- 1The Ohio State University Comprehensive Cancer Center and Arthur G. James Cancer Hospital, Columbus, OH, United States
- 2Division of Medical Oncology, Department of Internal Medicine, The Ohio State University College of Medicine, The Ohio State University, Columbus, OH, United States
Background: Randomized controlled phase III trials have reported significant improvements in disease response and survival with the addition of chemotherapy to androgen deprivation therapy for men presenting with metastatic prostate cancer. We examined the implementation of such knowledge and its impact within the Surveillance, Epidemiology, and End Results (SEER) database.
Method: The administration of chemotherapy for men with an initial presentation of metastatic prostate cancer from 2004 to 2018 in the SEER database and its association with survival outcomes was examined. Kaplan–Meier estimates were applied to compare survival curves. Cox proportion hazard survival models were used to analyze the association of chemotherapy and other variables with both cancer- specific and overall survival.
Result: A total of 727,804 patients were identified with 99.9% presenting with adenocarcinoma and 0.1% with neuroendocrine histopathology. Chemotherapy as initial treatment for men with de novo distant metastatic adenocarcinoma increased from 5.8% during 2004–2013 to 21.4% during 2014–2018. Chemotherapy was associated with a poorer prognosis during 2004–2013 but was associated with improved cancer-specific (hazard ratio (HR) = 0.85, 95% confidence interval (CI): 0.78–0.93, p=0.0004) and overall survival (HR= 0.78, 95% CI: 0.71–0.85, p < 0.0001) during 2014–2018. The improved prognosis during 2014–2018 was observed in patients with visceral or bone metastasis and most impactful for patients aged 71–80 years. These findings were confirmed by subsequent propensity score matching analyses. Furthermore, chemotherapy was consistently provided to 54% of patients with neuroendocrine carcinoma at diagnosis from 2004 to 2018. Treatment was associated with improved cancer-specific survival (HR= 0.62, 95% CI: 0.45–0.87, p=0.0055) and overall survival (HR= 0.69, 95% CI: 0.51–0. 94, p=0.0176) during 2014–2018 but not significant in earlier years.
Conclusion: Chemotherapy at initial diagnosis was increasingly employed in men with metastatic adenocarcinoma after 2014 and consistent with the evolution of National Comprehensive Cancer Network (NCCN) guidelines. Benefits for chemotherapy are suggested after 2014 in the treatment of men with metastatic adenocarcinoma. The use of chemotherapy for neuroendocrine carcinoma at diagnosis has remained stable, and outcomes have improved in more recent years. Further development and optimization of chemotherapy continues to evolve for men with de novo diagnosis of metastatic prostate cancer.
Introduction
The burden of metastatic prostate cancer to society is enormous, both in terms of health care resources and human suffering; thus, the implementation of knowledge derived from quality clinical trials to community practice is imperative (1). Prostate cancer continues to be the most frequently diagnosed non-cutaneous cancer in American men and the second leading cause of cancer-related death (1), suggesting a critical need for improved screening and early diagnosis at a curable stage, and enhanced efficacy of therapy for advanced metastatic disease. Following the US Preventive Services Task Force (USPSTF) report in 2012 (2), there was a significantly reduced utilization of prostate cancer screening with prostate-specific antigen (PSA) testing, resulting in a lower overall detection of prostate cancer, but an unfortunate increase has emerged in the proportion of men presenting at advanced stages (1, 3–5). For example, recent data show a significant 41% increase in metastatic prostate cancer from 2010 to 2018 in men aged 45 –75 (3). This report focuses upon the treatments provided to the subgroup of men presenting with de novo metastatic disease in the real-world setting.
For decades, suppression of testosterone by castration or androgen deprivation therapy (ADT) has been the cornerstone of life-prolonging therapy for metastatic prostate cancer (6) and continues to improve with newer agents targeting specific components of the androgen signaling pathway (7–9). Yet, metastatic disease is essentially incurable, and mortality is nearly 70% by 5 years after diagnosis (10–13). Sadly, the median survival for men with castrate-resistant prostate cancer (CRPC) ranges from 18 to 24 months in most studies (12, 14, 15). Cytotoxic chemotherapy emerged as a beneficial treatment modality for metastatic CRPC, initially in the management of pain with mitoxantrone (16) and subsequently with docetaxel prolonging survival in landmark phase III trials by 2004 (17, 18) and supported by subsequent studies (19, 20). Soon thereafter, cabazitaxel, a second-generation taxane, showed a survival benefit in docetaxel refractory CRPC (21). With success in CRPC in the metastatic setting, the potential of adding taxane chemotherapy to androgen deprivation therapy (ADT) for men who present at initial diagnosis with treatment-naive metastatic disease was investigated in studies demonstrating improved overall survival and improved secondary endpoints such as prostate−specific antigen (PSA) failure and time to recurrence, particularly for those with higher volume disease (10, 11, 22, 23). National Comprehensive Cancer Network (NCCN) guidelines recommend ADT with docetaxel for six cycles as one of several options for the initial treatment of castration-naive metastatic prostate adenocarcinoma and was first included in the 2014 update (10, 24).
Our objective is to assess how the studies of chemotherapy combined with hormone therapy over recent decades have translated into real-world clinical practice for men with a new diagnosis of metastatic prostate cancer. The present study provides a comprehensive and contemporary (2004 –2018) summary of the large Surveillance, Epidemiology, and End Results (SEER) database. We also report the impact of initial chemotherapy on survival based upon the histopathological subtype and a number of relevant clinical and demographic factors.
Methods
Data source
We employed the population-based SEER Research Plus Data, 18 registries (2000–2018) using the SEERStat 8.3.9 software to identify patients 18 and older with an initial diagnosis of prostate cancer. We included those diagnosed during 2004–2018 because SEER collected PSA information since 2004 and the modern chemotherapy regimens (e.g., docetaxel) for metastatic disease were supported by clinical trial results in 2004. Those with stage Tis or T0 (no indication of cancer), with unknown T, N, and M stages and unknown survival time were excluded from the study. The primary endpoints were prostate-cancer-specific survival and overall survival. Based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), we only included patients with prostate adenocarcinoma (8,140) or neuroendocrine carcinoma (8,012, 8,013, 8,041, 8,042, 8,045, 8,240, 8,241, 8,246, and 8,574) (25, 26). The SEER registries collect information on the first course of treatment. Chemotherapy data are categorized as either “yes— patient had chemotherapy” or “no/unknown— no evidence of chemotherapy was found in the medical records examined.” Patients with de novo distant metastatic disease were defined by M stage as 1. M stage was further grouped into M1a, M1b, M1c, and M1x. The following demographic and clinicopathological variables were included: age at diagnosis; PSA concentration; ethnicity (White, Black, Asian or Pacific Islander, and other); marital status; region of the US; Gleason score; T, N, and M stage; and treatments including surgery, radiotherapy, and chemotherapy. As the data are de-identified, institutional review board approval was not necessary for this project.
Statistical analysis
Continuous data were evaluated by T-test. Square root or log transformation of the original data was applied to satisfy the assumption of equal variances. Categorical data were compared using the Pearson’s chi‐square test. The trend for the proportion of patients receiving chemotherapy was examined by the Cochran–Armitage test. Survival curves were defined by Kaplan–Meier methodology and compared through log rank testing. Univariate and multivariate Cox proportional hazards regression analyses were utilized to examine the impact of chemotherapy and predictors on cancer- specific and overall survival. The multivariable model was constructed with a backward selection strategy with an entry level of 0.05 at every step. Only variables with a p-value < 0.10 in the univariate analyses were included, except that chemotherapy was always included in the multivariate analysis. To address potential disparities between patients treated with or without chemotherapy, impacts of chemotherapy on prognosis were determined in propensity score matching analyses. Matching variables included age; PSA; Gleason score; T, N, and M stages; race; marital status; region; and local treatment. All statistical tests were two-sided with p< 0.05 to be significant. Data analyses were performed using SAS 9.4 (Raleigh, NC).
Results
Patients with prostate adenocarcinoma or neuroendocrine carcinoma diagnosed during 2004–2018
A total of 727,804 patients diagnosed with prostate cancer during 2004–2018 (Supplementary Table S1) were identified with 727,133 (99.9%) having adenocarcinoma and 671 (0.1%) with neuroendocrine histopathology. Those with neuroendocrine cancer at presentation were older and exhibited higher PSA and greater Gleason grade. The proportion of men with metastatic adenocarcinoma at diagnosis was 3%, which was much lower than 57% of those with neuroendocrine histology (Supplementary Table S1).
Time trends for chemotherapy administration for metastatic prostate cancer at diagnosis during 2004–2018
As expected, the proportion of men with non-metastatic adenocarcinoma receiving chemotherapy was between 0.2% and 0.5% over time (p trend <0.0001) (Figure 1A). For men with metastatic adenocarcinoma at presentation, chemotherapy was provided to 5.8% during years 2004–2013 and increased to 21.4% during the years of 2014–2018 (p trend < 0.0001) (Figure 1A). In this population, the administration of chemotherapy was strongly age dependent after 2013 (all p trends < 0.0001) (Figure 1B; Table 1), with younger men more likely to receive chemotherapy. A much high proportion of men presenting with neuroendocrine cancer received chemotherapy (54%), and the proportion remained steady between 2004 and 2018, with year-to-year variation due to the overall smaller number of cases compared to adenocarcinoma (p trend=0.1349) (Figure 1C).
