- 1Department of Digestive Oncology, Cancer Center, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 2Department of Biochemistry and Molecular Biology, Shanxi Key Laboratory of Birth Defect and Cell Regeneration, Shanxi Medical University, Taiyuan, Shanxi, China
- 3Department of Gastroenterology, Shanxi Province Cancer Hospital/Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences/Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, Shanxi, China
Targeted therapy has been standardized in front-line therapies for metastatic colorectal cancer (mCRC), while explicit recommendations for third- or later-line are still lacking. This study evaluated the efficacy and safety of combining targeted therapy with chemotherapy in the third- or later-line treatment for mCRC via meta-analysis, providing evidence-based guidance for clinical or research practice. Comprehensive retrieval of related studies was conducted according to the PRISMA guideline. Studies were stratified with patient characteristics and pharmacological classification of the drugs. For the data available for quantitative analysis, pooled overall response rate, disease control rate, hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS), and adverse events rate with respective 95% confidence intervals (CIs) were calculated. A total of 22 studies (1,866 patients) were included in this meta-analysis. Data from 17 studies (1,769 patients) involving targets of epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) were extracted for meta-analyses. The overall response rates for monotherapy and combined therapy were 4% (95% CI: 3%, 5%) and 20% (95% CI: 11%, 29%). The pooled HRs (combined therapy vs. mono) for OS and PFS were 0.72 (95% CI: 0.53, 0.99) and 0.34 (95% CI: 0.26, 0.45). Another five studies were included in narrative depiction, involving targets of BRAF, HER-2, ROS1, and NTRK. The findings of this meta-analysis indicate that VEGF and EGFR inhibitors manifest promising clinical response rates and prolonged survival in the treatment of mCRC with acceptable adverse events.
Introduction
Colorectal cancer (CRC) is one of the most common malignancies worldwide; the estimated annual incidence and mortality are 19.7/100,000 and 8.9/100,000 (1, 2). Among patients diagnosed with CRC, 20% had metastatic colorectal cancer (mCRC) and 40% had recurrence after previous treatment of localized diseases (3, 4). Furthermore, prognosis remains poor after standard treatment for patients with mCRC, with a median 5-year survival rate of less than 20% (4).
At present, the standard first-line and second-line therapies for mCRC are a combination of doublet or cytotoxic triplet chemotherapy and targeted therapies, including anti-epidermal growth factor receptor (EGFR) or anti-vascular endothelial growth factor (VEGF) antibody, the choice of treatment is influenced by patient features, cancer characteristics, and molecular profiles (5–8). In addition, RAS and BRAF tests are recommended by the European Society for Medical Oncology (ESMO) and the United States (US) National Comprehensive Cancer Network (NCCN) guidelines before the initiation of first-line therapy (9, 10). The choice of second-line regimen depends on the first-line systemic treatment, and approximately two-thirds of mCRC patients received second-line treatment (11). Fluorouracil, folinic acid, and irinotecan (FOLFIRI) and fluorouracil, folinic acid, and oxaliplatin (FOLFOX) are typical second-line chemotherapy options for mCRC patients (12). However, the efficacy of chemotherapy is very low in the third-line treatment of CRC, and tumor shrinkage is rarely observed (13). Immunotherapy revolutionized the oncology landscape in the past 10 years, pembrolizumab or nivolumab are recommended as treatment options in second-line and beyond for patients with deficient MMR/MSI-high mCRC (11, 12). For CRC patients receiving third-line treatment, considering molecular cancer characteristics and clinical trial registration is an important aspect of management (12). Cetuximab or panitumumab is particularly effective for KRAS/NRAS wild-type mCRC patients not previously treated with EGFR antibodies and is recommended as the standard treatment for the third-line or later-line follow-up treatment (14, 15). Regorafenib is recommended in RAS wild-type patients previously treated with EGFR antibodies (10). Furthermore, receptor tyrosine kinase inhibitor (rTKI) has been shown to prolong progression-free survival (PFS) in refractory mCRC patients with acceptable tolerability (16). Agents targeting human epidermal growth factor receptor-2 (HER2), neurotrophic tyrosine receptor kinase (NTRK), and c-ros oncogene 1, receptor tyrosine kinase (ROS1) were used in the treatment of mCRC (17–19). Nevertheless, EGFR inhibitors are associated with toxicity, including rash and diarrhea in tissues expressing EGFR. Multi-kinase inhibitors can cause hand-foot skin reactions, rash, fatigue, diarrhea, and hypertension (20). Therefore, when the quality of life gains importance as a therapeutic goal, the difference in the mechanism of action and, more importantly, the safety of available third-line/later-line mCRC therapy may guide the treatment choices of individual patients.
Targeted therapy has been standardized in front-line therapies for mCRC, but explicit recommendations for third- or later-line are still lacking. As far as it is concerned, several studies reported the efficacy and safety of targeted treatment alone or combined chemotherapy (16, 21–28). This study aimed to conduct a meta-analysis through a synthesis of the evidence to generate a comprehensive assessment of efficacy and safety of third-line or later-line targeted treatment for patients with mCRC and subsequently to provide evidence and clues for clinical research and practice.
Materials and methods
Statements
This meta-analysis was conducted based on published citations that had declared ethical approvals, and no original clinical raw data of the published results were collected or utilized, thereby ethical approval was not warranted for this study. This study was based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) (29).
