- 1Unit for Research and Higher Education, Centre for Clinical Research Dalarna, Uppsala University, Region Dalarna, Falun, Sweden
- 2Pathology and Cytology Dalarna, County Hospital Falun, Region Dalarna, Falun, Sweden
- 3College of Health Sciences, Pan-European University, Banja Luka, Bosnia and Herzegovina
- 4Pathology Unit, Azienda Sanitaria Toscana Nord-Ovest, Pisa, Italy
Objective: HER2 status in breast cancer is an essential parameter in individual therapeutic decision-making and is routinely assessed in primary tumors in accordance with international recommendations. Reports of HER2 heterogeneity raise the question of basing treatment decisions on HER2 status in metastases, if present. We investigated the degree and clinical implications of HER2 heterogeneity in lymph node–positive breast cancer. Because of recent recognition of therapeutic opportunities in this group of tumors, we especially focused on cases involving low-level HER2 expression.
Methods: The HER2 status of primary tumors and of corresponding lymph node metastases was determined in archived material at the protein and gene levels using the gene– protein assay and interpreted in accordance with 2018 ASCO/CAP criteria. HER2-low status was defined as protein expression levels 1+ or 2+ with negative amplification status.
Results: We analyzed a series of 43 cases of primary infiltrating breast cancer, each with at least two axillary nodes harboring macrometastases (>2 mm), in total 206 such nodes. In 7% of cases, we detected intertumoral HER2 heterogeneity. Three of nine HER2-positive primary tumors were associated with HER2-negative metastases. No cases with HER2-negative primary tumors had HER2-positive metastases, but 55% (6/11) of HER2 0 primary tumors had HER2 1+ and/or 2+ metastases, and 19% (3/16) HER2 1+ cases had exclusively HER2 0 metastases. All metastases in HER2 2+ cases showed HER2-low protein expression levels. Internodal HER2 heterogeneity at low protein expression levels (presence of HER2 0, HER2 1+, and/or HER2 2+ metastatic deposits within the same axilla) was seen in 40% (17/43) of cases. We found no statistically significant association between HER2 heterogeneity and other tumor-related parameters. Survival data indicated worse outcomes in the HER2-low group compared with the rest of the cohort.
Conclusion: Our results indicate a substantial instability of HER2 protein expression, leading to considerable intertumoral and internodal HER2 heterogeneity in lymph node–positive breast carcinomas. This heterogeneity is particularly relevant in HER2-low tumors in which the corrective effects of HER2 gene copy number analysis definitionally is absent. Our findings suggest that determining HER2 status in metastatic lymph nodes may generate relevant information for therapeutic decision-making.
1 Introduction
Breast cancer is a heterogeneous disease (1), divided into several distinct subtypes. Expression levels of tumor biomarkers such as estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) are pivotal for treatment decisions and prognostication in breast cancer. Approximately 15%–20% of all breast cancers are HER2-positive, attributable to over-expression of the HER2 protein and/or increased copy number of the HER2 gene (amplification) (2). Findings suggest that expression levels of HER2 might vary within a single tumor focus (intratumoral heterogeneity) as well as between different tumor foci and between the primary tumor(s) and synchronous axillary lymph node metastases (intertumoral heterogeneity) (3–5). HER2 heterogeneity is a major challenge for accurate evaluation of HER2 status and affects both prognosis and treatment success (6–12). In clinical practice, prognosis and treatment decisions are regularly based on the evaluation of biomarkers in primary tumors, even in cases involving synchronous axillary metastases.
The group of HER2-low breast carcinomas represents a newly defined entity in the field, thanks to the observation that treatment with trastuzumab duocarmazine led to partial response in 28% and 40% of patients with HER2-low ER-positive and ER-negative breast cancer, respectively (13). This finding highlighted the need to delineate the group of patients who might benefit from a targeted therapy because the target is present in some tumor cells even in the absence of the amplified oncogene. The HER2-low category comprises a spectrum of carcinomas with levels of protein expression up to HER2 2+. These tumors are associated with a poorer prognosis than with HER2-negative cancers (14–16).
We previously showed that HER status may differ between the primary tumor and metastases in as many as 13.2% of cases (12). We also observed internodal HER2 heterogeneity of uncertain significance in our cases, which suggested the need for more studies with an expanded panel of metastases (12). Sapino et al. reported a high prevalence of HER2 heterogeneity in HER2-low tumors (17), which motivated us to focus our study on this tumor category.
2 Materials and methods
2.1 Breast cancer cases
We retrospectively identified patients diagnosed with primary invasive breast cancer at the Department of Pathology and Cytology Dalarna of the County Hospital Falun in Sweden between 2011 and 2015. To allow for analysis of internodal heterogeneity, only cases involving at least two axillary lymph node macrometastases were included, with macrometastasis defined as a metastatic deposit >2 mm within a lymph node (18). Patients were included if they had provided informed consent at the time of diagnosis, allowing us to use parts of their archived tumor material for this study. Patients with recurrent disease and those who received neoadjuvant treatment before surgery were excluded. Availability of tumor and metastatic material was a requirement for inclusion. This study was approved by the ethical review board in Uppsala, Sweden (registration number 2010/461, 2010/461/1), and by the Swedish Ethical Review Authority (registration number 2020-00310).
