
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 October 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1156647
 Yanping Lin1‡
Yanping Lin1‡ Long Chen2‡
Long Chen2‡ Rong Li1‡
Rong Li1‡ Xin Liu3
Xin Liu3 Quan Li4
Quan Li4 Jingjing Cai4
Jingjing Cai4 Yaxi Du3
Yaxi Du3 Guangqiang Zhao5
Guangqiang Zhao5 Xiaoxiong Wang3
Xiaoxiong Wang3 Zhenghai Shen3
Zhenghai Shen3 Yedan Liao1
Yedan Liao1 Yang Chen6
Yang Chen6 Lin Xie1*†
Lin Xie1*† Yongchun Zhou3*†
Yongchun Zhou3*† Yunchao Huang5*†
Yunchao Huang5*†Importance: Patients with EGFR mutations who have advanced-stage non-small cell lung cancer (NSCLC) already receive tyrosine kinase inhibitors (TKIs) as the standard first-line therapy. Notably, Yunnan is a regional high incidence area of lung cancer in the highlands with a high rate of rare EGFR mutations. Overall, lung cancer patients in Xuanwei may present a distinct subgroup globally. Recent studies suggested that the NSCLC cohort in Xuanwei harbored a significantly higher uncommon mutation rate. However, little was known about the clinicopathological features and treatment efficacy of EGFR-TKI in Yunnan NSCLC patients.
Objective: This study aimed to investigate the clinical impact of histologic type on the survival outcomes of patients with stage IIIB and IV NSCLC receiving EGFR-TKI treatment of Yunnan in southwestern China.
Methods: In this retrospective study, we enrolled advanced NSCLC patients (IIIB-IV) with EGFR mutations who were first diagnosed and treated at Yunnan Cancer hospital from January 2016 to December 2019. Sociodemographics, lifestyle, survival, and clinicopathological characteristics of the patients were collected. The Kaplan-Meier method was used to assess the OS and PFS of patients. An analysis of prognostic factors was conducted using Cox regression.
Results: A total of 468 eligible patients were included. The median progression-free survival (PFS) and overall survival(OS) were 11.30(95% CI, 10.12-12.48) months and 30.30(95% CI, 26.24-34.36) months. Based on survival analysis among all the patients,females(HR=0.815;95% CI:0.671-0.989; P=0.017), Xuanwei origin (HR=0.776; 95% CI: 0.609-0.989; P=0.040), sample types(HR=0.780; 95% CI: 0.642-0.947; P=0.012) had a longer PFS. Multivariable analysis showed that only the sample type was an independent factor on median PFS with EGFR-TKI therapy. Patients less than 60 years old (HR=1.433; 95% CI:1.134-1.812, P=0.003)had better OS, but objectives with BMI≥24kg/m2(HR=0.653; 95% CI: 0.500-0.864; P=0.002), females(HR=0.776; 95% CI:0.613-0.982; P=0.035)and patients with tissue sample type (HR=0.760; 95% CI:0.600-.0961; P=0.022) had better OS. Notably, subgroup analysis of our study also found that PFS was significantly better in patients with G719X, L861Q, S768I, G719X+L861Q, and G719X+S768I in Xuanwei than classical mutation ones, including 19-Del and L858R (median 22.7 vs. 12.0 months, HR=0.523, P=0.010), while PFS was inferior in patients with rare mutations of EGFR in non-Xuanwei than the classical mutation ones (median 5.10 vs. 11.10 months, HR=1.760, P=0.015).
Conclusion: NSCLC patients in Yunnan displayed a unique EGFR mutation profile, especially a higher prevalence of EGFR uncommon and compound mutations subtype. This study indicates prognostic factors of NSCLC treated with EGFR-TKI in Yunan and Xuanwei. This study will provide new clinical evidence for EGFR-TKI-targeted therapy in patients with rare EGFR mutations in China and worldwide. More researchs were needed for NSCLC EGFR-TKI therapy and medical insurance policy-making in Yunnan, Xuanwei area and uncommon especially.
The first and most common cause of cancer death worldwide is lung cancer, and NSCLC accounts for 80% to 85% of all lung cancer deaths (1). When a patient is initially diagnosed, they may have advanced stages, following the claim that epidermal growth factor receptor mutations drive NSCLC (2). Patients with EGFR mutations who have advanced-stage NSCLC already receive tyrosine kinase inhibitors (TKIs) as the standard first-line therapy (3).
However, patient groups encountered in clinical practice do not meet the stringent inclusion criteria required for participation in clinical trials. Therefore, the effectiveness of EGFR-TKIs in patients treated in the natural world setting remains unclear. Notably, Yunnan is a regional high incidence area of lung cancer in the highlands with a high rate of rare EGFR mutations. Overall, lung cancer patients in Xuanwei may present a distinct subgroup globally (4, 5). Recent studies suggested that the NSCLC cohort in Xuanwei harbored a significantly higher uncommon complexed mutation rate. Little was known about the clinicopathological features and treatment efficacy of EGFR-TKI in Yunnan NSCLC patients (6). Here, we explored the effectiveness of TKI in Yunan-advanced NSCLC patients with EGFR mutation. To the best of our knowledge, this study is currently the first real-world study related to EGFR-TKI for NSCLC in Yunnan.
For this retrospective cohort study, advanced NSCLC patients treated with TKI from January 2016 to August 2019 in the Molecular Diagnostic Center of Yunnan Cancer Hospital were enrolled. Inclusion criteria: ①First diagnosis and treatment in the hospital; ②Local residents; ③Age≥18 years; ④Clinical stage IIIB or IV; ⑤With EGFR mutation; ⑥Treatment with EGFR TKIs. Exclusion criteria: ①None first-line treatment; ②Cases with medical records were incomplete.
The medical records and EGFR genotype data of 3007 NSCLC patients were retrospectively collected from our hospital from 16 sites, including all of the sites in Yunnan. Clinical data were collected from the medical records of each patient. This included patients characteristics(date of NSCLC diagnosis, sex, age, histological diagnosis, clinical staging, distant metastasis organ, smoking history, drinking history, and type of EGFR mutation); survival data (status as of the end of April 2022, date of death or date of the last follow-up).
Formalin-fixed paraffin-embedded (FFPE) tumor tissues, fine-needle aspiration and core needle biopsies, pleural effusion cells, and plasma samples were used to detect mutations. Genomic DNA and total RNA were extracted from FFPE samples using the AmoyDx FFPE DNA/RNA extraction kit (Amoy Diagnostics, Xiamen, China) following the manufacturer’s protocols. For other types of models, an AmoyDx Tissue DNA/RNA extraction kit (Amoy Diagnostics) was used. An Amplification Refractory Mutation System Polymerase Chain Reaction (ARMS-PCR) and a Mutation Detection Kit (Amoy Diagnostics) were used to detect the EGFR mutations.
The clinical stage was evaluated according to the 8th edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) classification system. The tumor response to TKI was assessed based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The primary endpoints of this study were PFS, which was defined as the time from initiating EGFR-TKI treatment to the date of disease progression or the last follow-up. Overall survival (OS) was defined as the interval from the first dose of first-line treatment until the date of death. Time to disease progression and survival for all patients was obtained by the active follow-up (telephone follow-up) and passive follow-up (case database review and tumor registry database matching) for the study subjects with a follow-up deadline of April 30, 2022.
Descriptive statistics presented patients’ baseline characteristics. And the data were presented as a percentage for dichotomous variables and analyzed using a chi-square test or Fisher’s exact test. Kaplan-Meier method was used to calculate the curves for PFS between groups. The Cox proportional hazards regression model was used to evaluate the impact of collected variables on PFS. The log-rank test determined significant differences. A two-tailed with a P value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS® software, version 20.0 (IBM Corp, Armonk, NY, USA).
Between 1 January 2016 and 30 December 2019, 3007 patients received EGFR mutation detection, and 1398 patients were mutant. A total of 515(61.09%) patients with stage IIIB and IV received EGFR TKI therapy, but 18 cases lost follow-up, and 29 objectives were the non-first line to receive TKI. Treatment and survival details of 468 patients were enrolled from 16 sites, including all of the sites in Yunnan, Figure S1. Among these 468 patients who received TKI therapy, over half, 235(50.21%) of patients had tumors with an EGFR 19-Del mutation, and 181 (38.68%) had the L858R mutation. A total of 52 patients (11.11%) had uncommon mutations and these are detailed in Figure 1, included G719X(n=11), L861Q(n=3), 20-ins(n=3), S768I(n=2), G719X+L861Q(n=25), G719X+S768I(n=6), T790M(n=2). The patient’s identification flow charts are illustrated in Figure 1. The clinicopathological characteristics, including sex, age at diagnosis, smoking history, staging, ethnic, area, smoking history, drinking history, type of specimen, clinical stage, drugs and type of EGFR mutation, are listed in Table 1.
The median duration of follow-up was 38.29 months(95% CI, 37.31-39.27m). At the end of follow-up, 415 patients (88.68%) had disease progression or died (progressed: n=367, 78.42%; died: n=48, 10.25%), and 53 (11.32%) were censored. The median PFS was 11.30 months(95% CI,10.12-12.48m), Figure 2A.
The median PFS of females (12.50 months, 95%CI: 11.21-13.79m) was longer than males(12.50 vs.10.20 months, hazard ratio = 0.815, 95% CI 0.671 to 0.989, P = 0.017, Figure 3A). Again, the median PFS was longer in Xuanwei origins compared to non-Xuanwei origins(13.00 vs. 10.70months, hazard ratio =0.776, 95% CI 0.609 to 0.989, P=0.040, Figure 3B). PFS benefit longer for tissue samples patients(12.00 vs.10.50 months, hazard ratio=0.780, 95% CI 0.642 to 0.947, P =0.012, Figure 3C). No statistical difference in PFS between patients with classical EGFR mutations and those with rare mutations, P=0.135, Figure 3E. PFS analysis by age, nationality, BMI, smoking status, hypertension history, diabetes history, lung cancer family history, clinical stage, brain metastasis, and types of TKI drugs revealed differences without statistical significance(P>0.05), Table 2 and Figure 3.