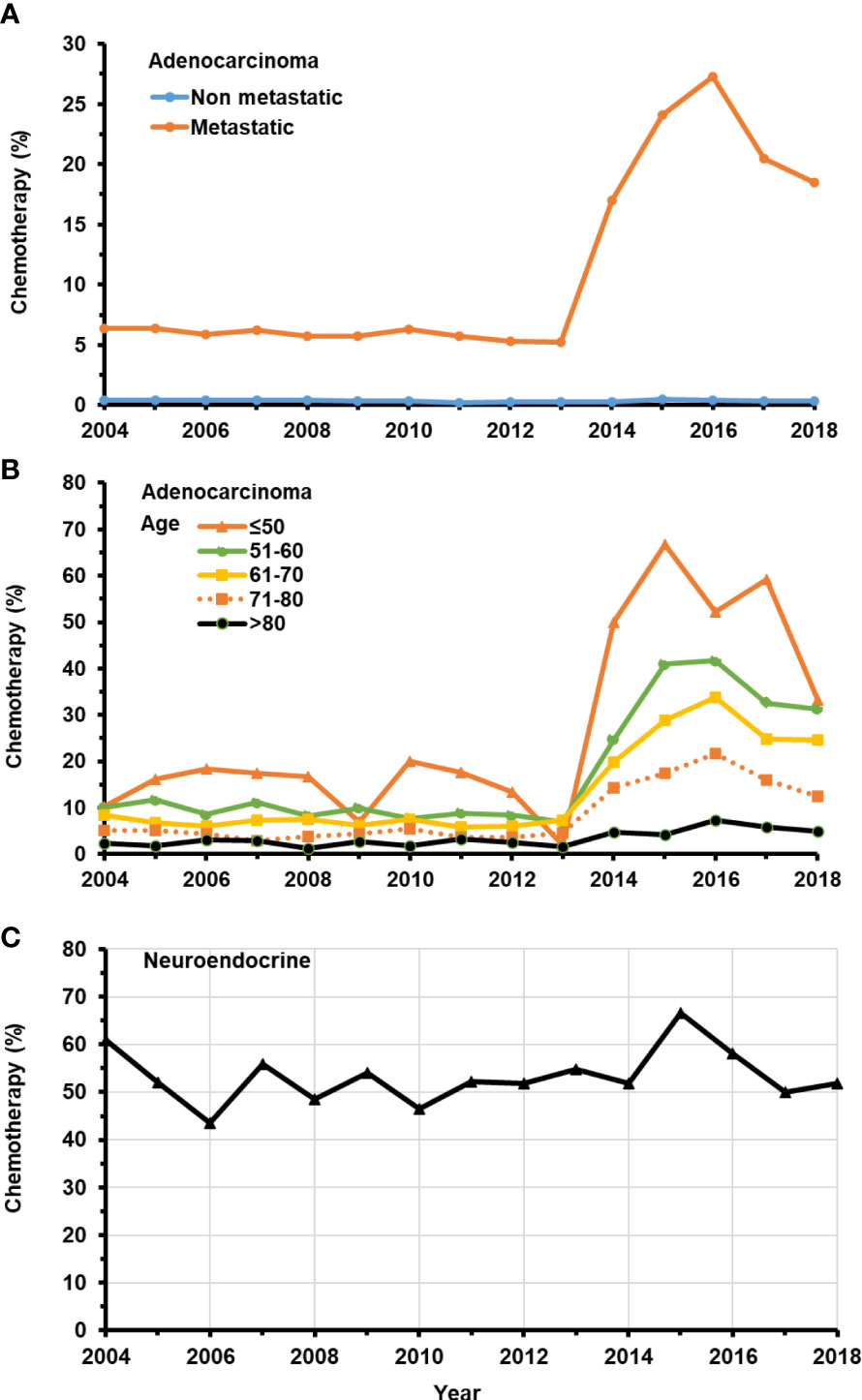
Figure 1 Temporal change in the percentage of patients with de novo metastatic prostate adenocarcinoma receiving chemotherapy from 2004–2018. (A) Patients with or without de novo metastatic prostate adenocarcinoma. (B) Patients in different age groups with de novo metastatic prostate adenocarcinoma. (C) All patients with prostate neuroendocrine carcinoma.
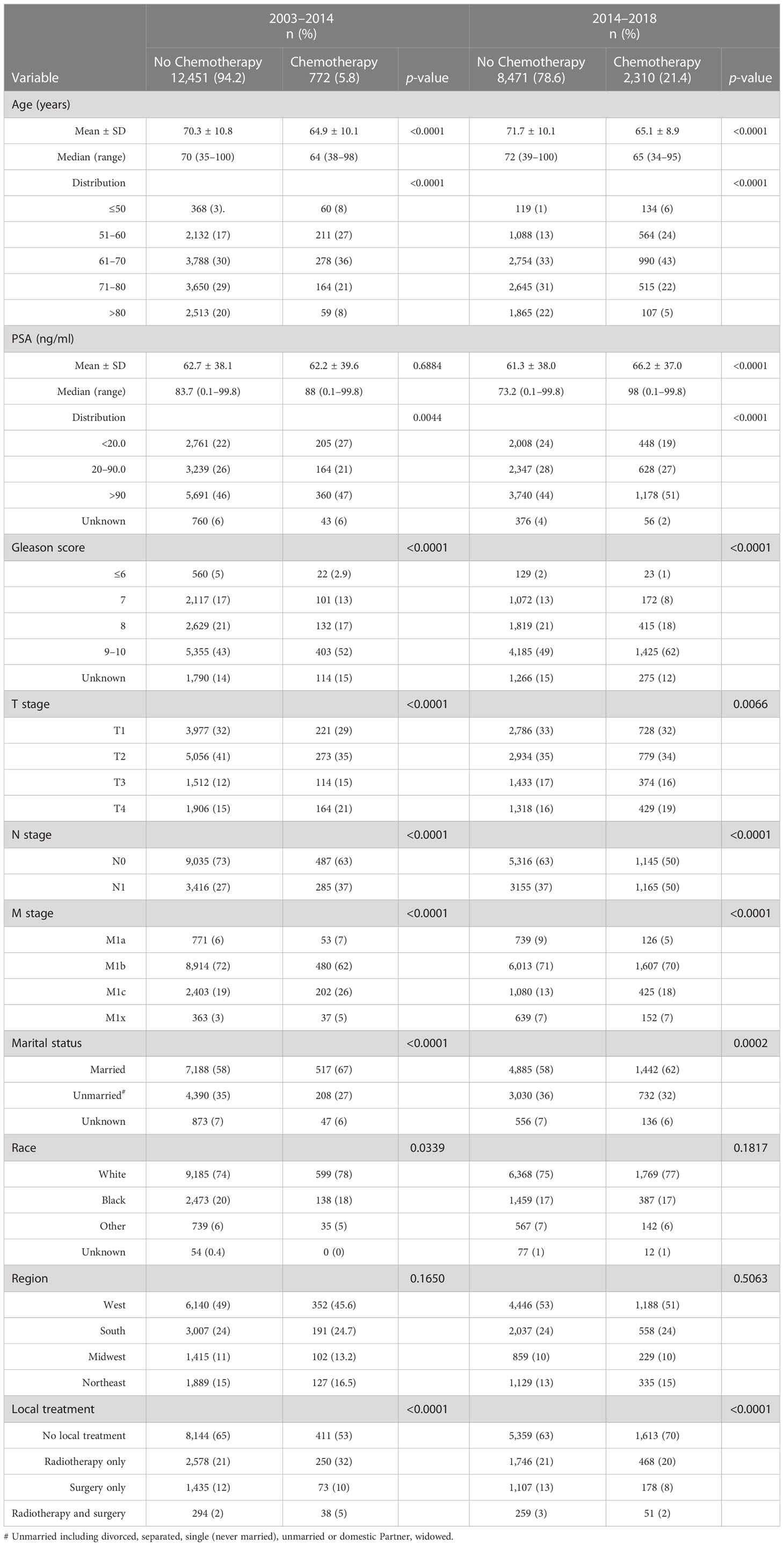
Table 1 Descriptive characteristics of patients with a de novo diagnosis of metastatic prostate adenocarcinoma who were initially treated with or without chemotherapy during 2004–2013 and 2014–2018.
Characteristics of patients with metastatic prostate adenocarcinoma at diagnosis and initially treated with or without chemotherapy during 2004–2013 and 2014–2018
Table 1 outlines factors contributing to the selection of chemotherapy for initial treatment of men presenting with metastatic prostate adenocarcinoma at primary diagnosis for the intervals of 2004–2013 (5.8% receiving chemotherapy) and 2014–2018 (21.4% receiving chemotherapy). Younger age at diagnosis was strongly associated with selection of initial chemotherapy particularly after 2013 (Table 1). Patients with cancers characterized by higher Gleason score (9, 10), more advanced T stage, positive lymph node metastasis, and more advanced M stage (M1c) were significantly more likely to receive chemotherapy in both periods. A higher PSA (> 90 ng/ml) emerged as a modest predictor for chemotherapy treatment during 2014–2018.
Impact of chemotherapy on cancer- specific and overall survival in patients presenting with metastatic prostate adenocarcinoma during 2004–2013 and 2014–2018
During 2004–2013, there were significantly higher proportions of both cancer-specific and overall deaths in metastatic patients receiving chemotherapy compared with those receiving no chemotherapy (Supplementary Table S2; Figure 2). In contrast, during 2014–2018, the proportion of overall death in patients receiving chemotherapy was significantly less than in those without chemotherapy, while cancer-specific death was not significantly impacted by chemotherapy selection (Supplementary Table S2). Survival curves illustrate that chemotherapy was associated with significantly worse cancer-specific and overall survival in patients with metastatic prostate adenocarcinoma carcinoma during 2004–2013 (Figures 2A, B) but was associated with significantly improved prognoses during 2014–2018 (Figures 2C, D).
