- 1Department of Laboratory Medicine, Wuhan Asian Heart Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, China
- 2School of Medicine, Wuhan University of Science and Technology, Wuhan, China
Congestion is the permanent mechanism driving disease progression in patients with acute heart failure (AHF) and also is an important treatment target. However, distinguishing between the two different phenotypes (intravascular congestion and tissue congestion) for personalized treatment remains challenging. Historically, carbohydrate antigen 125 (CA125) has been a frequently used biomarker for the screening, diagnosis, and prognosis of ovarian cancer. Interestingly, CA125 is highly sensitive to tissue congestion and shows potential for clinical monitoring and optimal treatment of congestive heart failure (HF). Furthermore, in terms of right heart function parameters, CA125 levels are more advantageous than other biomarkers of HF. CA125 is expected to become a new biological alternative marker for congestive HF and thereby is expected be widely used in clinical practice.
1 Introduction
According to the 2021 European Society of Cardiology (ESC) Guidelines, the underlying cause of cardiac insufficiency is important in the diagnosis of heart failure (HF). This is because understanding the specific pathophysiology can help determine appropriate treatment options (1). Congestion is the culprit in the progression of HF and end-stage organ damage, which can eventually lead to direct cytotoxicity, myocardial fibrosis, arrhythmia, and pump failure. Congestion symptoms mainly manifest at the pulmonary, systemic or mixed levels. With the increase in venous pressure, plasma gradually infiltrates into the interstitial space. The current research mainly focuses on two different types of congestion, including intravascular congestion and tissue congestion. The majority of patients with congestive HF frequently experience intravascular and tissue congestion (Figure 1) (2, 3). However, with the development of precision medicine, it cannot be denied that it is crucial to identify the main phenotype in the formulation of personalized treatment and the assessment of prognosis.
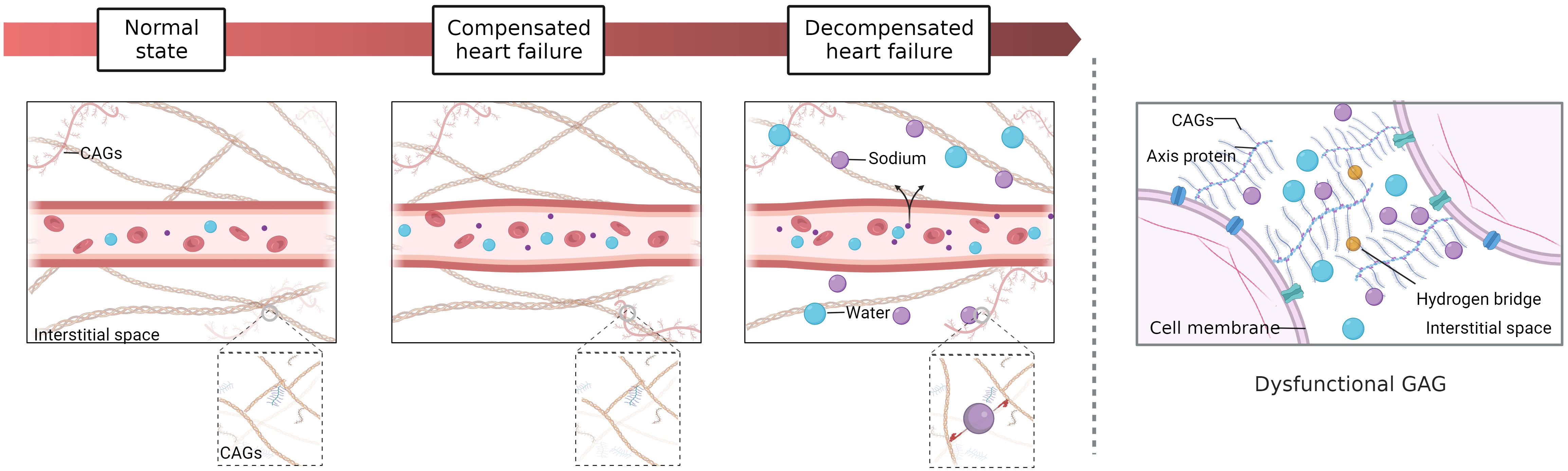
Figure 1 Tissue versus intravascular congestion. Interstitial fluid is formed by filtration through capillaries and subsequently drained by many lymphatic vessels. 1) Normal state: the hydrostatic pressure and osmotic pressure of capillaries are balanced. 2) Compensated HF (isolated circulatory congestion): with the increases in blood volume in capillaries, hydrostatic pressure rises and osmotic pressure decreases, but a balance is generally maintained by the compensatory mechanism. 3) Decompensated HF (circulatory congestion with tissue congestion): During the early stages of decompensated HF, there may be circulatory congestion with subclinical tissue congestion. At this point, apart from weight gain, there may not be any obvious symptoms or signs of congestion. As the condition progresses, there may be an increase in plasma volume and interstitial water, leading to circulatory congestion with tissue congestion and with development of symptoms during exercise and possibly interstitial lung edema (B lines). In the terminal stage, severe dyspnea at rest may even occur. Lymphatic drainage becomes less efficient, and the hydrostatic pressure and osmotic pressure of capillaries continue to increase so that many sodium and water molecules begin to accumulate in the interstitial space. The interstitial space contains a large number of glycosaminoglycans (GAGs), which form a strong network connected by hydrogen bonds and have important regulatory functions. However, high concentrations of sodium eventually alter the conformation of macromolecules in the GAG network, resulting in a loss of interstitial network integrity and buffering capacity. Hence, a slight increase in capillary blood pressure may cause interstitial fluid accumulation during this stage.
Classical theory suggests that N-terminal pro-B-type natriuretic peptide (NT-proBNP) is the gold standard for the diagnosis of intravascular congestion in patients with congestive HF. Nevertheless, existing studies indicate that NT-proBNP is not an appropriate marker in the assessment of tissue congestion (4–6). Since Nägele et al. (5) first observed in 1999 that the increase in serum carbohydrate antigen 125 (CA125) levels in HF patients was significantly correlated with clinical severity and filling pressure. Numerous studies have been carried out to explore the correlation between CA125 and HF clinical, neurohumoral and hemodynamic data (Tables 1, 2). Therefore, further studies are needed to identify a more comprehensive biomarker to guide treatment and inform prognosis to meet clinical needs. This article aims to provide a better understanding of the contributory role of CA125 in clinical application and shed light on a novel therapeutic target for congestive HF.
Table 2 Relationship between CA125 and the severity of symptoms, physical fitness and decompensation of heart failure.
2 Overview of the characteristics of CA125 and congestive heart failure
CA125 (also known as MUC16) is a transmembrane protein that is highly glycosylated and has a high molecular weight. It is mainly composed of three parts: the N-terminal domain, tandem repeat domain, and C-terminal domain (Figure 2) (27–29). Research shows that CA125 is not expressed in tumor cells but instead originates from the cell surfaces of various tissues of the coelomic epithelium (30). Its main function is to hydrate, lubricate and protect the surface of the epithelial cavity from physical pressure (24, 31, 32). CA125 is considered a valuable biomarker for diagnosing ovarian cancer and evaluating the therapeutic prognosis of patients (31–33).
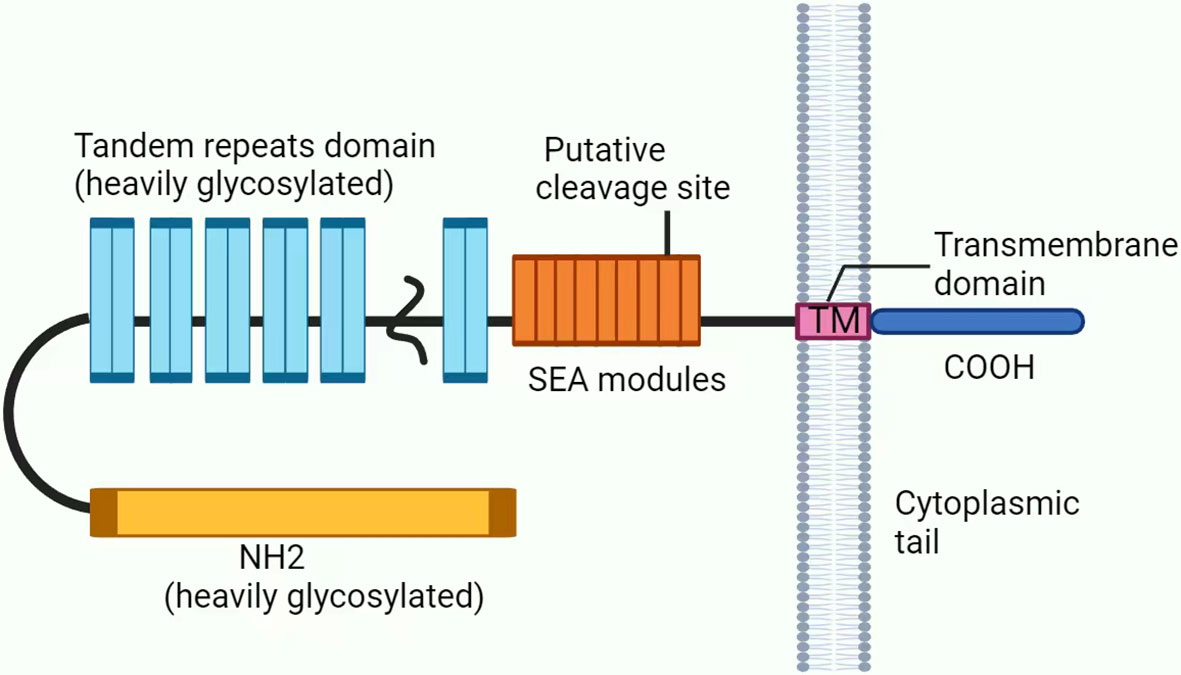
Figure 2 Structure of CA125/MUC16. CA125 is mainly composed of three parts: (1) The N-terminal domain (heavily glycosylated). (2) The tandem repeat domain, which is highly O-glycosylated, has repeating sequences that are high in serine, threonine and proline. (3) The C-terminal domain contains multiple extracellular SEA (sea urchin sperm protein, enterokinase, and agrin) modules, a transmembrane (TM) domain, and a short 31-amino acid cytoplasmic tail (CT). In addition, CA125 is thought to be putatively cleaved at a potential tyrosine phosphorylation site in SEA.
