
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol., 08 May 2023
Sec. Molecular and Cellular Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1162835
This article is part of the Research TopicDiverse roles of MAP4K4 in MAP Kinase signalling and its implication for cancer therapeuticsView all 5 articles
MAP4K4 is a serine/threonine kinase that belongs to the MAP kinase family and plays a critical role in embryogenesis and cellular migration. It contains approximately 1,200 amino acids and has a molecular mass of 140 kDa. MAP4K4 is expressed in most tissues where it has been examined and its knockout is embryonic lethal due to impaired somite development. Alterations in MAP4K4 function have a central role in the development of many metabolic diseases such as atherosclerosis and type 2 diabetes, but have recently been implicated in the initiation and progression of cancer. For example, it has been shown that MAP4K4 can stimulate the proliferation and invasion of tumor cells by activating pro-proliferative pathways (such as the c-Jun N-terminal kinase [JNK] and mixed-lineage protein kinase 3 [MLK3] pathways), attenuate anti-tumor cytotoxic immune responses, and stimulate cell invasion and migration by altering cytoskeleton and actin function. Recent in vitro experiments using RNA interference-based knockdown (miR) techniques have shown that inhibition of MAP4K4 function reduces tumor proliferation, migration, and invasion, and may represent a promising therapeutic approach in many types of cancer such as pancreatic cancer, glioblastoma, and medulloblastoma, among others. Over the last few years, specific MAP4K4 inhibitors such as GNE-495 have been developed but have not yet been tested in cancer patients. However, these novel agents may be useful for cancer treatment in the future.
MAP4K4 is a serine/threonine kinase that belongs to the mammalian family of Ste20 protein kinases. The members of this family can be divided into two groups based on the locations of their catalytic domains (1) and can be divided into eight subfamilies based on the structures of their non-catalytic regions (2). MAP4K4 is one of the four members of the germinal center-like kinase IV family (3). MAP4K4 kinase contains approximately 1,200 amino acids and has a molecular mass of approximately 140 kDa (4). The MAP4K4 gene is located at 2q11.2 (1). MAP4K4 has multiple physiological functions and is expressed in all cell types where it has been examined, but its expression is highest in testicular tissue and cells of the nervous system (5). MAP4K4 knockout is lethal due to altered embryonic development and impaired cell migration (6). Furthermore, MAP4K4 has critical roles in the regulation of cell adhesion (7) and inflammation (8), and has been implicated in the development of metabolic diseases such as type 2 diabetes (9) and atherosclerosis (10).
Recent research has shown that MAP4K4 plays a role in cancer development and its inhibition may be a novel treatment strategy for several types of cancer. In this mini-review, we will discuss the available evidence underlying the role of MAP4K4 in cancer and the experimental data supporting its inhibition as a new therapeutic strategy.
In recent years, several studies have reported that MAP4K4 plays a role in the initiation and progression of cancer. MAP4K4 is overexpressed in multiple tumor types such as pancreatic cancer (11), colorectal cancer (12), ovarian epithelial cancer (13), lung cancer (14), gastric cancer (15), and hepatocellular carcinoma (16). MAP4K4 contributes to cancer development in many ways but primarily acts through three main axes: activation of cell proliferation pathways, alteration of cytoskeleton function, and impairment of anti-tumor immune responses.
MAP4K4 can activate downstream pathways that promote tumor cell proliferation. A recent investigation demonstrated that MAP4K4-mediated phosphorylation and activation of mixed-lineage protein kinase 3 (MLK3) promoted pancreatic tumor proliferation, migration, and colony formation (17). Similarly, in an in vitro model of ovarian cancer, knockdown of MAP4K4 inhibited the migration of various cell types. The migration-promoting effect of MAP4K4 was mediated by c-Jun N-terminal kinase (JNK) and dependent on AP-1 activation (18). The ability of MAP4K4 to phosphorylate JNK was also demonstrated in an in vitro model of colorectal cancer (19). Additionally, knockdown of MAP4K4 inhibited the proliferation, growth, and migration of adenocarcinoma cells in vitro. Furthermore, In EGFR-mutated and erlotinib-treated lung adenocarcinoma cell lines, downregulation of MAP4K4 prevented ERK reactivation, suggesting that MAP4K4 is critical for maintaining tumor growth (20).
