- 1Department of Medical and Surgical Sciences and Translational Medicine - Sant’ Andrea Hospital, Sapienza University of Rome, Rome, Italy
- 2Radiotherapy, Santa Maria Goretti Hospita, Latina, Italy
- 3Radiation Oncology Unit, Gemelli Molise Hospital - Università Cattolica del Sacro Cuore, Campobasso, Italy
- 4Department of Radiation Oncology, Sant’ Andrea Hospital, Sapienza University of Rome, Rome, Italy
Introduction: Lymph node metastases (NMs) are a common site of tumor spread that can occur at different times of the disease. Stereotactic body radiation therapy (SBRT) can be a therapeutic option for the treatment of NMs in the setting of oligometastatic disease (OMD). The aim of this study was to evaluate as primary end points the local control (LC) and secondary end points the locoregional nodal control (LRNC), distant nodal control (DNC), distant metastasis-free survival (DMFS), progression-free survival (PFS), and overall survival (OS), and concurrently to assess the predictive factors of response.
Methods: This is a retrospective study that analyzes a group of patients treated with SBRT on NMs from different primary tumors, with a of maximum five metastasis. Treated lesions were divided into four groups: oligometastatics, oligorecurrents, oligoprogressives, and oligopersistents.
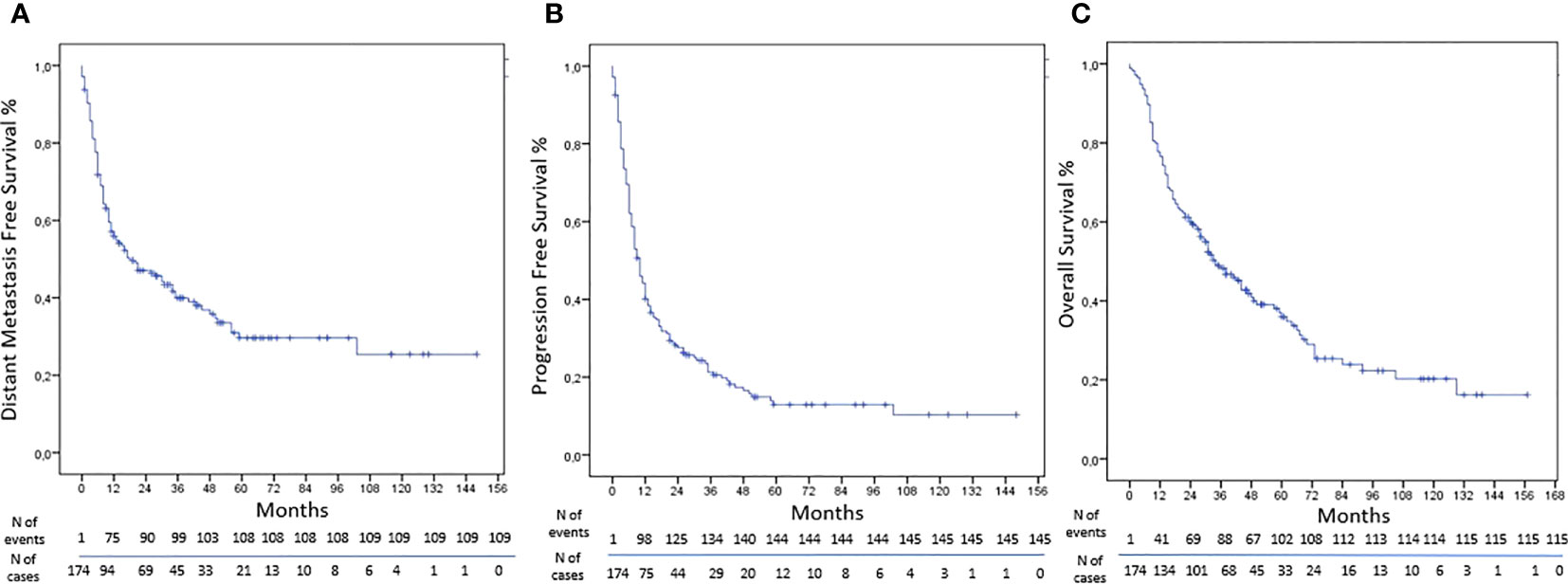
Results: From 2007 to 2021, 229 NMs were treated in 174 patients with different primary tumor. The schedule most represented was 30 Gy in five fractions. The LC was obtained in 90% of NMs treated by SBRT with rates at 1, 3, and 5 years of 93%, 86%, and 86%, respectively. The LRNC was reached in 84% of cases with rates at 1, 3, and 5 years of 88%, 83%, and 77%, respectively. The DNC was obtained in 87% of cases with rates at 1, 3, and 5 years of 92%, 82%, and 78%, respectively. The DMFS was obtained in 38% of cases with rates at 1, 3, and 5 years of 57%, 40%, and 30%, respectively. The rate of PFS were 44%, 23%, and 13% at 1, 3, and 5 years, respectively. The rates at 1, 3, and 5 years of OS were 78%, 48%, and 36%, respectively.
Conclusion: SBRT is an option for the treatment of NMS, with high rates of LC, improving survival, and with a good safety and tolerance. Tumor volume, tumor burden, lesion site, and doses can be predictive factors of response; however, multi-institutional studies with a greater number of patients could be helpful to better select patients and understand the right integrations between ablative treatment and systemic therapies.
Introduction
The oligometastatic disease (OMD) is an area of great interest, rapidly evolving in the characterization and treatment, with a large spectrum of presentation and behavior (1, 2). The first definition of OMD was introduced by Hellman and Weichselbaum in 1995 as an intermediate stage between limited disease and metastatic dissemination (3). In recent years, there has been a big effort to standardize the nomenclature; the proposal was the execution of reproducible trials and a better understanding of the disease. The European Society for Radiotherapy and Oncology (ESTRO) and the American Society for Radiation Oncology (ASTRO) have created a collaborative project to develop a consensus on patient identification and treatment (4–6).
Isolated lymph node metastases (NMs) represent sometimes the only site of disease, as an OMD. The lymph nodes are a common site of tumor spread that can occur at different times of the disease. The incidence of NMs is heterogeneous, and it depends on several factors, based on the primary tumor site, stage, histology, and grading. In the past, they were considered a marker of extensive disease, and systemic therapy was the standard of care; however, over recent years, a more radical approach is emerging in the oligometastatic setting for nodal lesions, with stereotactic body radiotherapy (SBRT) as one of the main weapons (7–10).
During recent decades, SBRT has become a new local treatment option for OMD as a metastasis direct therapy. This technique uses specialized technology able to give high doses of radiation in few fractions on the target, sparing normal tissue compared to conventional radiotherapy. In potentially operable cases, SBRT is more precise and conformed with less morbidity, invasiveness, and costs (11–13); however, lymph node metastases have some characteristics as the minimum organ motion, small volumes, well-defined contours, and a regular form for which they are an ideal target for SBRT treatments (14). This technique is very promising for OMD or in some primary tumors, with high rates of local control (LC) (15, 16). A Phase II trial, SABR-COMET, has indicated in OMD good results in terms of overall survival (OS) and progression-free survival (PFS). Long-term outcomes have shown an 8-year OS of 27.2% in the SBRT plus standard of care arm (SOC) vs. 13.6% in the SOC arm (17, 18).
The aim of this study was to evaluate in our cohort of patients treated at Sant’Andrea Hospital by SBRT on NMs as primary end points the rates of LC and secondary end points the rates of locoregional nodal control (LRNC), distant nodal control (DNC), distant metastasis-free survival (DMFS), progression-free survival (PFS), and OS, and concurrently to assess the predictive factors of response.
Materials and methods
This is a retrospective study that analyzes a group of patients treated by SBRT on NMs from different primary tumors. The cases were discussed and approved by an internal tumor board. The criteria of inclusion are the following: ECOG performance status ≤2; primary cancer disease under control; maximum of five metastases of which one is the lymph node treated by SBRT; and maximum lymph node diameter ≤5 cm. A written informed consent was obtained from all patients before SBRT treatments. In addition, concomitant treatments were allowed. OMD is defined as a disease with a limited number of metastases detected on imaging (from 1 to 5) and is classified according to the patient’s history of metastasis and systemic therapies. The classifications were as follows: oligometastatics in case of lesions present at the moment of diagnosis; oligorecurrents in case of lesions observed during a free interval from systemic therapy; oligoprogressives in case of isolated progression during systemic therapy; and oligopersistents in case of isolated persistence of disease after systemic therapy.
Treatment plan
All patients underwent a computed tomography (CT) simulation and were immobilized in the supine position. It was important that the position was stable, reproducible, and comfortable during the CT simulation and then during the irradiation. A 4DCT system was used to generate an internal target volume (ITV) to evaluate respiratory excursion when the localization of NMS needed the evaluation of excursion breathing. The ITV was created on the maximum intensity projection (MIP). The gross tumor volume (GTV) was the visible lesion on CT, but when the lesion was not well visible, we did an image fusion with diagnostic images, as an MR or CT/PET, in all scan sets. The sum of GTV plus ITV was expanded by 4 mm in all directions to create the planning tumor volume (PTV). In the other site, the clinical target volume (CTV) was equal to GTV, and PTV was the CTV plus 4 mm in all directions.
