- Neurosurgical Clinic, Department of Neurosurgery and Neurotechnology, Eberhard Karls University, Tuebingen, Germany
Objective: Observation, radiotherapy and surgery are treatment options in vestibular schwannomas (VS). Decision making differs between centers and is usually based on tumor characteristics (e.g., size) and the expected physical health (PH) outcome (i.e., hearing and facial function). However, mental health (MH) is often under-reported. The objective of the present study was to ascertain the impact of VS treatment on PH and MH.
Methods: PH and MH were assessed in a prospective cross-sectional study including 226 patients with unilateral sporadic VS before and after surgical removal (SURG). Quality-of-life (QoL) was estimated by self-rating questionnaires: general Short-Form Health Survey (SF-36), Penn Acoustic Neuroma Quality-of-Life Scale (PANQOL), Dizziness Handicap Inventory (DHI), Hearing Handicap Inventory (HHI), Tinnitus Handicap Inventory (THI), and Facial Disability Index (FDI). QoL changes over time as well as predictive factors were accessed by multivariate analyses of covariance (MANCOVA).
Results: In total, 173 preoperative and 80 postoperative questionnaires were analyzed. There was a significant PH deterioration related to facial function (FDI, PANQOL-face) after surgery. In line with facial rehabilitation, however, FDI improved within the first five years after surgery and did not differ compared to the preoperative patient cohort, eventually. In contrast, MH (i.e., PANQOL-anxiety) and general health (i.e., PANQOL-GH) improved with surgery and correlated with the extent-of-resection.
Conclusion: Physical and mental health is significantly influenced by VS surgery. While PH might decrease after surgery, MH potentially increases when patient is cured. Practitioners should take MH into account before advising an incompletely VS treatment (e.g., subtotal resection, observation or radiosurgery).
Introduction
Vestibular schwannomas (VS) are characterized by a progressive loss of cranial nerve (CN) functions (e.g., hearing, balance), affecting patient’s quality of life (QoL) (1–3). Total surgical removal of the tumor is usually providing a definite cure (4, 5). Concurrently, VS surgery implies an increased risk of additional harm to the CN (e.g., facial palsy) (5–10). Observation or radiosurgery are further treatment strategies and in recent years complete VS resection has been discouraged in large VS (11). Instead, current guidelines recommend partial resection (PR) with subsequent radiotherapy in these cases (11). The underlying rationale for this recommendation is to preserve CN function and QoL assuming a linear relationship between them. In fact, several studies report a deterioration of QoL by VS surgery (12–14) relating to hearing, vestibular and facial function (6–8). Radiosurgery or observation has been suggested to affect CN function and QoL to a lesser extent. However, there is increasing evidence that neither radiosurgery nor observation can preserve CN function (in particular hearing) on a long-term (2, 3, 15, 16). Furthermore, some symptoms might be accentuated in comparison to microsurgery (15, 16). Recent studies do not detect any QoL differences when comparing patients following different treatment strategies (14, 17, 18). However, most studies mainly relate to physical health (PH) aspects of QoL. Mental health (MH) referring to the emotional and psychological well-being is often under-reported (17, 19).
An important feature of treatment strategies avoiding a complete VS resection is that it turns a potentially curable disease into a chronic disease with a higher risk of recurrence. It is well known that chronic diseases (e.g., Parkinson’s, cancer) affect patient’s MH independent of their PH (20, 21). In line, two recent studies demonstrated that a gross total resection (GTR) in VS is associated with a better MH compared to partial VS resection (PR) (19). It has been hypothesized that microsurgery may confer an advantage with regard to patient’s MH, relating to the psychological benefit of “cure” from tumor removal (17). In general, however, QoL data in VS treatment differentiating between PH and MH is scarce (14, 17, 18, 22).
The present study aims to investigate the physical and mental health-related QoL in patients with non-treated (before surgery), incompletely (subtotal resection, STR) and completely treated VS (GTR).
Methods
Patient characteristics
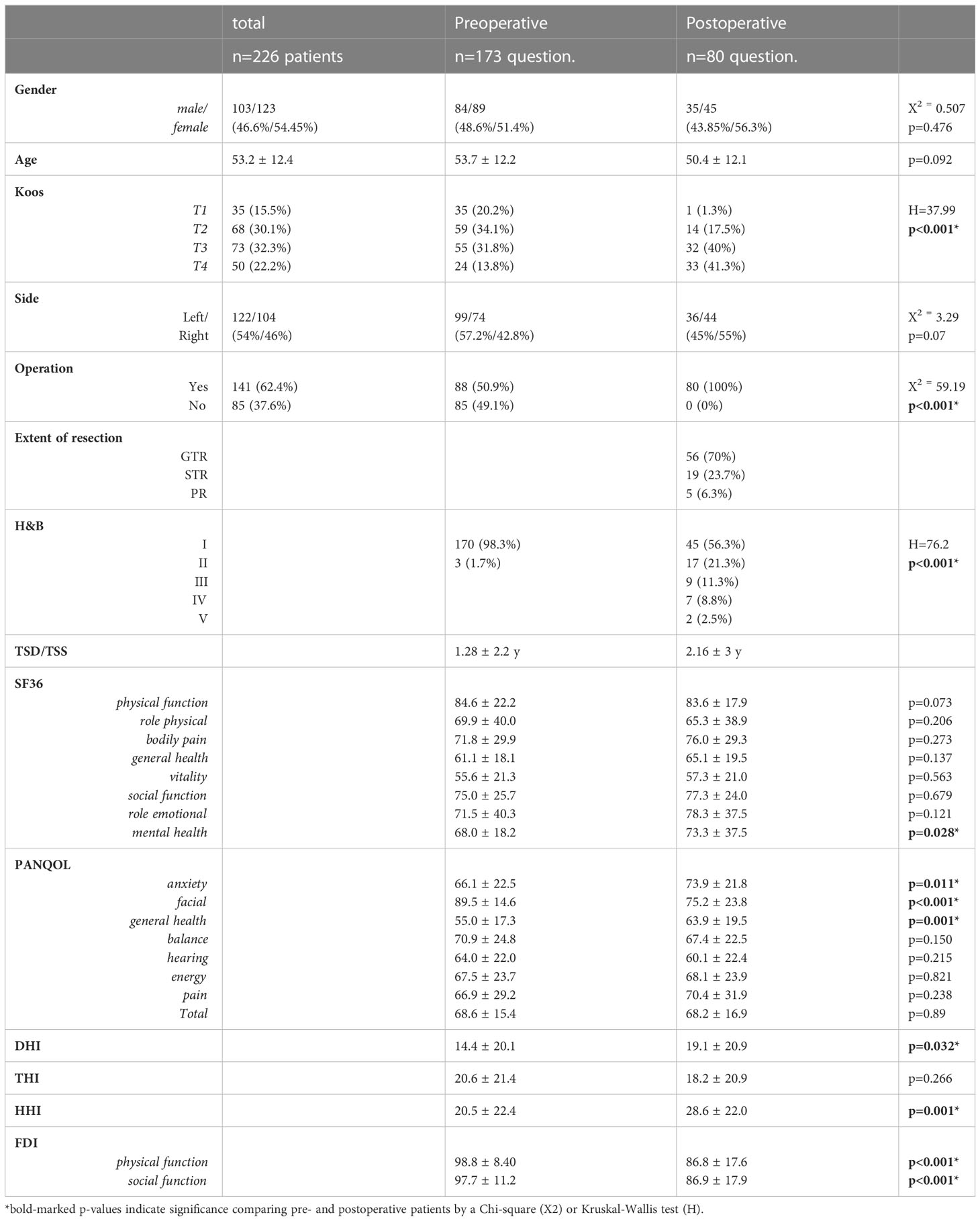
This prospective cross-sectional study included 226 patients (Table 1) with an unilateral VS who answered standardized questionnaires on QoL during their treatment at our Neurosurgical Department between 11/2019 and 09/2021. A total of 141/226 (62.5%) underwent surgical resection of the VS via a retrosigmoidal approach in a semi-sitting or supine position in that period (Figure 1). Patients with neurofibromatosis, previous VS surgery and incomplete questionnaires were excluded. The study was approved by the local Hospital Ethics Committee and conducted in accordance with the declaration of Helsinki.
