
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 12 April 2023
Sec. Cancer Epidemiology and Prevention
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1150303
This article is part of the Research TopicImmune Imbalance in Obesity-associated DiseasesView all 12 articles
Purpose: Obesity, especially the hidden type of obesity (central obesity), has been believed to be the major risk factor for developing and progressing non-communicable diseases, including cancers. However, there are limited studies regarding the issue in Ethiopia and the study area. Therefore, this study aimed to evaluate the magnitude of central obesity and its associated factors among cancer patients visited the oncology unit of the University of Gondar Comprehensive Specialized Hospital.
Methods: An institutional-based cross-sectional study was conducted from January 10 to March 10, 2021. A total of 384 study participants were enrolled using a systematic sampling technique. The data were collected using a semi-structured interviewer-administered questionnaire and were pretested to address the quality of assurance. The weight of the participants was assessed using body mass index (BMI) and central obesity. Both bivariate and multivariate logistic regressions were conducted to identify the factors associated with central obesity, and p-values less than 0.05 with multivariate were considered statistically significant associations.
Result: Most respondents (60.16%) were stage I cancer patients. The study found that about 19.27% of the participants were prevalent central obesity, and none of them were obese by body mass index (BMI) categorization criteria. However, about 12.24% and 7.03% of the participants were found to be underweight and overweight, respectively. The variables associated with central obesity were sex (AOR=14.40; 95% CI: 5.26 - 39.50), occupation (AOR=4.32; 95%CI: 1.10 - 17.01), and residency (AOR=0.30; 95% CI: 0.13 - 0.70).
Conclusion: A significant number of the respondents (19.27%) were centrally obese. Being female, urban residency and having an occupation other than a farmer, merchant, and governmental were the factors associated with central obesity. Hence, cancer patients may be centrally obese with average body weight.
Non-communicable diseases have become major public health problems worldwide (1). They are the leading cause of death in both developed and under-developing countries. Of these, cancers are the second most common prevalent disease next to cardiovascular diseases (CVDs) (2). Globally, around 18.1 million of the population is affected by cancer problems. Besides, about 9.6 million people are estimated to die yearly from cancer and related complications (3). The problem is becoming rapidly prevalent in low and middle-income countries (LMICs), especially in sub-Saharan African countries, and it has been estimated that in 2050, the majority (70%) of annual cancer case incidence will be located in LMICs (4). The absence of screening and failure to diagnose at an early stage of cancer cases makes it difficult to overcome the burden of cancer problems in LMICs. Moreover, treatment is usually compromised fordifferent reasons, such as limied of skilled manpower, facilities, and economic restriction (5). Therefore, screening for early identification of cancer cases and associated factors are believed to be an ideal way to limit the progression and development of adversative outcomes.
Substance abuse like cigarette smoking, alcohol drinking and chat chewing, frequent utilization of processed food, being overweight or obese, and being older are the most common factors associated with the development and proical characteristics of the study participants among adults gression of most cancers (6). Although the association between obesity and cancer development is still controversial, several reports have approved a strong positive association. For instance, studies done across the world showed that obesity has been directly associated with the occurrence of breast cancer (7), ovarian cancer (8), liver cancer (9), endometrial cancer (10), esophageal adenocarcinoma (11), kidney cancer (12), colorectal cancer, gallbladder cancer (13) and thyroid cancer (14). In particular, the hidden type of obesity (central or visceral obesity) is highly dangerous and is usually associated with the development of several NCDs, including cancers, than that of general obesity (identified by BMI) (15, 16). Currently, dietary modifications, controlling the accumulation of fat around the abdominal wall, weight control, and frequent exercise are recommended as a supplementary therapeutic option to limit the progression of cancer cases (17, 18). Globally, the magnitude of abnormal or excessive fat accumulation which is measured by BMI and/or central obesity among cancer patients, is becoming a remarkable problem in the last decades (19). Although the attribution of obesity for cancer incidence varies among each cancer type, it is estimated to reach up to 44% for esophageal adenocarcinoma and 54% for bladder cancer development (20). However, to the best of our search, there are limited studies regarding the issue in Ethiopia and the study area. Besides, the burdens of central obesity have not got equal attention as general obesity among cancer patients worldwide, and this gives great value to the novelty of our study. Consequently, central obesity is becoming one of the major public health problems for the general population of Ethiopia (21–25). As our study is the first among Ethiopian cancer patients that focused on the magnitude of central obesity, the findings will be filled the knowledge gap on the burden of central obesity for Ethiopian cancer patients and can act as a baseline for further researchers with large sample sizes and multi-centered study area towards the impact of central obesity among Ethiopian cancer patients. The finding of this study will also give valuable input for clinicians to give attainable attention to general and central obesity aimed at cancer patients. Hence, we aimed to evaluate the magnitude of central obesity and its associated factors among cancer patients visiting the Oncology ward of the University of Gondar Comprehensive Specialized Hospital.
An institution-based cross-sectional study was conducted among cancer patients from January to March 2021. It was accompanied by the University of Gondar Comprehensive Specialized Hospital (UoGCSH), Oncology ward. During the data collection period, the oncology ward has ten beds, serving more than 100 cancer patients annually.
All adult cancer patients who were ≥18 years coming to the Oncology ward were taken as the study’s source population. Of these, who were in the Oncology ward during the study period were recruited as study participants. Severely ill patients who were unable to communicate and did not have attendant, clinically confirmed pregnant women, and edematous and abdominal distension patients were excluded from the study. To determine the sample size of the study, we used a single population formula using a 50% expected proportion of central obesity, 95% confidence level, and a 5% margin of error. By considering the 5% non-response rate, the final size was computed to be 403. A systematic sampling technique with a skip interval of two was implemented to select the sample of the participants.
