- 12nd Department of Oncology, Faculty of Medicine, Comenius University and National Cancer Institute, Bratislava, Slovakia
- 2Institute of Molecular Biomedicine, Faculty of Medicine, Comenius University, Bratislava, Slovakia
- 3Translation Research Unit, Comenius University, National Cancer Institute, Bratislava, Slovakia
- 41st Department of Oncology, Faculty of Medicine Comenius University (FMCU) and St. Elizabeth Cancer Institute, Bratislava, Slovakia
- 5Department of Surgical Oncology, National Institute for Oncology, Bratislava, Slovakia
Background: Testicular cancer is the most common malignancy among young men. Vitamin D has pluripotent effects on cancer pathogenesis and plays a role in the metastatic cascade. The aim of this study is to analyze plasma vitamin D in association with clinico-pathological findings and prognosis in patients with germ-cell tumors (GCTs).
Methods: This study included 120 newly diagnosed and/or relapsed GCT patients treated from April 2013 to July 2020, for whom plasma was available in the biobank. Blood samples were drawn the 1st chemotherapy cycle as well as before the 2nd cycle. Plasma vitamin D was measured using ELISA and correlated with disease characteristics and the outcome. For survival analysis, the cohort was dichotomized into “low” and “high” based on median vitamin D.
Results: There was no significant difference in vitamin D plasma levels between healthy donors and GCT patients (p = 0.71). Vitamin D level was not associated with disease characteristics except for brain metastases, where patients with brain metastases had a vitamin D level that was 32% lower compared to patients without brain metastases, p = 0.03. Vitamin D was also associated with response to chemotherapy, with an approximately 32% lower value in patients with an unfavorable response compared to a favorable response, p = 0.02. Moreover, low plasma levels of vitamin D were significantly associated with disease recurrence and inferior progression-free survival (PFS), but not with overall survival (OS) (HR = 3.02, 95% CI 1.36–6.71, p = 0.01 for PFS and HR = 2.06, 95% CI 0.84–5.06, p = 0.14 for OS, respectively).
Conclusion: Our study suggests the prognostic value of pretreatment vitamin D concentrations in GCT patients. Low plasma vitamin D was associated with an unfavorable response to therapy and disease recurrence. However, it remains to be determined whether the biology of the disease confirms a causative role for low vitamin D and whether its supplementation affects the outcome.
Introduction
Testicular cancer is the most common cancer among young men below 34 years old; however, its overall contribution to all cancers among men is only 1% (1). Cure rates reach more than 90% (2), mostly depending on the clinical stage of the disease and associated patient symptoms (3). However, GCTs represent a heterogeneous group of patients, and the prognosis substantially varies according to the International Germ Cell Collaborative Group (IGCCCG) classification, with 5-year survival ranging from 98% in the good-risk group to 50%–70% in the poor-risk group (4, 5). Current prognostic biomarkers widely used are encompassed in the IGCCCG risk stratification model, such as serum tumor markers and extension of disease according to tumor, node, and metastasis status (6). Despite this, new biomarkers with further proper prognostic stratification in germ cell populations are still needed. Programmed death ligand-1 status as well as neutrophil–lymphocyte ratio or systemic inflammatory index were evaluated as prognostic biomarkers in several studies (7, 8), with favorable results as independent predictors of outcome in a selected population of germ cell tumors, but their usefulness in practice has not been established. Thus, understanding disease better with more precise identification of patients with an adverse prognosis is crucial to improving patient management, as might be disease monitoring.
Vitamin D is the precursor of the steroidal hormone calcitriol (9), which is currently investigated among various cancers such as gastrointestinal cancers, breast cancers, and urological cancers (10, 11). It is generally known that vitamin D plays its key role in the regulation of calcium metabolism (12); moreover, vitamin D is an inducer of cell differentiation and promotes inhibition of epithelial–mesenchymal transmission (13, 14); as well, vitamin D shares antiangiogenic properties (15, 16), regulates autophagy (17, 18), and induces apoptosis (19, 20). Vitamin D mediates its function via the vitamin D receptor (21), which is being expressed on immune cells (22), as well as in germ cell tumor tissue, unlike tumor-free testicular tissue (23). Therefore, immunity regulation is also affected by vitamin D in terms of shifting T-cell maturation from the Th1 to the Th2 phenotype as well as the polarization of tumor-associated macrophages from the M1 to the M2 type, where antitumor abilities are promoted (22). Moreover, inhibition of differentiation and mutation of dendritic cells, along with inhibition of B-cell proliferation, is caused by vitamin D (24). To conclude, suppression of pro-inflammatory cytokines and increased production of anti-inflammatory cytokines are mediated by vitamin D (24). The role of vitamin D in testicular germ cell tumors (TGCTs) was assessed in this study (23); a significant anti-proliferative effect on testicular germ cell tumor lines was found. Along with that, vitamin D also affects gene expression in TGCT lines, such as upregulation tumor suppressor genes along with upregulation of MAP kinase-activated protein kinase 2 (MAPKAP2) (23), which may play a role in cancer invasiveness (25). Moreover, differentiation of GCT is also affected by vitamin D. Downregulation of pluripotency genes as well as inducement of mesenchymal transmission toward an osteogenic phenotype in embryonal carcinoma cells were observed (26). Therefore, it seems that vitamin D may operate as an inhibitor of cell proliferation and most likely have an impact on metastasis. Thus, the question arises whether vitamin D might be associated with more favorable tumor features as well as with tumor behavior such as less aggressive behavior.
