- Department of Hematology and Oncology, China-Japan Union Hospital of Jilin University, Changchun, Jilin, China
Diagnosis and treatment of multiple primary malignancies are becoming a new challenge in clinical practice worldwide. The present study aimed to characterize the clinical and genetic features of multiple primary malignancies in patients with synchronous or metachronous lymphoma and another solid tumor. We retrospectively analyzed 11 cases with lymphoma and another solid tumor. The germline mutations in plasma cell-free DNA samples and somatic mutations in lymphoma and solid tumor tissue samples were identified using targeted next-generation sequencing. In the 11 lymphoma patients, the most common type of concurrent solid tumor was colon adenocarcinoma (case 3, 5, 9 11) followed by papillary thyroid carcinoma (case 1, 7, 10). Metachronous lymphoma and solid tumor in 6 patients were treated with corresponding standard therapy asynchronously. Chemotherapy for colon adenocarcinoma during the interval of lymphoma chemotherapy led to excellent outcome in two patients. Immediate chemotherapy for lymphoma plus elective surgery for synchronous papillary thyroid carcinoma also yielded good prognosis in two patients with synchronous double primaries. Interestingly, we found that 10 of 11 patients with lymphoma and another solid tumor harbored germline mutations in Fanconi anemia complementation group (FANC) genes, including FANCI, FANCA, FANCG, FANCL, FANCD1, FANCF, FANCJ, and FANCS. In summary, comprehensive study of the clinical and genetic features of patients with multiple primary malignancies may improve diagnosis and treatment in the future. Mutations in FANC genes might be a predisposition to tumorigenesis of lymphoma patients with a second solid malignancy.
Introduction
During the last three decades, substantial progress has been made in the prevention, early diagnosis, and treatment of malignancies. Advances in early detection and effective treatment have significantly improved overall survival in patients with malignant tumors, such as proteomics used in ovarian cancer (1). The five-year survival rate for all cancers is nearly 40.5% in China and 68% the United States (2, 3). As of January 2019, there were approximately 16.9 million cancer survivors in the United States (3). With the aging of the population, the number of cancer survivors is projected to be greater than 22.1 million by early 2030 (3). One of the new health challenges that cancer survivors may face is developing a second primary cancer. Patients with two or more histologically unrelated malignancies arising independently are diagnosed with multiple primary malignancies (4, 5).
Multiple primary malignancies are defined as more than one synchronous or metachronous cancers in which the second primary malignant tumor is diagnosed within or after 6 months of the primary one, respectively (4). Epidemiological studies reported that the frequency of multiple primary malignancies varies from 2% to 17% (6–9). The second primary cancer usually complicates the situation, and diagnosis and treatment for multiple primary malignancies have become a new challenge in clinical practice (10). Genetic and environmental factors may contribute to the development of multiple primary malignancies. Except for hereditary multiple cancer syndromes, such as Li-Fraumeni syndrome, the genetic variations responsible for the development of sporadic multiple primary malignancies are largely unknown. All survivors of lymphoma, including Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL), have an increased risk of developing a secondary cancer (11, 12). It has been traditionally thought that high-dose extensive radiation and chemotherapy with alkylating agents are linked with a higher risk of secondary cancer (13). Radiation is now given locally in lower doses. Moreover, second primary malignancies can emerge synchronously with lymphoma and thus are unlikely secondary to the treatment of lymphoma (14). The mechanisms underlying the development of lymphoma and other solid malignancies in the same individual are still unclear, and it is unknown whether genetic susceptibility contributes to the pathogenesis of such multiple malignancies.
The present study aimed to characterize the clinical and genetic features of multiple primary malignancies in lymphoma patients. We summarized the diagnosis, treatment, and prognosis of 11 patients with synchronous or metachronous lymphoma and another solid tumor. We also determined and compared the germline and somatic genetic mutations in both tumor tissues/cells.
Materials and methods
Patients and data collection
This study enrolled 11 lymphoma patients who were diagnosed with multiple primary malignancies and treated at the China-Japan Union Hospital of Jilin University. The diagnosis of lymphoma and other solid tumors was confirmed by biopsy. Multiple primary malignancies were defined as two or more histologically unrelated, independent primary malignancies in one individual and were diagnosed using the criteria proposed by the Surveillance Epidemiology and End Results (SEER) project and the International Association of Cancer Registries and International Agency for Research on Cancer (IACR/IARC). Synchronous multiple tumors were diagnosed in an interval of less than 6 months, while metachronous tumors were diagnosed in an interval of more than 6 months. Only patients with lymphoma (HL or NHL) and another solid cancer were included in the present study. Patients with more than two primary malignancies were excluded from this study. The demographic and clinical data were obtained by medical record review. The Institutional Review Board of the China-Japan Union Hospital of Jilin University (Jilin, China) approved the study protocol (#20220628020), and the project has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (5). All involved patients provided written informed consent.
