
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 March 2023
Sec. Breast Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1157789
 Tomomi Sanomachi1
Tomomi Sanomachi1 Hitomi Sumiyoshi Okuma1*
Hitomi Sumiyoshi Okuma1* Rui Kitadai1
Rui Kitadai1 Asuka Kawachi1
Asuka Kawachi1 Shu Yazaki1
Shu Yazaki1 Momoko Tokura1
Momoko Tokura1 Motoko Arakaki1
Motoko Arakaki1 Ayumi Saito1
Ayumi Saito1 Shosuke Kita1
Shosuke Kita1 Kasumi Yamamoto1
Kasumi Yamamoto1 Aiko Maejima1
Aiko Maejima1 Yuki Kojima1
Yuki Kojima1 Tadaaki Nishikawa1
Tadaaki Nishikawa1 Kazuki Sudo1
Kazuki Sudo1 Tatsunori Shimoi1
Tatsunori Shimoi1 Emi Noguchi1
Emi Noguchi1 Yasuhiro Fujiwara1
Yasuhiro Fujiwara1 Hirokazu Sugino2
Hirokazu Sugino2 Sho Shiino3
Sho Shiino3 Akihiko Suto3
Akihiko Suto3 Masayuki Yoshida2
Masayuki Yoshida2 Kan Yonemori1
Kan Yonemori1Introduction: Triple-negative breast cancer (TNBC) is negative for hormone receptors and human epidermal growth factor receptor 2 (HER2). In stage I TNBC, adjuvant therapy or follow-up are performed according to risk factors, but clinical trial data is scarce. In recent years, it has been reported that HER2-low cases (1+/2+ and in situ hybridization negative) have different prognoses than HER2-0 cases. However, the risk of recurrence and risk factors in this HER2-low population for stage I TNBC have not yet been investigated.
Methods: Herein, out of 174 patients with TNBC who underwent surgery from June 2004 to December 2009 at the National Cancer Center Hospital (Tokyo), we retrospectively examined 42 cases diagnosed as T1N0M0 TNBC after excluding those treated with preoperative chemotherapy.
Results: All patients were female, the median age was 60.5 years, and 11 cases were HER2-low and 31 cases were HER2-0. The median follow-up period was 121 months. Postoperative adjuvant therapy was administered in 30 patients and recurrence occurred in 8 patients. HER2-low cases showed a significantly shorter disease-free survival (HR: 7.0; 95% CI: 1.2– 40.2; P=0.0016) and a trend towards shorter overall survival (hazard ratio [HR]: 4.2, 95% confidence interval [CI]: 0.58–31.4) compared with that of HER2-0 cases. HER2 was also identified as a factor for poor prognosis from the point- estimated values in univariate and multivariate analyses after confirming that there was no correlation between the other factors.
Conclusion: For patients with stage I TNBC, the HER2-low population had a significantly worse prognosis than the HER2-0 population.
Breast cancer is the most prevalent cancer among women worldwide with more than 2 million new cases reported in 2020 (1). In Japan, the prevalence of breast cancer is lower than that in Western countries; however, it is still the most common cancer type among Japanese women (2). Breast cancer is divided into subtypes depending on hormone receptor (estrogen receptor [ER] and progesterone receptor [PgR]) and human epidermal growth factor receptor 2 (HER2) expression. ER-, PgR-negative, HER2-0, or HER2-low-expressing triple-negative breast cancer (TNBC) account for 15-20% of all breast cancers (3). Patients with TNBC have poor chemotherapy response rates due to tumor heterogeneity and frequent development of treatment resistance, resulting in a worse prognosis compared to that in other subtypes (4, 5). Although more than 90% of breast cancers do not metastasize at the time of diagnosis, metastasis is a key reason for poor prognosis in TNBC; its 5-year survival rate is 65% for local tumors and 11% for distant metastasis, which is worse than that for other subtypes (4). As TNBC is defined as both hormone receptor-negative and HER2 negative, neither hormone therapy nor HER2 targeted therapy can be applied, and chemotherapies are the main treatments. However, immune checkpoint inhibitors are treatment options for patients with TNBC (6). Both atezolizumab and pembrolizumab were approved in 2022 in Japan for treatment of the early stages of TNBC and in the metastatic setting (7). However, perioperative adjuvant therapy and follow-up are still performed for early-stage TNBC according to the individual risk factors. Owing to TNBC heterogeneity (8), there are no precision treatments for this subtype. Additionally, clinical trial data for these early-stage patients are still scarce, which impacts the assessment of therapy effectiveness and accurate predictive markers.
