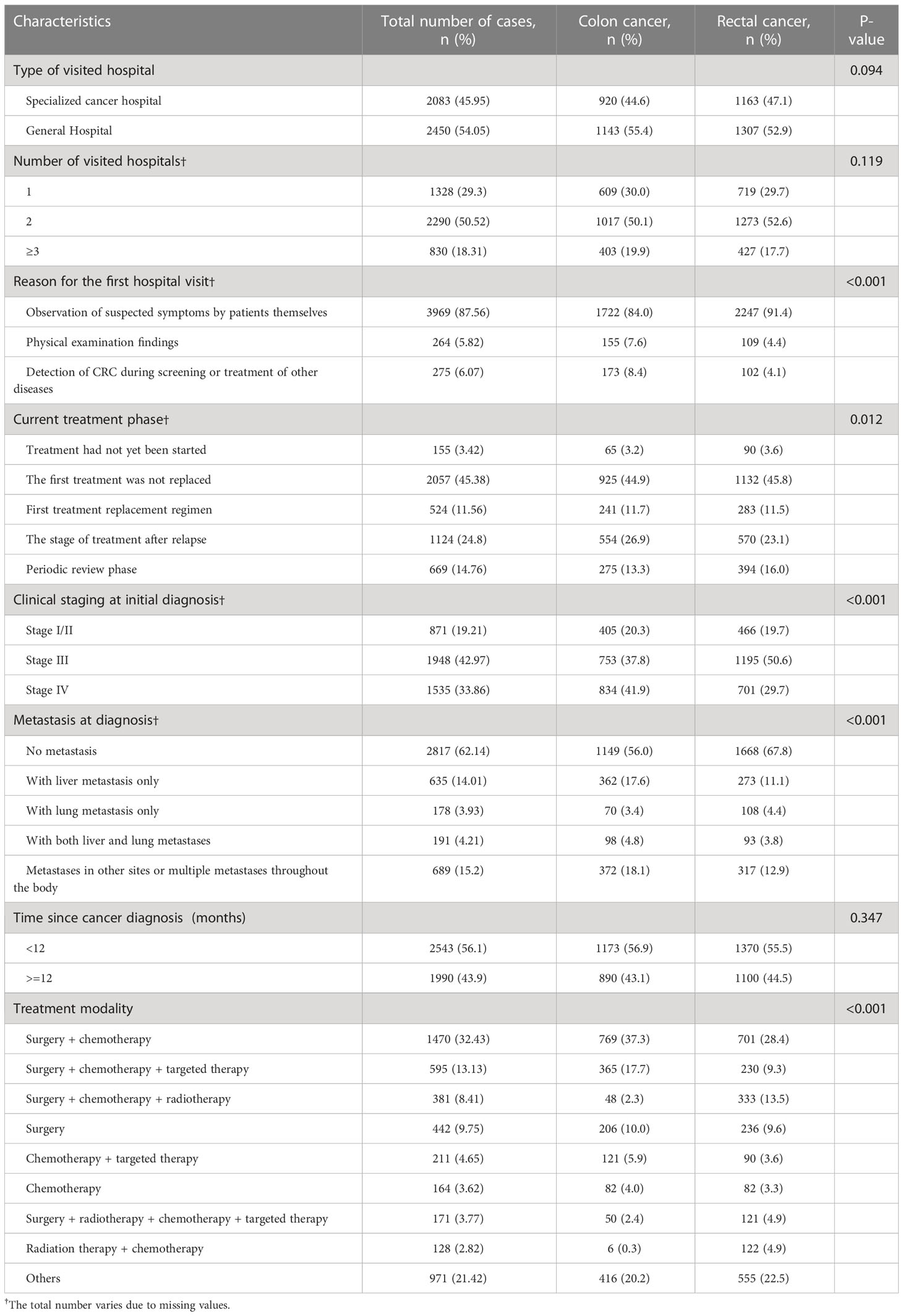
The clinical characteristics of the 4533 enrolled patients are listed in Table 2. Among them, 45.9% were recruited from specialized tumor hospitals and 54.1% were from general hospitals. 18.3% of the patients visited more than 3 hospitals to further confirm their disease status, 87.6% found suspected symptoms themselves, while only 5.8% had hospital visits based on abnormal results during regular health examinations. For tumor stage, 76.8% of the patients were classified as stage III or IV CRC, and 37.9% had metastasis at their first diagnosis, of whom 14.0% had liver metastasis. In terms of treatment methods, 32.4% of the patients received surgery combined with chemotherapy, 13.1% underwent surgery combined with chemotherapy and targeted therapy, and 9.8% were treated with surgery alone.
3.3 Involvement in treatment decision-making
In terms of patient involvement in treatment decision-making, 7.0% of patients had full responsibility for treatment decision-making throughout treatment, 47.0% of patients shared treatment decision-making with family members, 19% of the patients relied exclusively on their family members for decision-making, while 27% of the patients left the responsibility of treatment decision-making entirely to their physicians (Figure 1).