Targeted next-generation sequencing
Next-generation sequencing was carried out to detect germline mutations in plasma cell-free DNA samples and somatic mutations in lymphoma tissue and solid tumor tissue samples as previously described (15). Briefly, genomic DNA was extracted from the above-mentioned samples using the Maxwell FFPE DNA kit following the manufacturer’s instructions. The DNA libraries were prepared using the KAPA Hyper Prep Kit (KAPA Biosystems, Wilmington, MA, USA). Target enrichment was performed using the probes targeting 437 cancer-related genes (Geneseeq Prime panel) for detections of germline mutations and somatic mutations of solid tumors and using the probes targeting 475 key genes in hematologic malignancies (Hemasalus) for detections of somatic mutations in lymphoma tissues. The target-enriched libraries were amplified by quantitative polymerase chain reaction using the KAPA Library Quantification kit (KAPA Biosystems) and sequenced on a HiSeq 4000 sequencing platform (Illumina, San Diego, CA, USA). Sequencing data were processed and analyzed as previously described (15, 16). Briefly, germline variations were identified by comparing plasma cell-free DNA sequences with the hg19 reference genome, while somatic mutations were identified by comparing variations in tumor tissues with germline variations. Variants identified from plasma cell-free DNA sequencing with allele fractions greater than 50% were considered as germline variations which were also present in lymphoma tissues. Germline variations were compared with Fanconi Anemia Mutation Database (LOVD v.3.0) and NIH Single-Nucleotide Polymorphism database. Variations with less than 1% of frequency in the population were considered as mutations.
Results
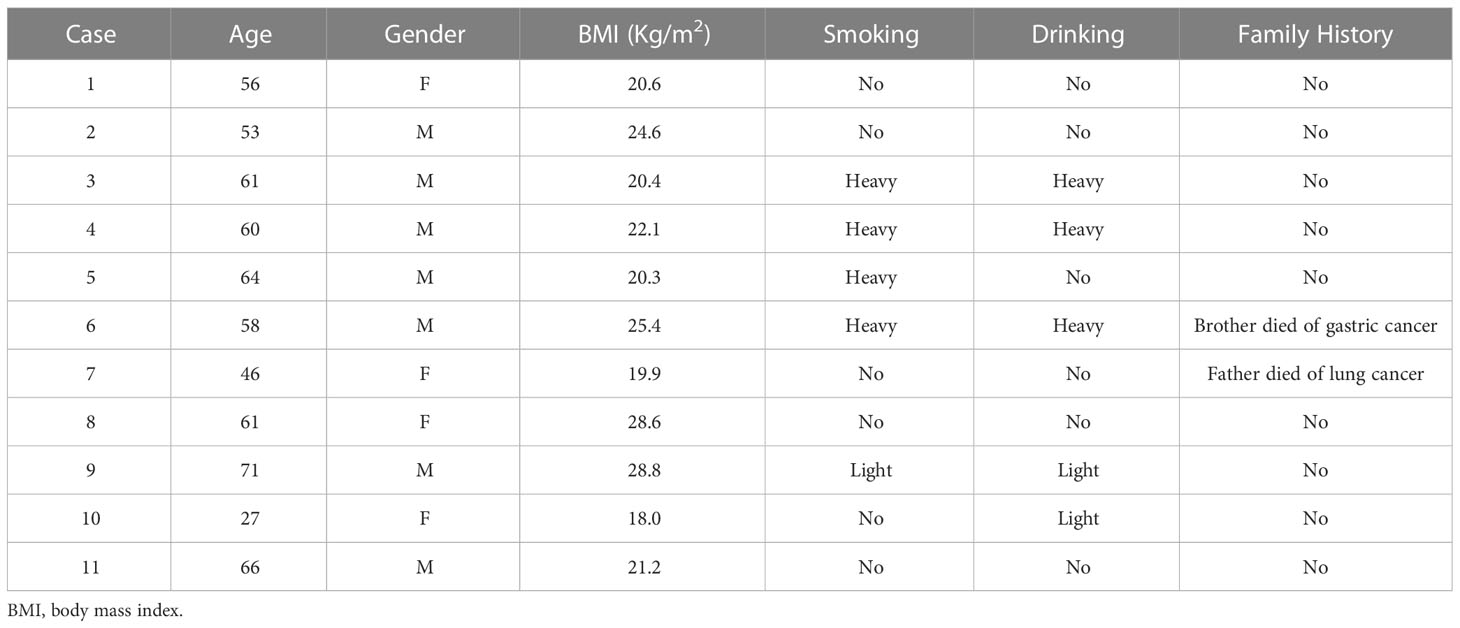
Among the 11 patients with an average age of 57 years old, four of them had a history of heavy smoking or drinking, and only two patients had a family history of cancer. The detailed demographic characteristics and medical history of the patients were summarized in Table 1.