The prognostic association of vitamin D serum levels was also studied (27–29). Studies have shown that low plasma levels of vitamin D in patients are seen in advanced stages of disease (30, 31). Moreover, a prognostic association between plasma levels of vitamin D and overall and/or progression-free survival was evaluated in cancers such as melanoma, colorectal, breast, and Hodgkin´s lymphoma (32–34), where a positive correlation between vitamin D plasma value with progression-free survival (PFS) and/or overall survival (OS) was established. Due to this date, there was no specific study that addressed the prognostic significance of vitamin D in testicular germ cell tumors.
Studies evaluating the function of vitamin D proposed that the metastatic cascade is affected by the vitamin D plasma serum level (35–38); therefore, we hypothesize that there might be an association between the site and/or presence of metastases and the vitamin D plasma level in germ cell tumor patients. Positive association between low vitamin D level and promotion of bone metastasis as well as lung metastases in mice with breast tumor models was well described (36, 39); thus, potential correlation between clinic-pathological variables in testicular germ cell tumor and vitamin D plasma level might be present as well.
The aim of this study was to assess the relationship between clinico-pathological characteristics and vitamin D plasma levels in patients with GCT, as well as to determine its impact on prognosis in specific subpopulations.
Methods
Study population
The study included 120 newly diagnosed and/or relapsed GCT patients treated with first-line or salvage chemotherapy from April 2013 to July 2020 at the National Cancer Institute and/or St. Elizabeth Cancer Institute, for whom plasma was available in the biobank. Vitamin D supplementation in the studied group was not assessed in this study. Data regarding tumor histology, chemotherapy regimens, stage of disease, type and site of metastasis, and other patient and tumor characteristics were recorded and correlated with vitamin D levels. The study was approved by the institutional review board of the National Cancer Institute of Slovakia. The study included 21 age-matched healthy donors (HD) as well. Each participant, including healthy donors, signed informed consent before the study was initiated.
Plasma sample collection
From each enrolled study subject, an atraumatic peripheral blood sample of 1 ml was collected at the antecubital fossa and transferred into EDTA-treated collection tubes (BD Vacutainer®) at baseline in the morning on day -1 or 0 of the first or later line (>1) of chemotherapy. Collected blood samples were centrifuged at 5,000 rpm for 10 min at room temperature within 2 h of venipuncture. Obtaining supernatants were aliquoted into 1-ml aliquots that were archived at −80°C until further analysis.
Vitamin D
A commercial ELISA kit was used for the quantification of 25-hydroxyvitamin D (catalog number DE1971, Demeditec Diagnostics, Kiel, Germany). With an analytical sensitivity of 3 ng/ml, the kit was chosen for the expected low concentrations in the collected samples. Technical variability expressed as intra- and inter-assay coefficients of variation was 3% and 10%, respectively.
Determination of leukocyte immunophenotypes
Leukocyte immunophenotypes were determined in peripheral blood samples collected from analyzed GCT patients in an EDTA-treated collection tube. Tested samples were processed within 24 h following collection, as described below.
Briefly, leukocytes were stained using fluorochrome-conjugated antibodies from BD Pharmingen, and subsequently, leukocytes with defined immunophenotypes were quantified using flow cytometry (Canto II Cytometer; Becton, Dickinson and Company, Franklin Lakes, NJ, USA). The antibody combinations used for the basic panel, the regulatory T-cell panel, the dendritic-cell (DC) panel, and the myeloid-derived suppressor-cell panel were used in the same scheme as that described by Kalavska et al. (40). Used antibodies are also listed in the supplementary material. A cocktail of applied antibodies was incubated with 300,000–500,000 white blood cells in 200 µl for 20 min at room temperature. For the assessment with a BD FACSCanto™ II flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA), a minimum of 100,000 leukocytes were utilized. KALUZA software (Beckman Coulter, Inc., Brea, CA, USA) was used for the analysis of the flow cytometry data. Forward scatter (FSC) and side scatter were used to exclude debris according to size and granularity, while the exclusion of doublets was performed using FSC-Height and FSC-Area. The number of gated cells considered the minimum for evaluation was 100.