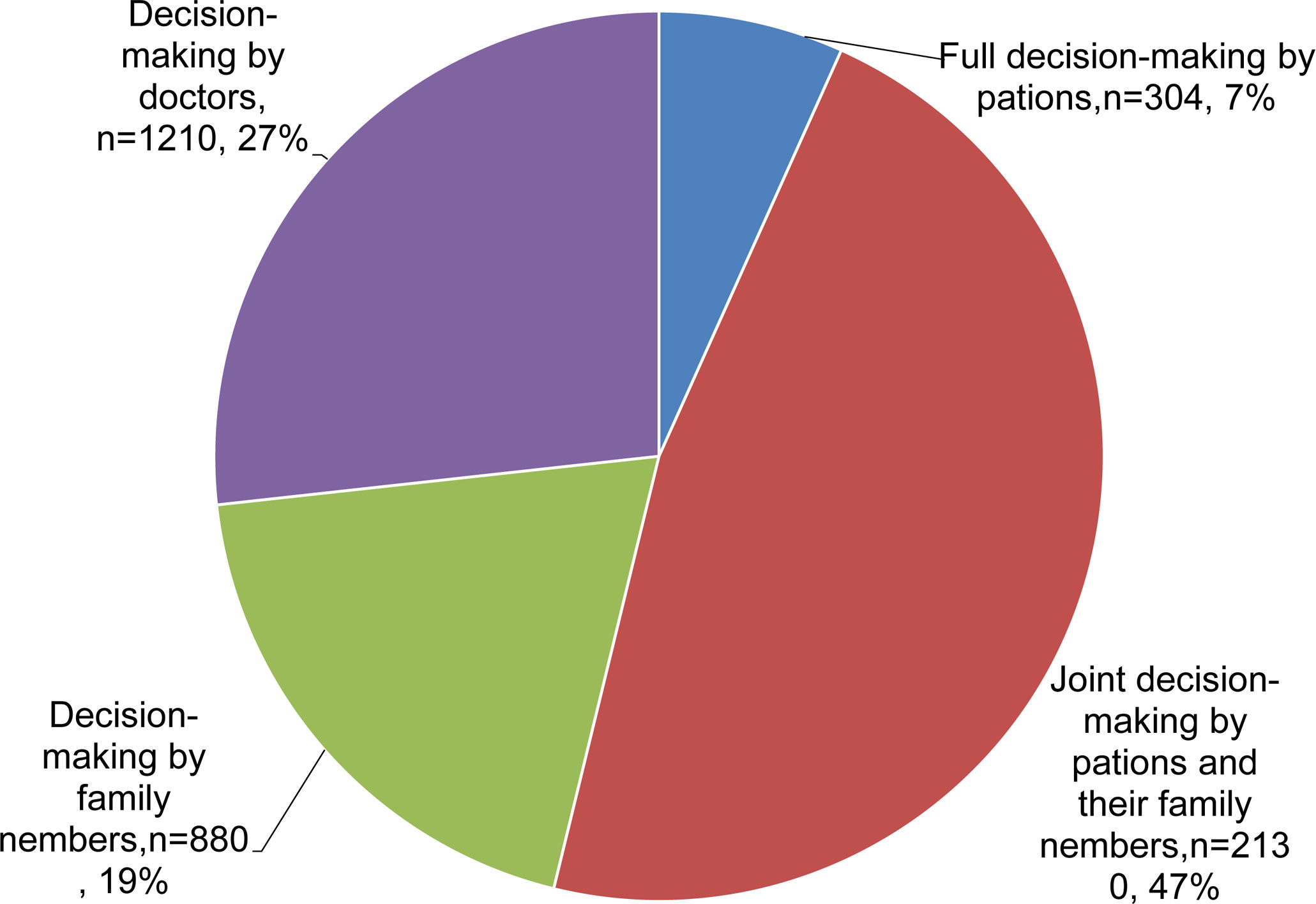
Figure 1 Pie chart showing the involvement in treatment decision-making in patients with advanced colorectal cancer.
3.4 Univariate and multinomial analysis of patient involvement in treatment decision-making
In univariate analysis, factors associated with involvement in treatment decision-making included gender, age at diagnosis, time since cancer diagnosis, occupation, marital status, education level, average annual family income, treatment cost burden, awareness of CRC-related factors, the type of visited hospital, the number of hospital visits, the clinical stage at diagnosis, and the treatment method (p<0.01 for all) (Table 3).
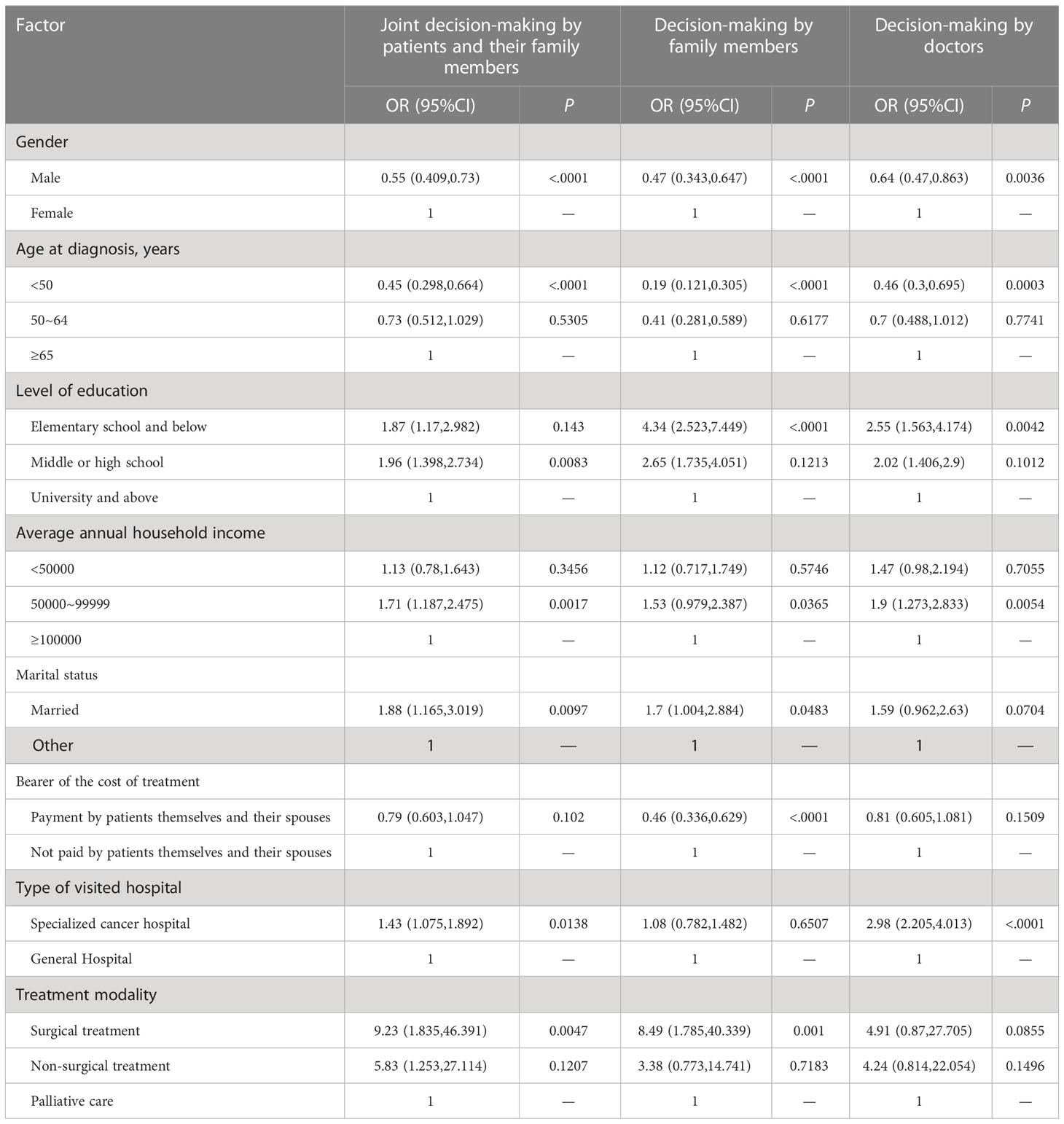
Multivariate logistic regression analysis was conducted by using full treatment decision-making by patients served as the reference. The results showed that gender, age, education level, annual family income, marital status, treatment cost burden, type of hospitals visited, and treatment methods were significantly related to involvement in treatment decision-making (Table 4). In detail, males were more likely to be solely responsible for treatment decision-making (OR 0.55 to 0.64). Patients under 50 years of age were more dominant in treatment decision-making compared to those over 65 years of age (OR 0.19 to 0.46). Compared with patients with an education level of university or above, patients with elementary school or less education level were less involved in making treatment decisions and were more likely to have family members or doctors make treatment decisions (OR 2.55 to 4.34). Patients with middle or high school education levels were more likely to make treatment decisions with family members (OR=1.96,95% CI=1.398-2.734). Patients with an average annual household income between 50,000 Yuan and 100,000 Yuan were more likely to make treatment decisions jointly with family members or by family members and physicians than patients with an