The data were collected using a semi-structured interviewer-administered questionnaire. It was prepared using related pieces of literature in an international language (English), then translated into the Ethiopian national language (Amharic), and then re-translated back to English to check the consistency. The questionnaire includes the general characteristics of the participants, substance abuse like smoking, alcohol drinking, and Khat chewing, and the factors that are believed to be associated with the independent outcome. The data were collected by trained Health Professionals such as Nurses and Public Health Professionals under the supervision of the principal investigator and oncologist. The completeness and consistency of the data were checked daily by the principal investigator. The substance abuse habits of the participants were assessed using a dichotomous yes and no questionnaire with expanded questions for those who responded “yes” about the amount, duration, and frequency of alcohol drinking, khat chewing, and cigarette smoking habits. Thus, alcohol drinkers were defined as any alcoholic product, including locally prepared alcoholic beverages intake at least twice per week for the last year regardless of the amount, otherwise defined as non-drinkers (26). Furthermore, those individuals who have smoked cigarettes for the last year were defined as smokers unless defined as non-smokers. Khat chewers were defined similarly to smokers (27). The physical activity of the participants was grouped based on the World Health Organization and international physical activities analysis guidelines as vigorous, moderate, and poor physical activities. Any activity that causes a substantial increase in breathing or heart rate (e.g., running, carrying, or lifting heavy loads, digging, or construction work) that continued for at least 30 min for a minimum of three days per week was defined as vigorous physical activity. Besides, any activity that causes a small increase in breathing or heart rate (brisk walking or carrying light loads) that continued for at least 30 min for at least three days per week or five or more days of these activities for at least 20 min per day or ≥3 days of vigorous-intensity activity per week of at least 20 min per day was defined as moderate physical activity. The respondents who did not fulfill both vigorous and moderate intensity activity were grouped under poor physical activators (28).
Anthropometric (physical) measurements such as weight, height, waist circumference, and blood pressure were measured through adjusted equipment. The weight and height of the participants were measured in kilograms and centimeters in barefoot, respectively to calculate body mass index (BMI). Consequently, BMI was grouped into underweight (BMI ≤18.5 kg/m2), normal weight (BMI between 18.5 and 24.9 kg/m2), overweight (BMI between 24.9 and 30 kg/m2), and obesity (BMI≥30 kg/m2) (29). The Waist circumference was measured to define independent outcome (central obesity). Waist circumference was measured in centimeters at the narrowest mid-point between the lower margin of the lowest palpable rib and the top of the iliac crest with flexible plastic tape without heavy outdoor closing. Then, the participants with a waist circumference of >94 cm for males and >80 cm for females were defined as centrally obese (30). The Blood pressure (BP) of the respondents was measured three times with at least a five-minute interval in each measurement in a sitting position using a standardized mercury sphygmomanometer with an appropriate cuff size that covers two-thirds of the upper arm. It was measured with at least five minutes or 30 minutes rest for those who take hot drinks like coffee on their left arm. Then, the average BP measurement was taken, and elevated BP (hypertension) was defined as if systolic blood pressure (SBP) is ≥140mg/dl or diastolic blood pressure (DBP) is ≥90mg/dl or current use of the anti-hypertensive drugs (31).
The data was entered into Epidata version 3.1 to check the completeness and analyzed using STATA 14 software. The participants’ socio-demographic, behavioral, and clinical characteristics were described through descriptive statistics and presented using tables and narration. The factors associated with the independent variable were identified using the binary logistic regression model. The multivariable logistic regression was implemented to detect the Adjusted Odds Ratio (AOR). The 95% confidence interval was estimated to show the strength of the associations. Hence, a p-value of less than 0.05 in the multivariable logistic regression analysis was used to declare the statistically significant association of the independent variables with central obesity. Hosmer and Lemeshow’s goodness of fit test was used to check the goodness of fit of the model.
A total of 384 study participants with a 95.3% response rate were completed and enrolled in the analysis. Of these, most of the participants were under the age range of 41-60 years (55.99%) and females (51.30%). Most of the participants were coming from a rural area (64.4%) and more than two-thirds of the participants were Orthodox Christianity followers (63.3%). Regarding bad behavioral practices, about 11.7%, 46.6%, and 26.8% of the study participants were current smokers, alcohol drinkers, and khat chewers, respectively. Out of the total participants, only 13.02% of them had vigorous physical activity (Table 1).
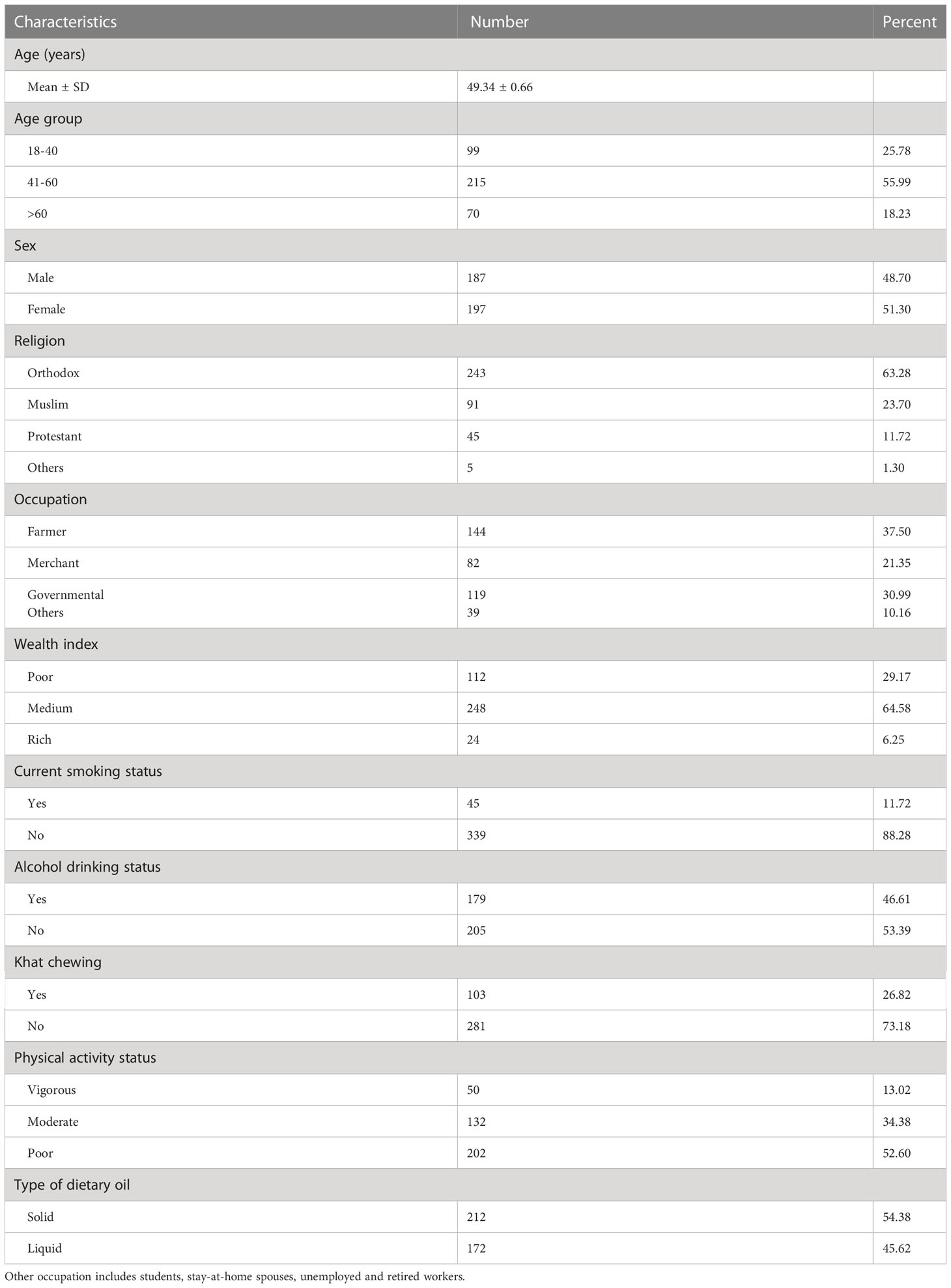
Table 1 Socio-demographic characteristics of the study participants among adults in urban areas of Northwest Ethiopia, 2022 (n=384).