The contour and plan were performed with Eclipse v16 treatment planning system, and the algorithm used was AAA. The SBRT plan has had an implementation over the years. In 2008, a plan was created with a 3D conformational technique; then from 2007 to 2018, 159 plans were created with IMRT, and from 2018 to 2021, 69 plans were done with Rapid-Arc. Cone-beam CT was obtained prior to each treatment to check correct pre-treatment positioning.
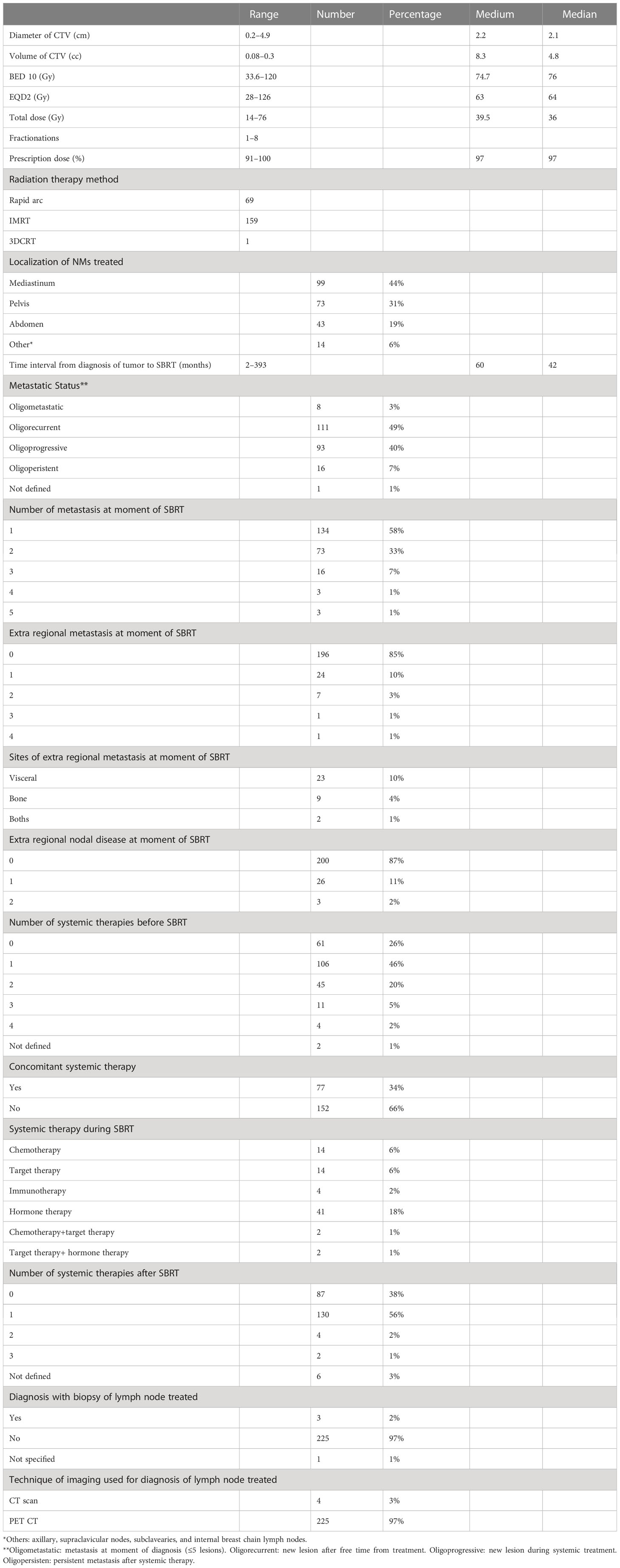
The choice of single vs. multiple fractions was based on the volume of lesions and on the proximity of the target to critical organs. The doses were measured also in terms of biological effective dose (BED) assuming that the α/β ratio is 10 for tumor. The characteristics of lesions, doses, and treatments are summarized in Tables 1 and 2.
Follow-up
The first clinical follow-up was at 2 months, then every 3 months for 2 years, and then every 6 months. Clinical evaluation and diagnostic imaging (CT or PET scan) were planned at physician’s choice. The criteria for radiological response evaluation were extrapolated from the RECIST 1.1 scale (Solid Tumors Response Evaluation Criteria). Toxicity was classified by the CTCAE scale v.4.4.
Statistical analysis
The first end point of our study was to assess the LC and predictive factors of response; the secondary end points were to evaluate the LRNC, DNC, DMFS, PFS, and overall survival, and concurrently the predictive factors of response. The LC was defined as the time from the beginning of SBRT to the date of in-field progression or last follow-up. The LRNC is defined as the time from SBRT to the appearance of new locoregional lymph node metastases or last follow-up. The DNC is defined as the time from SBRT to the appearance of new non-regional lymph node metastases. The DMFS is defined as the time from the treatment to the appearance of non-nodal metastases or last follow-up. The PFS is defined as the time from the treatment to the progression of disease or last follow-up. The OS is calculated from the SBRT to either death or last follow-up.
To analyze actuarial outcomes, the Kaplan–Meier method was used; differences among subgroups were evaluated by log-rank tests. Cox logistic regression was used to carry out the univariate and multivariate analysis of factors predicting LC and LRNC on “a per lesion” basis and DMFS, DNR, PFS, and OS on “a per patient” basis. The result of the logistic regression model was expressed as odds ratios with 95% confidence intervals. Statistically significance was obtained if p<0.05. Only the variables with a p < 0.2 at univariate analysis were selected for the multivariate analysis in order to select the most relevant variables for this analysis.
Statistical analysis was carried out by SPSS statistical software (IBM Corp., released 2011, IBM SPSS Statistics for Windows, Version 20.0, Armonk, NY: IBM Corp).
Results
Baseline characteristics
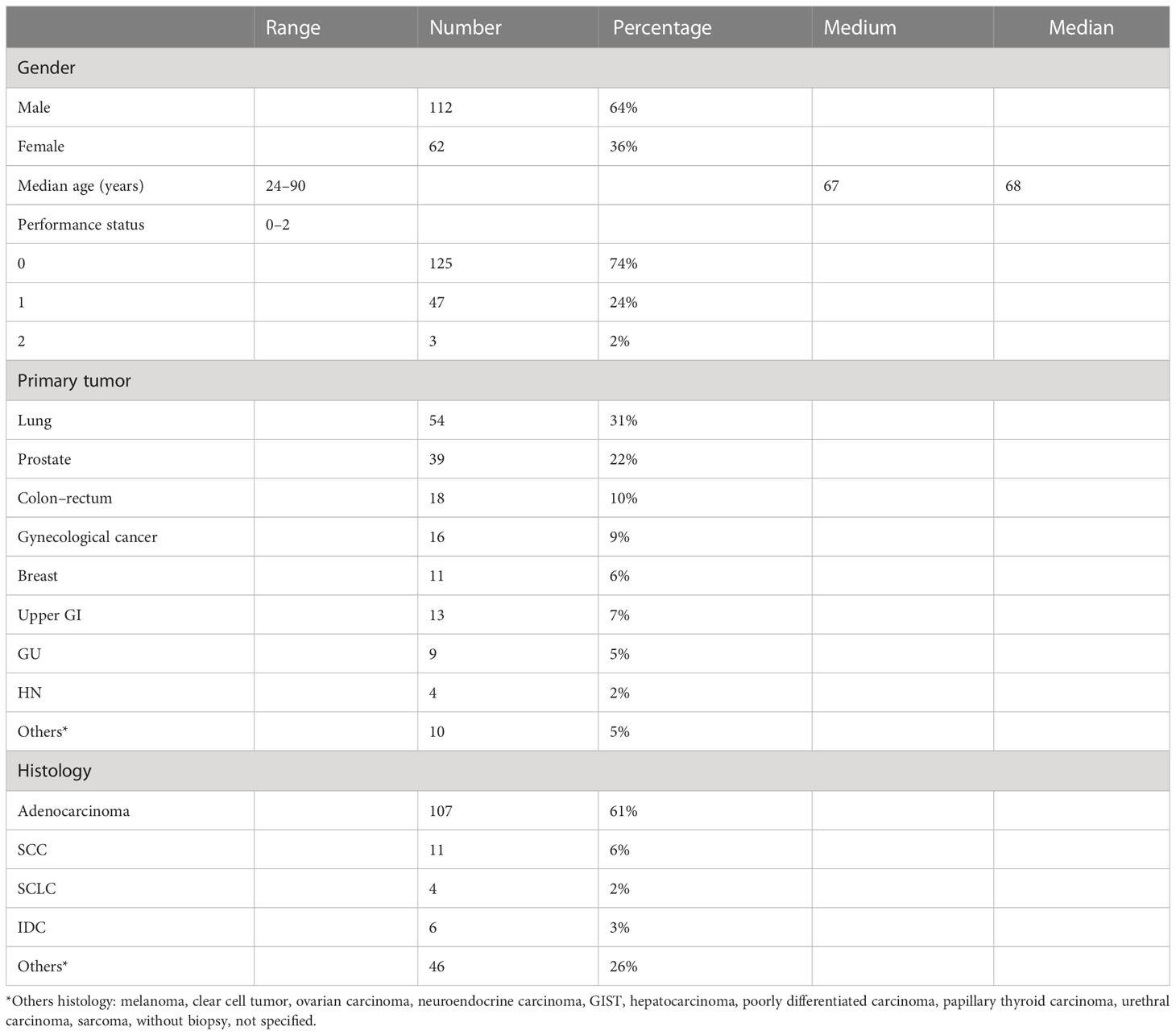
From November 2007 to September 2021, 229 NMs were treated in 174 patients. The median age was 68 years (range, 24–90 years). We had 64% male and 36% female patients. The primary tumor most represented was lung in 54 patients and prostate in 39 patients. We had various primary tumors and different histologies; the characteristics of the patients are reported in Table 3. We had 8 oligometastatic, 111 oligorecurrent, 93 oligoprogressive, and 16 oligopersistent lesions; one was not defined. In 34% of the treatment, concomitant treatment was allowed. The medium volume of lesions was 8.3 cc (range, 0.08–60.3 cc), the medium diameter was 2.2 cm (range, 0.2–4.9 cm) (Table 1). The single and multiple fractions were administered in 22% and 78% of cases, respectively. The schedule most represented was 30 Gy in five fractions, and the medium BED (10) of SBRT was 74.7 Gy (range, 33.6–120 Gy).
