- 1Department of Oncology and Palliative Medicine, Nordland Hospital, Bodø, Norway
- 2Department of Clinical Medicine, Faculty of Health Sciences, UiT – The Arctic University of Norway, Tromsø, Norway
- 3Department of Radiation Oncology, University Hospital Zurich, University of Zurich, Zurich, Switzerland
- 4Department of Radiation Oncology, Medical Center, Medical Faculty, University Freiburg, Freiburg, Germany
Background and objectives: The validated LabBM score (laboratory parameters in patients with brain metastases) represents a widely applicable survival prediction model, which incorporates 5 blood test results (serum lactate dehydrogenase (LDH), C-reactive protein (CRP), albumin, platelets and hemoglobin). All tests are classified as normal or abnormal, without accounting for the wide range of abnormality observed in practice. We tested the hypothesis that improved stratification might be possible, if more granular test results are employed.
Methods: Retrospective analysis of 198 patients managed with primary whole-brain radiotherapy in one of the institutions who validated the original LabBM score.
Results: For two blood tests (albumin, CRP), discrimination was best for the original dichotomized version (normal/abnormal). For two others (LDH, hemoglobin), a three-tiered classification was best. The number of patients with low platelet count was not large enough for detailed analyses. A modified LabBM score was developed, which separates the intermediate of originally 3 prognostic groups into 2 statistically significantly different strata, resulting in a 4-tiered score.
Conclusion: This initial proof-of-principle study suggests that granular blood test results might contribute to further improvement of the score, or alternatively development of a nomogram, if additional large-scale studies confirm the encouraging results of the present analysis.
1 Introduction
Brain metastases should not be regarded as universally fatal cancer manifestation anymore (1–4). Even if survival outcomes of less than one year are still common, especially in patients with large burden of extracranial metastases who lack effective systemic treatment options (5), survival beyond 5 or even 10 years can be achieved in a minority of patients (6–8). Better understanding of factors explaining these survival differences has led to prognostic scores, which might aid clinicians who have to choose between vastly different treatment options. The latter include surgical resection, radiosurgery, other radiotherapy, systemic treatment and best supportive care, if other approaches are unlikely to prolong survival or improve quality of life (1–4).
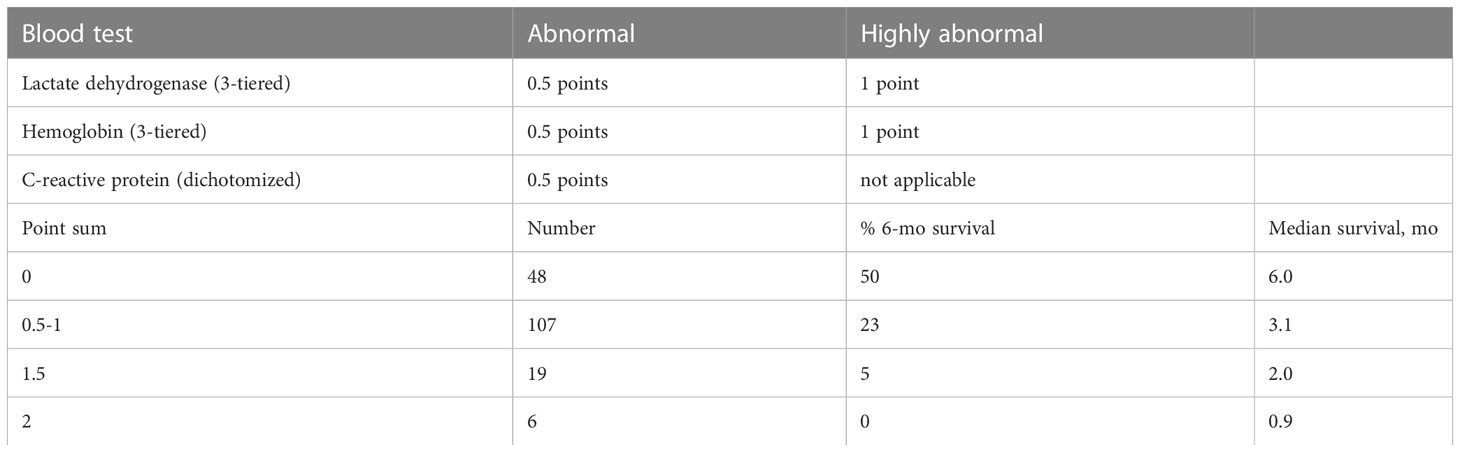
Prognostic scores come with often unique advantages and disadvantages and are not expected to provide perfectly accurate survival predictions (9, 10). Gradual improvement of scores, e.g. diagnosis-specific graded prognostic assessment (DS-GPA), has long been a focus of dedicated research groups (11, 12). The validated LabBM score (laboratory parameters in patients with brain metastases) represents one of the DS-GPA’s competitors and has been studied in Austria, Switzerland and Norway (13, 14). It covers all cancer types and is solely based on easy-to-evaluate blood test results, allowing for rapid clinical implementation also in busy practices. LabBM incorporates five inexpensive blood tests (serum lactate dehydrogenase (LDH), C-reactive protein (CRP), albumin, platelets and hemoglobin), which indirectly reflect disease burden and its impact on inflammatory processes in the body. Normal blood test results are expected in case of limited disease extent. In contrast, multiple extracranial metastases and large overall tumor burden typically cause abnormal test results. The latter were simply dichotomized (normal/abnormal) in the original and validation LabBM studies. Elevated LDH led to 1 point, elevated CRP to 1 additional point, low hemoglobin, albumin and platelets each added 0.5 points to the LabBM score. A maximum sum of 3.5 points can be achieved and patients are assigned to one of three prognostic groups (best survival: 0-1 points, intermediate: 1.5-2 points, short survival: 2.5-3.5 points). As repeatedly shown, even in patients irradiated for non-brain metastases (15), the LabBM score performs well. It shares a limitation with other scores, i.e. the relatively large subgroup of patients with intermediate prognosis.