Search strategy and selection criteria
We systematically searched the online electronic databases, PubMed, Scopus, and Embase, from the databases’ inception to June 16, 2022, with articles in English all considered. The following keywords and terms were used for the online database search: third-line, later-line, fruquintinib, famitinib, bevacizumab, ramucirumab, cetuximab, panitumumab, trastuzumab, pertuzumab, tucatinib, lapatinib, larotrectinib, entrectinib, encorafenib, vemurafenib, targeted therapy, VEGF, ALK, ROS1, EGFR, HER2, NTKR, BRAF, metastatic colorectal cancer, and mCRC. The search strategy was ((((((((((((((((((((((((third-line[Title/Abstract]) OR (later-line[Title/Abstract])) OR (fruquintinib[Title/Abstract])) OR (famitinib[Title/Abstract])) OR (bevacizumab[Title/Abstract])) OR (ramucirumab[Title/Abstract])) OR (cetuximab[Title/Abstract])) OR (panitumumab[Title/Abstract])) OR (trastuzumab[Title/Abstract])) OR (pertuzumab[Title/Abstract])) OR (tucatinib[Title/Abstract])) OR (lapatinib[Title/Abstract])) OR (larotrectinib[Title/Abstract])) OR (entrectinib[Title/Abstract])) OR (encorafenib[Title/Abstract])) OR (vemurafenib[Title/Abstract])) OR (targeted therapy[Title/Abstract])) OR (VEGF[Title/Abstract])) OR (ALK[Title/Abstract])) OR (ROS1[Title/Abstract])) OR (EGFR[Title/Abstract])) OR (HER2[Title/Abstract])) OR (NTKR[Title/Abstract])) OR (BRAF[Title/Abstract])) AND ((metastatic colorectal cancer[Title/Abstract]) OR (mCRC[Title/Abstract])) AND (english[Filter]). The references of related reviews and included articles were also searched to retrieve additional studies not previously identified in the initial literature search. Inclusion criteria were as follows: clinical trials or cohort studies evaluating the efficacy and safety of third-line or later-line targeted treatment of patients with mCRC and relevant outcomes regarding treatment effects and adverse events were reported or could be calculated from the available data in the citation. Exclusion criteria included conference abstracts, case reports or case series, reviews, news, and editorials.
Two independent investigators (Wen-Hui Xue and Xue-Wei Li) accomplished the literature search and conducted the process of study selection. A third author (Wen-Hui Yang) was involved if no consensus was achieved.
Data extraction and quality assessments
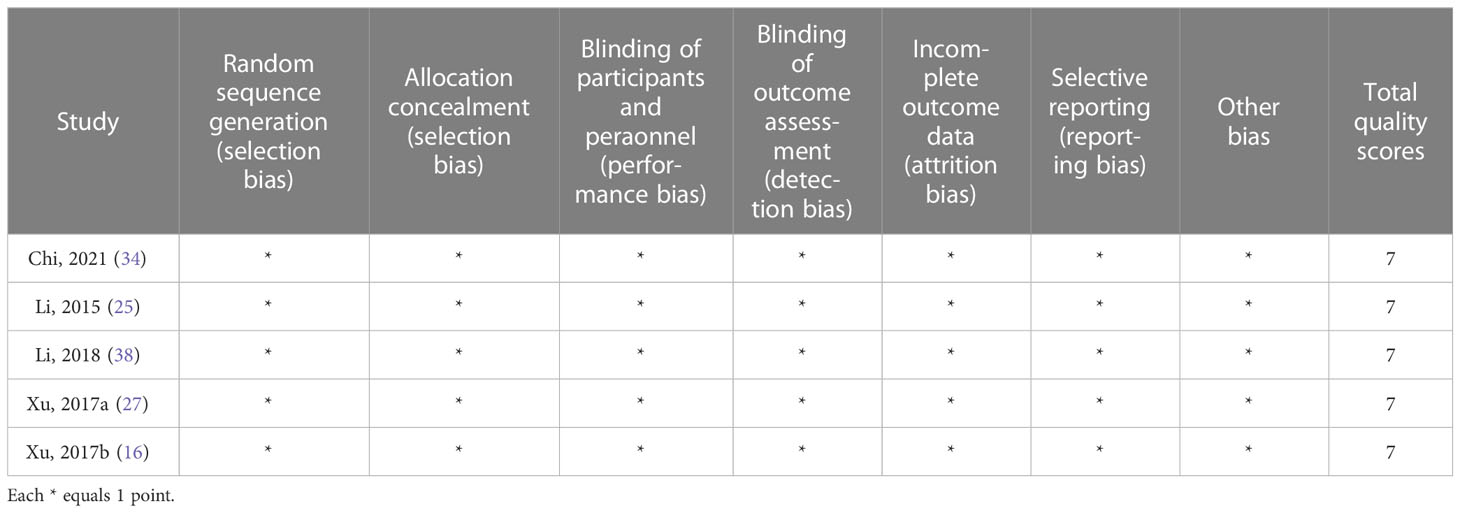
The following information was extracted from each study: name of the first author, year of publication, country, study design, number of patients, age of patients, percentage of females, patient performance, targeted molecule, lines of current treatment, therapy schedule, response rate, complete response rate (ORR), partial response rate, stable disease rate, disease progression rate, disease control rate, hazard ratios (HRs) for overall survival (OS) and progression-free survival (PFS), and adverse events rate. Clinical response and disease progression were assessed according to Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) (30). The Cochrane Collaboration tool was used to evaluate the risk of bias in randomized trials enrolled in this meta-analysis (31). The methodological index for non-randomized studies (MINORS) was used for single-arm studies (32).
Statistical analysis
The R (A language and environment for statistical computing. Version 3.6.1) was used for statistical analyses. Pooled rates and HRs with their respective 95% confidence intervals (CIs) were synthesized with a random or fixed-effects model. A random-effects model was used if the I² value was > 50%; otherwise, a fixed-effects model was used. The Cochran Q test was used to assess heterogeneity between studies, and the I² statistic was used to test the magnitude of the heterogeneity. Egger’s tests were performed to evaluate the publication bias in this meta-analysis. A p-value less than 0.05 was considered to be of statistical significance.