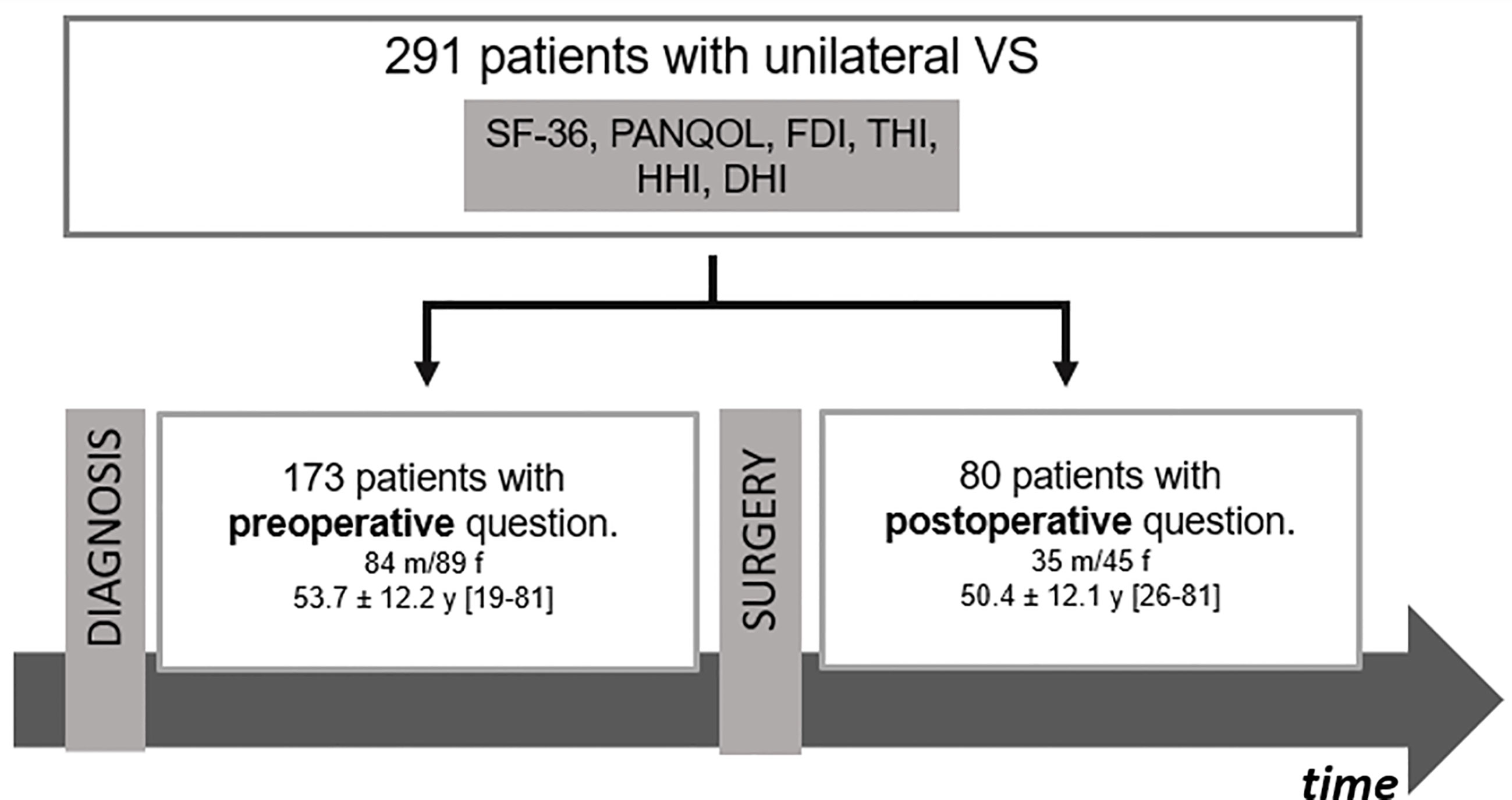
Figure 1 Flow chart of patients’ cohort. DHI, Dizziness Handicap Inventory; FDI, Facial Disability Index; HHI, Hearing Handicap Inventory; PANQOL, Penn Acoustic Neuroma Quality-of-Life Scale; SF-36: Short-Form Health Survey 36.