Clinical outcomes
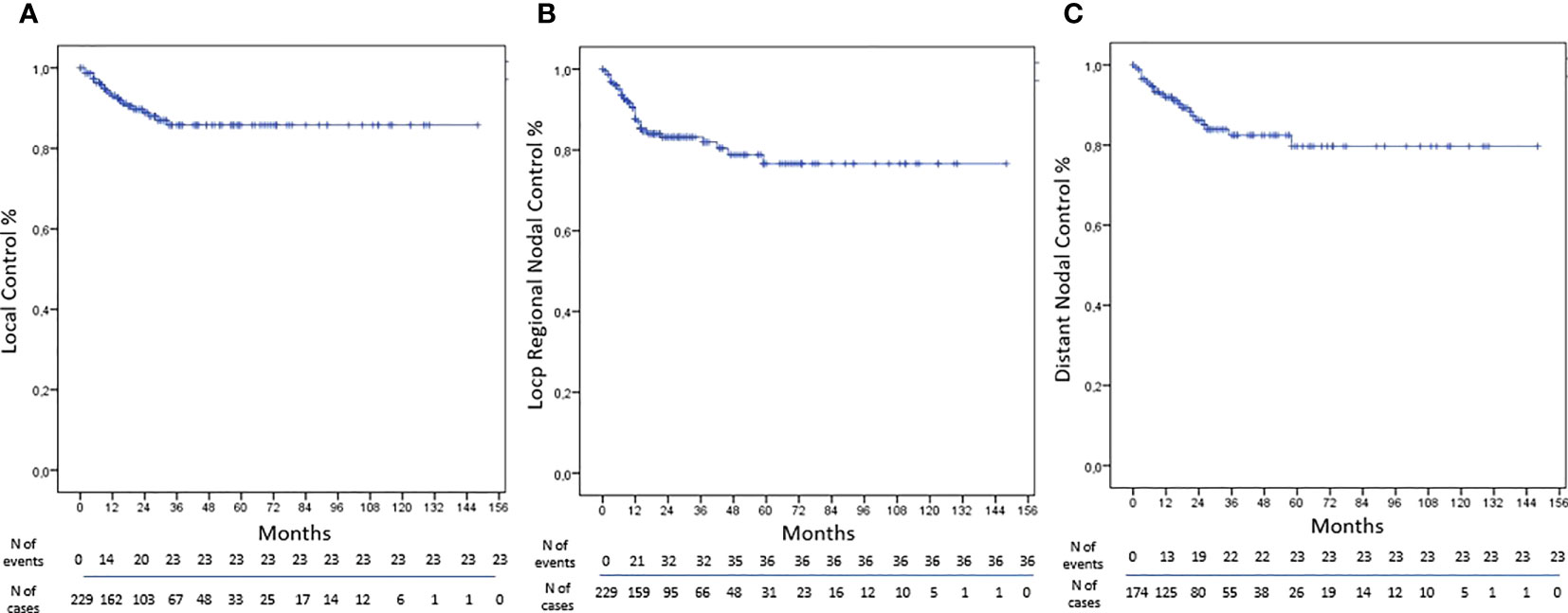
The LC was obtained in 90% of NMs treated by SBRT with rates at 1, 3, and 5 years of 93%, 86%, and 86%, respectively (Figure 1). At univariate analysis, only the total dose >30 Gy was a statistically significant negative prognostic factor for LC (HR, 3.361; 95%CI, 1.323–8.534; p-value, 0.011); however, these data were not confirmed at multivariate analysis (Table 4).
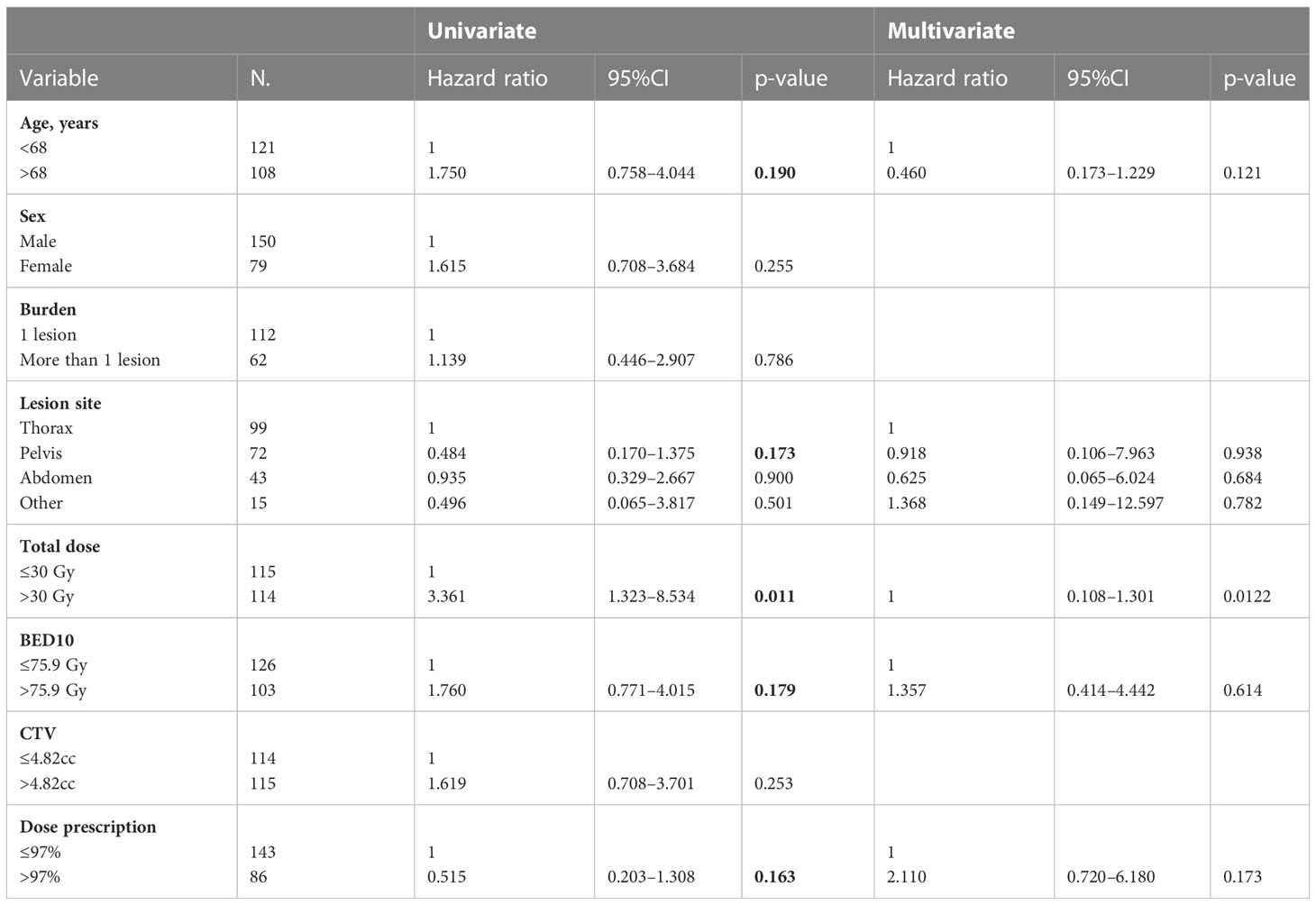
Table 4 Univariate and multivariate Cox regression analysis of variables predicting LC on “lesion” basis.
The LRNC was reached in 84% of cases with rates at 1, 3, and 5 years of 88%, 83%, and 77% respectively (Figure 1). At univariate analysis abdominal localization was a positive statistically significant prognostic factor for LRNC (HR, 0.170; 95%CI, 0.040–0.720; p-value, 0.016), but not confirmed at multivariate analysis (Table 5).