The exact biological mechanism of CA125 remains unclear, but it is believed that several biological pathways may be involved. (a) It induces the movement of tumor cells through the interaction between cadherin and epithelial-mesenchymal and combines with mesothelin to facilitate the invasion of cells to the peritoneum (34). (b) CA125 not only promotes the progression and metastasis of ovarian cancer but also acts as a barrier for trophoblasts to adhere to the endometrium, protects cancer cells from natural killer (NK) cell-mediated destruction, and controls red blood cell aggregation. (c) CA125 can also act as a barrier for bacterial and viral infections in the ocular epithelium (35, 36). Felder et al. (6) reported that the N-glycan structure presented in CA125 may have a role in regulating both innate and adaptive immune responses. Furthermore, CA125 can function as a lectin anti-receptor and has a strong affinity for galectin-1 and galectin-3 (37). Galectin-3 may also contribute to cardiac remodelling by regulating galectin activity and modifying the quality and hardness of the intercellular matrix.
In addition to malignant tumors, elevated CA125 levels can also be observed in various physiological or pathological conditions such as early pregnancy, menstruation, peritoneal injury and ascites of any cause (34, 38, 39), such as ascites due to cirrhosis (40). Subsequent studies have highlighted congestive HF as a special cause of increased serum CA125 levels. Moreover, CA125 levels are closely associated with the severity of congestion and are often accompanied by significant volume overload and fluid accumulation (41). Indeed, up to two-thirds of patients with AHF exhibit CA125 levels (35-200 U/mL) above the normal range (32, 42), and in patients with stable HF is mostly lower than 35 U/mL. In contrast, patients with ovarian cancer may exhibit serum concentrations as high as 2000-3000 U/mL (43, 44).
The mechanism behind the upregulation of serum CA125 in patients with congestive HF has not been confirmed. In the existing studies, mechanical stress and inflammatory stimulation are considered crucial factors in this process, and two hypotheses have been proposed (Figure 3): (1) Mesothelial cells are stimulated by the mechanical stress caused by tissue tension due to fluid overload, leading to the release of CA125 (45, 46). (2) The overexpression of CA125 in mesothelial cells is stimulated by activation of the inflammatory cytokine network (42). According to Colombo et al. (47, 48), venous congestion leads to endothelial activation, upregulation of inflammatory cytokines, hepatic dysfunction, and intestinal villus ischemia. Intestinal villus ischemia can eventually cause abnormal function and loss of barrier function in intestinal epithelial cells, allowing the lipopolysaccharides and endotoxins produced by gram-negative bacteria in the intestinal lumen to enter the circulation. It further aggravates the inflammatory environment that is already established by venous congestion and neurohormonal activity. Fluid overload and inflammatory processes interact to form a vicious cycle in congestive HF. Moreover, mechanical stress and inflammatory stimuli activate the JNK pathway within the cytoplasm of mesothelial cells and jointly initiate the synthesis of CA125 (49–51). There are data showing that interleukin-6 (IL-6), interleukin-10 (IL-10), and tumor necrosis factor-α (TNF-α) can promote CA125 synthesis by stimulating mesothelial cells in the case of HF (46, 48, 52, 53). In an in vitro model, Zeillemaker et al. demonstrated that the ability of mesothelial cells to synthesize CA125 is enhanced when they are stimulated (27, 54). It has been suggested that CA125 may act as a secondary cytokine, and its level could be increased due to the activation of primary cytokine networks such as TNF-α and IL-1, 4 (55). However, the relationship between the primary and secondary factors is not fully understood and requires further research. Myocardial remodelling can result in pathological cardiomyocyte hypertrophy and re-expression of embryonic genes. This process is accompanied by the activation of proto-oncogenes, which further stimulate growth factors in the embryo, leading to increased levels of CA125 (56).
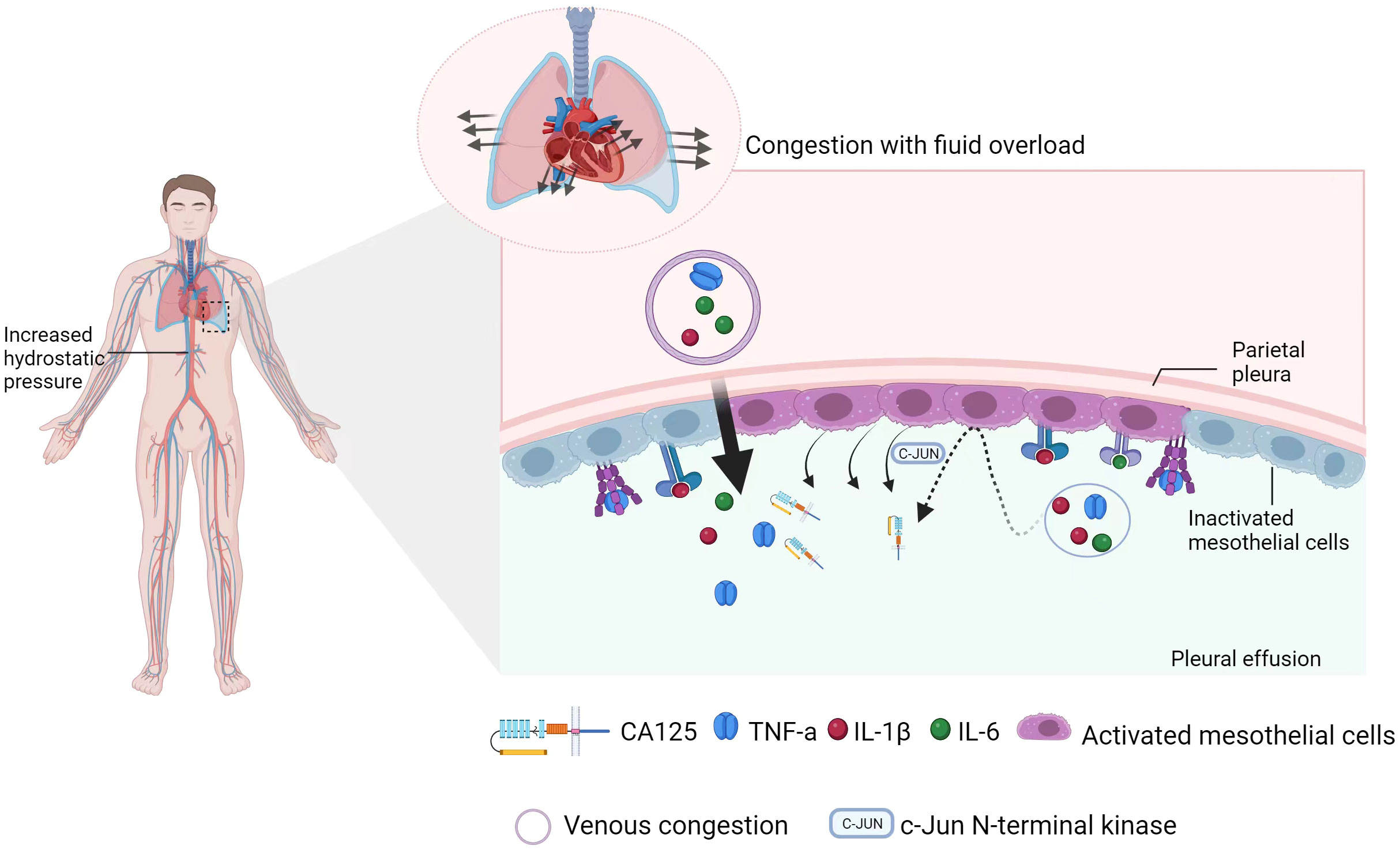
Figure 3 Schematic diagram of CA125 synthesis. On the one hand, intravascular congestion (venous congestion) can lead to endothelial activation, upregulation of inflammatory cytokines, liver dysfunction, and intestinal villus ischemia, which can promote the release of inflammatory factors. However, tissue congestion (pleural effusion) activates mesothelial cells in the pleura through mechanical stress and inflammatory stimuli. This activation initiates the synthesis of CA125 via the JNK pathway. At the same time, changes in cell morphology and membrane stability cause shedding of the extracellular domain of CA125 from mesothelial cells.
It is crucial to provide a comprehensive explanation for elevated CA125 levels to differentiate between ovarian cancer and HF accurately. While fluid retention can cause tissue damage and elevate CA125 levels in both ovarian cancer and HF patients, the level of CA125 is considerably higher in ovarian cancer patients compared to HF patients (57). Furthermore, it is important to note that CA125 alone is not an ideal diagnostic tool for either condition due to its limited specificity and sensitivity (57). Therefore, for better clinical effectiveness in screening and early detection, it should be combined with symptoms/signs, other biomarkers or ultrasound and other multimodal methods.
3 Potential clinical applications of CA125
3.1 Diagnostic utility of CA125 for heart failure and its LV phenotype
In cases of myocardial hypertrophy and failure, compensatory mechanisms, which mainly include the renin-angiotensin-aldosterone system (RAAS), vasopressin activation, and sympathetic nerve excitation, can cause water and sodium retention, as well as peripheral vasoconstriction. These mechanisms increase the cardiac pre- and postloads, leading to an increase in venous pressure. Additionally, an imbalance of capillary hydrostatic pressure and colloid osmotic pressure in the mesenchyme can result in peripheral edema, serosal cavity effusion, and an increase in the pulmonary capillary wedge pressure (2). B-type natriuretic peptide (BNP) is a neurohormone synthesized in ventricular myocardium that is released into the circulation during ventricular dilatation and pressure overload. As a result, increased circulating natriuretic peptides (NPs) may indicate intravascular and intracardiac congestion rather than tissue congestion. Symptoms and signs, such as ascites, rales, and peripheral edema, are established indicators of tissue congestion, but they are poorly sensitive to the diagnosis of interstitial edema in HF.