2.2 Tumor material
Clinico-pathological parameters such as tumor size, disease extent, growth pattern/lesion distribution, TNM stage, Nottingham histology grade, and biomarker profile/molecular phenotype were retrieved from the medical records (for the definitions of these parameters, see reference (19)). An experienced breast pathologist reexamined the primary tumors of the selected cases. Formalin-fixed, paraffin-embedded archived material from the metastatic lymph nodes was retrieved for this work. Sections were cut in 4 µm slices from the most representative paraffin blocks and stained as described below.
2.3 Gene–protein assay (GPA)
Newly sliced tumor material from metastatic lymph nodes was stained using the Roche GPA method, as previously described (20), with primary tumors re-stained only in cases for which GPA was unavailable from the time of diagnosis. In brief, the HER2 protein was stained using an HER2/neu rabbit monoclonal primary antibody (clone 4B5, Ventana Medical Systems, Inc., Tucson, AZ, USA). A dual chromogen in situ hybridization (ISH) was performed on this material to quantitatively detect the HER2 gene as well as the chromosome 17 centromere (CEN17). Silver ISH (SISH) was used to visualize the HER2 gene and chromogen red ISH (Red ISH) to visualize CEN17. The slides were counterstained with hematoxylin II and bluing reagent. These assays were performed using the BenchMark® XT (Ventana Medical Systems, Inc.), with NexES software and the INFORM HER2 Dual ISH DNA Probe Cocktail mix (Ventana Medical Systems, Inc.) in accordance with the manufacturer’s recommendation.
The stained slides were analyzed using a brightfield microscope (Olympus BX45) for HER2 status using a 60× objective. At least three separate distant foci of the tumor cells in a single metastatic lymph node were analyzed for HER2. Two experienced pathologists performed the analyses without awareness of the patient’s clinical status. HER2 status was assessed in 120 tumor cells (40 cells per focus) according to the 2018 ASCO/CAP guidelines (21).
Status was assessed as HER2-positive (1) if the immunohistochemical staining was rated 3+; (2) if the immunohistochemical staining was rated 2+, the HER2/CEP17 ratio was ≥2, and the average number of copies of the HER2 gene was >4.0; or (3) if the immunohistochemical staining was rated 2+, the HER2/CEP17 ratio was <2, and the average number of copies of the HER2 gene was ≥6.0. Status was assessed as HER2-negative if the tumor did not fulfill the above criteria. Primary tumors and metastases were defined as HER2-low if protein expression levels were rated HER21+ or HER2 2+ and were non- amplified (i. e., the HER2/CEP17 ratio was <2 and/or the average number of copies of the HER2 gene was ≤4.0). Tumors and metastases with HER2 protein expression level 0 were regarded as HER2-negative.
Intertumoral heterogeneity was defined as any discrepancy in HER2 protein expression levels and/or gene amplification status between the primary tumor and its lymph node metastases. Internodal heterogeneity was defined as the same discrepancy in HER2 levels being present between the metastases located to lymph nodes within the same axilla.
2.4 Statistical analysis
Numerical data were presented as means, standard deviations, and ranges. Categorical data were presented as counts (n) and proportions. Analysis of the statistical significance was performed using the independent samples t-test (student's t-test) or Fisher's exact test. A statistical result was considered significant when P < 0.05. Statistical analysis was performed using Jamovi software (22, 23). The potential impact of the results on prognosis and therapeutic decisions was estimated based on Swedish national recommendations (24).
3 Results
All included patients were women, with a mean age of 59.8 years. Extensive tumors (e.g., >40 mm) were found in 29 (67.4%) of the cases. The aggregate growth pattern was unifocal in 13 (30.2%), multifocal in 13 (30.2%), and diffuse in 17 (39.5%) of the cases. Of the 43 included cases, 10 (23.3%) were luminal A-like, 22 (51.2%) were luminal B-like, and 2 (4.6%) were triple negative, while 9 (20.9%) were HER2-overexpressing. The number of macrometastases in the ipsilateral lymph nodes ranged from 2 to 23. In total, 206 macrometastases and 43 primary tumors were analyzed for HER2 status. Details of the clinico-pathological parameters in this cohort are shown in Table 1.
