
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 21 June 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1166035
Background: This study evaluates the diagnostic accuracy of ultrasound-guided fine needle aspiration (US-FNA) and core needle biopsy (US-CNB) for detecting axillary lymph nodes in women with breast cancer.
Methods: Eligible studies and pertinent literature resources were identified in Cochrane, PubMed, Embase, CNKI, VIP, and Wanfang databases using subject-specific keywords. Study outcomes were tested for heterogeneity, and meta-analyses were performed to estimate sensitivity, specificity, and diagnostic odds ratios (DORs). The summary receiver operating characteristic (SROC) curve analysis was also performed.
Results: A total of 22 studies involving 3,548 patients were included to evaluate the diagnostic accuracy of US-FNA and 11 studies involving 758 patients were included to evaluate the diagnostic accuracy of US-CNB in identifying axillary lymph nodes in women with breast cancer. The accuracy of US-FNA in identifying suspicious axillary lymph nodes was as follows: overall sensitivity, 79% (95% CI: 73%–84%); global specificity, 96% (95% CI: 92%–98%); overall positive likelihood ratio, 18.55 (95% CI: 10.53–32.69); overall negative likelihood ratio, 0.22 (95% CI: 0.17–0.28); DOR, 71.68 (95% CI: 37.19–138.12); and the area under the SROC curve, 0.94 (95% CI: 0.92–0.96). The accuracy of US-CNB in identifying suspicious axillary lymph nodes was as follows: overall sensitivity, 85% (95% CI: 81%–89%); global specificity, 93% (95% CI: 87%–96%); overall positive likelihood ratio, 11.88 (95% CI: 6.56–21.50); overall negative likelihood ratio, 0.16 (95% CI: 0.12–0.21); overall DOR, 66.83 (95% CI: 33.28–134.21), and the area under SROC curve 0.96 (95% CI: 0.94–0.97).
Conclusions: The results indicate that both US-FNA and US-CNB have high accuracy for suspicious axillary lymph nodes.
Axillary lymph node (ALN) metastasis is an important factor in the clinical evaluation of the prognosis of breast cancer. Conventional ALN dissection (ALND) plays a critical role in the staging of breast cancer, but it is associated with serious postoperative complications that affect postoperative recovery. Sentinel lymph node biopsy (SLNB) is also used for the assessment of the ALN stage and the formulation of planning of treatment. However, SLNB requires accurate preoperative positioning and pathological diagnosis results, which is also prone to false-negative results. Therefore, developing a simple and effective diagnostic method is of pivotal importance. Imaging examination as a non-invasive examination method is frequently applied in clinical diagnosis and treatment and can help to effectively diagnose ALN and avoid unnecessary SLNB (1–4).
Ultrasound is a commonly used imaging method to evaluate the properties of ALN. With its real-time dynamics, simple operation, and non-invasiveness, ultrasound can explore ALN from multiple angles and directions. In recent years, ultrasound-guided needle biopsy, contrast-enhanced ultrasound, and elastography have enriched the ultrasonic diagnosis of ALN. Compared with conventional ultrasound, ultrasound-guided percutaneous biopsy results are more accurate and effective in the diagnosis of ALN metastasis (5–8). By identifying relevant studies from scientific literature, the present study aimed to evaluate the accuracy of ultrasound-guided fine needle aspiration (US-FNA) and core needle biopsy (US-CNB) in detecting suspicious ALNs in women with breast cancer.
All the eligible studies analyzing the diagnostic accuracy of US-FNA and US-CNB in detecting ALNs in women with breast cancer were searched in Cochrane, PubMed, Embase, CNKI, VIP, and Wanfang databases. Other related correlational studies or referenced data were also retrieved. Two researchers independently retrieved the articles, and a third researcher was involved to resolve any disagreements.
The criteria for study inclusion were as follows: (1) cohort or cross-sectional research design; (2) evaluated the diagnostic accuracy of suspicious ALNs in women with breast cancer; (3) suspicious ALNs were diagnosed using US-FNA and/or US-CNB; (4) reported true positive (TP), false positive (FP), false negative (FN), and true negative (TN) data; and (5) publication language was English or Chinese. The criteria for exclusion were as follows: (1) duplicate articles, or articles with the same results; (2) case reports, theoretical studies, conference presentations, review of literature, meta-analysis, expert commentary, or analyses; (3) research articles without results relevant to this study; and (4) without clinical outcomes of TP, FP, FN, or TN. Two researchers decided whether the article was to be included with a third researcher helping to resolve any disagreements.
Extraction of data was performed independently by two researchers with the help of a third researcher who was involved to resolve any disagreement. For the clinical outcomes, 2 × 2 diagnostic table (TP, FP, FN, and TN) data were sought for each of the included articles. Sensitivity, specificity, and likelihood ratios were computed. Accuracy of US-FNA and US-CNB in diagnosis was measured with the diagnostic odds ratio (DOR). When DOR equaled one, it suggested no distinguishing ability, whereas a higher value indicated a higher correlation of the evaluated diagnostic tool.
Stata 10.0 (TX, USA) was used for all statistical analyses. Statistical heterogeneity was determined with the chi-squared test and I2 values. If the p-value of the chi-squared test was equal to or lower than 0.05 and I2 was higher than 50%, the random-effects model was chosen for meta-analysis; otherwise, the fixed-effects model was used. Based on the correlation analysis (Spearman’s) between the logarithm of sensitivity and the logarithm of [1 − specificity], the presence or absence of the effect of threshold was checked to further investigate the heterogeneity. In the presence of the threshold effect, there should be a negative correlation between the sensitivity and specificity (or a positive correlation between sensitivity and [1 − specificity]). A strong positive correlation between sensitivity and [1 − specificity] indicates the effect of the threshold. The summary receiver operating characteristic (SROC) curve was analyzed when the heterogeneity was caused by the effect of the threshold. The overestimated overall values of sensitivity and specificity were evaluated by this method. The publication bias was assessed using Deeks’ Funnel Asymmetry Plot.
A total of 511 publications were identified by searching keywords. After initial screening, 427 publications were excluded after reviewing the title or abstract, and 84 publications were subjected to further assessment. Fifty-nine publications failed to meet the inclusion criteria because they were theoretical research (6), reports without clinical outcomes (9), and studies without comparative diagnostic methods (10). Finally, 25 studies (9–34) with 4,354 patients were included in this meta-analysis. The flow path is shown in Figure 1. Important characteristics of these studies are given in Table 1.