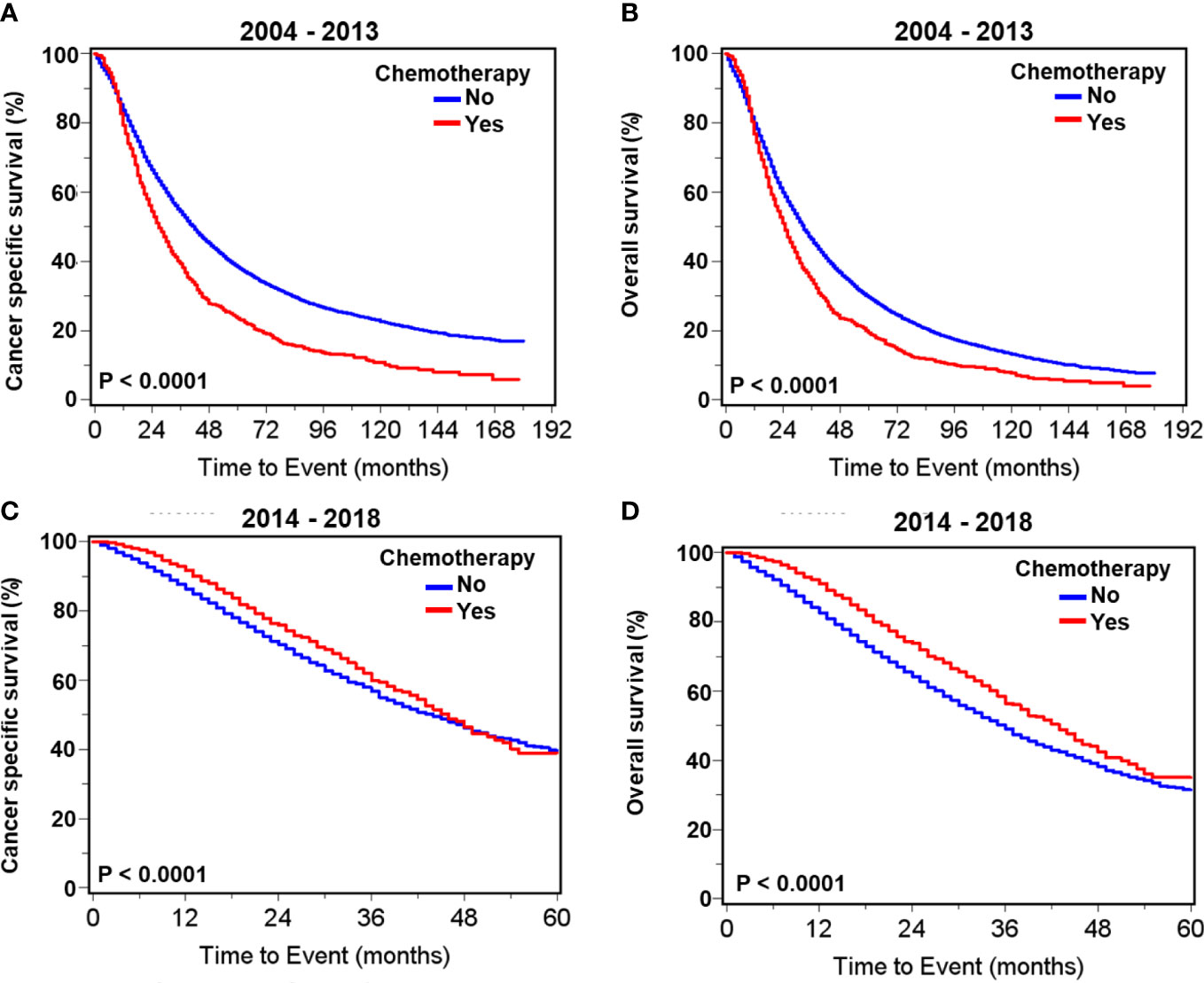
Figure 2 Kaplan–Meier survival curves for cancer- specific and overall survival in all patients with de novo metastatic prostate adenocarcinoma with or without chemotherapy. For patients diagnosed during 2004–2013, curves of cancer- specific survival (A) and overall survival (B). For patients diagnosed during 2014–2018, curves of cancer- specific survival (C) and overall survival (D).
Table 2 presents multivariate survival analyses showing greater depth of insight with reduced bias. Chemotherapy was associated with significantly improved cancer-specific survival (HR= 0.85, 95% CI: 0.78 –0.93, p=0.0004) and overall survival (HR= 0.78, 95% CI: 0.71 –0.85, p<0.0001) in patients with metastatic prostate adenocarcinoma diagnosed during 2014–2018. In comparison, during the period of 2004–2013, chemotherapy was associated with significantly worse cancer-specific survival (HR =1.48, 95% CI: 1.37 –1.61, p<0.0001) and overall survival (HR =1.39, 95% CI: 1.28 –1.50, p<0.0001) (Table 2). Other factors significantly predicting poor outcomes in both time intervals were greater age, higher PSA, T4 stage, extensive metastasis beyond M1a, and higher Gleason score. The inclusion of radiotherapy or surgery to the initial treatment plan was not associated with a change in cancer-specific or overall survival during either time period. However, combined radiotherapy and surgery was associated with significantly worse survival during the earlier time frame of 2004–2013 (Table 2). Men who were married, as an indicator of support systems, fared significantly better than those who were not by 18% –25%. Men with metastatic prostate cancer living in the South fared significantly worse than in other regions regardless of treatment and time interval.
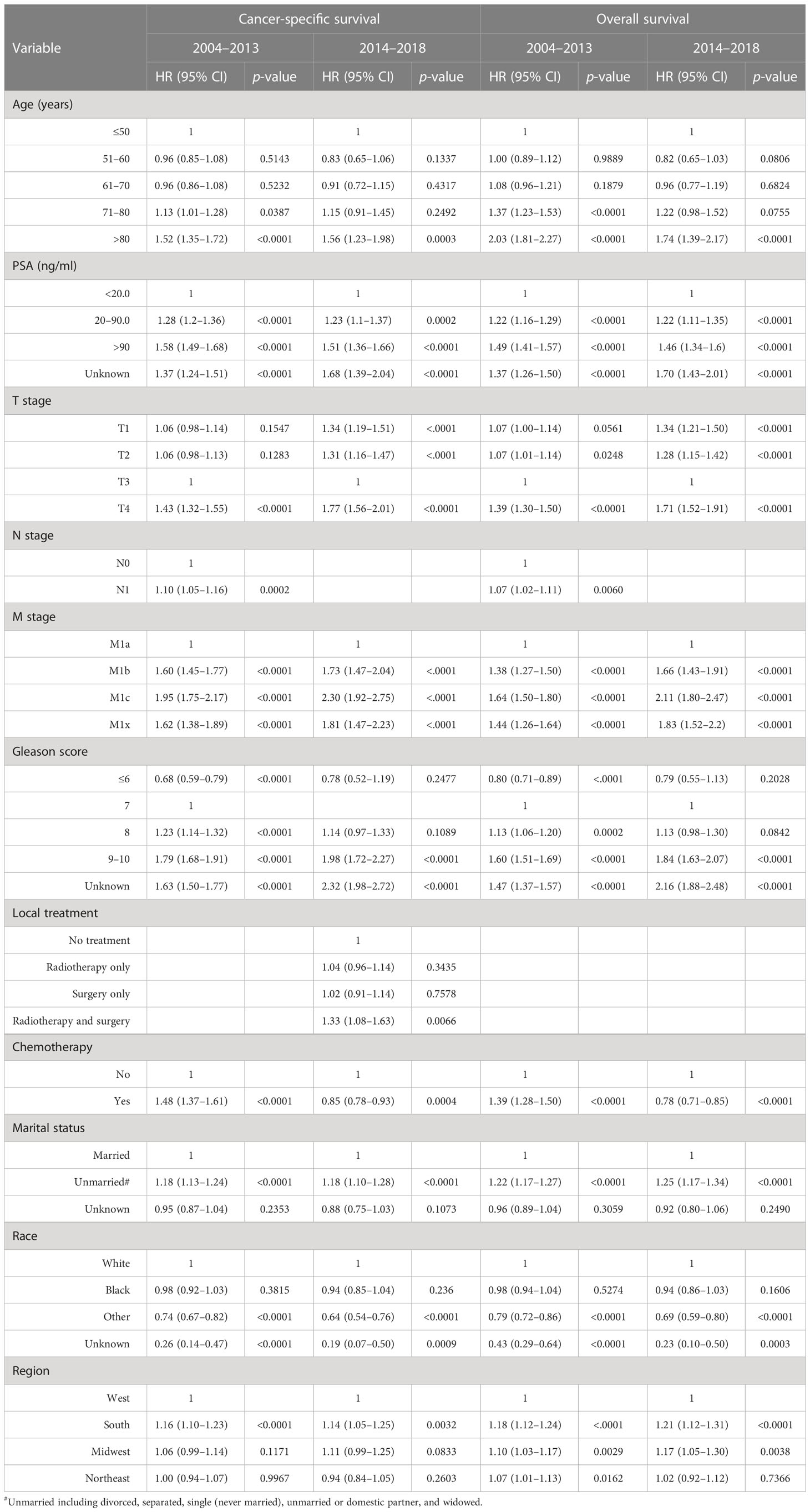
Table 2 Multivariate survival analyses of variables associated with survival in patients with metastatic prostate adenocarcinoma diagnosed during 2004–2013 and 2014–2018.
Age at diagnosis, which clearly impacted selection of chemotherapy, appears to be associated with survival response to chemotherapy. We observed no significant improvement in overall or cancer-specific survival for younger men <70 years or for those over 80. In contrast, improved cancer- specific and overall survival were seen among men aged 71–75 and 76–80 years (Table 3).