An important question has not been evaluated in the previous studies: do dichotomized blood test results provide optimum information or should we account for magnitude of deviation from normal values? It cannot be taken for granted that, e.g., a LDH level of 275 U/L provides the same prognostic information as a level of 550 U/L or 1,100 U/L. The purpose of the present study was to test the role of non-dichotomized blood test results in comparison to the original version of the LabBM score.
2 Materials and methods
2.1 Study population and data collection
This initial study (proof-of-principle or pilot study) was performed in a limited, but homogeneously treated population to minimize confounding factors, and speed up the process of signal detection, i.e. results that would justify large-scale analyses. A pre-existing database (14) with dichotomized blood test results was expanded to include the actual level of deviation in patients with abnormal results. All patients had received palliative whole-brain radiotherapy (WBRT, 10 fractions of 3 Gy, no preceding surgery or other brain metastases therapy) for multiple brain metastases at Nordland Hospital (time period 2007-2021, consecutive patients). Systemic treatment and salvage for progressive brain metastases, e.g. radiosurgery, were administered as indicated. The blood tests were part of routine oncological assessment approximately 1-2 weeks before WBRT. Normal values: hemoglobin 11.7-15.3 g/dl (females) and 13.4-17.0 g/dl (males); platelets 130-400 x109; albumin 34-45 g/l; LDH <205 U/l; CRP <5 mg/l.
2.2 Statistical analysis
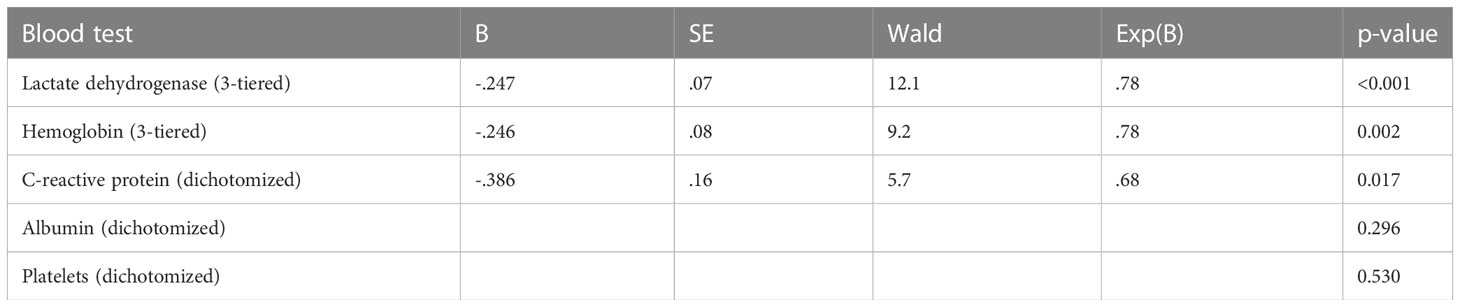
The original LabBM score (dichotomized blood test results) was calculated as outlined in the Introduction. Then, the underlying, now non-dichotomized blood test results were analyzed in univariate Cox regression analyses for continuous variables to test their prognostic impact. Next, optimum cut-off points were identified to group the results (maximum 4 strata per blood test, log-rank tests for actuarial overall survival curves, focus on best possible