average annual household income greater than 100,000 Yuan (OR 1.53-1.9). Married patients preferred shared treatment decision-making with family members or full treatment decision-making by family members than unmarried, divorced, or widowed patients (OR 1.7-1.88). Patients who paid treatment costs by themselves and their spouses were less likely to let family members make treatment decisions (OR=0.46, 95% CI=0.336-0.692). Patients from specialized cancer hospitals were more likely to share treatment decision-making with family members or follow physicians’ treatment decisions compared to those from general hospitals (OR=1.43-2.98). Patients with surgical treatment were more likely to make treatment decisions jointly with or solely by family members compared to those with palliative care (OR=8.49-9.23).
3.5 Self-reported efficacy
Self-reported efficacy evaluations were available for 3824 patients. For the first treatment, 76.5% of patients reported efficacy as good, 14.8% reported a stable condition, and 8.7% reported poor efficacy (Figure 2). Regarding the efficacy of the second treatment, the percentage of patients with self-reported good efficacy, stable condition, and poor efficacy was 61.7%, 26.2%, and 12.1%, respectively. Regarding the treatment efficacy for patients who had recurrence and metastasis, the percentage of patients with self-reported good efficacy, stable condition, and poor efficacy was 43.2%, 38.4%, and 18.4%, respectively (Figure 2).
3.6 Univariate and multinomial analysis of factors affecting self-reported efficacy
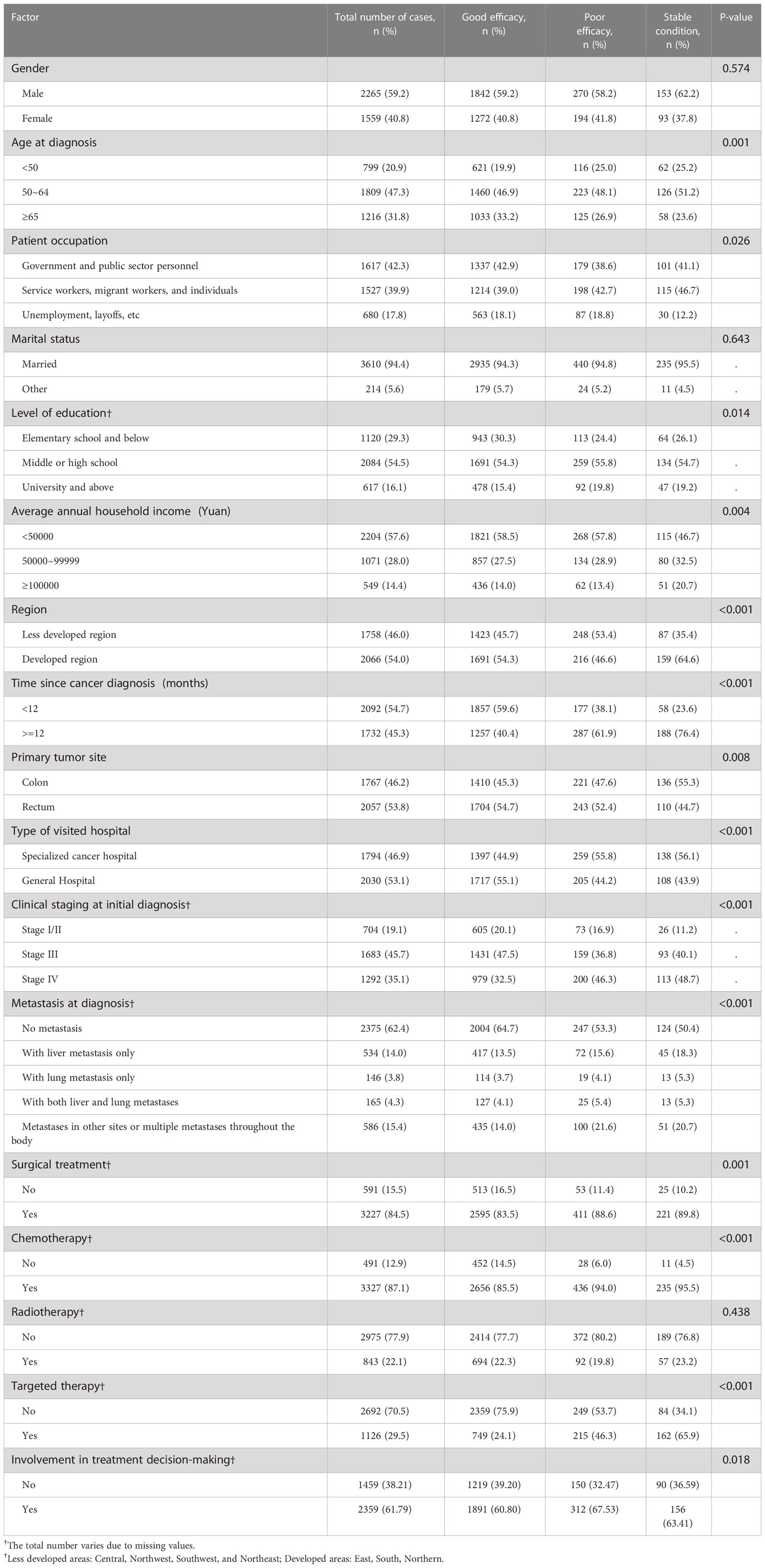
In univariate analysis, factors associated with self-reported efficacy included age at diagnosis, occupation, education level, average annual family income, region, time since cancer diagnosis, primary site, type of hospital, clinical stage at diagnosis, metastasis status, surgical treatment, chemotherapy, targeted therapy, and involvement in treatment decision-making (Table 5).