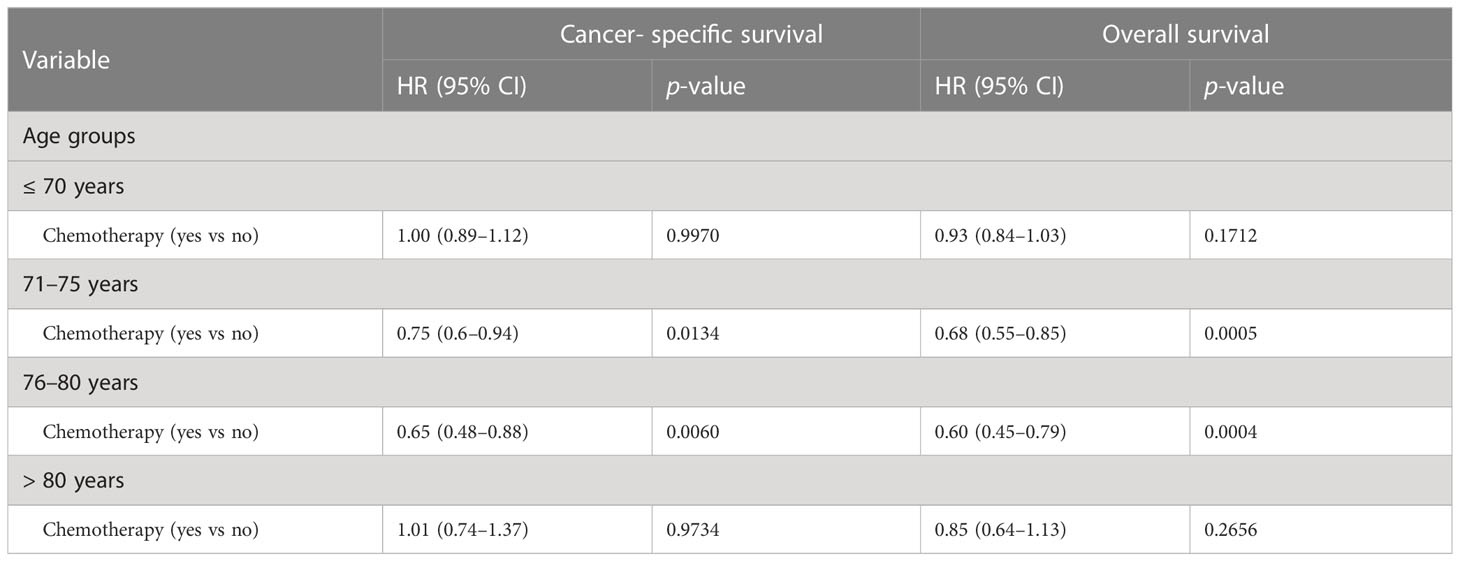
Table 3 Multivariate survival analyses of impacts of chemotherapy on survival in patients with de novo metastatic prostate adenocarcinoma among different age groups during 2014–2018.
The benefits of chemotherapy are related to metastatic disease burden at diagnosis. Our data showed that chemotherapy was associated with significantly improved cancer- specific and overall survival in patients with metastasis to either visceral (liver, lung, or brain), or bone alone. In contrast, chemotherapy was not associated with improvements in cancer-specific survival or overall survival in patients presenting with only distant lymph node metastasis (Table 4).
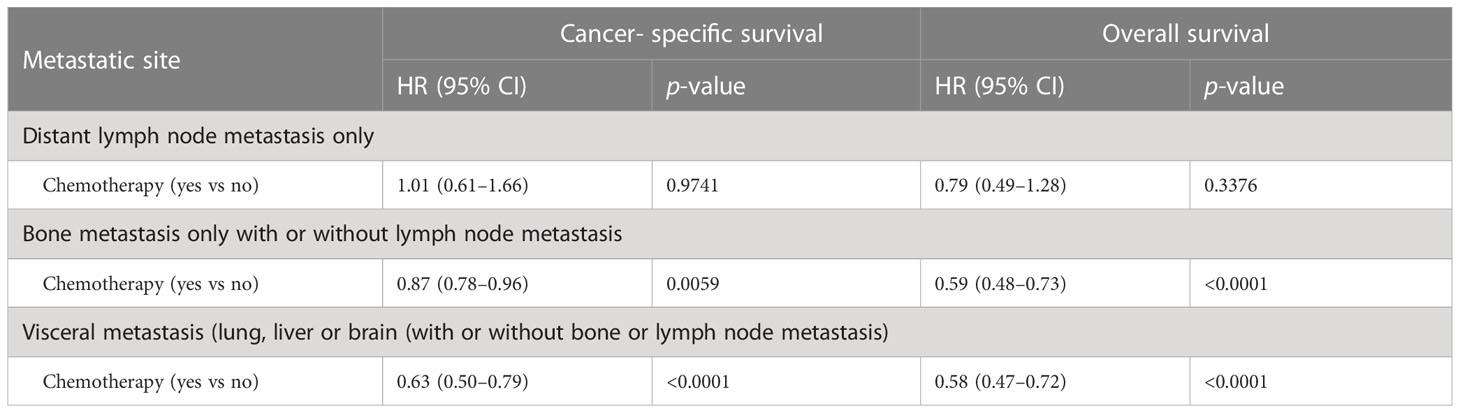
Table 4 Multivariate survival analyses of impacts chemotherapy on survival in patients with de novo metastatic prostate adenocarcinoma at varied metastatic sites during 2014–2018.
Propensity score matching analyses of impact of chemotherapy on prognosis in patients presenting with metastatic prostate adenocarcinoma during 2004–2013 and 2014–2018
After the propensity score matching, equal numbers of patients with comparable features treated with or without chemotherapy were selected in those having metastatic adenocarcinoma during 2004–2013 and 2014–2018 (Supplementary Table S3). We observed similar and consistent patterns of death and survival curves with propensity score matching. (Supplementary Table S4; Figures S1A–D). Most importantly, we confirmed that in patients with metastatic prostate adenocarcinoma, chemotherapy was associated with worse prognoses during 2004–2013 but improved cancer-specific and overall survival during 2014–2018 (Table 5).
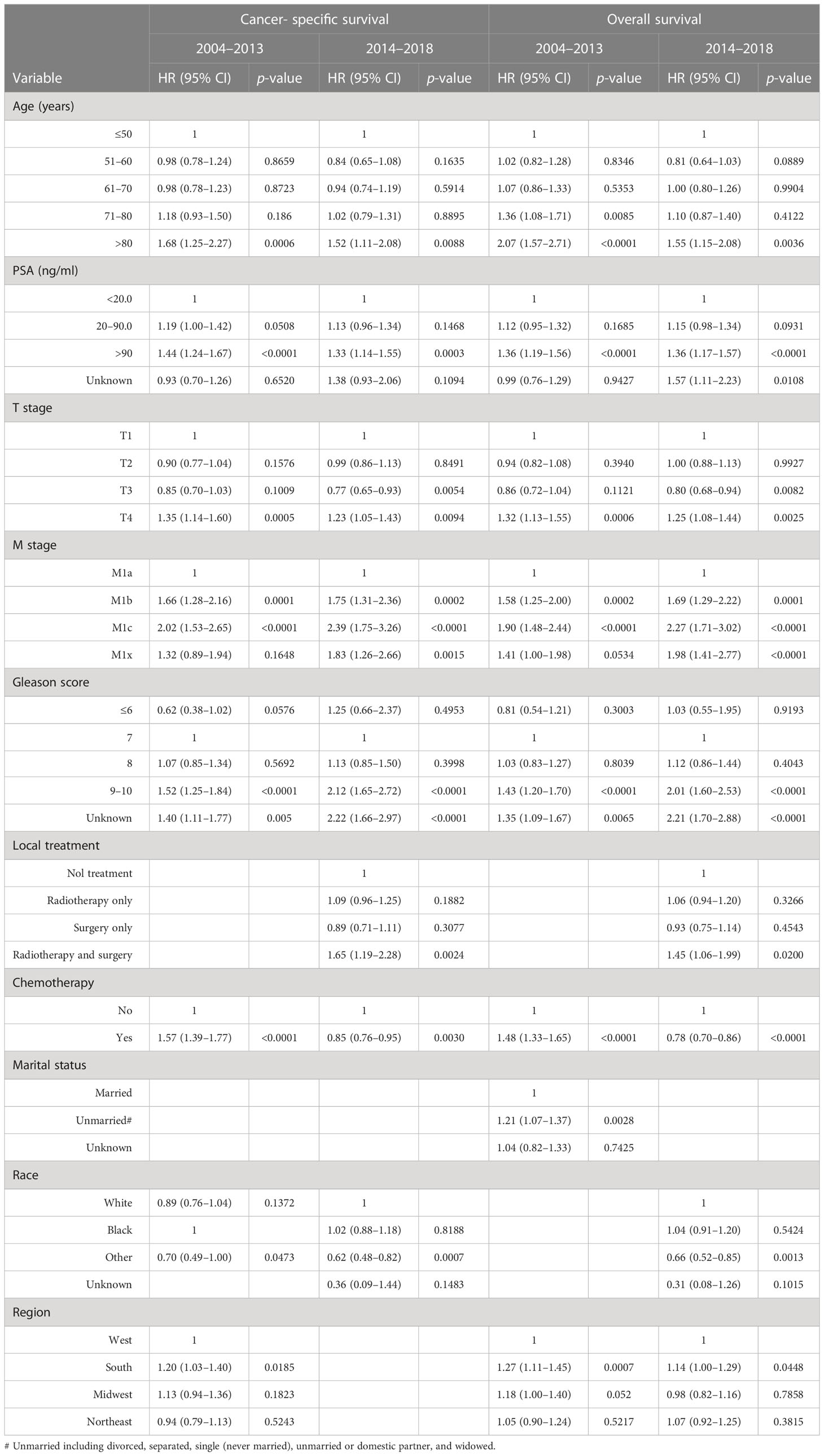
Table 5 Multivariate analyses of risk factors correlated with survival in propensity score matched patients with metastatic prostate adenocarcinoma diagnosed during 2004–2013 and 2014–2018.
