- 1Department of Internal Medicine, University of Kentucky, Lexington, KY, United States
- 2Markey Cancer Center, University of Kentucky, Lexington, KY, United States
- 3Department of Pathology and Laboratory Medicine, University of Kentucky, Lexington, KY, United States
- 4Liberty University College of Osteopathic Medicine, Lynchburg, VA, United States
- 5University of Pikeville- Kentucky College of Osteopathic Medicine, Pikeville, KY, United States
- 6Division of Thoracic Surgery, University of Kentucky, Lexington, KY, United States
- 7Department of Internal Medicine, Division of Medical Oncology, University of Kentucky, Lexington, KY, United States
Primary mediastinal seminomas are exceedingly rare tumors, often localized to the anterior mediastinum. They may present with numerous complications, including superior vena cava syndrome, chylothorax, and pericardial effusions. Less commonly, they may present with paraneoplastic encephalitis. In this report we describe a case of a 19-year-old male with no significant past medical history who presented with bilateral hearing loss, progressive neuropathy, and ataxia. Subsequently the patient was found to have mediastinal mass with a high-titer anti-Hu antibody. To our knowledge, only one other case of mediastinal seminoma presenting with anti-Hu antibodies has been described in the literature. In this report, we describe a rare case of mediastinal seminoma, describe treatment options, and discuss additional known cases presenting with paraneoplastic encephalitis.
Introduction
First described in 1955 by Wolner et. al, primary mediastinal seminomas (PMS) are exceptionally rare germ cell tumors that account for 0.5-5% of mediastinal tumors (1, 2). We present here a unique case of PMS with paraneoplastic encephalitis. To our knowledge, only six additional cases have been previously described around the world. This is likely due to the rarity of the tumor, but also because this tumor type can be difficult to diagnose. Prior to full histologic evaluation, these tumors are often mistaken for thymic or thyroid neoplasms (3–5). For example, seminoma tumor cells have a CD117+ immunohistochemical (IHC) phenotype, a pattern also seen in thymic tumors (6). Selective IHC stain patterns are emerging and may be able to assist in effective tumor identification (7). In a recent study of PMS published by Fichtner et. al, staining for OCT3/4, D2-40, and TdT along with CD117+ were specific for seminoma histology identification (7). Cellular histology of seminomas demonstrates epithelioid cells with glycogen-rich cytoplasm and nucleoli that resemble primordial germ cells (6). Histology of PMS is identical to that of testicular seminomas.
Despite presenting as a diagnostic challenge, PMS can present with distinct complications often related to mass effect. Known common complications of mediastinal tumors include superior vena cava (SVC) syndrome (8), chylothorax (9), pericardial effusion (10), and lymphoid hyperplasia (5, 11). When planning for surgical removal, these tumors require remarkable precision, skill, and planning due to the nature of their location and adjacency to surrounding tissues. This tumor type’s response to surgery as well as chemotherapy and/or radiation has been shown to be favorable. Even under circumstances where these tumors are invading nearby structures, PMS have been shown to have excellent survival rates (12, 13). Overall survival rates at 5 and 10-year timepoints range from 87% to 100% and from 75% to 100% (14). Rarely, these tumors can present with the aforementioned complications as well as paraneoplastic encephalitis. The specific survival outcomes of patients with PMS and paraneoplastic encephalitis are not well-defined, as these tumors present a complex clinical picture requiring extensive neurologic, oncologic, and often surgical treatments.