However, HER2 expression may be another factor to be considered when treating early-stage TNBC. HER2 is a receptor involved in cell proliferation and is amplified or overexpressed in approximately 18–20% of primary invasive breast cancers (9). Additionally, HER2-low expression, defined as HER2 1+, HER2 2+, or HER2-in situ hybridization (ISH)-negative, has been shown to influence prognosis (10), and a number of HER2-low cases are now included in conventional TNBC diagnosis. The prognosis differs between HER2-low and HER2-0 cases (11), and for HER2-low cases, a clinical trial (DESTINY-Breast04) has shown that the anti-HER2 drug trastuzumab deruxtecan is highly effective in metastatic HER2-low breast cancer (12). Therefore, it is hypothesized that the risk of recurrence and therapeutic effects may differ between HER2-low and HER2-0 cases of early-stage TNBC. Despite previous reports focusing on this population (13) these included all subtypes at different stages. Thus, the recurrence risk and prognostic relationship between HER2-low/0 expression and stage I TNBC have not yet been investigated. Clarifying the relationship between low HER2 expression, recurrence risk, and prognostic factors in HER2-low stage I TNBC will enable predictions of therapeutic response and new therapeutic strategies. These results may contribute to personalized treatments for the TNBC population. Therefore, in this retrospective study, we investigated the involvement of low HER2 expression in recurrence risk and prognosis of patients with stage I TNBC.
We initially included patients who were diagnosed with TNBC and underwent surgery at the National Cancer Center Hospital (NCCH) (Tokyo, Japan) between June 2004 and December 2009 (N=174). According to UICC TNM classification 8th, stageI breast cancer is T0-1N0-1miM0. We further selected patients diagnosed with stage I TNBC, excluding those who underwent preoperative chemotherapy, and no axillary lymph node metastasis who were ER-negative, PgR-negative, and HER2-0, 1+, 2+, plus HER2-ISH negative (N=42). All 42 cases were female. Negative ER/PgR status was defined as less than 1% or 0% according to the American Society of Clinical Oncology/College of American Pathologists 2018 guidelines (14). Disease-free survival (DFS), overall survival (OS), and patient background (age, tumor size, nuclear grade, HER2/ER/PgR status, surgical procedure, administration of adjuvant radiotherapy, and adjuvant chemotherapy) were obtained from the electronic medical records. The clinical data were retrospectively evaluated, and we examined the predictors of DFS, OS, recurrence risk, and prognosis based on different HER2 expressions in stage I TNBC cases. This study was conducted in compliance with the research protocol established by the Department of Medical Oncology of NCCH. This study was approved by the ethical review board of the NCCH (No. 2014092) and conducted in full compliance with the Declaration of Helsinki.
Pathological examinations were performed using formalin-fixed paraffin-embedded specimens. For HER2 evaluation, specimens prepared at the surgical admission were used. The specimens were provided by the Biobank, in accordance with the National Cancer Research Center Biobank Specimen Usage Detailed Regulations. The specimens were assigned anonymized numbers and provided to two pathologists at the NCCH. The pathologists re-evaluated the specimens and determined the HER2 status according to the American Society of Clinical Oncology/College of American Pathologists 2018 guidelines. HER2 status on representative tumor cut surfaces of surgical resection specimens was examined. Sections were stained using a HercepTest II (DakoCytomation, Glostrup, Denmark), strictly following the manufacturer’s guidelines, or CB11 (BioGenex, San Ramon, CA, USA). In IHC 2+ cases, HER2 FISH testing was performed using PathVysion (Abbott Molecular Inc., Des Plaines, Illinois, USA). For hormone receptors (ER and PgR), an Allred score of 2 or less was considered negative.
After dividing stage I TNBC into HER2-low cases, defined as HER2 1+ or 2+ (ISH negative), and HER2-0 cases, we compared DFS and OS. We also examined factors that could affect DFS in HER2-low cases and HER2-0 cases; age, tumor size, surgery, adjuvant chemotherapy, adjuvant therapy, histological grade, nuclear grade, estrogen receptor, progesterone receptor, and HER2 status. Univariate analyses of continuous and nominal variables and multivariate analyses were performed using the Wald test in the Cox proportional-hazards model. The significance level of the P value was set at 0.05. OS was defined as the interval from the date of surgery to the date of any relevant event, which included death or loss to follow-up. DFS was time-limited by death, recurrence, or second cancer, whichever occurred first. For those lost to follow-up (N=37), the date of censoring was the date of final confirmation of survival. Survival analysis was performed by plotting Kaplan–Meier curves using the log-rank test. JMP 14.0.0 (SAS Institute Japan Corp. Japan) and GraphPad Prism version 8.4.3 (MDF Co., Ltd. Japan) software were used for statistical analysis.