3.1 HER2 status according to the 2018 ASCO/CAP criteria
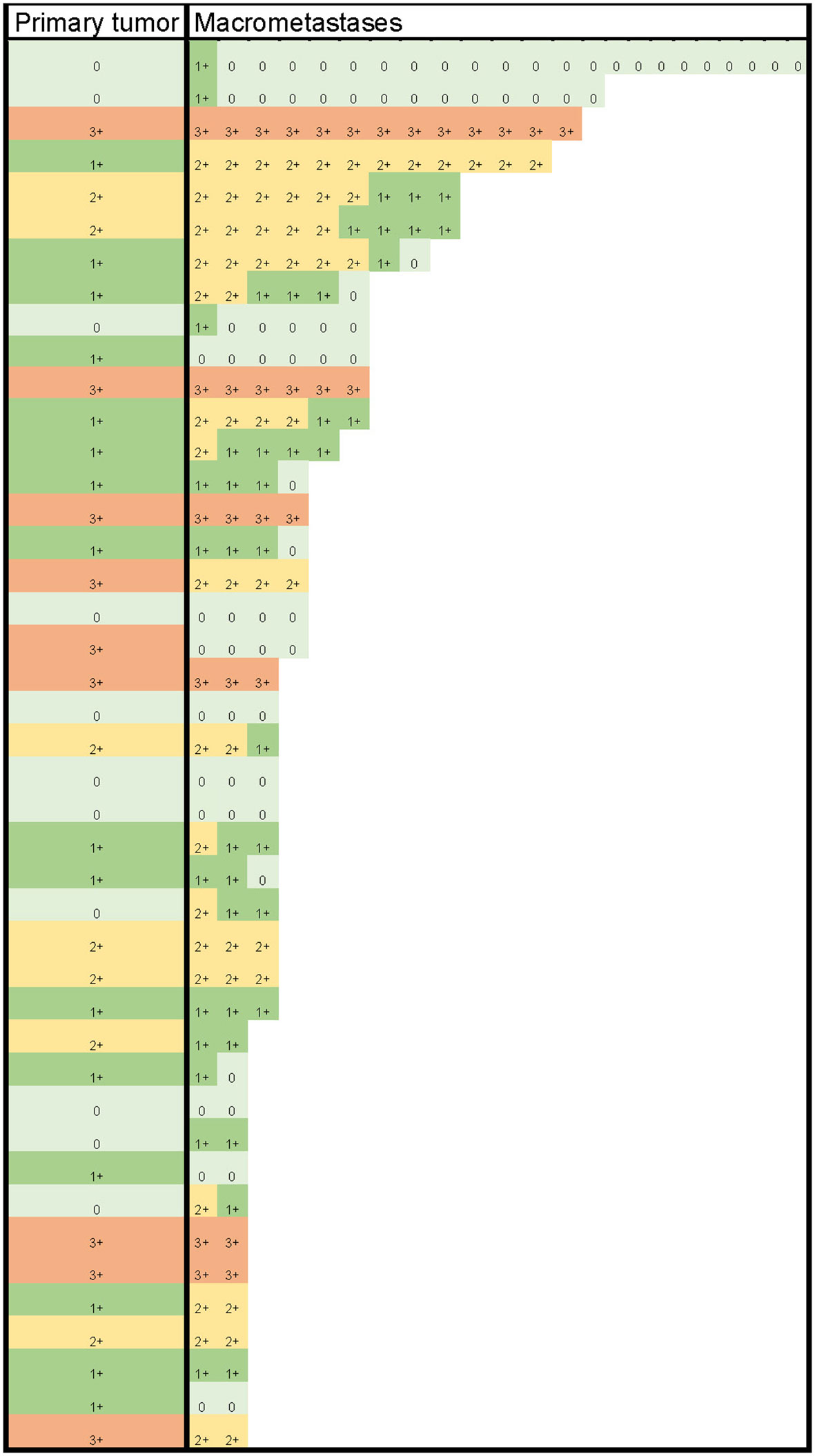
HER2 status (negative/positive) was determined based on HER2 protein levels, HER2/CEP17 ratio, and average HER2 copy number, according to the 2018 ASCO/CAP guidelines (21). None of the 34 cases with a primary tumor diagnosed as HER2-negative displayed HER2-positive macrometastases. Of the nine cases with HER2-positive primary tumors, three (33.3%) had HER2-negative macrometastases, with no signs of internodal heterogeneity among the positive lymph nodes. The nine cases with HER2-positive primary tumors collectively had 30 (75.0%) HER2-positive and 10 (25.0%) HER2-negative macrometastases. The details from the comparison of HER2 levels in primary tumors with those in macrometastases are presented in Table 2 and Figure 1.