Statistical analysis
The data were tabulated. Characteristics were summarized using the mean or median (range) for continuous variables and the frequency (percentage) for categorical variables, respectively. Statistical analysis was performed using non-parametric tests as the distribution of vitamin D expression was with non-normal distribution (Shapiro–Wilk test). The non-parametric Kruskal–Wallis test was used for the analysis of the association of serum vitamin D expression with clinico-pathological variables between two and more than two groups of patients. The independence of two group variables with dichotomized vitamin D levels (“low” vs “high” based on median value) was compared by the Fischer test. PFS was calculated from the date of starting treatment with chemotherapy to the date of progression, death, or the date of the last adequate follow-up. OS was calculated from the date of starting chemotherapy treatment to the date of death or last follow-up. A univariate Kaplan–Meier statistical approach was used to assess the outcome of survival data in conjunction with vitamin D status (“high,” defined as above the median vs. “low,” below the median) in certain populations among our studied group. Moreover, vitamin D plasma levels, which were available before the 1st and 2nd cycles of chemotherapy (CTx) in the same patients, were stratified into four groups based on the dichotomized value of vitamin D (Low-Low (LL), Low-High (LH), High-Low (HL), and High-High (HH)) and compared in univariate Kaplan–Meier models for PFS and OS as well. All p-values presented are two-sided, and associations were considered significant if the p-value was less than or equal to 0.05. Statistical analyses were performed with NCSS 2022 statistical software.
Results
Patients’ characteristics
From April 2013 to January 2020, the study encompasses 120 patients with diagnosed GCTs and 21 age-matched healthy donors. Most of the patients were treatment naïve 105 (90%), while 12 (10%) were treated with salvage chemotherapy. The median age of the studied population was 34 years, compared to a median age of 35 years for healthy donors (p = 1.00). Table 1 summarizes patient and/or disease characteristics. The majority of patients had non-seminoma histology, prevailing at clinical stage III, and were mostly represented as having a low-risk disease, according to the IGCCCG classification. The most common site of metastases was the retroperitoneum, followed by the lungs, while brain metastases were revealed in only six patients.