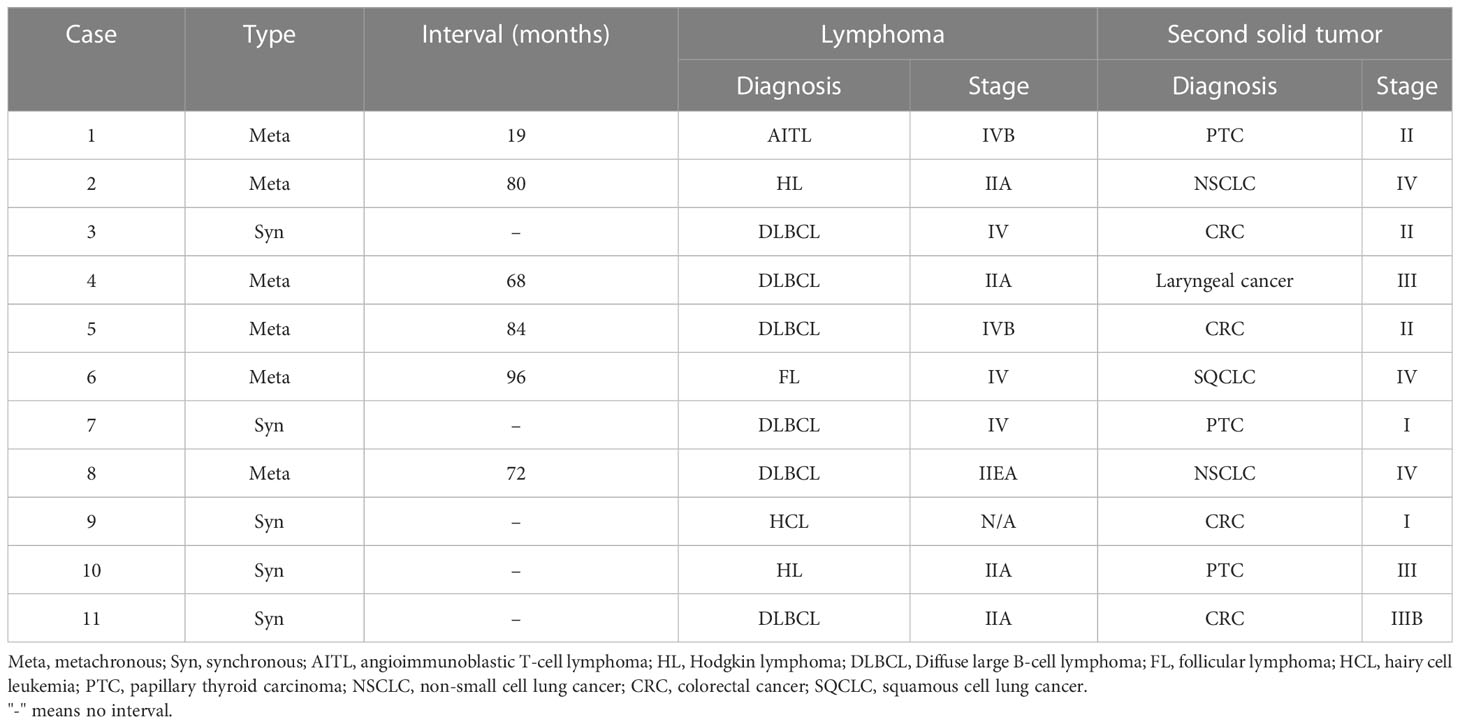
Six of the 11 lymphoma patients suffered diffuse large B cell lymphoma (DLBCL), and all were non-germinal center B-cell (non-GCB) subtype. Five patients had synchronous while six had metachronous multiple primary malignancies. The interval of metachronous multiple primary malignancies ranged from 19 to 96 months. Lymphoma and colon adenocarcinoma are the most common combination of synchronous multiple primary malignancies, which occurred in three patients. Two patients had HL while nine had NHL with the most common type being non-GCB DLBCL in our patients. In the 11 lymphoma patients, the most common type of concurrent solid tumor was colon adenocarcinoma followed by papillary thyroid carcinoma. The diagnosis and staging of lymphoma and the concurrent solid tumor were listed in Table 2.
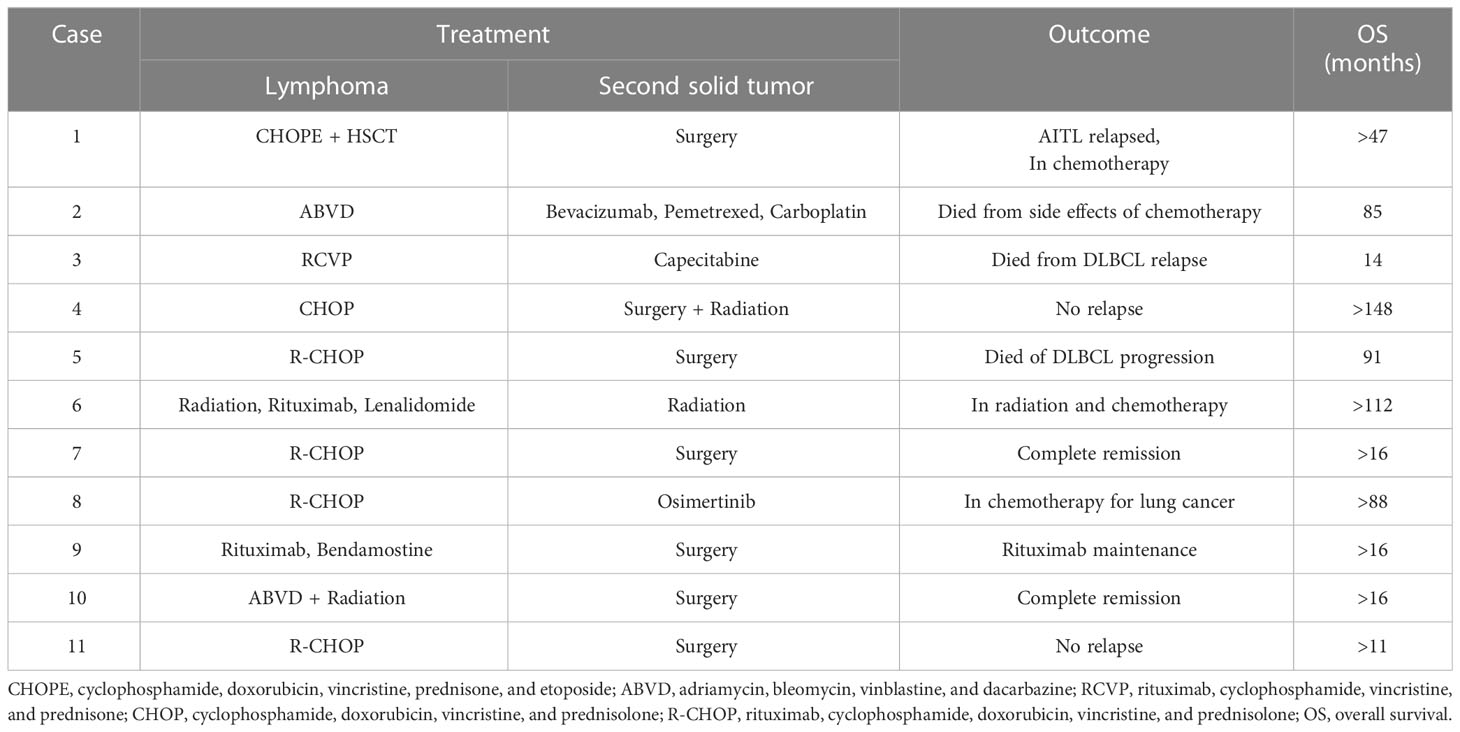
Patients with HL were treated with ABVD (adriamycin, bleomycin, vinblastine, dacarbazine) chemotherapy while the majority of patients with NHL were treated with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisolone)-based regimes (Table 3). The second solid tumors were treated with surgery, radiation, or chemotherapy. Two patients succumbed to DLBCL relapse and progression, and one patient died from side effects of chemotherapy more than 12 months after the diagnosis of their first malignancy, whereas other patients are still alive without cancer or under ongoing treatments. In patients with metachronous multiple primary malignancies, their lymphoma and another solid tumor