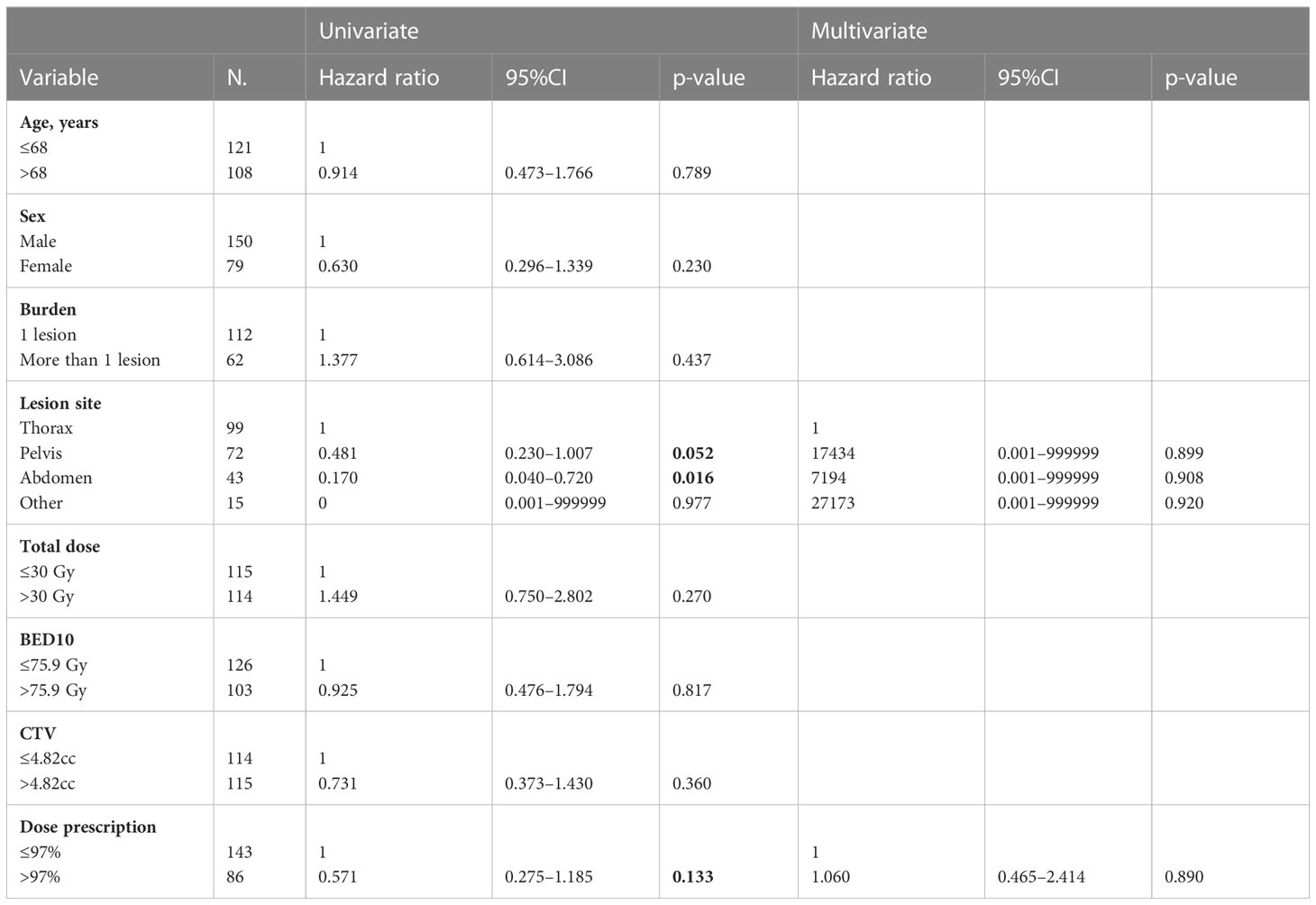
Table 5 Univariate and multivariate Cox regression analysis of variables predicting LRNC on “lesion” basis.
The DNC was obtained in 87% of cases with rates at 1, 3, and 5 years of 92%, 82%, and 78%, respectively (Figure 1). At univariate analysis, the abdominal lesion site was a positive statistically significant prognostic factor for DNC (HR, 3.143; 95%CI, 1.138–8.679; p-value, 0.027), but not confirmed at the multivariate one. Furthermore, at univariate analysis, the volume of CTV > 4.82 cc was a statistically significant positive factor of DNC (HR, 0.310; 95%CI, 0.122–0.789; p-value, 0.014), and the multivariate analysis has confirmed these data (HR, 0.299; 95%CI, 0.111–0.805; p-value, 0.017) (Table 6).
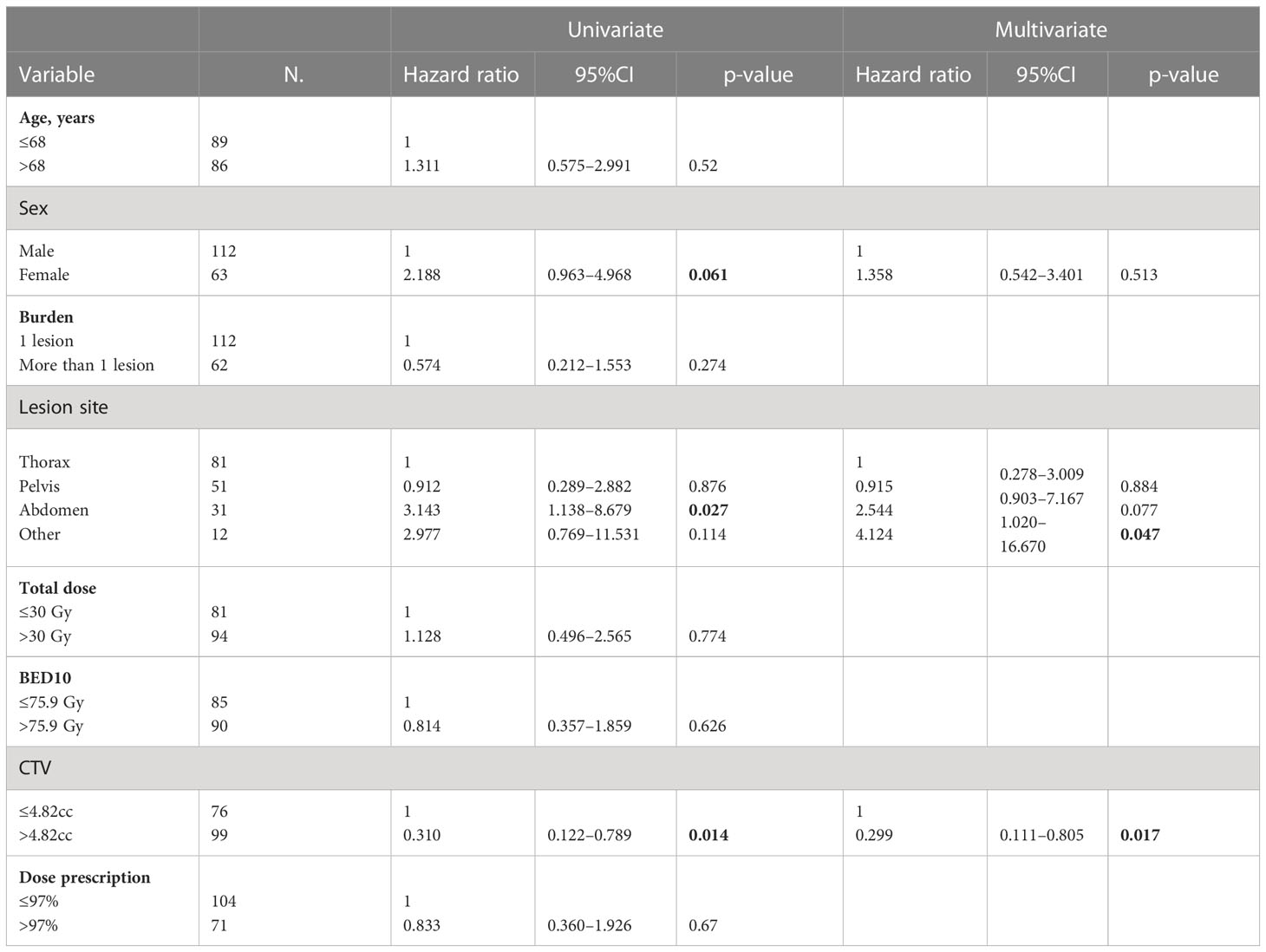
Table 6 Univariate and multivariate Cox regression analysis of variables predicting DNC on “patient” basis.
The DMFS was obtained in 38% of cases with rates at 1, 3, and 5 years of 57%, 40%, and 30%, respectively (Figure 2). At univariate analysis, a higher tumor burden was a statistically significant predictor of worse results (HR, 1.738; 95%CI, 1.184–2.552; p-value, 0.005), and the same was confirmed at multivariate analysis (HR, 1.897; 95%CI, 1.273–2.828; p-value, 0.002). At univariate analysis, the CTV>4.82cc (HR, 1.689; 95%CI, 1.146–2.489; p-value, 0.008) and the total dose> 30 Gy (HR, 1.559; 95%CI, 1.064–2.285; p-value, 0.023) were statistically significant unfavorable prognostic factors for DMFS, but at multivariate analysis, only the BED>75.9Gy was a statistically significant negative prognostic factor (HR, 1.681; 95%CI, 1.132–2.497; p-value, 0.010) (Table 7).