separation of the curves). Actuarial overall survival was calculated (Kaplan-Meier method) from the first day of WBRT, including also discontinued courses of WBRT. Date of death was known in almost all patients, while 5 were still alive at the time of analysis. Their median follow-up was 45 months (minimum 17 months). After these univariate analyses, the optimally stratified blood test variables were entered into a multivariate forward stepwise Cox regression analysis. Test results with significant impact on survival were then employed to assign a modified LabBM score, adhering to the original strategy described by Berghoff et al. (13) (0.5 or 1 point, depending on a factor’s regression coefficient B, or in other words impact on survival). P-values ≤0.05 were considered statistically significant. Analyses were performed in SPSS 28, IBM Corp., Armonk, NY, USA
2.3 Ethical statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As a retrospective quality of care analysis employing an established database, no approval from the Regional Committee for Medical and Health Research Ethics (REK Nord) was necessary. This research project was carried out according to our institutions’ guidelines and with permission to access the patients’ data. Written informed consent was received from all patients.
3 Results
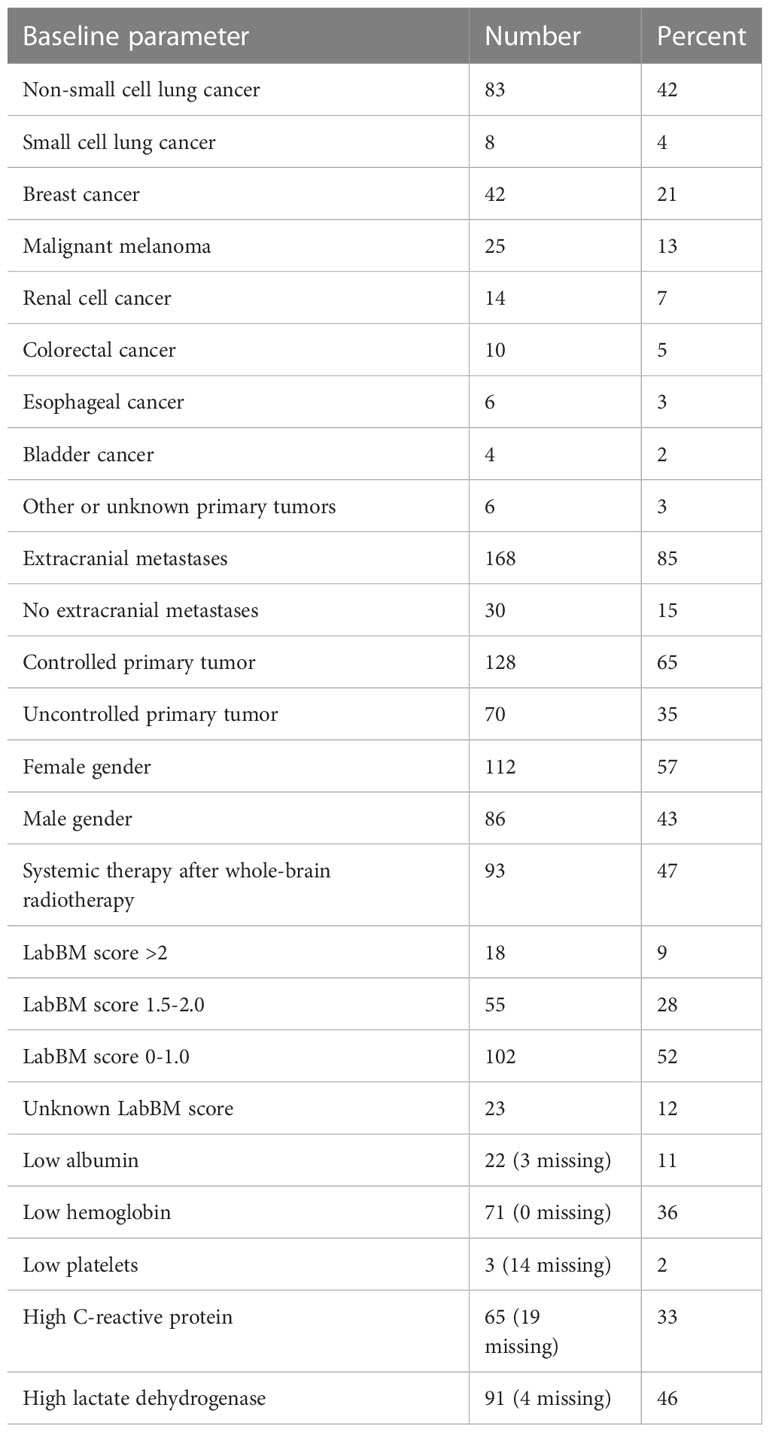
The study included 198 patients (193 death events) whose baseline characteristics are shown in Table 1. Not all patients had complete blood test results available. Due to low numbers, continuous variable Cox regression analysis was not feasible for patients with low platelet count (n=3). Figure 1 shows the results of the analyses for the remaining 4 blood tests. For two of these (albumin, CRP), discrimination was best for the original dichotomized version (normal/abnormal). For the two others (LDH, hemoglobin), a three-tiered classification was best (Figures 2, 3).
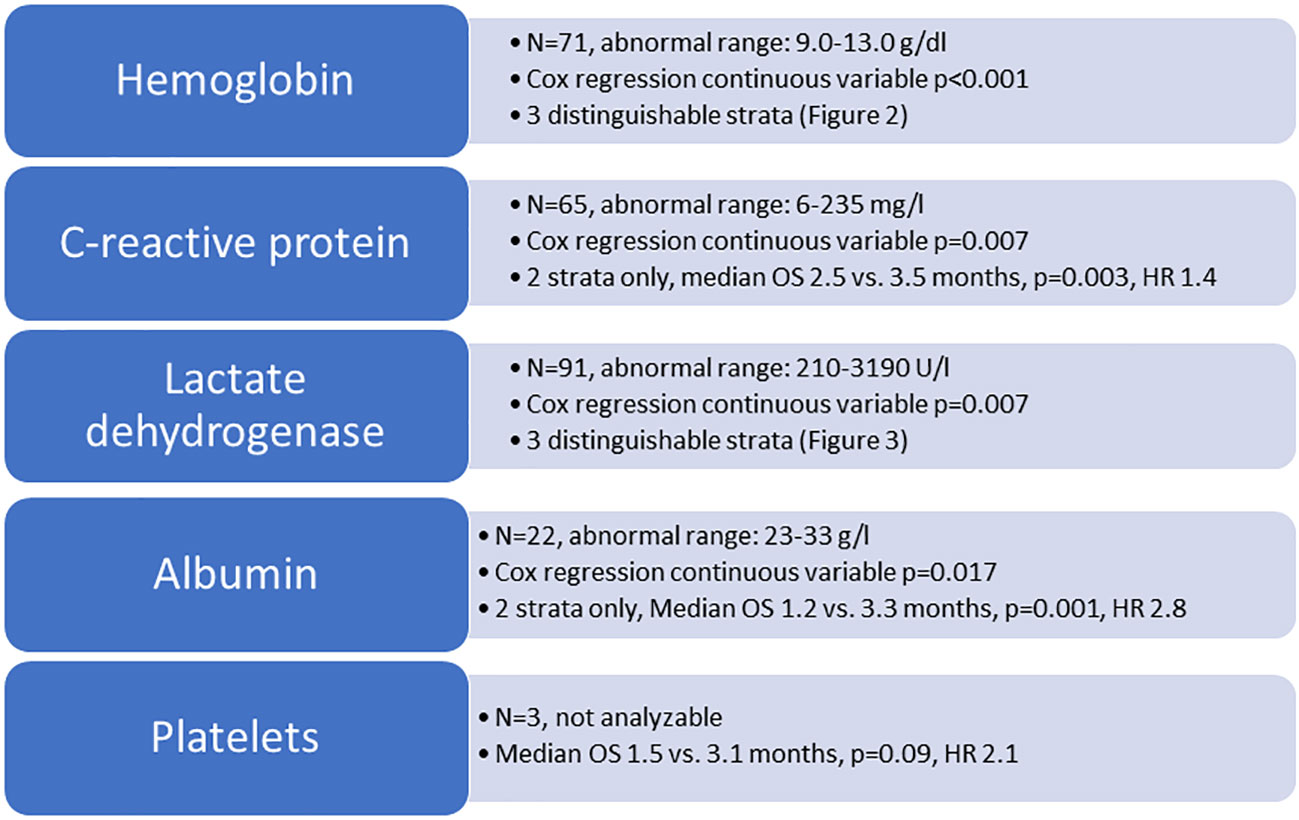
Figure 1 Overview of blood test analyses. OS, overall survival (Kaplan-Meier analysis, log-rank test); HR, hazard ratio.