were treated asynchronously. The treatment of synchronous lymphoma and another solid tumor is challenging. For the third case with synchronous DLBCL and colon adenocarcinoma, the patient was taking capecitabine orally for colon cancer while receiving RCVP (rituximab, cyclophosphamide, vincristine, prednisone) chemotherapy for DLBCL. The patient had intermittent hematochezia during the treatment and eventually succumbed to the disease. For the seventh and tenth cases with synchronous DLBCL and papillary thyroid carcinoma, the two patients received surgery for thyroid cancer after complete remission of DLBCL with R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone)-chemotherapy or after complete remission of HL with ABVD chemotherapy. For the ninth case with synchronous hairy cell leukemia and colon adenocarcinoma, the patient received surgery for colon cancer during the interval between targeted therapies with rituximab for hairy cell leukemia. Similarly, the eleventh patient with synchronous DLBCL and colon adenocarcinoma received surgery for colon cancer during the interval between chemotherapies with the R-CHOP regimes.
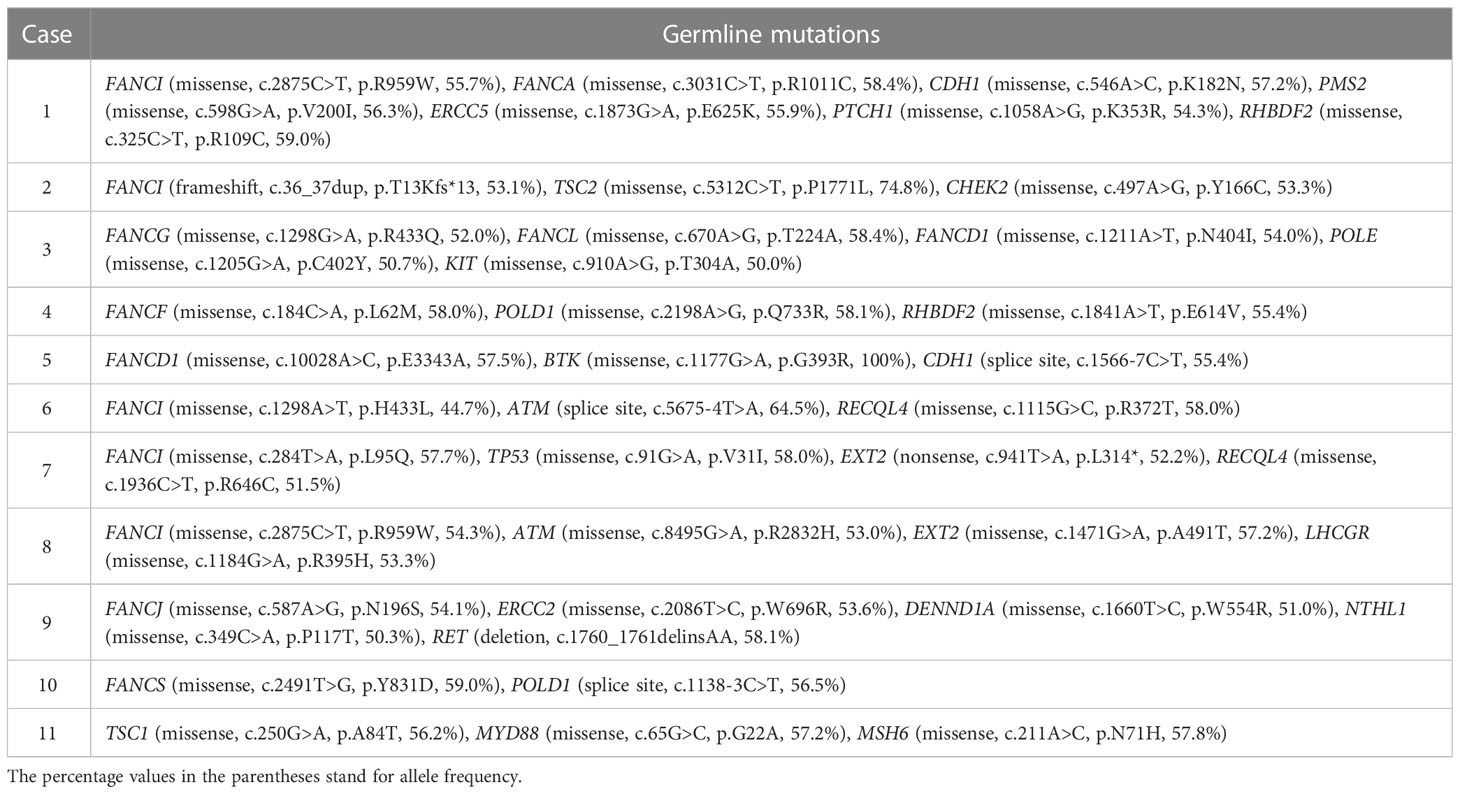
Ten of 11 patients had germline mutations in genes belonging to the Fanconi anemia complementation group (FANC), including FANCI, FANCA, FANCG, FANCL, FANCD1, FANCF, FANCJ, and FANCS (Table 4), although none of the patients had clinical manifestations of Fanconi anemia or its pathologic mutations. Other common mutations were found in the genes of POLD1, ATM, EXT2, and TSC1.