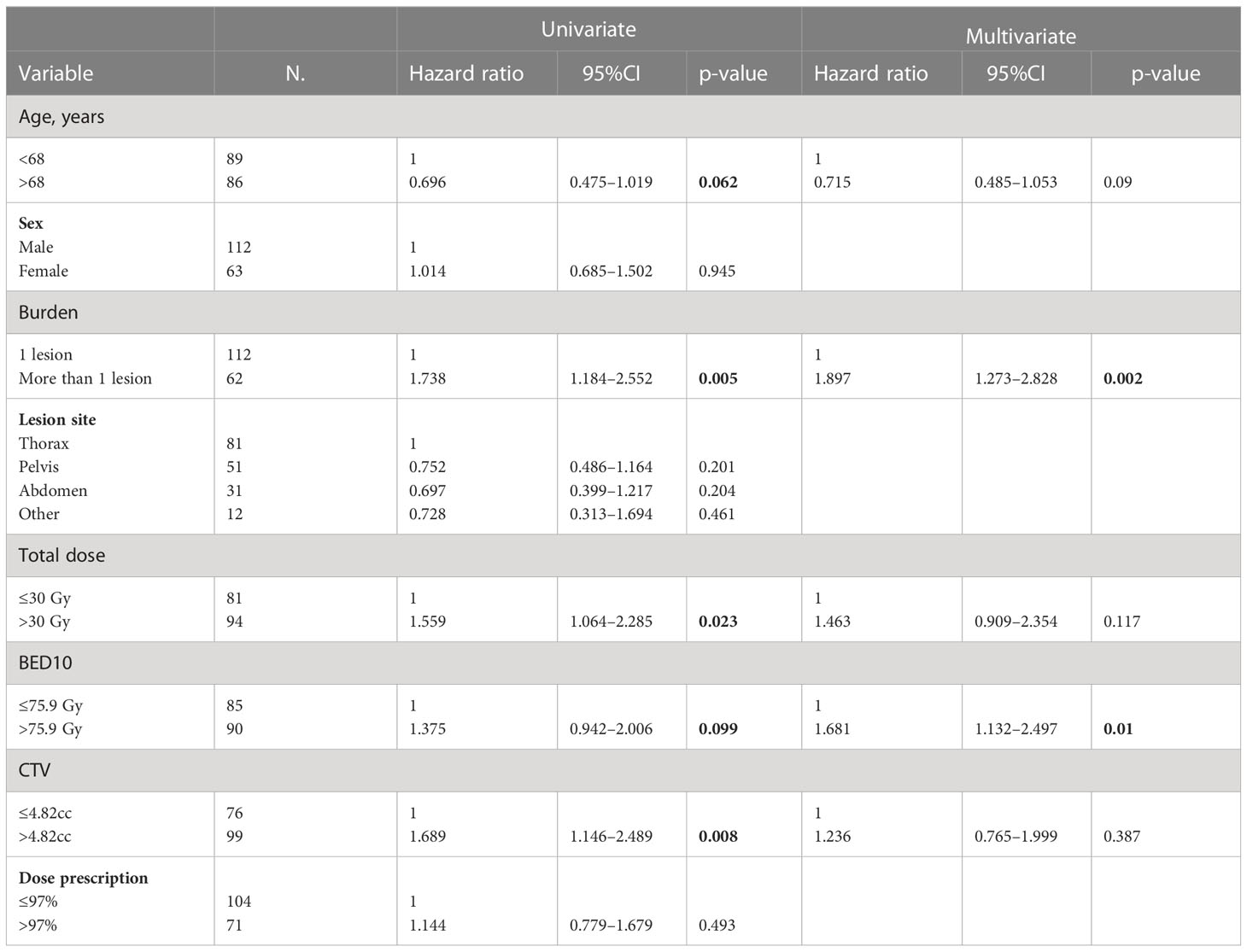
Table 7 Univariate and multivariate Cox regression analysis of variables predicting DMFS on “patient” basis.
The rate of PFS were 44%, 23%, and 13% at 1, 3, and 5 years, respectively (Figure 2). At univariate analysis, a pelvic lesion site was a favorable prognostic factor (HR, 0.565; 95%CI, 0.381–0.839; p-value, 0.005), but the data were not confirmed at multivariate analysis. On the other hand, a CTV>4.82cc was a negative statistically significant prognostic factor for PFS at univariate analysis (HR, 1.422; 95%CI, 1.021–1.980; p-value, 0.037), but it was not confirmed at multivariate analysis. On the other hand, the total dose >30 Gy was a statistically significant negative prognostic factor at univariate analysis (HR, 1.633; 95%CI, 1.174–2.274; p-value, 0.004) and at multivariate analysis (HR, 1.584; 95%CI, 1.032–2.431; p-value, 0.035) (Table 8).
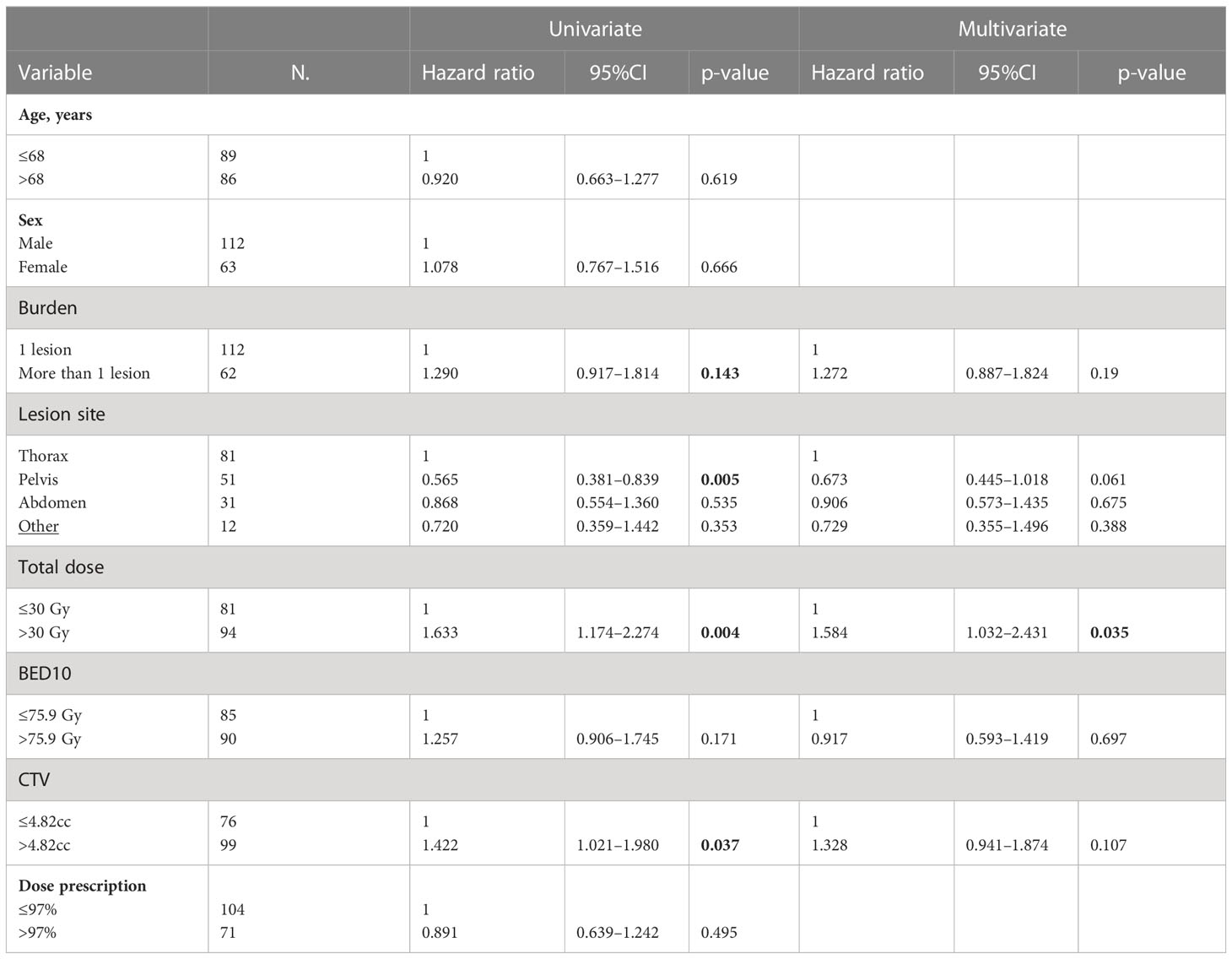
Table 8 Univariate and multivariate Cox regression analysis of variables predicting PFS on “patient” basis.