Comparison of vitamin D plasma level between GCT and healthy donors
In our studied group, we did not observe a significant difference in vitamin D plasma level between healthy donors and GCT patients (mean ± standard error of the mean was 15.7 ± 1.6 ng/ml vs. 15.9 ± 0.7 ng/ml, p = 0.71). The vitamin D plasma level of healthy donors was also compared to the vitamin D level in GCT patients in a month-matched manner to exclude differences due to sun exposure; no significant difference was observed (p = 0.23).
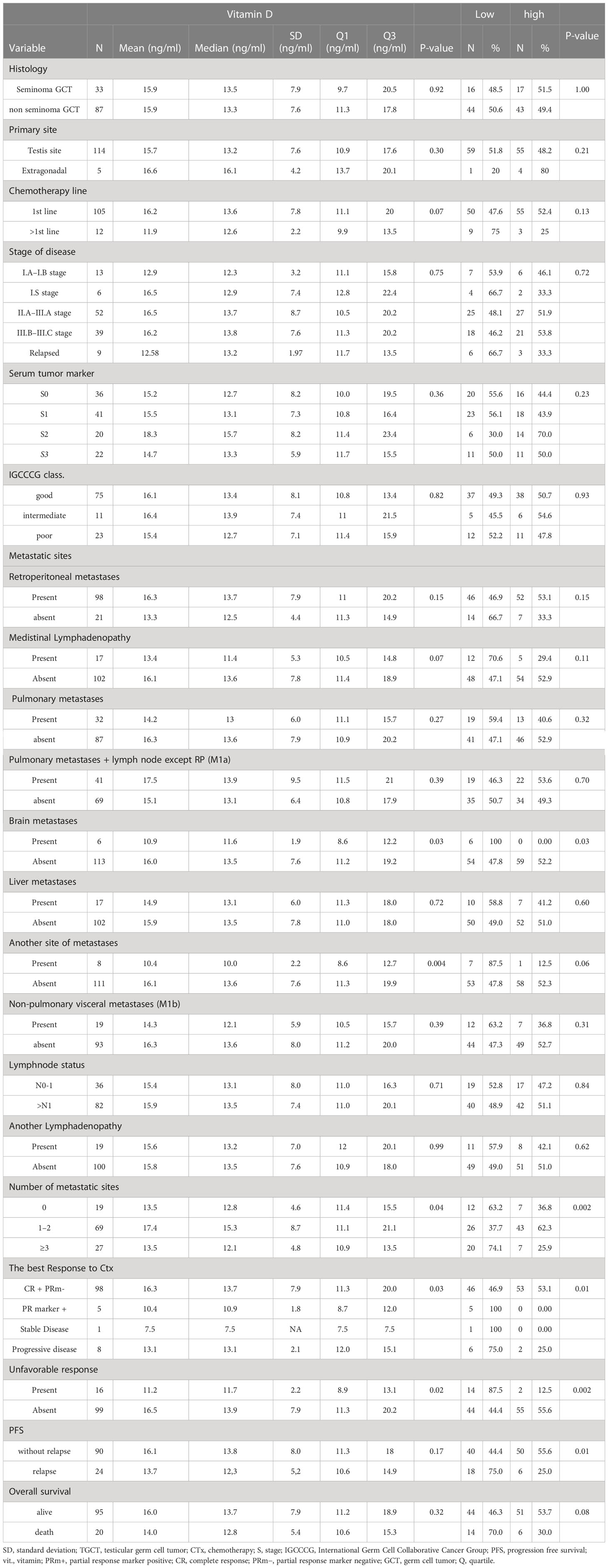
Association between mean plasma vitamin D level and patients and/or tumor characteristics
The mean plasma level of vitamin D ± SEM was compared between groups of patients with different clinico-pathological variables (Table 2). There was no difference in plasma level and patient and/or tumor characteristics except for the number of metastatic sites (>3) and brain metastases, which were inversely associated with vitamin D level. A significant difference was also retained when comparing healthy donors to GCT patients with brain metastases (10.9 ± 2.4 ng/ml vs 15.7 ± 1.3 ng/ml, p = 0.03); however, the number of patients with brain metastases was only six. We were not able to confirm similar results in GCT patients with metastatic sites (>3) (p = 0.13). We also compared vitamin D values among GCT patients in the 1st-line chemotherapy setting to patients with relapse, where a significant difference in vitamin D value was not observed (p = 0.06).
There was a significant difference in the mean vitamin D level depending on response to the chemotherapy regimen. High levels of vitamin D were observed in patients with complete responses and partial response markers negative compared to patient in whom partial response markers were positive stable or progressive disease was stated (p = 0.03). Similarly, a significantly lower mean vitamin D value was associated with patients in whom an unfavorable response (other than complete remission and/or partial remission with negative serum tumor markers) was experienced (p = 0.02). Moreover, a significant difference in vitamin D