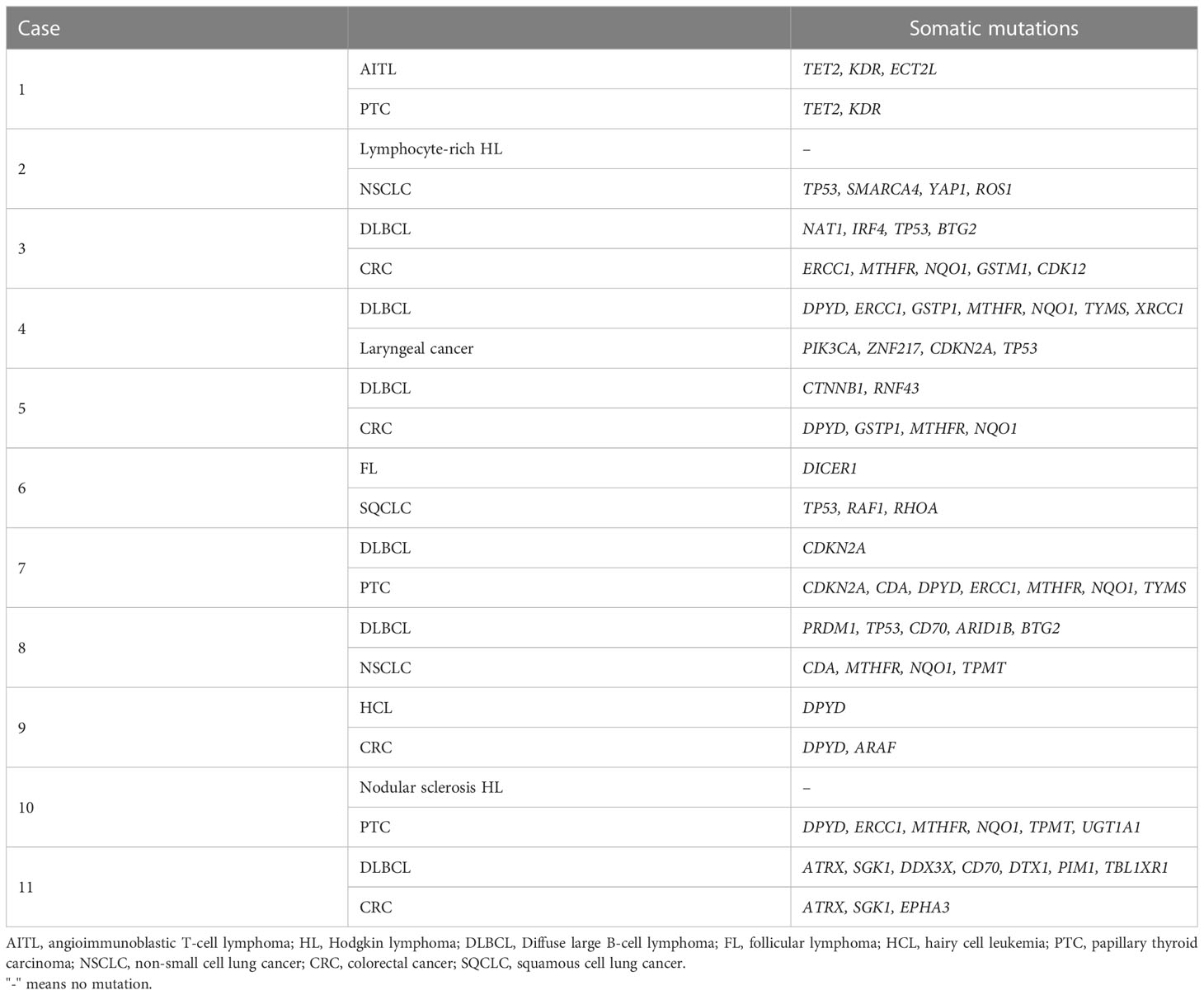
In terms of somatic mutations, none of the patients had identical genetic variation profiles between their lymphoma and the other solid tumor. However, there were different somatic mutations in common genes in lymphoma and solid tumor tissues in the same patient (Table 5). For example, mutations in the TET2 and KDR genes were identified in both angioimmunoblastic T-cell lymphoma and her papillary thyroid carcinoma tissues (Table 5). Somatic mutations in the common genes between lymphoma and solid tumor were also found in cases 7, 9, and 11 (Table 5).