The rates at 1, 3, and 5 years of OS were 78%, 48%, and 36%, respectively (Figure 2). At univariate analysis, having more than one lesion (HR, 1.513; 95%CI, 1.029–2.224; p-value, 0.035), a total dose>30Gy (HR, 1.488; 95%CI, 1.026–2.159; p-value, 0.036), and a CTV>4.82cc (HR, 2.028; 95%CI, 1.378–2.983; p-value, <0.001) were statistically significant negative prognostic factors, but only CTV volume was confirmed at the multivariate analysis (HR, 1.942; 95% CI, 1.294–2.916; p-value, 0.001). On the other hand, a lesion site in the pelvis was a positive statistically significant prognostic factor (HR, 0.455; 95%CI, 0.283–0.730; p-value, 0.001), confirmed at multivariate analysis (HR, 0.544; 95%CI, 0.331–0.894; p-value, 0.016) (Table 9).
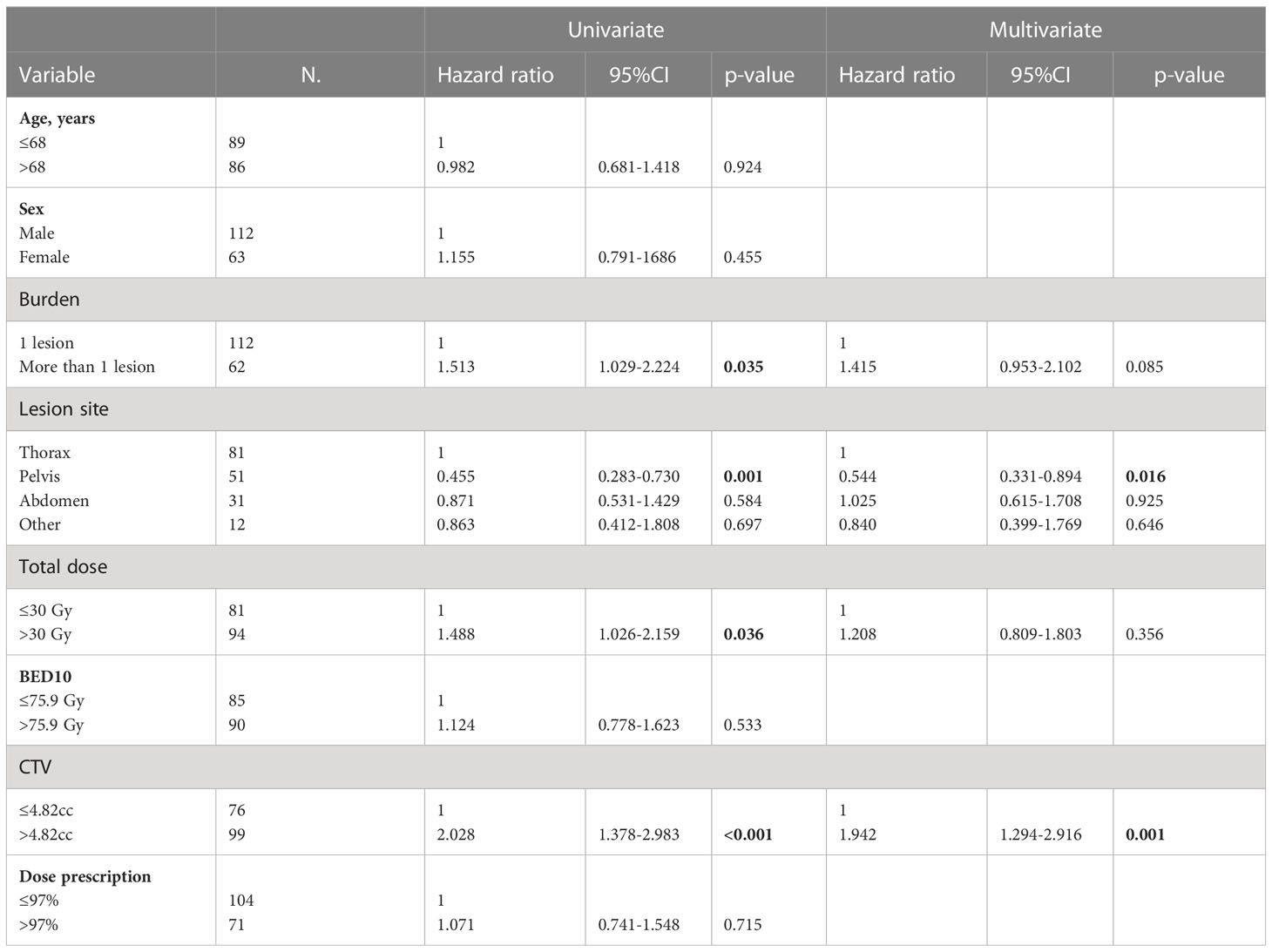
Table 9 Univariate and multivariate Cox regression analysis of variables predicting OS on “patient” basis.
Toxicity. Treatment was well tolerated. The acute toxicities were nine (4%) cases G1, in the form of pain, fatigue, nausea, vomiting, and cough; one case (0.44%) of anemia G2; and one case (0.44%) of death caused by esophageal bleeding, G5, which happened few days after the treatment on the subcarenal lymph nodes treated with a dose of 45 Gy in three fractions. The only late toxicity was a case G2 (0.44%) in the form of dyspnea.
Discussion
Today, there is a new stage that is OMD, and emerging data suggest that ablative therapy may improve outcomes and provide durable disease control in this clinical setting (19, 20). This is a study with a large group of patients with OMD treated on NMs by SBRT. The first aim of our study was to evaluate the LC, which was obtained in 90% of cases with rates at 1, 3, and 5 years of 93%, 86%, and 86%, respectively. Comparing these results with the current literature, we can observe an important heterogeneity: 1-year LC rate can range from 95% in the experience reported by Burkon et al. (21), to 82% in the one described by Jereczek-Fossa (22) or 75% in the one reported by Franzese (23). Similar results were reported in terms of 2, 3, or 4 years LC (21–24). These differences in results may depend on various factors, such as the different proportion in terms of primary, radiosensitive vs. radio resistant lesion, and differences in terms of treatments. For example, in the study of Jereczek-Fossa et al., there was a high proportion of colorectal cancers, which are considered less sensitive to radiotherapy; in addiction, the concomitant systemic therapy was not allowed as also in the study of Burkon. In our cohort, the primaries most represented are lung and prostate cancer. In the first case, a better synergic action between SBRT and systemic therapy played a major role; in the second case, we have a series of lesions more radiosensitive in nature (10, 21, 22). Another consideration is that in the study of Franzese et al., the oligoprogressive lesions were at higher risk of local progression following SBRT vs. oligorecurrents. The inferior response in oligoprogressive disease could be potentially correlated to a resistance acquired from previous treatments. In our cohort, the lesions were treated mostly in an oligorecurrence status (23).
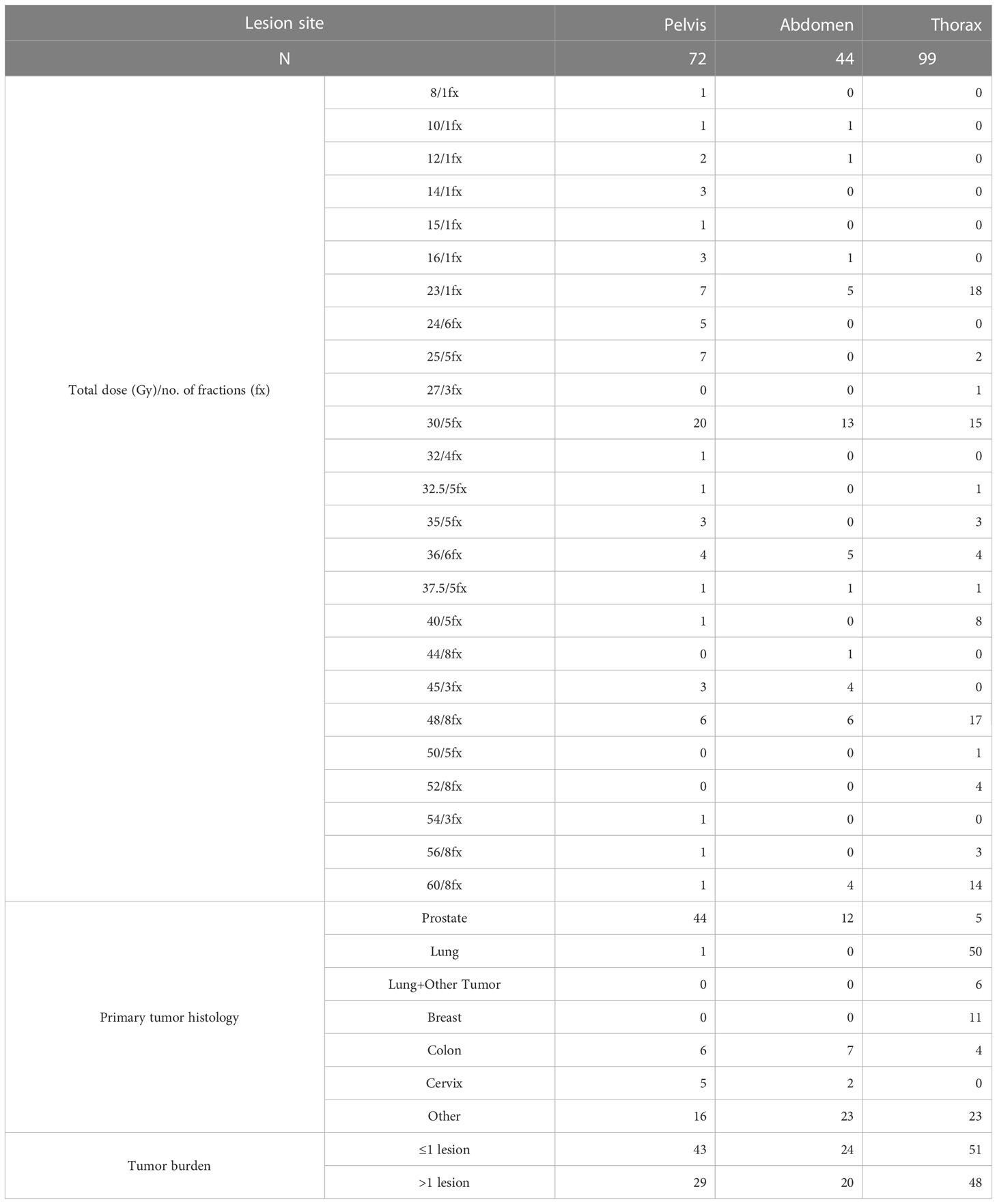
In terms of prognostic factors, we registered worse results at univariate analysis in terms of LC with a total dose > 30 Gy. On the contrary, previous studies showed that with higher doses of SBRT, the LC was also higher (25, 26). This result could be explained with our over-mentioned population heterogeneity: as shown in Table 2, the lesions in the pelvic or abdominal district were generally treated with a total dose <30 Gy and with prostate as primary tumor. On the other hand, lesions treated in the thoracic district were generally treated with higher doses, and the primary was lung cancer, whose natural history is generally worse than prostatic cancer. These differences could have heavily influenced the univariate and multivariate analysis for LC and other outcomes, explaining some of these unprecedented results.