MAP4K4 can also alter cytoskeletal function. c-Met is a tyrosine kinase receptor aberrantly expressed in some types of medulloblastoma. It has been shown that MAP4K4 can control c-Met endocytosis and integrin-B1 activation, which are associated with invasive phenotypes in this tumor type (21). Similarly, Kumar et al. demonstrated that MAP4K4 mediated c-Met-induced invasive cell phenotypes by controlling actin dynamics in the cytoskeleton (22). Finally, experiments with CRISPR-Cas9 in an in vitro model of glioblastoma multiforme demonstrated that MAP4K4 was involved in cell motility and tumor invasion (23).
MAP4K4 can also impair anti-tumor immune responses. For example, genetic deletion of MAP4K4 has been shown to increase the expression of lymphocyte-associated antigen 1 (LFA1) on CD8+ T lymphocytes and improves their adherence to antigen-presenting cells (24). MAP4K4 deletion also increases CD8+ T lymphocyte activity, cytokine production, and cytotoxic activity. The interaction between MAP4K4 and LFA1 is mediated by ERM proteins (ezrin, radixin, and moesin) and could represent a new therapeutic opportunity in tumors with primary or acquired immunotherapy resistance. Further studies should address the role of MAP4K4 inhibition in the anti-tumor effects of immunotherapy. Table 1 shows a summary about these 3 main ways by which MAP4K4 can stimulate tumor growth.
Upstream control of MAP4K4 in tumor cells is exerted by striatin (STRN) family proteins (members of the STRIPAK family). In an in vitro model of medulloblastoma, MAP4K4 interacted with STRN3/4 and stimulated cell growth. Accordingly, STRN3/4 depletion could reduce the invasive capacity of medulloblastoma cells (25). The interaction between MAP4K4 and STRN may be mediated by protein phosphatase 2A (PP2A). Recent experiments have demonstrated that STRN4 promotes MAP4K4 inactivation through the phosphatase activity of PP2A. Therefore, low levels of PP2A activity may contribute to cancer development (26). Other important upstream regulators of MAP4K4 function in tumor cells are TNFR, c-MET, PYK2, RAP2 among many others (27)
Evidence pertaining to the anti-neoplastic role of MAP4K4 inhibition is early but promising. Most studies in this area have been in vitro investigations leveraging RNA interference to block MAP4K4 function.
In a murine model of pancreatic cancer, specific pharmacological inhibition of MAP4K4 with GNE-495 inhibited pancreatic cell growth and tumor migration (17). MAP4K4 is also overexpressed in patients with pancreatic cancer and could represent a biomarker associated with poor clinical prognosis (17). In parallel, in vitro results demonstrated that inhibition of MAP4K4 (through the use of RNA interference with miR-98-5p) reduced the proliferation of pancreatic cancer cells (28).
In an in vitro model of glioblastoma multiforme, MAP4K4 inhibition reduced the invasion of tumor cells (23). MAP4K4 also reduced the chemosensitivity of cervical cancer cells to platinum therapy by regulating SOX6-induced autophagy (29). These results suggest that MAP4K4 inhibitors or specific autophagy inhibitors may increase the sensitivity of cervical cancer to chemotherapy. Additionally, the use of RNA interference (miR-200c) to block MAP4K4 in cervical cancer diminished the invasive behavior of the cancer cells in vitro. These results reinforce the idea that MAP4K4 inhibition may be a therapeutic target for cervical cancer treatment (30).
In colorectal cancer, MAP4K4 inhibition via miR-141 increased tumor cell chemosensitivity and diminished their proliferation, invasion, and migration (31). In breast cancer, miR-141 inhibited tumor cell proliferation by suppressing MAP4K4 expression, which was associated with an increase in tumor infiltration by CD4+ T lymphocytes (32). Thus, MAP4K4 inhibition could have anti-neoplastic effects in breast cancer by increasing immune cell infiltration. Furthermore, MAP4K4 inhibition provoked tumor cell apoptosis (by increasing the Bax/Bcl-2 ratio) and inhibited tumor cell proliferation in a gastric cancer model (15).