Case presentation
A 19-year-old male with no significant past medical history presented with a chief complaint of hearing loss. Initial otolaryngology work-up revealed severe bilateral sensorineural hearing loss, and he was fitted with hearing aids. Shortly thereafter he began to experience progressive dizziness, neuropathy, ataxia, hand tremor, and generalized seizures. He had no family history of testicular cancer. No past medical history of Klinefelter syndrome. Physical exam was significant for symptoms of cerebellar impairment, including wide-based gait with varying stride length, and prolonged finger-to-nose testing. Extensive work up was performed including serum neoplastic antibody panel: ANNA1 (Anti-Hu), ANNA2S (Anti-Ri), ANNA3S, P/Q-type Calcium Channel, CRMP-5-IgG, PCA2, PCA1,PCA-TR, Neuronal Voltage-Gated K+ Channel, AGNA-1, Amphiphysin, MOGFS Myelin Oligodendrocyte Glycoprotein (MOG-IgG1), NMO/AQP4 FACS, and Muscle-Specific Kinase (MuSK) were normal except Anti-Hu (titer: 1:3840); tumor markers: serum beta-hCG, and alpha fetoprotein were normal. LDH also was within normal limits at 196 (normal 116 – 250). No lumbar puncture or CSF studies were performed, as antineoplastic antibodies were detected prior to procedure. Computed tomography (CT) head with contrast was unremarkable. Magnetic resonance imaging (MRI) head performed with and without IV contrast demonstrated midline cerebellar atrophy. No restricted diffusion noted on MRI. No mass lesions noted. Video electroencephalogram performed was normal without evidence of epileptiform activity. CT chest with contrast revealed a heterogeneous anterior mediastinal soft tissue nodule located within the thymus (Figure 1). Initial CT-guided core mediastinal mass biopsy demonstrated findings suggestive of thymic neoplasm. Subsequently, the patient underwent median sternotomy with radical thymectomy. Pathology revealed seminoma with lymphoid hyperplasia