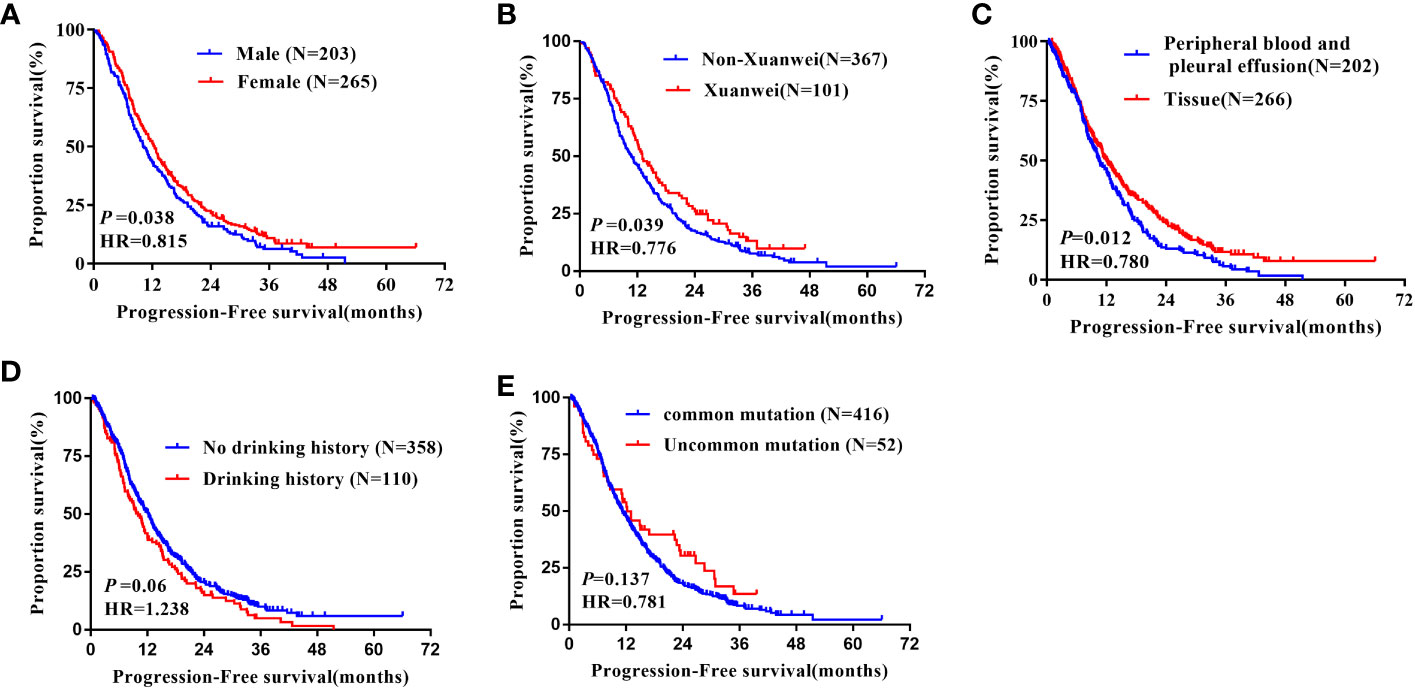
Figure 3 Kaplan-Meier curves showing the PFS of the sequential EGFR-TKI treatment in patients with distinct clinical subgroups. (A) Genders; (B) Region; (C) Types of genetic testing specimens; (D) Drinking history; (E) Type of EGFR mutation.
Multivariate analysis indicated that EGFR gene test sample type was an independent factor affecting PFS in patients treated with EGFR-TKI (HR=0.814, 95%CI: 0.669-0.991; P=0.040). However, gender and regional distribution of patients (Xuanwei origins versus non-Xuanwei origins) were not independent factors affecting PFS in patients treated with EGFR-TKI, Table 3.
In addition, the median OS was 30.30 months (95% CI, 26.24-34.36m, Figure 2B). During follow-up for OS, 280 (59.83%) patients died, and 188 patients were censored (40.17%). Reasons for censoring included: regular end of study (n=158, 84.04%); lost to follow-up (n=20,10.64%); patient’s wish (n=10, 5.32%).
In the analysis of overall survival, there was a significant difference with gender, age, BMI, and the specimen types of the EGFR mutation test. Median OS was longer in females versus males (32.50 vs.21.90 months, hazard ratio =0.776, 95% CI 0.613 to 0.982, P =0.035, Figure 4A). Significantly longer OS was noted in patients with less than 60 years old group than more than 60 ones(34.60 vs21.90 months, hazard ratio=1.433, 95% CI 1.134 to 1.812, P =0.003, Figure 4B), and in those patients whose BMI more than 24kg/m2 had longer OS than others (39.50 vs.24.60 months, hazard ratio=0.653, 95% CI 0.500 to 0.864, P =0.002, Figure 4C). What’s more, in terms of the sample types, tissue samples tested for PFS were longer than other sample types (31.50 vs.25.10 months, hazard ratio=0.760, 95% CI 0.600 to 0.961, P =0.022, Figure 4G). However, factors such as ethnicity, smoking history, alcohol consumption, hypertension, diabetes, family history of lung cancer, disease stage, EGFR mutation type, and EGFR-TKI drug type were not associated with OS of patients after EGFR-TKI treatment, not statistically significant (P>0.05, Figure 4D–F, H), Table 2.
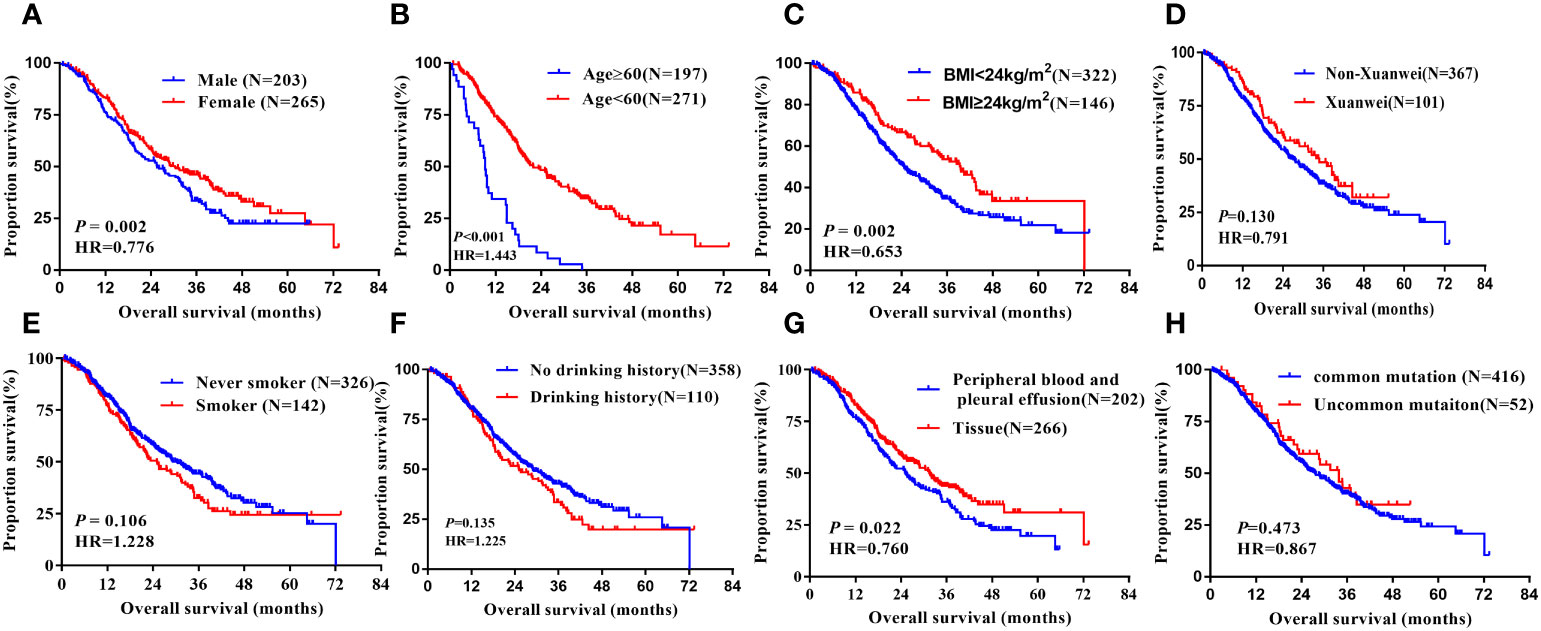
Figure 4 Kaplan-Meier curves showing the OS of the sequential EGFR-TKI treatment in patients with distinct clinical subgroups. (A) Genders; (B) Ages; (C) BMI; (D) Country; (E) Smoking history; (F) Drinking history; (G) Types of genetic testing specimens; (H) Type of EGFR mutation.
In multivariate analyses using multiple Cox proportional hazards models, we observed that gender(HR=0.778, 95% CI:0.614-0.986, P=0.038), age(HR=1.391, 95% CI:1.099-1.760, P=0.006), BMI (HR=0.658, 95% CI:0.503-0.861, P=0.002), types of specimen for EGFR mutation test (HR=0.787, 95% CI:0.622-0.997, P=0.047) were the independent prognostic factors for OS, Table 3.