Figure 1 shows the flow diagram for patient inclusion or exclusion. Table 1 shows the baseline characteristics of the 42 patients. These patients had a median follow-up of 121 months (range: 2-210 months), a median age of 60.5 years (range: 33–84), and tumor sizes of T1a/T1b/T1c = 2/11/29. Adjuvant therapy was administered to 30 patients, including 26 (61.9%) who received chemotherapy and 12 (28.6%) who received radiotherapy. Pathological features were grade 3 in both the histological grade (HG) and nuclear grade (NG) in the majority of cases (Grade 3 HG/NG = 73.8%/76.2%), and all cases were negative for ER and PgR. Stage I TNBCs usually include a variety of histologic types, such as apocrine carcinoma, adenoid cystic carcinoma, and dysplastic carcinoma, as well as invasive ductal carcinoma of the breast. In this study, we did not arbitrarily exclude these cases, but all cases were invasive ductal carcinoma. There were 11 (26.2%) HER2-low cases and 31 (73.8%) HER2-0 cases. There were eight cases of recurrence in the patient population. A comparison of HER2-low and HER2-0 cases showed differences in the surgical procedures. HER2 1+/2+ (ISH-negative) positivity was compared between the medical records and pathologist diagnosis (Supplementary Table 1), and the agreement rate was higher than in previous studies (15). Figure 2 shows the Kaplan–Meier curves for DFS in HER2-low and HER2-0 cases. The HER2-low group had a shorter median DFS than the HER2-0 group (HER2-low: 118 months; HER2-0: 185 months) (hazard ratio [HR]: 7.0; 95% confidence interval [CI]: 1.2–40.2; P=0.0016). Figure 3 shows the Kaplan–Meier curves for OS in HER2-low and HER2-0 cases. HER2-low cases had shorter OS than HER2-0 cases (HER2-low: 166 months; HER2-0: not reached) (HR: 4.2; 95% CI: 0.58–31.4; P=0.082). Adverse events during treatment were within the known limits in all cases, and there were no treatment-related deaths. Tables 2, 3 shows the results of univariate and multivariate analyses of DFS. In the univariate analysis, adjuvant therapy, NG, and HER2 status were identified as prognostic factors affecting DFS. Confirming that there was no correlation between the other factors, based on the univariate and multivariate point estimates, HER2-low status was the factor contributing the most to poor patient prognosis.
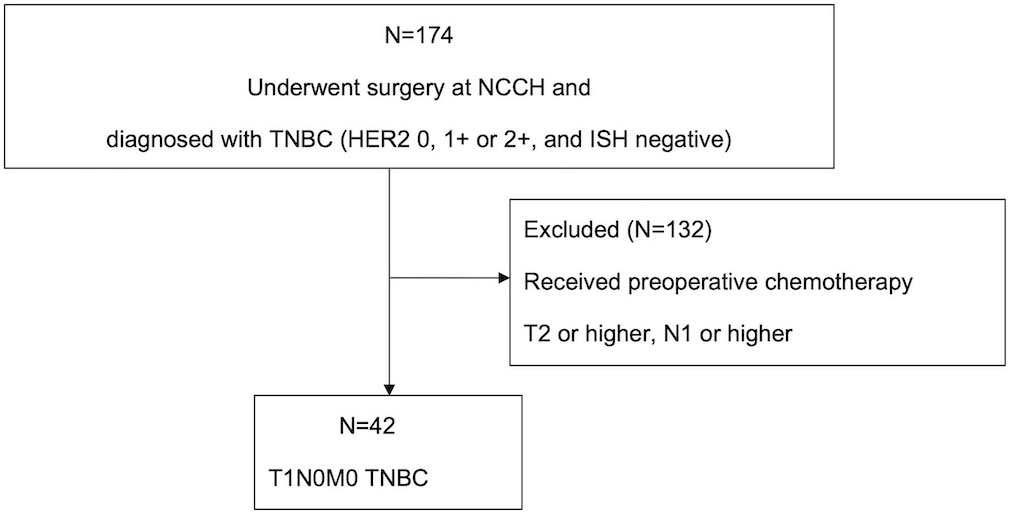
Figure 1 Flow diagram of the patient selection process. In total, 174 patients with TNBC were considered as candidates. Of these, 132 patients who underwent preoperative chemotherapy and those with stage T2 or higher N1 or higher were excluded from the analysis. Finally, 42 T1N0M0 TNBC were included in the analysis and followed up until October 2022. NCCH, National Cancer Center Hospital; TNBC, triple-negative breast cancer; ISH, in situ hybridization.