A total of 22 studies with 3,548 patients were included to estimate the diagnostic accuracy of US-FNA in identifying ALNs in women with breast cancer. The random-effects model was selected for meta-analysis based on the presence of high heterogeneity (p < 0.001, I2 = 59.2%). The correlation result (Spearman’s R = −0.2331, p = 0.2965) between the logarithm of sensitivity and the logarithm of [1 − specificity] indicated that there was no effect of the threshold.
The global sensitivity was 79% (95% CI: 73%–84%), and the global specificity was 96% (95% CI: 92%–98%). The overall positive and negative likelihood ratios were 18.55 (95% CI: 10.53–32.69) and 0.22 (95% CI: 0.17–0.28), respectively, which suggested that US-FNA increased 18.6-fold the odds of the diagnosis of suspicious ALNs and decreased 0.22-fold the odds of the false-positive results. The overall DOR was 71.68 (95% CI: 37.19–138.12), which showed that the odds were 71.7-fold higher for the positive result among positive ALNs when compared with negative ALNs. The area under the SROC curve was 0.94 (95% CI: 0.92–0.96). All the data are shown in Figures 2–5.
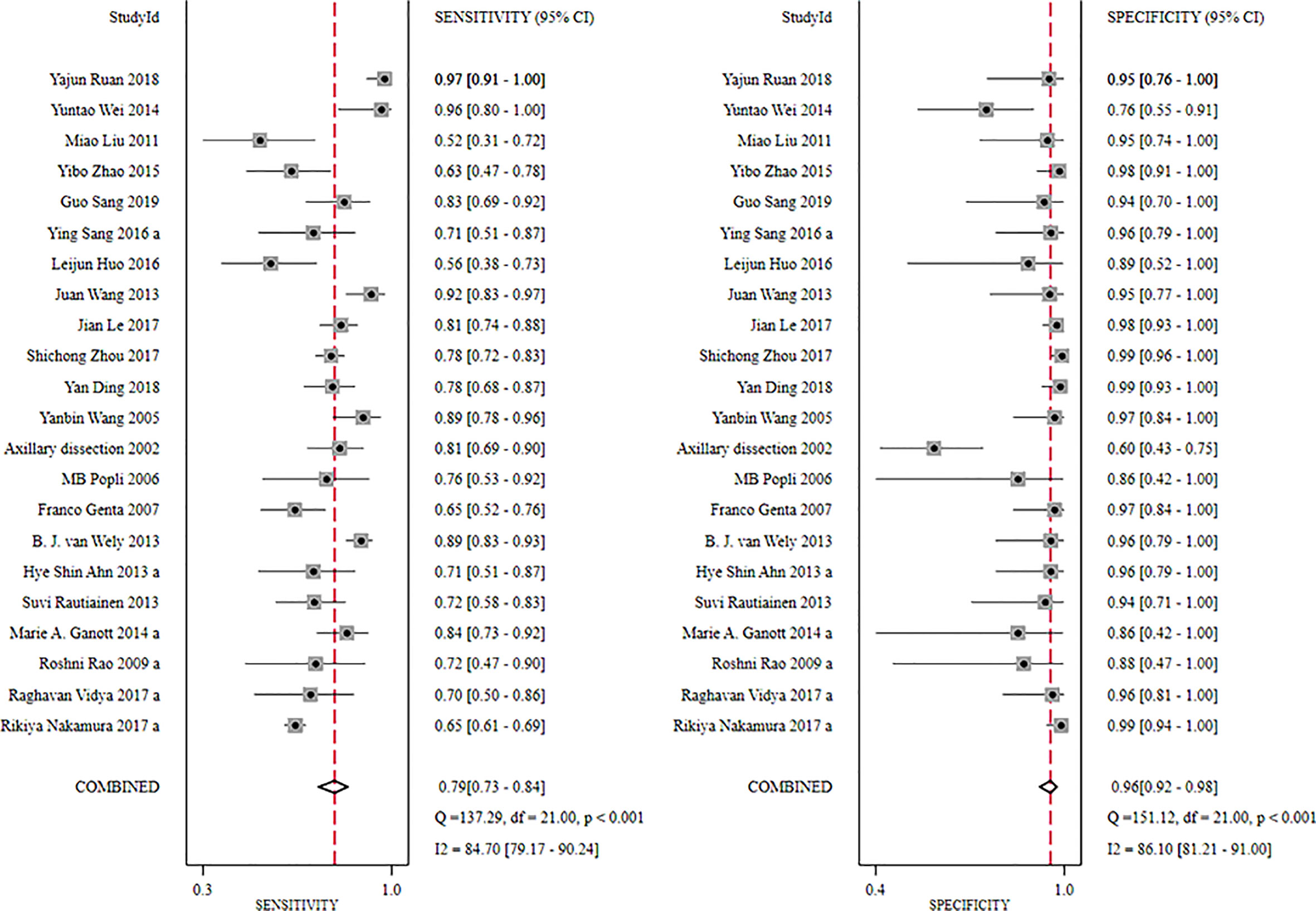
Figure 2 Forest plot showing the sensitivity and specificity values of US-FNA for suspicious axillary lymph nodes.