Our group has long been engaged in research on the etiology, prevention, and treatment of lung cancer in Xuanwei, Yunnan Province (7, 8). Previous studies (5, 9)found that the incidence and mortality rates of lung cancer in Xuanwei are significantly higher than in other regions. It is characterized by a high rate of rare mutations and compound mutations in EGFR. Meanwhile, univariate analysis in this study suggested that there is a significant difference in PFS between Xuanwei and non-Xuanwei patients with non-small cell lung cancer after first-line treatment with EGFR-TKI, so we performed a subgroup analysis to explore the possible causes of the difference.
Notably, subgroup analysis based on the patients’ region of this study, Table 4, also found that, in the Xuanwei group, the PFS of patients with uncommon EGFR mutations was significantly better than classical mutations patients (median 22.70 vs. 12.00 months, HR=0.523, 95% CI 0.318 to 0.862, P =0.011, Figure 5F). Similarly, we found that the OS was longer in uncommon EGFR mutations vs. common ones (median 38.50 vs. 27.30 months, HR=0.577, 95% CI 0.302 to 1.103, P=0.096, Figure 6F).
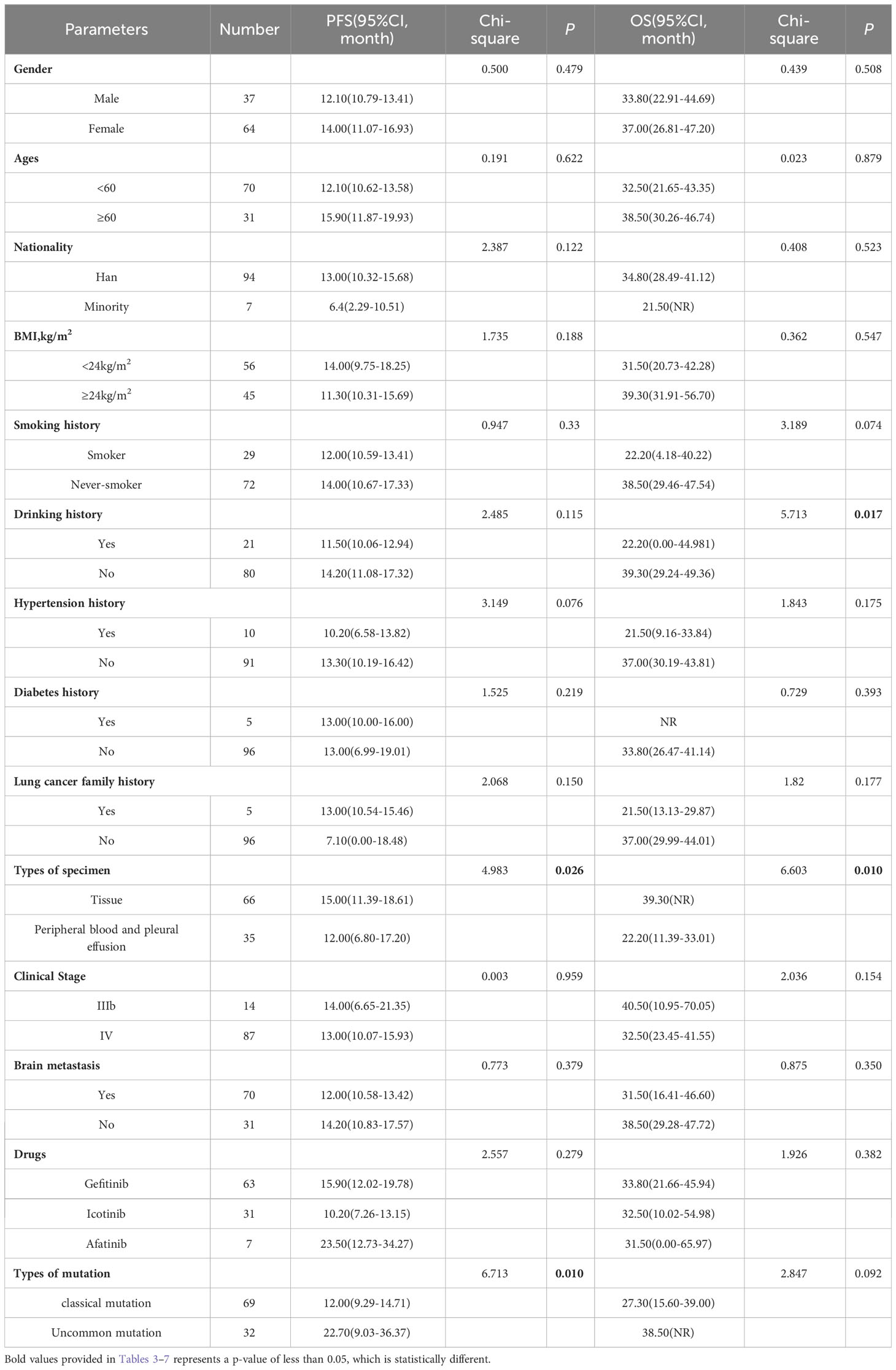
Table 4 Progress free survival and overall survival: univariate analysis of Xuanwei county lung cancer patients.
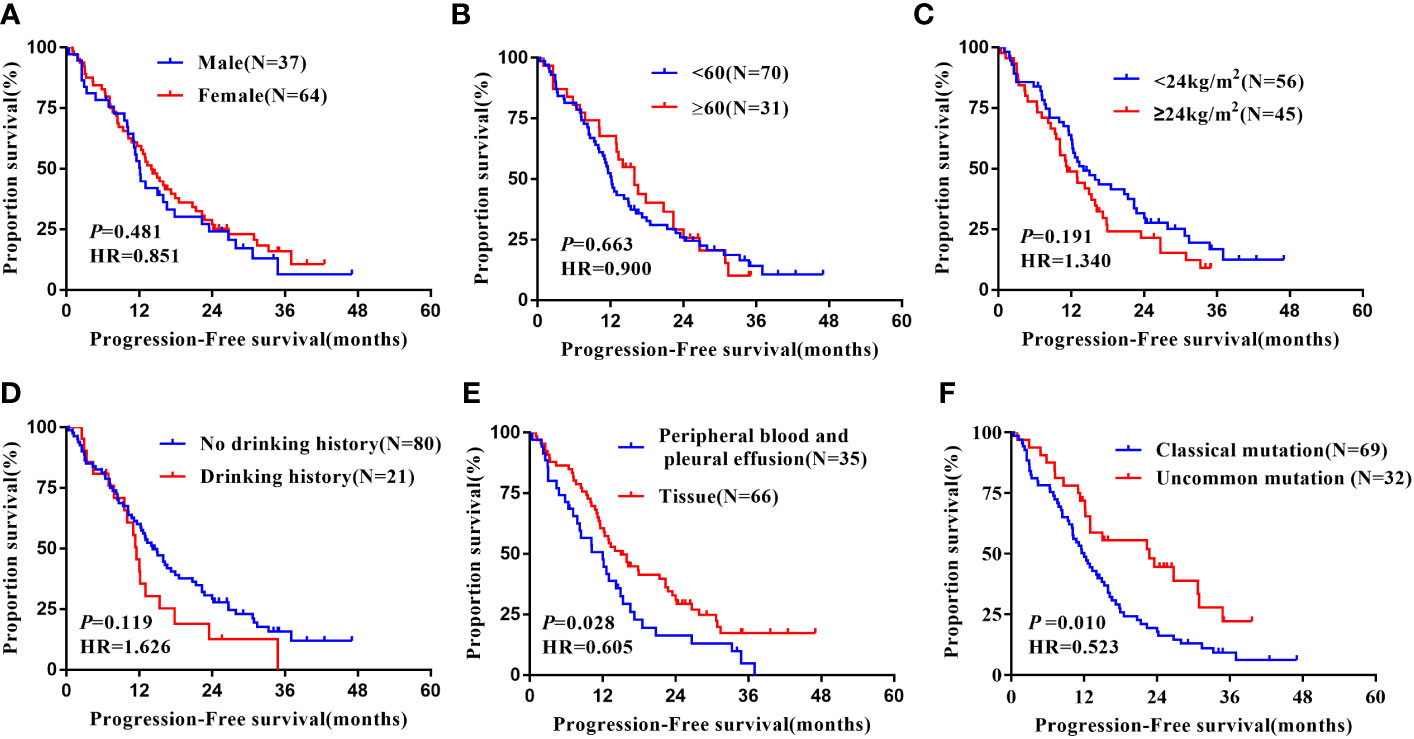
Figure 5 Subgroup analysis in Xuanwei lung cancer patients of mPFS. (A) Genders; (B) Ages; (C) BMI; (D) Drinking history; (E) Types of genetic testing specimens; (F) Type of EGFR mutation.