The multinomial analysis was conducted by using the poor self-reported efficacy as a reference. The results showed that occupation in government and public institutions (OR=1.44, 95%CI=1.044 -1.999), primary education and below (OR=1.57-2.18), annual family income of more than 100,000 Yuan (OR=0.59, 95%CI=0.341-1.005), living in less developed regions (OR=0.51-0.78), time since cancer diagnosis for less than 12 months (OR=1.94, 95%CI=1.547-2.43), admission in general hospitals (OR=0.7, 95%CI=0.558-0.881), clinical stage III (OR=1.41, 95%CI=0.961-2.078), surgery (OR=, 1.72, 95%CI=1.225-2.414), chemotherapy (OR=1.69, 95%CI= 1.101-2.598), targeted therapy (OR=2.03, 95%CI=1.585-2.593), and involvement in treatment decision-making (OR=1.33, 95%CI=1.061-1.665) were significantly associated with good self-reported treatment outcomes (all P<0.05, Table 6).
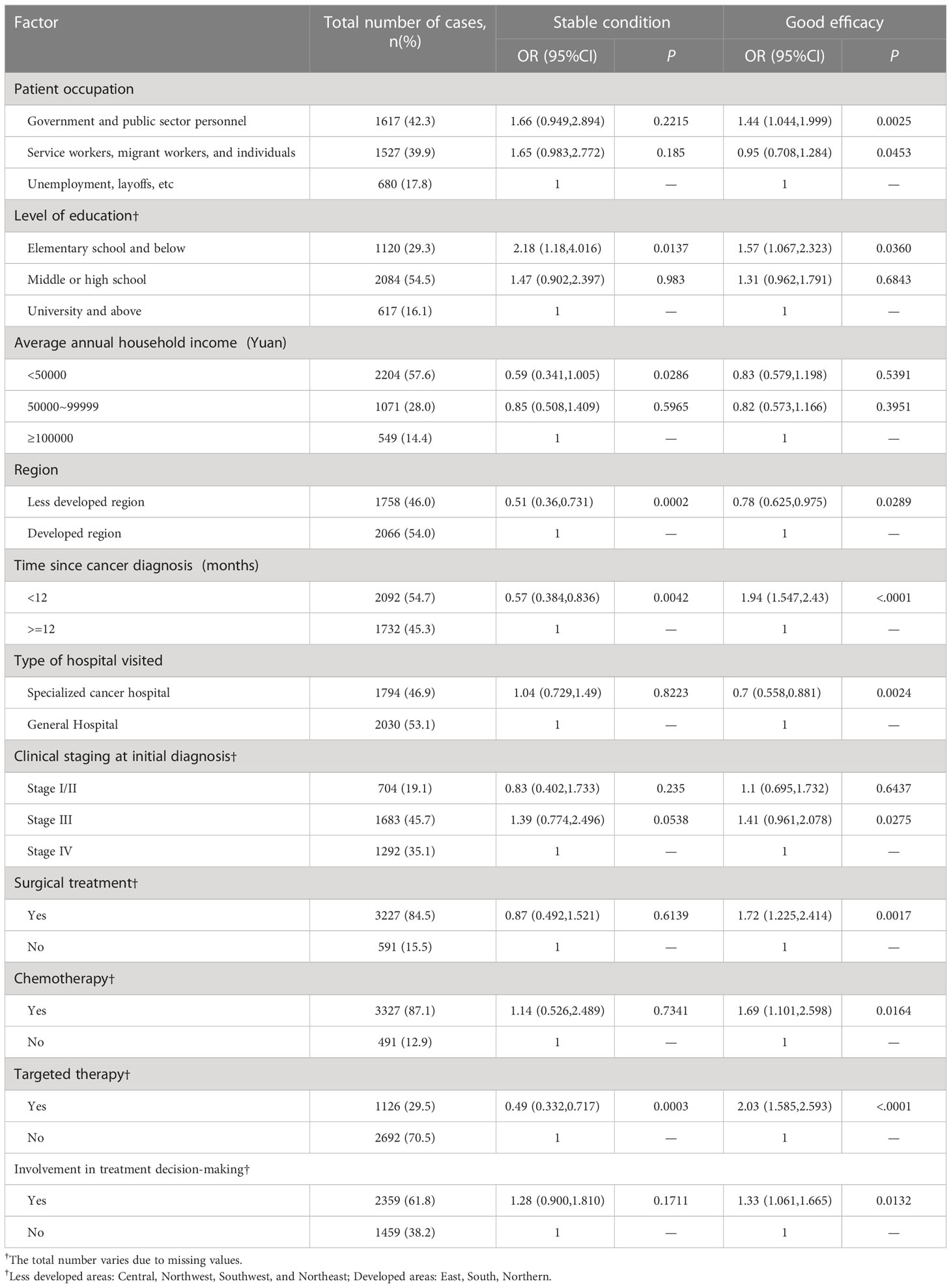
Table 6 Multinomial analysis of factors affecting self-reported treatment efficacy (with poor efficacy as a reference).
4 Discussion
For the first time, we conducted a nationwide multicenter hospital-based survey of patients with advanced CRC. Our results showed that the awareness of CRC-related knowledge such as risk factors, screening methods, and treatment methods was poor in patients with advanced CRC before diagnosis, which was similar to previous studies. For example, Amlani et al. showed that more than half of 2500 people from five European countries who had never received a colonoscopy were unaware that colonoscopy was a screening and prevention tool (14). Mueller et al. showed that only 36.0% of the respondents knew the starting age of CRC screening, and only 8.0% of the respondents answered all the screening knowledge correctly (19).