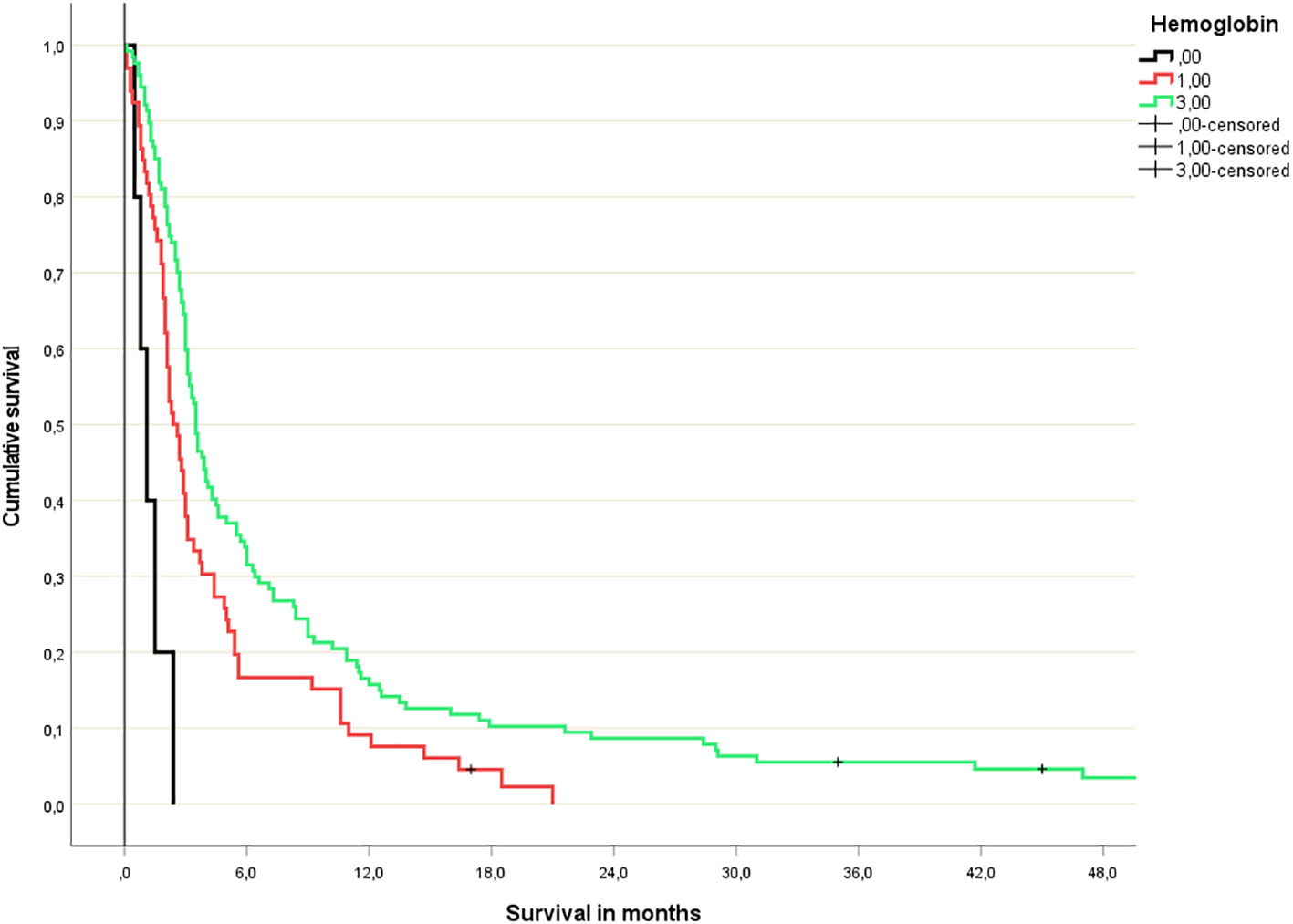
Figure 2 Hemoglobin-based 3-tiered survival prediction (Kaplan-Meier analysis; n=5 (hemoglobin <10 g/dl), 66 (hemoglobin 10-13 g/dl) and 127 (normal hemoglobin), respectively; median survival 1.1, 2.4 and 3.5 months, respectively; p=0.03 or better for all comparisons; HR 3.2 and 1.5, respectively).