Another major concern in this clinical setting is the progression of disease in others lymph nodes or in others organ; in the current literature, the authors described similar parameters with slightly different definitions. For example, in our series, we have analyzed LRNC and DNC in the same way of the retrospective study of Franzese et al., and also in this case, we have noticed a heterogeneity of results. In fact, at 1 year, Franzese et al. have recorded a rate of 71% vs. 88% of our study for LRNC and 82% vs. 92%, respectively, for DNC. On the contrary, analyzing the predictive factors of response in our study, the volume of CTV was a statistically significant positive factor of DNC at univariate and at multivariate analysis as the study of Franzese et al. (23).
For the parameter DMFS in our study, the rates at 1 and 3 years of DMF were 57% and 40%, respectively. In the current literature, we have appreciated at 1-year rates that ranged from 67% to 82% (23, 27), and at 3 years, Franzese et al. have showed a rate of 58% (27). In our analysis, the volume and the tumor burden with more than one lesion were a statistically significant negative predictive factor of response in terms of DMFS at univariate and multivariate analyses. Similar results were found in other retrospective and prospective studies, where the increased disease load, as volume and/or as number of lesions, can affect increased metastatic cascade and the risk is significant (28, 29).
The rates of PFS in our study at 1 and 3 years were 44% and 23%, respectively; in the literature, described rates ranged between 28% and 95% at 1 year and from 17% and 22% at 3 years (13). In our analysis, pelvic lesions were correlated with better results in terms of PFS and OS; the same was recorded by Franzese et al. who treated abdominal-pelvic NMs with SBRT with a rate of PFS at 5 years of 73%. In our analysis, we had better LRNC and DNC when the lesion site was in the abdomen (8). As described above in Table 2, the differences in terms of histology could have played an important role in our analysis.
In our study, the rates at 1, 3, and 5 years of OS were 78%, 48%, and 36%, respectively; data were almost confirmed in some studies with rates at 1, 3, and 5 years of 76%, 49%, and 41%, respectively (7, 22, 30). In our analysis, the volume and the tumor burden with more than one lesion were a statistically significant negative predictive factor of response in terms of OS at the univariate analysis, and similar results were found in others studies (29, 30).
In general, the most challenging site for NMs SBRT seemed to be the thorax with worst LC, PFS, and a major risk of fatal complication (3–34). In fact, also in our study, we had a case of death for an NM treated in the mediastinum. In the systematic review of Deodato et al., they concluded that considering all the patients included in the studies, the rates of G ≥ 3 were 2% and G5 toxicities were 0.2%, respectively (13). In our study, except for a toxicity G5, we had no G3 toxicity; therefore, we can conclude that SBRT is a treatment well tolerated.
This experience represents one of the largest populations in this clinical setting to the best of our knowledge, even though its retrospective nature and its heterogeneous population in terms of primary and lesion sites represent an important limit. It is probably due to this high level of heterogeneity, especially in terms of doses and fractionations, that no relation between BED and outcomes was found. This underlines the necessity of the patient selection for this type of treatment and the definition of the ablative doses, which are crucial. Technology is also an important bias that must be considered in this type of experiences; however, in this series, the patients were treated with the same type of technology from 2007 up to the end of 2022.
A metastasis direct therapy as SBRT on NMS is a very important therapeutic strategy especially with the advent of new systemic therapies whose synergy could give great results. Maybe in the future, with the introduction of circulating DNA, we could have more information about residual tumor, which will help us in choosing an ideal tailored treatment (35).
In conclusion, SBRT is an option for the treatment of NMS, with high rates of LC, improving the data of survival, with a good safety and tolerance. However, prospective studies are necessary to better understand the right cohort of patients to be treated and eventually the integration with systemic therapy.
Data availability statement
Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The Internal Review Board of S. Andrea Hospital—La Sapienza University of Rome approved our study. Considering the retrospective design, analyzing existing data, ethical review and approval code from the Ethics Committee was not required in accordance with the local legislation and institutional requirements. Informed consent was obtained from all subjects involved in the study. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization of the study: DC and VDS. Data curation: DC, RS, and MR. Formal analysis: DP, DC, and PB. Performed methodology: DC, DP, and PB. Supervised the study: DC, PB, MO and VDS. Wrote the article: DC. Reviewed the manuscript: DP, PB, and DC. Critical revision and Final approval of the version to be published: DC and VDS. PB and DP authors share second authorship. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
OMD, oligometastatic disease; ESTRO, European Society for Radiotherapy and Oncology; ASTRO, American Society for Radiation Oncology; NMs, lymph node metastases; SBRT, stereotactic body radiation therapy; LC, local control; OS, overall survival; PFS, progression-free survival; SOC, standard of care; LRNC, locoregional nodal control; DNC, distant nodal control; DMFS, distant metastasis-free survival; ECOG, Eastern Cooperative Oncology Group; CT, computed tomography; 4DCT, 4-dimensional computed tomography; ITV, internal target volume; MIP, maximum intensity projection; GTV, gross tumor volume; MR, magnetic resonance; CT/PET, computed tomography/positron emission tomography; mm, millimeter; PTV, planning tumor volume; CTV, clinical target volume; 3D, 3-dimensional; IMRT, intensity modulated radiation therapy; BED, biological effective dose; HR, odds ratio; CI, confidence interval; cc, centimeter cubic; G, grade.