In this study, only 5.82% of the patients had hospital visits based on abnormal health examination results, while 87.6% found suspected symptoms themselves. Regarding tumor stage, 76.8% of the patients had stage III or IV CRC, and 37.5% had metastasis. These results were consistent with previous studies (20, 21). It has been shown that in countries with long-term and sustainable screening programs for CRC, CRC-related mortality is largely reduced and the diagnostic rate of early-stage CRC is increased (22, 23). Losurdo et al. showed that screening could significantly increase the rate of early diagnosis and surgery in CRC, reduce the incidence of complications, and improve survival outcomes (24). Kanth et al. showed that most of the CRC screening in the United States was based on opportunistic screening, and the goal of the screening rate reached 80.0% in 2018 (25). In China, Urban Cancer Early Diagnosis and Early Treatment Project was carried out in 2012 to screen high-risk groups for CRC in urban areas (26).
In this study, the treatment methods for advanced CRC were mainly surgery, surgery combined with chemotherapy, and, surgery combined with chemotherapy, radiotherapy, and targeted therapy. According to the China guideline for diagnosis and comprehensive treatment of colorectal liver metastases (2020 edition) (27) and Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology (28), the conventional treatment for CRC is surgery combined with chemotherapy or radiotherapy. Approximately 66.0% and 61.0% of stage II and III colon and rectal patients, respectively, received further treatment with adjuvant chemotherapy and/or radiotherapy (29). For advanced unresectable metastatic CRC, the mainstay of treatment is systemic therapy, such as targeted therapy and immunotherapy (30). In 2021, the American Society of Clinical Oncology (ASCO) and the European Society of Medical Oncology (ESMO) announced many research advances in immune and targeted therapy for advanced CRC (31–34), which greatly improved the survival rate of patients with advanced CRC.
In this study, only 7.0% of patients were solely responsible for treatment decision-making, 47.0% of patients shared treatment decision-making with their family members, and 46.0% of patients had treatment decision-making by solely family members or doctors. These results are consistent with the results of previous studies conducted on Asian patients from Taiwan and the United States (35–37). There are also findings showing that patients are willing to be involved in treatment decision-making, but most patients prefer to make treatment decisions with or by their physicians (38–40). However, it also has been shown that patients in the United States and other developed countries are more willing to make treatment decisions (41, 42). This may be related to the traditional Chinese concept of family and the paternalistic style of doctors. Chinese patients have high trust in doctors, believe that “doctors know the best”, and are willing to have their doctors make treatment decisions (43).
In this study, we found that gender, age at diagnosis, education level, family economic income, marital status, bearer of treatment expenses, type of hospital, and treatment method were independent factors affecting patient involvement in treatment decision-making. Males tended to be more likely to make treatment decisions by themselves, which reflects the dominance of males in the family. Younger patients, those with higher levels of education, and those with higher family income were more independent in making treatment decisions, which was similar to previous studies (44, 45), suggesting that younger and more educated patients are more likely to acquire disease-related information and to be more involved in treatment decision-making. Patients with wealthy families do not have to worry too much about the financial burden of treatment and are willing to have more personal control over their treatment decisions (46). Married patients are more involved in treatment decision-making than those in other marital statuses, which further reflects that Asians have a heavier family concept (37). For patients who paid for treatment costs by themselves and their spouses, family members were less likely to make treatment decisions, mainly because these patients had economic dominance. Compared with general hospitals, doctors from specialized cancer hospitals were more involved in treatment decision-making, which may be related to patients’ higher trust in oncologists. It has been shown that the lack of knowledge of patients and the imbalance of the doctor-patient relationship are the main obstacles for patients to participate in treatment decision-making (47). Clinicians should timely provide information about diseases to patients from the perspective of patients, which has a great impact on patient involvement in treatment decision-making (48, 49). Good and efficient doctor-patient communication is a major factor in improving treatment decision-making satisfaction, treatment compliance, and improved treatment outcomes.
Patient-Reported Outcomes (PROs) refer to any outcomes directly reported by patients, including information related to health, life quality, and functional status (50). PROs may more accurately reflect the physical functioning and emotional well-being of an individual, which cannot be affected by physician interpretation and prejudice (51) and are superior predictors of survival compared with functional status (52). The application of PROs can reduce the symptom burden of CRC patients and improve patients’ quality of life and survival. The evaluation of PRO is mainly through questionnaires (53), including short form-36, European Organization for Research and Treatment of Cancer, the quality of life questionnaire (EORTCQLQC30), and, the European Organization for Research and Treatment of colorectal cancer, the quality of life questionnaire (EORTCQLQ-CR38), etc. (54). In this study, we did not use scales to evaluate PROs. Instead, we used self-reported efficacy, i.e. patients’ subjective feelings after treatment, which was divided into good efficacy, poor efficacy, and stable condition. A total of 3824 patients, mainly those receiving the first treatment, the second treatment, and the treatments following recurrence and metastasis, submitted self-reported efficacy assessments during treatment. With the increase in the number of treatment times, patients reported an increased number of unsatisfactory treatment outcomes, which is also in line with the progression of the disease. Generally, the patient’s quality of life decreases with longer disease duration and advanced disease stages (55).
In this study, occupation, education level, average annual family income, economic level of the region, time since cancer diagnosis, hospital type, clinical stage at diagnosis, surgical treatment, chemotherapy, targeted therapy, and involvement in treatment decision-making were all significant factors affecting self-reported efficacy in patients with advanced CRC. Belachew. et al. found that higher education levels and economic income were associated with higher quality of life (56). McCombie et al. showed that CRC patients over the age of 80 years were always satisfied with the outcome of surgical treatment (57). Moreover, gender, ethnicity, medical insurance, tumor location, stage, metastasis, and other factors are all prognostic factors of CRC (58, 59). In addition, appropriate surgical treatment is essential to control tumor recurrence and metastasis, thus achieving improved survival rates (60). However, in this study, tumor location and metastasis were not independent prognostic factors, which may be related to the fact that all the study participants included in the study were with advanced CRC. In addition, patients who participated in treatment decision-making reported better self-reported treatment outcomes than patients who did not participate in treatment decision-making, possibly because patients who participated in treatment decision-making acquired more knowledge of tumors and had a better quality of life (61).