About 4.17%, 14.32%, 8.59%, 6.77%, 15.63%, 15.10%, 3.39%, and 32.03% of the participants were diagnosed with lung, breast, cervical, ovarian, hematologic, gastrointestinal, skin, and another type of cancers, respectively. Near to two-thirds of the participants were diagnosed with stage I cancer (60.16%) and 9.11% of them were under an advanced stage or stage IV. The majority of study participants (46.4%) were diagnosed with a duration of three to seven months, and more than two-thirds of the participants (68.8%) were not characterized by cancer metastasis. Most of the participants (82.6%) were already under treatment; of these about 25.24%, 38.8%, and 35.96% were treated with chemotherapy, surgery, and combined chemotherapy & surgery, respectively. Moreover, 36.2% and 16.67% of the study participants had hypertension and diabetes comorbidities, respectively (Table 2).
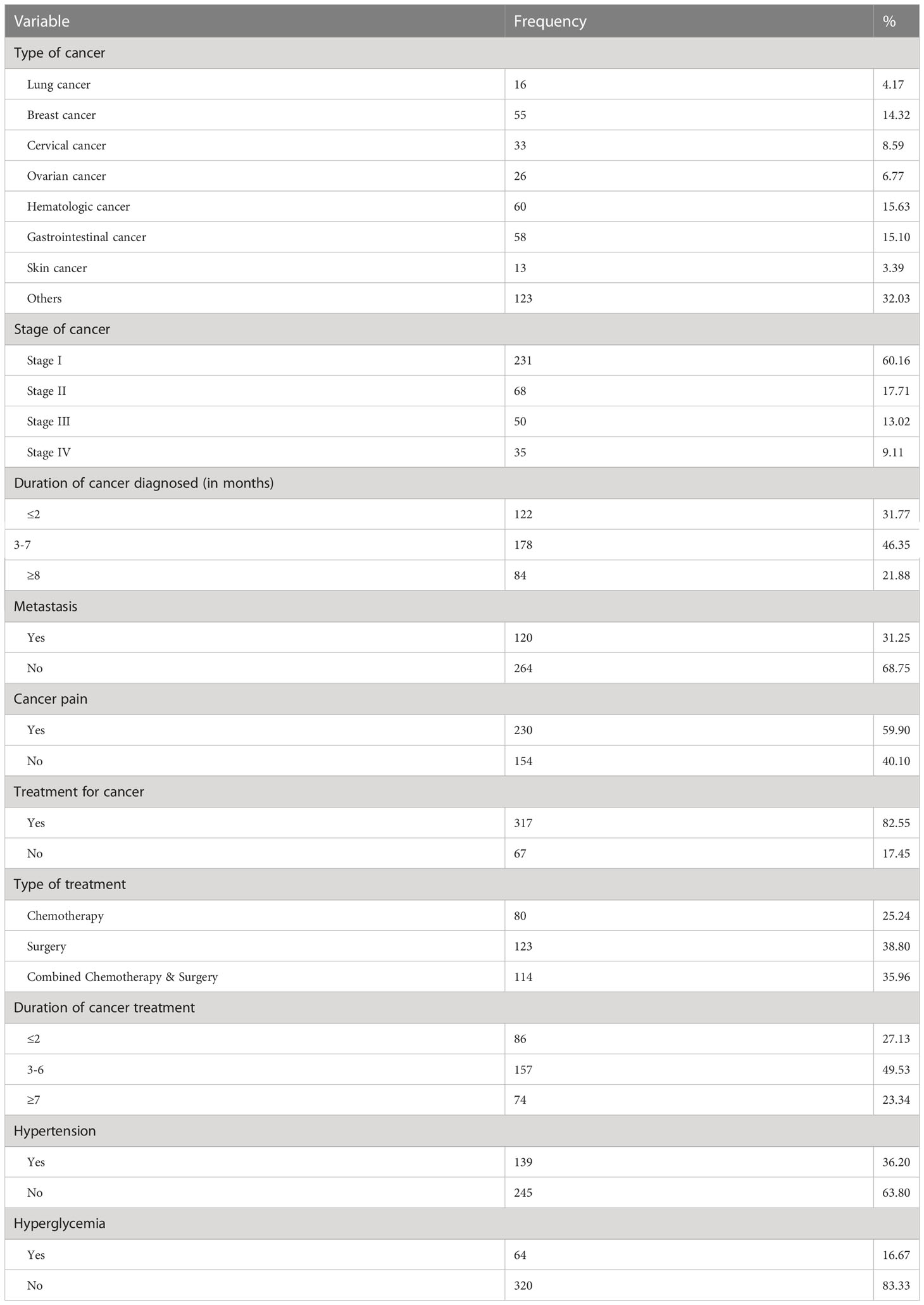
Table 2 Clinical characteristics of the study participants among adults in urban areas of Northwest Ethiopia, 2022 (n=384).