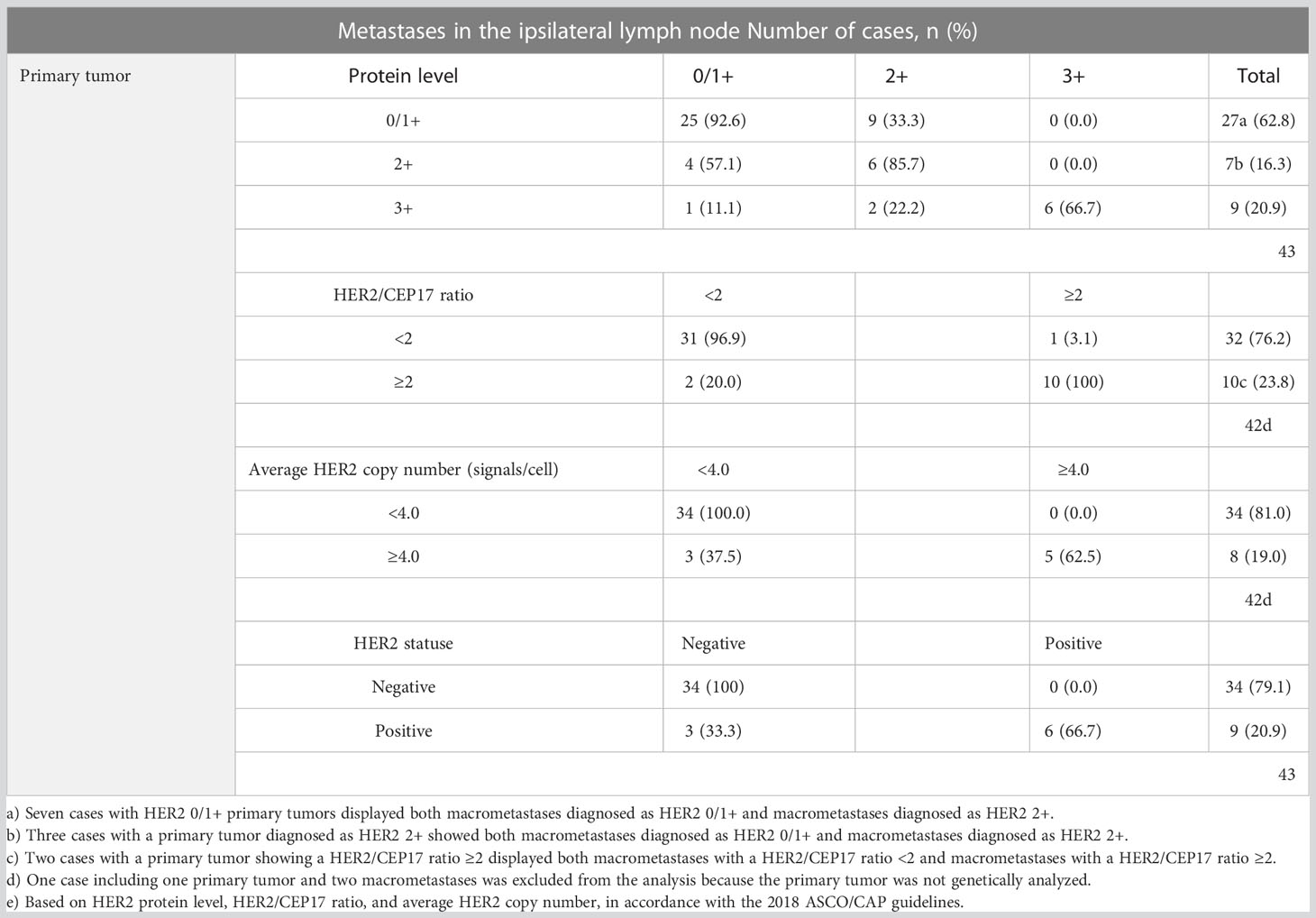
Table 2 HER2 status of primary tumors compared to HER2 status in macrometastases in ipsilateral lymph nodes.
3.2 Intertumoral and internodal heterogeneity in HER2-low tumors
Of the cases with HER2-negative (HER2 0) primary tumors, 55% (6/11) had macrometastases with HER2 1+ and/or 2+ protein expression levels in at least one positive lymph node, qualifying the metastases as being HER2-low. In 19% (3/16) of cases, HER2 1+ primary tumors lost their protein expression, becoming HER2 0 in all of their metastatic deposits. All seven HER2 2+ primary tumors had HER2-low lymph node deposits. Internodal HER2 heterogeneity was found at the protein level in 40% (17/43) of cases, as follows: in seven cases with both HER2 0 and HER2 1+ metastases, in eight cases with both HER2 1+ and 2+, and in two cases with HER2 0, 1+, and 2+ metastases within the same axilla. None of the axillae simultaneously contained HER2-low and HER2 3+ tumor deposits. The details of the comparisons of HER2 in macrometastases to HER2 in the primary tumor are shown in Table 3 and Figure 1.