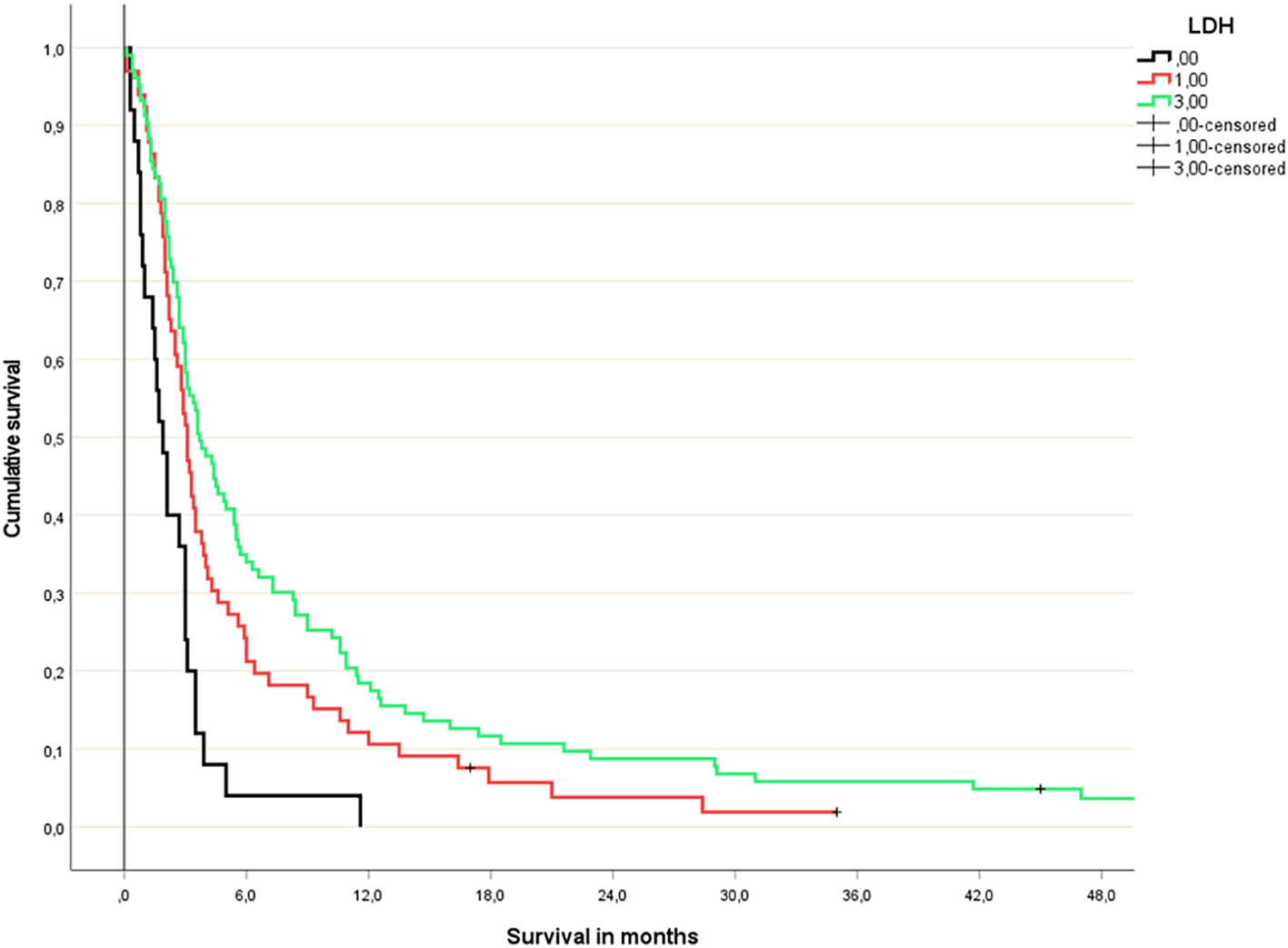
Figure 3 Lactate dehydrogenase-based 3-tiered survival prediction (Kaplan-Meier analysis; n=25 (LDH >400 U/l), 66 (LDH 210-399 U/l) and 103 (normal LDH), respectively; median survival 1.9, 3.1 and 3.7 months, respectively; p=0.04 or better for all comparisons; HR 1.9 and 1.2, respectively).
The multivariate Cox regression analysis with all 5 blood tests (normal/abnormal albumin, CRP, platelets; 3-tiered hemoglobin and LDH) is shown in Table 2. All 3 significant predictors of survival were employed to assign a modified LabBM score, which is displayed in Table 3. Figure 4 shows the Kaplan-Meier survival curves (4 strata were possible) and Figure 5 shows the original three-tiered LabBM score. The original LabBM score with 5 blood tests could not be converted into a 4-tiered score, because all intermediate survival curves were overlapping, as shown in Figure 6. Both versions were identical in terms of patients with 0 points (n=48, median survival 6 months) and patients with highest point sum (n=6 with 3 points (original), n=6 with 2 points (modified), median survival 0.9 months).
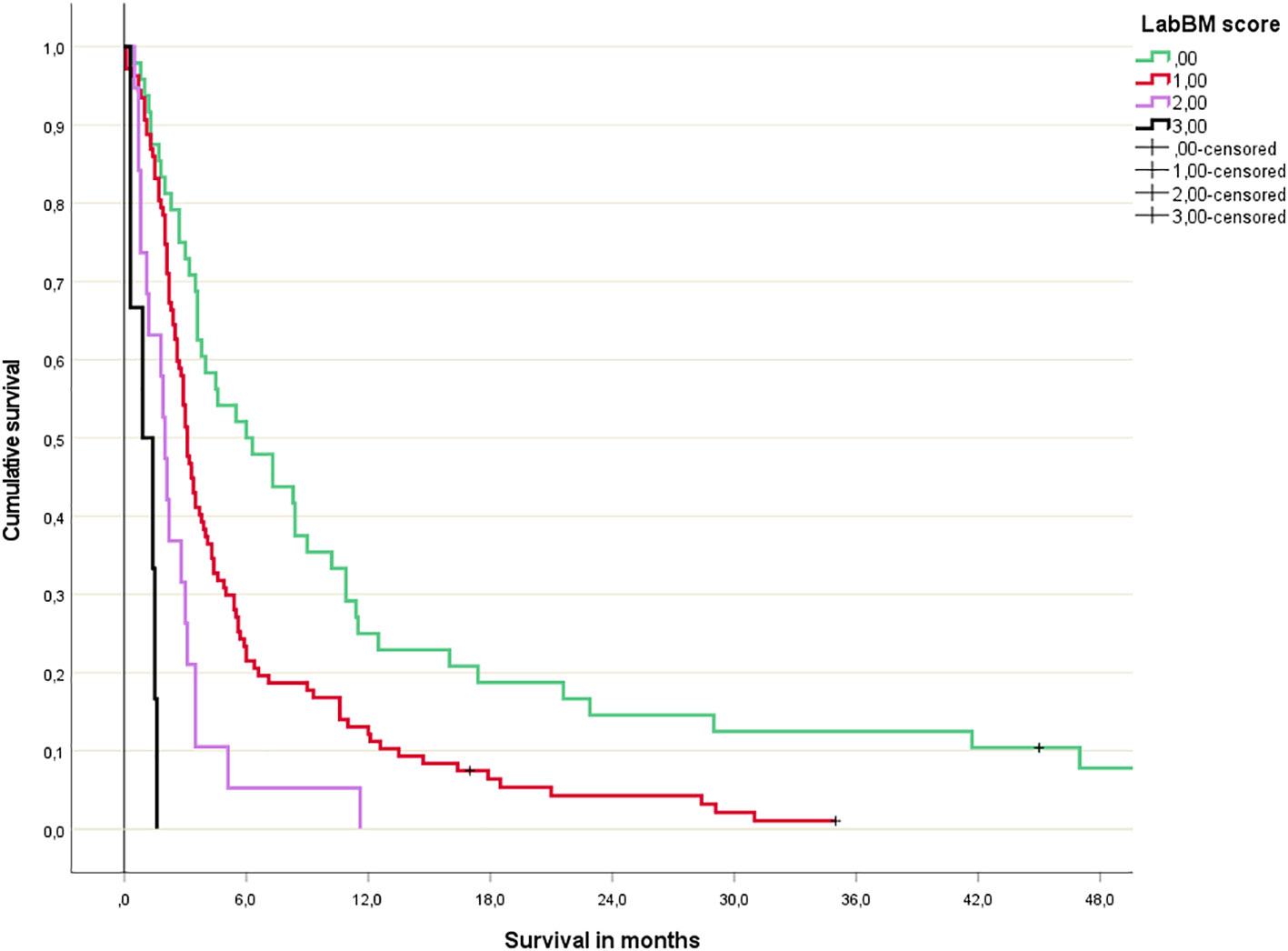
Figure 4 Modified LabBM score 4-tiered survival prediction (Kaplan-Meier analysis; for further details please see Table 3; p=0.01 or better for all comparisons).