Results
Study selection and characteristics
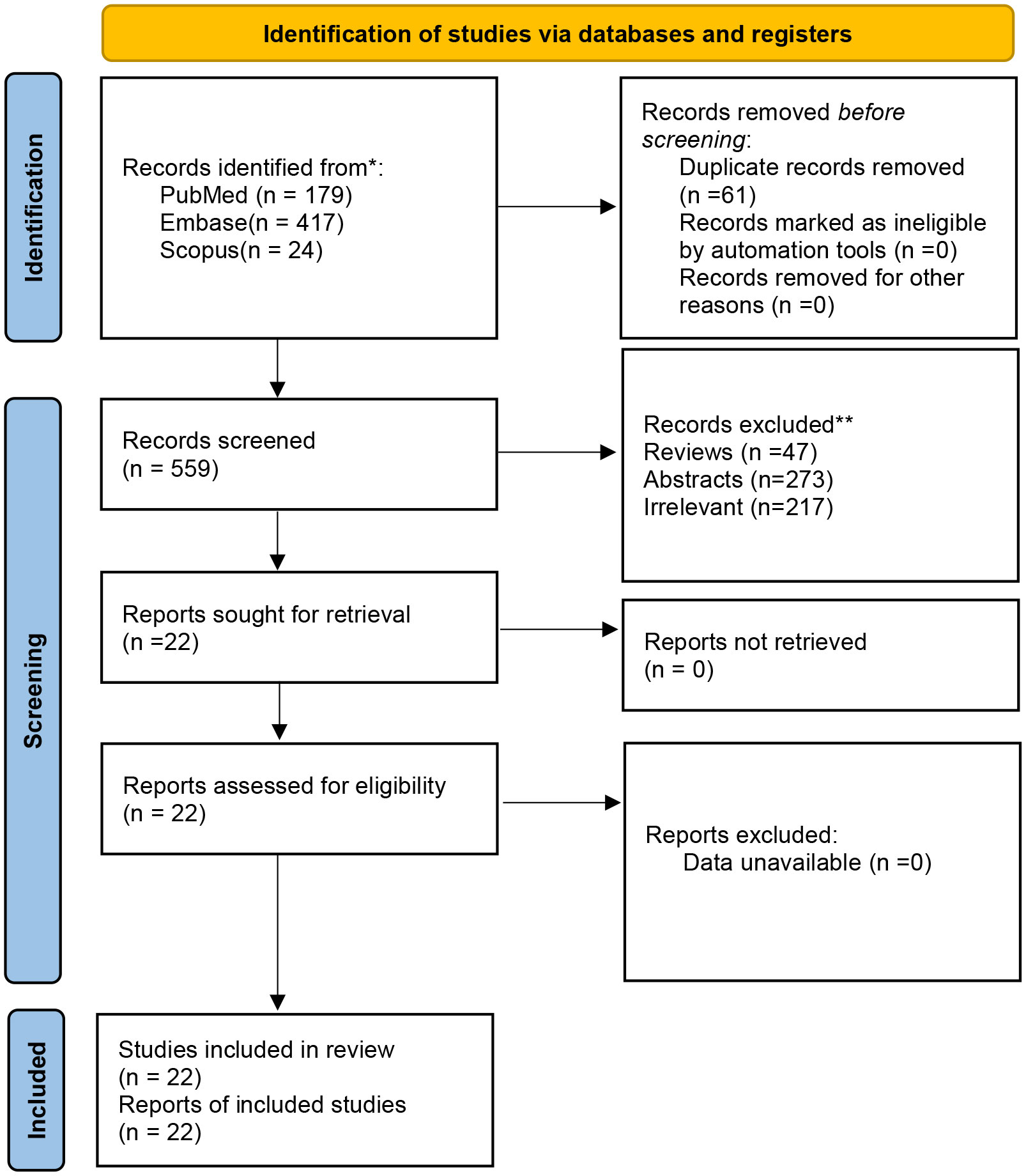
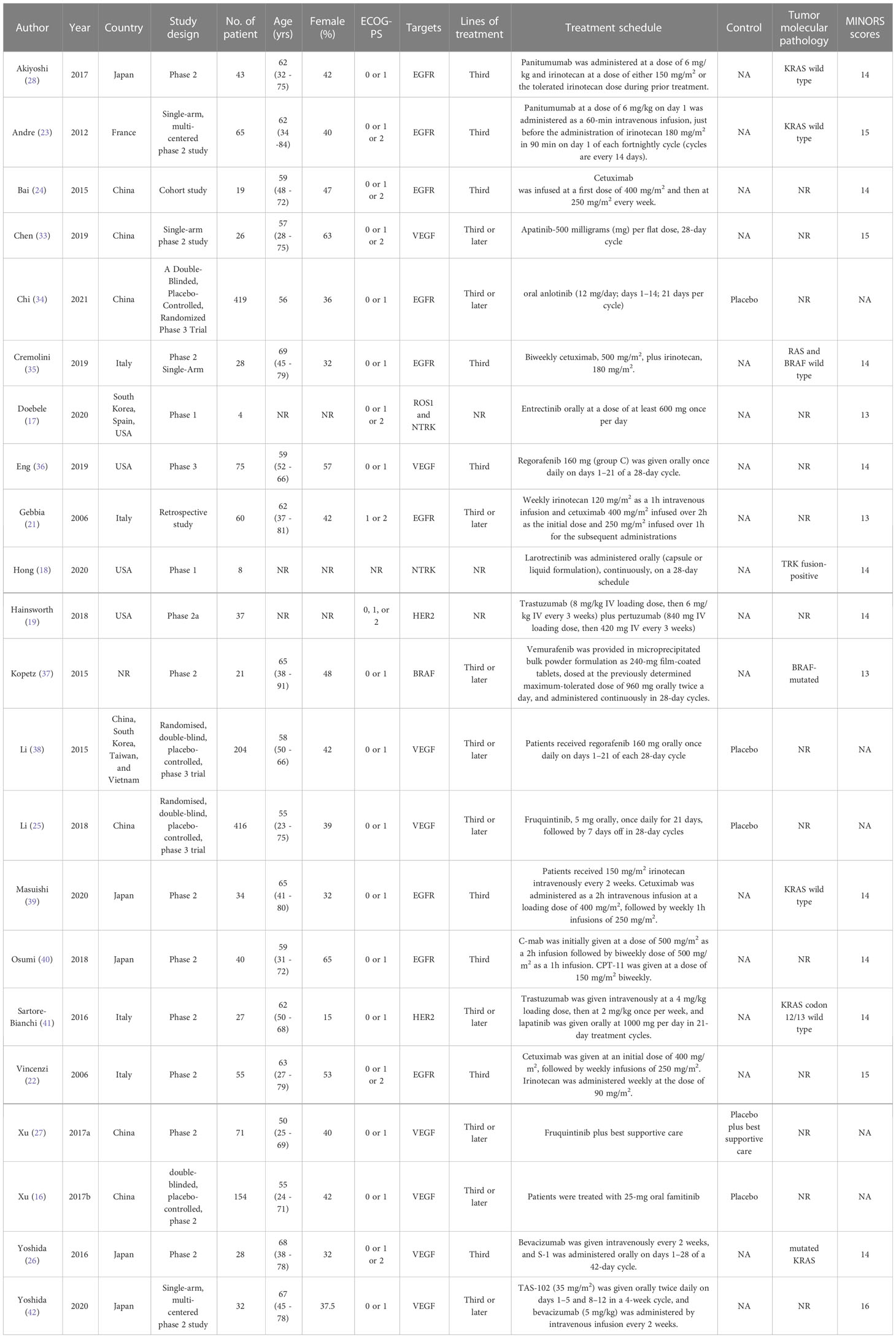
A total of 620 articles were identified from the databases searched. Sixty-one duplicates were eliminated, and 537 studies were excluded through an initial screening. After a full-text assessment for eligibility of the remaining 22 articles, 17 studies were eligible for inclusion in this meta-analysis, and five studies were narratively depicted. No additional studies were identified through reference screening of the included papers and relevant reviews. Figure 1 shows details on the literature search and study selection. The enrolled 22 citations contained 1,866 patients with confirmed mCRC and reported relevant eligible outcomes for data synthesis. Twenty studies were clinical trials, and two studies were cohort studies. These studies were conducted in China, the United States, Italy, South Korea, Vietnam, France, Spain, and Japan. Table 1 shows detailed characteristics of the included studies. The quality of included studies was rated as high based on the Cochrane Collaboration tool and the MINORS scale (Tables 1, 2).
Treatment response
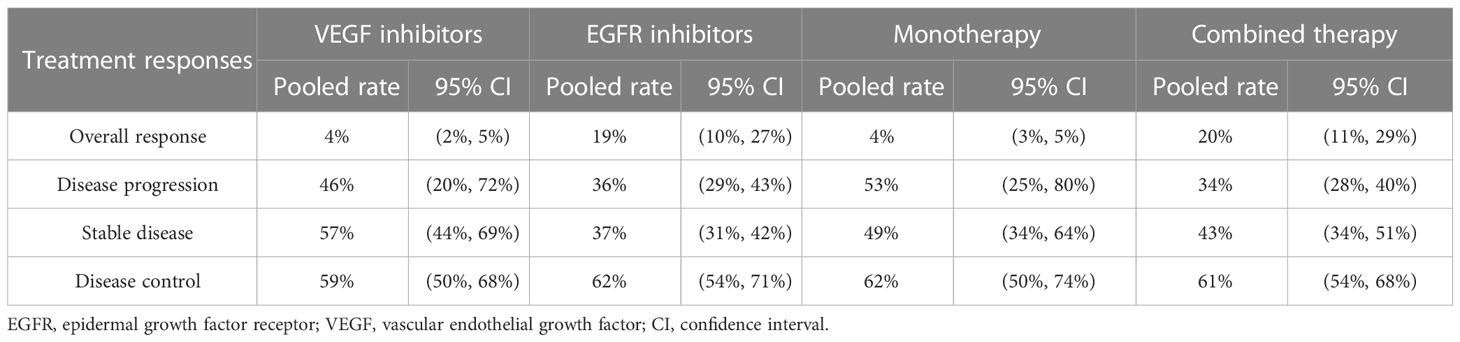