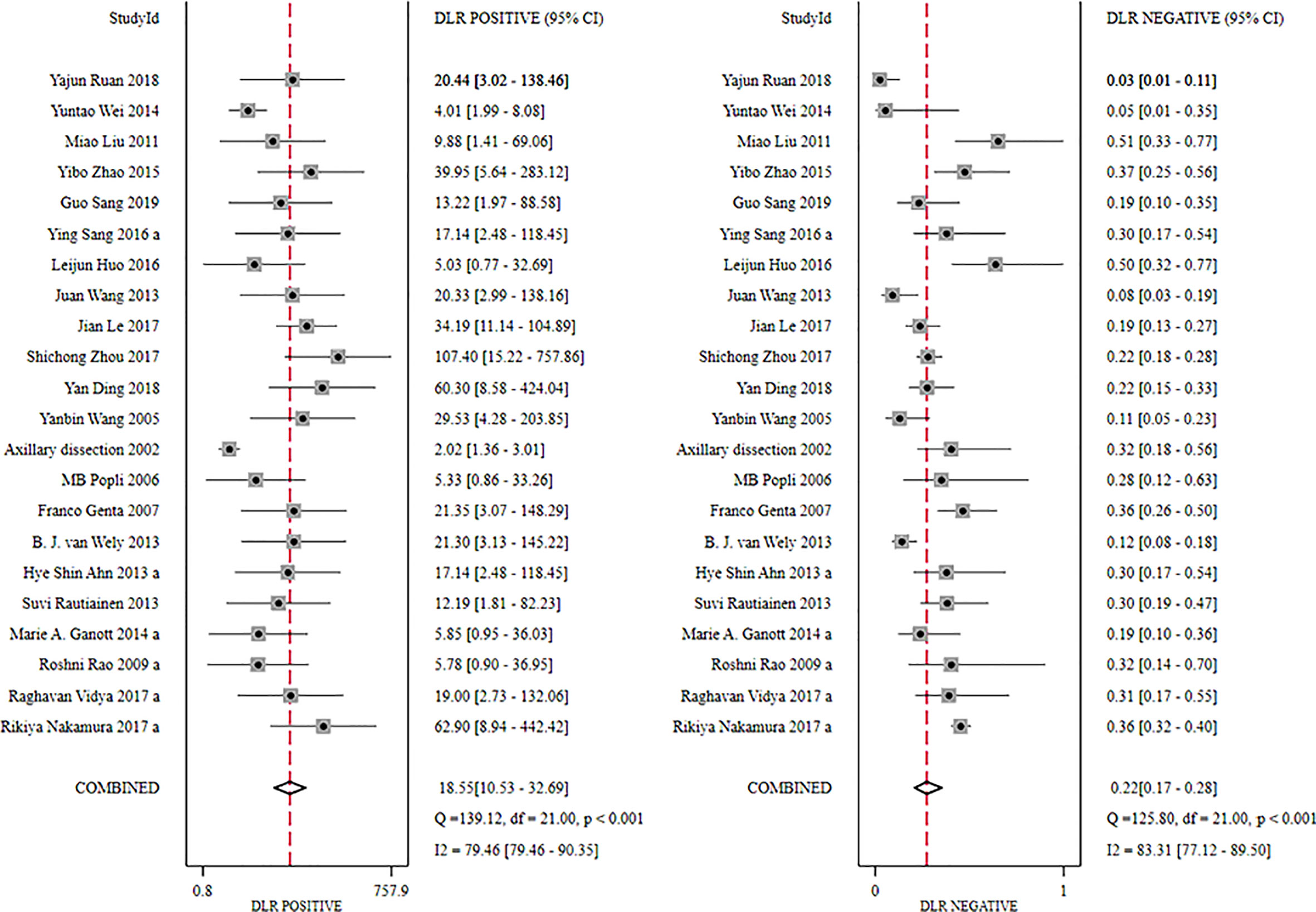
Figure 3 Forest plot showing the positive and negative likelihood ratio of US-FNA for suspicious axillary lymph nodes.
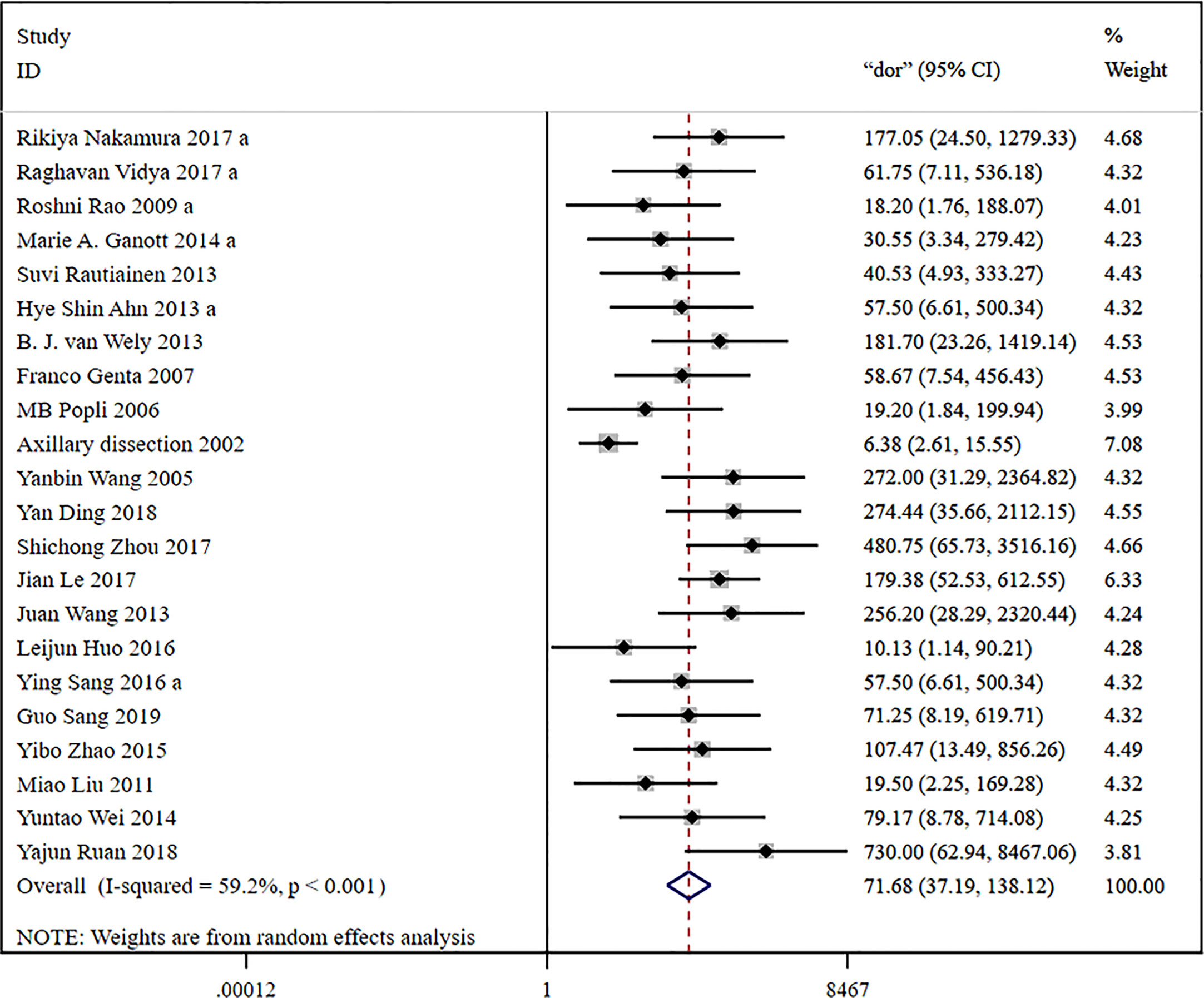
Figure 4 Forest plot showing the diagnostic odds ratio of US-FNA for suspicious axillary lymph nodes.