level was observed when poor responders (patients with an unfavorable response to chemotherapy) were compared to healthy donors (10.8 ng/ml vs 15.7 ng/ml, p = 0.0002). The vitamin D plasma value was also compared before the 1st cycle of chemotherapy and again after the 2nd cycle among all studied patients; however, this difference was not statistically significant.
Association between vitamin D plasma level and specific immune cell subpopulations
Statistical analysis of a possible link between the level of vitamin D in the plasma of GCT patients and changes in the percentage of evaluated innate immune cells revealed that the level of vitamin D in the plasma positively correlated with the number of eosinophils (p = 0.009) and the percentage of CD4-positive NKT cells, (p = 0.02). On the other hand, an inverse association was determined between plasma vitamin D and CD16+ HLADR+ Lin− dendritic cells (DCs) (p = 0.01).
Assessing selected adaptive immune cells subpopulations, we found no significant link between value of vitamin D in plasma of GCT patients and adaptive immune cells percentage.
Prognostic value of vitamin D level and patients’ outcome
The median follow-up of all patients was 22.6 months (ranging from 0.1 to 100.4 months); 24 patients (20%) experienced disease relapse, and 19 patients (16.67%) experienced death. Patients who experienced disease relapse had a lower vitamin D level compared to patients without recurrence (13.7 ± 1.5 ng/ml vs 16.1 ± 0.8 ng/ml, p = 0.01).
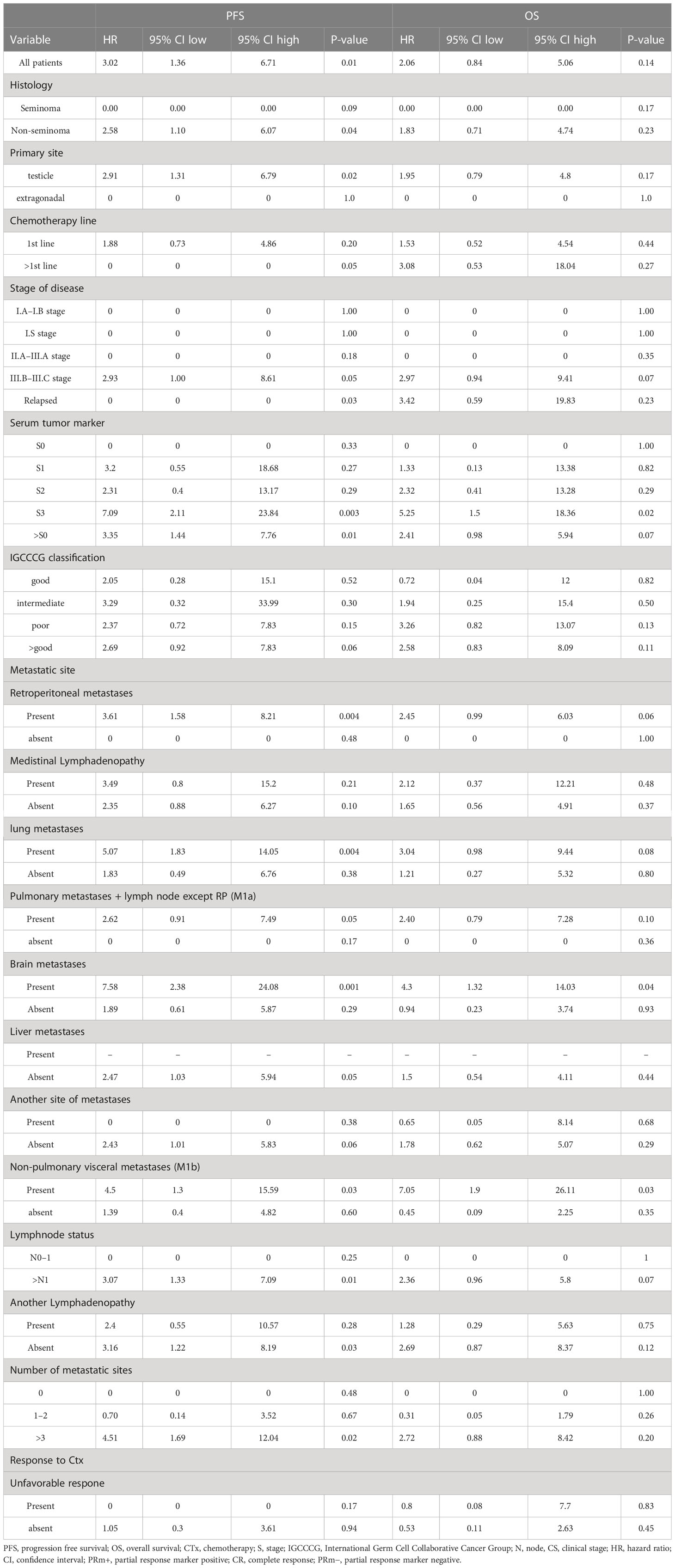
Patients with “low” plasma vitamin D level had significantly inferior PFS but not OS compared to patients with “high” plasma vitamin D level (hazard ratio [HR] = 3.02, 95% CI (1.36–6.71), p = 0.01 for PFS and [HR] = 2.06, 95% CI (0.84–5.06), p = 0.14 for OS, respectively) (Figures 1, 2). The prognostic impact of vitamin D level in PFS was observed in non-seminoma histology as well as in patients with clinical stages III.B–III.C. Metastatic disease (>3) retains prognostic significance, and metastatic sites such as liver metastases and retroperitoneal lymphadenopathy, as well as patients with non-pulmonary visceral metastases, were significantly associated with the prognostic impact between PFS and vitamin D plasma level (Table 3). Subgroup analysis revealed the prognostic value of plasma