and cystic change with IHC positive for pancytokeratin, CD117, octomer-binding transcription factor 3/4 (Oct3/4), placental alkaline phosphatase (PLAP), and spalt like transcription factor 4 (SALL4) (Figure 2). He was diagnosed with rhombencephalitis, paraneoplastic syndrome secondary to PMS. CT abdomen/pelvis was completed for staging and did not demonstrate any evidence of malignancy or metastatic disease. He was given intravenous (IV) methylprednisolone with mild improvement in his neurological symptoms. He is currently undergoing intravenous immune globulin (IVIG) treatment for his rhombencephalitis. No reported seizures and his neuropathy remains stable. Regarding his cancer, he is under surveillance as his repeat post-operative CT chest did not identify any new suspicious nodules and tumor markers were normal. He works with physical and occupational therapists and is now able to walk with walker (Figure 3).
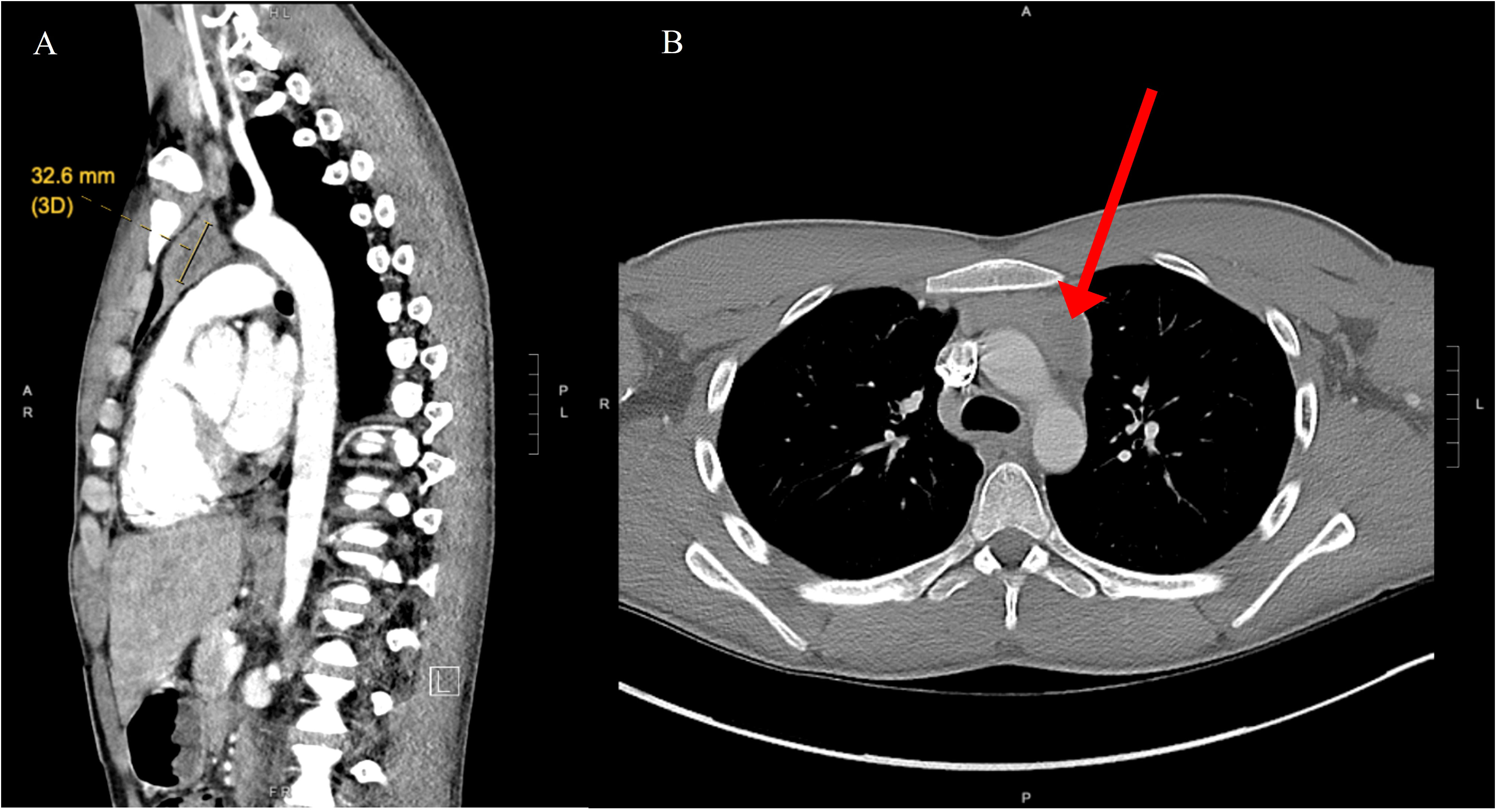
Figure 1 (A) Nodular area with central low density in the left side of the thymus, measuring roughly 32 mm long axis indicative of mediastinal seminoma (yellow indicator). (B) Computed tomography of axial chest demonstrates anterior mediastinal mass (red arrow).
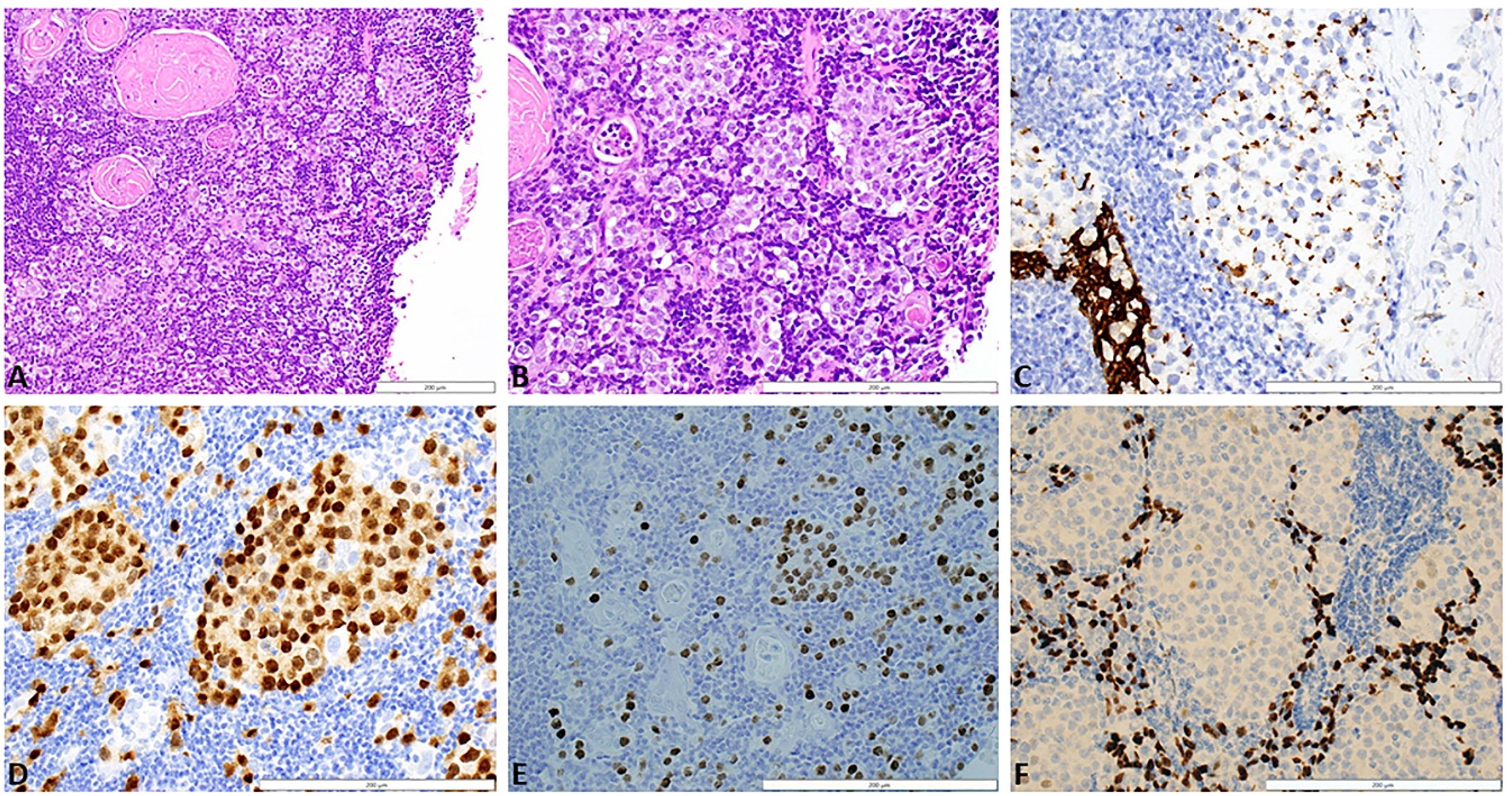
Figure 2 (A) Section of thymus shows cystic degeneration at the right side of the image (white space) and a rim of thymus showing intact, normal Hassel's corpuscles and a diffusely infiltrative malignant process. Tumor cells are present as single, dispersed cells, as well as cords and clusters, in a background of lymphocytes and thymic epithelial cells. Although there is cystic degeneration, the diffusely infiltrative growth pattern results in more of thickening of the thymic epithelium rather than a discrete mass. H&E stain, 20x magnification. (B) Higher magnification showing tumor infiltrative as single cells, cords, and clusters between lymphocytes, thymic