Discussion
In the present study, we reported the demographic, clinical, and genetic characteristics of 11 lymphoma patients with a synchronous or metachronous second solid malignancy. We found that the coexistence of DLBCL and colon adenocarcinoma are common in patients with multiple primary malignancies. Synchronous lymphoma and solid tumor could be treated simultaneously or sequentially. Interestingly, we found that most patients with multiple primary malignancies had germline mutations in genes of the FANC family. Although genetic variation profiles of lymphoma and concurrent solid tumor were not identical in any patient, there were common somatic mutations in lymphoma and solid tumor tissues in the same patient.
In the two categories of multiple primary malignancies, synchronous multiple primaries are less common than metachronous tumors. According to previous observational studies, adenocarcinomas and squamous cell carcinomas were the most common types in multiple primary malignancies, while hematological malignancies such as lymphomas were the least frequent type (17). Therefore, synchronous combination of lymphoma and other solid carcinomas are considered as a rare condition (14, 18–21). A few cases of synchronous double primary tumors of DLBCL and digestive carcinoma have been reported as rare case reports (22–24). In our five cases of synchronous double primary malignancies, two patients had synchronous DLBCL and colon adenocarcinoma. The coexistence of DLBCL and colon adenocarcinoma has also been found in metachronous double primary malignancies in the present study. Interestingly, all six patients with DLBCL had the non-GCB subtype, suggesting that the non-GCB subtype may be more likely involved in multiple primary malignancies. The gastrointestinal tract is the most common location for the extranodal involvement of NHL, accounting for 20-40% of all extranodal disease and 10-15% of all NHL, with stomach being the most common site of involvement (60-75%) followed by the small bowel, ileum, cecum, colon, and rectum (25). However, primary colonic lymphoma is a rare malignancy (26). prevalence of GI in lymphoma is relatively common Therefore, it is challenging to differentiate coexistent colon adenocarcinoma from colonic involvement in patients with lymphoma. Comprehensive analyses of clinical features, image studies, and pathologic findings could be helpful for accurate diagnosis (18). Biopsy and pathological examinations are crucial for the confirmation of secondary malignancy in lymphoma patients with suspicious or clinically atypical lesions.
Metachronous multiple primary malignancies are usually treated separately and asynchronously. There is no standard treatment for synchronous lymphoma and solid tumors, and the choice of treatment could be difficult to make. Previous studies suggest that the malignancy that primarily affects the patient’s prognosis and outcome should be treated as a priority in patients with synchronous lymphoma and solid tumors (27). For instance, papillary thyroid carcinoma is usually less aggressive than most types of lymphoma. Specifically, DLBCL, which is considered as an aggressive lymphoma and being fatal without treatment, should be treated immediately after diagnosed. In our two patients with synchronous lymphoma and papillary thyroid carcinoma, lymphomas (DLBCL and HL) were treated first with chemotherapy while papillary thyroid carcinoma was treated later with elective surgery. Both patients achieved complete remission and have been tumor-free for 16 months so far. Their excellent outcome suggests that chemotherapy plus elective surgery should be recommended to patients with synchronous lymphoma and papillary thyroid carcinoma. Besides the types of malignancies, we should also consider the cancer staging. The present study involved three patients with synchronous lymphoma and colon adenocarcinoma. The two patients with relatively early-stage lymphoma received surgery for colon cancer during the interval between chemotherapies for lymphoma, resulting in a good prognosis and outcome. However, the patient with synchronous stage IV DLBCL and stage II colon adenocarcinoma died from DLBCL relapse and progression despite simultaneous chemotherapy for both malignancies. A previous case series study of patients with synchronous lymphoma and digestive system carcinoma reported that neoadjuvant chemotherapy for controlling colon carcinoma growth and metastasis during the interval of chemotherapy for lymphoma should be considered as this strategy has acceptable tolerance and low toxicity (18).
The etiology and pathogenetic mechanisms for multiple primary malignancies are largely unknown. Genetic, lifestyle, hormonal, and environmental factors may contribute to the development of multiple cancers in the same patient. Except for hereditary cancer syndromes, increasing evidence demonstrate that genetic mutations play an important role in sporadic multiple primary malignancies (28). A study reported that 21% of patients with multiple primary tumors had pathogenic or likely pathogenic mutations (29). One of the important findings of the present study was that most of our patients with double lymphoma and solid tumor had germline mutations in FANC genes. FANC proteins are involved in DNA inter-strand cross-link repair and maintenance of chromosome stability (30). Homozygous germline mutations in 22 FANC genes lead to Fanconi anemia while heterozygous mutations in FANC genes significantly increase malignancy susceptibility without causing Fanconi anemia (30). We have compared the identified FANC gene variations with Fanconi Anemia Mutation Database and confirmed that all the variations we found are not pathogenic mutations which cause Fanconi anemia and that their clinical significances are uncertain. The incidence of germline mutations in homologous recombination repair genes is 11–33% among men with metastatic castration resistant prostate cancer, and the most common DNA damage repair aberration in prostate cancer is FANCD1 (also known as BRCA2), followed by FANCA (31). Although germline mutations in FANC genes have been reported to increase risk for developing both hematological and solid cancers (32–34), their roles in tumorigenesis of multiple primary malignancies are largely unknown. A previous case study demonstrated that a germline FANCA mutation (p.Q405*) might be a predisposition to double primary malignancies of thyroid cancer gastric adenocarcinoma (35). These findings suggest that FANCA mutations may be a risk factor for multiple primary malignancies. One of the limitations of the present study is that it is difficult to confirm if the identified mutations in FANC genes are pathological mutations, which needs further investigation. In addition, the mutations identified in second generation sequencing in the present study need to be validated using polymerase chain reaction or Sanger sequencing in the future.