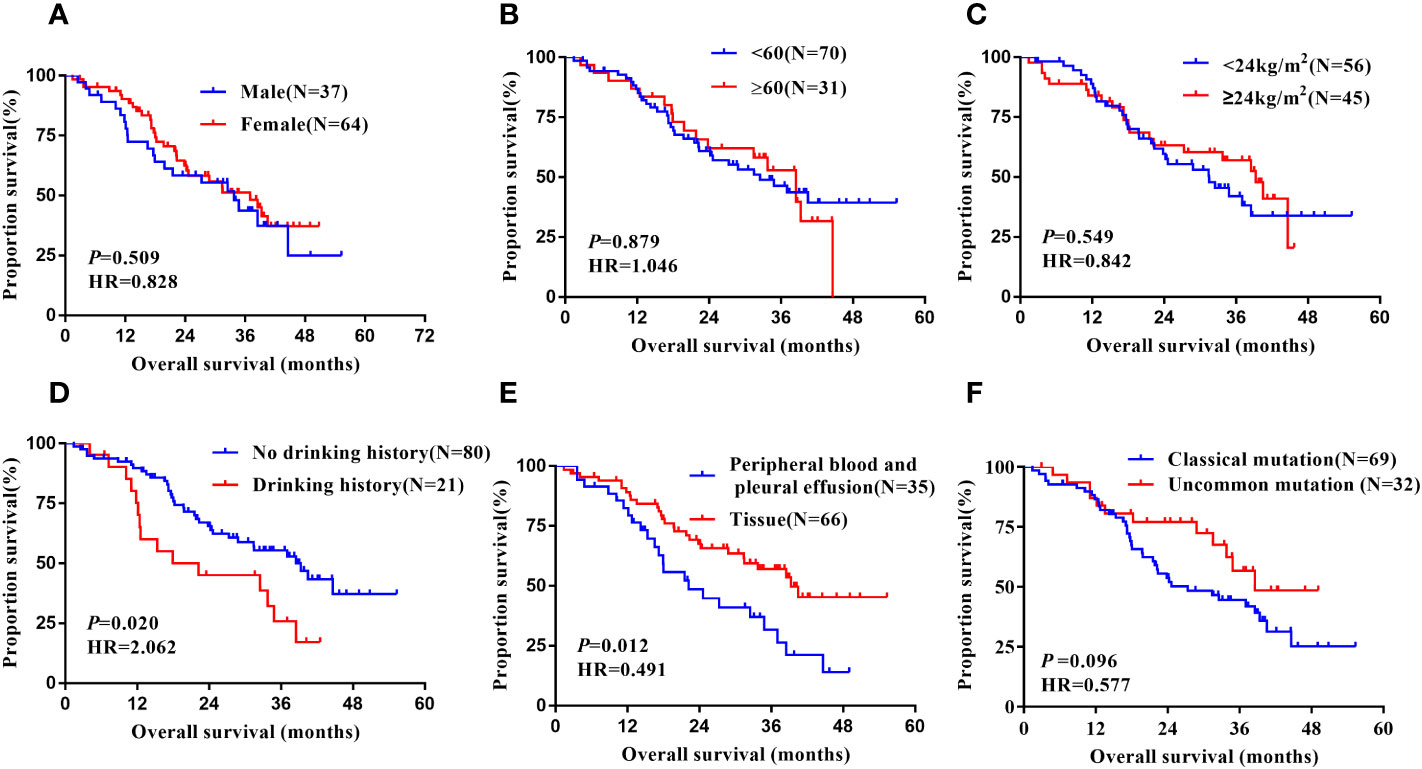
Figure 6 Subgroup analysis in Xuanwei lung cancer patients of mOS. (A) Genders; (B) Ages; (C) BMI; (D) Drinking history; (E) Types of genetic testing specimens; (F) Type of EGFR mutation.
In the Xuanwei lung cancer patients subgroup, OS was significantly prolonged in patients with no history of alcohol consumption compared to those with a history of alcohol consumption(median 39.30 vs. 22.20 months, HR=2.062, 95% CI 1.123 to 3.785, P=0.020, Figure 5D). In addition, the study also showed that patients with tissue samples had better PFS(median 15.00 vs. 12.00 months, HR=0.523, 95% CI 0.318 to 0.862, P=0.011, Figure 5E) and OS (median 39.30 vs. 22.20 months, HR=0.491, 95% CI 0.282 to 0.855, P=0.012, Figure 6E) than other sample ones. In contrast, gender, age, ethnicity, smoking history, and family history of lung cancer were not associated with PFS and OS of TKI therapy in patients with non-small cell lung cancer in this region (P>0.05, Figures 5A–C, 6A–D). Multifactorial analysis, Table 5, EGFR mutation type, which is divided into classical and uncommon mutation types, was an independent factor influencing PFS of TKI treatment in patients in Xuanwei (HR=0.523,95% CI 0.318-0.862, P=0.011). In addition the specimen types (HR=0.520, 95% CI 0.297-0.909, P=0.022) and history of alcohol consumption (HR=1.911, 95% CI 1.036-3.524, P=0.038) were independent influencing factors for OS.
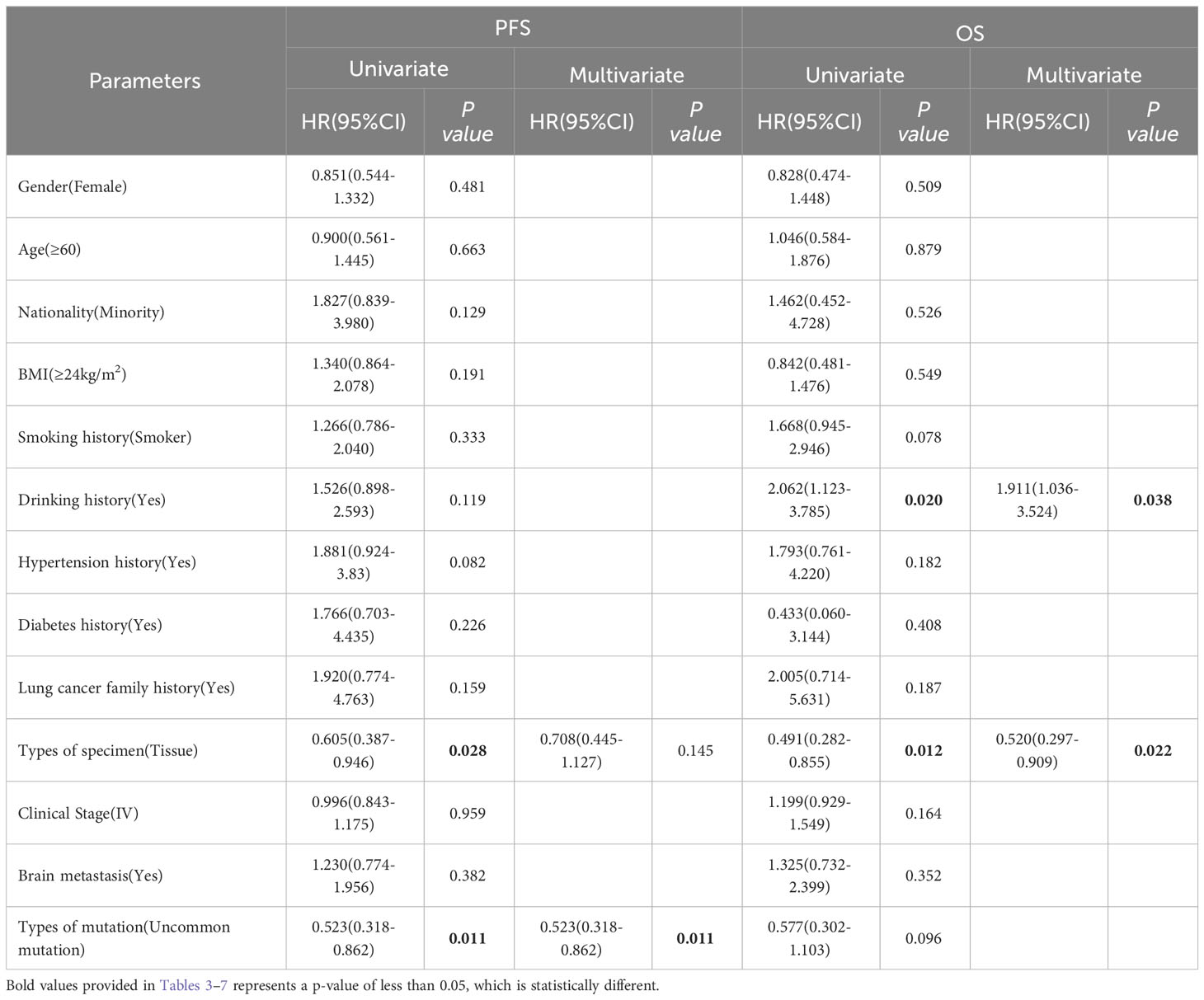
Table 5 Progress free survival and overall survival: univariate and multivariate analysis in Xuanwei lung cancer subgroup.
In the non-Xuanwei group, Table 6, the mPFS of patients with uncommon EGFR mutations in non-Xuanwei was significantly lower than classical mutation ones(median 5.10 vs.11.10 months, HR=1.760, 95% CI 1.106 to 2.800, P=0.017, Figure 7F). Similarly, we found that the OS was longer in non-Xuanwei origins with uncommon mutation patients vs. Classical mutation ones(median 19.10 vs. 28.80 months, HR=1.490,95% CI 0.895 to 2.479, P=0.125). Still, no statistical difference was reached, Figure 8F. In the subgroup of patients from non-Xuanwei origins, the univariate analysis suggested that age was associated with patient OS, and OS in the <60 years age group was significantly better than that in the≥60 years age group (median 32.70 vs. 20.20 months, HR=1.508, 95% CI 1.164 to 1.955, P=0.002, Figure 8B). OS was significantly better in patients with BMI≥24 kg/m2 than in patients with BMI<24 kg/m2(median 39.50 vs. 23.30 months, HR=0.618, 95% CI 0.453 to 0.843, P=0.002, Figure 8C, while gender, ethnicity, history of smoking, history of hypertension, history of diabetes mellitus, family history of lung cancer, sample types, and clinical stage were not associated with TKI efficacy in NSCLC patients in this region (P>0.05, Figures 7A–E, 8A, D, E). Multifactorial analysis showed that in Table 7, mutation type was an independent factor influencing PFS of TKI treatment in non-Xuanwei area patients (HR:1.760, 95% CI 1.106 to 2.800, P=0.017), and age (HR:1.501, 95% CI 1.158 to 1.946, P=0.002) and BMI (HR:0.621,95% CI 0.456 to 0.848, P=0.003) were independent factors affecting OS of TKI treatment in non-Xuanwei patients.