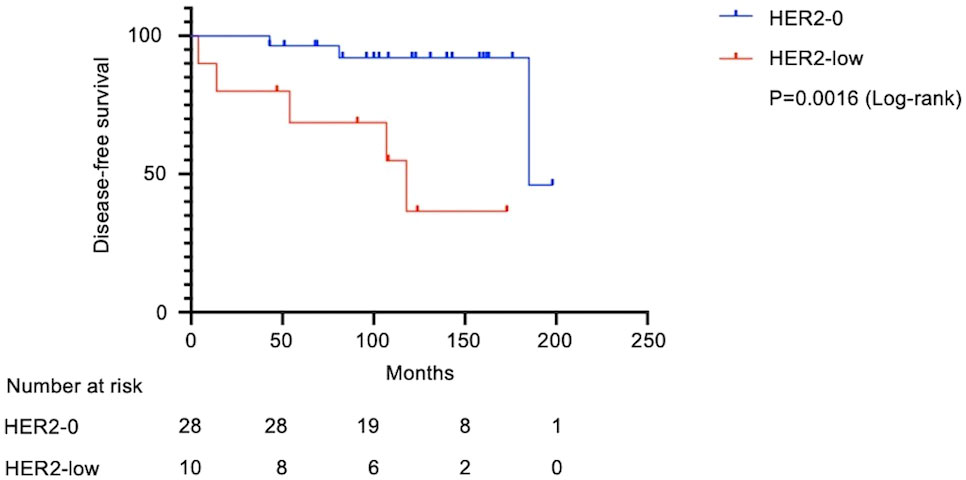
Figure 2 Kaplan–Meier survival curves for disease-free survival in HER2-low and HER2-0 cases. Of the 42 patients with stage I TNBC, 38 patients were analyzed after excluding 4 patients whose recurrence time was unclear in the medical records. Median survival was 118 months in the HER2-low group and 185 months in the HER2-0 group. The HER2-low group had significantly shorter DFS (Log-rank test, HR: 7.0; 95% CI: 1.2–40.2; P=0.0016). DFS, disease-free survival; HR, hazard ratio; CI, confidence interval.
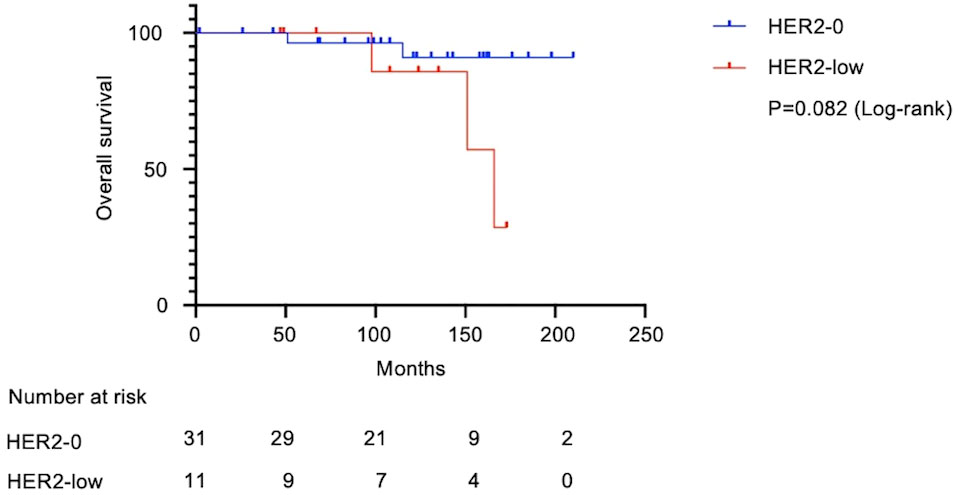
Figure 3 Kaplan–Meier survival curve of overall survival in HER2-low and HER2-0 cases. In total, 42 patients with Stage I TNBC were analyzed. Median survival was 166 months in the HER2-low group and undefined in the HER2-0 group. The HER2-low group had shorter OS (Log-rank test, HR: 4.2; 95% CI: 0.58–31.4; P=0.082). OS, overall survival; HR, hazard ratio; CI, confidence interval.
In a cohort of patients with stage I TNBC, we found that low HER2 expression was a negative prognostic factor. HER2-low expressed TNBC showed a DFS of 118 months compared to 185 months for HER2-0 TNBC.