There are several limitations in this study. First, the role of doctors and nurses in patient involvement in treatment decision-making was not analyzed. Second, this is a cross-sectional study without long-term follow-up. The causal relationship between treatment effects and associated factors cannot be determined. Third, there are some missing values for some variables, which might cause some potential bias. Considering that the highest missing rate of a specific variable was lower than 5%, and the missing rates of most variables were less than 1%, we did not make further adjustments. Last but not least, the treatment effects were self-reported and were only evaluated based on subjective feelings, without using appropriate scales, which may lead to certain biases. In the follow-up work, we will collect more data and conduct a more in-depth analysis of the patient involvement in treatment decision-making and the self-reported efficacy of CRC patients.
In this study, we conducted a nationwide multi-center hospital-based survey of patients with advanced CRC and found that the involvement of patients in treatment decision-making was poor. The vast majority of treatment decisions were made jointly with family members or by family members/physicians. Effective communication between physicians and patients should be further improved. Thus, patients can obtain timely information on CRC and then participate in treatment decision-making. The use of patient self-reported outcomes in clinical practice in China is in its infancy and lacks appropriate measurement tools, which should be further improved in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was reviewed and approved by the Medical Ethics Committee of Henan Cancer Hospital (No. 2019273). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design: X-FG, H-FX, Y-LQ, LM, and RR. Administrative support: Y-LQ. Provision of study materials or patients: X-FG, H-FX, LL, Y-QY, XZ, X-HW, W-JW, L-BD, S-XD, H-LC, Y-QZ, Y-YL, J-XH, JC, Y-PF, C-YF, X-ML, J-CD, and LM. Collection and assembly of data: Y-QY, XZ, and H-FX. Data analysis and interpretation: X-FG, YL, and H-FX. Manuscript writing: X-FG, H-FX. Final approval of manuscript: All authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Beijing Love Book Cancer Foundation and Merck Serono Co. Ltd.The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li N, Lu B, Luo C, Cai J, Lu M, Zhang Y, et al. Incidence, mortality, survival, risk factor and screening of colorectal cancer: a comparison among China, Europe, and northern America. Cancer Lett (2021) 522:255–68. doi: 10.1016/j.canlet.2021.09.034
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Zhou J, Zheng R, Zhang S, Zeng H, Wang S, Chen R, et al. Colorectal cancer burden and trends: comparison between China and major burden countries in the world. Chin J Cancer Res (2021) 33(1):1–10. doi: 10.21147/j.issn.1000-9604.2021.01.01
4. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and united states, 2022: profiles, trends, and determinants. Chin Med J (Engl) (2022) 135(5):584–90. doi: 10.1097/CM9.0000000000002108
5. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
6. Wu C, Gu K, Gong Y, Zheng R, Wang S, Chen R, et al. Analysis of incidence and mortality of colorectal cancer in China, 2015. China Oncol (2020) 30(4):241–5. doi: 10.19401/j.cnki.1007-3639.2020.04.001
7. Shi JF, Wang L, Ran JC, Wang H, Liu CC, Zhang HZ, et al. Clinical characteristics, medical service utilization, and expenditure for colorectal cancer in China, 2005 to 2014: overall design and results from a multicenter retrospective epidemiologic survey. Cancer (2021) 127(11):1880–93. doi: 10.1002/cncr.33445
8. Shen L, Li Q, Wang W, Zhu L, Zhao Q, Nie Y, et al. Treatment patterns and direct medical costs of metastatic colorectal cancer patients: a retrospective study of electronic medical records from urban China. J Med Econ (2020) 23(5):456–63. doi: 10.1080/13696998.2020.1717500
9. Wang W, Yin P, Liu Y-N, Liu J-M, Wang L-J, Qi J-L, et al. Mortality and years of life lost of colorectal cancer in China, 2005–2020: findings from the national mortality surveillance system. Chin Med J (Engl) (2021) 134(16):1933–40. doi: 10.1097/CM9.0000000000001625
10. Cao W, Chen H-D, Yu Y-W, Li N, Chen W-Q. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) (2021) 134(7):783–91. doi: 10.1097/CM9.0000000000001474
11. Brown R, Butow P, Wilson-Genderson M, Bernhard J, Ribi K, Juraskova I. Meeting the decision-making preferences of patients with breast cancer in oncology consultations: impact on decision-related outcomes. J Clin Oncol (2012) 30(8):857–62. doi: 10.1200/JCO.2011.37.7952
12. Ohki S, Okayama H, Chida S, Sakamoto W, Fujita S, Saito M, et al. [Significance and treatment strategy of Glasgow prognostic score in high risk stage II colorectal cancer]. Gan Kagaku Ryoho (2021) 48(13):1770–3.
13. Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol (2022) 7(3):262–74. doi: 10.1016/S2468-1253(21)00426-X
14. Amlani B, Radaelli F, Bhandari P. A survey on colonoscopy shows poor understanding of its protective value and widespread misconceptions across Europe. PloS One (2020) 15(5):e0233490. doi: 10.1371/journal.pone.0233490
15. Tian c-x, ZHao L. Epidemiological charecteristics of colorectal cancer and colorectal livermetasctasi. Chin J Cancer Prevtreat (2021) 28(1):1033–8. doi: 10.16073/j.cnki.cjcpt.2021.13.12
16. Martini G, Dienstmann R, Ros J, Baraibar I, Cuadra-Urteaga JL, Salva F, et al. Molecular subtypes and the evolution of treatment management in metastatic colorectal cancer. Ther Adv Med Oncol (2020) 12:1758835920936089. doi: 10.1177/1758835920936089
17. Sun R, Yao J, Yang Z, Wang J, Ma J. Clinical characteristics and prognostic factors of 52 patients with colorectal cancer. Chin J Clin Oncol Rehabil (2015) 22(12):1420–2. doi: 10.13455/j.cnki.cjcor.2015.12.04
18. Xu HF, Gu XF, Wang XH, Wang WJ, Du LB, Duan SX, et al. Knowledge and awareness of colorectal cancer risk factors, screening, and associated factors in advanced colorectal cancer patients: a multicenter cross-sectional study in China. Ann Transl Med (2022) 10(6):354. doi: 10.21037/atm-22-1019
19. Mueller NM, Hyams T, King-Marshall EC, Curbow BA. Colorectal cancer knowledge and perceptions among individuals below the age of 50. Psycho-Oncology (2022) 31(3):436–41. doi: 10.1002/pon.5825
20. Zhuang Y, Wang H, Jiang D, Li Y, Feng L, Tian C, et al. Multi gene mutation signatures in colorectal cancer patients: predict for the diagnosis, pathological classification, staging and prognosis. BMC Cancer (2021) 21(1):380. doi: 10.1186/s12885-021-08108-9
21. Ma B, Li Y, Meng Q. The predictive and prognostic value of sex in localized colorectal cancer: a seer-based analysis. Transl Cancer Res (2021) 10(5):2108–19. doi: 10.21037/tcr-20-3421
22. Meester RG, Doubeni CA, Zauber AG, Goede SL, Levin TR, Corley DA, et al. Public health impact of achieving 80% colorectal cancer screening rates in the united states by 2018. Cancer (2015) 121(13):2281–5. doi: 10.1002/cncr.29336
23. Cardoso R, Guo F, Heisser T, Hackl M, Ihle P, De Schutter H, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol (2021) 22(7):1002–13. doi: 10.1016/S1470-2045(21)00199-6
24. Losurdo P, Giacca M, Biloslavo A, Fracon S, Sereni E, Giudici F, et al. Colorectal cancer-screening program improves both short- and long-term outcomes: a single-center experience in Trieste. Updates Surg (2020) 72(1):89–96. doi: 10.1007/s13304-020-00703-y
25. Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. BMJ (2021) 374:n1855. doi: 10.1136/bmj.n1855
26. Chen W-q, Li N, Shi J-f, Ren J-s, Chen H-d, Li J, et al. Progress of cancer screening program in urban China. China Cancer (2019) 28(1):23–5. doi: 10.11735/j.issn.1004-0242.2019.01.A003
27. Chinese College of Surgeons, Chinese Medical Doctor Association. China Guideline for diagnosis and comprehensive treatment of colorectal liver metastases ( 2020 edition). J Clin Hepatol (2021) 37(3):543–53. doi: 10.3969/j.issn.1001-5256.2021.03.009
28. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, et al. Colon cancer, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(3):329–59. doi: 10.6004/jnccn.2021.0012
29. Johdi NA, Sukor NF. Colorectal cancer immunotherapy: options and strategies. Front Immunol (2020) 11:1624. doi: 10.3389/fimmu.2020.01624
30. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. JAMA (2021) 325(7):669–85. doi: 10.1001/jama.2021.0106
31. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C, et al. Pembrolizumab in microsatellite-Instability-High advanced colorectal cancer. N Engl J Med (2020) 383(23):2207–18. doi: 10.1056/NEJMoa2017699
32. Dong C, Ding Y, Weng S, Li G, Huang Y, Hu H, et al. Update in version 2021 of csco guidelines for colorectal cancer from version 2020. Chin J Cancer Res (2021) 33(3):302–7. doi: 10.21147/j.issn.1000-9604.2021.03.02
33. Weiss J, Yaeger RD, Johnson ML, Spira A, Klempner SJ, Barve MA, et al. Lba6 krystal-1: adagrasib (Mrtx849) as monotherapy or combined with cetuximab (Cetux) in patients (Pts) with colorectal cancer (Crc) harboring a Krasg12c mutation. Ann Oncol (2021) 32:S1294. doi: 10.1016/j.annonc.2021.08.2093
34. Kopetz S, Guthrie KA, Morris VK, Lenz HJ, Magliocco AM, Maru D, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in braf-mutant metastatic colorectal cancer (Swog S1406). J Clin Oncol (2021) 39(4):285–94. doi: 10.1200/jco.20.01994
35. Lin ML, Pang MC, Chen CH. Family as a whole: elective surgery patients' perception of the meaning of family involvement in decision making. J Clin Nurs (2013) 22(1-2):271–8. doi: 10.1111/j.1365-2702.2012.04194.x
36. Hobbs GS, Landrum MB, Arora NK, Ganz PA, van Ryn M, Weeks JC, et al. The role of families in decisions regarding cancer treatments. Cancer (2015) 121(7):1079–87. doi: 10.1002/cncr.29064
37. Zhai H, Lavender C, Li C, Wu H, Gong N, Cheng Y. Who decides? shared decision-making among colorectal cancer surgery patients in China. Support Care Cancer (2020) 28(11):5353–61. doi: 10.1007/s00520-020-05391-3
38. Josfeld L, Keinki C, Pammer C, Zomorodbakhsch B, Hübner J. Cancer patients' perspective on shared decision-making and decision aids in oncology. J Cancer Res Clin Oncol (2021) 147(6):1725–32. doi: 10.1007/s00432-021-03579-6
39. Sankar SD, Dhanapal B, Shankar G, Krishnaraj B, Karra S, Natesan V. Desire for information and preference for participation in treatment decisions in patients with cancer presenting to the department of general surgery in a tertiary care hospital in India. J Glob Oncol (2018) 4:1–10. doi: 10.1200/jgo.17.00144
40. Xiao L, Peng M, Liu Y, Zhang L. Information, deliberation, and decisional control preferences for participation in medical decision-making and its influencing factors among Chinese cancer patients. Health Expect (2021) 24(5):1725–36. doi: 10.1111/hex.13312
41. Singh JA, Sloan JA, Atherton PJ, Smith T, Hack TF, Huschka MM, et al. Preferred roles in treatment decision making among patients with cancer: a pooled analysis of studies using the control preferences scale. Am J Manag Care (2010) 16(9):688–96.