In chronic HF patients, those with serous cavity effusion (SCE) had significantly higher serum CA125 levels than those without SCE (22). ROC curve analyses of CA125 and NT-proBNP showed that NT-proBNP was more suitable for predicting the presence of chronic HF than CA125. However, CA125 was found to be a better predictor of chronic HF patients with SCE than NT-proBNP. Additionally, CA125 was identified as an important predictor of AHF with pleural effusion and peripheral edema (21, 32). The increase in CA125 is influenced by the mechanical extension of mesothelial cells from SCE (45, 46). It is not yet clear whether the serum CA125 threshold is still less than 35 U/mL in HF. In HF patients with aortic stenosis (AS), the area under the ROC curve was 0.85 for CA125 and 0.78 for BNP. The best cut-offs were determined to be 10.3 U/mL and 254.64 pg/mL, respectively (58). In patients with chronic obstructive pulmonary disease (COPD), CA125 levels can predict the presence of right ventricular failure with an AUC of 0.902. High levels of CA125 (≥35 U/mL) increase the risk of having a diagnosis of right ventricular failure by echocardiography by 51-fold (13). It is worth noting that during the process of diagnosing HF, several confounding factors need to be ruled out. These include malignant tumors, active inflammatory diseases, and women who are menstruating or pregnant, as these conditions may lead to conflicting results (34, 38, 39). In addition, it is necessary to adjust for the interference of age, sex, obesity, and atrial fibrillation (AF) on the level of CA125 (13, 59, 60). Therefore, a comprehensive evaluation should be conducted before making any clinical explanations.
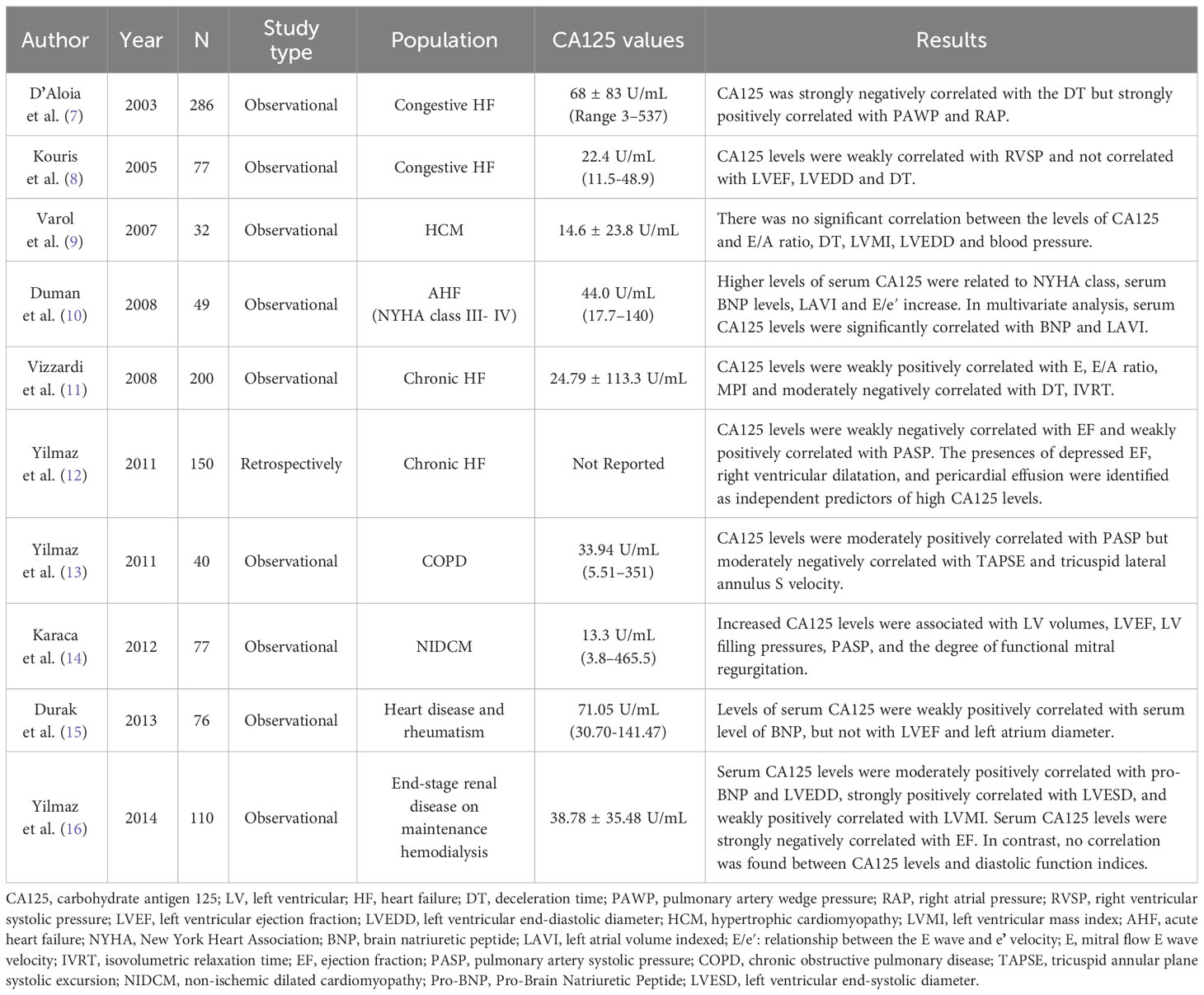
The relationship between CA125 levels and echocardiographic parameters has produced conflicting results in some studies, particularly in regards to left ventricular function (Table 1). In a study of 77 patients with congestive HF, CA125 levels were weakly correlated with right ventricular systolic pressure (RVSP) and were not correlated with Doppler echocardiography E wave deceleration time(DT), left ventricular ejection fraction (LVEF), or left ventricular end diastolic diameter (LVEDD) (8). Duman et al. (8, 10, 18) analyzed the relationship between serum CA125 levels and left ventricular (LV) filling pressure parameters in patients with advanced HF. They also measured plasma BNP and used the left atrial volume index (LAVI) to reflect the severity and duration of LV diastolic dysfunction. The study found a positive correlation between ln CA125 and ln BNP, LAVI, and the ratio of mitral inflow early diastolic velocity to annulus velocity (E/e). However, there was no correlation with ejection fraction (EF), mitral deceleration time (DT), isovolumic relaxation time (IVRT), mitral E-wave velocity, or the ratio of mitral inflow early to late (A) diastolic velocity (E/A). In a study by Varol et al. (9), CA125 was not found to be associated with left ventricular functional parameters. However, Yilmaz et al. (12) discovered that CA125 levels were positively correlated with pulmonary systolic pressure and negatively correlated with ejection fraction. Furthermore, a decrease in EF, right ventricular dilatation, and pericardial effusion were independent predictors of high CA125 levels. Although the relationship between CA125 and left ventricular function parameters is controversial in different studies, the level of CA125 is always related to pulmonary artery wedge pressure and right atrial pressure. This suggests that CA125 may be advantageous in evaluating right ventricular function.
3.2 CA125: symptom severity, physical capacity and decompensation
As a biomarker reflecting congestion, CA125 is related to disease severity, hemodynamics and echocardiographic parameters. Patients with HF had a much higher serum level of CA125 than healthy controls (Table 2). In a study of 529 patients with AHF, the average plasma level of CA125 was 7-fold higher than that of a control group of asymptomatic HF patients matched for age, sex and cardiovascular risk factor (60). Durak et al. (15) found that the median serum CA125 level was significantly higher in patients with decompensated HF than in those with compensated HF. Kouris et al. (8) used the New York Heart Association (NYHA) classification method and found that the serum CA125 values were higher in patients with NYHA Class III/IV than in those with NYHA Class I/II. Meanwhile, 44 patients with HF were prospectively evaluated, whose CA125 levels were positively correlated with NYHA functional class (61), and the subsequent findings were also consistent with this finding (15). In addition, CA125 levels are also associated with right ventricular enlargement and dysfunction. For example, Nägele et al. (5) found that CA125 values were positively correlated with right atrial pressure and pulmonary capillary wedge pressure in patients with advanced HF receiving heart transplantation. In patients with COPD, CA125 levels were negatively correlated with tricuspid annular plane systolic excursion and tricuspid lateral annulus S velocity but positively correlated with the severity of tricuspid regurgitation (TR) and right atrial size (13). Therefore, CA125 can serve as a useful surrogate index for assessing the severity of HF.