Baseline characteristics of patients with prostate neuroendocrine carcinoma with or without chemotherapy during 2004–2013 and 2014–2018
Younger patients with neuroendocrine carcinoma with lower serum PSA levels, more advanced T stage, lymph node and distant metastasis, and radiotherapy were more likely to receive chemotherapy during both periods. During 2004–2013, patents of 51–60 years old compared to older individuals were more likely to receive chemotherapy. In addition, during 2014–2018, patients of 61–70 years and from West and Northeast regions were more likely to receive chemotherapy (Table 6). Detailed treatments provided to these patients is presented in Supplementary Table S5.
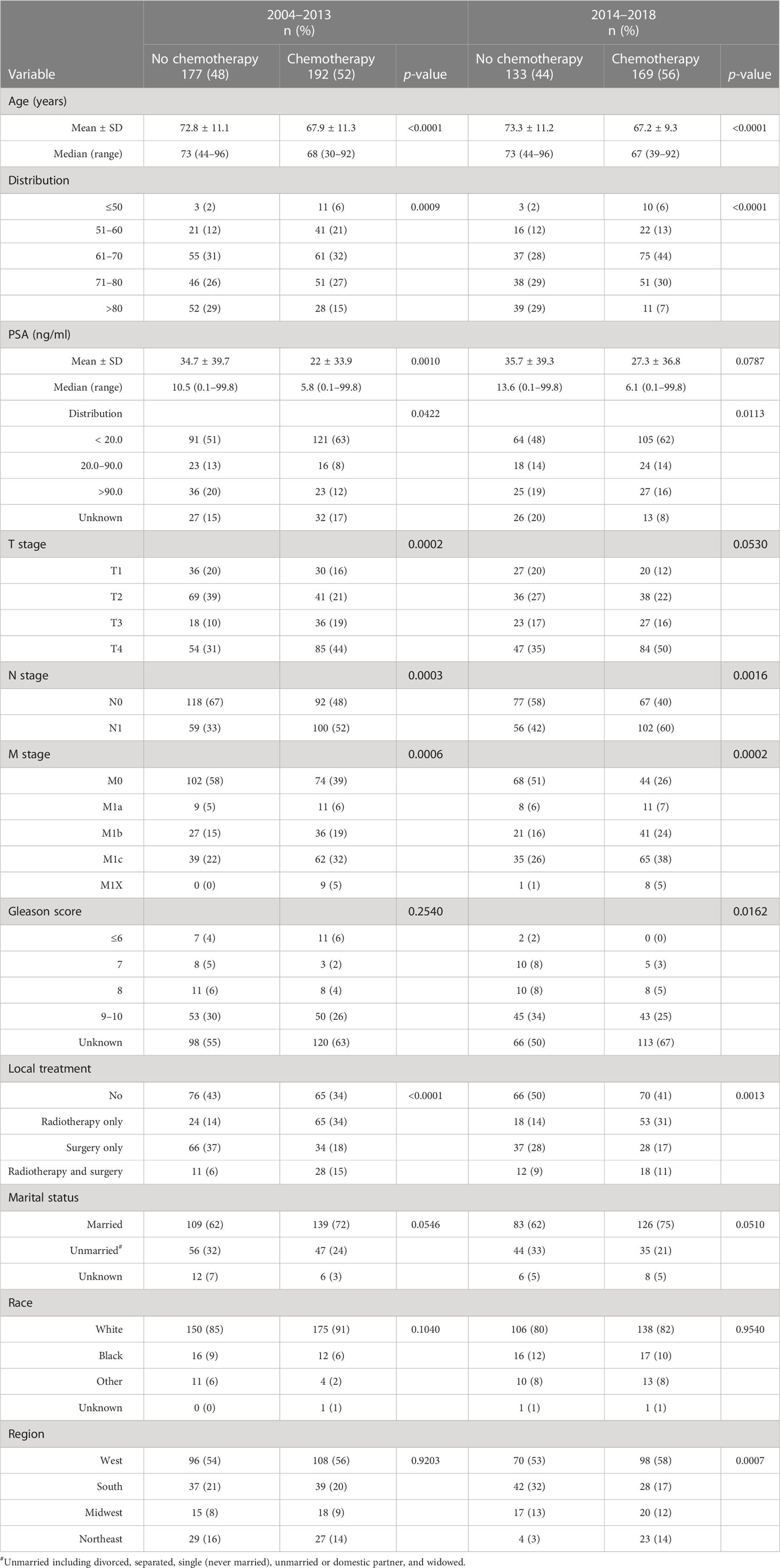
Table 6 Descriptive characteristics of patients with a de novo diagnosis of prostate neuroendocrine carcinoma who were initially treated with or without chemotherapy during 2004–2013 and 2014–2018.
Impact of chemotherapy on survival in patients with neuroendocrine carcinoma
Patients with prostate neuroendocrine carcinoma receiving chemotherapy had significantly higher proportions of cancer- specific death and overall death compared to those without chemotherapy during 2004–2013. During 2014–2018, the proportions of cancer- specific and overall deaths were comparable between chemotherapy and no-chemotherapy groups (Supplementary Table S6). Survival curves showed that chemotherapy was associated with slightly worse cancer- specific survival (p=0.0225) but not overall survival (p=0.5559) in patients with neuroendocrine carcinoma during 2004–2013 (Figures 3A, B). Chemotherapy was not associated with cancer- specific (p=0.1060) and overall (p=0.1011) survival during 2014–2018 (Figures 3C, D). Multivariate survival analyses showed that chemotherapy was associated with improved cancer- specific survival (HR= 0.62, 95% CI: 0.45–0.87, p=0.0055) and overall survival (HR=0.69, 95% CI: 0.51–0.94, p = 0.0176) in patients with neuroendocrine carcinoma during 2014–2018. Conversely, chemotherapy was not significantly associated with cancer- specific survival (HR = 0.99, 95% CI: 0.76–1.29, p = 0.9138) and survival (HR= 0.89, 95% CI: 0.70–1.14, p=0.3540) during 2004–2013 (Table 7).
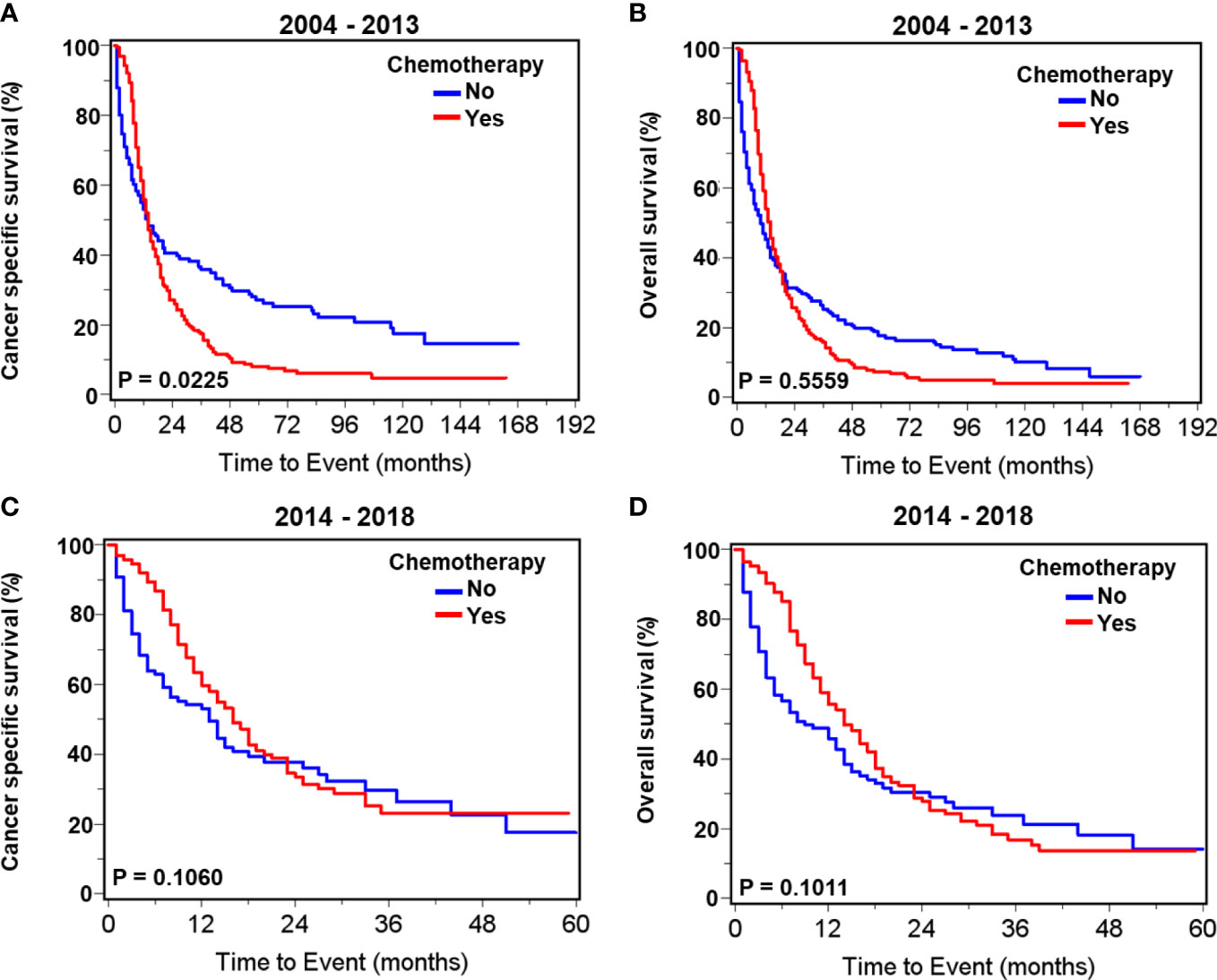
Figure 3 Kaplan–Meier survival curves for cancer-specific and overall survival in patients with de novo neuroendocrine prostate carcinoma with or without chemotherapy. For patients diagnosed during 2004–2013, curves of cancer- specific survival (A) and overall survival (B). For patients diagnosed during 2014–2018, curves of cancer- specific survival (C) and overall survival (D).