The Deek’s funnel plot for DOR of US-FNA was asymmetric, indicating a significant publication bias (Figure 6, p = 0.002). The funnel plot revealed an apparent asymmetry, which suggested the presence of potential publication bias and inflated estimates due to methodological design flaws in small studies, and/or lack of publication of small trials with non-robust results.
A total of 11 studies with 758 patients were included to estimate the accuracy of US-CNB in diagnosing ALNs in women with breast cancer. The fixed-effects model was selected for meta-analysis due to the absence of heterogeneity (p = 0.985, I2 = 0.0%). The correlation result (Spearman’s R = −0.7963, p = 0.0034) between the logarithm of sensitivity and the logarithm of specificity indicated the presence of the effect of the threshold.
The global sensitivity was 85% (95% CI: 81%–89%), and the global specificity was 93% (95% CI: 87%–96%). The overall positive and negative likelihood ratios were 11.88 (95% CI: 6.56–21.50) and 0.16 (95% CI: 0.12–0.21), respectively. Thus, US-FNA increased 11.9-fold the odds of the diagnosis of suspicious ALNs and decreased 0.16-fold the odds of the false-positive result. The overall DOR was 66.83 (95% CI: 33.28–134.21), showing that the odds were 66.8-fold higher for the positive US-FNA result among positive ALNs when compared with negative ALNs. The area under the SROC curve was 0.96 (95% CI: 0.94–0.97), which indicated that the combined diagnosis was effective. All the data are shown in Figures 7–10.

Figure 7 Forest plot showing the sensitivity and specificity values of US-CNB for suspicious axillary lymph nodes.
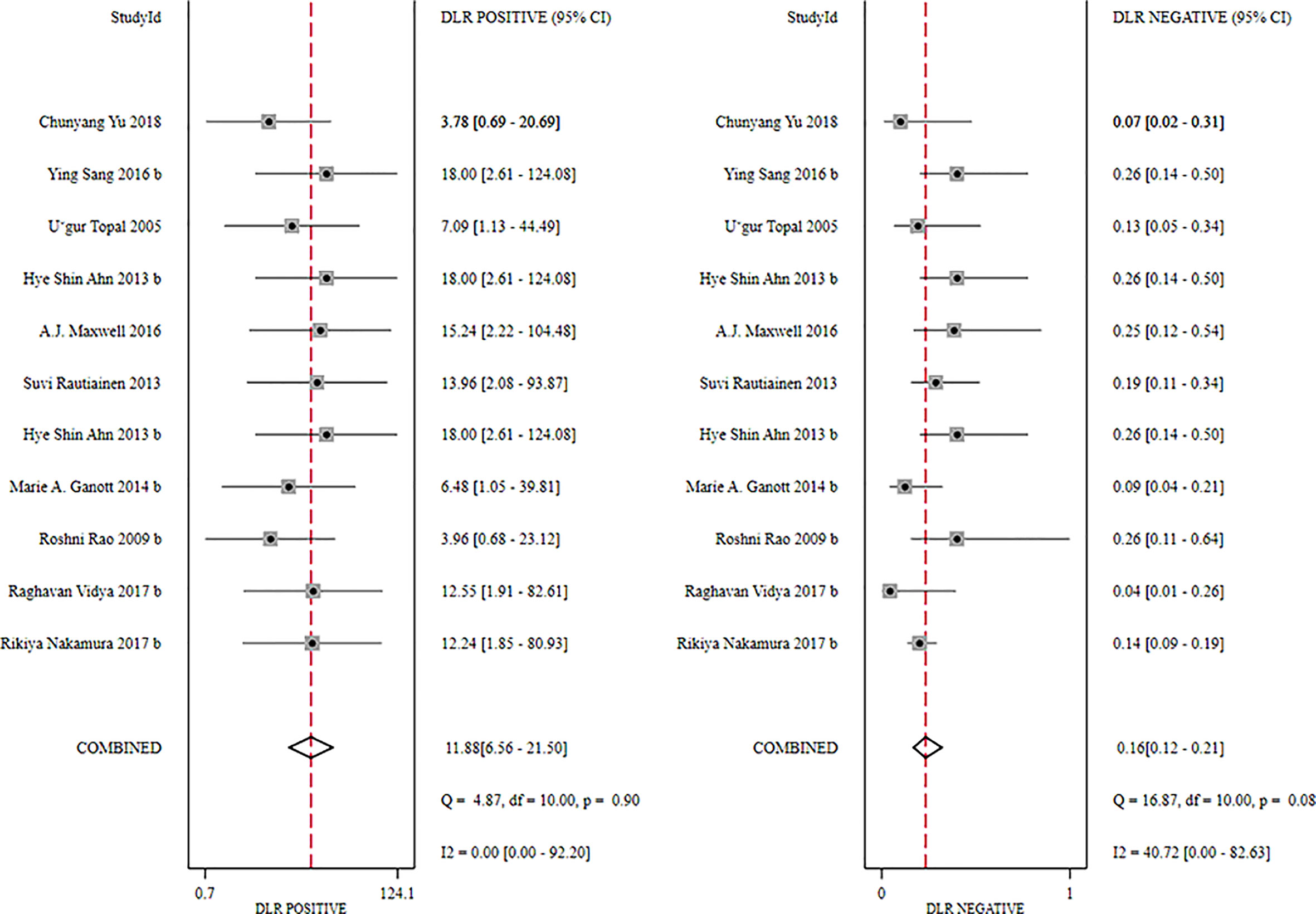
Figure 8 Forest plot showing the positive and negative likelihood ratios of US-CNB for suspicious axillary lymph nodes.