3.3 CA125 and prognosis
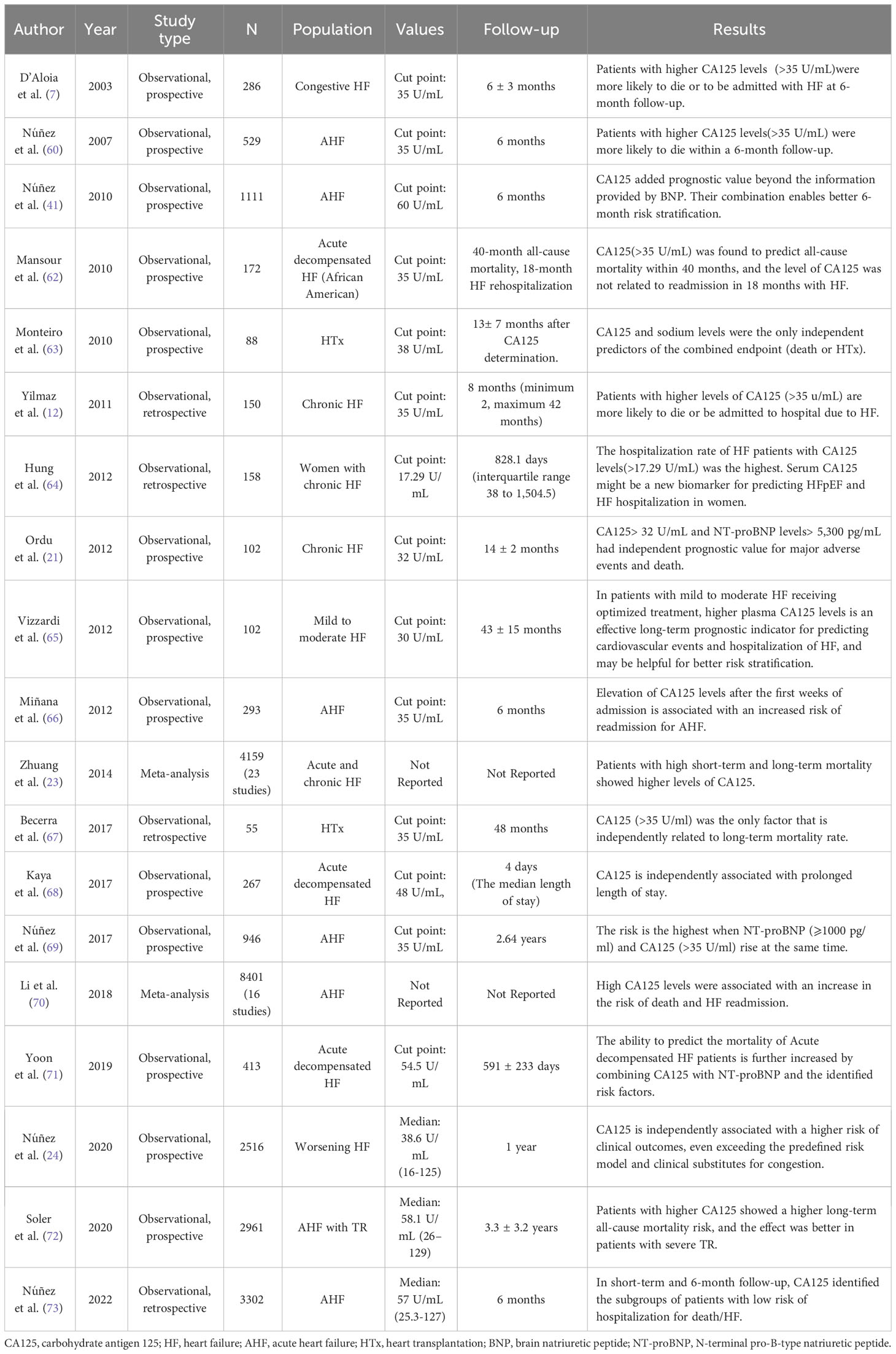
CA125 has been shown to be associated with poor outcomes in patients with AHF (Table 3). A study found that repeated measurements of serum CA125 (3402 observations) over the long term can independently predict the risk of long-term death in a group of 946 patients who were discharged from AHF. Patients with normal serum CA125 levels (median of 31 days) at their first outpatient visit had the lowest risk of death, while those with reduced but not normal levels were at intermediate risk. The changes in serum CA125 levels can be used to predict the long-term prognosis of patients with HF and improve risk stratification (69). The current study found that CA125 was significantly associated with the risk of death and readmission in multiple AHF regimens (7, 32, 42, 69). The BioStat-CHF cohort study found a strong association between CA125 levels and a combined risk of 1-year all-cause death, HF all-cause death, and hospitalization (24). A study of 1111 cases of AHF found that a higher level of CA125 (> 60 U/mL) was associated with increased mortality at 6 months. Another study by Hung et al. (64) reported that adding CA125 to NT-proBNP improved the prediction of hospitalization for female HF patients with preserved ejection fraction (HFpEF). In conclusion, CA125 is a useful short- and long-term prognostic factor for HF patients. When paired with other biomarkers, it can enhance the precision of predicting adverse events.
3.4 CA125: response to treatment
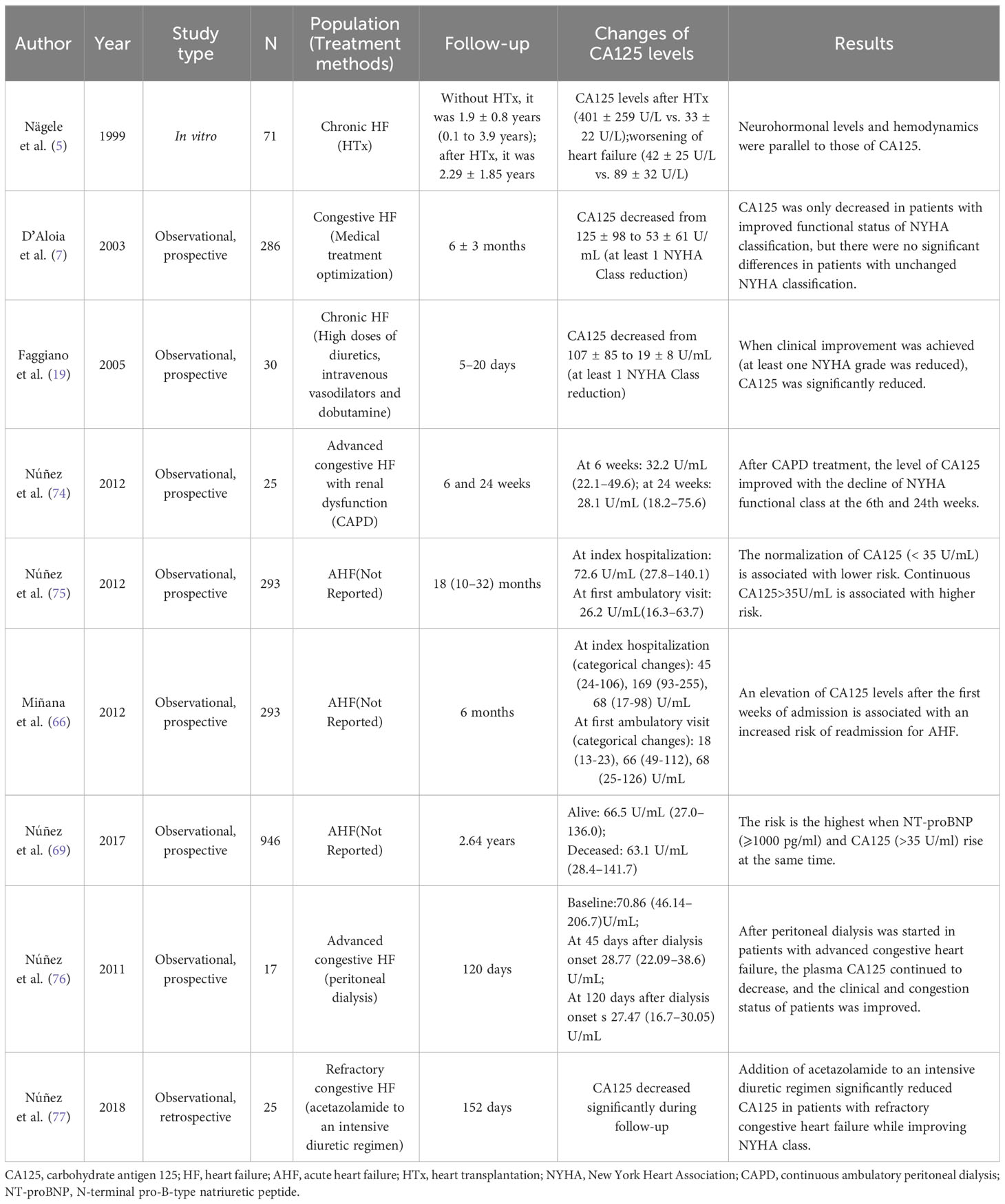
Recent studies indicate that an alteration of CA125 is in line with the evolution of clinical status (Table 4). For instance, a study involving 77 patients who underwent heart transplantation revealed that the concentration of CA125 in individuals with a notable improvement in NYHA class after the procedure decreased. Conversely, the level of CA125 in subjects with deteriorating HF increased (5). Similarly, Núñez et al. (76) reported that peritoneal dialysis resulted in a sustained decrease in plasma CA125 levels in patients with advanced congestive HF. This decrease was accompanied by improvements in the patient’s clinical and congestion status, despite the serosal irritation caused by infusion of the osmotic solution into the peritoneum. Further studies have also observed a decrease in CA125 levels with enhanced diuretic therapy (74, 77, 78). Therefore, after effective medication or surgical treatment, serum CA125 decreased with the improvement of clinical symptoms, and vice versa (79).
3.5 CA125 for guiding therapy for heart failure
Diuretics have been shown to alleviate symptoms of pulmonary and systemic venous congestion (1). Paradoxically, studies have shown that the use of higher doses of loop diuretics is associated with a poor prognosis (80, 81), a view shared by Ahmed et al. (82) López-Vilella et al. (78) found that the combination of diuretics required to improve patients with decompensated HF is associated with the CA125 level, site of congestion, renal function, and right ventricular functionality. Compared with patients with pulmonary congestion who used a single diuretic, those with systemic congestion with more diuretic combinations had higher levels of CA125. However, the optimal use boundary of loop diuretics relies heavily on subjective empirical assessment rather than evidence-based guidance. This highlights the need for further research in this area. Notably, CA125 has the potential to be a useful tool in monitoring and guiding diuretic therapy following episodes of AHF (75, 83). A prospective randomized multicenter trial aimed to assess treatment effects based on a series of measurements of serum CA125 levels in HF patients (84). These include adjusting the dose of diuretics based on serum CA125 levels, increasing the number of outpatient visits, administering intravenous iron in patients with iron deficiency, upregulating of statins in those with high serum CA125 levels, and initiating mineralocorticoid antagonists, with the goal of maintaining serum CA125 levels below 35 U/ml. The results suggest that the CA125-guided regimen is superior to the standard regimen and that its effect is primarily driven by a significantly lower rate of readmissions rather than mortality. The study also found that the effect of CA125-guided therapy was time-dependent, with the effect being more pronounced in the first 6 months, after which the Kaplan-Meier curve tended to converge. The study indicates that monitoring serum CA125 levels in patients can aid in detecting and treating congestion early, potentially preventing rehospitalization for HF. However, the serum CA125-guided approaches focus on empirical and sometimes controversial interventions. It is still noteworthy that these therapeutic options regimens for reducing serum CA125 are not specific to relieving congestion. Statins seem to reduce the expression of CA125 by inhibiting the inflammatory response (85, 86), while Cleland et al. (87) found that statins appeared to be ineffective in patients with HF and more severe congestion. From this, it may be inferred that an elevated CA125 was likely to indicate that statins would be ineffective. Likewise, more direct evidence is needed to verify the value of CA125 in optimizing diuretics to relieve congestion.
A significant proportion of patients admitted with AHF exhibit renal dysfunction or experience worsening of renal function during hospitalization. These conditions contribute significantly to prolonged hospital stays and poor prognosis (88, 89). The kidneys play a key role in congestion. Traditionally, reduced cardiac output leads to decreased glomerular filtration and increased tubular reabsorption, resulting in water and sodium retention (69). However, the kidneys themselves may contribute to HF-related congestion before the neurohormonal system intervention (88). Therefore, further consideration should be given to the clinical effectiveness of CA125 levels in adjusting the intensity of diuretic therapy in patients with cardiorenal syndrome. In a randomized study involving 160 patients with AHF and renal dysfunction, a CA125-guided diuretic strategy was found to be more effective than conventional treatment in improving short-term renal function in these patients (90). Other uses for CA125 in cardiovascular medicine.