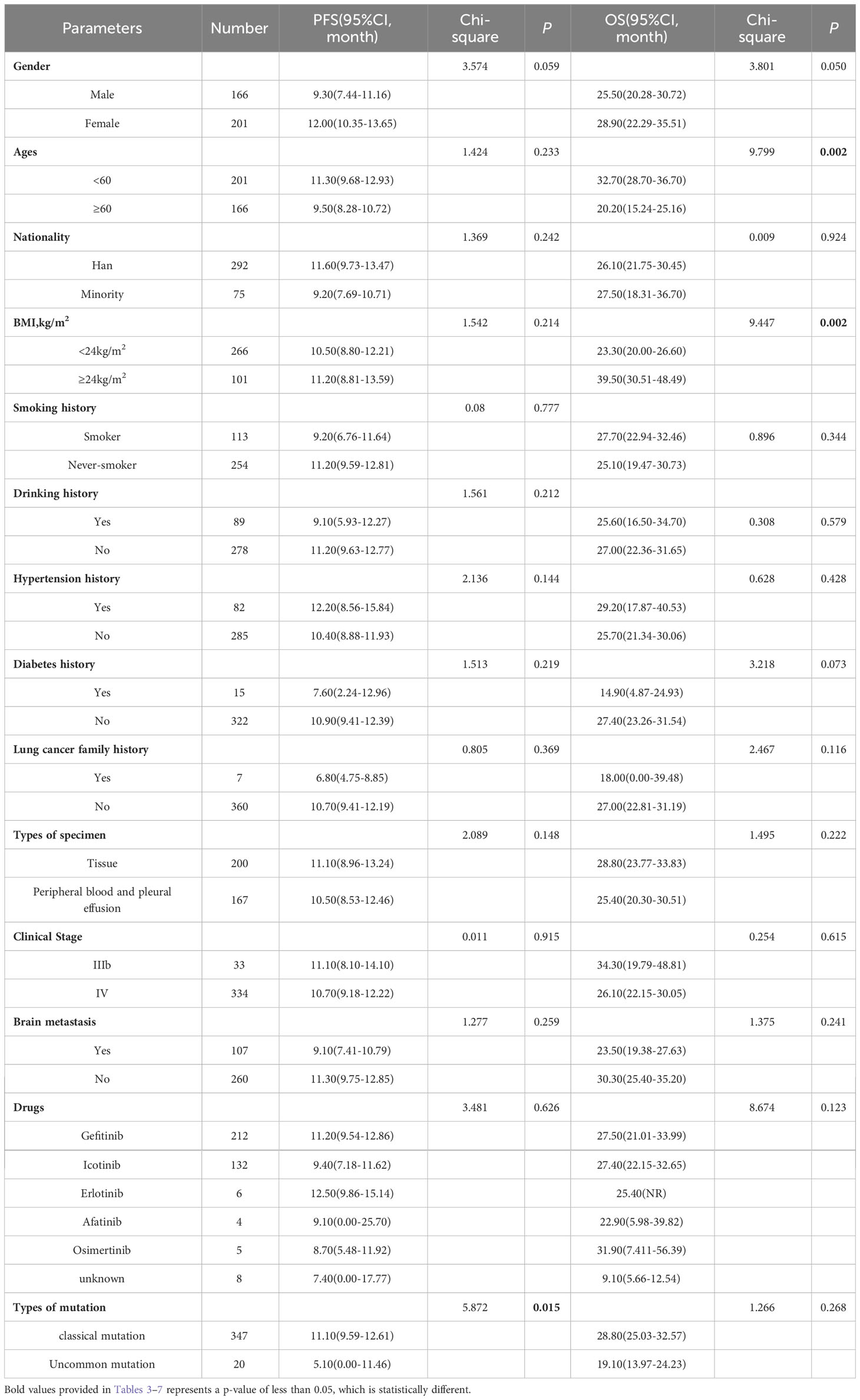
Table 6 Progress free survival and overall survival: univariate analysis of Non-Xuanwei lung cancer patients.
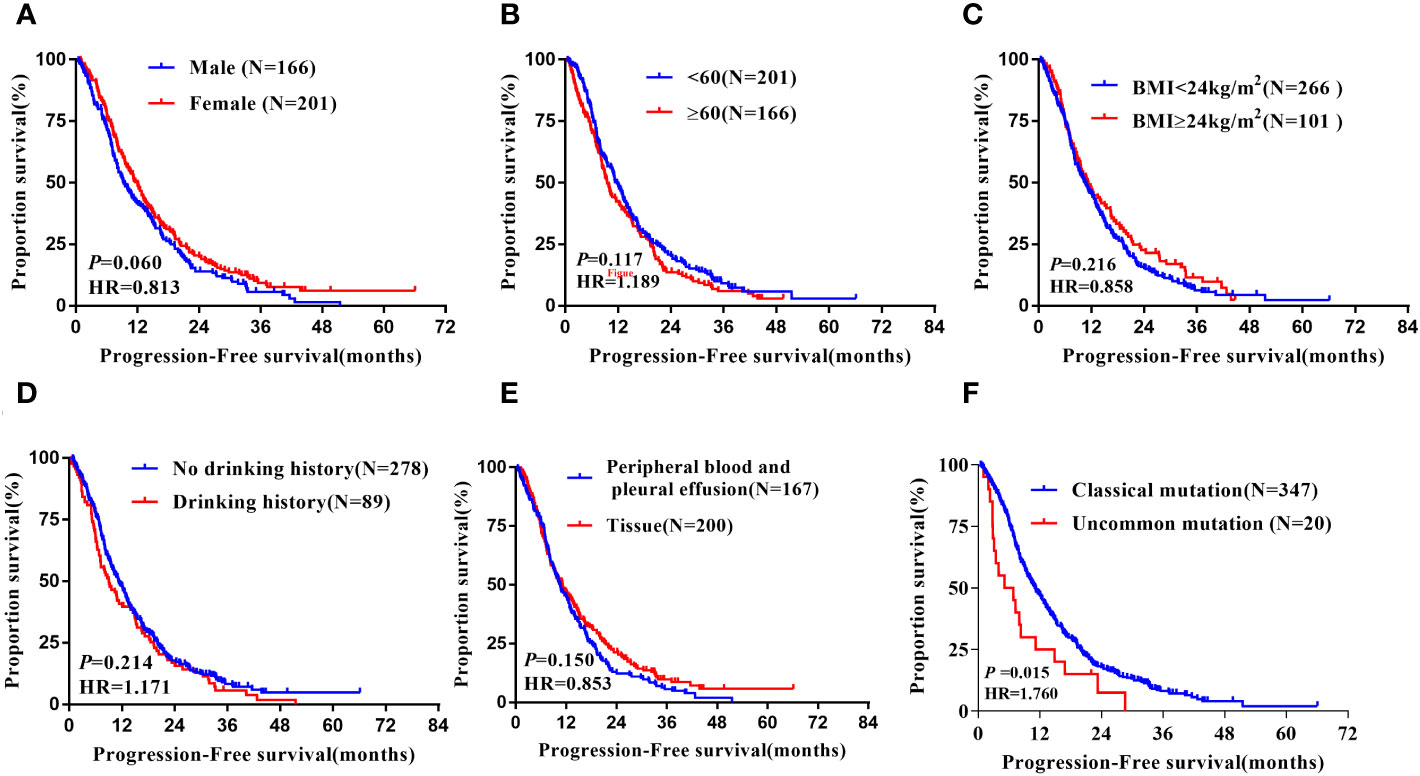
Figure 7 Subgroup analysis in Non-Xuanwei lung cancer patients of mPFS. (A) Genders; (B) Ages; (C) BMI; (D) Drinking history; (E) Types of genetic testing specimens; (F) Type of EGFR mutation.
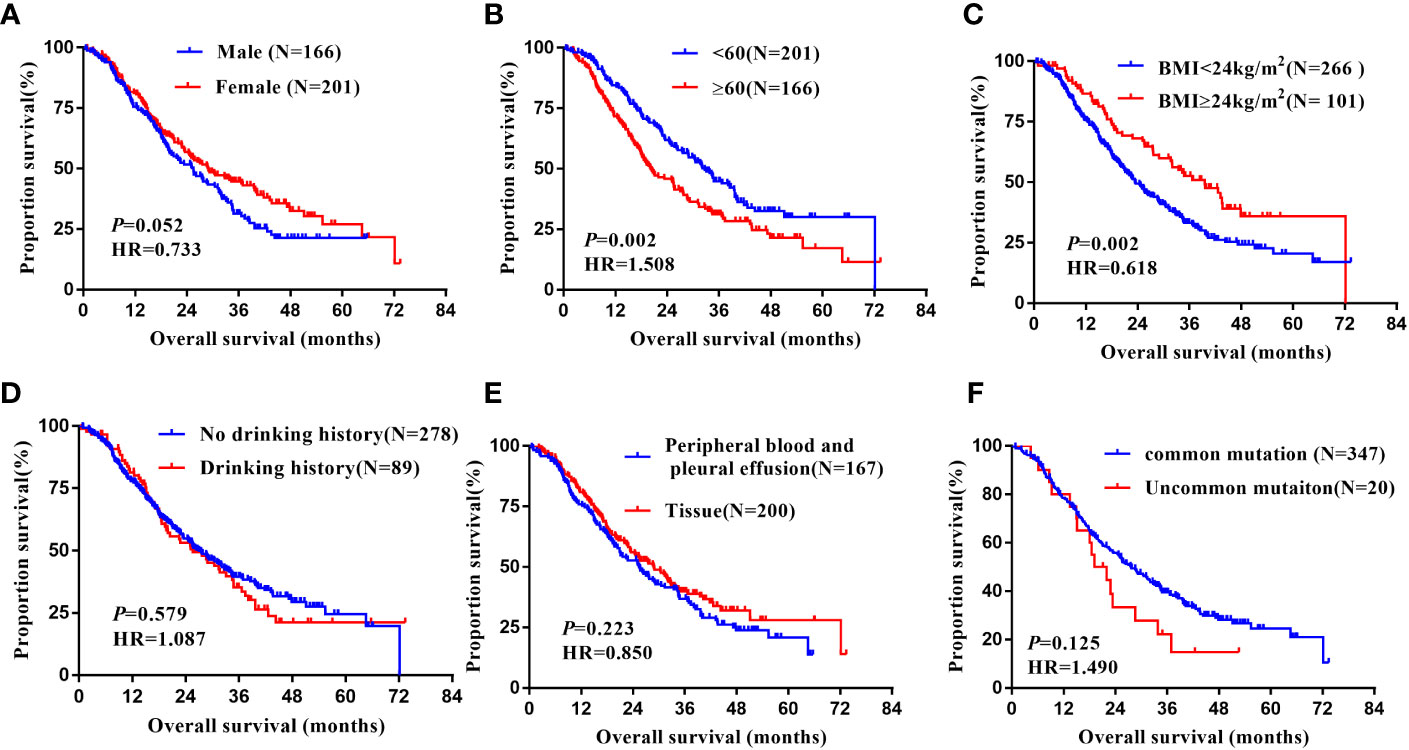
Figure 8 Subgroup analysis in Non-Xuanwei lung cancer patients of mOS. (A) Genders; (B) Ages; (C) BMI; (D) Drinking history; (E) Types of genetic testing specimens; (F) Type of EGFR mutation.