New treatment strategies are limited for early-stage TNBC, and the prognosis remains poor compared to that of other subtypes (16). According to the National Comprehensive Cancer Network guidelines (17), adjuvant chemotherapy is recommended for TNBC tumors larger than 1 cm, but there is no consensus for smaller tumors. However, despite their size, these tumors are life-threatening. To characterize this cohort with stage I TNBC tumors, we evaluated patient DFS, OS, and background (age, tumor size, NG, HER2/ER/PgR status, surgical procedure, and whether adjuvant radiotherapy or chemotherapy was administered). Among these breast cancer-related factors, HER2 1+, 2+, and ISH negative were termed HER2-low, which has been reported to have different characteristics from the HER2-0 group (18). Therefore, we focused on HER2-low, which is present in some TNBC cases, as a breast cancer-related factor affecting recurrence risk and treatment choice for patients with TNBC. In our cohort, we identified that 26% of patients with stage I TNBC were HER2-low, consistent with the expected proportion (19, 20). Both univariate and multivariate analyses were performed for factors expected to affect the risk of recurrence, such as age (21), NG (22), and adjuvant therapy. HER2 was identified as a negative prognostic factor in both univariate and multivariate analyses of DFS in stage I TNBC. In addition, the Kaplan–Meier curves for DFS demonstrated that HER2-low cases had a statistically worse prognosis.
There are two explanations for this worse prognosis of HER2-low patients compared to that of HER2-0 patients: IHC false-negative results and molecular subtype differences.
In breast cancer, the definitions of hormone receptor and HER2 status have changed several times since 2004. In this study, we reexamined hormone receptor expression and HER2 status using the 2022 criteria and found a high concordance rate. However, we did not perform molecular analysis, such as mRNA testing, which may indicate discordance between the molecular and protein levels of these tumors. However, this molecular testing is beyond today’s clinical practice. With these limitations in mind, we believe that there is a molecular difference between HER2-low and HER2-0 subtypes. Recently, Ziteng Li et al. (23) showed that as a pan-cancer predictive biomarker for HER2-targeted therapy, the HER2 index, combined with HER2 multi-omics capabilities, can help identify potential candidates for such therapy in multiple tumor types, including breast cancer. Incorporating transcription patterns into the assessment of HER2 status is expected to lead to better patient selection, which should be taken into account for “future work”.
TNBC, an already heterogenous cancer, can be further subdivided by molecular subtypes and HER2 status using microarray assays (24). According to PAM50 analysis, approximately 60–80% of TNBC cases are basal-like, but approximately 10% are classified as HER2-enriched, which has been reported to have a worse prognosis (25). Lehmann et al. used histopathological quantification and laser capture microdissection to classify TNBC into seven subtypes, including the subtype luminal androgen receptor (LAR) (26, 27). HER2-enriched TNBC constitutes 74% of the LAR subtype (28) and the LAR subtype has been reported to have a significantly lower pathological complete response rate than that of other subtypes receiving preoperative chemotherapy (29). It has been suggested that the LAR subtype is inherently insensitive to chemotherapy. These and our results suggest that HER2-low may be a predictor of poor prognosis as a surrogate marker for the HER2-enriched subtype in TNBC.
In TNBC, the recurrence risk peaks within 3 years after diagnosis, and recurrence events associated with poor prognosis are more common within 5 years. Additionally, mortality increases 5 years after diagnosis (30). In this study, a statistically significant difference was observed in DFS between HER2-low and HER2-0 cases by the log-rank test, but the difference in OS was not statistically significant. This outcome is thought to be because appropriate post-treatment reduced the risk of death. In breast cancer, cases of late recurrence are often encountered. For this reason, we extended the analysis period to more than 10 years, making it more reliable.