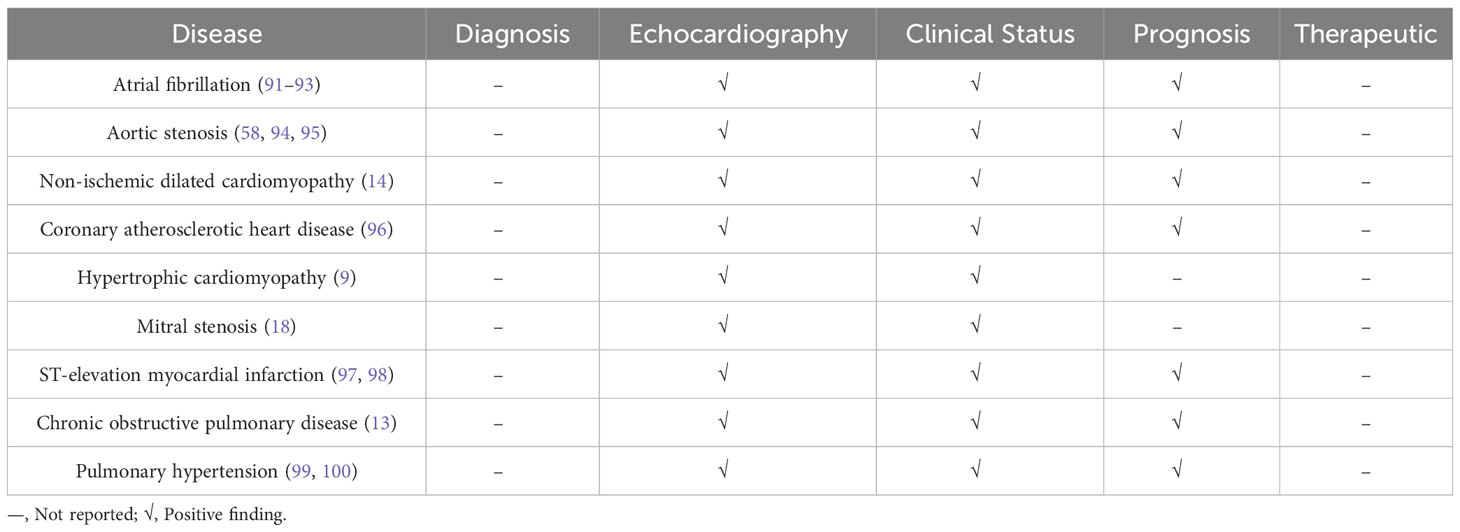
Given that CA125 is not a specific cardiac biomarker, Table 5 summarizes the current clinical applications of CA125 in various cardiovascular diseases. Among them, some studies have found that elevated levels of CA125 were associated with an increased risk of new-onset AF in HF patients (101), and the inflammatory response associated with AF may be a key factor in inducing serum CA125 synthesis (91, 102–104). Serum CA125 can independently predict the recurrence of AF after radiofrequency ablation, and patients with serum CA125 levels above 13.75 U/mL had a higher risk of AF recurrence than patients with serum CA125 levels below the critical value (102). A study conducted on 228 patients with severe aortic valve disease revealed that the longitudinal history of CA125 levels can be used to predict adverse clinical outcomes after transcatheter aortic valve implantation (94). When assessing the prognosis of AHF patients with functional TR, the use of CA125 and NT-proBNP yielded different results. That study found that NT-proBNP was significantly associated with mortality in nonsevere TR patients, while elevated levels of CA125 were significantly associated with mortality risk in all patients, with a greater impact on severe TR patients (72). Recent studies have indicated that CA125 may have a positive impact on patients with COPD and PAH who have impaired right heart function (99). Therefore, in addition to aid in diagnosis and treatment, CA125 levels have been found to be correlated with the severity and prognosis of various cardiovascular diseases.
4 Value of CA125 compared to other cardiovascular biomarkers
4.1 Added value of CA125 over NPs
Secretion of B-type natriuretic peptides (BNP) is stimulated by increasing atrial and ventricular wall tension, reflecting the degree of congestion. Accordingly, increased plasma concentrations of BNP/NT-proBNP are associated with more severe symptoms of HF and a worse prognosis, and they are also useful markers of therapeutic response (105). CA125 was positively correlated with NT-proBNP, although the correlation were mostly weak (24, 26, 41, 42). However, from a clinical point of view, CA125 was positively correlated with parameters of congestion (serous effusion, peripheral edema, inferior vena cava pressure (106), pulmonary wedge pressure, and severity of TR) (8, 41, 42, 60, 72, 84, 107). That study found that NT-proBNP values were only positively correlated with pleural effusion and IVC diameter, and the correlation was weaker compared to CA125 (69, 77, 83). Another study showed that neither clinical parameters of congestion nor surrogate echocardiographic parameters of right ventricular dysfunction were associated with higher values of NT-proBNP (25). It can be inferred that CA125 is more appropriate to evaluate patients with right ventricular dysfunction. Additionally, studies have reported that CA125 provides better prognostic information than NPs. Notably, as a pathophysiological biomarker different from NT-proBNP, CA125 also complements NT-proBNP in other ways. First, the effectiveness of NT-proBNP in guiding HF therapy occasionally leads to contradictory result, especially in elderly patients, despite their NT-proBNP being apparently improve (26), whereas serum CA125 production was not influenced by age, sex or renal function, which bypasses many of the limitations of NT-proBNP (41). Second, the average half-life of BNP is short, approximately 20 minutes, and its responses to initial therapy vary significantly. CA125, typically having a half-life of more than one week, is a biomarker of delayed response to acute hemodynamic changes. Combining acute hemodynamic information from the initial therapy (provided by BNP) with information on the chronicity of HF (provided by CA125) provides better prognosis and risk stratification (21, 41), which is similar to blood sugar and glycated hemoglobin in diabetic patients. Therefore, for patients with decompensated HF, CA 125 is a reliable surrogate that reflects fluid status over the past few weeks (20, 41, 42).
4.2 CA125 and other biomarkers
In addition to the association between proinflammatory cytokines (TNF-α and IL-6) and NPs, CA125 also shows a high affinity for Galectin-1 and Galectin-3. A study (108) reported that Gal-3 was closely associated with a higher risk of long-term death and repeated hospitalization but only in AHF patients with CA125 values greater than 67 U/ml. Furthermore, CA125 levels are also positively correlated with biomarkers that represent filling pressure/congestion, such as BIO-ADM (109). ADM is expressed by various cells including vascular endothelial cells, cardiomyocytes, leukocytes, and vascular smooth muscle cells, and it plays a vital role in maintaining endothelial barrier function. Increased levels of plasma ADM may suggest excessive fluid overload and are significantly related to symptoms and adverse consequences in patients with congestive HF (110–112). Serum BIO-ADM, soluble CD146, and CA125 were categorized together by Boorsma et al. (2) As valuable biomarkers for evaluating tissue congestion, this result provides a new method for evaluating congestion in multiple parameters. However, further research is still needed to compare this marker with the other recognized HF markers.
5 Problems and prospects
Undeniably, the following challenges still exist: (1) The pathophysiological role of CA125 upregulation in HF is not clear, and most of the relevant studies remain speculative at the moment. Whether the overexpression of CA125 is a consequence of mechanical stress and inflammatory stimulation caused by congestion or appears as a reason for exacerbating congestion is still being debated. (2) The threshold of CA125 for diagnosing HF has not yet been clarified yet. (3) There was a high level of heterogeneity between studies, and larger prospective studies and more controlled studies are needed to confirm the clinical value of CA125. (4) CA125 does not have organ specificity or disease specificity. In the absence of a diagnosis of HF, elevated CA125 may indicate a wide range of heterogeneous diseases, and thus a comprehensive clinical evaluation is needed.
6 Conclusions
The close relationship between CA125 and congestive HF is a surprising discovery, which further distinguishes between intravascular congestion and tissue congestion. To a certain extent, it compensates for the lack of a standardized biomarker for assessing congestion in daily clinical practice. CA125 has the capacity to predict beyond NT-proBNP and has important prognostic, risk stratification, monitor, and treatment guidance functions. Furthermore, its wide availability and low cost facilitate its application in clinical practice.