Quality of life questionnaires
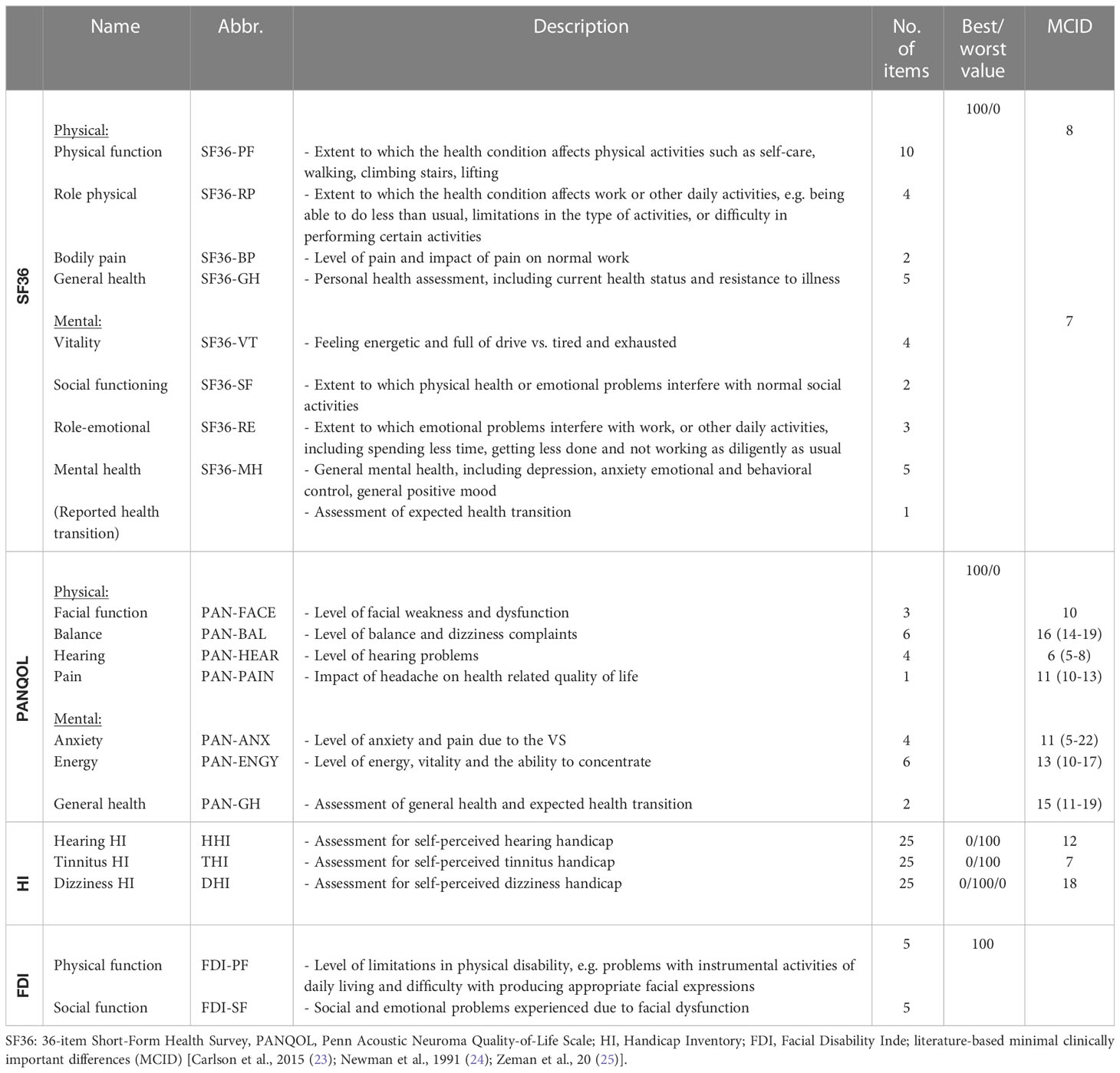
Several QoL questionnaires were completed during the treatment period: the general Short-Form Health Survey (SF-36), Penn Acoustic Neuroma Quality-of-Life Scale (PANQOL), Dizziness Handicap Inventory (DHI), Hearing Handicap Inventory (HHI), Tinnitus Handicap Inventory (THI), and Facial Disability Index (FDI) (Table 2) (23–25).
The SF-36 is the most common health-related QoL questionnaire. Its 36 items can be divided into physical and mental classes with 4 domains each: physical function (SF36-PF), role-physical (SF36-RP), bodily pain (SF36-BP), general health (SF36-GH), vitality (SF36-VT), social functioning (SF36-SF), role-emotional (SF36-RE) and mental health (SF36-MH). Each domain is scored from 0-100, with a higher score corresponding to a better QoL.
The PANQOL is a disease-specific questionnaire containing 26 questions which are divided into the domains anxiety (PAN-ANX), facial function (PAN-FACE), general health (PAN-GH), balance (PAN-BAL), hearing (PAN-HEAR), energy (PAN-ENGY) and pain (PAN-PAIN). A total score (PAN-TTL) is calculated from the individual scores. The response options are classified on a Likert scale from strong disagreement (1) to strong agreement (5), whereby the values are normalized to a scale of 0-100 points to determine the domain scores. A score of 100 corresponds to the best possible QoL, a score of 0 to the lowest QoL.
The HHI, THI and DHI are symptom-specific questionnaires for dizziness, hearing function and tinnitus. Each questionnaire contains 25 self-assessment items, which can be answered by yes (2), sometimes (1) or no (0). Item scores result in a total score of 0-100, whereby a higher score corresponds to greater impairment by the symptom. The FDI were administered to all patients with facial paresis. The FDI contains 10 questions, which are divided into the domains physical function (-25=worst to 100=best function; FDI-PF) and social function (0=worst to 100=best function; FDI-SF).
Disease-specific data
In addition, we analyzed numerous disease specific information. Magnetic resonance images (MRI) were retrospectively analyzed to determine the tumor size according to Koos classification (1: purely intrameatal, 2: intra- and extrameatal, 3: filling the cerebellopontine cistern, 4: compressing or shifting the brainstem) (26) and tumor side. The extent of resection (EOR) after surgery (GTR: complete resection; STR: minimal residual tumor on the facial nerve or exclusively in the internal auditory canal; PR: great tumor volume) was determined by MRI and the surgical record. Medical records of patients were reviewed to define the time between diagnosis and QoL survey (time since diagnosis, TSD; preoperatively), time between preoperative QoL survey and surgery (time before surgery, TBS; preoperatively; only patients who answered the preoperative survey and underwent VS resection at a later time during the evaluation period), time between surgery and postoperative survey (time since surgery, TSS; postoperatively) as well as the facial function according to the House-Brackmann scale (H&B) (27). The H&B classifies overall facial function into ranges from 1 (normal) to 6 (total paralysis) based on the assessment of e.g. eye closure and mouth movement.
Statistics
Statistical tests were performed using SPSS (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.). Group differences in distribution of clinical characteristics (e.g., EOR) were determined by Chi-squared or Kruskal-Wallis tests. In a first step, (multivariate) analyses of covariance ((M)ANCOVAs) were performed to evaluate the effects of surgery (SURG) and gender (SEX) on QoL scores. Secondary, a (M)ANCOVA-based evaluation of the impact of TSD, TSS and EOR on QoL was performed. In order to ensure that results were not influenced by assumption violations, data were checked for outliers, homogeneity of variance–covariance matrices (Box’s M test) and homogeneity of variances (Levene’s test). In this context, a MANCOVA is a two-step process. In the first step, the overall hypothesis is tested, i.e. whether there is a difference between different groups. If this test is significant, in the second step the MANCOVA was followed by post-hoc tests (i.e. univariate ANOVAs) to explain the group differences. Furthermore, we performed a secondary subcohort analysis of patients who completed the questionnaires in both the pre- and postoperative period. To estimate differences in QoL scores before and after surgery we performed a repeated measures ANOVA. Statistical significance was considered at p < 0.05 for all statistical tests.
Results
Patient cohort
A total of 226 patients (53.2 ± 12.4 years; 123 female) completed all QoL questionnaires. 103/226 (45.6%) of the VS corresponded to a tumor size Koos 1/2 and 123/226 (54.4%) to a grade 3/4 (Table 1). VS were resected in 141/226 (62.4%) of the patients, while 85/226 (37.6%) had not undergone surgery at the time of evaluation (Figure 1). In summary, 27/226 (11.9%) patients completed questionnaires pre- and postoperatively (Supplementary Table 1), whereas 146/226 (64.6%) and 53/226 (23.5%) were surveyed only pre- or postoperatively, respectively.