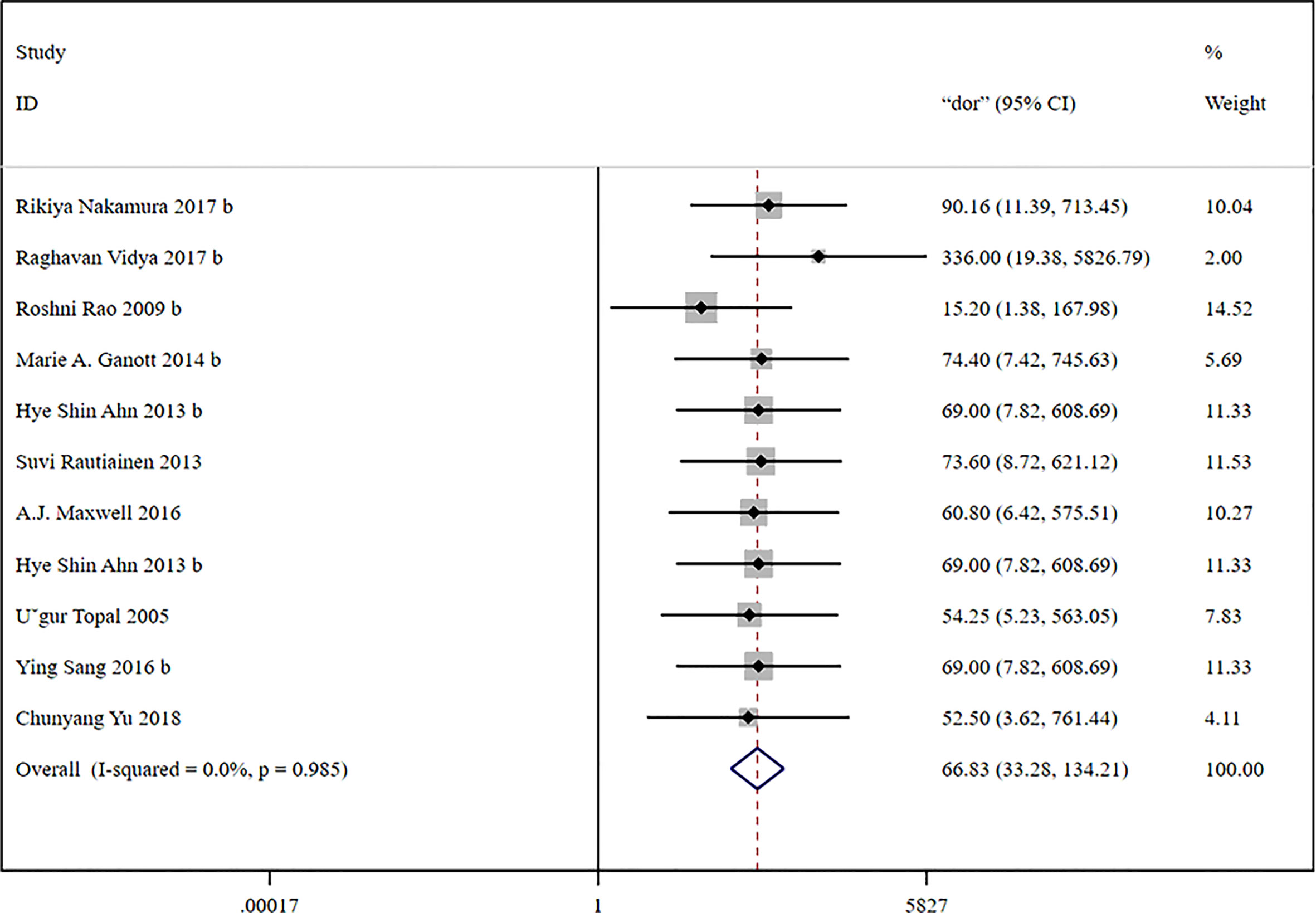
Figure 9 Forest plot showing the diagnostic odds ratio of US-CNB for suspicious axillary lymph nodes.
The Deek’s funnel graph for DOR of US-CNB was symmetric, indicating no significant bias of publication (Figure 11, p = 0.31).
This meta-analysis found that both US-FNA and US-CNB have high diagnostic accuracy in detecting ALN metastasis in patients with breast cancer. For detecting ALN metastasis, the sensitivity and specificity of US-FNA were 79% and 96%, whereas the sensitivity and specificity of US-CNB were 85% and 93%, respectively. The area under the SROC curve was 0.94 for US-FNA and 0.96 for US-CNB.
In the early stages of ALN metastasis of breast cancer, tumorous cells are first implanted in the marginal lymph node sinus by lymphatic infusion and then spread into the medullary sinus. At a later stage, the lymph node is completely occupied by the cancerous cells, and the cancer continues to develop. Cancer cells break through the capsule and adhere to the surrounding tissues, accompanied by the proliferation of the surrounding interstitial fibrous tissues, resulting in poor mobility of the lymph nodes, increase in stiffness, and less deformation by compression leading to an enlarged blue range in the elastogram.
Clinical palpation (PE), mammography (MMG), ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), SLNB, FNA, and CNB are used to determine ALN status. PE examines superficial lymph nodes, but it is not meant for detecting metastasis as it has been found to be associated with considerably high false positives or false negatives and the reported sensitivity is 30% in detecting ALN metastasis in women with breast cancer (35). MMG can only detect the anterior ALNs and cannot completely cover the entire ALN area. MMG has a limited ability in distinguishing between benign tumors and malignancies in lymph nodes. In detecting metastasis in lymph nodes in patients with breast cancer, the sensitivity and specificity of MMG have been reported to be 21% and 80%, respectively (36).
CT is not much used in the detection of ALN metastasis in women with breast cancer. However, its use in estimating the extent of disease in advanced cases is more important (36). A sensitivity of 72% and a specificity of 40% of CT is reported in diagnosing ALN metastases in breast cancer patients who received neoadjuvant chemotherapy (37). MRI has a strong soft tissue resolution without radioactive damage, but the examination procedure is complicated, time-consuming, and expensive. MRI has no major role in the diagnosis of ALNs in breast cancer patients because of its limited ability to visualize the axilla although dedicated protocols have led to the attainment of high sensitivity (84%) and specificity (95%), which are not feasible in routine clinical practice (38). PET has a low spatial resolution and yields a considerable false-negative rate. The sensitivity of PET is low for smaller metastases and is unreliable for micro-metastases, and therefore, it is not usually recommended for ALN metastasis detection (39). The US is the preferential method used for the detection of ALN metastasis, which not only can help in visualizing the dimensions and contours of lymph nodes but also can detect the changes in cortical shape and texture indicative of the presence of metastasis (15, 38).