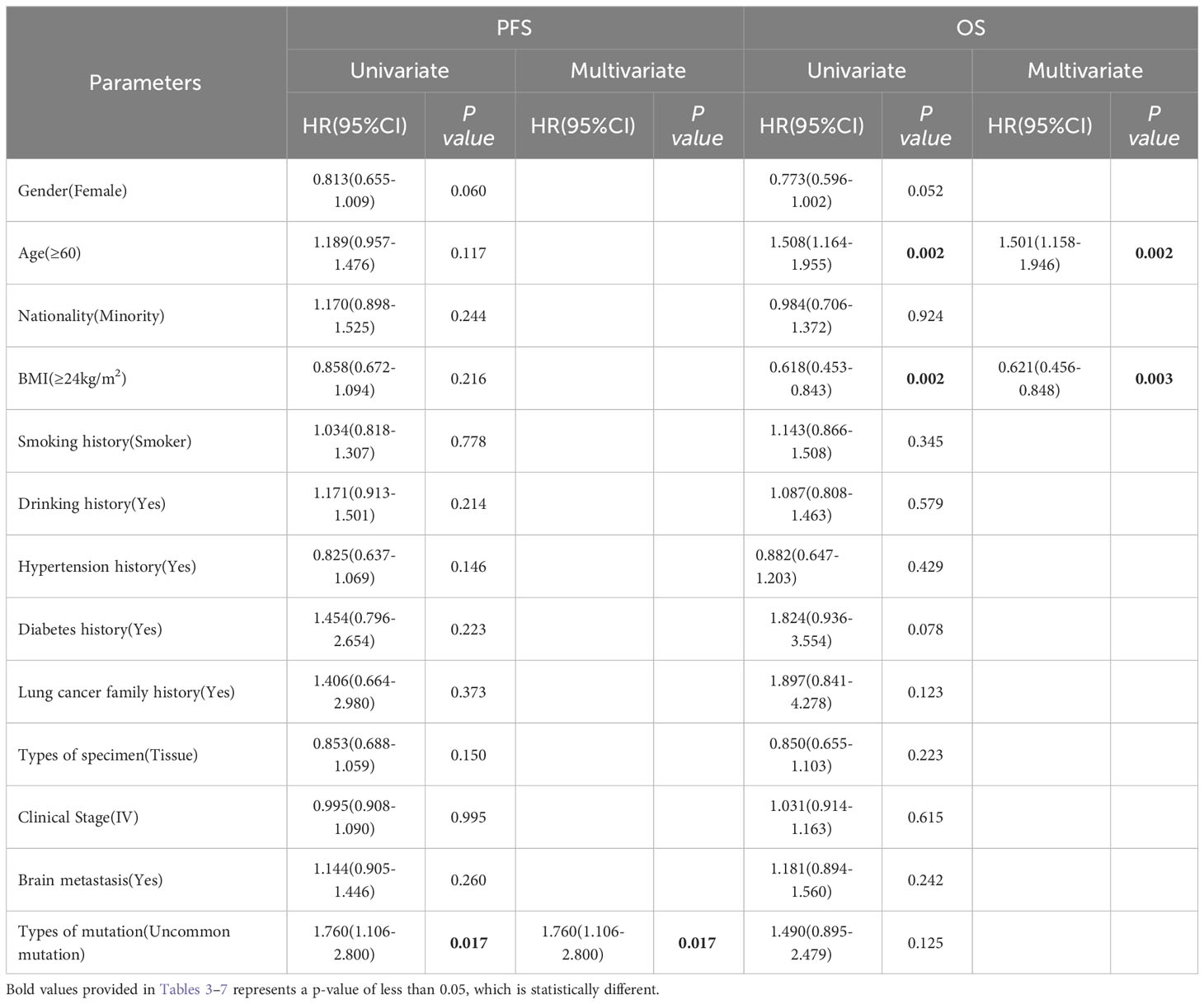
Table 7 Progress free survival and overall survival: univariate and multivariate analysis in Non-Xuanwei lung cancer subgroup.
As shown in Table 6, compared with the other study, our study had much longer PFS and OS in patients with EGFR uncommon mutation, especially in the Xuanwei subgroup, but had a little difference in PFS and OS in typical mutation patients.
Compared with the FLAURA study, patients with EGFR classical mutation in our study had a little longer in PFS(11.20m vs. 10.20m) and a little shorter in OS(27.50m vs. 31.80m), the same as Xuanwei county subgroup (12.00m vs. 10.20m) and (27.30m vs. 31.80m), Table 8 (9). Compared with the national multicenter real-world study of UpSwinG, we had a longer PFS (12.20m vs. 10.70m) and much longer OS(33.80m vs. 25.60m) in overall objectives with EGFR uncommon mutation (10). Again, in the Xuanwei subgroup, there was a much longer PFS(22.70m vs. 10.70m) and OS(38.50m vs. 25.60m), but there were shorter PFS(5.10m vs. 10.70m) and OS(19.10m vs. 25.60m) in Non-Xuanwei subgroup, Table 8, Figure 9.
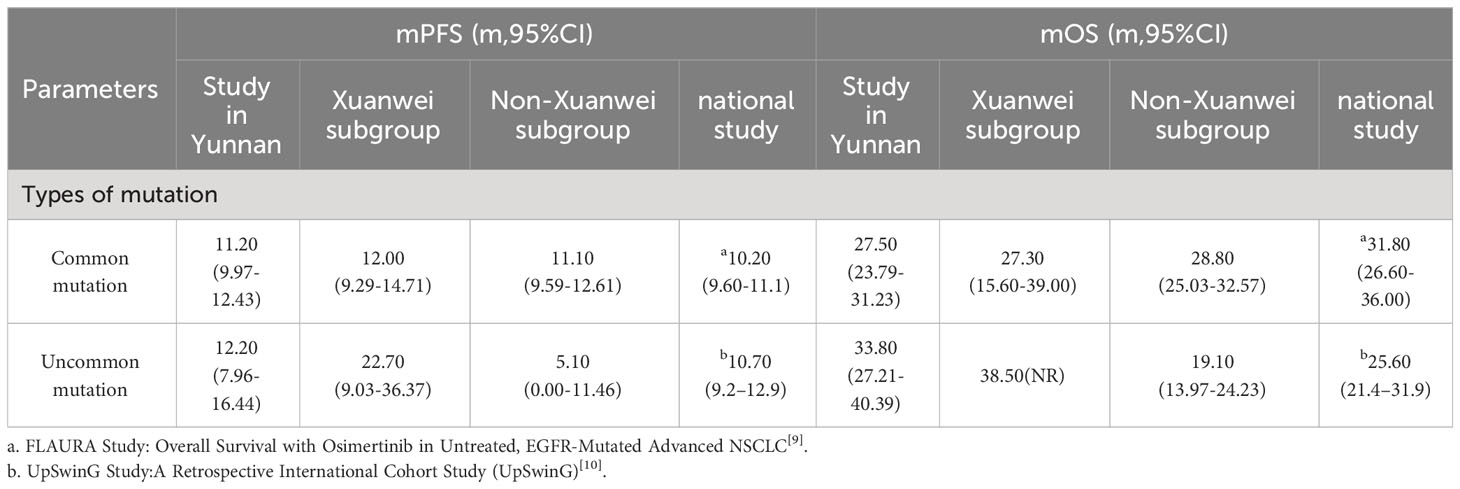
Table 8 Comparisons of PFS and OS among the study in Yunnan, Xuanwei subgroup and other national muticenter study.