Common health-related QoL: SF36
A MANCOVA was applied to SF36 subdomains in order to determine the effect of surgery (SURG) on QoL while controlling for SEX, AGE and tumor size (SIZE) (Figure 2A). Neither SURG (F(8,240)=1.09, p=0.374) nor SEX (F(8,240)=1.37, p=0.21) had a significance effect on QoL. In contrast, MANOVA depicted a significant effect of AGE on SF36 (F(8,240)=3.72, p<0.001). Follow-up ANOVAs confirmed a significant impact of AGE on PH as depicted by SF36-PF (F(1,247)=12.74, p<0.001) and SF36-GH (F(1,247)=5.50, p=0.020). Independent of the other covariates, PH items (SF36-PF and SF36-GH) decreased with age (r =-0.2, p=0.001 and r=-0.15, p=0.016).
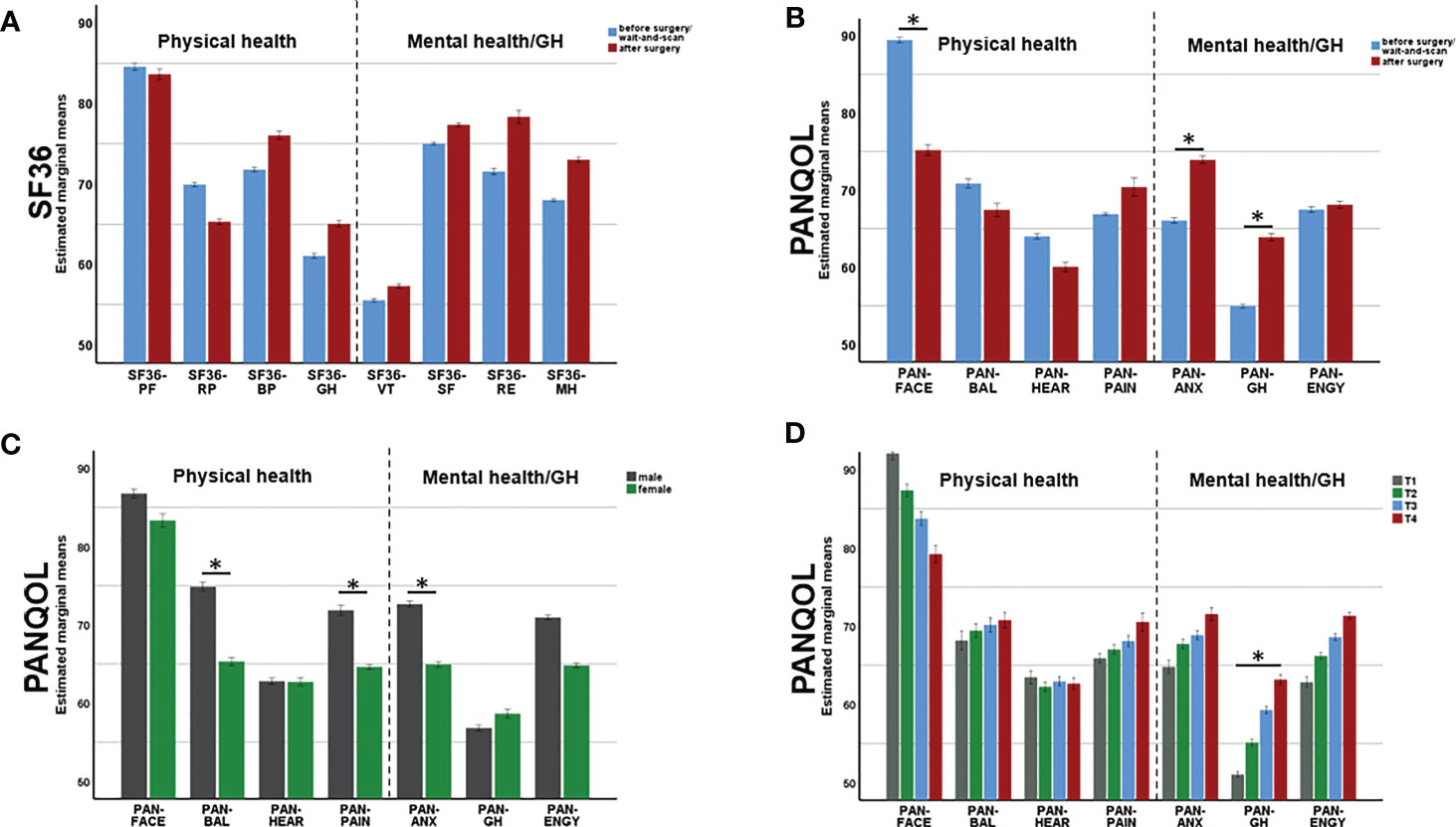
Figure 2 Changes of physical (PH) mental health (MH) after surgery. While SF36 (A) did not depict any surgery-related changes in QoL, PANQOL (B) showed a decline of PH (related to the facial function, PAN-FACE) after surgery. At the same time, surgery improved MH related to anxiety (PAN-ANX) and general health (PAN-GH). Multivariate analysis also depicted an effect of gender (C) and tumor size (D) on QoL. Bars in (C, D) demonstrate data from both, pre- and postoperatively. PANQOL, Penn Acoustic Neuroma Quality-of-Life Scale; PAN-ANX, PANQOL anxiety; PAN-ENGY, PANQOL energy; PANQOL-GH, PANQOL general health; PAN-FACE, PANQOL facial; PAN-BAL, PANQOL balance; PAN-HEAR, PANQOL hearing; PAN-PAIN, PANQOL pain; SF36-PF: SF36 physical function; SF36-RP: SF36 role physical; SF36-BP: SF36 bodily pain; SF36-GH: SF36 general health; SF36-VT: SF36 vitality; SF36-SF: SF36 social functioning; SF36-RE: SF36 role emotional; SF36-MH: SF36 mental health. Significance is indicated by an asterisk (*; p<0.05, MANOVA).
Disease-specific QoL: PANQOL
A MANCOVA was performed to estimate the effect of SURG on PANQOL subdomains while controlling for SEX, AGE and SIZE. There was a significant multivariate main effect of SURG (F(7,241)=7.75, p<0.001; Figure 2B). SURG improved mental and general health in the subdomains PAN-GH (63.9 ± 19.5 vs 55.0 ± 17.3; F(1,247)=4.86, p=0.028) and PAN-ANX (73.9 ± 21.8 vs 66.1 ± 22.5; F(1,247)=5.68, p=0.018). In contrast, PH relating to facial function (PAN-FACE) decreased postoperatively on a group level (75.2 ± 23.8 and 89.5 ± 14.6; F(1,247)=27.35, p<0.001) (Figure 3A).