US-FNA is an efficient method of detecting lymph node metastasis in the axilla of breast cancer patients with high potential for predicting positive cases that can help in the initial staging of the tumor and deciding appropriate management strategies such as SLNB or ALND (15, 40). SLNB is a minimally invasive method with high diagnostic accuracy for ALN status determination that can be used to avoid ALND, which is associated with serious complications leading to a significant morbidity and compromised quality of life of patients (41–43). Histopathological examination after ALND is a reliable method for the diagnosis of ALN metastasis. However, ALND may cause many complications such as lymphatic reflux disorder, neuropathy, and shoulder stiffness in the affected upper arm after operation, thus affecting the upper limb function of patients. SLNB helps determine the nature of ALNs in breast cancer, and ALND is not required for negative SLNB results. The false negative rate of SLNB is about 5%–10%, with less trauma and fewer complications, but there may also be local effusion, sensory nerve injury, lymphedema, and other complications, and the occurrence of complications is closely related to the surgeon’s proficiency.
US-CNB of breast masses is a highly valuable technique. Any ultrasound-detected lesion can be subjected to CNB. US-CNB has several advantages including good needle control in real time, accessibility to problematic positions such as the axilla, acquisition of samples from multiple lesions, comfort to patients and radiologists, and cost-effectiveness (44). US-CNB has been found to yield better diagnostic accuracy than US-FNA in detecting axillary node metastasis in breast cancer patients (45). Based on the histological results from the puncture biopsy, the appropriate treatment for breast lesions can be determined. Under the guidance of ultrasound, CNB and FNA cytology play important roles in the diagnosis of breast lesions to improve the early diagnosis rate of breast cancer, which can improve the prognosis. FNA and CNB, which are the most widely used minimally invasive breast biopsy technologies, are mainly characterized by high accuracy, fast speed, small wound, few complications, and lower costs.
Neoadjuvant chemotherapy has been identified as a factor affecting the discrepancy between the initial and final staging of axillary nodes (26). A study found a significantly different rate of neoadjuvant chemotherapy between patients with false-negative results and those correctly diagnosed with percutaneous biopsy (21). Another study reported better sensitivity of US-CNB than US-FNA (92.5% vs 85%) in neoadjuvant chemotherapy-treated and (76.9% vs 65.4%) in no chemotherapy-treated patients. Patients treated with neoadjuvant chemotherapy had a higher level of abnormal appearance of nodes and had thicker cortices (12). A meta-analysis of 16 studies found 94% sensitivity and 6% false-negative rate of SLNB after neoadjuvant chemotherapy in staging axillary nodes in breast cancer patients who were node-negative initially (46) whereas another meta-analysis of 19 studies found 91% identification rate and 13% false-negative rate of SLNB after neoadjuvant chemotherapy in breast cancer patients who were node-positive initially (47).
There are certain limitations of the present study: (1) only English and Chinese articles were included due to logistic and technical difficulties resulting in a selection bias, which might have some influence on the overall outcomes; (2) there can be some impact of the variabilities in the operation of procedure on the outcomes of individual studies; (3) the presence of benign or non-cancerous lesions can also affect the results; and (4) a limited number of histological specimens may have influenced the accuracy of biopsy results of individual studies. However, the meta-analysis tends to moderate such effects by pooling the outcomes of several studies and entertaining their individual heterogeneities and thence minimizes the impacts of such factors. In our meta-analyses, statistical heterogeneity was not high, which may indicate the some influence of clinical and methodological heterogeneity; (5) only pooled data were analyzed, as individual patient data were not available, and this precluded more in-depth analyses.
Both US-CNB and US-FNA have good diagnostic accuracy in identifying suspicious ALNs in patients with breast cancer. With 85% sensitivity, 93% specificity, and the area under the SROC curve of 0.96, US-CNB appears to have better diagnostic efficiency than US-FNA, which is found to have 79% sensitivity, 96% specificity, and an area under the SROC curve value of 0.94.
The datasets presented in this study can be obtained from the corresponding author upon a reasonable request.
Substantial contributions to conception and design: HZ, RZ, and WW. Data acquisition, data analysis, and interpretation: XL, XW, CW, and YR. Drafting the article or critically revising it for important intellectual content: HZ, RZ, and WW. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: All authors. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ALN, axillary lymph node; ALND, conventional ALN dissection; CNB, core needle biopsy; CT, computed tomography; DOR, diagnostic odds ratio; FN, false negative; FNA, fine needle aspiration; FP, false positive; MMG, mammography; MRI, magnetic resonance imaging; PE, clinical palpation; PET, positron emission tomography; SLNB, sentinel lymph node biopsy; SROC, summary receiver operating characteristic; TN, true negative; TP, true positive; US, ultrasonography.
1. Arnedos M, Nerurkar A, Osin P, A'Hern R, Smith IE, Dowsett M. Discordance between core needle biopsy (CNB) and excisional biopsy (EB) for estrogen receptor (ER), progesterone receptor (PgR) and HER2 status in early breast cancer (EBC). Ann Oncol (2009) 20(12):1948–52. doi: 10.1093/annonc/mdp234
2. Saffar B, Bennett M, Metcalf C, Burrows S. Retrospective preoperative assessment of the axillary lymph nodes in patients with breast cancer and literature review. Clin Radiol (2015) 70(9):954–9. doi: 10.1016/j.crad.2015.04.019
3. Wright AA, Zhang B, Keating NL, Weeks JC, Prigerson HG. Associations between palliative chemotherapy and adult cancer patients' end of life care and place of death: prospective cohort study. BMJ (Clinical Res ed) (2014) 348:g1219. doi: 10.1136/bmj.g1219
4. Zhao Y, Dong X, Li R, Song J, Zhang D. Correlation between clinical-pathologic factors and long-term follow-up in young breast cancer patients. Trans Oncol (2015) 8(4):265–72. doi: 10.1016/j.tranon.2015.05.001
5. Bruening W, Fontanarosa J, Tipton K, Treadwell JR, Launders J, Schoelles K. Systematic review: comparative effectiveness of core-needle and open surgical biopsy to diagnose breast lesions. Ann Internal Med (2010) 152(4):238–46. doi: 10.7326/0003-4819-152-1-201001050-00190
6. Fung AD, Collins JA, Campassi C, Ioffe OB, Staats PN. Performance characteristics of ultrasound-guided fine-needle aspiration of axillary lymph nodes for metastatic breast cancer employing rapid on-site evaluation of adequacy: analysis of 136 cases and review of the literature. Cancer Cytopathology (2014) 122(4):282–91. doi: 10.1002/cncy.21384
7. Hafiz F, Choudhury T, Kamal M, Banu SG. The spectrum of pathological changes in breast cancer following neoadjuvant chemotherapy. Mymensingh Med J (2014) 23(2):272–80.