This study found that 19.27% (95% CI; 15.61- 23.54) of the cancer patients were centrally obese; of these, the majority (91.89%) were females. However, only 7.03% (95% CI; 4.86 - 10.07) of the study participants were overweight and none of them were obese based on the BMI categorization criteria. Besides, about 12.24% (95% CI; 9.31 -15.93) of the study participants were found to be underweight (Table 3).
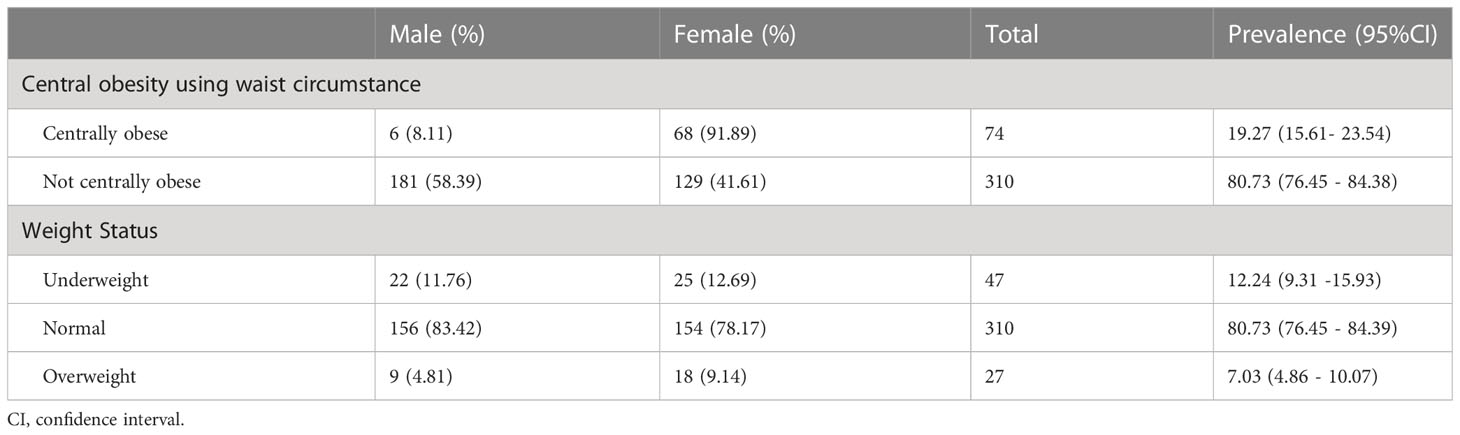
Table 3 Prevalence of central obesity and weight status of the cancer patients at the University of Gondar Specialized Comprehensive Hospital, Northwest Ethiopia, 2022 (n=384).
This study has shown the distribution of central obesity across cancer patients. Accordingly, we found that about 1 out of 15 lung cancer patients, 20 out of 35 breast cancer patients, 12 out of cervical cancer patients, 6 out of 20 0varian cancer patients, 8 out of 52 hematologic cancer patients, 5 out of 53 gastrointestinal cancer patients, 2 out of skin cancer patients, and 20 out of 103 other types of cancer patients were centrally obese (Figure 1).
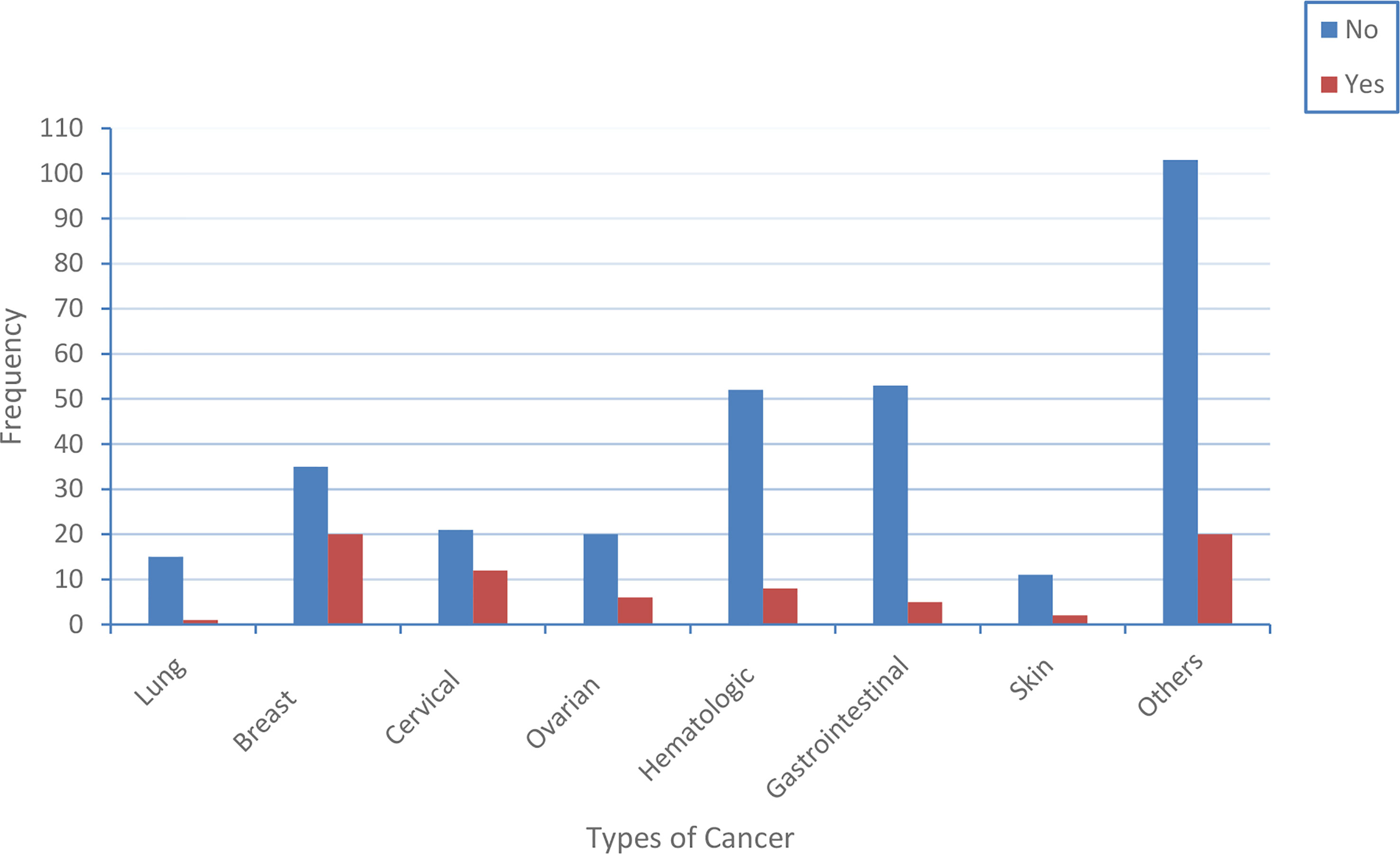
Figure 1 The distribution of central obesity across the type of cancer patients. The blue and red color indicates the proportion of cancer patients that have central obesity and do not have central obesity, respectively.