42. Schaede U, Mahlich J, Nakayama M, Kobayashi H, Takahashi Y, Saito K, et al. Shared decision-making in patients with prostate cancer in Japan: patient preferences versus physician perceptions. J Glob Oncol (2018) 4:1–9. doi: 10.1200/jgo.2016.008045
43. Salloch S, Ritter P, Wäscher S, Vollmann J, Schildmann J. Medical expertise and patient involvement: a multiperspective qualitative observation study of the patient's role in oncological decision making. Oncologist (2014) 19(6):654–60. doi: 10.1634/theoncologist.2013-0268
44. Brom L, Hopmans W, Pasman HR, Timmermans DR, Widdershoven GA, Onwuteaka-Philipsen BD. Congruence between patients' preferred and perceived participation in medical decision-making: a review of the literature. BMC Med Inform Decis Mak (2014) 14:25. doi: 10.1186/1472-6947-14-25
45. Spooner K. Self-reported preferences for patient and provider roles in cancer treatment decision-making in the united states. Family Med Community Health (2017) 5(1):43–55. doi: 10.15212/FMCH.2017.0102
46. Viklund P, Lagergren J. A care pathway for patients with oesophageal cancer. Eur J Cancer Care (Engl) (2007) 16(6):533–8. doi: 10.1111/j.1365-2354.2007.00790.x
47. Joseph-Williams N, Elwyn G, Edwards A. Knowledge is not power for patients: a systematic review and thematic synthesis of patient-reported barriers and facilitators to shared decision making. Patient Educ Couns (2014) 94(3):291–309. doi: 10.1016/j.pec.2013.10.031
48. Fowler FJ Jr., Levin CA, Sepucha KR. Informing and involving patients to improve the quality of medical decisions. Health Aff (Millwood) (2011) 30(4):699–706. doi: 10.1377/hlthaff.2011.0003
49. Milliron KJ, Griggs JJ. Advances in genetic testing in patients with breast cancer, high-quality decision making, and responsible resource allocation. J Clin Oncol (2019) 37(6):445–7. doi: 10.1200/jco.18.01952
50. Weldring T, Smith SM. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights (2013) 6:61–8. doi: 10.4137/HSI.S11093
51. Kerrigan K, Patel SB, Haaland B, Ose D, Weinberg Chalmers A, Haydell T, et al. Prognostic significance of patient-reported outcomes in cancer. JCO Oncol Pract (2020) 16(4):e313–e23. doi: 10.1200/JOP.19.00329
52. Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol (2008) 26(8):1355–63. doi: 10.1200/JCO.2007.13.3439
53. Taibi A, Geyl S, Salle H, Salle L, Mathonnet M, Usseglio J, et al. Systematic review of patient reported outcomes (Pros) and quality of life measures after pressurized intraperitoneal aerosol chemotherapy (Pipac). Surg Oncol (2020) 35:97–105. doi: 10.1016/j.suronc.2020.08.012
54. Zhang L, Liu Y, Chen L, Peng L, Li W, Li X. Patient-reported outcomes in clinical research of colorectal cancer: a literature review. Modern Clin Nurs (2021) 20(10):79–84. doi: 10.3969/j.issn.1671-8283.2021.09.014
56. Belachew AA, Reyes ME, Ye Y, Raju GS, Rodriguez MA, Wu X, et al. Patterns of Racial/Ethnic disparities in baseline health-related quality of life and relationship with overall survival in patients with colorectal cancer. Qual Life Res (2020) 29(11):2977–86. doi: 10.1007/s11136-020-02565-8
57. McCombie AM, Frampton CM, Frizelle FA. Quality of life preferences in colorectal cancer patients aged 80 and over. ANZ J Surg (2021) 91(9):1859–65. doi: 10.1111/ans.16739
58. Zheng C, Jiang F, Lin H, Li S. Clinical characteristics and prognosis of different primary tumor location in colorectal cancer: a population-based cohort study. Clin Trans Oncol (2019) 21(11):1524–31. doi: 10.1007/s12094-019-02083-1
59. Tang M, Wang H, Cao Y, Zeng Z, Shan X, Wang L. Nomogram for predicting occurrence and prognosis of liver metastasis in colorectal cancer: a population-based study. Int J Colorectal Dis (2021) 36(2):271–82. doi: 10.1007/s00384-020-03722-8
60. Chan KM, Wu TH, Wang YC, Lee CF, Wu TJ, Chou HS, et al. Clinical relevance of oncologic prognostic factors in the decision-making of pre-hepatectomy chemotherapy for colorectal cancer hepatic metastasis: the priority of hepatectomy. World J Surg Oncol (2018) 16(1):24. doi: 10.1186/s12957-018-1322-9
Keywords: colorectal cancer, treatment, decision making, self-reported efficacy, China
Citation: Gu X-F, Xu H-F, Liu Y, Li L, Yu Y-Q, Zhang X, Wang X-H, Wang W-J, Du L-B, Duan S-X, Cao H-L, Zhao Y-Q, Liu Y-Y, Huang J-X, Cao J, Fan Y-P, Feng C-Y, Lian X-M, Du J-C, Rezhake R, Ma L and Qiao Y-L (2023) Involvement in treatment decision-making and self-reported efficacy among patients with advanced colorectal cancer: a nationwide multi-center cross-sectional study. Front. Oncol. 13:1168078. doi: 10.3389/fonc.2023.1168078
Received: 17 April 2023; Accepted: 22 June 2023;
Published: 26 July 2023.
Edited by:
Samuel Aguiar Junior, A.C.Camargo Cancer Center, BrazilReviewed by:
Celso Mello, A.C.Camargo Cancer Center, BrazilMarkus Andret Cavalcante Gifoni, Federal University of Ceara, Brazil
Bruna Elisa Catin Kupper, A.C.Camargo Cancer Center, Brazil
Copyright © 2023 Gu, Xu, Liu, Li, Yu, Zhang, Wang, Wang, Du, Duan, Cao, Zhao, Liu, Huang, Cao, Fan, Feng, Lian, Du, Rezhake, Ma and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Remila Rezhake, UmVtaWxhQHhqbXUuZWR1LmNu; Li Ma, bWFsaV9sZWxlQHNpbmEuY29t; You-Lin Qiao, cWlhb3lAY2ljYW1zLmFjLmNu
†These authors have contributed equally to this work
 Xiao-Fen Gu
Xiao-Fen Gu Hui-Fang Xu
Hui-Fang Xu Yin Liu
Yin Liu Li Li
Li Li Yan-Qin Yu
Yan-Qin Yu Xi Zhang
Xi Zhang Xiao-Hui Wang6
Xiao-Hui Wang6 Ling-Bin Du
Ling-Bin Du Yu-Qian Zhao
Yu-Qian Zhao Yun-Yong Liu
Yun-Yong Liu Yan-Ping Fan
Yan-Ping Fan Xue-Mei Lian
Xue-Mei Lian Remila Rezhake
Remila Rezhake Li Ma
Li Ma You-Lin Qiao
You-Lin Qiao