It is important to consider some caveats about the potential use of MAP4K4 inhibition as a therapeutic agent in cancer, because MAP4K4 has a role in multiple fundamental signaling systems, including NFκB activation, regulation of small GTPases and the Hippo cascade (33). In this line, a remarkable finding is the antiproliferative activity of MAP4K4 through activation of Hippo tumor suppressor signaling, which could lead to increased proliferation due to shutdown of Hippo signaling (34). Also, it is important to consider the potential adverse effects of a MAP4K4 inhibitor therapy. In this line, preclinical studies have reported that MAP4K inhibitors could be associated with weight loss, increased body temperature, tachycardia, among others (35). Thus, this new and promising area of research raises key questions that need to be addressed in the future before being implemented as a therapy.
Figure 1 shows a flow chart about the main ways by which MAP4K4 can stimulate tumor growth and invasion and the functions that specific MAP4K4 inhibitors could play.
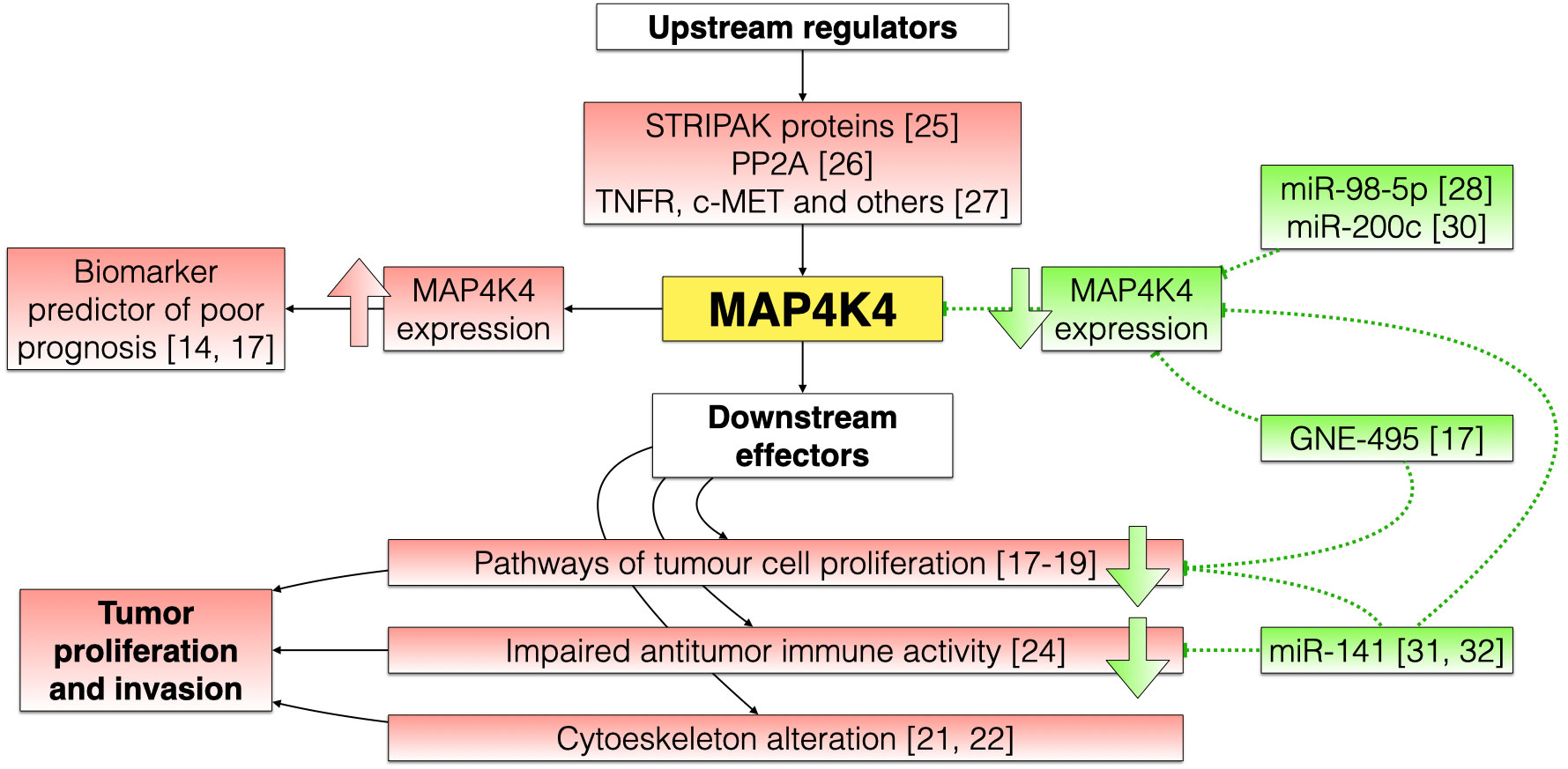
Figure 1 Schematic flow chart showing the possible pathways by which MAP4K4 can induce the initiation and progression of cancer, and the possible mechanisms by which MAP4K4 inhibitors could exert their antitumor effect. Oncogenic pathways are marked in red, and the mechanisms of MAP4K4 inhibitors are marked in green.