The study analyzed the multinomial logistic analysis for central obesity to determine its association with the sociodemographic, behavioral, and clinical characteristics of cancer patients. Thus, being female (p<0.001), having urban residency (p=0.002), and having occupations other than a farmer, merchant, and governmental (p=0.036) were found to be the factors associated with central obesity. The probability of having central obesity was 14 times higher among female participants compared to that of male cancer participants (AOR=14.40; 95 CI: 5.26 - 39.50). The odds of having central obesity were 4 times higher among study participants with an occupation other than a farmer, merchant, and governmental as compared to that of the farmers (AOR=4.32; 95%CI: 1.10 - 17.01). Urban residents were less likely to have central obesity by 30% compared to rural residents (AOR=0.30; 95CI: 0.13 - 0.70) (Table 4).
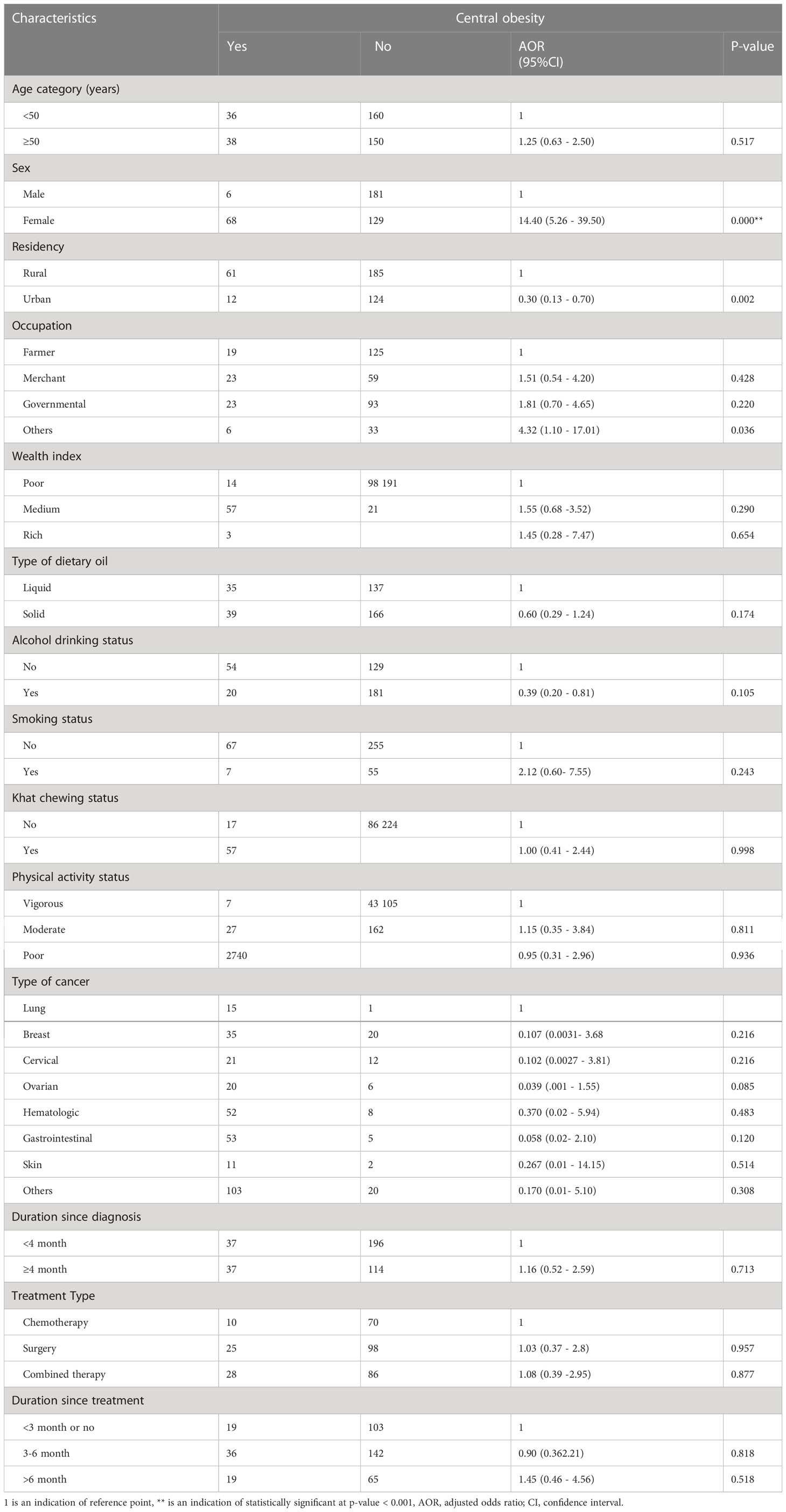
Table 4 The multivariable logistic regression analysis that identified the factors associated with central obesity among cancer patients at the University of Gondar Specialized Comprehensive Hospital, Northwest Ethiopia, 2022 (n=384) .