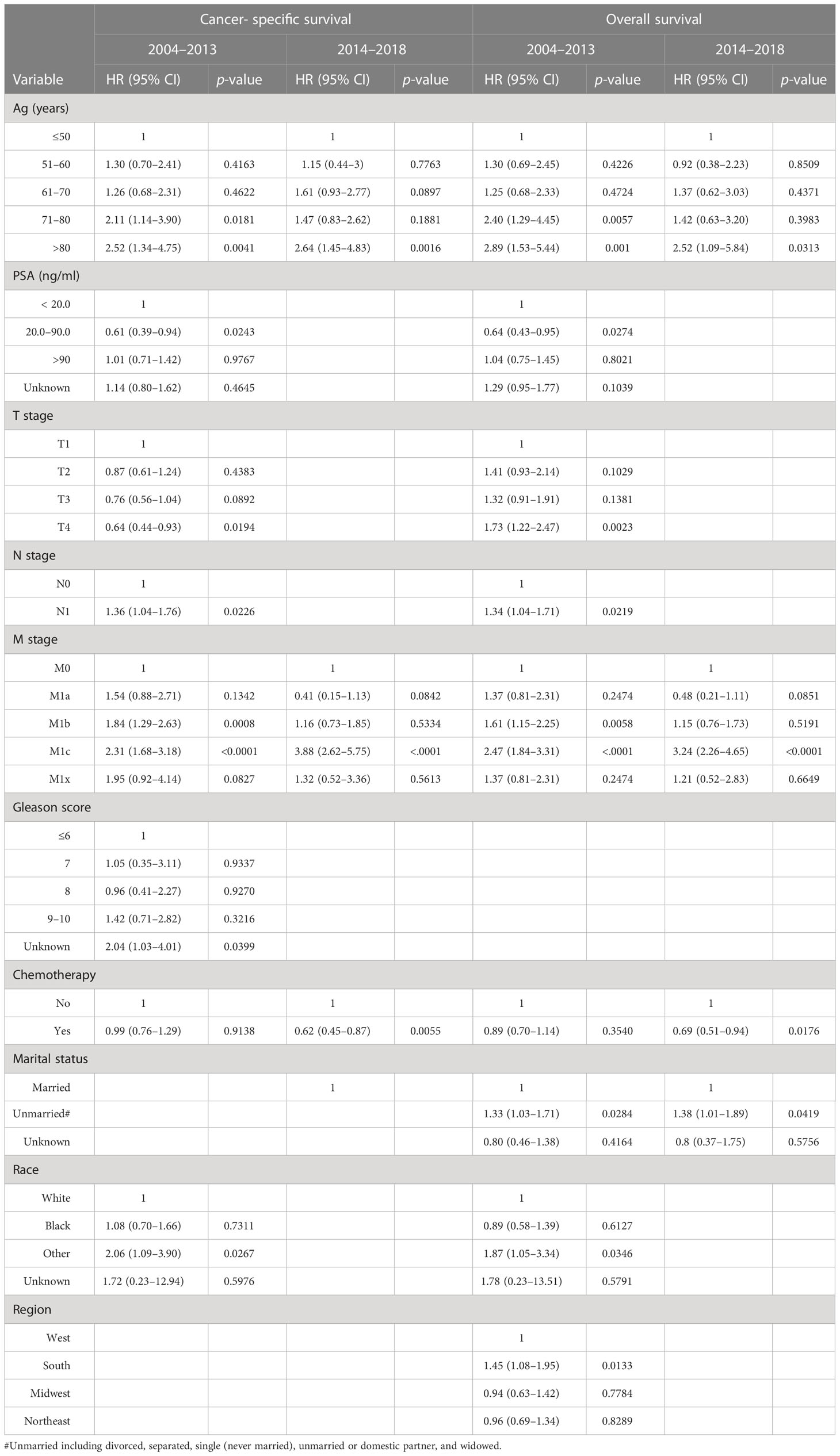
Table 7 Multivariate analyses of risk factors related to survival in patients with a de novo diagnosis of prostate neuroendocrine carcinoma during 2004–2013 and 2014–2018.
Discussion
The SEER 18 database, capturing approximately 28% of the total United States population, provides a valuable resource to assess patterns of care for prostate cancer. We specifically examined the utilization of systemic cytotoxic chemotherapy for men with metastatic disease at an initial diagnosis from 2004 to 2018, a period when results of clinical trials suggested new strategies for care. As expected, few men not showing metastatic disease received initial chemotherapy throughout the period. During 2004–2013, the proportion of patients with de novo metastatic adenocarcinoma receiving chemotherapy was low (5.8%) but significantly increased to an average of 21.4% during 2014 –2018. The pattern observed likely represents a shared decision- making process (27) between the patient and provider throughout the interval (2004–2018) with utilization of chemotherapy increasingly being offered as an option following publication of new clinical trial results approximately 2014 (10, 11, 22, 23). By 2014, National Comprehensive Cancer Network (NCCN) guidelines recommended ADT plus docetaxel for six cycles as one of several options for the initial treatment of castration- naive metastatic prostate adenocarcinoma (24). Multivariate survival analysis of data from 2004 to 2013 showed that chemotherapy in men with distant metastasis was associated with worse cancer-specific and overall survival; by contrast, it was associated with improved prognosis during 2014–2018.
This is most likely related to patient selection, with chemotherapy being used for men with the most ominous presentation. In contrast to adenocarcinoma, men with neuroendocrine prostate carcinoma (0.1% of all prostate cancer) show an average of 54% of patients receiving chemotherapy with no clear directional change over the entire period. Interestingly, chemotherapy was associated with improved cancer-specific and overall survival in neuroendocrine carcinoma patients during 2014–2018 but not during 2004–2013, perhaps due to improvements in supportive care and patient selection.
For decades, studies of cytotoxic chemotherapy failed to demonstrate benefits for men with metastatic prostate cancer due to challenges in objectively measuring response for a disease dominated by nodal and bone metastasis coupled with a lack of efficacy (28–30). The approval of mitoxantrone for pain control established chemotherapy as an option for men with advanced metastatic disease in 1999 (16, 31). By 2004, a landmark series of studies showed that docetaxel-based therapy for the first time demonstrated improved survival for men with metastatic- castration-resistant prostate adenocarcinoma (17, 18, 31). Newer studies refined our knowledge and documented benefits dependent upon dose intensity (32) and that taxane analogues may prolong benefits (21). Such progress led investigators to consider moving docetaxel chemotherapy into initial treatment strategies for newly diagnosed hormone-sensitive metastatic prostate cancer. Trials showing improved biochemical progression-free survival with the addition of docetaxel to ADT in metastatic hormone-naive prostate cancer patients were emerging by 2013 (23), and by 2015, studies were showing that upfront docetaxel chemotherapy improved overall survival, failure-free survival, and progression-free survival (10, 22, 23). Our study examines how this knowledge impacted therapy for prostate cancer patients in the non-protocol standard practice over the time frame that these results became available (33).
As expected, our study revealed that chemotherapy for those with metastatic adenocarcinoma at diagnosis was sparingly employed at 5.8% prior to 2014 followed by a dramatic rise to nearly 30% from 2014 to 2016. The rapid change was certainly driven by the enthusiasm derived from the new studies and guidelines (23). Although it is possible that the wider insurance coverage becoming available at this time resulting from the American Afford Care Act (34) contributed somewhat, we suspect that the main driving force of adoption was the published research and NCCN guidelines. Yet, it may be surprising to some that only 20%–30% are receiving upfront chemotherapy after 2013–2014. We suspect that both providers and patients contribute to these findings. Our analysis indicates that practitioners are using a range of criteria in patient selection for early chemotherapy, which of course is then modulated by patient preferences after considering risks and benefits. Men receiving initial chemotherapy are younger, typically married (perhaps a marker of support systems), with more advanced T stage, higher Gleason score, positive lymph node metastasis, and more advanced M staging. Thus, it is likely that patients perceived to have a more aggressive phenotype based upon established risk factors are more likely to be offered chemotherapy by practitioners. Of course, we do not have data regarding the initial discussion of options for these men and what percentage were offered chemotherapy and declined. One additional factor may be referral patterns with the urologist typically serving as the initial focal point for diagnosis and perhaps not having medical oncology engaged at the time of the initial treatment plan. Clearly, with only 30% of men receiving chemotherapy, the perceived benefits do not exceed risks in the minds of practitioners and or patients.