In the present study, somatic mutations in different tumor tissues in the same patient were not identical. Different mutations in common genes were identified in solid tumors and lymphoma tissues in a few patients. The role of these mutations in the development of multiple primary malignancies is unclear. However, identifying common genes in multiple primary malignancy tissues might help to find out potential targets for immunochemotherapy with monoclonal antibodies.
Taken together, the present study characterized the clinical and genetic features of patients with lymphoma and a second primary solid tumor. Mutations in FANC genes are associated with multiple primary malignancies, however, their causative role in the tumorigenesis of multiple primaries warrants further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by The Institutional Review Board of the China-Japan Union Hospital of Jilin University (Jilin, China), (#20220628020). The patients/participants provided their written informed consent to participate in this study.
Author contributions
DZ, LH and CJ collected the data, performed the experiments, analyzed the data, authored or reviewed drafts of the paper, and approved the final draft. LB conceived and designed the experiments, prepared tables, authored or reviewed drafts of the paper, and approved the final draft. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Division of Social science and Technology Development, Science and Technology Department of Jilin Province, China (Project Number: 20210203067SF).
Acknowledgments
We appreciate Geneseeq Research Institute for help in targeted next-generation sequencing and result interpretation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1222716/full#supplementary-material
References
1. Ghose A, Gullapalli SVN, Chohan N, Bolina A, Moschetta M, Rassy E, et al. Applications of proteomics in ovarian cancer: dawn of a new era. Proteomes (2022) 10:16. doi: 10.3390/proteomes10020016
2. Li J, Pang H, Sun Z, Zhao L, Bai C. Health status of middle-aged and older cancer survivors: a nationwide cross-sectional study from the China health and retirement longitudinal study (CHARLS). Ann Transl Med (2020) 8:183. doi: 10.21037/atm.2020.01.105
3. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin (2019) 69:363–85. doi: 10.3322/caac.21565
4. Vogt A, Schmid S, Heinimann K, Frick H, Herrmann C, Cerny T, et al. Multiple primary tumours: challenges and approaches, a review. ESMO Open (2017) 2:e000172. doi: 10.1136/esmoopen-2017-000172
5. Copur MS, Manapuram S. Multiple primary tumors over a lifetime. Oncol (Williston Park) (2019) 33:629384.
6. Coyte A, Morrison DS, McLoone P. Second primary cancer risk - the impact of applying different definitions of multiple primaries: results from a retrospective population-based cancer registry study. BMC Cancer (2014) 14:272. doi: 10.1186/1471-2407-14-272
7. Weir HK, Johnson CJ, Thompson TD. The effect of multiple primary rules on population-based cancer survival. Cancer Causes Control (2013) 24:1231–42. doi: 10.1007/s10552-013-0203-3
8. Rosso S, De Angelis R, Ciccolallo L, Carrani E, Soerjomataram I, Grande E, et al. Multiple tumours in survival estimates. Eur J Cancer (2009) 45:1080–94. doi: 10.1016/j.ejca.2008.11.030
9. Irimie A, Achimas-Cadariu P, Burz C, Puscas E. Multiple primary malignancies–epidemiological analysis at a single tertiary institution. J Gastrointestin Liver Dis (2010) 19:69–73.
10. Gokyer A, Kostek O, Hacioglu MB, Erdogan B, Kodaz H, Turkmen E, et al. Clinical features of the patient with multiple primary tumors: single center experience. North Clin Istanb (2017) 4:43–51. doi: 10.14744/nci.2017.67044
11. Tadmor T, Liphshitz I, Silverman B, Polliack A. Incidence and epidemiology of non-Hodgkin lymphoma and risk of second malignancy among 22 466 survivors in Israel with 30 years of follow-up. Hematol Oncol (2017) 35:599–607. doi: 10.1002/hon.2302
12. Schaapveld M, Aleman BM, van Eggermond AM, Janus CP, Krol AD, van der Maazen RW, et al. Second cancer risk up to 40 years after treatment for hodgkin’s lymphoma. N Engl J Med (2015) 373:2499–511. doi: 10.1056/NEJMoa1505949
13. Dracham CB, Shankar A, Madan R. Radiation induced secondary malignancies: a review article. Radiat Oncol J (2018) 36:85–94. doi: 10.3857/roj.2018.00290
14. Al-Gahmi A, Alhuthali M, Alrehaili M, Baltow B, Tashkandi E. Unusual synchronous association of solid tumors with hematological malignancies in multiple primary cancers: case series and literature review. Case Rep Oncol (2021) 14:352–64. doi: 10.1159/000514147
15. Li B, Qu H, Zhang J, Pan W, Liu M, Yan X, et al. Genomic characterization and outcome evaluation of kinome fusions in lung cancer revealed novel druggable fusions. NPJ Precis Oncol (2021) 5:81. doi: 10.1038/s41698-021-00221-z