This study has three limitations. First, the HER2 mRNA level and the IHC/FISH results in HER2-0 cases were not compared, and we cannot rule out false-negative test results. Second, the number of cases was limited because this study was conducted at a single institution. Additionally, there were few recurrent events, and the univariate and multivariate models were not highly stable. The small sample size of this study hinders additional statistical models or adjustments. In univariate analysis, for DFS, adjuvant therapy (either or both chemotherapy and radiation therapy) was a significant prognostic factor, whereas adjuvant chemotherapy alone was not. According to NCCN guideline, in T1-3, N0-1, M0 breast cancer, radiation therapy is mandatory for partial mastectomy, total mastectomy should be treated with radiation therapy according to the needs of the individual case. The present study included 22 cases of Lumpectomy and 16 cases of Mastectomy, which may have been influenced by the presence of a certain percentage of cases requiring additional adjuvant radiation therapy in addition to adjuvant chemotherapy. In this study, the median age of the target patients was 60.5 years old, and 16 patients (38.1%) aged 65 years or older were included. In a previous report, 35% of patients older than 65 years with TNBC were HER2-enriched, and age may be an important predictor in larger patient population sizes (31). Considering our limited sample size, previous reports (32, 33) showed that HER2-low cases had a better prognosis than HER2-0 cases, a result different from our own. However, none of these reports included hormone positivity/negativity- or stage-specific factors. Denkert et al. (33) and de Moura et al. (34) included cases of preoperative adjuvant therapy, which were excluded in our study. Our study focused only on stage I TNBCs, which may have led to different results because of the purity of the population. Another factor may be the discrepancy in the HER2-low expression degree in TNBC between early- and advanced-stage samples (35). Tarantino et al. reported that as breast cancers develop into advanced stages, in most part, they have shifts from HER2-0 to HER2-low; the vice versa being less frequent. This result suggests that previous clinical trials that were not stage-specific and/or included a large number of advanced-stage cases may have had an expanded HER2-low population, leading to different results. However, HER2-low expression instability in early-stage TNBC remains unclear. Although several reports related to HER2-low breast cancer prognosis differ from ours, a recently published report supports our results (36). Di Cosimo et al. found that although HER2-low cases tended to have a worse prognosis than HER2-0 cases, there was no difference in DFS. As in our study, the HER2 status of the included cases was determined by the American Society of Clinical Oncology/College of American Pathologists 2018 criteria rather than extracted from medical records, as has been done in previous reports. However, this study only included breast tumors ≥ 2 cm (T2 or larger), which does not clarify the role of HER2-low as a prognostic factor in stage I TNBC. Third, since this was a retrospective study with a small number of cases, verification in prospective clinical trials is necessary.
Recent clinical trials related to HER2-low include the NSABP B-47/NRG and DESTINY-Breast04 trials and the drugs used in these trials have been approved by the FDA. The NSABP B-47/NRG trial is a randomized phase III study of the add-on effect of trastuzumab, a humanized HER2 monoclonal antibody, on two standard regimens for HER2-low, high-risk, primary invasive breast cancers. This treatment change showed no significantly increase in invasive DFS or OS in the subgroup analysis classified by hormone receptor expression level (13). However, hormone-positive breast cancer accounted for 82.7% of all patients, and this trial was not stage-specific. The results of the DESTINY-Breast04 trial were published in 2022, which was an international multicenter joint phase 3 study targeting patients with HER2-low metastatic/recurrent breast cancer who had received pretreatment with chemotherapy (12). In this study, trastuzumab deruxtecan, which is trastuzumab conjugated to a camptothecin derivative with topoisomerase I inhibitory effects, was used as the tested drug. Encouragingly, it significantly prolonged PFS, which was the primary endpoint; however, this study included hormone receptor-positive, unresectable, metastatic, and recurrent HER2-low breast cancers. The effect of DS-8201a/trastuzumab deruxtecan on early HER2-low breast cancer and TNBC (HER2-low) is currently under investigation. However, based on the results of this study, we expect that this drug will be effective for stage I TNBC with low HER2 expression. According to a previous report by Tolaney et al., reported in a single-group, uncontrolled trial examining the efficacy of adjuvant paclitaxel and trastuzumab for stage T1-2 HER2- positive breast cancer (37). In this study, 91% of patients with T1mic-c cancers were included, indicating that this combination therapy will be useful for stage I HER2-positive breast cancer. The patient outcomes exceeded the 3-year survival rate for patients without invasive disease (37). In this study, 91% of patients with T1a-c cancers were included, indicating that this combination therapy will be useful for stage I HER2-positive breast cancer. However, the effect remains unclear as more than half of these cases were hormone receptor-positive, and HER2 status was not classified as HER2-low or -0. Therefore, it is desirable to further investigate postoperative adjuvant therapy, including anti-HER2 therapeutic agents, in preventing recurrence in patients with stage I TNBC with low HER2 expression.