MANCOVA also proved a significant main effect of SEX on PANQOL (F(7,241)=3.49, p=0.001; Figure 2C). Females had significant worse PAN-ANX (64.9 ± 22.0 vs 72.6 ± 22.5; F(1,247)=7.44, p=0.007), PAN-BAL (65.3 ± 24.8 vs 74.9 ± 22.4; F(1,247)=9.47, p=0.002) and PAN-PAIN (64.6 ± 31.3 vs 71.9 ± 28.1; F(1, 247)=7.56, p=0.006) scores independent of the actual VS treatment. Furthermore, both covariates AGE (F(7,241)=5.93, p<0.001) and SIZE (F(7,241)=2.13, p=0.04) had significant impact on PANQOL. Increasing AGE had a negative effect on facial function (PAN-FACE; r=-0.18, p=0.005; Spearman’s), balance (PAN-BAL; r= -0.15, p=0.001; Spearman’s) and hearing (PAN-HEAR; r=-0.15, p=0.016; Spearman’s). PAN-GH was significantly impacted by the SIZE (F(1,247)=5.47, p = 0.02) with better values in Koos 3/4 compared to Koos 1/2 tumors (H=14.75, p=0.001; Kruskal-Wallis) (Figure 2D).
Symptom-specific QoL: HHI, THI, DHI and FDI
In the MANCOVA, there was a significant effect of SURG (F(3,245)=4.96, p=0.002), SEX (F(3,245)=7.55, p<0.001) and AGE (F(3,245)=3.94, p=0.009) on the handicap inventories (i.e., DHI, HHI and THI). In the follow-up ANOVAs, both SURG (20.5 ± 22.4 vs 28.6 ± 22.0; F(1,247)=7.55, p=0.006) and AGE (F(1,247) =3.89, p=0.05) had negative impact on hearing perception (HHI). In contrast, females suffered from dizziness (DHI) more frequently than males regardless of VS treatment (20.4 ± 22.4 and 10.8 ± 16.6; F(1,247)=13.47, p<0.001). Tinnitus perception (THI) was unaffected by SURG, SEX or AGE in the present cohort.
Both FDI subscores representing the physical (FDI-PH) and social handicap (FDI-SH) of a facial palsy were negatively affected by SURG (F(1,247)=36.9, p<0.001 and F(1,247)=23.25, p<0.001). The covariate SIZE had impact only on FDI-PH (F(1, 247) = 4.51, p=0.035). FDI-PF and FDI-SF correlated significantly with H&B (r=-0.88, p<0.001 and r=-0.85, p<0.001; Spearman’s.
Impact of time since diagnosis, time before/since surgery as and extent of resection on patients’ QoL
In order to evaluate the impact of timing of the survey after VS diagnosis and before surgery on mental health, the association between PAN-GH and PAN-ANX as well as TSD and TBS was analyzed. Among the 173 preoperative questionnaires, there was no significant correlation between TSD and PAN-ANX or PAN-GH. Furthermore, in the 88/173 patients who completed a questionnaire in the observation phase and underwent surgery later during the evaluation period, no correlation between TBS and mental health was found either (PAN-ANX: r=0.021, p=0.843; PAN-GH: r=-0.18, p= 0.093; Spearman’s).
As the functional status after surgery is constantly changing due to rehabilitation mechanisms, we ought to evaluate health-related QoL depending on the TSS. In fact, for both FDI-PF (H=56.65, p<0.001; Kruskal Wallis) and FDI-SF(H=53.93, p<0.001; Kruskal Wallis) there was a significant decline of QoL directly after surgery which improved during the postoperative course in line with facial rehabilitation (Figure 3B). After a TSS of approx. 5 years, there was no significant difference in neither FDI-PF (H=36.16, p=0.122; Kruskal Wallis) nor FDI-SF (H=33.17, p=0.240; Kruskal Wallis) when compared to the preoperative situation (Figure 3B). There was no comparable effect for the disability inventories THI, HHI, DHI or PAN-ANX and PAN-GH (Figures 3C, D). In contrast, postoperative mental and general health parameters (PAN-ANX and PAN-GH) were associated with the EOR. Kruskal-Wallis test revealed a significant better PAN-ANX (H=6.81, p=0.033) and PAN-GH (H=10.63, p=0.005) in GTR and STR in comparison to PR (Figures 4A, B).
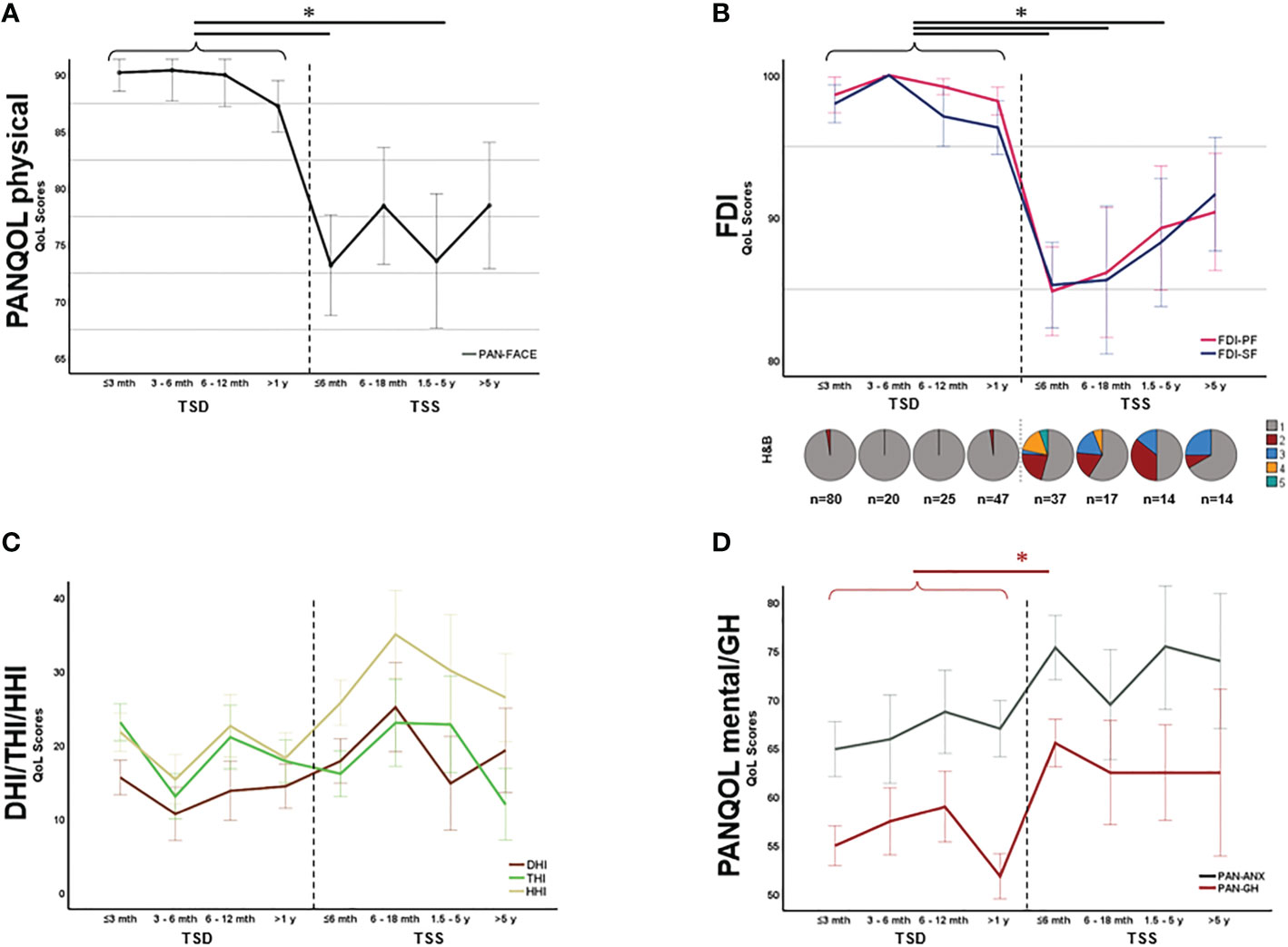
Figure 3 Symptom-specific quality of life (QoL) over time. PAN-FACE (A) and FDI scores (B) improved after surgery in line with the facial rehabilitation (see distribution of H&B scores in the inlay). After 5 years, PAN-FACE and FDI differed significant in comparison to the preoperative patient cohort. In contrast, neither DHI, THI and HHI (C) nor PAN-ANX/PAN-GH (D) changed during follow-up. The numbers under the pie charts in (B) indicate the total number of patients in each time period. DHI, dizziness handicap inventory; THI, tinnitus handicap inventory; HHI, hearing handicap inventory; FDI-PF, Facial disability index - physical function; FDI-SF, Facial disability index – social function; TSD, time-since-diagnosis; TSS, time-since-surgery. Significance is highlighted by an asterisk (*; p<0.05, Dunn’s test, corrected).