8. Zhao Y, Dong X, Li R, Ma X, Song J, Li Y, et al. Evaluation of the pathological response and prognosis following neoadjuvant chemotherapy in molecular subtypes of breast cancer. OncoTargets Ther (2015) 8:1511–21. doi: 10.2147/OTT.S83243
9. Zhou S, Le J, Hu N, Gao Y, Chang. C. The study of ultrasound guided fine needle aspiration of axillary lymph nodes in breast cancer. Chin J Ultrasonography (2017) 26(6):527–30. doi: 10.7863/ultra.32.12.2177
10. Nakamura R, Yamamoto N, Miyaki T, Itami M, Shina N, Ohtsuka M. Impact of sentinel lymph node biopsy by ultrasound-guided core needle biopsy for patients with suspicious node positive breast cancer. Breast Cancer (Tokyo Japan) (2018) 25(1):86–93. doi: 10.1007/s12282-017-0795-7
11. Ahn HS, Kim SM, Jang M, La Yun B, Kim SW, Kang E, et al. Comparison of sonography with sonographically guided fine-needle aspiration biopsy and core-needle biopsy for initial axillary staging of breast cancer. J Ultrasound Med (2013) 32(12):2177–84. doi: 10.7863/ultra.32.12.2177
12. Boone BA, Huynh C, Spangler ML, Sumkin J, Johnson R, McGuire KP, et al. Axillary lymph node burden in invasive breast cancer: a comparison of the predictive value of ultrasound-guided needle biopsy and sentinel lymph node biopsy. Clin Breast Cancer (2015) 15(5):e243–8. doi: 10.1016/j.clbc.2015.03.011
13. Ding Y, Zhou F, Hu L, Liu X, Qin A. Ultrasound-guided fine-needle aspiration biopsy combined with cytokeratin 19-fragments 21-1 in diagnosis of axillary lymph node metastasis in breast cancer. Chin J Interventional Imaging Ther (2018) 15(10):601–4. doi: 10.1007/s00268-007-9009-3
14. Ganott MA, Zuley ML, Abrams GS, Lu AH, Kelly AE, Sumkin JH, et al. Ultrasound guided core biopsy versus fine needle aspiration for evaluation of axillary lymphadenopathy in patients with breast cancer. ISRN Oncol (2014) 2014:703160. doi: 10.1155/2014/703160
15. Genta F, Zanon E, Camanni M, Deltetto F, Drogo M, Gallo R, et al. Cost/accuracy ratio analysis in breast cancer patients undergoing ultrasound-guided fine-needle aspiration cytology, sentinel node biopsy, and frozen section of node. World J Surg (2007) 31(6):1155–63. doi: 10.1007/s00268-007-9009-3
16. Huo L, Wu K, Zhang Y, Zhang J, Gao H. Application of ultrasound-guided fine needle aspiration cytology in diagnosis of axillary lymph node metastasis in breast cancer. Guangdong Med J (2016) 37(S1):110–3.
17. Krishnamurthy S, Sneige N, Bedi DG, Edieken BS, Fornage BD, Kuerer HM, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer (2002) 95(5):982–8. doi: 10.1002/cncr.10786
18. Le J, Zhou S, Hu N, Gao Y, Chen M, Chang C. The study of the correlation between the results of ultrasound-guided aspiration and axillary lymph node load in breast cancer. Chin J Ultrasound Med (2017) 33(12):1067–70. doi: 10.1016/j.crad.2016.02.024
19. Liu M, Liu P, Xie F, Yang D. Ultrasound-guided fine-needle aspiration for the evaluation of axillary lymph node metastasis in breast cancer. Chin J Gen Surg (2011) 26(1):25–8. doi: 10.1007/s12282-017-0795-7
20. Maxwell AJ, Bundred NJ, Harvey J, Hunt R, Morris J, Lim YY. A randomised pilot study comparing 13 G vacuum-assisted biopsy and conventional 14 G core needle biopsy of axillary lymph nodes in women with breast cancer. Clin Radiol (2016) 71(6):551–7. doi: 10.1016/j.crad.2016.02.024
21. Popli MB, Sahoo M, Mehrotra N, Choudhury M, Kumar A, Pathania OP, et al. Preoperative ultrasound guided fine-needle aspiration cytology for axillary staging in breast carcinoma. Australas Radiol (2006) 50(2):122–6. doi: 10.1111/j.1440-1673.2006.01545.x
22. Rao R, Lilley L, Andrews V, Radford L, Ulissey M. Axillary staging by percutaneous biopsy: sensitivity of fine-needle aspiration versus core needle biopsy. Ann Surg Oncol (2009) 16(5):1170–5. doi: 10.1245/s10434-009-0421-9
23. Rautiainen S, Masarwah A, Sudah M, Sutela A, Pelkonen O, Joukainen S, et al. Axillary lymph node biopsy in newly diagnosed invasive breast cancer: comparative accuracy of fine-needle aspiration biopsy versus core-needle biopsy. Radiology (2013) 269(1):54–60. doi: 10.1148/radiol.13122637
24. Ruan Y, Pan D, HangYanzhu, Shen Z, Zheng L. The diagnostic value of ultrasound-guided fine needle aspiration of axillary lymph node metastasis in breast cancer. Chin J Prev Control Chronic Dis (2018) 26(10):763–6.
25. Sang G, He Y, Lu C, Sun R. Utilization of preoperative ultrasound-guided fine needle aspiration cytology for the evaluation of axillary lymph nodes status in patients with early stage breast cancer. Chin J Endocrinological Surg (2019) 13(3):241–4.
26. Sang Y, Xie H. Application value of ultrasound-guided fine-needle and core-needle aspiration biopsy for axillary staging of breast cancer. Guangxi Med J (2016) 38(11):1544–6. doi: 10.1016/j.ejrad.2005.05.018
27. Topal U, Punar S, Tasdelen I, Adim SB. Role of ultrasound-guided core needle biopsy of axillary lymph nodes in the initial staging of breast carcinoma. Eur J Radiol (2005) 56(3):382–5. doi: 10.1016/j.ejrad.2005.05.018
28. van Wely BJ, de Wilt JH, Schout PJ, Kooistra B, Wauters CA, Venderinck D, et al. Ultrasound-guided fine-needle aspiration of suspicious nodes in breast cancer patients; selecting patients with extensive nodal involvement. Breast Cancer Res Treat (2013) 140(1):113–8. doi: 10.1007/s10549-013-2624-9
29. Vidya R, Iqbal FM, Bickley B. Pre-operative axillary staging: should core biopsy be preferred to fine needle aspiration cytology? Ecancermedicalscience (2017) 11:724. doi: 10.3332/ecancer.2017.724
30. Wang J, Wang W, Wei C, Gu Z. Diagnosis of axillary lymph node metastasis in breast cancer by ultrasound-guided needle biopsy. Chin J Ultrasound Med (2013) 15(3):203–4.