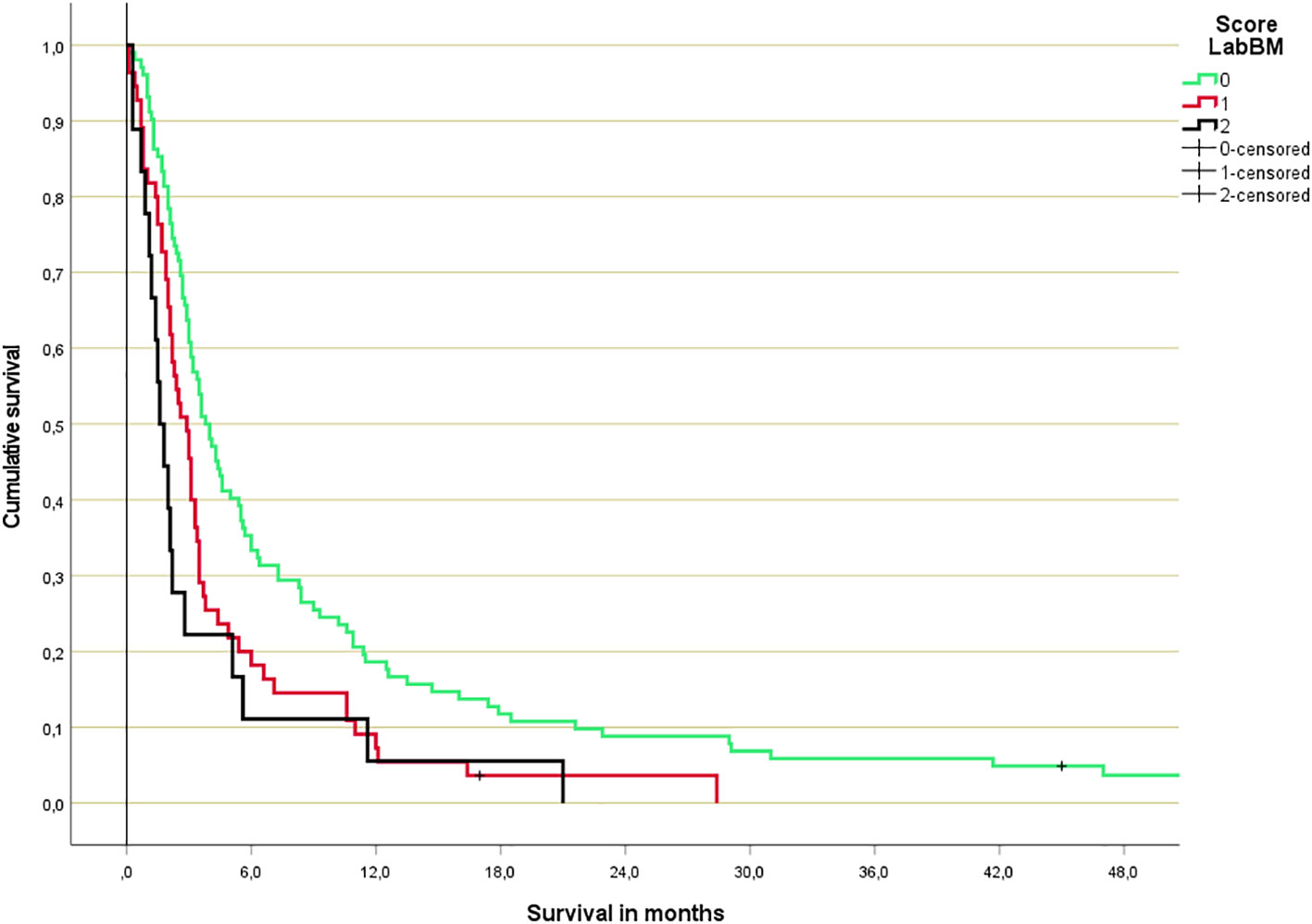
Figure 5 Original LabBM score 3-tiered survival prediction (Kaplan-Meier analysis; p<0.001 over all strata; median in months: 3.8 (0-1 points), 2.8 (1.5-2 points), 1.6 (2.5-3.5 points); n=102, 55, 18).