Interestingly, we found that from 2004 to 2013, the administration of chemotherapy was associated with worse prognosis compared with no chemotherapy. As chemotherapy became more widespread, we observed a significantly improved cancer- specific and overall survival during 2014–2018. Notably, these findings are verified in subsequent propensity score matching analyses. The poorer outcomes in 2004–2013 likely represents the use of initial chemotherapy for men with greater cancer volume and more aggressive disease, a subgroup destined to have a shorter life expectancy. The lack of a standard of care chemotherapy regimen (agents, dose, and duration) for de novo distant metastatic patients may also attribute to the worse outcome during the period. Multiple factors, such as cancer grade, distribution of lesions and volume of disease, PSA, age, and other variables impact offering the chemotherapy option. A previous publication, employing an older version of SEER database (2014–2015) with far less data, also found that chemotherapy-exposed prostate cancer patients exhibited significantly better overall survival (HR=0.82, 95% CI: 0.72–0.96, p=0.01) compared to their chemotherapy- naive counterparts (35). The finding was confirmed in propensity score matching analyses (multivariable HR: 0.77, 95% CI: 0.66-0.90, p<0.001) (35). Utilizing a large national cancer database in the United States (2014–2015), another retrospective cohort study revealed that upfront chemotherapy was associated with longer overall survival (HR= 0.78, 95% CI: 68–0.89, p < 0.001) in men with de novo, treatment- naive metastatic prostate cancer after adjusting for patient and clinical variables (36).
Due to lack of precise data in the SEER database regarding lesion numbers, size, and locations of metastasis, it is not possible to precisely define the high-volume disease. Our multivariate survival analysis suggests that chemotherapy displayed a more beneficial impact for men with potentially higher volume. Men with the nodal metastasis experience no benefit, whereas those with bone metastasis fare better with chemotherapy, and the greatest benefit is seen in men with the visceral disease with or without bone and nodal metastasis.
Not surprisingly, age is shown to be a risk factor for death and particularly strong over the age of 80 years. This and other studies suggest that chemotherapy is less often prescribed for older patients with metastatic prostate cancers (37, 38) and likely due perceived risks associated with frailty, accumulating comorbidities, and poor resilience. Interestingly, we find the benefit of chemotherapy to be best in the ages of 70 –80 years, both on cancer- specific and overall survival. Those younger than 70 and older than 80 tended to gain little or no benefit from chemotherapy. Perhaps, younger men with de novo metastatic prostate cancer have a more aggressive disease that is less sensitive to therapy, while older men have comorbidities impacting resilience and tolerability. Similar to our finding, another report suggests that chemotherapy plus ADT, compared to ADT alone, was associated with improved overall survival in de novo metastatic prostate cancer patients ≥70 years but not in patients <70 years (39).
Our multivariate survival analyses quantitate the impact of several relevant variables on survival in this cohort. We have limited data on the impact of integrating radiotherapy and surgery but do see a worse outcome noted for those receiving both. It is possible that men with significant and symptomatic local disease have a more aggressive phenotype or medical complications that impact survival. We see a clear trend for married men doing better in survival, perhaps a marker for stronger support systems and compliance with care plans. We did not detect a difference in response based on black vs. white race, but the Southern region of the United States consistently shows poorer survival than the Midwest, with the West and Northeast being similar, perhaps reflecting the impact of social and economic issues on health care access and quality (40, 41). Higher grade cancers and greater disease burden, as indicated by PSA, TNM staging, and Gleason scoring, were strongly related to poor outcomes for those presenting with de novo metastatic adenocarcinoma.
Neuroendocrine carcinoma is the rare histological type of prostate cancer with the worst prognosis (25, 42). The disease is typically defined histopathologically and often characterized by lower PSA secretion, higher risk of metastasis, an inferior response to ADT, and poor prognosis (26, 43–45). Small early studies supported the use of chemotherapy with agents often used for cancers of other tissue origins with neuroendocrine features (46). Chemotherapy, radiotherapy, or combinations are associated with improved overall survival compared with palliative therapy (47). We observe that an average of 54% of patients with neuroendocrine prostate cancer are treated with first-line chemotherapy and is steady from 2004 to 2018. Chemotherapy is associated with improved cancer-specific and overall survival during 2014–2018, but not during 2004–2013, perhaps due to improved supportive care plans and better patient selection. Hence, our findings may support the use of chemotherapy for both de novo and treatment-emergent neuroendocrine prostate cancer due to the potential survival benefits.
This retrospective study has limitations. Our study is subject to the constraints of the SEER database, including the precision of data collection and the number of variables collected. There is a lack of information on the specific chemotherapy regime, dose of drug, compliance, and dose intensity including the number of cycles. The database has no information on concurrent ADT and the types or duration of agents provided. Indeed, we suspect that the increased use of effective agents impacting androgen receptor signaling may reduce the frequency of selecting taxane-based chemotherapy in the up-front approach to de novo metastatic disease since 2016 (Figure 1). There is no information on other important outcomes such as toxicities of chemotherapy, quality of life, recurrence of cancer and additional therapy, and the dynamic change in PSA. Our study is limited by the inherent challenges of a retrospective cohort design. For example, it is likely that patients with better performance status were selected for chemotherapy, which may contribute to better survival outcomes. Hence, selection bias is inevitable but is clearly a component of practice decisions. The strength of this study is the very large sample size providing accurate insight into practice patterns in a real world setting during a period when new data were emerging.
Conclusions
Chemotherapy has been increasingly employed in the community for men with de novo metastatic adenocarcinoma at diagnosis following a series of publications in 2013–2014, yet for less than one-third of men. Our data suggest that both medical practitioners and patients may be carefully considering the risks and benefits for each individual based upon age, histopathological features, PSA, staging criteria, comorbidities, and a number of factors such as overall performance status. Findings of this study support the initial treatment with chemotherapy in men in the 70–80 age group presenting de novo with more aggressive features or greater volume of metastatic disease. Clearly, our work suggests that shared decision making is the strategy in the community for men presenting with metastatic adenocarcinoma who are mostly seniors and often with comorbidities. In contrast, the treatment of neuroendocrine prostate cancer with initial chemotherapy has been stable at approximately 50%, but with improving outcomes in recent years.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Concept and design: SW and SC. Data acquisition and analysis: SW and SC. Interpretation of data: all authors. Drafting of the manuscript: SW and SC. Critical revision of the manuscript for important intellectual content: all authors. Supervision: SC. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Prostate Cancer Prevention Development Fund supported by the Karlsberger Family (SKC, 302024) and The Biostatistics Shared Resource supported by The Ohio State University Comprehensive Cancer Center (NIH P30CA016058).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1165188/full#supplementary-material
References
1. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: Cancer J Clin (2023) 73(1):17–48. doi: 10.3322/caac.21763
2. Moyer VA, Force USPST. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med (2012) 157(2):120–34. doi: 10.1059/0003-4819-157-2-201207170-00459
3. Desai MM, Cacciamani GE, Gill K, Zhang J, Liu L, Abreu A, et al. Trends in incidence of metastatic prostate cancer in the US. JAMA Netw Open (2022) 5(3):e222246. doi: 10.1001/jamanetworkopen.2022.2246
4. Jemal A, Fedewa SA, Ma J, Siegel R, Lin CC, Brawley O, et al. Prostate cancer incidence and PSA testing patterns in relation to USPSTF screening recommendations. JAMA (2015) 314(19):2054–61. doi: 10.1001/jama.2015.14905
5. Eapen RS, Herlemann A, Washington SL 3rd, Cooperberg MR. Impact of the united states preventive services task force 'D' recommendation on prostate cancer screening and staging. Curr Opin Urol (2017) 27(3):205–9. doi: 10.1097/MOU.0000000000000383
6. Huggins C HC. Studies on prostatic cancer. i. the effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 93–7. Cancer Res (1941) 1:293–97.
7. Chi KN, Agarwal N, Bjartell A, Chung BH, Pereira de Santana Gomes AJ, Given R, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med (2019) 381(1):13–24. doi: 10.1056/NEJMoa1903307
8. de Wit R, de Bono J, Sternberg CN, Fizazi K, Tombal B, Wulfing C, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med (2019) 381(26):2506–18. doi: 10.1056/NEJMoa1911206
9. James ND, de Bono JS, Spears MR, Clarke NW, Mason MD, Dearnaley DP, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med (2017) 377(4):338–51. doi: 10.1056/NEJMoa1702900
10. Sweeney CJ, Chen YH, Carducci M, Liu G, Jarrard DF, Eisenberger M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. New Engl J Med (2015) 373(8):737–46. doi: 10.1056/NEJMoa1503747
11. Kyriakopoulos CE, Chen YH, Carducci MA, Liu G, Jarrard DF, Hahn NM, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer: long-term survival analysis of the randomized phase III E3805 CHAARTED trial. J Clin Oncol (2018) 36(11):1080–7. doi: 10.1200/JCO.2017.75.3657
12. Siegel DA, O'Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and Race/Ethnicity - united states, 2001-2017. MMWR Morbidity mortality weekly Rep (2020) 69(41):1473–80. doi: 10.15585/mmwr.mm6941a1
13. Armstrong AJ, Lin P, Tombal B, Saad F, Higano CS, Joshua AM, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol (2020) 78(3):347–57. doi: 10.1016/j.eururo.2020.04.061
14. Halabi S, Lin CY, Kelly WK, Fizazi KS, Moul JW, Kaplan EB, et al. Updated prognostic model for predicting overall survival in first-line chemotherapy for patients with metastatic castration-resistant prostate cancer. J Clin Oncol (2014) 32(7):671–7. doi: 10.1200/JCO.2013.52.3696
15. Tangen CM, Hussain MH, Higano CS, Eisenberger MA, Small EJ, Wilding G, et al. Improved overall survival trends of men with newly diagnosed M1 prostate cancer: a SWOG phase III trial experience (S8494, S8894 and S9346). J Urol (2012) 188(4):1164–9. doi: 10.1016/j.juro.2012.06.046
16. Kantoff PW, Halabi S, Conaway M, Picus J, Kirshner J, Hars V, et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group b 9182 study. J Clin Oncol (1999) 17(8):2506–13. doi: 10.1200/JCO.1999.17.8.2506
17. Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med (2004) 351(15):1502–12. doi: 10.1056/NEJMoa040720
18. Petrylak DP, Tangen CM, Hussain MH, Lara PN Jr., Jones JA, Taplin ME, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med (2004) 351(15):1513–20. doi: 10.1056/NEJMoa041318
19. Armstrong AJ, Carducci MA. Chemotherapy for advanced prostate cancer: results of new clinical trials and future studies. Curr Oncol Rep (2005) 7(3):220–7. doi: 10.1007/s11912-005-0077-y
20. Berry W, Eisenberger M. Achieving treatment goals for hormone-refractory prostate cancer with chemotherapy. Oncologist (2005) 10 Suppl 3:30–9. doi: 10.1634/theoncologist.10-90003-30
21. de Bono JS, Oudard S, Ozguroglu M, Hansen S, Machiels JP, Kocak I, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet (2010) 376(9747):1147–54. doi: 10.1016/S0140-6736(10)61389-X
22. Clarke NW, Ali A, Ingleby FC, Hoyle A, Amos CL, Attard G, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results from the STAMPEDE trial. Ann Oncol (2019) 30(12):1992–2003. doi: 10.1093/annonc/mdz396
23. Gravis G, Fizazi K, Joly F, Oudard S, Priou F, Esterni B, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol (2013) 14(2):149–58. doi: 10.1016/S1470-2045(12)70560-0
24. Mohler JL, Kantoff PW, Armstrong AJ, Bahnson RR, Cohen MB, D'Amico AV, et al. NCCN clinical practice guidelines in oncology, in: Prostate cancer. version 1 (2014). NCCNorg (Accessed Novemver 08, 2022).