Figure 4 Relationship between mental health and extent-of-resection (EOR). Both PAN-ANX (A) and PAN-GH (B) correlated with EOR. Patients with significant residual tumor (partial resection, PR) claimed higher level of anxiety and a reduced level of general health in comparison to patients undergoing a gross total resection (GTR) or subtotal resection (STR). Significance is highlighted by an asterisk (*; p<0.05, Dunn’s test, corrected).
Discussion
The present study evaluated main determinants of physical and mental health in patients with VS. While PH and MH did not change after diagnosis, deterioration of PH was detected postoperatively - mainly caused by an occurrence of facial nerve palsy and the deterioration of hearing function. However, PH related to facial function improved within the first years after surgery. Furthermore, mental and general health improved postoperatively and correlated with the EOR. The decision on therapy is therefore a consideration between MH and PH and must be made on a patient-specific basis.
Current guidelines for VS advise observation or radiotherapy and discourage complete VS resection to preserve CN function (11). The present study documents a significant postoperatively deterioration of facial and hearing QoL scores with a similar or even less pronounced extent compared to previous studies (13, 28). The retrosigmoid approach in this context may have resulted in less hearing loss compared to studies applying a translabyrinthine approach. However, physical limitations should not only be compared pre- and postoperatively, but also functional recovery after surgery should be considered when deciding on treatment. Our findings elicit an improvement of FDI-PF and FDI-SF over time after microsurgery. Nevertheless, our study could not detect a significant effect of TSS alone on facial function. This could be attributed to a data bias, since patients without physical complaints usually no longer present themselves in our outpatient clinic after approx. 3 years. Thus, an overrepresentation of patients with impairing facial palsy must be assumed in our postoperative cohort. In fact, previous studies show heterogenous results regarding longitudinal facial palsy-specific QoL (29, 30). Further longitudinal studies are necessary to assess the frequency, course of recovery and subjective limitation of facial palsies after VS resection.
Tinnitus and dizziness are symptoms often associated with VS. Nevertheless, they are often underrated when deciding on the treatment strategy. Tinnitus and vertigo, however, can significantly worsen QoL in VS patients (23, 31). Thus, we determined tinnitus-related QoL by the THI. Tinnitus-related discomfort tended to improve slightly although previous reported “minimal clinically important difference” (MCID) could not be reached (25). This is concordant with previous studies demonstrating postoperative improvement in patients with preoperative tinnitus, while patients without preoperative tinnitus can develop a new-onset tinnitus after surgery in ~20% (32–34). Consequently, patients with preoperative severe tinnitus could be offered microsurgical resection of the VS, as radiotherapy may worsen tinnitus-related discomfort (35). The results of studies investigating pre- and postoperative dizziness in VS are ambiguous (36). Our study could not reveal pre- and postoperative differences of DHI and PAN-BAL. Instead, more dizziness was associated with female gender and higher age.
The relevance of MH on overall health is often underestimated in the treatment of benign tumors. While there are numerous studies on MH in meningiomas (37–39), data on MH in VS are scarce. The present study could not demonstrate an effect of surgery on mental or physical SF-36 scores, confirming the assumption about low predictability of QoL in VS by the SF-36 (40, 41). However, despite the deterioration in PH the PANQOL findings demonstrated a significant increase of mental and general health post-surgically. In contrast, during the preoperative observational phase there was a deterioration of mental scores over time. This suggests that patients experience relief from treatment, whereas knowledge of the presence of a VS without treatment leads to a state of anxiety. These result supports the hypothesis of Carlson et al. which suggests that microsurgery may improve patient’s MH when the tumor is “cured” after complete surgical removal (17). While previous studies could demonstrate a reduced QoL of VS patients in comparison to age and sex matched normative data already before surgery, conversely, they could not prove a significant difference of MH between observational, microsurgery and radiotherapy groups (14, 17, 18, 28, 42–44). However, factors affecting the results (e.g., EOR, gender) are not taken into account in these studies. While multivariate statistics could not demonstrate an effect of EOR on MH scores, univariate analysis demonstrated a significantly worse PAN-GH in partial resections compared to STR and GTR. This is concordant with studies comparing GTR with incomplete resection or combined radio- and microsurgery (19, 28). Since other studies furthermore demonstrated a significant regrowth rate with a tumor residue of >0.7 cm3 and a higher MIB-1 index (45, 46), general recommendation for PR in large VS should be avoided. Instead, multicenter studies, that prospectively assign patients to different intention-to-treat groups (i.e. intended GTR, intended PR), are necessary.
Limitations
The present study is limited due to the lack of comparison with radiosurgery or other surgical procedures (e.g., translabyrinthine surgery), a non-tumor cohort and the absence of longitudinality. The small number of postoperative controls can lead to a selection bias of the health status, since at long-term, patients with persistent complaints continue to present themselves in the consultation, while patients with good health no longer present themselves.
Conclusion
In VS patients, the trading of MH and PH is essential for treatment decision making. While mental health in particular is impaired preoperatively, patients are impaired postoperatively, especially due to physical problems related to cranial nerve dysfunction. Attending physicians should take this into account during treatment decision making.
Data availability statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics committee of the medical faculty of Eberhard Karls University Tübingen and conducted in accordance with the declaration of Helsinki.
Author contributions
Study conception and design: KM, GN. Data acquisition: KM, LL, SW. Data analysis: GN, KM. Data interpretation: KM, SW, MT, GN. Statistical analysis: KM, GN. writing of the first draft: KM, GN. Review of the final manuscript: KM, MT, GN. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1152833/full#supplementary-material
Supplementary Table 1 | QoL values of patients who completed the questionnaires both in the pre- and postoperative period. *p-values indicate significance pre-and postoperatively by a repeated measures ANOVA.