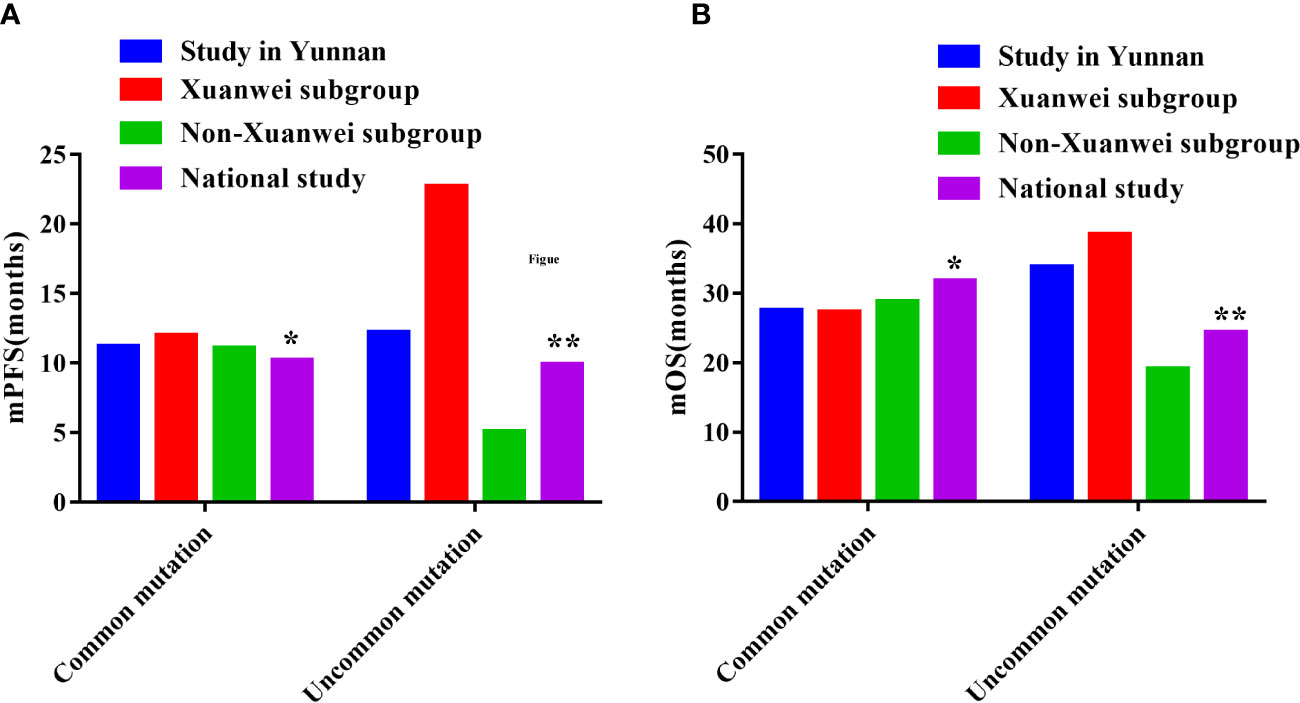
Figure 9 Comparisons of PFS and OS among the study in Yunnan, Xuanwei subgroup and other national muticenter study. (A) Comparisons of PFS. (B) Comparisons of OS. *FLAURA Study: Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC (9) ** UpSwing Study: A Retrospective International Cohort Study (UpSwinG) (10).
As the incidence rate and mortality of lung cancer in Xuanwei, YunnanProvince, China, is higher than that in the whole country and the rest the world, andseveral previous studies have demonstrated the genetic mutation characteristics oflung cancer patients with rare EGFR mutations, compound mutation rates, and RASmutation rates in Xuanwei (4, 5, 8). Therefore, conducting a comprehensive study onthe TKI treatment results in this region may provide a clinical basis forpersonalized clinical precision treatment of rare EGFR mutations in specific cancerhigh-risk areas and even other regions of the world.
Previous studies have suggested that EGFR-TKI is significantly more effectivein treating classical mutations than rare mutations (11, 12). In contrast, our overallstudy population showed no significant difference in efficacy between the twogroups.It is worth noting that univariate analysis revealed differences in PFSbetween regions, with Xuanwei lung cancer patients achieving longer mPFS thannon-Xuanwei lung cancer patients. However, analysis of patients in the Xuanweisubgroup showed that, in contrast, patients with rare mutations had better outcomesthan those with common mutations (5, 13). This may be related to the different typesand proportions of rare mutations in patients from different regions in each subgroup.Our previous studies showed that among rare mutations, the mutation rates ofEGFR-sensitive mutations such as G719X, G719X+L861Q, G719X+S768I, andS768I in Xuanwei were significantly higher than that in non-Xuanweiregions (13–15).
In addition, we compared the differences in PFS and OS between patients in ourstudy with FLAURA (9). It is difficult for us to make original comparison withoutthe raw data so a direct comparison between them was conducted. Generallyspeaking, the difference in PFS was not significant (11.2 m vs 10.2 m), while thedifference in OS was so apparent (27.5 m vs 31.8 m), with an averaged reduction of4.3 months. Previous in vitro studies found that the 19Del mutation has a higheraffinity for EGFR-TKI and thus may have a better effect on downstream signaling,while the 21 L858R mutation has a relatively low affinity for EGFR TKI and may beslightly less selective for EGFR TKI (15). Clinical study also discovered that patientswith 19Del mutation have better efficacy and better PFS and OS when treated withEGFR TKI compared to patients with 21 L858R mutation (16, 17). In our study,56.49% (235/416) of patients with 19-Del and 43.51% (207/416) of patients withL858R were included , while FLAURA study included 62.77% (349/556) and37.23% (207/556) accordingly, which possibly result in the different PFS. In recentyears, investigators have also explored the efficacy and prognosis of EGFR-TKItreatment for each 19Del subtype and found that different EGFR-TKI treatmentefficacy in patients with different Del- 19 subtypes was associated with differentsurvival, with longer PFS and OS for del E746 compared with del E746-A750, so wespeculate that there may be 19Del subtype differences or some specific unknownmutant loci to be further investigated in depth subsequently (18).
Regarding the EGFR rare mutation study population, the EGFR rare mutationpopulation in this study achieved longer PFS and OS compared to the globalUpSwinG multicenter study (10), with a prolonged mPFS of 1.5 months and aprolonged mOS of 8.2 months. The UpSwinG multicenter study included 246patients from 9 countries and regions worldwide, of which 83.7% were Asian and9.3% were Caucasian; the rare mutation types were common rare mutations (G719X,L861Q, S768I) accounting for 72.8%, and compound rare mutations accounting for32.8%. The majority of compound rare mutations were combinations of major raremutations. Interestingly, the UpSwinG study included subjects with similar raremutation types as the present study, with the difference that the proportion ofcompound rare mutations was higher in our study (59.6%), especially the highestproportion of patients with rare compound mutations in Xuanwei region. It is wellacknowledged that patients with compound mutations have improved outcome (13, 14), which may also be the potent reason for the longer mPFS and mOS obtainedin our study one of the most important reasons.
With the promotion of liquid biopsy in genetic testing, more and more studieshave confirmed that liquid biopsy can be an important complement to tissue biopsyin molecular testing. Studies clearly indicate that the positive detection rate of liquidbiopsy in EGFR gene testing is significantly lower than that of tissue samples, butstudies on whether there is a difference in the effect of subsequent TKI treatment indifferent sample testing populations are still lacking (5, 19). Our study suggests thatthe mPFS and mOS for EGFR-TKI therapy is different between different sampletypes, and the prognosis of the tissue sample delivery population is significantlybetter than that of the liquid biopsy and pleural effusion cytology deliverypopulations. However, a retrospective study (20) that included 59 samples showed nodifference in PFS and OS between patients with blood-delivered samples and thosewith tissue-delivered samples, which may be related to the small sample size and thehigh number of censored data described in the discussion of that study. We speculatethat this may be related to some differences in the accuracy of detection of fluid andplasma cavity effusion cytology specimens versus tissue samples. Interestingly, astudy that predicted the risk of TKI resistance by detecting EGFR mutations inplasma samples before and after TKI treatment suggested that a high rate of EGFRmutations was detected in the resistant patient population before clinical evaluationof resistance, which may suggest a relationship between plasma EGFR mutationsand TKI efficacy and resistance, but further studies are needed.
The NEJ002 study suggested that gefitinib, the first-generation TKI, was lesseffective than common mutation region in treating rare mutations in EGFR (21). However, a post hoc analysis of the LUX-Lung2, LUX-Lung3, and LUX-Lung6clinical studies showed that afatinib, the second-generation TKI yielded relativelygood data in patients with rare mutations such as L861Q, G719X, and S768I, with aPFS of up to 13.8 months and an OS of 26.9 months, with a sensitivity similar to thatof the common EGFR mutations, but its study sample size was only 75 cases (22). The German nNGM real-world study included 856 NSCLC cases with atypicalEGFR mutations (including co-mutations) from 12 centers and clinical follow-updata from 260 patients treated with different EGFR-TKI, chemotherapy, and immunecheckpoint inhibitors showed that patients with predominantly rare EGFR mutations(G719X, S7681, L861Q and above modifications coexisted) patients were treatedwith TKI, and 88.68% (415/468) of patients in this study population had a PFS of12.2 months with a generation TKI, suggesting that patients with rare mutations inthis region can benefit from the first generation TKI, and the benefit was moresignificant in the Xuanwei subgroup (23). This study will provide evidence-basedtreatment options for patients with rare EGFR mutations worldwide.
This study has its advantages. Sample size has always been difficult in raremutation population studies. Yunnan, especially Xuanwei, has a high rate of rareEGFR mutations and is an advantageous region for studying rare mutations. TheFLAURA study showed that the use of triple TKI axitinib in the first-line treatmentof advanced EGFR mutated NSCLC can achieve longer PFS and OS (24), andclinical guidelines have been approved as a Class IA evidence level 1recommendation and included in health insurance reimbursement (25). Unfortunately,however, patients with rare mutations have not yet been included in clinical trialstudies. It is also worthwhile to expect whether the third generation TKI can achievebetter efficacy in patients with rare EGFR mutations. The advantage of our study isthe inclusion of patients with rare mutations.