Author contributions
RF drafted this manuscript. ZLZ and QKF revised the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors thank Litao Zhang, Ling Li, and Yifan Bao for revising and enhancing the review. All figures were created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J (2021) 42(36):3599–726. doi: 10.1093/eurheartj/ehab368
2. Boorsma EM, Ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nat Rev Cardiol (2020) 17(10):641–55. doi: 10.1038/s41569-020-0379-7
3. Cleland JGF, Pfeffer MA, Clark AL, Januzzi JL, McMurray JJV, Mueller C, et al. The struggle towards a Universal Definition of Heart Failure-how to proceed? Eur Heart J (2021) 42(24):2331–43. doi: 10.1093/eurheartj/ehab082
4. Girerd N, Seronde M, Coiro S, Chouihed T, Bilbault P, Braun F, et al. Integrative assessment of congestion in heart failure throughout the patient journey. JACC Heart Failure (2018) 6(4):273–85. doi: 10.1016/j.jchf.2017.09.023
5. Nägele H, Bahlo M, Klapdor R, Schaeperkoetter D, Rödiger W. CA125 and its relation to cardiac function. Am Heart J (1999) 137(6):1044–9. doi: 10.1016/S0002-8703(99)70360-1
6. Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, et al. MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer (2014) 13:129. doi: 10.1186/1476-4598-13-129
7. D'Aloia A, Faggiano P, Aurigemma G, Bontempi L, Ruggeri G, Metra M, et al. Serum levels of carbohydrate antigen 125 in patients with chronic heart failure: relation to clinical severity, hemodynamic and Doppler echocardiographic abnormalities, and short-term prognosis. J Am Coll Cardiol (2003) 41(10):1805–11. doi: 10.1016/S0735-1097(03)00311-5
8. Kouris NT, Zacharos ID, Kontogianni DD, Goranitou GS, Sifaki MD, Grassos HE, et al. The significance of CA125 levels in patients with chronic congestive heart failure. Correlation with clinical and echocardiographic parameters. Eur J Heart Fail (2005) 7(2):199–203. doi: 10.1016/j.ejheart.2004.07.015
9. Varol E, Ozaydin M, Altinbas A, Aslan SM, Dogan A, Dede O. Elevated carbohydrate antigen 125 levels in hypertrophic cardiomyopathy patients with heart failure. Heart vessels (2007) 22(1):30–3. doi: 10.1007/s00380-006-0938-9
10. Duman D, Palit F, Simsek E, Bilgehan K. Serum carbohydrate antigen 125 levels in advanced heart failure: relation to B-type natriuretic peptide and left atrial volume. Eur J Heart Fail (2008) 10(6):556–9. doi: 10.1016/j.ejheart.2008.04.012
11. Vizzardi E, Nodari S, D'Aloia A, Chiari E, Faggiano P, Metra M, et al. CA 125 tumoral marker plasma levels relate to systolic and diastolic ventricular function and to the clinical status of patients with chronic heart failure. Echocardiography (Mount Kisco NY) (2008) 25(9):955–60. doi: 10.1111/j.1540-8175.2008.00714.x
12. Yilmaz M, Zorlu A, Tandogan I. Plasma CA-125 level is related to both sides of the heart: a retrospective analysis. Int J Cardiol (2011) 149(1):80–2. doi: 10.1016/j.ijcard.2009.12.003
13. Yilmaz MB, Zorlu A, Dogan OT, Karahan O, Tandogan I, Akkurt I. Role of CA-125 in identification of right ventricular failure in chronic obstructive pulmonary disease. Clin Cardiol (2011) 34(4):244–8. doi: 10.1002/clc.20868
14. Karaca O, Guler GB, Guler E, Gunes HM, Alizade E, Agus HZ, et al. Serum carbohydrate antigen 125 levels in nonischemic dilated cardiomyopathy: a useful biomarker for prognosis and functional mitral regurgitation. Congestive Heart failure (Greenwich Conn) (2012) 18(3):144–50. doi: 10.1111/j.1751-7133.2011.00260.x
15. Durak-Nalbantic A, Resic N, Kulic M, Pecar E, Zvizdic F, Dzubur A, et al. Serum level of tumor marker carbohydrate antigen-CA125 in heart failure. Med Arch (Sarajevo Bosnia Herzegovina) (2013) 67(4):241–4. doi: 10.5455/medarh.2013.67.241-244
16. Yilmaz H, Gürel OM, Celik HT, Sahiner E, Yildirim ME, Bilgiç MA, et al. CA 125 levels and left ventricular function in patients with end-stage renal disease on maintenance hemodialysis. Renal failure (2014) 36(2):210–6. doi: 10.3109/0886022X.2013.859528
17. Turk HM, Pekdemir H, Buyukberber S, Sevinc A, Camci C, Kocabas R, et al. Serum CA 125 levels in patients with chronic heart failure and accompanying pleural fluid. Tumour Biol (2003) 24(4):172–5. doi: 10.1159/000074425
18. Duman C, Ercan E, Tengiz I, Bozdemir H, Ercan HE, Nalbantgil I. Elevated serum CA 125 levels in mitral stenotic patients with heart failure. Cardiology (2003) 100(1):7–10. doi: 10.1159/000072385
19. Faggiano P, D'Aloia A, Brentana L, Bignotti T, Fiorina C, Vizzardi E, et al. Serum levels of different tumour markers in patients with chronic heart failure. Eur J Heart Fail (2005) 7(1):57–61. doi: 10.1016/j.ejheart.2004.04.009
20. Varol E, Ozaydin M, Dogan A, Kosar F. Tumour marker levels in patients with chronic heart failure. Eur J Heart Fail (2005) 7(5):840–3. doi: 10.1016/j.ejheart.2004.12.008
21. Ordu S, Ozhan H, Alemdar R, Aydin M, Caglar O, Yuksel H, et al. Carbohydrate antigen-125 and N-terminal pro-brain natriuretic peptide levels: compared in heart-failure prognostication. Tex Heart Inst J (2012) 39(1):30–5.
22. Huang F, Zhang K, Chen J, Cai Q, Liu X, Wang T, et al. Elevation of carbohydrate antigen 125 in chronic heart failure may be caused by mechanical extension of mesothelial cells from serous cavity effusion. Clin Biochem (2013) 46(16):1694–700. doi: 10.1016/j.clinbiochem.2013.09.008
23. Zhuang J, Faggiano P, Li Q, Pradelli D, Med V, Peng W, et al. Insights into the clinical implications of carbohydrate antigen 125 as a biomarker of heart failure: a meta-analysis and systematic review of published studies. J Cardiovasc Med (Hagerstown) (2014) 15(12):864–72. doi: 10.2459/JCM.0000000000000051
24. Núñez J, Bayés-Genís A, Revuelta-López E, Ter Maaten JM, Miñana G, Barallat J, et al. Clinical role of CA125 in worsening heart failure: A BIOSTAT-CHF study subanalysis. JACC Heart Fail (2020) 8(5):386–97. doi: 10.1016/j.jchf.2019.12.005
25. Miñana G, de la Espriella R, Mollar A, Santas E, Núñez E, Valero E, et al. Factors associated with plasma antigen carbohydrate 125 and amino-terminal pro-B-type natriuretic peptide concentrations in acute heart failure. Eur Heart J Acute Cardiovasc Care (2020) 9(5):437–47. doi: 10.1177/2048872620908033
26. Llàcer P, Gallardo M, Palau P, Moreno MC, Castillo C, Fernández C, et al. Comparison between CA125 and NT-proBNP for evaluating congestion in acute heart failure. Med Clin (Barc) (2021) 156(12):589–94. doi: 10.1016/j.medcli.2020.05.063
27. Zeillemaker A, Verbrugh H, Hoynck van Papendrecht A, Leguit P. CA 125 secretion by peritoneal mesothelial cells. J Clin Pathol (1994) 47(3):263–5. doi: 10.1136/jcp.47.3.263
28. Yin B, Dnistrian A, Lloyd K. Ovarian cancer antigen CA125 is encoded by the MUC16 mucin gene. Int J Cancer (2002) 98(5):737–40. doi: 10.1002/ijc.10250
29. Yin B, Lloyd K. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem (2001) 276(29):27371–5. doi: 10.1074/jbc.M103554200
30. Camera A, Villa M, Rocco S, De Novellis T, Costantini S, Pezzullo L, et al. Increased CA 125 serum levels in patients with advanced acute leukemia with serosal involvement. Cancer (2000) 88(1):75–8. doi: 10.1002/(SICI)1097-0142(20000101)88:1<75::AID-CNCR11>3.0.CO;2-#
31. Vizzardi E, D'Aloia A, Curnis A, Dei Cas L. Carbohydrate antigen 125: a new biomarker in heart failure. Cardiol Rev (2013) 21(1):23–6. doi: 10.1097/CRD.0b013e318265f58f
32. Núñez J, Miñana G, Núñez E, Chorro F, Bodí V, Sanchis J. Clinical utility of antigen carbohydrate 125 in heart failure. Heart failure Rev (2014) 19(5):575–84. doi: 10.1007/s10741-013-9402-y
33. Meyer T, Rustin GJ. Role of tumour markers in monitoring epithelial ovarian cancer. Br J Cancer (2000) 82(9):1535–8. doi: 10.1054/bjoc.2000.1174
34. Epiney M, Bertossa C, Weil A, Campana A, Bischof P. CA125 production by the peritoneum: in-vitro and in-vivo studies. Hum Reprod (Oxford England) (2000) 15(6):1261–5. doi: 10.1093/humrep/15.6.1261
35. Fraser CC, Jia B, Hu G, Johani LI, Fritz-Klaus R, Ham JD, et al. Ovarian cancer ascites inhibits transcriptional activation of NK cells partly through CA125. J Immunol (Baltimore Md. 1950) (2022) 208(9):2227–38. doi: 10.4049/jimmunol.2001095
36. Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, et al. N-Glycosylation affects the stability and barrier function of the MUC16 mucin. J Biol Chem (2017) 292(26):11079–90. doi: 10.1074/jbc.M116.770123
37. Bottoni P, Scatena R. The role of CA 125 as tumor marker: biochemical and clinical aspects. Adv Exp Med Biol (2015) 867:229–44. doi: 10.1007/978-94-017-7215-0_14
38. Yilmaz MB, Nikolaou M, Cohen Solal A. Tumour biomarkers in heart failure: is there a role for CA-125? Eur J Heart Fail (2011) 13(6):579–83. doi: 10.1093/eurjhf/hfr022
39. Dochez V, Caillon H, Vaucel E, Dimet J, Winer N, Ducarme G. Biomarkers and algorithms for diagnosis of ovarian cancer: CA125, HE4, RMI and ROMA, a review. J Ovarian Res (2019) 12(1):28. doi: 10.1186/s13048-019-0503-7
40. Devarbhavi H, Kaese D, Williams A, Rakela J, Klee G, Kamath P. Cancer antigen 125 in patients with chronic liver disease. Mayo Clinic Proc (2002) 77(6):538–41. doi: 10.4065/77.6.538