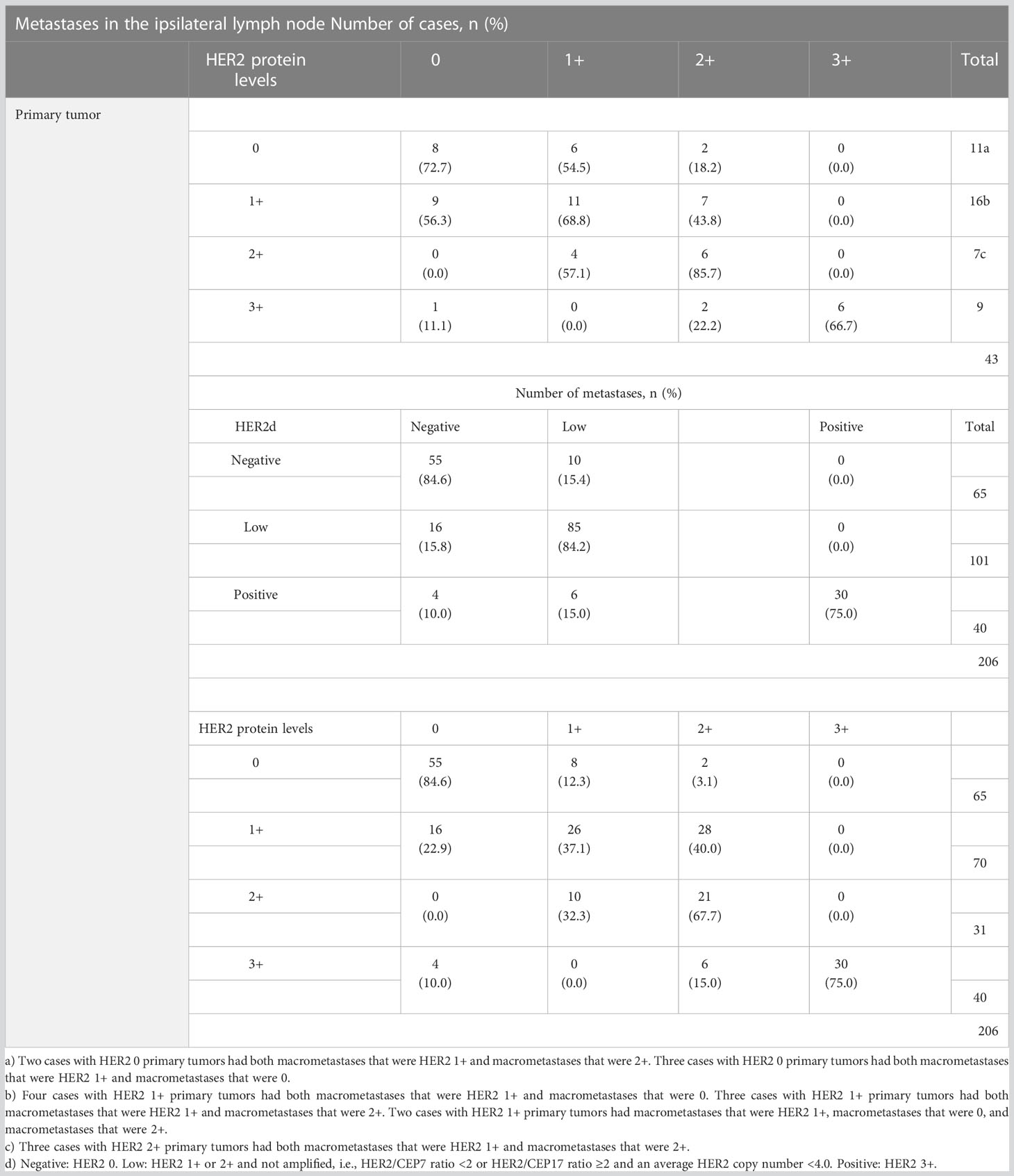
Table 3 Results from comparing HER2 levels in primary tumors and macrometastases with regard to HER2-low.
3.3 Breast cancer–specific survival data
Of the 43 patients represented in this work, 10 died of breast cancer during the observation period up to 13 October 2021, half of them within 5 years after diagnosis. The survival time of the 10 patients who died varied from 1202 to 3626 days (mean, 2196 days). No statistically significant differences were found between surviving group and the group that died of the disease when comparing their ER and PR status, tumor size, pathological T-stage and N-stage and number of metastatic lymph nodes (Supplementary Table S1). The average number of positive nodes among the fatal cases was 13.6 (41/3) in the HER2 0 group, 3.7 (60/16) in the HER2 1+ group, and 4.4 (31/7) in the HER2 2+ group. None of the patients included in this studies had distant metastases at the time of diagnosis. None of the patients with HER2-positive primary tumors died of the disease during this period. Three of eleven patients with HER2-negative primary tumors (HER2 0) had a fatal outcome, as did 7 of 23 patients with tumors showing low HER2 protein expression levels (4/16 HER2 1+, 3/7 HER2 2+). Two of the three HER2 0 patients with a fatal outcome had a HER2 1+ lymph node deposit in one of their metastases, so that all but one of the fatal cases involved HER2-low protein expression levels in the primary or metastatic tumors or both.