Studies published over the last five years have shown that MAP4K4 plays a role in the initiation and progression of cancer, primarily by activating intracellular proliferative signaling (such as the JNK and MLK3 pathways), impairing cytoskeleton function, and reducing anti-tumor immune responses. However, the functions of MAP4K4 in cancer are just beginning to be described and could be more diverse than current data indicate. Multiple experiments with RNA interference reinforced the notion that this molecule is susceptible to pharmacological inhibition with specific inhibitors such as GNE-495. Is MAP4K4 ready for the main stage as a cancer therapy target? Although the current evidence is still early, it is promising. Further studies are needed to elucidate the efficacy of specific pharmacological MAP4K4 inhibition in cancer patients and to test the safety of its pharmacological blockade.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Delpire E. The mammalian family of sterile 20p-like protein kinases. Pflugers Arch (2009) 458(5):953–67. doi: 10.1007/s00424-009-0674-y
2. Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol (2001) 11(5):220–30. doi: 10.1016/s0962-8924(01)01980-8
3. Nakano K, Yamauchi J, Nakagawa K, Itoh H, Kitamura N. NESK, a member of the germinal center kinase family that activates the c-jun n-terminal kinase pathway and is expressed during the late stages of embryogenesis. J Biol Chem (2000) 275(27):20533–9. doi: 10.1074/jbc.M001009200
4. Su YC, Han J, Xu S, Cobb M, Skolnik EY. NIK is a new Ste20-related kinase that binds NCK and MEKK1 and activates the SAPK/JNK cascade via a conserved regulatory domain. EMBO J (1997) 16(6):1279–90. doi: 10.1093/emboj/16.6.1279
5. Wright JH, Wang X, Manning G, LaMere BJ, Le P, Zhu S, et al. The STE20 kinase HGK is broadly expressed in human tumor cells and can modulate cellular transformation, invasion, and adhesion. Mol Cell Biol (2003) 23(6):2068–82. doi: 10.1128/MCB.23.6.2068-2082.2003
6. Vitorino P, Yeung S, Crow A, Bakke J, Smyczek T, West K, et al. MAP4K4 regulates integrin-FERM binding to control endothelial cell motility. Nature (2015) 519(7544):425–30. doi: 10.1038/nature14323
7. Yue J, Xie M, Gou X, Lee P, Schneider MD, Wu X. Microtubules regulate focal adhesion dynamics through MAP4K4. Dev Cell (2014) 31(5):572–85. doi: 10.1016/j.devcel.2014.10.025
8. Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, et al. Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature (2009) 458:1180–4. doi: 10.1038/nature07774
9. Chuang HC, Sheu WH, Lin YT, Tsai CY, Yang CY, Cheng YJ, et al. HGK/MAP4K4 deficiency induces TRAF2 stabilization and Th17 differentiation leading to insulin resistance. Nat Commun (2014) 5:4602. doi: 10.1038/ncomms5602
10. Roth Flach RJ, Skoura A, Matevossian A, Danai LV, Zheng W, Cortes C, et al. Endothelial protein kinase MAP4K4 promotes vascular inflammation and atherosclerosis. Nat Commun (2015) 6:8995. doi: 10.1038/ncomms9995
11. Badea L, Herlea V, Dima SO, Dumitrascu T, Popescu I. Combined gene expression analysis of whole-tissue and microdissected pancreatic ductal adenocarcinoma identifies genes specifically overexpressed in tumor epithelia. Hepatogastroenterology (2008) 55(88):2016–27.