epithelium, and Hassel's corpuscles. The tumor cells are large, contain clear to eosinophilic cytoplasm, and have large, round nuclei with prominent nucleoli. H&E stain, 40x magnification. (C) Pancytokeratin stain shows diffuse staining in the normal thymic epithelium in the lower left portion of the image. In contast, clusters of tumor cells show focal, punctuate staining. AE1/AE3 stain, 40x magnification. (D) Clusters and isolated seminoma cells show nuclear posibility with the germ cell marker OCT3/4. OCT3/4 stain, 40x magnification. (E) Clusters and isolated seminoma cells show nuclear positivity with the germ cell marker SALL4. SALL4 stain, 40x magnification. (F) Background thymic epithelium stain positively with p63 while the seminoma cells that are present in clusters are negative. p63 stain, 40x magnification. H&E, Hematoxylin and eosin; AE1/AE3, cytokeratin stain; OCT 3/4, octamer-binding transcription factor 4; SALL4, spalt like transcription factor 4.

Figure 3 A detailed diagnosis and treatment timeline of mediastinal seminoma with paraneoplastic anti-Hu encephalitis.
Discussion
Anti-Hu associated paraneoplastic rhombencephalitis is uncommonly associated with mediastinal germ cell tumors. Also known as antineuronal nuclear antibody (ANNA-1), presence of anti-Hu antibodies can result in varying neurologic disturbances, such as psychoses, seizures, hemiparesis, unexplained fevers, visual disturbance, and hearing loss. As the symptoms of paraneoplastic syndromes that occur secondary to PMS are nonspecific, diagnosis of these tumors can be difficult to ascertain. The mediastinum is also common location for thymomas, thyroid carcinomas, and T-cell lymphoma (4). Thyroid carcinoma and seminoma tissue have similar histologic features, which can result in misdiagnoses. Staining for markers such as SALL4, PLAP, and OCT3/4 are key in the process of clarifying a mediastinal seminoma diagnosis (4, 7).