41. Núñez J, Sanchis J, Bodí V, Fonarow GC, Núñez E, Bertomeu-González V, et al. Improvement in risk stratification with the combination of the tumour marker antigen carbohydrate 125 and brain natriuretic peptide in patients with acute heart failure. Eur Heart J (2010) 31(14):1752–63. doi: 10.1093/eurheartj/ehq142
42. Llàcer P, Bayés-Genís A, Núñez J. Carbohydrate antigen 125 in heart failure. New era in the monitoring and control of treatment. Med Clin (Barc) (2019) 152(7):266–73. doi: 10.1016/j.medcli.2018.08.020
43. Molina R, Filella X, Bruix J, Mengual P, Bosch J, Calvet X, et al. Cancer antigen 125 in serum and ascitic fluid of patients with liver diseases. Clin Chem (1991) 37(8):1379–83. doi: 10.1093/clinchem/37.8.1379
44. Zhang M, Zhang Y, Fu J, Zhang L. Serum CA125 levels are decreased in rectal cancer but increased in fibrosis-associated diseases and in most types of cancers. Prog Mol Biol Transl Sci (2019) 162:241–52. doi: 10.1016/bs.pmbts.2018.12.012
45. Yura Y, Sano S, Walsh K. Clonal hematopoiesis: A new step linking inflammation to heart failure. JACC Basic to Trans Sci (2020) 5(2):196–207. doi: 10.1016/j.jacbts.2019.08.006
46. Kosar F, Aksoy Y, Ozguntekin G, Ozerol I, Varol E. Relationship between cytokines and tumour markers in patients with chronic heart failure. Eur J Heart Fail (2006) 8(3):270–4. doi: 10.1016/j.ejheart.2005.09.002
47. Colombo P, Onat D, Harxhi A, Demmer R, Hayashi Y, Jelic S, et al. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. Eur Heart J (2014) 35(7):448–54. doi: 10.1093/eurheartj/eht456
48. Núñez J, de la Espriella R, Miñana G, Santas E, Llácer P, Núñez E, et al. Antigen carbohydrate 125 as a biomarker in heart failure: a narrative review. Eur J Heart Fail (2021) 23(9):1445–57. doi: 10.1002/ejhf.2295
49. Leard L, Broaddus VJR. Mesothelial cell proliferation and apoptosis. Respirology (2004) 9(3):292–9. doi: 10.1111/j.1440-1843.2004.00602.x
50. Colombo P, Ganda A, Lin J, Onat D, Harxhi A, Iyasere J, et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev (2012) 17(2):177–90. doi: 10.1007/s10741-011-9261-3
51. Huang F, Chen J, Liu Y, Zhang K, Wang J, Huang H. New mechanism of elevated CA125 in heart failure: the mechanical stress and inflammatory stimuli initiate CA125 synthesis. Med Hypotheses (2012) 79(3):381–3. doi: 10.1016/j.mehy.2012.05.042
52. Turgut O, Tandogan I, Yilmaz M, Gul I, Gurlek A. CA125 levels among patients with advanced heart failure: an emerging independent predictor for survival. Int J Cardiol (2010) 145(1):71. doi: 10.1016/j.ijcard.2009.04.030
53. Yalta K, Yilmaz A, Turgut O, Erselcan T, Yilmaz M, Karadas F, et al. Evaluation of tumor markers CA-125 and CEA in acute myocardial infarction. Adv Ther (2006) 23(6):1052–9. doi: 10.1007/BF02850225
54. Topatan B, Basaran A. CA-125 and heart failure: déjà vu or "still to be seen". Int J Cardiol (2010) 145(3):626–9. doi: 10.1016/j.ijcard.2010.09.060
55. Cheung A, Gong M, Bellanti R, Ali-Hasan-Al-Saegh S, Li G, Roig E, et al. Cancer antigen-125 and risk of atrial fibrillation: a systematic review and meta-analysis. Heart Asia (2018) 10(1):e010970. doi: 10.1136/heartasia-2017-010970
56. Rheude T, Pellegrini C, Schmid H, Trenkwalder T, Mayr N, Joner M, et al. Comparison of carbohydrate antigen 125 and N-terminal pro-brain natriuretic peptide for risk prediction after transcatheter aortic valve implantation. Am J Cardiol (2018) 121(4):461–8. doi: 10.1016/j.amjcard.2017.11.020
57. Molina R, Filella X, Jo J, Agusti C, Ballesta AM. CA 125 in biological fluids. Int J Biol Markers (1998) 13(4):224–30. doi: 10.1177/172460089801300410
58. Antonini-Canterin F, Popescu B, Popescu A, Beladan C, Korcova R, Piazza R, et al. Heart failure in patients with aortic stenosis: clinical and prognostic significance of carbohydrate antigen 125 and brain natriuretic peptide measurement. Int J Cardiol (2008) 128(3):406–12. doi: 10.1016/j.ijcard.2007.05.039
59. Hamdy NM. Relationship between pro-anti-inflammatory cytokines, T-cell activation and CA 125 in obese patients with heart failure. Med Sci monitor (2011) 17(3):Cr174–179. doi: 10.12659/MSM.881453
60. Núñez J, Núñez E, Consuegra L, Sanchis J, Bodí V, Martínez-Brotons A, et al. Carbohydrate antigen 125: an emerging prognostic risk factor in acute heart failure? Heart (British Cardiac Society) (2007) 93(6):716–21. doi: 10.1136/hrt.2006.096016
61. Voors A, Anker S, Cleland J, Dickstein K, Filippatos G, van der Harst P, et al. A systems BIOlogy Study to TAilored Treatment in Chronic Heart Failure: rationale, design, and baseline characteristics of BIOSTAT-CHF. Eur J Heart Fail (2016) 18(6):716–26. doi: 10.1002/ejhf.531
62. Mansour IN, Napan S, Tarek Alahdab M, Stamos TD. Carbohydrate antigen 125 predicts long-term mortality in African American patients with acute decompensated heart failure. Congestive Heart failure (Greenwich Conn) (2010) 16(1):15–20. doi: 10.1111/j.1751-7133.2009.00110.x
63. Monteiro S, Franco F, Costa S, Monteiro P, Vieira H, Coelho L, et al. Prognostic value of CA125 in advanced heart failure patients. Int J Cardiol (2010) 140(1):115–8. doi: 10.1016/j.ijcard.2008.11.023
64. Hung CL, Hung TC, Liu CC, Wu YJ, Kuo JY, Hou CJ, et al. Relation of carbohydrate antigen-125 to left atrial remodeling and its prognostic usefulness in patients with heart failure and preserved left ventricular ejection fraction in women. Am J Cardiol (2012) 110(7):993–1000. doi: 10.1016/j.amjcard.2012.05.030
65. Vizzardi E, D'Aloia A, Pezzali N, Bugatti S, Curnis A, Dei Cas L. Long-term prognostic value of CA 125 serum levels in mild to moderate heart failure patients. J Card Fail (2012) 18(1):68–73. doi: 10.1016/j.cardfail.2011.09.012
66. Miñana Escrivá G, Núñez J, Sanchis J, Bodi V, Núñez E, Chorro FJ, et al. Carbohydrate antigen 125 serial measurements after an admission for acute heart failure and risk of early readmission. Med Clin (Barc) (2012) 139(11):479–86. doi: 10.1016/j.medcli.2011.05.029
67. Becerra-Muñoz VM, Sobrino-Márquez JM, Rangel-Sousa D, Fernández-Cisnal A, Lage-Gallé E, García-Pinilla JM, et al. Long-term prognostic role of CA-125 in noncongestive patients undergoing a cardiac transplantation. Biomarkers Med (2017) 11(3):239–43. doi: 10.2217/bmm-2016-0247
68. Kaya H, Kurt R, Beton O, Zorlu A, Yucel H, Gunes H, et al. Cancer antigen 125 is associated with length of stay in patients with acute heart failure. Tex Heart Inst J (2017) 44(1):22–8. doi: 10.14503/THIJ-15-5626
69. Núñez J, Núñez E, Bayés-Genís A, Fonarow GC, Miñana G, Bodí V, et al. Long-term serial kinetics of N-terminal pro B-type natriuretic peptide and carbohydrate antigen 125 for mortality risk prediction following acute heart failure. Eur Heart J Acute Cardiovasc Care (2017) 6(8):685–96. doi: 10.1177/2048872616649757
70. Li K, Gong M, Li G, Baranchuk A, Liu T, Wong M, et al. Cancer antigen-125 and outcomes in acute heart failure: a systematic review and meta-analysis. Heart Asia (2018) 10(2):e011044. doi: 10.1136/heartasia-2018-011044
71. Yoon JY, Yang DH, Cho HJ, Kim NK, Kim CY, Son J, et al. Serum levels of carbohydrate antigen 125 in combination with N-terminal pro-brain natriuretic peptide in patients with acute decompensated heart failure. Korean J Internal Med (2019) 34(4):811–8. doi: 10.3904/kjim.2017.313
72. Soler M, Minana G, Santas E, Núñez E, de la Espriella R, Valero E, et al. CA125 outperforms NT-proBNP in acute heart failure with severe tricuspid regurgitation. Int J Cardiol (2020) 308:54–9. doi: 10.1016/j.ijcard.2020.03.027
73. Núñez J, Bayés-Genís A, Revuelta-López E, Miñana G, Santas E, Ter Maaten JM, et al. Optimal carbohydrate antigen 125 cutpoint for identifying low-risk patients after admission for acute heart failure. Rev Esp Cardiol (Engl Ed) (2022) 75(4):316–24. doi: 10.1016/j.rec.2021.02.002
74. Núñez J, González M, Miñana G, Garcia-Ramón R, Sanchis J, Bodí V, et al. Continuous ambulatory peritoneal dialysis as a therapeutic alternative in patients with advanced congestive heart failure. Eur J Heart Fail (2012) 14(5):540–8. doi: 10.1093/eurjhf/hfs013
75. Núñez J, Núñez E, Miñana G, Bodí V, Fonarow G, Bertomeu-González V, et al. Differential mortality association of loop diuretic dosage according to blood urea nitrogen and carbohydrate antigen 125 following a hospitalization for acute heart failure. Eur J Heart failure (2012) 14(9):974–84. doi: 10.1093/eurjhf/hfs090
76. Núñez J, Miñana G, González M, Garcia-Ramón R, Sanchis J, Bodí V, et al. Antigen carbohydrate 125 in heart failure: not just a surrogate for serosal effusions? Int J Cardiol (2011) 146(3):473–4. doi: 10.1016/j.ijcard.2010.12.027
77. Núñez J, Heredia R, Payá A, Sanchis I, Del Prado S, Miñana G, et al. Use of acetazolamide in the treatment of patients with refractory congestive heart failure. Cardiovasc Ther (2018) 36(6):e12465. doi: 10.1111/1755-5922.12465