Abbreviations
CN, cranial nerve; DHI, Dizziness Handicap Inventory; FDI, Facial Disability Index; GH, general health; GTR, gross total resection; HHI, Hearing Handicap Inventory; MCID, minimal clinically important difference; MH, mental health; PANQOL, Penn Acoustic Neuroma Quality-of-Life Scale; PH, physical health; TBS, time before surgery; TSD, time since diagnosis; TSS, time since surgery, PR, partial resection; QoL, quality of life; STR, sub-total resection; THI, Tinnitus Handicap Inventory; VS, vestibular schwannoma.
References
1. Nilsen KS, Lund-Johansen M, Nordahl SHG, Finnkirk M, Goplen FK. Long-term effects of conservative management of vestibular schwannoma on dizziness, balance, and caloric function. Otolaryngol - Head Neck Surg (United States) (2019) 161:846–51. doi: 10.1177/0194599819860831
2. Sughrue ME, Yang I, Aranda D, Lobo K, Pitts LH, Cheung SW, et al. The natural history of untreated sporadic vestibular schwannomas: a comprehensive review of hearing outcomes - clinical article. J Neurosurg (2010) 112:163–7. doi: 10.3171/2009.4.JNS08895
3. Sughrue ME, Kane AJ, Kaur R, Barry JJ, Rutkowski MJ, Pitts LH, et al. A prospective study of hearing preservation in untreated vestibular schwannomas: clinical article. J Neurosurg (2011) 114:381–5. doi: 10.3171/2010.4.JNS091962
4. Nakatomi H, Jacob JT, Carlson ML, Tanaka S, Tanaka M, Saito N, et al. Long-term risk of recurrence and regrowth after gross-total and subtotal resection of sporadic vestibular schwannoma. J Neurosurg (2020) 133:1052–8. doi: 10.3171/2016.11.JNS16498
5. Tatagiba M, Ebner FH, Nakamura T, Naros G. Evolution in surgical treatment of vestibular schwannomas. Curr Otorhinolaryngol Rep (2021) 9:467–76. doi: 10.1007/s40136-021-00366-2
6. Falcioni M, Fois P, Taibah A, Sanna M. Facial nerve function after vestibular schwannoma surgery: clinical article. J Neurosurg (2011) 115:820–6. doi: 10.3171/2011.5.JNS101597
7. Matthies C, Samii M. Management of 1000 vestibular schwannomas (acoustic neuromas): clinical presentation. Neurosurgery (1997) 40:1–9. doi: 10.1097/00006123-199701000-00001
8. Ebner FH, Tatagiba M. Update on diagnostics and microsurgical treatment of vestibular schwannoma. Nervenarzt (2019) 90:578–86. doi: 10.1007/s00115-019-0721-7
9. Acioly MA, Gharabaghi A, Liebsch M, Carvalho CH, Aguiar PH, Tatagiba M. Quantitative parameters of facial motor evoked potential during vestibular schwannoma surgery predict postoperative facial nerve function. Acta Neurochir (Wien) (2011) 153:1169–79. doi: 10.1007/s00701-011-0995-4
10. Rizk AR, Adam A, Gugel I, Schittenhelm J, Tatagiba M, Ebner FH. Implications of vestibular schwannoma consistency: analysis of 140 cases regarding radiologic and clinical features. World Neurosurg (2017) 99:159–63. doi: 10.1016/j.wneu.2016.11.082
11. Goldbrunner R, Weller M, Regis J, Lund-Johansen M, Stavrinou P, Reuss D, et al. Eano guideline on the diagnosis and treatment of vestibular schwannoma. Neuro Oncol (2020) 22:31–45. doi: 10.1093/neuonc/noz153
12. Soulier G, van Leeuwen BM, Putter H, Jansen JC, Malessy MJA, van Benthem PPG, et al. Quality of life in 807 patients with vestibular schwannoma: comparing treatment modalities. Otolaryngol - Head Neck Surg (United States) (2017) 157:92–8. doi: 10.1177/0194599817695800
13. Pruijn IMJ, Kievit W, Hentschel MA, Mulder JJS, Kunst HPM. What determines quality of life in patients with vestibular schwannoma? Clin Otolaryngol (2021) 46:412–20. doi: 10.1111/coa.13691
14. Miller LE, Brant JA, Naples JG, Bigelow DC, Lee JYK, Ruckenstein MJ. Quality of life in vestibular schwannoma patients: a longitudinal study. Otol Neurotol (2020) 41:e256–61. doi: 10.1097/MAO.0000000000002445
15. Murphy ES, Barnett GH, Vogelbaum MA, Neyman G, Stevens GHJ, Cohen BH, et al. Long-term outcomes of gamma knife radiosurgery in patients with vestibular schwannomas: clinical article. J Neurosurg (2011) 114:432–40. doi: 10.3171/2009.12.JNS091339
16. Boari N, Bailo M, Gagliardi F, Franzin A, Gemma M, del Vecchio A, et al. Gamma knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg (2014) 121:123–42. doi: 10.3171/2014.8.GKS141506
17. Carlson ML, Barnes JH, Nassiri A, Patel NS, Tombers NM, Lohse CM, et al. Prospective study of disease-specific quality-of-Life in sporadic vestibular schwannoma comparing observation, radiosurgery, and microsurgery. Otol Neurotol (2021) 42:e199–208. doi: 10.1097/MAO.0000000000002863
18. Neve OM, Jansen JC, Koot RW, de RM, Paul G. van Benthem P, AM S, et al. Long-term quality of life of vestibular schwannoma patients: a longitudinal analysis. Otolaryngol - Head Neck Surg (United States) (2022) 168(2):210–7. doi: 10.1177/01945998221088565
19. Link MJ, Lund-Johansen M, Lohse CM, Driscoll CLW, Myrseth E, Tveiten OV, et al. Quality of life in patients with vestibular schwannomas following gross total or less than gross total microsurgical resection: shouldwe be taking the entire tumor out? Clin Neurosurg (2018) 82:541–7. doi: 10.1093/neuros/nyx245
20. Voinov B, Richie WD, Bailey RK. Depression and chronic diseases: it is time for a synergistic mental health and primary care approach. Prim Care Companion J Clin Psychiatry (2013) 15(2):PCC.12r01468. doi: 10.4088/PCC.12r01468
21. Chen CM, Mullan J, Su YY, Griffiths D, Kreis IA, Chiu HC. The longitudinal relationship between depressive symptoms and disability for older adults: a population-based study. Journals Gerontol - Ser A Biol Sci Med Sci (2012) 67:A:1059–1067. doi: 10.1093/gerona/gls074
22. Windisch P, Tonn JC, Fürweger C, Ehret F, Wowra B, Kufeld M, et al. Longitudinal changes of quality of life and hearing following radiosurgery for vestibular schwannoma. Cancers (Basel) (2021) 13:1–10. doi: 10.3390/cancers13061315
23. Carlson ML, Tveiten OV, Driscoll CL, Goplen FK, Neff BA, Pollock BE, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and nontumor controls. J Neurosurg (2015) 122:833–42. doi: 10.3171/2014.11.JNS14594