4 Discussion
In the present study 37% (16/43) of the cases had at least one metastasis with HER2 protein levels diverse from those in the primary tumor. This finding is in line with results of previously published studies. A review of similar studies comparing HER2 protein expression in primary tumors and metastases described discordances of up to 33.2% (25). Other studies, however, have shown a high concordance of HER2 status between primary tumor and metastases, with discrepancy rates as low as 2% (26) and 3.4% (4). Analysis of HER2 status at the gene level in our study showed markedly higher concordance than results at the protein level, with only 7% (3/43) of cases (i.e., 3 HER2-positive tumors) losing their HER2-positive status in metastases. Cho et al. reported similar findings, reporting a discordance of 18% in protein expression levels and 6% with chromogenic ISH when comparing the HER2 status of primary breast cancer and paired metastatic lymph nodes (27). The same tendency was seen in the study showing a 3.4% rate of discordant protein expression but concordant gene amplification status in all cases (4). Another study using FISH demonstrated concordant HER2 amplification status between the primary tumor and synchronous axillary metastases in all analyzed cases (3).
The HER2-low tumor category is a newly defined group of breast carcinomas that express low levels of HER2 protein in the membrane of the tumor cells up to HER2 2+ level but show no amplification of the HER2 gene (28, 29). These tumors may partially respond to modern anti-HER2 treatment, and their delineation from HER2-negative breast carcinomas is essential (12) because these patients may potentially benefit from anti-HER2 targeted therapy. However, delineating HER2 1+ carcinomas from HER2 0 tumors is a real challenge in everyday practice, and intratumoral heterogeneity, intraobserver and interobserver variations, and technical issues represent the main obstacles (29). To avoid these potential technical pitfalls, we reassessed the HER2 slides of the primary tumors and stained the sections from the metastases in batches in the same instrument, using identical protocols and reagent kits. By analyzing 120 cells (i.e., three times the recommended 40 cells), we tried to eliminate the influence of intratumoral heterogeneity. Analysis was done by two experienced pathologists unaware of the patient’s clinical status. The GPA method is particularly useful in assessing the HER2 status of heterogeneous tumors (20) and in our case, also in assessing HER2-low status.
As noted, HER2 protein expression levels may vary in a considerable proportion of breast carcinomas, and HER2-low tumors, defined by their protein expression levels, are known for their intratumoral heterogeneity (11). In addition, HER2-low heterogeneity is evident in a high proportion of multifocal cancers, between the multiple simultaneous invasive tumors within the same breast (11). Our study also demonstrated a substantial intertumoral heterogeneity in HER2-low tumors, with discrepancies in HER2 protein expression levels between the primary tumor and its metastases in a third of HER2-negative primary tumor cases (HER2 0, 1+, and 2+, non-amplified). We found that 55% (6/11) of HER2 0 tumors gained HER2-low status (HER2 1+ and/or 2+) in at least one lymph node metastasis and 19% (3/16) of HER2-low (HER2 1+) cases lost protein expression, showing HER2 0 expression in all metastases. All HER2 2+ primary tumors had HER2-low lymph node deposits.
Discordance in HER2 status between two or more metastatic foci has been more sparsely studied. Gancberg et al. reported an 18% discordance in different metastatic sites (30). We analyzed cases with two or more macrometastases in the ipsilateral lymph nodes and showed that lymph node metastases displaying diverse HER2 protein levels were common, seen in 40% of our HER2-low cases.
We found no statistically significant association between HER2-low tumor status and age, histological tumor grade, molecular tumor phenotype, tumor size, disease extent, or lesion distribution. In contrast, Baez-Navarro et al. found in a much larger cohort that HER2-low tumors were significantly associated with histologic subtype, a higher ER, and lower PR expression in their ER+ cohort, whereas within the ER- cohort, HER2-low tumors were associated with a lower tumor grade (11).
HER2-low tumors seem to carry a poorer prognosis compared with HER2-negative cancers (14–16). Our results also indicate that HER2-low status impacts survival, with 9 of 10 fatal outcomes in this study occurring in the group showing HER2-low primary tumors, HER2-low metastases, or both. The patients with fatal HER2-negative (HER2 0) primary tumors had a much larger metastatic tumor burden than those with fatal HER2-low tumors, which may explain the fatal outcome in HER2 0 cases. No HER2-positive patients died of the disease in our study, which may be related to efficient targeted therapy. The large number of fatal HER2-low cases may be attributable to the fact that these patients, in accordance with international and national recommendations during the studied period, did not receive anti-HER2 therapy. A recent large retrospective study with a limited follow-up time showed no evidence of significant differences in overall survival associated with HER2-low and HER2-0 tumors (11).
Weaknesses of this study include its retrospective character and the limited number of patients included after application of rigorous selection criteria. These limitations preclude conclusions regarding potential differences between the clinicopathological parameters in the HER2 subgroups.