Nine studies assessed the efficacy of EGFR inhibitors monotherapy or combining chemotherapy as third-line or later-line treatment for mCRC. The other eight studies evaluated the efficacy of VEGF antibodies in treating mCRC. The pooled ORRs for monotherapy and combined therapy were 4% (95% CI: 3%, 5%) and 20% (95% CI: 11%, 29%). In the subgroup analysis of molecule targets, the pooled ORRs for VEGF and EGFR inhibitors were 4% (95% CI: 2%, 5%) and 19% (95% CI: 10%, 27%). The pooled disease progression rates for monotherapy and combined therapy were 53% (95% CI: 25%, 80%) and 34% (95% CI: 28%, 40%), respectively. The respective pooled disease progression rates for VEGF and EGFR inhibitors were 46% (95% CI: 20%, 72%) and 36% (95% CI: 29%, 43%). Concerning stable disease rates, the pooled rates for monotherapy and combined therapy were 49% (95% CI: 34%, 64%) and 43% (95% CI: 34%, 51%), and the pooled rates for VEGF and EGFR inhibitors were 57% (95% CI: 44%, 69%) and 37% (95% CI: 31%, 42%). The pooled disease control rates for monotherapy and combined therapy were 62% (95% CI: 50%, 74%) and 61% (95% CI: 54%, 68%), respectively. The pooled disease control rates for VEGF and EGFR inhibitors were 59% (95% CI: 50%, 68%) and 62% (95% CI: 54%, 71%) (Table 3).