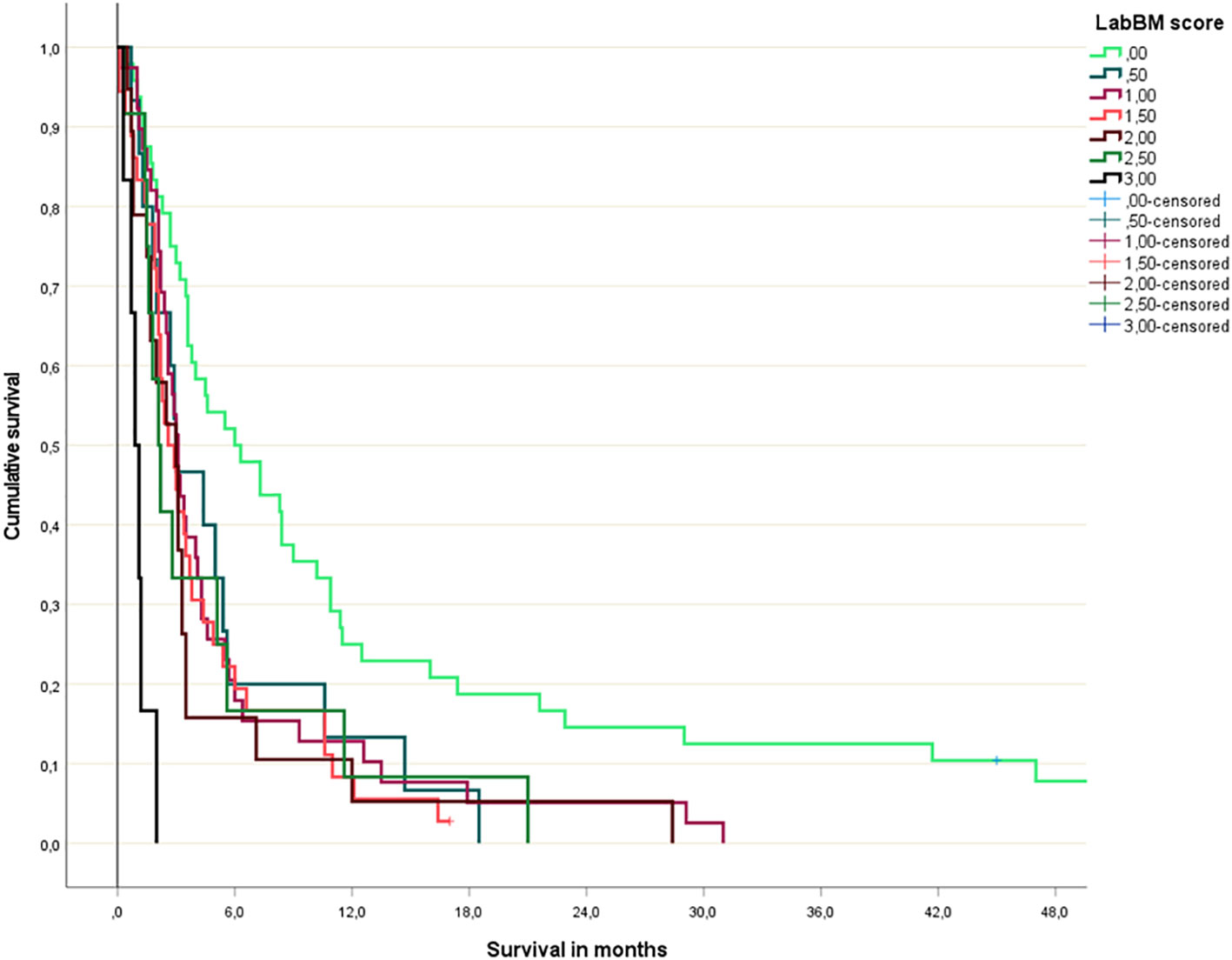
Figure 6 Original LabBM score (Kaplan-Meier analysis; p=0.04 or better for all comparisons involving patients with 0 points; p=0.003 or better for all comparisons involving patients with 3 points).
4 Discussion
The purpose of the present proof-of-principle study was to test the role of non-dichotomized blood test results in comparison to the original version of the LabBM score with dichotomized blood tests. A homogeneously treated patient population with few censored events was selected, which was characterized by poor prognostic features such as multiple brain metastases and typically also extracranial metastases (85% of patients). Due to these features, WBRT was selected as primary modality, while our institution preferred radiosurgery for patients with better prognosis.
We showed that analyses of abnormal blood tests as continuous variables, i.e. the truly observed distribution, are feasible, but not uniformly associated with a gain in prognostic information. For albumin and CRP, dichotomized values continued to represent the preferred strategy. For LDH and hemoglobin, no more than 3 strata were needed to separate the cohort in the best possible fashion. Unfortunately, detailed analyses of low platelet counts were not possible. The multivariate analysis demonstrated that changes in variable stratification, i.e. 3 strata rather than 2, led to changes of the resulting score components, e.g., reduction to 3 significant predictors of survival (LDH, hemoglobin, CRP). The modified LabBM score was both, easier to assign (albumin and platelets were no longer needed) and better able to discriminate or subclassify intermediate class patients, i.e. those not having the minimum or maximum point sum.
It is not justified to claim that the modified LabBM score represents the best possible score that can be developed with the 5 underlying blood tests, because of several limitations of this preliminary study. Small subgroups, unknown test results in some patients, and the risk of overfitting statistical models in the absence of validation strategies have to be mentioned here. However, we felt that a moderately sized study might represent a useful first step before one allocates lots of resources to a large analysis, without knowing that positive signals support this avenue of research. Large-scale analyses are also necessary to study the potential for nomogram development, based on very granular blood test differences, which eventually might provide more information than the present categories.