vitamin D level for OS and for PFS in patients with highly elevated serum tumor markers (S3); additionally, prognostic significance for OS was observed in patients with non-pulmonary visceral metastases as well as in a subgroup of patients with brain metastases. Vitamin D plasma level retains its prognostic significance in OS and in patients stratified according to treatment response, particularly in those who did not reach a complete response.
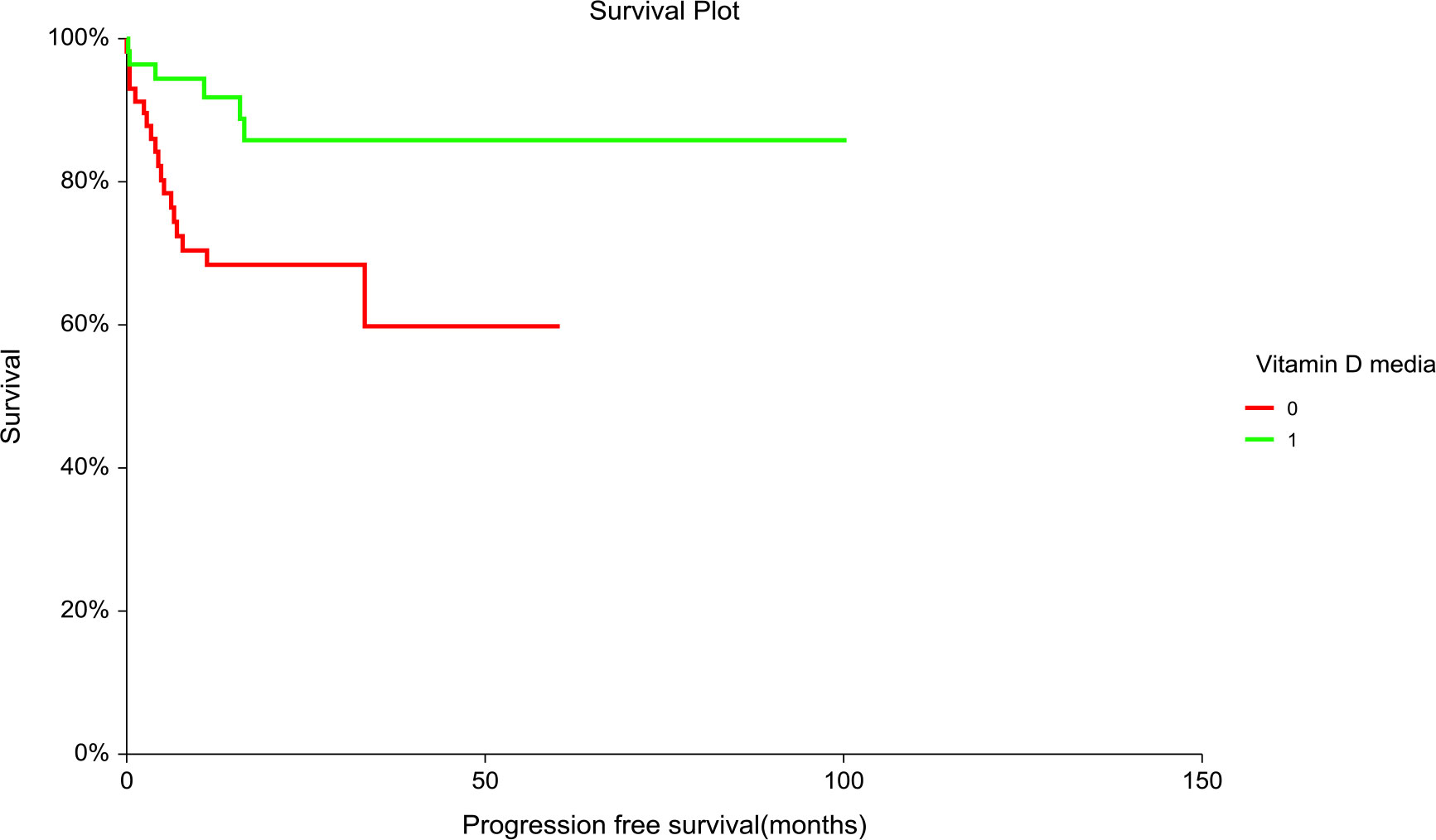
Figure 1 Kaplan–Meier estimates of probabilities of progression-free survival according to plasma vitamin D concentration in TGCT patients (N = 120). Patients with plasma vitamin D concentration above median had significantly better PFS as compared to patients with lower vitamin D concentration [HR] = 3.02, 95% CI (1.36–6.71), p = 0.014; 0 = plasma vitamin D concentration below median, 1 = plasma vitamin D concentration above median). HR, hazard ratio; TCGT, testicular germ cell tumor; PFS, progression free survival; 0, plasma vitamin D concentration below median; 1, plasma vitamin D concentration above median.
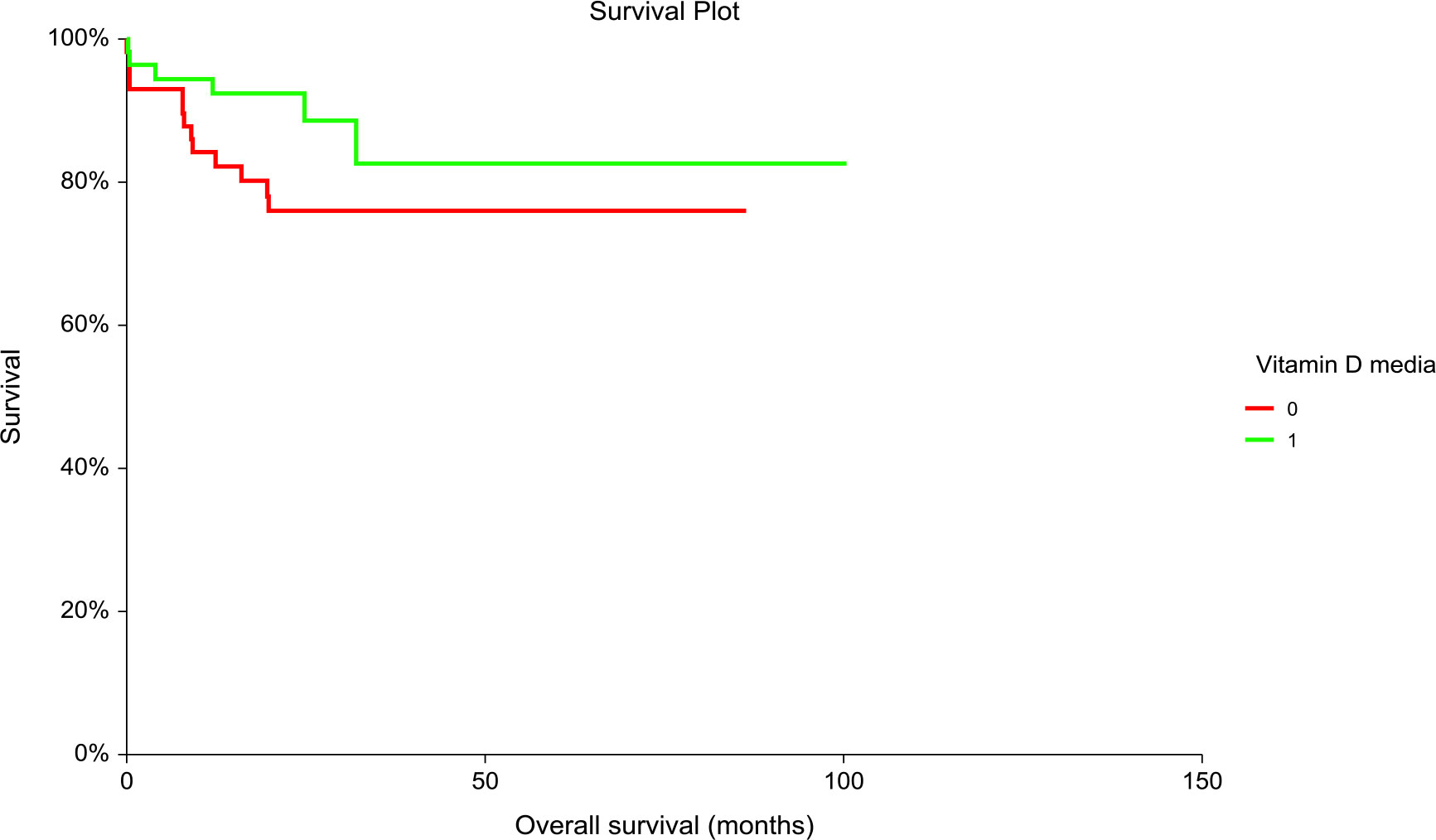
Figure 2 Kaplan–Meier estimates of probabilities of overall survival according to plasma vitamin D levels in TGCT patients (N = 120). Patients with plasma vitamin D concentration above median had non-significantly better OS as compared to patients with lower vitamin D concentration, [HR] = 2.06, 95% CI (0.84–5.06), p = 0.135; 0 = plasma vitamin D concentration below median, 1 = plasma vitamin D concentration above median). HR, hazard ratio; TCGT, testicular germ cell tumor; OS, overall survival; 0, plasma vitamin D concentration below median; 1, plasma vitamin D concentration above median.
We also looked for the kinetics of vitamin D levels between the 1st and 2nd cycles of chemotherapy, where patients with “low-low” compared to “high-high” had worse PFS (HR = 1.19, 95% CI 0.4–3.53, p = 0.03) and OS (HR = 1.05, 95% CI 0.38–2.96, p = 0.04).
Discussion