16. Zhang S, Xu Y, Zhao P, Bao H, Wang X, Liu R, et al. Integrated analysis of genomic and immunological features in lung adenocarcinoma with micropapillary component. Front Oncol (2021) 11:652193. doi: 10.3389/fonc.2021.652193
17. Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, et al. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Med (Baltimore) (2017) 96:e6799. doi: 10.1097/MD.0000000000006799
18. Meng J, Pan H, Li X, Liu T, Liu Z, Li Q, et al. Diagnosis and treatment of synchronous lymphoma and digestive system carcinoma: report of four cases and literature review. Front Oncol (2019) 9:1367. doi: 10.3389/fonc.2019.01367
19. Ren Y, Chen Z, Su C, Tong H, Qian W. Diffuse large B-cell lymphoma in colon confounded by prior history of colorectal cancer: a case report and literature review. Oncol Lett (2016) 11:1493–5. doi: 10.3892/ol.2016.4078
20. Tanba K, Chinen Y, Uchiyama H, Uoshima N, Shimura K, Fuchida S, et al. Prognostic impact of a past or synchronous second cancer in diffuse large B cell lymphoma. Blood Cancer J (2018) 8:1. doi: 10.1038/s41408-017-0043-6
21. Lee DY, Hong SW, Chang YG, Lee WY, Lee B, Kang YK. Synchronous T-cell lymphoma in patient with colon cancer: a case report. J Korean Surg Soc (2012) 83:60–4. doi: 10.4174/jkss.2012.83.1.60
22. Pliakou E, Lampropoulou D, Soupos N, Aravantinos G. Synchronous gastric adenocarcinoma and diffuse Large B-cell lymphoma in the pelvis: a rare case presentation. Case Rep Gastrointestinal Med (2022) 2022:1–3. doi: 10.1155/2022/7535036
23. Dayer N, Fasquelle F, Salati E, Dietrich G. Multiple primary malignancies: synchronous lymphoma, pancreatic neuroendocrine tumour and colorectal cancer. BMJ Case Rep (2021) 14:e241938. doi: 10.1136/bcr-2021-241938
24. Wang W, Li P. Coexistence of colon adenocarcinoma, diffuse large B-cell lymphoma, and myelodysplastic syndrome: a case report. Med (Baltimore) (2019) 98:e16742. doi: 10.1097/MD.0000000000016742
25. Boussios S, Zerdes I, Vassou A, Bareta E, Seraj E, Papoudou-Bai A, et al. Extranodal diffuse large B-cell lymphomas: a retrospective case series and review of the literature. Hematol Rep (2018) 10:7070. doi: 10.4081/hr.2018.7070
26. Pandey M, Swain J, Iyer HM, Shukla M. Primary lymphoma of the colon: report of two cases and review of literature. World J Surg Oncol (2019) 17:18. doi: 10.1186/s12957-018-1548-6
27. Namikawa T, Munekage E, Fukudome I, Maeda H, Kitagawa H, Togitani K, et al. Clinicopathological characteristics and therapeutic outcomes of synchronous gastric adenocarcinoma and gastric lymphoma. Anticancer Res (2014) 34:5067–74.
28. Nyqvist J, Kovacs A, Einbeigi Z, Karlsson P, Forssell-Aronsson E, Helou K, et al. Genetic alterations associated with multiple primary malignancies. Cancer Med (2021) 10:4465–77. doi: 10.1002/cam4.3975
29. Cadoo KA, Mukherjee S, Khurram A, Kemel Y, Tkachuk K, Liu YL, et al. Characterization of patients with multiple primary tumors. J Clin Oncol (2020) 38:1. doi: 10.1200/JCO.2020.38.15_suppl.1502
30. Niraj J, Farkkila A, D’Andrea AD. The fanconi anemia pathway in cancer. Annu Rev Cancer Biol (2019) 3:457–78. doi: 10.1146/annurev-cancerbio-030617-050422
31. Boussios S, Rassy E, Moschetta M, Ghose A, Adeleke S, Sanchez E, et al. BRCA mutations in ovarian and prostate cancer: bench to bedside. Cancers (Basel) (2022) 14:3888. doi: 10.3390/cancers14163888
32. Mathew CG. Fanconi anaemia genes and susceptibility to cancer. Oncogene (2006) 25:5875–84. doi: 10.1038/sj.onc.1209878
33. Degrolard-Courcet E, Sokolowska J, Padeano MM, Guiu S, Bronner M, Chery C, et al. Development of primary early-onset colorectal cancers due to biallelic mutations of the FANCD1/BRCA2 gene. Eur J Hum Genet (2014) 22:979–87. doi: 10.1038/ejhg.2013.278
34. Garcia MJ, Fernandez V, Osorio A, Barroso A, Fernandez F, Urioste M, et al. Mutational analysis of FANCL, FANCM and the recently identified FANCI suggests that among the 13 known fanconi anemia genes, only FANCD1/BRCA2 plays a major role in high-risk breast cancer predisposition. Carcinogenesis (2009) 30:1898–902. doi: 10.1093/carcin/bgp218
Keywords: multiple primary malignancies, mutation, lymphoma, FANC, germline mutation, somatic mutation
Citation: Zhou D, Han L, Jin C and Bi L (2023) Clinical and genetic characteristics in lymphoma patients with a second solid malignancy. Front. Oncol. 13:1152290. doi: 10.3389/fonc.2023.1152290
Received: 30 January 2023; Accepted: 03 July 2023;
Published: 21 July 2023.
Edited by:
Clement Yedjou, Florida Agricultural and Mechanical University, United StatesReviewed by:
Nicholas Pavlidis, University of Ioannina, GreeceRalf Küppers, University of Duisburg-Essen, Germany
Copyright © 2023 Zhou, Han, Jin and Bi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lintao Bi, YmlsdEBqbHUuZWR1LmNu
†These authors have contributed equally to this work
 Di Zhou†
Di Zhou† Chanjuan Jin
Chanjuan Jin Lintao Bi
Lintao Bi