This is the first study that revealed the most dangerous type of obesity or hidden type of obesity (central obesity) and its association among cancer patients in Ethiopia and the study area.
The study found that a significant proportion of study participants (19.27%) were centrally obese among cancer patients that follow at UOGSCH. However, none of the study participants were found to be obese according to the BMI definition criteria of obesity. Even the prevalence of overweight (7.03%) was lower than compared to that of central obesity, and about 12.24% of the participants were confirmed to be underweight. Most researchers are advised to check fat accumulation around the abdominal wall, which is usually identified through waist circumference measurement, than that of general or peripheral obesity (via BMI) to know the exact pathogenic cause of obesity for the initiation and progression of inflammatory associated chronic diseases, including cancers (32–34). With normal or low BMI levels, people may develop central obesity since BMI can be affected by muscular mass (16, 35). Thus, our finding also revealed that the high burden of central obesity was encountered when compared to that of general obesity among the study participants. Abdominal/visceral obesity (identified by WC) is usually associated with metabolic changes which could affect the pathogenesis of tumor cells. However, the association between obesity and cancers is controversial yet. Some study finding proved their strong positive association, and some others have found no association. However, it has been evidenced that weight gain or obesity is responsible for around 20% of all cancer cases (20). Moreover, several studies and review articles have publicized that over-accumulation of fat around the abdominal wall is the main cause of inflammation cascade initiation, which is one of the most common problems encountered in cancer pathogenesis (36–38). In an individual with visceral obesity, adiposity can be directly encapsulated for tumor development through response to the obesity which is usually associated with the direct release of pro-inflammatory cytokines, hormones (peptide and steroid hormones), and growth factors (39).
The prevalence of central obesity in this study is extremely lower than that of a study done by L.A. Healy et al. (40). This may be due to the study population variation since the current study includes all cancer patients attending the hospital regardless of the metabolic syndrome categorization, whereas the L.A. Healy et al. initially categorizes the study participants as metabolic and non-metabolic participants. As central obesity is one of the major components of metabolic syndrome, the prevalence can be significantly elevated in their study finding. The finding of central obesity was also lower than a study conducted in Africa (41). The discrepancy may be due to the restriction of the study population. The study done among the African population was focused on male cancer patients affected by prostate cancer. The study design may also be different from the current study. Several studies have also been conducted on the association of having central obesity and the progression of tumors (40–42). Although the prevalence of central obesity is a double-digit in the current study without being obese based on BMI classification criteria, it is much lower than studies done in Ethiopia among the general population (21–25). These discrepancies are due to the variation of study participants between studies. Our study focused on cancer patients with disease or medication associated with weight loss. Most studies done about abdominal obesity and cancer targeting their association which makes difficult for further comparison of our finding with other similar studies. This finding will bring for the investigation of further studies and attention for central obesity among cancer patients.
In the current study, the probability of having central obesity was 14 times higher among female participants compared to its counterpart. Although the exact molecular link between being female and central obesity development is not exactly clear, different studies have reported similar findings, even among non-cancer study participants (21, 43, 44). This might be justified as females are naturally fattier than that of males, which may contribute to the re-distribution of fat accumulation towards the visceral area. Consequently, the accumulation of this fat will end up with central obesity. Another possible reason may be due to females may have a sedentary lifestyle and less access to different leaflets that showed the precautions of central obesity for health (45). Most of the population living in developing countries; including Ethiopia do not have enough awareness about central obesity as they estimate their weight using BMI. However, central obesity found among normal BMI groups is the most dangerous as mentioned earlier. It is not also surprising that females living in developing countries have less access to work outside the home, making it more difficult to get different leaflets. Besides, most females have focused on technical activities rather than labor work, and central obesity is frequently associated with those individuals who didn’t undergo physical exercise or labor work (45–47).
The study also revealed that occupation was statistically associated with central obesity among cancer patients. The odds of having central obesity were 4.3 times higher among study participants with occupations other than a farmer, merchant, and governmental than the farmers. We used farmers as reference groups because farmers are less likely to fall victim to central obesity or cancer due to the reason that farmers have routine activities on the farm, which is energy-demanding work that lowers the risk of getting central obesity (48). This can be logically justified as the type of occupation is usually associated with energy expenditure per day. More physical activity brings less fat accumulation on the abdominal wall as lipids or fats are one of the most common energy precursors, especially during the exercise period. Thus, in our study who are regarded as other than a farmer, merchants and government include those participants having no work, are retired, students, and housewives. These study participants may be directly or indirectly favorable for not having labor work or regular exercise. Therefore, early screening of central obesity is vital for all cancer patients to limit its adverse outcomes regardless of their BMI level. It is also supported by a study which reported that centrally obese individuals have poor prognostic outcomes among breast cancer study participants (49).
This study also found that those living in urban areas were less likely to have central obesity by 30% compared to rural residents. This may be due to the reason that accessibility of media, which creates awareness to maintain their weight status, and the probability of getting physician advice is more for urban residents compared to rural residents in Ethiopia (50). Thus, urban residents may undergo regular exercise and modify their diet (which can lower the risk of central obesity and also help to improve the problem). Additionally, cancer patients living in rural areas have less knowledge about the association of central obesity with cancer outcomes. The disparities in cancer risk factors, incidence, mortality, and associated metabolic diseases are also greatly affected by the residency of the patients (51). Therefore, it will be encroached to have health education focused on central obesity for cancer patients.
This study identifies the magnitude of the hidden type of obesity called central or visceral or abdominal obesity and found that its prevalence was 19.27% among cancer patients attending at oncology unit of the University of Gondar Specialized Comprehensive Hospital. This magnitude was significantly higher among female study participants than that males. Being female, having urban residency, and having occupations other than farmer, merchant, and government were found to be the factors associated with central obesity. Based on our findings, we encouraged screening for early identification of central obesity among cancer patients, and it will be good to manage it, especially for those participants who belong to the associated factors.