78. López-Vilella R, Jover Pastor P, Donoso Trenado V, Sánchez-Lázaro I, Martínez Dolz L, Almenar Bonet L. Clinical phenotypes according to diuretic combination in acute heart failure. Hellenic J Cardiol HJC = Hellenike kardiologike epitheorese (2023) 73:1–7. doi: 10.1016/j.hjc.2023.03.009
79. Costanzo M. Verdict in: congestion guilty! JACC Heart failure (2015) 3(10):762–4. doi: 10.1016/j.jchf.2015.06.004
80. Preiss D, Campbell R, Murray H, Ford I, Packard C, Sattar N, et al. The effect of statin therapy on heart failure events: a collaborative meta-analysis of unpublished data from major randomized trials. Eur Heart J (2015) 36(24):1536–46. doi: 10.1093/eurheartj/ehv072
81. Pellicori P, Cleland JGF, Zhang J, Kallvikbacka-Bennett A, Urbinati A, Shah P, et al. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther (2016) 30(6):599–609. doi: 10.1007/s10557-016-6697-7
82. Ahmed A, Husain A, Love T, Gambassi G, Dell'Italia L, Francis G, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J (2006) 27(12):1431–9. doi: 10.1093/eurheartj/ehi890
83. Núñez J, Llàcer P, Núñez E, Ventura S, Bonanad C, Bodí V, et al. Antigen carbohydrate 125 and creatinine on admission for prediction of renal function response following loop diuretic administration in acute heart failure. Int J Cardiol (2014) 174(3):516–23. doi: 10.1016/j.ijcard.2014.04.113
84. Núñez J, Llàcer P, Bertomeu-González V, Bosch M, Merlos P, García-Blas S, et al. Carbohydrate antigen-125-guided therapy in acute heart failure: CHANCE-HF: A randomized study. JACC. Heart failure (2016) 4(11):833–43. doi: 10.1016/j.jchf.2016.06.007
85. Tousoulis D, Oikonomou E, Siasos G, Stefanadis CJP. therapeutics. Statins in heart failure–With preserved and reduced ejection fraction. An update. Pharmacol Ther (2014) 141(1):79–91. doi: 10.1016/j.pharmthera.2013.09.001
86. Niazi M, Galehdar N, Jamshidi M, Mohammadi R, Moayyedkazemi A. A review of the role of statins in heart failure treatment. Curr Clin Pharmacol (2020) 15(1):30–7. doi: 10.2174/1574884714666190802125627
87. Cleland JG, McMurray JJ, Kjekshus J, Cornel JH, Dunselman P, Fonseca C, et al. Plasma concentration of amino-terminal pro-brain natriuretic peptide in chronic heart failure: prediction of cardiovascular events and interaction with the effects of rosuvastatin: a report from CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure). J Am Coll Cardiol (2009) 54(20):1850–9. doi: 10.1016/j.jacc.2009.06.041
88. McKie P, Schirger J, Costello-Boerrigter L, Benike S, Harstad L, Bailey K, et al. Impaired natriuretic and renal endocrine response to acute volume expansion in pre-clinical systolic and diastolic dysfunction. J Am Coll Cardiol (2011) 58(20):2095–103. doi: 10.1016/j.jacc.2011.07.042
89. Garcia-Blas S, Bonanad C, Llacer P, Ventura S, Núñez JM, Sánchez R, et al. Diuretic strategies in acute heart failure and renal dysfunction: conventional vs carbohydrate antigen 125-guided strategy. Clinical trial design. Rev Esp Cardiol (Engl Ed) (2017) 70(12):1067–73. doi: 10.1016/j.rec.2017.02.028
90. Núñez J, Llàcer P, García-Blas S, Bonanad C, Ventura S, Núñez J, et al. CA125-guided diuretic treatment versus usual care in patients with acute heart failure and renal dysfunction. Am J Med (2020) 133(3):370–380.e374. doi: 10.1016/j.amjmed.2019.07.041
91. Sekiguchi H, Shimamoto K, Takano M, Kimura M, Takahashi Y, Tatsumi F, et al. Cancer antigen-125 plasma level as a biomarker of new-onset atrial fibrillation in postmenopausal women. Heart (2017) 103(17):1368–73. doi: 10.1136/heartjnl-2016-310272
92. De Gennaro L, Brunetti ND, Montrone D, De Rosa F, Cuculo A, Di Biase M. Inflammatory activation and carbohydrate antigen-125 levels in subjects with atrial fibrillation. Eur J Clin Invest (2012) 42(4):371–5. doi: 10.1111/j.1365-2362.2011.02592.x
93. Yucel H, Kaya H, Zorlu A, Yıldırımlı K, Sancakdar E, Gunes H, et al. Cancer antigen 125 levels and increased risk of new-onset atrial fibrillation. Herz (2015) 40(2):119–24. doi: 10.1007/s00059-014-4148-4
94. Husser O, Núñez J, Núñez E, Holzamer A, Camboni D, Luchner A, et al. Tumor marker carbohydrate antigen 125 predicts adverse outcome after transcatheter aortic valve implantation. JACC Cardiovasc Interv (2013) 6(5):487–96. doi: 10.1016/j.jcin.2013.02.006
95. Varol E, Yücel H, Arslan A, Ozaydın M, Erdoğan D, Doğan A. Elevated carbohydrate antigen 125 levels in patients with aortic stenosis: relation to clinical severity and echocardiographic parameters. Turk Kardiyol Dern Ars (2012) 40(4):309–15. doi: 10.5543/tkda.2012.87894
96. Li X, He M, Zhu J, Yao P, Li X, Yuan J, et al. Higher carbohydrate antigen 125 levels are associated with increased risk of coronary heart disease in elderly chinese: a population-based case-control study. PloS One (2013) 8(11):e81328. doi: 10.1371/journal.pone.0081328
97. Xu K, Wu M, Huang M, Zhuo X, Weng Y, Chen X. Carbohydrate antigen 125 combined with N-terminal pro-B-type natriuretic peptide in the prediction of acute heart failure following ST-elevation myocardial infarction. Medicine (2022) 101(48):e32129. doi: 10.1097/MD.0000000000032129
98. Falcao FJA, Oliveira FRA, Cantarelli F, Cantarelli R, Brito-Junior P, Lemos H, et al. Carbohydrate antigen 125 predicts pulmonary congestion in patients with ST-segment elevation myocardial infarction. Braz J Med Biol Res (2019) 52(12):e9124. doi: 10.1590/1414-431x20199124
99. Zhang Y, Jin Q, Zhao Z, Zhao Q, Yu X, Yan L, et al. Carbohydrate antigen 125 is a biomarker of the severity and prognosis of pulmonary hypertension. Front Cardiovasc Med (2021) 8:699904. doi: 10.3389/fcvm.2021.699904
100. Sahin A, Kaya H, Avci O. Cancer antigen-125 is a predictor of mortality in patients with pulmonary arterial hypertension. Clin Biochem (2021) 89:58–62. doi: 10.1016/j.clinbiochem.2020.12.010
101. Kaya H, Zorlu A, Yucel H, Tatlisu M, Kivrak T, Coskun A, et al. Higher cancer antigen 125 level is associated with the presence of permanent atrial fibrillation in systolic heart failure patients. Acta Cardiol (2016) 71(1):61–6. doi: 10.1080/AC.71.1.3132099
102. Wang Q, Dang C, Liu H, Hui J. Plasma carbohydrate antigen-125 for prediction of atrial fibrillation recurrence after radiofrequency catheter ablation. BMC Cardiovasc Disord (2021) 21(1):400. doi: 10.1186/s12872-021-02207-y
103. den Uijl D, Delgado V, Bertini M, Tops L, Trines S, van de Veire N, et al. Impact of left atrial fibrosis and left atrial size on the outcome of catheter ablation for atrial fibrillation. Heart (2011) 97(22):1847–51. doi: 10.1136/hrt.2010.215335
104. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol (2012) 60(22):2263–70. doi: 10.1016/j.jacc.2012.04.063
105. Mueller C, McDonald K, de Boer R, Maisel A, Cleland J, Kozhuharov N, et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail (2019) 21(6):715–31. doi: 10.1002/ejhf.1494
106. Josa-Laorden C, Giménez-López I, Rubio-Gracia J, Ruiz-Laiglesia F, Garcés-Horna V, Pérez-Calvo JI. Prognostic value of measuring the diameter and inspiratory collapse of the inferior vena cava in acute heart failure. Rev clinica espanola (2016) 216(4):183–90. doi: 10.1016/j.rceng.2015.11.005
107. Miñana G, Núñez J, Sanchis J, Bodí V, Núñez E, Llàcer A. CA125 and immunoinflammatory activity in acute heart failure. Int J Cardiol (2010) 145(3):547–8. doi: 10.1016/j.ijcard.2010.04.081
108. Núñez J, Rabinovich GA, Sandino J, Mainar L, Palau P, Santas E, et al. Prognostic value of the interaction between galectin-3 and antigen carbohydrate 125 in acute heart failure. PloS One (2015) 10(4):e0122360. doi: 10.1371/journal.pone.0122360
109. Ter Maaten J, Kremer D, Demissei B, Struck J, Bergmann A, Anker S, et al. Bio-adrenomedullin as a marker of congestion in patients with new-onset and worsening heart failure. Eur J Heart Fail (2019) 21(6):732–43. doi: 10.1002/ejhf.1437
110. Pandhi P, Ter Maaten J, Emmens J, Struck J, Bergmann A, Cleland J, et al. Clinical value of pre-discharge bio-adrenomedullin as a marker of residual congestion and high risk of heart failure hospital readmission. Eur J Heart Fail (2020) 22(4):683–91. doi: 10.1002/ejhf.1693
111. Voors A, Kremer D, Geven C, Ter Maaten J, Struck J, Bergmann A, et al. Adrenomedullin in heart failure: pathophysiology and therapeutic application. Eur J Heart Fail (2019) 21(2):163–71. doi: 10.1002/ejhf.1366
Keywords: carbohydrate antigen 125, congestive heart failure, heart failure, N-terminal Pro-B-type natriuretic peptide, tissue congestion
Citation: Feng R, Zhang Z and Fan Q (2023) Carbohydrate antigen 125 in congestive heart failure: ready for clinical application? Front. Oncol. 13:1161723. doi: 10.3389/fonc.2023.1161723
Received: 08 February 2023; Accepted: 16 October 2023;
Published: 31 October 2023.
Edited by:
Jun-ichi Abe, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Julio Nunez, Hospital Clínico Universitario de Valencia, SpainDuska Glavas, Monzino Cardiology Center (IRCCS), Italy
John Cleland, University of Glasgow, United Kingdom
Copyright © 2023 Feng, Zhang and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenlu Zhang, emhlbmx1emhhbmd3aEAxNjMuY29t
 Rui Feng
Rui Feng Zhenlu Zhang
Zhenlu Zhang Qingkun Fan1
Qingkun Fan1