In addition to our documented case of PMS with paraneoplastic rhombencephalitis, there are six additional reported cases as described in Table 1. All of the patients were male with ages ranging from 19 to 49 years old. Similar to our patient, a 45-year-old male in Israel presented with bilateral sensorineural hearing loss (17). He was found to have anti-KLH11 antibodies which have been described previously in Kelch-like protein 11 antibody-associated paraneoplastic neurological (KELCH) syndrome. Anti-KLH11, first described in 2019 as a new marker for paraneoplastic encephalitis, has been postulated as more likely to be associated with germ cell tumors such as seminoma (21). Cases reviewed in a literature review by Li et al. documented that patients with anti-KLH11 also presented with other paraneoplastic antibodies such as anti-leucine zipper 4, anti-Ma2, and anti-Hu (21). In an additional PMS case, a 21-year- male from Germany who presented with panhypopituitarism was found to have Anti-Ma2 antibodies (19). Another case describes a 19-year-old male, also with PMS and Anti-Ma2 antibodies (16). In France, a 49-year-old male presented with signs of panhypopituitarism as well as progressive behavioral disorder, right hemiparesis, and malignant fever. He was found to have PMS with Anti-Ri antibodies (15). A 20-year-old male with PMS, psychosis, and suicide attempts was found to have Anti-SS DNA 189 u/ml antibodies (18). A 32-year-old male from Germany with PMS was found to have concurrent anti-Hu and anti-NMDA receptor antibodies (20).This is the only additional reported case of PMS presenting with anti-Hu paraneoplastic encephalitis.

Table 1 In addition to our documented case of paraneoplastic encephalitis with primary mediastinal seminoma there are six additional documented cases.
Patients in each of these cases underwent a variety of treatments, including chemotherapy, plasmapheresis, IVIG, and surgical removal. A recently published study performed by Zhai et al. compared outcomes of patients with PMS who underwent surgical treatment versus chemotherapy +/- radiation. This single-center retrospective study in 27 patients showed that there was no significant difference in 5-year overall survival, cancer-specific survival, and progression-free survival between patients with and without surgery (1). In contrast, a population-based study performed in China with 476 patients sought to predict the survival of patients with PMS (22). One factor found to negatively impact overall survival was not undergoing surgery (22).
While outcomes of patients with PMS are well-described in the literature, outcomes of patients with PMS and concurrent paraneoplastic encephalitis are not articulated. The paucity of cases involving PMS with paraneoplastic encephalitis makes it difficult to assess which treatments are the most effective at benefitting the patient’s mortality and improving patients’ symptoms. In reference to the cases discussed here, neurologic recovery and patient outcomes differed on a case-by-case basis in PMS patients with paraneoplastic encephalitis. One case of a 22-year-old male with Anti-SS DNA 189 u/ml antibodies reported a nearly full recovery of cognitive functions with treatment (18), while the patient with anti-KLHL11 reported stabilization of neurologic symptoms (17). It appears that even after surgical removal of the patients’ tumors and immunosuppressive treatment, neurological symptoms persisted in most patients. Our patient was treated with IV and oral prednisone for a short course and is currently undergoing IVIG treatment. His neurological symptoms have mildly improved. He was wheelchair bound and is now able to walk with walker, but his weakness and neuropathy have persisted. The patient with both anti-Hu and anti-NMDA receptor antibodies was treated with a combination of prednisolone, IVIG, and plasmapheresis, however, it was reported that symptoms of generalized seizures, oculomotor and auditory disturbances did not improve (20). The patient with Anti-Ri antibodies underwent tumor resection along with carboplatin, corticosteroids, and hormone replacement therapy. His paraneoplastic fever disappeared, however his abnormal behaviors remained unchanged (15). The 21-year-old male from Germany with Anti-Ma2 was treated with prednisolone reportedly rapidly improved (16). The report did not specify what improvements the patient experienced, or if the patient underwent resection of primary tumor.