12. Hao JM, Chen JZ, Sui HM, Si-Ma XQ, Li GQ, Liu C, et al. A five-gene signature as a potential predictor of metastasis and survival in colorectal cancer. J Pathol (2010) 220(4):475–89. doi: 10.1002/path.2668
13. Urzúa U, Roby KF, Gangi LM, Cherry JM, Powell JI, Munroe DJ. Transcriptomic analysis of an in vitro murine model of ovarian carcinoma: functional similarity to the human disease and identification of prospective tumoral markers and targets. J Cell Physiol (2006) 206(3):594–602. doi: 10.1002/jcp.20522
14. Qiu MH, Qian YM, Zhao XL, Wang SM, Feng XJ, Chen XF, et al. Expression and prognostic significance of MAP4K4 in lung adenocarcinoma. Pathol Res Pract (2012) 208(9):541–8. doi: 10.1016/j.prp.2012.06.001
15. Liu YF, Qu GQ, Lu YM, Kong WM, Liu Y, Chen WX, et al. Silencing of MAP4K4 by short hairpin RNA suppresses proliferation, induces G1 cell cycle arrest and induces apoptosis in gastric cancer cells. Mol Med Rep (2016) 13(1):41–8. doi: 10.3892/mmr.2015.4510
16. Liu AW, Cai J, Zhao XL, Jiang TH, He TF, Fu HQ, et al. ShRNA-targeted MAP4K4 inhibits hepatocellular carcinoma growth. Clin Cancer Res (2011) 17(4):710–20. doi: 10.1158/1078-0432.CCR-10-0331
17. Singh SK, Kumar S, Viswakarma N, Principe DR, Das S, Sondarva G, et al. MAP4K4 promotes pancreatic tumorigenesis via phosphorylation and activation of mixed lineage kinase 3. Oncogene (2021) 40(43):6153–65. doi: 10.1038/s41388-021-02007-w
18. Collins CS, Hong J, Sapinoso L, Zhou Y, Liu Z, Micklash K, et al. A small interfering RNA screen for modulators of tumor cell motility identifies MAP4K4 as a promigratory kinase. Proc Natl Acad Sci USA (2006) 103(10):3775–80. doi: 10.1073/pnas.0600040103
19. Lin JC, Lee YC, Tan TH, Liang YC, Chuang HC, Fann YC, et al. RBM4-SRSF3-MAP4K4 splicing cascade modulates the metastatic signature of colorectal cancer cell. Biochim Biophys Acta Mol Cell Res (2018) 1865(2):259–72. doi: 10.1016/j.bbamcr.2017.11.005
20. Gao X, Chen G, Gao C, Zhang DH, Kuan SF, Stabile LP, et al. MAP4K4 is a novel MAPK/ERK pathway regulator required for lung adenocarcinoma maintenance. Mol Oncol (2017) 11(6):628–39. doi: 10.1002/1878-0261.12055
21. Tripolitsioti D, Kumar KS, Neve A, Migliavacca J, Capdeville C, Rushing EJ, et al. MAP4K4 controlled integrin β1 activation and c-met endocytosis are associated with invasive behavior of medulloblastoma cells. Oncotarget (2018) 9(33):23220–36. doi: 10.18632/oncotarget.25294
22. Santhana Kumar K, Tripolitsioti D, Ma M, Grählert J, Egli KB, Fiaschetti G, et al. The Ser/Thr kinase MAP4K4 drives c-met-induced motility and invasiveness in a cell-based model of SHH medulloblastoma. Springerplus (2015) 4:19. doi: 10.1186/s40064-015-0784-2
23. Prolo LM, Li A, Owen SF, Parker JJ, Foshay K, Nitta RT, et al. Targeted genomic CRISPR-Cas9 screen identifies MAP4K4 as essential for glioblastoma invasion. Sci Rep (2019) 9(1):14020. doi: 10.1038/s41598-019-50160-w