In this translational study, we observed for the first time that vitamin D has a prognostic impact on patients with diagnosed germ cell tumors. The prognostic significance of vitamin D plasma level and progression-free survival was statistically significant among all studied patients; unfortunately, overall survival among all studied patients and its association with vitamin D plasma level failed to prove statistical significance. This observation is consistent with studies evaluating the prognostic impact of vitamin D in different types of cancer (41–43), whereas studies addressing the prognostic impact of vitamin D plasma level in testicular cancer are currently lacking. In our study group, the prognostic impact of vitamin D plasma level on overall survival was demonstrated in patients with highly elevated serum tumor markers (S3) and in patients with non-pulmonary and liver metastases. Thus, this prognostically significant observation in overall survival is mostly prominent in patients with advanced stages of disease. The prognostic impact of vitamin D in PFS, unlike in OS, among all studied patients might be explained by the fact that vitamin D levels might change over time during the course of the disease. Moreover, subsequent salvage treatment approaches might affect the prognostic value of vitamin D on OS as well. Interestingly, we also observed a significant association between vitamin D plasma levels and patient and tumor characteristics. Brain metastases as well as the number of metastatic sites (except for brain, liver, lung, and lymph nodes) were associated with a lower plasma vitamin D level. This observation is in concordance with studies (44, 45), which found a significant association between vitamin D plasma level and adverse clinico-pathological features in cancer, such as breast cancer or melanoma (44, 45). However, due to the limited number of patients with brain metastases, those results are hypothesis-generating and deserve further validation in a larger cohort of patients that includes patients with brain metastases. Those findings are also attributed to the fact that a low value of vitamin D is mostly associated with an advanced stage of disease in cancer (4, 45), which is in concordance with our study, where we observed a lower level of vitamin D in higher clinical stages of disease (III.B–III.C). Another significant fact observed among the studied group was that response to treatment in terms of partial response marker negative (PRm−) and complete response (CR) as well as patients without relapse were associated with higher plasma levels of vitamin D. Those observations raised several new questions.