In conclusion, among stage I TNBC cases, HER2-low cases had a significantly shorter DFS and a trend towards shorter OS compared to HER2-0 cases. HER2 was also identified as a factor for most poor prognosis from the point-estimated values in univariate and multivariate analyses after confirming that there was no correlation between the other factors. HER2-low may be a predictor of poor prognosis as a surrogate marker for the HER2-enriched subtype in stage I TNBC cases.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the NCCH (No. 2014092). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TS and HO. The first draft of the manuscript was written by TS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
The authors thank Ms. Sachiko Miura, Ms. Toshiko Sakaguchi, and Mr. Hiroki Kakishima for their skillful technical assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1157789/full#supplementary-material
1. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer-epidemiology, risk factors, classification, prognostic markers, and current treatment strategies-an updated review. Cancers (Basel) (2021) 13. doi: 10.3390/cancers1317428
2. Uchida K, Ohashi H, Kinoshita S, Nogi H, Kato K, Toriumi Y, et al. Breast cancer screening and the changing population pyramid of Japan. Breast Cancer (2015) 22:172–6. doi: 10.1007/s12282-013-0470-6
3. Li Y, Zhang H, Merkher Y, Chen L, Liu N, Leonov S, et al. Recent advances in therapeutic strategies for triple-negative breast cancer. J Hematol Oncol (2022) 15:121. doi: 10.1186/s13045-022-01341-0
4. Won KA, Spruck C. Triple−negative breast cancer therapy: Current and future perspectives (Review). Int J Oncol (2020) 57:1245–61. doi: 10.3892/ijo.2020.5135
5. Bianchini G, De Angelis C, Licata L, Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol (2022) 19:91–113. doi: 10.1038/s41571-021-00565-2
6. Keenan TE, Tolaney SM. Role of immunotherapy in triple-negative breast cancer. J Natl Compr Canc Netw (2020) 18:479–89. doi: 10.6004/jnccn.2020.755
7. Kwapisz D. Pembrolizumab and atezolizumab in triple-negative breast cancer. Cancer Immunol Immunother (2021) 70:607–17. doi: 10.1007/s00262-020-02736-z
8. So JY, Ohm J, Lipkowitz S, Yang L. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol Ther (2022) 237:108253. doi: 10.1016/j.pharmthera.2022.108253
9. de Melo Gagliato D, Jardim DL, Marchesi MS, Hortobagyi GN. Mechanisms of resistance and sensitivity to anti-HER2 therapies in HER2+ breast cancer. Oncotarget (2016) 7:64431–46. doi: 10.18632/oncotarget.7043
10. Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers (Basel) (2021) 13. doi: 10.3390/cancers1305101
11. Almstedt K, Heimes AS, Kappenberg F, Battista MJ, Lehr HA, Krajnak S, et al. Long-term prognostic significance of HER2-low and HER2-zero in node-negative breast cancer. Eur J Cancer (2022) 173:10–9. doi: 10.1016/j.ejca.2022.06.012
12. Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med (2022) 387:9–20. doi: 10.1056/NEJMoa220369
13. Fehrenbacher L, Cecchini RS, Geyer CE Jr., Rastogi P, Costantino JP, Atkins JN, et al. NSABP b-47/NRG oncology phase III randomized trial comparing adjuvant chemotherapy with or without trastuzumab in high-risk invasive breast cancer negative for HER2 by FISH and with IHC 1+ or 2. J Clin Oncol (2020) 38:444–53. doi: 10.1200/jco.19.01455
14. Allison KH, Hammond MEH, Dowsett M, McKernin SE, Carey LA, Fitzgibbons PL, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol (2020) 38:1346–66. doi: 10.1200/jco.19.0230
15. Xu B, Shen J, Guo W, Zhao W, Zhuang Y, Wang L. Impact of the 2018 ASCO/CAP HER2 guidelines update for HER2 testing by FISH in breast cancer. Pathol Res Pract (2019) 215:251–5. doi: 10.1016/j.prp.2018.10.03
16. Cosar R, Sut N, Ozen A, Tastekin E, Topaloglu S, Cicin I, et al. Breast cancer subtypes and prognosis: Answers to subgroup classification questions, identifying the worst subgroup in our single-center series. Breast Cancer (Dove Med Press) (2022) 14:259–80. doi: 10.2147/bctt.S380754
17. Network NCC. NCCN clinical practice guidelines in oncology (NCCN guidelines®) breast cancer version 5 (2020). Available at: https://www2.tri-kobe.org/nccn/guideline/breast/index.html (Accessed 09.05, 2022).