The efficacy of BRAF inhibitor monotherapy for mCRC is not promising, with 0% to 5% ORRs (37). The anti-HER2 antibody trastuzumab and the dual EGFR/HER2 kinase inhibitor lapatinib were used in a phase 2 trial performed at four Italian academic cancer centers; the results were as follows: ORR of 30%, DCR of 74%, with 22% of Grade 3 toxicity (41). In addition, results of the MyPathway Study revealed that trastuzumab plus pertuzumab showed an ORR of 38% (95% CI: 23% to 55%) in 37 mCRC patients (19). In the study of Hong et al., four in eight patients with TRK fusion-positive colon cancer demonstrated a response to larotrectinib with a median response duration of 3.7 months (18). Doebele et al. reported that one in four patients with CRC responded to entrectinib, an ROS1 and NTRK inhibitor (17).
Survival
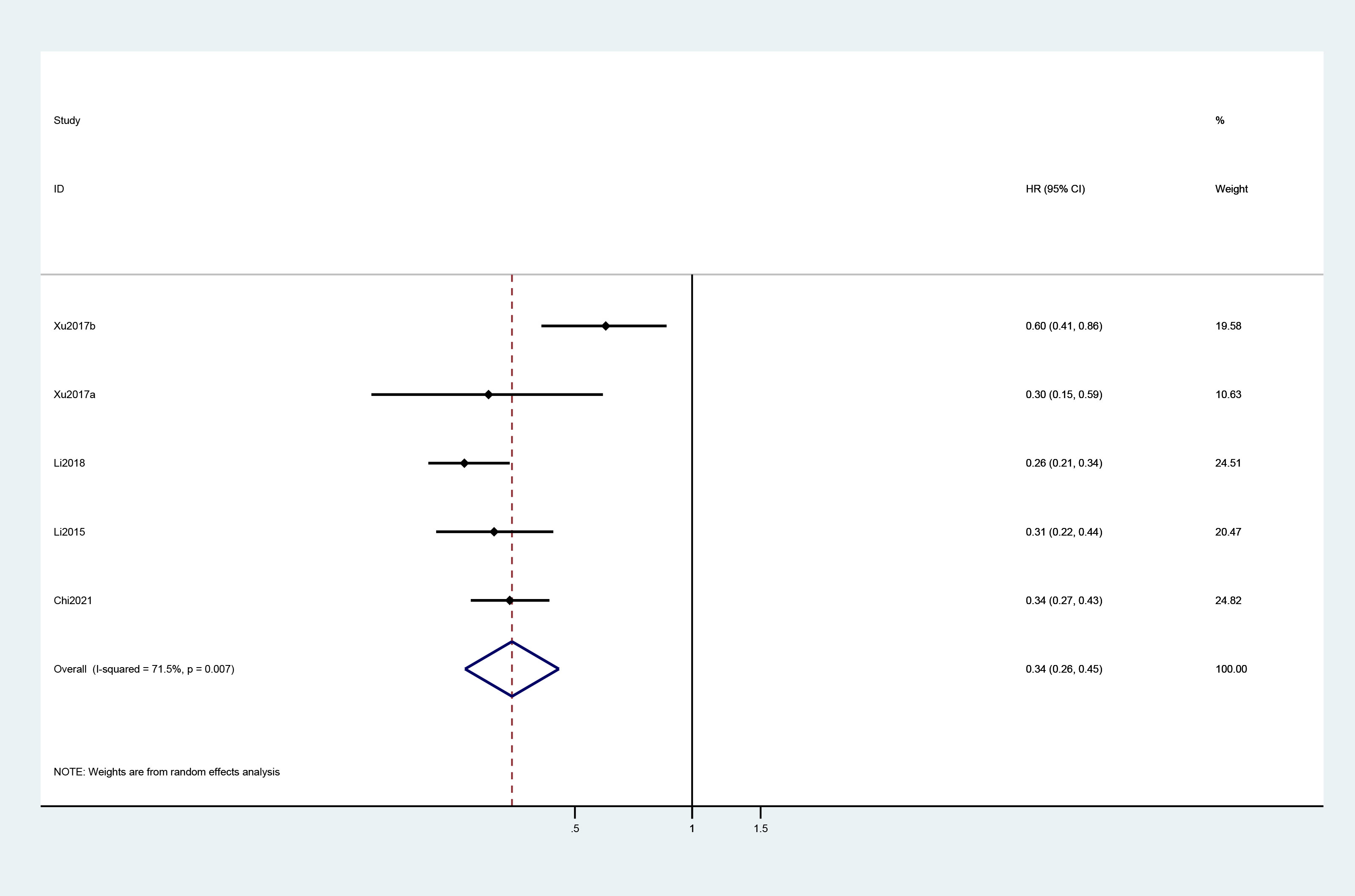
A total of four studies reported the Kaplan–Meier estimates of overall survival in the treatment and control groups. The pooled HR was 0.72 (95% CI: 0.53, 0.99) (Figure 2). For PFS, the pooled HR of five trials was 0.34 (95% CI: 0.26, 0.45) (Figure 3).