25. Bronkema C, Arora S, Sood A, Dalela D, Keeley J, Borchert A, et al. Rare histological variants of prostate adenocarcinoma: a national cancer database analysis. J Urol (2020) 204(2):260–6. doi: 10.1097/JU.0000000000001011
26. Yao J, Liu Y, Liang X, Shao J, Zhang Y, Yang J, et al. Neuroendocrine carcinoma as an independent prognostic factor for patients with prostate cancer: a population-based study. Front Endocrinol (Lausanne) (2021) 12:778758. doi: 10.3389/fendo.2021.778758
27. Kashaf MS, McGill E. Does shared decision making in cancer treatment improve quality of life? a systematic literature review. Med Decis Making (2015) 35(8):1037–48. doi: 10.1177/0272989X15598529
28. Rosenthal SA, Hunt D, Sartor AO, Pienta KJ, Gomella L, Grignon D, et al. A phase 3 trial of 2 years of androgen suppression and radiation therapy with or without adjuvant chemotherapy for high-risk prostate cancer: final results of radiation therapy oncology group phase 3 randomized trial NRG oncology RTOG 9902. Int J Radiat Oncol Biol Phys (2015) 93(2):294–302. doi: 10.1016/j.ijrobp.2015.05.024
29. Bouman-Wammes EW, van den Berg HP, de Munck L, Beeker A, Smorenburg CH, Vervenne WL, et al. A randomised phase II trial of docetaxel versus docetaxel plus carboplatin in patients with castration-resistant prostate cancer who have progressed after response to prior docetaxel chemotherapy: the RECARDO trial. Eur J Cancer (2018) 90:1–9. doi: 10.1016/j.ejca.2017.11.021
30. Fléchon A, Pouessel D, Ferlay C, Perol D, Beuzeboc P, Gravis G, et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French genito-urinary tumor group (GETUG) P01 trial. Ann Oncol (2011) 22(11):2476–81. doi: 10.1093/annonc/mdr004
31. Berthold DR, Pond GR, Soban F, de Wit R, Eisenberger M, Tannock IF. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J Clin Oncol (2008) 26(2):242–5. doi: 10.1200/JCO.2007.12.4008
32. Kushnir I, Mallick R, Ong M, Canil C, Bosse D, Koczka K, et al. Docetaxel dose-intensity effect on overall survival in patients with metastatic castrate-sensitive prostate cancer. Cancer Chemother Pharmacol (2020) 85(5):863–8. doi: 10.1007/s00280-020-04063-7
33. Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, et al. Update on systemic prostate cancer therapies: management of metastatic castration-resistant prostate cancer in the era of precision oncology. Eur Urol (2019) 75(1):88–99. doi: 10.1016/j.eururo.2018.03.028
34. Zhao J, Mao Z, Fedewa SA, Nogueira L, Yabroff KR, Jemal A, et al. The affordable care act and access to care across the cancer control continuum: a review at 10 years. CA: Cancer J Clin (2020) 70(3):165–81. doi: 10.3322/caac.21604
35. Hoeh B, Wurnschimmel C, Flammia RS, Horlemann B, Sorce G, Chierigo F, et al. Effect of chemotherapy on overall survival in contemporary metastatic prostate cancer patients. Front Oncol (2021) 11:778858. doi: 10.3389/fonc.2021.778858
36. Weiner AB, Ko OS, Li EV, Vo AX, Desai AS, Breen KJ, et al. Survival following upfront chemotherapy for treatment-naive metastatic prostate cancer: a real-world retrospective cohort study. Prostate Cancer Prostatic Dis (2021) 24(1):261–7. doi: 10.1038/s41391-020-00278-0
37. Jha GG, Anand V, Soubra A, Konety BR. Challenges of managing elderly men with prostate cancer. Nat Rev Clin Oncol (2014) 11(6):354–64. doi: 10.1038/nrclinonc.2014.71
38. Oh WK, Tully P, Kantoff PW, Regan MM. Physician attitudes toward cytotoxic chemotherapy use in patients with advanced prostate carcinoma. Cancer. (2003) 97(9):2171–9. doi: 10.1002/cncr.11344
39. Lage DE, Michaelson MD, Lee RJ, Greer JA, Temel JS, Sweeney CJ. Outcomes of older men receiving docetaxel for metastatic hormone-sensitive prostate cancer. Prostate Cancer Prostatic Dis (2021) 24(4):1181–8. doi: 10.1038/s41391-021-00389-2
40. Oates GR, Jackson BE, Partridge EE, Singh KP, Fouad MN, Bae S. Sociodemographic patterns of chronic disease: how the mid-south region compares to the rest of the country. Am J Prev Med (2017) 52(1S1):S31–S9. doi: 10.1016/j.amepre.2016.09.004
41. Islami F, Guerra CE, Minihan A, Yabroff KR, Fedewa SA, Sloan K, et al. American Cancer society's report on the status of cancer disparities in the united states, 2021. CA Cancer J Clin (2022) 72(2):112–43. doi: 10.3322/caac.21703
42. Alanee S, Moore A, Nutt M, Holland B, Dynda D, El-Zawahry A, et al. Contemporary incidence and mortality rates of neuroendocrine prostate cancer. Anticancer Res (2015) 35(7):4145–50.
43. Zaffuto E, Pompe R, Zanaty M, Bondarenko HD, Leyh-Bannurah SR, Moschini M, et al. Contemporary incidence and cancer control outcomes of primary neuroendocrine prostate cancer: a SEER database analysis. Clin Genitourin cancer (2017) 15(5):e793–800. doi: 10.1016/j.clgc.2017.04.006
44. Zhu J, Liang X, Wu D, Chen S, Yang B, Mao W, et al. Clinicopathological characteristics and survival outcomes in neuroendocrine prostate cancer: a population-based study. Med (Baltimore) (2021) 100(15):e25237. doi: 10.1097/MD.0000000000025237
45. Conteduca V, Oromendia C, Eng KW, Bareja R, Sigouros M, Molina A, et al. Clinical features of neuroendocrine prostate cancer. Eur J Cancer (2019) 121:7–18. doi: 10.1016/j.ejca.2019.08.011
46. Moore SR, Reinberg Y, Zhang G. Small cell carcinoma of prostate: effectiveness of hormonal versus chemotherapy. Urology (1992) 39(5):411–6. doi: 10.1016/0090-4295(92)90235-O
47. Wang HT, Yao YH, Li BG, Tang Y, Chang JW, Zhang J. Neuroendocrine prostate cancer (NEPC) progressing from conventional prostatic adenocarcinoma: factors associated with time to development of NEPC and survival from NEPC diagnosis-a systematic review and pooled analysis. J Clin Oncol (2014) 32(30):3383–90. doi: 10.1200/JCO.2013.54.3553
Keywords: prostate cancer, chemotherapy, SEER, adenocarcinoma, neuroendocrine
Citation: Wang S, Yin M, Wang P, Folefac E, Monk JP, Tabung FK and Clinton SK (2023) Chemotherapy for the initial treatment of metastatic prostate adenocarcinoma and neuroendocrine carcinoma at diagnosis: real world application and impact in the SEER database (2004 –2018). Front. Oncol. 13:1165188. doi: 10.3389/fonc.2023.1165188
Received: 13 February 2023; Accepted: 18 May 2023;
Published: 09 June 2023.
Edited by:
Sifeng Qu, Shandong University, ChinaReviewed by:
Che-Kai Tsao, Icahn School of Medicine at Mount Sinai, United StatesAhmed El-Zawahry, University of Toledo, United States
Copyright © 2023 Wang, Yin, Wang, Folefac, Monk, Tabung and Clinton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihua Wang, U2hpaHVhLldhbmdAb3N1bWMuZWR1; Steven K. Clinton, U3RldmVuLmNsaW50b25Ab3N1bWMuZWR1
 Shihua Wang
Shihua Wang Ming Yin
Ming Yin Peng Wang1,2
Peng Wang1,2 Edmund Folefac
Edmund Folefac J. Paul Monk
J. Paul Monk Fred K. Tabung
Fred K. Tabung