24. Esen E, Sergin I, Jesudason R, Himmels P, Webster JD, Zhang H, et al. MAP4K4 negatively regulates CD8 T cell-mediated antitumor and antiviral immunity. Sci Immunol (2020) 5(45):eaay2245. doi: 10.1126/sciimmunol.aay2245
25. Migliavacca J, Züllig B, Capdeville C, Grotzer MA, Baumgartner M. Cooperation of striatin 3 and MAP4K4 promotes growth and tissue invasion. Commun Biol (2022) 5(1):795. doi: 10.1038/s42003-022-03708-y
26. Kim JW, Berrios C, Kim M, Schade AE, Adelmant G, Yeerna H, et al. STRIPAK directs PP2A activity toward MAP4K4 to promote oncogenic transformation of human cells. Elife (2020) 9:e53003. doi: 10.7554/eLife.53003
27. Jovanovic D, Yan S, Baumgartner M. The molecular basis of the dichotomous functionality of MAP4K4 in proliferation and cell motility control in cancer. Front Oncol (2022) 12:1059513. doi: 10.3389/fonc.2022.1059513
28. Fu Y, Liu X, Chen Q, Liu T, Lu C, Yu J, et al. Downregulated miR-98-5p promotes PDAC proliferation and metastasis by reversely regulating MAP4K4. J Exp Clin Cancer Res (2018) 37(1):130. doi: 10.1186/s13046-018-0807-2
29. Huang H, Han Q, Zheng H, Liu M, Shi S, Zhang T, et al. MAP4K4 mediates the SOX6-induced autophagy and reduces the chemosensitivity of cervical cancer. Cell Death Dis (2021) 13(1):13. doi: 10.1038/s41419-021-04474-1
30. Mei J, Wang DH, Wang LL, Chen Q, Pan LL, Xia L. MicroRNA-200c suppressed cervical cancer cell metastasis and growth via targeting MAP4K4. Eur Rev Med Pharmacol Sci (2018) 22(3):623–31. doi: 10.26355/eurrev_201802_14286
31. Wang F, Zhao L, Zhang J, Meng Z, Zhou C, Wang G, et al. Chemotherapy-induced miR-141/MAP4K4 signaling suppresses progression of colorectal cancer. Biosci Rep (2018) 38(6):BSR20180978. doi: 10.1042/BSR20180978
32. Zhang Q, Xin H, Fen T. Function of microRNA−141 in human breast cancer through cytotoxic CD4+ T cells regulated by MAP4K4 expression. Mol Med Rep (2018) 17(6):7893–901. doi: 10.3892/mmr.2018.8814
33. Virbasius JV, Czech MP. Map4k4 signaling nodes in metabolic and cardiovascular diseases. Trends Endocrinol Metab (2016) 27(7):484–92. doi: 10.1016/j.tem.2016.04.006
34. Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, Hansen CG, Hong AW, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the hippo pathway. Nat Commun (2015) 6:8313–57. doi: 10.1038/ncomms9357
Keywords: MAP4K4, MAP kinases, cytoskeleton, cancer, RNA interference
Citation: González-Montero J, Rojas CI and Burotto M (2023) MAP4K4 and cancer: ready for the main stage? Front. Oncol. 13:1162835. doi: 10.3389/fonc.2023.1162835
Received: 10 February 2023; Accepted: 06 April 2023;
Published: 08 May 2023.
Edited by:
Ruchi Roy, University of Illinois at Chicago, United StatesReviewed by:
Paramita Bhattacharya, National Institute of Biomedical Genomics (NIBMG), IndiaCopyright © 2023 González-Montero, Rojas and Burotto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mauricio Burotto, bWJ1cm90dG9AYnJhZGZvcmRoaWxsLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.