18. Lai HZ, Han JR, Fu X, Ren YF, Li ZH, You FM. Targeted approaches to HER2-low breast cancer: Current practice and future directions. Cancers (Basel) (2022) 14. doi: 10.3390/cancers14153774
19. Won HS, Ahn J, Kim Y, Kim JS, Song JY, Kim HK, et al. Clinical significance of HER2-low expression in early breast cancer: A nationwide study from the Korean breast cancer society. Breast Cancer Res (2022) 24:22. doi: 10.1186/s13058-022-01519-x
20. Gampenrieder SP, Rinnerthaler G, Tinchon C, Petzer A, Balic M, Heibl S, et al. Landscape of HER2-low metastatic breast cancer (MBC): Results from the Austrian AGMT_MBC-registry. Breast Cancer Res (2021) 23:112. doi: 10.1186/s13058-021-01492-x
21. Aapro M, Wildiers H. Triple-negative breast cancer in the older population. Ann Oncol (2012) 23 Suppl 6:vi52–5. doi: 10.1093/annonc/mds189
22. Bilik R, Mor C, Wolloch Y, Dintsman M. Histopathologic high risk factors influencing the prognosis of patients with early breast cancer (T1N0M0). Am J Surg (1986) 151:460–4. doi: 10.1016/0002-9610(86)90103-0
23. Li Z, Chen S, Feng W, Luo Y, Lai H, Li Q, et al. A pan-cancer analysis of HER2 index revealed transcriptional pattern for precise selection of HER2-targeted therapy. EBioMedicine (2020) 62:103074. doi: 10.1016/j.ebiom.2020.103074
24. Derakhshan F, Reis-Filho JS. Pathogenesis of triple-negative breast cancer. Annu Rev Pathol (2022) 17:181–204. doi: 10.1146/annurev-pathol-042420-093238
25. Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast (2015) 24 Suppl 2:S26–35. doi: 10.1016/j.breast.2015.07.008
26. Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest (2011) 121:2750–67. doi: 10.1172/jci45014
27. Lehmann BD, Jovanović B, Chen X, Estrada MV, Johnson KN, Shyr Y, et al. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PloS One (2016) 11:e0157368. doi: 10.1371/journal.pone.0157368
28. Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol (2014) 232:142–50. doi: 10.1002/path.4280
29. Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res (2013) 19:5533–40. doi: 10.1158/1078-0432.Ccr-13-0799
30. Boyle P. Triple-negative breast cancer: Epidemiological considerations and recommendations. Ann Oncol (2012) 23 Suppl 6:vi7–12. doi: 10.1093/annonc/mds187
31. Gulbahce HE, Bernard PS, Weltzien EK, Factor RE, Kushi LH, Caan BJ, et al. Differences in molecular features of triple-negative breast cancers based on the age at diagnosis. Cancer (2018) 124:4676–84. doi: 10.1002/cncr.31776
32. Horisawa N, Adachi Y, Takatsuka D, Nozawa K, Endo Y, Ozaki Y, et al. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer (2022) 29:234–41. doi: 10.1007/s12282-021-01303-3
33. Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, et al. Clinical and molecular characteristics of HER2-low-positive breast cancer: Pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol (2021) 22:1151–61. doi: 10.1016/s1470-2045(21)00301-6
34. de Moura Leite L, Cesca MG, Tavares MC, Santana DM, Saldanha EF, Guimarães PT, et al. HER2-low status and response to neoadjuvant chemotherapy in HER2 negative early breast cancer. Breast Cancer Res Treat (2021) 190:155–63. doi: 10.1007/s10549-021-06365-7
35. Tarantino P, Gandini S, Nicolò E, Trillo P, Giugliano F, Zagami P, et al. Evolution of low HER2 expression between early and advanced-stage breast cancer. Eur J Cancer (2022) 163:35–43. doi: 10.1016/j.ejca.2021.12.022
36. Di Cosimo S, La Rocca E, Ljevar S, De Santis MC, Bini M, Cappelletti V, et al. Moving HER2-low breast cancer predictive and prognostic data from clinical trials into the real world. Front Mol Biosci (2022) 9:996434. doi: 10.3389/fmolb.2022.996434
Keywords: stage I triple-negative breast cancer, HER2-low, HER2-0, prognosis, prognostic factor
Citation: Sanomachi T, Okuma HS, Kitadai R, Kawachi A, Yazaki S, Tokura M, Arakaki M, Saito A, Kita S, Yamamoto K, Maejima A, Kojima Y, Nishikawa T, Sudo K, Shimoi T, Noguchi E, Fujiwara Y, Sugino H, Shiino S, Suto A, Yoshida M and Yonemori K (2023) Low HER2 expression is a predictor of poor prognosis in stage I triple-negative breast cancer. Front. Oncol. 13:1157789. doi: 10.3389/fonc.2023.1157789
Received: 03 February 2023; Accepted: 13 March 2023;
Published: 27 March 2023.
Edited by:
Petra Tesarova, Charles University, CzechiaReviewed by:
Valentina Zavala, University of California, Davis, United StatesCopyright © 2023 Sanomachi, Okuma, Kitadai, Kawachi, Yazaki, Tokura, Arakaki, Saito, Kita, Yamamoto, Maejima, Kojima, Nishikawa, Sudo, Shimoi, Noguchi, Fujiwara, Sugino, Shiino, Suto, Yoshida and Yonemori. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hitomi Sumiyoshi Okuma, aHN1bWl5b3NAbmNjLmdvLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.