5 Conclusions
Our results demonstrate a substantial instability of HER2 protein expression leading to considerable intratumoral and internodal HER2 heterogeneity in lymph node–positive breast carcinomas. This finding is particularly relevant for HER2-low tumors which definitionally lack the corrective effects of HER2 gene copy number analysis. Although the clinical impact of these findings remains unclear and warrants larger studies, our results suggest that determining HER2 status in metastatic lymph nodes may generate relevant information for therapeutic decision-making.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study involving human participants was reviewed and approved by The Ethical Committee of the Uppsala–Örebro Region in Sweden, EPN No 2010/461, and the Swedish Ethical Review Authority, No 2020-00310 and 2019-01739. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: UP and TT; Methodology: UP, TT, and AB; Material preparation and data collection: UP, CF, IB, AB, and TT; Formal analysis and investigation: AB, CF, IB, and UP; Writing - original draft preparation: UP and TT; Writing - review and editing: all authors; Acquisition of funding: UP and TT. All authors contributed to the article and approved the submitted version.
Funding
The study was supported by grants to UP and to TT from the Centre for Clinical Research Dalarna, Uppsala University, Falun, Sweden. Grant numbers 9712 and 9761.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1167567/full#supplementary-material
References
1. Lüönd F, Tiede S, Christofori G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br J Cancer. (2021) 125(2):164–75. doi: 10.1038/s41416-021-01328-7
2. Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist (2009) 14(4):320–68. doi: 10.1634/theoncologist.2008-0230
3. Norton N, Advani PP, Serie DJ, Geiger XJ, Necela BM, Axenfeld BC, et al. Assessment of tumor heterogeneity, as evidenced by gene expression profiles, pathway activation, and gene copy number, in patients with multifocal invasive lobular breast tumors. PloS One (2016) 11(4):e0153411. doi: 10.1371/journal.pone.0153411
4. Ataseven B, Gologan D, Gunesch A, Kehl V, Hoegel B, Beer M, et al. HER2/neu, topoisomerase 2a, estrogen and progesterone receptors: discordance between primary breast cancer and metastatic axillary lymph node in expression and amplification characteristics. Breast Care (Basel). (2012) 7(6):465–70. doi: 10.1159/000345467
5. Pusztai L, Viale G, Kelly CM, Hudis CA. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. Oncologist (2010) 15(11):1164–8. doi: 10.1634/theoncologist.2010-0059
6. Shen T, Nitta H, Wei L, Parwani AV, Li Z. HER2 intratumoral heterogeneity is independently associated with distal metastasis and overall survival in HER2-positive breast carcinomas. Breast Cancer Res Treat (2020) 181(3):519–27. doi: 10.1007/s10549-020-05650-1
7. Shafi H, Astvatsaturyan K, Chung F, Mirocha J, Schmidt M, Bose S. Clinicopathological significance of HER2/neu genetic heterogeneity in HER2/neu non-amplified invasive breast carcinomas and its concurrent axillary metastasis. J Clin Pathol (2013) 66(8):64954. doi: 10.1136/jclinpath-2012-201403
8. Song H, Kim TO, Ma SY, Park JH, Choi JH, Kim JH, et al. Intratumoral heterogeneity impacts the response to anti-neu antibody therapy. BMC Cancer. (2014) 14:647. doi: 10.1186/1471-2407-14-647
9. Zhou X, Zhang J, Yun H, Shi R, Wang Y, Wang W, et al. Alterations of biomarker profiles after neoadjuvant chemotherapy in breast cancer: tumor heterogeneity should be taken into consideration. Oncotarget (2015) 6(34):36894–902. doi: 10.18632/oncotarget.5050
10. Hosonaga M, Arima Y, Sampetrean O, Komura D, Koya I, Sasaki T, et al. HER2 heterogeneity is associated with poor survival in HER2-positive breast cancer. Int J Mol Sci (2018) 19(8). doi: 10.3390/ijms19082158
11. Baez-Navarro X, van Bockstal MR, Andrinopoulou E-R, van Deurzen CHM. HER2-low breast cancer: incidence, clinicopathologic features and survival outcomes from real-world data of a large nationwide cohort. Modern Pathol (2023) 100087. doi: 10.1016/j.modpat.2022.100087
12. Baroš IV, Tanasković N, Pellas U, Eri Ž, Tadić Latinović L, Tot T. Internodal HER2 heterogeneity of axillary lymph node metastases in breast cancer patients. Bosn J Basic Med Sci (2019) 19(3):242–8. doi: 10.17305/bjbms.2019.3970