This is the first study that focused on the burden of central obesity and its associated factors in Ethiopia. The study area that fills the knowledge gap on it is taken as the study’s main strength. Being a cross-sectional study design that did not show the cause-effect relationship of the variables, the inability to address the dietary habit of the participants, and the inability to use hip to waist circumference ratio to define central obesity were the study’s limitations. Besides, this study was focused at a single institute that did not generalize the overall cancer patient of Ethiopia, which was another limitation of the study.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki. Ethical approval was obtained from the University of Gondar Ethical Review Committee. Before running the data collection, written informed consent was obtained from each study participants. All the way through the process of the study, confidentiality was kept for all study participants. The patients/participants provided their written informed consent to participate in this study.
All authors contributed to the study’s conception and design. All authors performed material preparation, data collection, and analysis. The first draft of the manuscript was written by MM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Our deepest gratitude goes to Robel Hussen Kabthymer for his major contribution for the edition of language and grammatical errors of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. WHO. Noncommunicable diseases progress monitor. Geneva: World Health Organization (2017). Available at: https://www.who.int/publications/i/item/9789241513029.
2. Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet (2018) 392(10159):1736–88. doi: 10.1016/S0140-6736(18)32203-7
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
4. Kingham TP, Alatise OI, Vanderpuye V, Casper C, Abantanga FA, Kamara TB, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol (2013) 14(4):e158–e67. doi: 10.1016/S1470-2045(12)70472-2
5. Farmer P, Frenk J, Knaul FM, Shulman LN, Alleyne G, Armstrong L, et al. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet (2010) 376(9747):1186–93. doi: 10.1016/S0140-6736(10)61152-X
6. Moussas G, Papadopoulou A. Substance abuse and cancer. Psychiatriki J (2017) 28(28):234–41. doi: 10.22365/jpsych.2017.283.234
7. Munsell MF, Sprague BL, Berry DA, Chisholm G, Trentham-Dietz A. Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiol Rev (2014) 36(1):114–36. doi: 10.1093/epirev/mxt010
8. medicine CGoESoOC. Ovarian cancer and body size: individual participant meta-analysis including 25,157 women with ovarian cancer from 47 epidemiological studies. PLOS Med (2012) 9(4):. doi: 10.1371/journal.pmed.1001200
9. Campbell PT, Newton CC, Freedman ND, Koshiol J, Alavanja MC, Freeman LEB, et al. Body mass index, waist circumference, diabetes, and risk of liver cancer for US adults. Cancer Res (2016) 76(20):6076–83. doi: 10.1158/0008-5472.CAN-16-0787
10. Dougan MM, Hankinson SE, Vivo ID, Tworoger SS, Glynn RJ, Michels KB. Prospective study of body size throughout the life-course and the incidence of endometrial cancer among premenopausal and postmenopausal women. Int J Res (2015) 137(3):625–37. doi: 10.1002/ijc.29427
11. Hoyo C, Cook MB, Kamangar F, Freedman ND, Whiteman DC, Bernstein L, et al. Body mass index in relation to oesophageal and oesophagogastric junction adenocarcinomas: a pooled analysis from the international BEACON consortium. Int J Epidemiol (2012) 41(6):1706–18. doi: 10.1093/ije/dys176
12. Wang F, Xu Y. Body mass index and risk of renal cell cancer: a dose-response meta-analysis of published cohort studies. Int J Res (2014) 135(7):1673–86. doi: 10.1002/ijc.28813
13. Li L, Gan Y, Li W, Wu C, Lu Z. Overweight, obesity and the risk of gallbladder and extrahepatic bile duct cancers: a meta-analysis of observational studies. Obesity (2016) 24(8):1786–802. doi: 10.1002/oby.21505
14. Kitahara CM, McCullough ML, Franceschi S, Rinaldi S, Wolk A, Neta G, et al. Anthropometric factors and thyroid cancer risk by histological subtype: pooled analysis of 22 prospective studies. Thyroid (2016) 26(2):306–18. doi: 10.1089/thy.2015.0319
15. Mooney SJ, Baecker A, AG R. Comparison of anthropometric and body composition measures as predictors of components of the metabolic syndrome in a clinical setting. Obes Res Clin Pract (2013) 7(1):e55–66. doi: 10.1016/j.orcp.2012.10.004
16. Park J, Lee ES, Kim J, Park SE, Park C-Y, Lee W-Y, et al. Waist circumference as a marker of obesity is more predictive of coronary artery calcification than body mass index in apparently healthy Korean adults: the kangbuk Samsung health study. Endocrinol Metab (2016) 31(4):559–66. doi: 10.3803/EnM.2016.31.4.559
17. Jones LW, Demark-Wahnefried W. Diet, exercise, and complementary therapies after primary treatment for cancer. Lancet Oncol (2006) 7(12):1017–26. doi: 10.1016/S1470-2045(06)70976-7
18. Brown JK, Byers T, Doyle C, Courneya KS, Demark-Wahnefried W, Kushi LH, et al. Nutrition and physical activity during and after cancer treatment: an American cancer society guide for informed choices. CA Cancer J Clin (2003) 53(5):268–91. doi: 10.3322/canjclin.53.5.268
19. Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin (2019) 69(2):88–112. doi: 10.3322/caac.21499
20. Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist (2010) 15(6):556–65. doi: 10.1634/theoncologist.2009-0285
21. Molla MD, Wolde HF, Atnafu A. Magnitude of central obesity and its associated factors among adults in urban areas of Northwest Ethiopia. Diabetes Metab Syndr Obes: Targets and Therapy (2020) 13:4169. doi: 10.2147/DMSO.S279837
22. Dagne S, Menber Y, Petrucka P, Wassihun Y. Prevalence and associated factors of abdominal obesity among the adult population in woldia town, northeast Ethiopia, 2020: Community-based cross-sectional study. PLOS ONE (2021) 16(3):. doi: 10.1371/journal.pone.0247960
23. Janakiraman B, Abebe SM, Chala MB, Demissie SF. Epidemiology of general, central obesity and associated cardio-metabolic risks among university employees, Ethiopia: a cross-sectional study. Diabetes Metab Syndr Obes: Targets and Therapy (2020) 13:343. doi: 10.2147/DMSO.S235981
24. Geto Z, Challa F, Lejisa T, Getahun T, Sileshi M, Nagasa B, et al. Cardiometabolic syndrome and associated factors among Ethiopian public servants, Addis Ababa, Ethiopia. Sci Rep (2021) 11(1):20635. doi: 10.1038/s41598-021-99913-6
25. Biru B, Tamiru D, Taye A, Regassa Feyisa B. Central obesity and its predictors among adults in nekemte town, West Ethiopia. SAGE Open Med (2021) 9:20503121211054988. doi: 10.1177/20503121211054988
26. Lintonen TP, Konu AI. Adolescent alcohol beverage type choices reflect their substance use patterns and attitudes. J Youth Adolesc (2003) 32(4):279–89. doi: 10.1023/A:1023084927465
27. Tesfaye F, Byass P, Wall S, Berhane Y, Bonita R. Peer reviewed: Association of smoking and khat (Catha edulis forsk) use with high blood pressure among adults in Addis Ababa, Ethiopia, 2006. Prev Chronic Dis (2008) 5(3). Available at: http://www.cdc.gov/pcd/issues/2008/jul/07_0137.htm.
28. Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, et al. The world health organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health (2016) 106(1):74–8. doi: 10.2105/AJPH.2015.302962
30. IDF Epidemiology Task Force Consensus Group. International Diabetes Federation: The IDF consensus worldwide definition of the metabolic syndrome. Diabetes Voice (2005). Available at: http://www.idf.org/webdata/docs/Metabolic_syndrome_def.pdf.2005.
31. Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, et al. European Society of hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens (2014) 32(7):1359–66. doi: 10.1097/HJH.0000000000000221
32. Lee M-J, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med J (2013) 34(1):1–11. doi: 10.1016/j.mam.2012.10.001
33. Upadhyay J, Farr O, Perakakis N, Ghaly W, Mantzoros C. Obesity as a disease. Med Clinics (2018) 102(1):13–33. doi: 10.1016/j.mcna.2017.08.004
34. Vickers N. Animal communication: when i’m calling you, will you answer too? Curr Biol (2017) 27(14):R713–R5. doi: 10.1016/j.cub.2017.05.064
35. Wu C-H, Heshka S, Wang J, Pierson R, Heymsfield S, Laferrere B, et al. Truncal fat in relation to total body fat: influences of age, sex, ethnicity and fatness. Int J Obes (2007) 31(9):1384–91. doi: 10.1038/sj.ijo.0803624
36. Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol (2012) 56(3):704–13. doi: 10.1016/j.jhep.2011.09.020
37. Riondino S, Roselli M, Palmirotta R, Della-Morte D, Ferroni P, Guadagni F. Obesity and colorectal cancer: role of adipokines in tumor initiation and progression. World J Gastroenterol (2014) 20(18):5177. doi: 10.3748/wjg.v20.i18.5177
38. Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci (2014) 1311(1):57–76. doi: 10.1111/nyas.12416
39. Bianchini F, Kaaks R, Vainio H. Weight control and physical activity in cancer prevention. (2002) 3(1):5–8. doi: 10.1046/j.1467-789X.2002.00046.x
40. Healy L, Ryan A, Carroll P, Ennis D, Crowley V, Boyle T, et al. Metabolic syndrome, central obesity and insulin resistance are associated with adverse pathological features in postmenopausal breast cancer. Clin Oncol (2010) 22(4):281–8. doi: 10.1016/j.clon.2010.02.001
41. Agalliu I, Lin W-KJ, Zhang JS, Jacobson JS, Rohan TE, Adusei B, et al. Overall and central obesity and prostate cancer risk in African men. Cancer Causes Control (2022) 1:1–17. doi: 10.1007/s10552-021-01515-0
42. Nam GE, Cho KH, Han K, Kim CM, Han B, Cho SJ, et al. Obesity, abdominal obesity and subsequent risk of kidney cancer: a cohort study of 23.3 million East asians. Br J Cancer (2019) 121(3):271–7. doi: 10.1038/s41416-019-0500-z
43. de Marins VR, Almeida RV, Pereira R, Barros M. Factors associated with overweight and central body fat in the city of Rio de Janeiro: results of a two-stage random sampling survey. Elsevier Public health (2001) 115(3):236–42. doi: 10.1038/sj.ph.1900763
44. Al-Riyami AA, Afifi MM. Prevalence and correlates of obesity and central obesity among omani adults. Saudi Med J (2003) 24(6):641–6.
45. Rathnayake KM, Roopasingam T, Dibley MJ. High carbohydrate diet and physical inactivity associated with central obesity among premenopausal housewives in Sri Lanka. BMC Res Notes (2014) 7(1):1–7. doi: 10.1186/1756-0500-7-564
46. Belavý D, Möhlig M, Pfeiffer A, Felsenberg D, Armbrecht G. Preferential deposition of visceral adipose tissue occurs due to physical inactivity. Int J Obes (2014) 38(11):1478–80. doi: 10.1038/ijo.2014.26
47. Jackson AS, Stanforth PR, Gagnon J, Rankinen T, Leon AS, Rao D, et al. The effect of sex, age and race on estimating percentage body fat from body mass index: The heritage family study. Lancet (2002) 26(6):789–96. doi: 10.1038/sj.ijo.0802006
48. Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev (2017). doi: 10.1152/physrev.00019.2016
49. Chen H-l, Ding A, Wang M-l. Impact of central obesity on prognostic outcome of triple negative breast cancer in Chinese women. SpringerPlus (2016) 5(1):1–8. doi: 10.1186/s40064-016-2200-y
50. BeLue R, Okoror TA, Iwelunmor J, Taylor KD, Degboe AN, Agyemang C, et al. An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global Health (2009) 5:1–12. doi: 10.1186/1744-8603-5-10
Keywords: cancer, central obesity, body mass index, associated factors, behavioral factors
Citation: Molla MD, Wolde HF, Tafesse Teferi E and Kibret AA (2023) Central obesity and its associated factors among cancer patients at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Front. Oncol. 13:1150303. doi: 10.3389/fonc.2023.1150303
Received: 07 February 2023; Accepted: 29 March 2023;
Published: 12 April 2023.
Edited by:
Tuo Deng, Second Xiangya Hospital, Central South University, ChinaCopyright © 2023 Molla, Wolde, Tafesse Teferi and Kibret. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meseret Derbew Molla, bWVzc2lkcm0xOUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.