The study confirmed the previously reported limited survival after primary WBRT, which has resulted in increasing utilization of radiosurgery also in patients with multiple brain metastases (16–18). Due to its relatively low biologically effective radiation dose, WBRT is not expected to provide long-lasting local control of visible brain metastases larger than few millimeters. However, long-lasting control becomes increasingly important if systemic therapy improves and, thus, provides durable extracranial disease stabilization. Utilization of the LabBM score, or blood biomarkers in a broader sense, renders comprehensive radiological assessment of primary tumor control and extracranial metastases unnecessary and is a low-cost intervention. Implementation of the LabBM score in clinical routine is feasible and might improve decision making and prediction of short survival. Possible modifications of the score beyond the current strategy have already been proposed and include assessment of tumor markers such as carcinoembryonic antigen (19).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CN: collected the related data, contributed to analysis of the data, investigated the study results, and wrote the manuscript. NA: interpreted data, and reviewed the manuscript. AG: interpreted data and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Open access publication fee funded by UiT - The Arctic University of Norway.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Suh JH, Kotecha R, Chao ST, Ahluwalia MS, Sahgal A, Chang EL. Current approaches to the management of brain metastases. Nat Rev Clin Oncol (2020) 17:279–99. doi: 10.1038/s41571-019-0320-3
2. Palme JD, Trifiletti DM, Gondi V, Chan M, Minniti G, Rusthoven CG, et al. Multidisciplinary patient-centered management of brain metastases and future directions. Neurooncol Adv (2020) 2(1):vdaa034. doi: 10.1093/noajnl/vdaa034
3. Brenner AW, Patel AJ. Review of current principles of the diagnosis and management of brain metastases. Front Oncol (2022) 12:857622. doi: 10.3389/fonc.2022.857622
4. Li AY, Gaebe K, Jerzak KJ, Cheema PK, Sahgal A, Das S. Intracranial metastatic disease: Present challenges, future opportunities. Front Oncol (2022) 12:855182. doi: 10.3389/fonc.2022.855182
5. Buecker R, Hong ZY, Liu XM, Jaenke G, Lu P, Schaefer U. Risk factors to identify patients who may not benefit from whole brain irradiation for brain metastases - a single institution analysis. Radiat Oncol (2019) 14(1):41. doi: 10.1186/s13014-019-1245-9
6. Mazzola R, Corradini S, Gregucc F, Figlia V, Fiorentino A, Alongi F. Role of radiosurgery/stereotactic radiotherapy in oligometastatic disease: brain oligometastases. Front Oncol (2019) 9:206. doi: 10.3389/fonc.2019.00206
7. Lanier CM, Pearce J, Isom S, Xing F, Lo HW, Whitlow CT, et al. Long term survivors of stereotactic radiosurgery for brain metastases: do distant brain failures reach a plateau and what factors are associated with a brain metastasis velocity of zero? J Neurooncol (2022) 160(3):643–8. doi: 10.1007/s11060-022-04183-5
8. Nieder C, Hintz M, Popp I, Bilger A, Grosu AL. Long-term survival results after treatment for oligometastatic brain disease. Rep Pract Oncol Radiother (2020) 25:307–11. doi: 10.1016/j.rpor.2020.03.001
9. Nieder C, Mehta MP, Guckenberger M, Gaspar LE, Rusthoven CG, Sahgal A, et al. Assessment of extracranial metastatic disease in patients with brain metastases: how much effort is needed in the context of evolving survival prediction models? Radiother Oncol (2021) 159:17–20. doi: 10.1016/j.radonc.2021.02.038
10. Nieder C, Mehta MP. Prognostic indices for brain metastases–usefulness and challenges. Radiat Oncol (2009) 4:10. doi: 10.1186/1748-717X-4-10
11. Sperduto PW, Chao ST, Sneed PK, Luo X, Suh J, Roberge D, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys (2010) 77:655–61. doi: 10.1016/j.ijrobp.2009.08.025
12. Sperduto PW, Mesko S, Li J, Cagney D, Aizer A, Lin NU, et al. Survival in patients with brain metastases: summary report on the updated diagnosis-specific graded prognostic assessment and definition of the eligibility quotient. J Clin Oncol (2020) 38:3773–84. doi: 10.1200/JCO.20.01255
13. Berghoff AS, Wolpert F, Holland-Letz T, Koller R, Widhalm G, Gatterbauer B, et al. Combining standard clinical blood values for improving survival prediction in patients with newly diagnosed brain metastases-development and validation of the LabBM score. Neuro Oncol (2017) 19:1255–62. doi: 10.1093/neuonc/now290
14. Nieder C, Dalhaug A, Pawinski A. External validation of the LabBM score in patients with brain metastases. J Clin Med Res (2019) 11:321–25. doi: 10.14740/jocmr3746
15. Nieder C, Dalhaug A, Haukland E. The LabBM score is an excellent survival prediction tool in patients undergoing palliative radiotherapy. Rep Pract Oncol Radiother (2021) 26:740–6. doi: 10.5603/RPOR.a2021.0096
16. Rades D, Dziggel L, Nagy V, Segedin B, Lohynska R, Veninga T, et al. A new survival score for patients with brain metastases who received whole-brain radiotherapy (WBRT) alone. Radiother Oncol (2013) 108:123–7. doi: 10.1016/j.radonc.2013.06.009
17. Gondi V, Bauman G, Bradfield L, Burri SH, Cabrera AR, Cunningham DA, et al. Radiation therapy for brain metastases: An ASTRO clinical practice guideline. Pract Radiat Oncol (2022) 12:265–82. doi: 10.1016/j.prro.2022.02.003
18. Garsa A, Jang JK, Baxi S, Chen C, Akinniranye O, Hall O, et al. Radiation therapy for brain metastases: A systematic review. Pract Radiat Oncol (2021) 11:354–65. doi: 10.1016/j.prro.2021.04.002
Keywords: brain metastases, prognostic model, score, biomarkers, tumor markers
Citation: Nieder C, Andratschke NH and Grosu AL (2023) Personalized radiotherapy of brain metastases: survival prediction by means of dichotomized or differentiated blood test results? Front. Oncol. 13:1156161. doi: 10.3389/fonc.2023.1156161
Received: 01 February 2023; Accepted: 29 March 2023;
Published: 11 April 2023.
Edited by:
Chunzhi Zhang, Tianjin Hospital, ChinaReviewed by:
John E. Mignano, Tufts University, United StatesDavid Kaul, Charité University Medicine Berlin, Germany
Copyright © 2023 Nieder, Andratschke and Grosu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carsten Nieder, Y2Fyc3Rlbi5uaWVkZXJAbmxzaC5ubw==
 Carsten Nieder
Carsten Nieder Nicolaus H. Andratschke
Nicolaus H. Andratschke Anca L. Grosu
Anca L. Grosu