Adverse events
Hematological adverse events were the most frequently reported in included studies. The occurrence rates of anemia for VEGF and EGFR inhibitors were 26% (95% CI: 7%, 44%) and 42% (95% CI: 3%, 87%). The pooled occurrence rates of leucopenia for VEGF and EGFR inhibitors were 36% (95% CI: 9%, 63%) and 33% (95% CI: 6%, 60%). With regard to neutropenia, pooled occurrence rates for VEGF and EGFR inhibitors were 34% (95% CI: 9%, 60%) and 47% (95% CI: 24%, 71%). The occurrence rates of thrombocytopenia for VEGF and EGFR inhibitors were 25% (95% CI: 14%, 36%) and 18% (95% CI: 12%, 23%).
Publication bias
P-values of Egger’s tests for publication bias were < 0.001, 0.129, 0.001, 0.052, 0.588, 0.622, 0.078 in the pooled analyses of overall response, stable disease, disease progression, disease control, HR for OS, HR for PFS, and adverse events, respectively.
Discussion
CRC is one of the most important tumors, with high incidence and mortality rates worldwide (43). Many patients are diagnosed at the metastatic stage of the disease; for these patients, treatment is mainly based on chemotherapy (44). Maintaining the quality of life is the primary goal and urgent need of mCRC patients undergoing third-line or later-line treatment (8). However, few insights are gained to guide the selection and sequencing of treatments for these patients (10, 14). Recently, prolonged OS in patients with mCRC has been observed through targeted therapies, such as antibodies against EGFR and VEGF (44).
In the meta-analysis, 17 published articles containing 1,769 patients with diagnosed mCRC and treated with targeted therapies were included. This meta-analysis showed that the pooled ORRs for VEGF and EGFR inhibitors were 4% and 19% in the third-line or later-line treatment of mCRC. Targeted therapy combined with chemotherapy demonstrated favorable ORR and disease control rate with less disease progression than target monotherapy. The results corroborated the findings from previous clinical trials. Furthermore, targeted therapy revealed increased OS and PFS; the goal of the third-line or later-line treatment is to prolong survival and prevent tumor progression without affecting the quality of life. The molecular type of mCRC in included studies was not specified. It is reported that the benefit in PFS and OS was observed only in the KRAS wild-type patients for both cetuximab and panitumumab (45, 46). Moreover, the NCCN clinical practice guideline recommended that regorafenib could be utilized in fit patients with the refractory disease after standard chemotherapy including fluoropyrimidine, oxaliplatin, and irinotecan and anti-VEGF or anti-EGFR therapies (RAS wild type) (47).
However, hematological adverse events, including anemia, neutropenia, leucopenia, and thrombocytopenia, were commonly observed in included studies. In addition, the evidence on clinical trials of other targeted therapies, namely, BRAF inhibitors, HER2 inhibitors, anti-NTRK agents, and ROS1 inhibitors, was limited, so we could not pool these outcomes. Therapies with HER2, NTRK, and ROS1 blockade have shown significant antitumor activity, and more well-designed clinical trials are needed to verify the efficacy and safety of these agents. It is recommended in HER2-positive patients with mCRC, treatment with HER2 dual blockade is optionally recommended, especially in RAS WT tumors (48).
This meta-analysis was conducted at the population level, because individual patient data cannot be obtained. In the current study, a comprehensive literature search in English was performed to increase the probability of obtaining all relevant included studies. Data extraction was conducted by two independent reviewers using a pre-designed form. In addition, we assessed the quality of enrolled studies using the Cochrane Collaboration tool and the MINORS scale. The quality of included studies was rated as high. We assessed the heterogeneity between the studies. Results showed significant heterogeneities in the analyses of OS and PFS. The heterogeneity may be attributed to differences in patient characteristics, study design, drug compliance, prior lines of therapy in each study, and other relevant factors. Due to heterogeneity between third-line or later-line treatment regimens, it is difficult for us to determine the specific subgroups, and therefore subgroup analysis based on regimens was not performed. Meta-regression was not performed due to a limited number of studies in each subgroup. Furthermore, the findings of Egger’s tests indicated that publication bias might not be neglected in analyzing several indicators. Albeit with the heterogeneity and publication bias in included studies and limitations of this meta-analysis, the results may provide evidence-based information on the efficacy and safety of third-line or later-line target therapy for patients with mCRC.
Based on the outcomes of this meta-analysis, we may conclude that targeted therapies, including VEGF and EGFR inhibitors, showed promising clinical response rates and prolonged survival in the treatment of mCRC patients with progression after first- and second-line therapy. Targeted therapy for mCRC patients with biomarker selection may improve marginal prognosis but is unlikely to change the treatment pattern of most patients significantly. Incidences of hematological adverse events were durable and acceptable. However, the pathogenesis of these adverse events remains poorly understood (49). Personalized treatment or combined therapy was recommended based on the feature of mCRC. It is expected that well-designed clinical trials, as well as real-world studies, should be conducted to address issues on the evaluation of efficacy and safety of VEGF and EGFR inhibitors and other targets in the treatment of mCRC. Very preliminary evidence was found regarding the targets of HER2, NTRK, and BRAF. Further studies are needed to investigate if such targets may perform an essential role as VEGF and EGFR in the later line management of mCRC.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
W-HY and JX conceived and designed this study. W-HX, X-WL, Y-QD, NW, B-BP, and X-YM were responsible for the collection, extraction, and analysis of the data. W-HX were responsible for writing the paper. W-HY and JX performed the quality evaluation and completed data analysis. W-HY polished the English language. All authors and participants reviewed the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 82002619), Key Medical Research Projects of Shanxi Province (No. 2020XM55), Talent introduction scientific research start-up fund of Shanxi Bethune Hospital (No. 2020RC006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
mCRC, metastatic colorectal cancer; HRs, hazard ratios; OS, overall survival; CIs, confidence intervals; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; ESMO, European Society for Medical Oncology; US, United States; NCCN, National Comprehensive Cancer Network; FOLFIRI, fluorouracil, folinic acid, and irinotecan; FOLFOX, fluorouracil, folinic acid, and oxaliplatin; rTKI, receptor tyrosine kinase inhibitor;
PFS, progression-free survival; HER2, human epidermal growth factor receptor-2; NTRK, neurotrophic tyrosine receptor kinase; ROS1, receptor tyrosine kinase; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-analysis.