13. Banerji U, van Herpen CML, Saura C, Thistlethwaite F, Lord S, Moreno V, et al. Trastuzumab duocarmazine in locally advanced and metastatic solid tumours and HER2-expressing breast cancer: a phase 1 dose-escalation and dose-expansion study. Lancet Oncol (2019) 20(8):1124–35. doi: 10.1016/S1470-2045(19)30328-6
14. Rossi V, Sarotto I, Maggiorotto F, Berchialla P, Kubatzki F, Tomasi N, et al. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist (2012) 17(11):1418–25. doi: 10.1634/theoncologist.2012-0194
15. Eggemann H, Ignatov T, Burger E, Kantelhardt EJ, Fettke F, Thomssen C, et al. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer. (2015) 22(5):725–33. doi: 10.1530/ERC-15-0335
16. Ménard S, Balsari A, Tagliabue E, Camerini T, Casalini P, Bufalino R, et al. Biology, prognosis and response to therapy of breast carcinomas according to HER2 score. Ann Oncol (2008) 19(10):170612. doi: 10.1093/annonc/mdn369
17. Sapino A, Goia M, Recupero D, Marchiò C. Current challenges for HER2 testing in diagnostic pathology: state of the art and controversial issues. Front Oncol (2013) 3:129. doi: 10.3389/fonc.2013.00129
18. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin (2017) 67(2):93–9. doi: 10.3322/caac.21388
20. Nitta H, Kelly BD, Padilla M, Wick N, Brunhoeber P, Bai I, et al. A gene-protein assay for human epidermal growth factor receptor 2 (HER2): brightfield tricolor visualization of HER2 protein, the HER2 gene, and chromosome 17 centromere (CEN17) in formalin-fixed, paraffin-embedded breast cancer tissue sections. Diagn Pathol (2012) 7:60. doi: 10.1186/1746-1596-7-60
21. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical Oncology/College of American pathologists clinical practice guideline focused update. J Clin Oncol (2018) 36(20):2105–22. doi: 10.1200/JCO.2018.77.8738
22. Jamovi. The jamovi project (2021). version 2. 2nd ed. Sydney, Australia: jamovi (2021). Available at: https://www.jamovi.org.
23. Team RC. R: a language and environment for statistical computing. version 4.0 . Available at: https://cran.r-project.org.
24. (RCC) RCC. Bröstcancer. nationellt vårdprogram. Available at: https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/var dprogram/2022.
25. Arslan C, Sari E, Aksoy S, Altundag K. Variation in hormone receptor and HER-2 status between primary and metastatic breast cancer: review of the literature. Expert Opin Ther Targets. (2011) 15(1):21–30. doi: 10.1517/14656566.2011.537260
26. Cardoso F, Di Leo A, Larsimont D, Gancberg D, Rouas G, Dolci S, et al. Evaluation of HER2, p53, bcl-2, topoisomerase II-alpha, heat shock proteins 27 and 70 in primary breast cancer and metastatic ipsilateral axillary lymph nodes. Ann Oncol (2001) 12(5):615–20. doi: 10.1023/A:1011182524684
27. Cho EY, Han JJ, Choi YL, Kim KM, Oh YL. Comparison of Her2, EGFR and cyclin D1 in primary breast cancer and paired metastatic lymph nodes: an immunohistochemical and chromogenic in situ hybridization study. J Korean Med Sci (2008) 23(6):1053–61. doi: 10.3346/jkms.2008.23.6.1053
28. Marchiò C, Annaratone L, Marques A, Casorzo L, Berrino E, Sapino A. Evolving concepts in HER2 evaluation in breast cancer: heterogeneity, HER2-low carcinomas and beyond. Semin Cancer Biol (2021) 72:123–35. doi: 10.1016/j.semcancer.2020.02.016
29. Baez-Navarro X, Salgado R, Denkert C, Lennerz JK, PenaultLlorca F, Viale G, et al. Selecting patients with HER2-low breast cancer: getting out of the tangle. Eur J Cancer. (2022) 175:187–92. doi: 10.1016/j.ejca.2022.08.022
Keywords: breast cancer, human epidermal growth factor receptor 2, HER2, heterogeneity, HER2-low, lymph node, metastasis, Gene-Protein Assay (GPA)
Citation: Pellas U, Bauer A, Baroš IV, Fattorini C and Tot T (2023) HER2-low metastases of HER2-negative primary tumors: a single institution analysis of intertumoral and internodal heterogeneity in node-positive breast cancer. Front. Oncol. 13:1167567. doi: 10.3389/fonc.2023.1167567
Received: 16 February 2023; Accepted: 19 June 2023;
Published: 07 July 2023.
Edited by:
Charles Theillet, Institut du Cancer de Montpellier (ICM), FranceReviewed by:
Marianna Giampaglia, Ospedale San Carlo, ItalySungchan Gwark, Ewha Womans University, Republic of Korea
Copyright © 2023 Pellas, Bauer, Baroš, Fattorini and Tot. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ulrika Pellas, VWxyaWthLlBlbGxhc0ByZWdpb25kYWxhcm5hLnNl
†ORCID: Ulrika Pellas, orcid.org/0000-0002-5690-5293
Caterina Fattorini, orcid.org/0000-0002-2377-0169
 Ulrika Pellas
Ulrika Pellas Annette Bauer2
Annette Bauer2 Ilija Vladimir Baroš
Ilija Vladimir Baroš