References
1. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J ClinOncol (2020) 38:2830–8. doi: 10.1200/JCO.20.00818
2. Niibe Y, Hayakawa K. Oligometastases and oligo-recurrence: the new era ofCancer therapy. Jpn J ClinOncol (2010) 40:107–11. doi: 10.1093/jjco/hyp167
3. Hellman S, Weichselbaum RR. Oligometastases. J ClinOncol (1995) 13:8–10. doi: 10.1200/JCO.1995.13.1.8
4. Nevens D, Jongen A, Kindts I, Billiet C, Deseyne P, Joye I, et al. Completeness of reporting oligometastatic disease characteristics in the literature and influence on oligometastatic disease classification using the ESTRO/EORTC nomenclature. Int J Radiat Oncol Biol Phys (2022) 114(4):587–95. doi: 10.1016/j.ijrobp.2022.06.067
5. Guckenberger M, Lievens Y, Bouma AB, Collette L, Dekker A, deSouza NM, et al. Characterisation and classification of oligometastatic disease: a European society for radiotherapy and oncology and European organisation for research and treatment of cancer consensus recommendation. Lancet Oncol (2020) 21(1):e18–28. doi: 10.1016/S1470-2045(19)30718-1
6. Lievens Y, Guckenberger M, Gomez D, Hoyer M, Iyengar P, Kindts I, et al. Defining oligometastatic disease from a radiation oncology perspective: an ESTRO-ASTRO consensus document. Radiother Oncol (2020) 148:157–66. doi: 10.1016/j.radonc.2020.04.003
7. Franceschini D, De Rose F, Fogliata A, Navarria P, Ascolese AM, Franzese C, et al. Volumetric modulated arc therapy for thoracic node metastases: a safe and effective treatment for a neglected disease. Oncotarget (2016) 7(33):53321–9. doi: 10.18632/oncotarget.10826
8. Franzese C, Cozzi L, Franceschini D, D’Agostino G, Comito T, De Rose F, et al. Role of stereotactic body radiation therapy with volumetric-modulated arcs and high-intensity photon beams for the treatment of abdomino-pelvic lymph-node metastases. Cancer Invest (2016) 34(7):348–54. doi: 10.1080/07357907.2016.1197235
9. Seo YS, Kim M-S, Yoo H-J, Jang W-I. Stereotactic body radiotherapy for oligo-recurrence within the nodal area from colorectal cancer. World J Gastroenterol (2014) 20(8):2005–13. doi: 10.3748/wjg.v20.i8.2005
10. Pezzulla D, Macchia G, Cilla S, Buwenge M, Ferro M, Bonome P, et al. Stereotactic body radiotherapy to lymph nodes in oligoprogressive castration-resistant prostate cancer patients: a post hoc analysis from two phase I clinical trials. Clin Exp Metastasis (2021) 38(6):519–26. doi: 10.1007/s10585-021-10126-7
11. Burkoň P, Slavik M, Kazda T, Pospíšil P, Prochazka T, Vrzal M, et al. Stereotactic body radiotherapy - current indications. Klin Onkol (2019) 32(1):10–24. doi: 10.14735/amko201910
12. Chang JS, Sethi RA, Barani IJ. ExtracranialOligometastases. In: Handbook of evidence-based stereotactic radiosurgery and stereotactic body radiotherapy. Cham: Springer International Publishing (2016). p. 203–20. doi: 10.1007/978-3-319-21897-7_12
13. Macchia G, Lazzari R, Colombo N, Laliscia C, Capelli G, D’Agostino GR, et al. Multicenter, retrospective study on efficacy and safety of stereotactic body radiotherapy (SBRT) in oligometastatic ovarian cancer (MITO RT1 study): a collaboration of MITO, AIRO GYN, and MaNGO groups. Oncologist (2020) 25(2):e311–20. doi: 10.1634/theoncologist.2019-0309
14. Deodato F, Macchia G, Buwenge M, Bonetti M, Cilla S, Zamagni A, et al. Systematic review of stereotactic body radiotherapy for nodal metastases. Clin Exp Metastasis (2021) 38(1):11–29. doi: 10.1007/s10585-020-10071-x
15. Rwigema J-C, King C, Wang P-C, Kamrava M, Kupelian P, Steinberg ML, et al. Stereotactic body radiation therapy for abdominal and pelvic oligometastases: dosimetric targets for safe and effective local control. Pract Radiat Oncol (2015) 5(3):e183–91. doi: 10.1016/j.prro.2014.09.006
16. Gomez DR, Tang C, Zhang J, Blumenschein GR Jr., Hernandez M, Lee JJ, et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-Small-Cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol (2019) 37(18):1558–65. doi: 10.1200/JCO.19.00201
17. Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet (2019) 393(10185):2051–8. doi: 10.1016/S0140-6736(18)32487-5
18. Harrow S, Palma DA, Olson R, Gaede S, Louie AV, Haasbeek C, et al. Stereotactic radiation for the comprehensive treatment of oligometastases (SABR-COMET): extended long-term outcomes. Int J Radiat Oncol Biol Phys (2022) 114(4):611–6. doi: 10.1016/j.ijrobp.2022.05.004
19. Ost P, Jereczek-Fossa BA, Van As N, Zilli T, Tree A, Henderson D, et al. Pattern of progression after stereotactic body radiotherapy for oligometastatic prostate cancer nodal recurrences. ClinOncol (2016) 28:e115–20. doi: 10.1016/j.clon.2016.04.040
20. Turchan WT, Pitroda SP, Weichselbaum RR. Beyond the visible spectrum: considering the oligometastatic hypothesis in the light of a new era. Int J Radiat Oncol Biol Phys (2022) 114(4):581–6. doi: 10.1016/j.ijrobp.2022.04.015
21. Burkon P, Selingerova I, Slavik M, Pospisil P, Bobek L, Kominek L, et al. Stereotactic body radiotherapy for lymph node oligometastases: real-world evidence from 90 consecutive patients. Front Oncol (2021) 10:616494. doi: 10.3389/fonc.2020.616494
22. Jereczek-Fossa BA, Piperno G, Ronchi S, Catalano G, Fodor C, Cambria R, et al. Linac-based stereotactic body radiotherapy for oligometastatic patients with single abdominal lymph node recurrent cancer. Am J ClinOncol Cancer Clin Trials (2014) 37(3):227–33. doi: 10.1097/COC.0b013e3182610878
23. Franzese C, Badalamenti M, Comito T, Franceschini D, Clerici E, Navarria P, et al. Assessing the role of stereotactic body radiation therapy in a large cohort of patients with lymph node oligometastases: does it affect systemic treatment’s intensification? Radiother Oncol (2020) 150:184–90. doi: 10.1016/j.radonc.2020.06.029
24. Loi M, Frelinghuysen M, Klass ND, Oomen-De Hoop E, Granton PV, Aerts J, et al. Locoregional control and survival after lymph node SBRT in oligometastatic disease. Clin Exp Metastasis (2018) 35(7):625–33. doi: 10.1007/s10585-018-9922-x
25. Onishi H, Araki T, Shirato H, Nagata Y, Hiraoka M, Gomi K, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer (2004) 101(7):1623–31. doi: 10.1002/cncr.20539
26. Jingu K, Matsushita H, Yamamoto T, Umezawa R, Ishikawa Y, Takahashi N, et al. Stereotactic radiotherapy for pulmonary oligometastases from colorectal cancer: a systematic review and meta-analysis. Technol Cancer Res Treat (2018) 17:1533033818794936. doi: 10.1177/1533033818794936
27. Franzese C, Comito T, Tripoli A, Franceschini D, Clerici E, Navarria P, et al. Phase II trial of high dose stereotactic body radiation therapy for lymph node oligometastases. Clin Exp Metastasis (2020) 37(5):565–73. doi: 10.1007/s10585-020-10047-x
28. Baker S, Jiang W, Mou B, Lund CR, Liu M, Bergman AM, et al. Progression-free survival and local control after SABR for up to 5 oligometastases: an analysis from the population-based phase 2 SABR-5 trial. Int J Radiat Oncol Biol Phys (2022) 114(4):617–26. doi: 10.1016/j.ijrobp.2022.05.033
29. Kim J, Chang JD, Sung W, Kim JS, Kim TH, Choi SH, et al. A comparison of 2 disease burden assessment methods (3D volume versus the number of lesions) for prognostication of survival in metastatic melanoma: implications for the characterization of oligometastatic disease. Int J Radiat Oncol Biol Phys (2022) 114(5):883–91. doi: 10.1016/j.ijrobp.2022.08.040
30. Pembroke CA, Fortin B, Kopek N. Comparison of survival and prognostic factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiother Oncol (2018) 127(3):493–500. doi: 10.1016/j.radonc.2018.04.022
31. Seo YS, Kim MS, Cho CK, Yoo HJ, Jang WI, Kim KB, et al. Stereotactic body radiotherapy foroligometastases confined to the para-aortic region: clinical outcomesand the significance of radiotherapy field and dose. Cancer Invest (2015) 33(5):180–7. doi: 10.3109/07357907.2015.1019678
32. Horne ZD, Richman AH, Dohopolski MJ, Clump DA, Burton SA, Heron DE. Stereotactic body radiation therapy for isolatedhilar and mediastinal non-small cell lung cancers. Lung Cancer (2018) 115:1–4. doi: 10.1016/j.lungcan.2017.10.014
33. Jereczek-Fossa BA, Muto M, Durante S, Ferrari A, Piperno G, Fodor C, et al. Stereotactic body radiation therapy for mediastinal lymphnode metastases: how do we fly in a ‘no-fly zone’? Acta Oncol (2018) 57:1532–9. doi: 10.1080/0284186X.2018.1486040
34. Bignardi M, Navarria P, Mancosu P, Cozzi L, Fogliata A, Tozzi A, et al. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys (2011) 81:831–8. doi: 10.1016/j.ijrobp.2010.05.032
Keywords: SBRT, lymph nodes metastasis, oligometastatic disease, ablative radiotherapy, metastasis direct therapy
Citation: Caivano D, Bonome P, Pezzulla D, Rotondi M, Sigillo RC, De Sanctis V, Valeriani M and Osti MF (2023) Stereotactic body radiation therapy for the treatment of lymph node metastases: a retrospective mono-institutional study in a large cohort of patients. Front. Oncol. 13:1163213. doi: 10.3389/fonc.2023.1163213
Received: 10 February 2023; Accepted: 29 June 2023;
Published: 03 August 2023.
Edited by:
Ferenc Lakosi, Somogy County Kaposi Mór Teaching Hospital, HungaryReviewed by:
Vanessa Panettieri, Peter MacCallum Cancer Centre, AustraliaDavide Franceschini, University of Milan, Italy
Copyright © 2023 Caivano, Bonome, Pezzulla, Rotondi, Sigillo, De Sanctis, Valeriani and Osti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Donatella Caivano, donatella.caivano@gmail.com
†These authors share second authorship
 Donatella Caivano
Donatella Caivano