24. Newman CW, Weinstein BE, Jacobson GP, Hug GA. Test-retest reliability of the hearing handicap inventory for adults. Ear Hear (1991) 12:355–7. doi: 10.1097/00003446-199110000-00009
25. Zeman F, Koller M, Figueiredo R, Aazevedo A, Rates M, Coelho C, et al. Tinnitus handicap inventory for evaluating treatment effects: which changes are clinically relevant? Otolaryngol - Head Neck Surg (2011) 145:282–7. doi: 10.1177/0194599811403882
26. Erickson NJ, Schmalz PGR, Agee BS, Fort M, Walters BC, McGrew BM, et al. Koos classification of vestibular schwannomas: a reliability study. Clin Neurosurg (2019) 85:409–14. doi: 10.1093/neuros/nyy409
27. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol - Head Neck Surg (1985) 93:146–7. doi: 10.1177/019459988509300202
28. Carlson ML, Tombers NM, Kerezoudis P, Celda MP, Lohse CM, Link MJ. Quality of life within the first 6 months of vestibular schwannoma diagnosis with implications for patient counseling. Otol Neurotol (2018) 39:e1129–36. doi: 10.1097/MAO.0000000000001999
29. Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg (2007) 133(1):56–60. doi: 10.1001/archotol.133.1.56
30. Lee S, Seol HJ, Park K, Lee J, Nam DH, Kong DS, et al. Functional outcome of the facial nerve after surgery for vestibular schwannoma: prediction of acceptable long-term facial nerve function based on immediate postoperative facial palsy. World Neurosurg (2016) 89:215–22. doi: 10.1016/j.wneu.2016.01.038
31. Kojima T, Oishi N, Nishiyama T, Ogawa K. Severity of tinnitus distress negatively impacts quality of life in patients with vestibular schwannoma and mimics primary tinnitus. Front Neurol (2019) 10:389. doi: 10.3389/fneur.2019.00389
32. Wang JJ, Feng YM, Wang H, Wu YQ, Shi HB, Chen ZN, et al. Changes in tinnitus after vestibular schwannoma surgery. Sci Rep (2019) 9:1–12. doi: 10.1038/s41598-019-38582-y
33. Trakolis L, Ebner FH, Machetanz K, Sandritter J, Tatagiba M, Naros G. Postoperative tinnitus after vestibular schwannoma surgery depends on preoperative tinnitus and both pre- and postoperative hearing function. Front Neurol (2018) 9:136. doi: 10.3389/fneur.2018.00136
34. Trakolis L, Bender B, Ebner FH, Ernemann U, Tatagiba M, Naros G. Cortical and subcortical gray matter changes in patients with chronic tinnitus sustaining after vestibular schwannoma surgery. Sci Rep (2021) 11:8411. doi: 10.1038/s41598-021-87915-3
35. Park SH, Oh HS, Jeon JH, Lee YJ, Moon IS, Lee W-SS. Change in tinnitus after treatment of vestibular schwannoma: microsurgery vs. Gamma knife radiosurgery. Yonsei Med J (2014) 55:19–24. doi: 10.3349/ymj.2014.55.1.19
36. Inoue Y, Ogawa K, Kanzaki J. Quality of life of vestibular schwannoma patients after surgery. Acta Otolaryngol (2001) 121:59–61. doi: 10.1080/000164801300006281
37. Maurer R, Daggubati L, Ba DM, Liu G, Leslie D, Goyal N, et al. Mental health disorders in patients with untreated meningiomas: an observational cohort study using the nationwide marketscan database. Neuro-Oncology Pract (2020) 7:507–13. doi: 10.1093/nop/npaa025
38. Wagner A, Shiban Y, Lange N, Joerger AK, Hoffmann U, Meyer B, et al. The relevant psychological burden of having a benign brain tumor: a prospective study of patients undergoing surgical treatment of cranial meningiomas. J Neurosurg (2019) 131:1840–7. doi: 10.3171/2018.8.JNS181343
39. Kalasauskas D, Keric N, Ajaj SA, von CL, Ringel F, Renovanz M. Psychological burden in meningioma patients under a wait-and-watch strategy and after complete resection is high–results of a prospective single center study. Cancers (Basel) (2020) 12:1–13. doi: 10.3390/cancers12123503
40. Godefroy WP, Kaptein AA, Vogel JJ, van der Mey AGL. Conservative treatment of vestibular schwannoma. Otol Neurotol (2009) 30:968–74. doi: 10.1097/MAO.0b013e3181b4e3c9
41. Adegboyega G, Jordan C, Kawka M, Chisvo N, Toescu SM, Hill C. Quality of life reporting in the management of posterior fossa tumours: a systematic review. Front Surg (2022) 9:970889. doi: 10.3389/fsurg.2022.970889
42. McLaughlin EJ, Bigelow DC, Lee JYK, Ruckenstein MJ. Quality of life in acoustic neuroma patients. Otol Neurotol (2015) 36:653–6. doi: 10.1097/MAO.0000000000000674
43. Chweya CM, Tombers NM, Lohse CM, Link MJ, Carlson ML. Disease-specific quality of life in vestibular schwannoma: a national cross-sectional study comparing microsurgery, radiosurgery, and observation. Otolaryngol - Head Neck Surg (United States) (2021) 164:639–44. doi: 10.1177/0194599820941012
44. Bender M, Tatagiba M, Gharabaghi A. Quality of life after vestibular schwannoma surgery: a question of perspective. Front Oncol (2022) 11:770789. doi: 10.3389/fonc.2021.770789
45. Fukuda M, Oishi M, Hiraishi T, Natsumeda M, Fujii Y. Clinicopathological factors related to regrowth of vestibular schwannoma after incomplete resection: clinical article. J Neurosurg (2011) 114:1224–31. doi: 10.3171/2010.11.JNS101041
Keywords: vestibular schwannoma (VS), quality of life, mental health, physical health, extent of resection (EOR)
Citation: Machetanz K, Lee L, Wang SS, Tatagiba M and Naros G (2023) Trading mental and physical health in vestibular schwannoma treatment decision. Front. Oncol. 13:1152833. doi: 10.3389/fonc.2023.1152833
Received: 28 January 2023; Accepted: 08 June 2023;
Published: 26 June 2023.
Edited by:
Arianna Rustici, University of Bologna, ItalyReviewed by:
Daniele Bagatto, University Hospital of Udine, ItalyLuciano Mastronardi, Ospedale San Filippo Neri, Italy
Giulia Cossu, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2023 Machetanz, Lee, Wang, Tatagiba and Naros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Georgios Naros, Z2Vvcmdpb3MubmFyb3NAbWVkLnVuaS10dWViaW5nZW4uZGU=
 Kathrin Machetanz
Kathrin Machetanz Larissa Lee
Larissa Lee Sophie S. Wang
Sophie S. Wang Marcos Tatagiba
Marcos Tatagiba Georgios Naros
Georgios Naros