However, this study also had some limitations. First, this was a retrospectivestudy that includes only data from a single center. In addition, PFS survival datawere mature in this study. Still, the overall survival analysis outcome event has notyet reached more than 80%, which is 59.83%, which may be one of the reasons whysome variables in OS analysis (e.g, declared versus non-declared regions, classicalversus rare mutations, single versus compound mutations, and subgroup analysis)showed a trend of difference in values but did not reach statistical difference.Nevertheless, our study showed expected results that have not been reported before.We will continue to follow up and unveil the findings ofOS maturity data as soon aspossible.
In conclusion, this study reported the prognosis of EGFR-TKI treatment forNSCLC patients with different EGFR mutation types in Yunnan firstly, providingnew clinical evidence for EGFR-TKI-targeted therapy in patients with rare EGFRmutations in this region and worldwide. Prospective multicenter clinical studies areneeded to validate these observations, and further clinical studies are required. Welook forward to the participation of interested researchers from all over the world.We are also pleased to contribute more rare mutation cases from our region to otherresearch centers.
NSCLC patients in Yunnan displayed a unique EGFR mutation profile, especially a higher prevalence of EGFR uncommon and compound mutations subtype. This study indicates prognostic factors of NSCLC treated with EGFR-TKI in Yunan and Xuanwei. This study will provide new clinical evidence for EGFR-TKI-targeted therapy in patients with rare EGFR mutations in China and worldwide. More research is needed for NSCLC EGFR-TKI therapy and medical insurance policy-making in Yunnan, Xuanwei area and uncommon especially.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YPL, LC and RL contributed to conception and design of the study. XL, QL, JC, YD, GZ, XW, ZS, YDL and YC contributed to the acquisition, analysis, or interpretation of data for the work. YPL wrote the first draft of the manuscript. Then, LX, YZ and YH critically revised this report. All authors contributed to the article and approved the submitted version.
This study was partially supported by The National Natural Science Fund (No. 81860513), Key Project of Applied Basic Research in Yunnan Province(No.202101AS070004), and Joint Special Project of Applied Basic Research of Yunnan Provincial Science and Technology Department and Kunming Medical University (No.202001AY070001-073).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1156647/full#supplementary-material
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2018) 29(Suppl 4):iv192–237. doi: 10.1093/annonc/mdy275
3. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced EGFR mutation-positive non-Small-Cell lung cancer (Eurtac): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13(3):239–46. doi: 10.1016/s1470-2045(11)70393-x
4. Chen G, Sun X, Ren H, Wan X, Huang H, Ma X, et al. The mortality patterns of lung cancer between 1990 and 2013 in xuanwei, china. Lung Cancer (Amsterdam Netherlands) (2015) 90(2):155–60. doi: 10.1016/j.lungcan.2015.08.006
5. Zhou YC, Lin YP, Li Q, Ma LY, Liu X, Wang XX, et al. Clinical characteristics analysis of epidermal growth factor receptor gene mutations in lung cancer patients in yunnan province. Chin J Oncol (2020) 42(9):729–34. doi: 10.3760/cma.j.cn112152-20200313-00201
6. Chen Y, Ye L, Stanford RR, Zhang D, Zhang X, Wei W. Distinct epithelial growth factor receptor mutation profile in non-Small-Cell lung cancer patients from the xuanwei area of china. Mol Clin Oncol (2016) 4(5):749–55. doi: 10.3892/mco.2016.805
7. Lan Q, He X, Shen M, Tian L, Liu LZ, Lai H, et al. Variation in lung cancer risk by smoky coal subtype in xuanwei, china. Int J Cancer (2008) 123(9):2164–9. doi: 10.1002/ijc.23748
8. Zhou Y, Ge F, Du Y, Li Q, Cai J, Liu X, et al. Unique profile of driver gene mutations in patients with non-Small-Cell lung cancer in qujing city, yunnan province, southwest china. Front Oncol (2021) 11:644895. doi: 10.3389/fonc.2021.644895
9. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. New Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
10. Popat S, Hsia TC, Hung JY, Jung HA, Shih JY, Park CK, et al. Tyrosine kinase inhibitor activity in patients with NSCLC harboring uncommon EGFR mutations: A retrospective international cohort study (Upswing). Oncologist (2022) 27(4):255–65. doi: 10.1093/oncolo/oyac022
11. Xu J, Jin B, Chu T, Dong X, Yang H, Zhang Y, et al. EGFRTyrosine kinase inhibitor (TKI) in patients with advanced non-small cell lung cancer (NSCLC) harboring uncommon EGFR mutations: A real-world study in china. Lung Cancer (Amsterdam Netherlands) (2016) 96:87–92. doi: 10.1016/j.lungcan.2016.01.018
12. Brückl WM, Reck M, Griesinger F, Schäfer H, Kortsik C, Gaska T, et al. Afatinib as first-line treatment in patients with EGFR-mutated non-small cell lung cancer in routine clinical practice. Ther Adv Med Oncol (2021) 13:17588359211012361. doi: 10.1177/17588359211012361
13. Okamoto I, Morita S, Tashiro N, Imamura F, Inoue A, Seto T, et al. Real world treatment and outcomes in EGFR mutation-positive non-small cell lung cancer: Long-term follow-up of a large patient cohort. Lung Cancer (Amsterdam Netherlands) (2018) 117:14–9. doi: 10.1016/j.lungcan.2018.01.005
14. Yap WK, Shih MC, Kuo C, Pai PC, Chou WC, Chang KP, et al. Development and validation of a nomogram for assessing survival in patients with metastatic lung cancer referred for radiotherapy for bone metastases. JAMA Network Open (2018) 1(6):e183242. doi: 10.1001/jamanetworkopen.2018.3242
15. Da Cunha Santos G, Shepherd FA, Tsao MS. EGFR mutations and lung cancer. Annu Rev Pathology-Mechanisms Dis (2011) 6:49–69. doi: 10.1146/annurev-pathol-011110-130206
16. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi: 10.1056/NEJMoa0810699
17. Mok TSK, Cheng Y, Zhou XD, et al. Updated overall survival in a randomized study comparing dacomitinib with gefitinib as first-line treatment in patients with advanced non-Small-Cell lung cancer and EGFR-activating mutations. Drugs (2021) 81(2):257–66. doi: 10.1007/s40265-020-01441-6
18. Wu S-G, Gow C-H, Chen Y-L, Liu Y-N, Tsai M-F, Shih J-Y. Different treatment efficacies and T790M acquisition of EGFR-TKIs on NSCLC patients with variable del-19 subtypes of EGFR. Int J Cancer (2023) 153(2):352–63. doi: 10.1002/ijc.34507
19. Lin YP, Ma LY, Ma J, Zhou H, Lu YN, Wu M, et al. Characterization of EGFR gene mutations in patients with non-small cell lung cancer. Chin J Cancer Control (2021) 28(15):1119–24. doi: 10.16073/j.cnki.cjcpt.2021.15.01
20. Dan Q. Correlation between tissue and plasma EGFR gene mutation in patients with non-small cell lung cancer and the efficacy of EGFR-TKI[D]. Third Military Medical University (2016).
21. Miyauchi E, Inoue A, Kobayashi K, Maemondo M, Sugawara S, Oizumi S, et al. Efficacy of chemotherapy after first-line gefitinib therapy in EGFR mutation-positive advanced non-small cell lung cancer-data from a randomized phase iii study comparing gefitinib with carboplatin plus paclitaxel (NEJ002). Japanese J Clin Oncol (2015) 45(7):670–6. doi: 10.1093/jjco/hyv054
22. Yang JC, Sequist LV, Geater SL, Tsai CM, Mok TS, Schuler M, et al. Clinical activity of afatinib in patients with advanced non-Small-Cell lung cancer harbouring uncommon egfr mutations: A combined post-hoc analysis of lux-lung 2, lux-lung 3, and lux-lung 6. Lancet Oncol (2015) 16(7):830–8. doi: 10.1016/s1470-2045(15)00026-1
23. Janning M, Süptitz J, Albers-Leischner C, Delpy P, Tufman A, Velthaus-Rusik JL, et al. Treatment outcome of atypical egfr mutations in the german national network genomic medicine lung cancer (NEJM). Ann Oncol (2022) 33(6):602–15. doi: 10.1016/j.annonc.2022.02.225
24. Yu XQ, Yap ML, Cheng ES, Ngo PJ, Vaneckova P, Karikios D, et al. Evaluating prognostic factors for sex differences in lung cancer survival: Findings from a large australian cohort. J Thorac Oncol (2022) 17(5):688–99. doi: 10.1016/j.jtho.2022.01.016
Keywords: lung cancer, non-small cell lung cancer, EGFR, TKI, uncommon mutation, Yunnan, Xuanwei
Citation: Lin Y, Chen L, Li R, Liu X, Li Q, Cai J, Du Y, Zhao G, Wang X, Shen Z, Liao Y, Chen Y, Xie L, Zhou Y and Huang Y (2023) Survival analysis of patients with advanced non-small cell lung cancer receiving EGFR-TKI treatment of Yunnan in southwestern China: a real-world study. Front. Oncol. 13:1156647. doi: 10.3389/fonc.2023.1156647
Received: 01 February 2023; Accepted: 03 August 2023;
Published: 10 October 2023.
Edited by:
Sharon R. Pine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Mauricio Cuello, Hospital de Clinicas, UruguayCopyright © 2023 Lin, Chen, Li, Liu, Li, Cai, Du, Zhao, Wang, Shen, Liao, Chen, Xie, Zhou and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lin Xie, eGllbGlueWFuZ2hhbkAxNjMuY29t; Yongchun Zhou, Y2h1bmd1aTc2MjVAMTYzLmNvbQ==; Yunchao Huang, aHVhbmd5Y2gyMDAxQGFsaXl1bi5jb20=
†These authors have contributed equally to this work
‡These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.