In a 2022 published report by Budhram et al, 27 patients with anti-Hu extralimbic encephalitis were identified and patient outcomes were described (23). None of the patients in this study had PMS, however ~80% of the patients did have solid organ malignancy. Many of the patients received chemotherapy and/or immunotherapy as well as immunosuppressive treatment. The majority of patients were placed on steroids. Overall, there was notably good response to treatment with anti-seizure therapy and either steroids or other immunotherapies (23). Most adults (10 out of 19) survived and remained seizure free post-treatment while other adults (8 out of 19) died. Half of the adults who survived and remained seizure-free had persistent neurologic symptoms including dysautonomia, gait ataxia, and hemiparesis.
Conclusion
In conclusion, PMS is a rare tumor, and presentation with paraneoplastic encephalitis is even more infrequent. A multidisciplinary approach should be considered in the setting of paraneoplastic symptoms. There is a need for more frequent documentation of unique cases in order to better establish treatment approaches that may improve patient outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CW: drafting of the article and final approval of the manuscript. ZM: drafting of the article, acquisition of data, and final approval of the manuscript. DA: providing histopathology pictures and final approval of the manuscript. AC and RB: drafting of the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhai Y, Chen B, Feng X, Liu K, Wang S, Hui Z, et al. Chemoradiotherapy is an alternative choice for patients with primary mediastinal seminoma. Radiat Oncol (2022) 17(1):58. doi: 10.1186/s13014-022-02013-6
2. Woolner LB, Jamplis RW, Kirklin JW. Seminoma (germinoma) apparently primary in the anterior mediastinum. N Engl J Med (1955) 252(16):653–7. doi: 10.1056/NEJM195504212521602
3. Liu H, Chen Y. Mediastinal seminoma mimicking invasive thymoma on 18F-FDG PET/CT. J Nucl Cardiol (2021) 28(4):1788–90. doi: 10.1007/s12350-020-02127-y
4. Murshed K, Al-Bozom I, Vattoth S, Akhtar M. Mediastinal seminoma presenting as a neck mass falsely diagnosed as anaplastic thyroid carcinoma: A case report. Diagn Cytopathol. (2019) 47(4):334–6. doi: 10.1002/dc.24090
5. Holmes C, Loo PS, Barnard S. Primary mediastinal seminoma with florid follicular lymphoid hyperplasia: a case report and review of the literature. Diagn Pathol (2021) 16(1):76. doi: 10.1186/s13000-021-01137-9
6. Pini GM, Colecchia M. Mediastinal germ cell tumors: a narrative review of their traits and aggressiveness features. Mediastinum (2022) 6:5. doi: 10.21037/med-21-22
7. Fichtner A, Richter A, Filmar S, Kircher S, Rosenwald A, Kuffer S, et al. Primary mediastinal germ cell tumours: an immunohistochemical and molecular diagnostic approach. Histopathology (2022) 80(2):381–96. doi: 10.1111/his.14560
8. Rampengan VRC, Bakhtiar A. A case of primary mediastinal seminoma with superior vena cava syndrome and large intracardiac thrombus. Int J Surg Case Rep (2022) 97:107478. doi: 10.1016/j.ijscr.2022.107478
9. Caronia FP, Di Miceli G, Macaluso A, Librizzi D, Sgalambro F, Fiorelli A. Retroaortic closure of thoracic duct in the management of persistent chylothorax: a case report. J Cardiothorac Surg (2019) 14(1):100. doi: 10.1186/s13019-019-0917-8
10. Cao J, Zhou Y, Zou F, Ma JA, Hu C. Intensity modulated radiation therapy to treat primary female mediastinal seminoma and massive pericardial effusion: A case report. Oncol Lett (2017) 13(3):1299–302. doi: 10.3892/ol.2017.5555