31. Wang Y, Gao W, Yin S, Zhang L, Zhang H, Yan K, et al. The diagnostic value of ultrasound and ultrasound-guided needle biopsy for axillary lymph nodes in breast cancer. Chin J Gen Surg (2005) 20(5):322–3.
32. Wei Y, Li Y, Zhang Q, Jiang. D. The value of preoperative ultrasound guide fine needle aspiration for axillary lymph node metastasis in breast cancer. Modern Oncol (2014) 22(03):569–71.
33. Yu C, Chen X, Fan Q, Chen J, Wang J, Ling. Z. A retrospective study on the effect of ultrasound guided core needle biopsy in breast cancer patients after neoadjuvant chemotherapy. J Harbin Med Univ (2018) 52(05):491–5.
34. Zhao Y, Bai L, Tang Y, Liu W, Hao X, Chen C, et al. Clinical value of ultrasound-guided core needle biopsy for detecting axillary lymph node metastasis in breast cancer. Military Med Sci (2015) 39(07):541–5.
35. Majid S, Tengrup I, Manjer J. Clinical assessment of axillary lymph nodes and tumor size in breast cancer compared with histopathological examination: a population-based analysis of 2,537 women. World J Surg (2013) 37(1):67–71. doi: 10.1007/s00268-012-1788-5
36. Choi HY, Park M, Seo M, Song E, Shin SY, Sohn YM. Preoperative axillary lymph node evaluation in breast cancer: current issues and literature review. Ultrasound Q (2017) 33(1):6–14. doi: 10.1097/RUQ.0000000000000277
37. Cheung YC, Chen SC, Hsieh IC, Lo YF, Tsai HP, Hsueh S, et al. Multidetector computed tomography assessment on tumor size and nodal status in patients with locally advanced breast cancer before and after neoadjuvant chemotherapy. Eur J Surg Oncol (2006) 32(10):1186–90. doi: 10.1016/j.ejso.2006.03.026
38. Marino MA, Avendano D, Zapata P, Riedl CC, Pinker K. Lymph node imaging in patients with primary breast cancer: concurrent diagnostic tools. Oncologist (2020) 25(2):e231–242. doi: 10.1634/theoncologist.2019-0427
39. Cooper KL, Harnan S, Meng Y, Ward SE, Fitzgerald P, Papaioannou D, et al. Positron emission tomography (PET) for assessment of axillary lymph node status in early breast cancer: a systematic review and meta-analysis. Eur J Surg Oncol (2011) 37(3):187–98. doi: 10.1016/j.ejso.2011.01.003
40. Alkuwari E, Auger M. Accuracy of fine-needle aspiration cytology of axillary lymph nodes in breast cancer patients. a study of 115 cases with cytologic-histologic correlation. Cancer Cytopahology (2008) 114(2):89–93. doi: 10.1002/cncr.23344
41. Sclafani L, Baron R. Sentinel lymph node biopsy and axillary dissection: added morbidity of the arm, shoulder and chest wall after mastectomy and reconstruction. Cancer J (2008) 14(4):216–22. doi: 10.1097/PPO.0b013e31817fbe5e
42. Del Bianco P, Zavagno G, Burelli P, Scalco G, Barutta L, Carraro P, et al. Morbidity comparison of sentinel lymph node biopsy versus conventional axillary lymph node dissection for breast cancer patients: results of the sentinella–GIVOM Italian randomised clinical trial. Eur J Surg Oncol (2008) 34(5):P508–513. doi: 10.1016/j.ejso.2007.05.017
43. Chen J, Huang X, Liu Z, Chen T, Cheng J, Yang W, et al. Sentinel node biopsy and quality of life measures in a Chinese population. Eur J Surg Oncol (2009) 35(9):P921–927. doi: 10.1016/j.ejso.2009.01.009
44. Apesteguía L, Pina LJ. Ultrasound-guided core-needle biopsy of breast lesions. Insights into Imaging (2011) 2:493–500. doi: 10.1007/s13244-011-0090-7
45. Balasubramanian I, Fleming CA, Corrigan MA, Redmond HP, Kerin MJ, Lowery AJ. Meta-analysis of the diagnostic accuracy of ultrasound-guided fine-needle aspiration and core needle biopsy in diagnosing axillary lymph node metastasis. Br J Surg (2018) 105(10):1244–53. doi: 10.1002/bjs.10920
46. Geng C, Chen X, Pan X, Li J. The feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemotherapy: a systematic review and meta-analysis. PloS One (2016) 11(9):e0162605. doi: 10.1371/journal.pone.0162605
47. El Hage Chehade H, Headon H, El Tokhy O, Heeney J, Kasem A, Mokbel K. Is sentinel lymph node biopsy a viable alternative to complete axillary dissection following neoadjuvant chemotherapy in women with node-positive breast cancer at diagnosis? an updated meta-analysis involving 3,398 patients. Am J Surg (2016) 212(5):969–81. doi: 10.1016/j.amjsurg.2016.07.018
Keywords: meta-analysis, fine-needle aspiration, core needle biopsy, axillary lymph nodes, diagnostic accuracy
Citation: Zheng H, Zhao R, Wang W, Liu X, Wang X, Wen C and Ren Y (2023) The accuracy of ultrasound-guided fine-needle aspiration and core needle biopsy in diagnosing axillary lymph nodes in women with breast cancer: a systematic review and meta-analysis. Front. Oncol. 13:1166035. doi: 10.3389/fonc.2023.1166035
Received: 16 February 2023; Accepted: 30 May 2023;
Published: 21 June 2023.
Edited by:
Jiefu Jin, Johns Hopkins University, United StatesReviewed by:
Xiao Sun, Shandong First Medical University & Shandong Academy of Medical Science, ChinaCopyright © 2023 Zheng, Zhao, Wang, Liu, Wang, Wen and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haining Zheng, emhlbmdoYWluaW5nMDEwQDE2My5jb20=; Chaoyang Wen, d2VuY3lwa3VpaEAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.