References
1. Kreidieh M, Mukherji D, Temraz S, Shamseddine A. Expanding the scope of immunotherapy in colorectal cancer: current clinical approaches and future directions. BioMed Res Int (2020) 2020:9037217. doi: 10.1155/2020/9037217
2. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. nature reviews. Gastroenterol Hepatol (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
3. Kahi CJ, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colonoscopy surveillance after colorectal cancer resection: recommendations of the US multi-society task force on colorectal cancer. Gastroenterology (2016) 150:758–768.e711. doi: 10.1053/j.gastro.2016.01.001
4. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: a review. Jama (2021) 325:669–85. doi: 10.1001/jama.2021.0106
5. Wrobel P, Ahmed S. Current status of immunotherapy in metastatic colorectal cancer. Int J Colorectal Dis (2019) 34:13–25. doi: 10.1007/s00384-018-3202-8
6. Morse MA, Hochster H, Benson A. Perspectives on treatment of metastatic colorectal cancer with immune checkpoint inhibitor therapy. Oncologist (2020) 25:33–45. doi: 10.1634/theoncologist.2019-0176
8. Bekaii-Saab T, Kim R, Kim TW, O'Connor JM, Strickler JH, Malka D, et al. Third- or later-line therapy for metastatic colorectal cancer: reviewing best practice. Clin Colorectal Cancer. (2019) 18:e117–29. doi: 10.1016/j.clcc.2018.11.002
9. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
10. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. Rectal cancer, version 2.2018, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network JNCCN. (2018) 16:874–901. doi: 10.6004/jnccn.2018.0061
11. De Falco V, Napolitano S, Roselló S, Huerta M, Cervantes A, Ciardiello F, et al. How we treat metastatic colorectal cancer. ESMO Open (2020) 4:e000813. doi: 10.1136/esmoopen-2020-000813
12. Modest DP, Pant S, Sartore-Bianchi A. Treatment sequencing in metastatic colorectal cancer. Eur J Cancer (Oxford Engl 1990). (2019) 109:70–83. doi: 10.1016/j.ejca.2018.12.019
13. Nielsen DL, Palshof JA, Larsen FO, Jensen BV, Pfeiffer P. A systematic review of salvage therapy to patients with metastatic colorectal cancer previously treated with fluorouracil, oxaliplatin and irinotecan +/- targeted therapy. Cancer Treat Rev (2014) 40:701–15. doi: 10.1016/j.ctrv.2014.02.006
14. Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol (2007) 25:1658–64. doi: 10.1200/JCO.2006.08.1620
15. Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. Cetuximab for the treatment of colorectal cancer. N Engl J Med (2007) 357:2040–8. doi: 10.1056/NEJMoa071834
16. Xu RH, Shen L, Wang KM, Wu G, Shi CM, Ding KF, et al. Famitinib versus placebo in the treatment of refractory metastatic colorectal cancer: a multicenter, randomized, double-blinded, placebo-controlled, phase II clinical trial. Chin J Cancer. (2017) 36:97. doi: 10.1186/s40880-017-0263-y
17. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol (2020) 21:271–82. doi: 10.1016/S1470-2045(19)30691-6
18. Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol (2020) 21:531–40. doi: 10.1016/S1470-2045(19)30856-3
19. Hainsworth JD, Meric-Bernstam F, Swanton C, Hurwitz H, Spigel DR, Sweeney C, et al. Targeted therapy for advanced solid tumors on the basis of molecular profiles: results from MyPathway, an open-label, phase IIa multiple basket study. J Clin Oncol (2018) 36:536–42. doi: 10.1200/JCO.2017.75.3780
20. Piawah S, Venook AP. Targeted therapy for colorectal cancer metastases: a review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer. (2019) 125:4139–47. doi: 10.1002/cncr.32163
21. Gebbia V, Del Prete S, Borsellino N, Ferraù F, Tralongo P, Verderame F, et al. Efficacy and safety of cetuximab/irinotecan in chemotherapy-refractory metastatic colorectal adenocarcinomas: a clinical practice setting, multicenter experience. Clin Colorectal Cancer. (2006) 5:422–8. doi: 10.3816/CCC.2006.n.013
22. Vincenzi B, Santini D, Rabitti C, Coppola R, Zobel BB, Trodella L, et al. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: a single centre phase II trial. Br J Cancer. (2006) 94:792–7. doi: 10.1038/sj.bjc.6603018
23. André T, Blons H, Mabro M, Chibaudel B, Bachet JB, Tournigand C, et al. Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann Oncol (2013) 24:412–9. doi: 10.1093/annonc/mds465
24. Bai M, Deng T, Han R, Zhou L, Ba Y. Gemcitabine plus s-1 versus cetuximab as a third-line therapy in metastatic colorectal cancer: an observational trial. Int J Clin Exp Med (2015) 8:21159–65.
25. Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol (2015) 16:619–29. doi: 10.1016/S1470-2045(15)70156-7
26. Yoshida M, Takagane A, Miyake Y, Shimada K, Nagata N, Sato A, et al. A phase II study of third-line combination chemotherapy with bevacizumab plus s-1 for metastatic colorectal cancer with mutated KRAS (SAVIOR study). Oncology (2016) 91:24–30. doi: 10.1159/000446372
27. Xu RH, Li J, Bai Y, Xu J, Liu T, Shen L, et al. Safety and efficacy of fruquintinib in patients with previously treated metastatic colorectal cancer: a phase ib study and a randomized double-blind phase II study. J Hematol Oncol (2017) 10:22. doi: 10.1186/s13045-016-0384-9
28. Akiyoshi K, Hamaguchi T, Yoshimura K, Takahashi N, Honma Y, Iwasa S, et al. A prospective, multicenter phase II study of the efficacy and feasibility of 15-minute panitumumab infusion plus irinotecan for oxaliplatin- and irinotecan-refractory, KRAS wild-type metastatic colorectal cancer (Short infusion of panitumumab trial). Clin Colorectal Cancer (2018) 17:e83–9. doi: 10.1016/j.clcc.2017.10.004
29. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg (London England). (2010) 8:336–41. doi: 10.1016/j.ijsu.2010.02.007
30. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J cancer. (2009) 45:228–47. doi: 10.1016/j.ejca.2008.10.026
31. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed.) (2011) 343:d5928. doi: 10.1136/bmj.d5928
32. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J surgery. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
33. Chen X, Qiu T, Zhu Y, Sun J, Li P, Wang B, et al. A single-arm, phase II study of apatinib in refractory metastatic colorectal cancer. Oncologist (2019) 24:883–e407. doi: 10.1634/theoncologist.2019-0164
34. Chi Y, Shu Y, Ba Y, Bai Y, Qin B, Wang X, et al. Anlotinib monotherapy for refractory metastatic colorectal cancer: a double-blinded, placebo-controlled, randomized phase III trial (ALTER0703). Oncologist. (2021) 26:e1693–703. doi: 10.1002/onco.13857
35. Cremolini C, Rossini D, Dell'Aquila E, Lonardi S, Conca E, Del Re M, et al. Rechallenge for patients with RAS and BRAF wild-type metastatic colorectal cancer with acquired resistance to first-line cetuximab and irinotecan: a phase 2 single-arm clinical trial. JAMA Oncol (2019) 5:343–50. doi: 10.1001/jamaoncol.2018.5080
36. Eng C, Kim TW, Bendell J, Argilés G, Tebbutt NC, Di Bartolomeo M, et al. Atezolizumab with or without cobimetinib versus regorafenib in previously treated metastatic colorectal cancer (IMblaze370): a multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol (2019) 20:849–61. doi: 10.1016/S1470-2045(19)30027-0
37. Kopetz S, Desai J, Chan E, Hecht JR, O'Dwyer PJ, Maru D, et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J Clin Oncol (2015) 33:4032–8. doi: 10.1200/JCO.2015.63.2497
38. Li J, Qin S, Xu RH, Shen L, Xu J, Bai Y, et al. Effect of fruquintinib vs placebo on overall survival in patients with previously treated metastatic colorectal cancer: the FRESCO randomized clinical trial. Jama. (2018) 319:2486–96. doi: 10.1001/jama.2018.7855
39. Masuishi T, Tsuji A, Kotaka M, Nakamura M, Kochi M, Takagane A, et al. Phase 2 study of irinotecan plus cetuximab rechallenge as third-line treatment in KRAS wild-type metastatic colorectal cancer: JACCRO CC-08. Br J cancer. (2020) 123:1490–5. doi: 10.1038/s41416-020-01042-w
40. Osumi H, Shinozaki E, Mashima T, Wakatsuki T, Suenaga M, Ichimura T, et al. Phase II trial of biweekly cetuximab and irinotecan as third-line therapy for pretreated KRAS exon 2 wild-type colorectal cancer. Cancer science. (2018) 109:2567–75. doi: 10.1111/cas.13698
41. Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol (2016) 17:738–46. doi: 10.1016/S1470-2045(16)00150-9
42. Yoshida Y, Yamada T, Kamiyama H, Kosugi C, Ishibashi K, Yoshida H, et al. Combination of TAS-102 and bevacizumab as third-line treatment for metastatic colorectal cancer: TAS-CC3 study. Int J Clin Oncol (2021) 26:111–7. doi: 10.1007/s10147-020-01794-8
43. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
44. Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol (2021) 32:959–67. doi: 10.1016/j.annonc.2021.03.206
45. Amado RG, Wolf M, Peeters M, Van Cutsem E, Siena S, Freeman DJ, et al. Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol (2008) 26:1626–34. doi: 10.1200/JCO.2007.14.7116
46. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-Ras mutations and benefit from cetuximab in advanced colorectal cancer. New Engl J Med (2008) 359:1757–65. doi: 10.1056/NEJMoa0804385
47. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Cancer Network JNCCN (2018) 16:359–69. doi: 10.6004/jnccn.2018.0021
48. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2023) 34:10–32. doi: 10.1016/j.annonc.2022.10.003
Keywords: efficacy, safety, metastatic colorectal cancer, meta-analysis, targeted therapy
Citation: Xue W-H, Li X-W, Ding Y-Q, Wu N, Pei B-B, Ma X-Y, Xie J and Yang W-H (2023) Efficacy and safety of third-line or later-line targeted treatment for patients with metastatic colorectal cancer: a meta-analysis. Front. Oncol. 13:1165040. doi: 10.3389/fonc.2023.1165040
Received: 20 February 2023; Accepted: 17 May 2023;
Published: 31 May 2023.
Edited by:
Alessandro Passardi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Eleonora Lai, University Hospital and University of Cagliari, ItalyAlfonso De Stefano, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2023 Xue, Li, Ding, Wu, Pei, Ma, Xie and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen-Hui Yang, yangwenhui-10012@163.com; Jun Xie, junxie@sxmu.edu.cn
†These authors have contributed equally to this work
 Wen-Hui Xue
Wen-Hui Xue