11. Lee HI, Jang IS, Jeon KN, Ko GH, Lee JS, Kim DC, et al. Thymoma and synchronous primary mediastinal seminomas with florid follicular lymphoid hyperplasia in the anterior mediastinum: A case report and review of the literature. J Pathol Transl Med (2017) 51(2):165–70. doi: 10.4132/jptm.2016.08.24
12. Bokemeyer C, Nichols CR, Droz JP, Schmoll HJ, Horwich A, Gerl A, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol (2002) 20(7):1864–73. doi: 10.1200/JCO.2002.07.062
13. Mead GM, Stenning SP. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol (1997) 15(2):594–603. doi: 10.1200/JCO.1997.15.2.594
14. Napieralska A, Majewski W, Osewski W, Miszczyk L. Primary mediastinal seminoma. J Thorac Dis (2018) 10(7):4335–41. doi: 10.21037/jtd.2018.06.120
15. Launay M, Bozzolo E, Venissac N, Delmont E, Fredenrich A, Thomas P. Encéphalite limbique paranéoplasique avec anticorps anti-RI et séminome médiastinal [Paraneoplastic limbic encephalitis with positive anti-RI antibodies and mediastinal seminoma]. Rev Neurol (Paris). (2008) 164(6-7):612–9. doi: 10.1016/j.neurol.2008.02.044
16. Bosemani T, Huisman TA, Poretti A. Anti-Ma2-associated paraneoplastic encephalitis in a male adolescent with mediastinal seminoma. Pediatr Neurol (2014) 50(4):433–4. doi: 10.1016/j.pediatrneurol.2013.12.020
17. Krivitski D, Alcalay Y, Peer M, Paran Y, Eisenstein O, Davidson T, et al. Bilateral hearing loss preceding rhomboencephalitis - a hint for Kelch-like 11 syndrome. Neurol Sci (2023) 44(1):369–72. doi: 10.1007/s10072-022-06369-1
18. Plioplys S, Beedle D. A case of acute psychosis and mediastinal seminoma. Psychosomatics (1998) 39(6):559–61. doi: 10.1016/S0033-3182(98)71294-0
19. Bergner CG, Lang C, Spreer A, Bähr M, Mohr A, Simons M. Teaching NeuroImages: Ma2 encephalitis presenting as acute panhypopituitarism in a young man. Neurology (2013) 81(19):e146–7. doi: 10.1212/01.wnl.0000435307.33389.f7
20. Pohley I, Roesler K, Wittstock M, Bitsch A, Benecke R, Wolters A. NMDA-receptor antibody and anti-Hu antibody positive paraneoplastic syndrome associated with a primary mediastinal seminoma. Acta Neurol Belg. (2015) 115(1):81–3. doi: 10.1007/s13760-014-0296-9
21. Li EC, Lai QL, Cai MT, Zheng Y, Fang GL, Fang W, et al. Kelch-like protein 11 antibody-associated paraneoplastic neurological syndrome: A state-of-the-art review. Clin Immunol (2022) 241:109074. doi: 10.1016/j.clim.2022.109074
22. Huang W, Luo J, Zhou X, Zhao Y, Zhang T, Ma X. Nomograms for predicting prognosis of primary mediastinal seminoma: A population-based study. J Oncol (2021) 2021:9048375. doi: 10.1155/2021/9048375
Keywords: primary mediastinal seminoma, anti-Hu, paraneoplastic encephalitis, ANNA-1, bilateral hearing loss, rhombencephalitis
Citation: Williams CM, Allison DB, Coleman AB, Bardhan R, Miller JD and Myint ZW (2023) Primary mediastinal seminoma presenting with paraneoplastic anti-Hu encephalitis: a case report and literature review. Front. Oncol. 13:1156566. doi: 10.3389/fonc.2023.1156566
Received: 10 July 2023; Accepted: 29 August 2023;
Published: 15 September 2023.
Edited by:
Anand Sharma, Mount Vernon Cancer Centre, United KingdomReviewed by:
Atsuto Katano, The University of Tokyo Hospital, JapanBicky Thapa, Medical College of Wisconsin, United States
Copyright © 2023 Williams, Allison, Coleman, Bardhan, Miller and Myint. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zin W. Myint, emluLm15aW50QHVreS5lZHU=
 Chelsey M. Williams
Chelsey M. Williams Derek B. Allison
Derek B. Allison Adam B. Coleman
Adam B. Coleman Roshmita Bardhan5
Roshmita Bardhan5 Zin W. Myint
Zin W. Myint