Surprisingly, we did not observe a significant difference in vitamin D plasma level between GCT and healthy donors; moreover, we did not observe a significant difference in the month-matched analysis of vitamin D plasma value between HD and GCT patients. The mean vitamin D level among all studied patients was observed in the range of hypovitaminosis; thus, most patients were characterized by vitamin D insufficiency; likewise, the mean vitamin D level in HD was also in the range of hypovitaminosis (46). There is evidence that GCT survivors are associated with an insufficient level of vitamin D after treatment of primary cancer (47–50), with a significant difference compared to healthy donors (48, 49). An interesting study, which encompassed healthy donors as well as GCT patients, was outdone, where measurements of vitamin D values in GCT patients were assessed in longitudinal follow-up (>24 months) (51). In this study, decrement of vitamin D in GCT patients after orchiectomy was only transient, with a nadir at 6 months (mean), and thereafter, values comparable to healthy donors were observed (51). To sum it up, the mentioned studies proposed conflicting results: that vitamin D level in GCT patients and/or survivors is associated with hypovitaminosis, which could be transient, however, whether the potential explanation of hypovitaminosis might arise from its high prevalence in the general population is up for debate. Those conflicting results were also confirmed by a systematic review (52), where selection bias and different study designs could have an impact on the variability of results (52). Therefore, prospective studies to assess vitamin D variability and GCT patients according to treatment approaches and comparison with aged and month-matched blood draws from healthy donors are needed to establish its relationship. It is known that cholecalciferol is also produced in the skin and is mostly dependent on solar radiation (53), which is highest at latitudes below 40° (54). Moreover, dietary habits play an additional role as a source of vitamin D (55); thus, both factors may contribute to the high prevalence of vitamin D hypovitaminosis among populations in middle Europe, such as the Slovak population, which counts for more than 40% (56, 57). Therefore, we can conclude that GCT patients could be associated with an insufficient level of vitamin D after orchiectomy; however, a significant difference in vitamin D level between TGCT patients and the healthy population might not be observed, which is also in accordance with our results. Thus, hypovitaminosis in our cohorts might be due to the high prevalence of vitamin D insufficiency in the general population, which participated in our results. Moreover, we found out that a significant difference in vitamin D level was observed when poor responders were compared to HD. This observation could lead to the hypothesis that vitamin D might mirror a more aggressive disease.
We suggest that one possible explanation for why we observed a tendency of inverse association of vitamin D with brain metastases and the number of metastatic sites might arise from C-X-C motif chemokine ligand 12 and its receptor, C-X-C chemokine receptor type 4 (CXCL12-CXCR4), which are associated with the metastatic cascade (58). In a mouse breast cancer model, vitamin D deficiency increases lung expression of CXCL-12; therefore, CXCR-4-positive tumor cells follow the CXCL-12 gradient (36). Similar pathogenic pathways could run in GCT with brain metastases (59), with strengthened evidence, where the CXCL12-CXCR4 gradient was proposed as responsible for metastasis in the in vitro GCT model (60). Moreover, hypoxemia-induced factor 1-a (HIF-1a) is also affected by vitamin D, which plays a role in epithelial–mesenchymal transmission, migrations and has an impact on vascular proliferation (41); however, no study addressing the role of vitamin D and HIF-1a in germ cell tumors currently exists. Another significant role of vitamin D is the mediation of inhibition via the Wnt/β-catenin pathway (61), while it is already known that high β-catenin levels are associated with poor clinico-pathological features in germ cell tumors (62, 63). Another possible conjunction of vitamin D and progressive disease might be due to low expression of MAPKAPK2, which is already known to play a role in cancer invasion (64, 65). Higher MAPKAPK2 mRNA levels were observed in TGCT cell lines after vitamin D stimulation (23); thus, a potential low value of MAPKAPK2 due to vitamin D deficiency may enhance the activity of matrix metalloproteinase and its sequential invasion (25).
The possible explanation of the correlation between vitamin D and response to chemotherapy treatment in terms of PRm− or CR might be driven by study (66), where the authors conclude, that co-treatment of vitamin D and cisplatin on the germ cell line NTera2 in an in vitro model potentiated its effect. However, this observation was not proved by an in vivo model (66). Moreover, another mechanism is proposed by the Wnt/β-catenin pathway, which could contribute to cisplatin resistance (67–69), along with the fact that vitamin D is a Wnt/β-catenin pathway inhibitor (61). We could postulate hypothesis about why PRm− and CR were mostly seen among vitamin D proficient patients. However, we did not observe a significant relationship between vitamin D and cisplatin sensitivity among the studied group (p = 0.92); unfortunately, our cohort included only two patients with cisplatin-resistant disease, which may contribute to false negative results. The hypothesis that vitamin D could ameliorate cisplatin resistance in germ cell tumors is still up for research, while it is already known that vitamin D reduces cisplatin resistance in other cancers such as oral squamous cell carcinoma (70) and esophageal cancer (71). Therefore, we hypothesize that there might be an additional effect of vitamin D on cisplatin chemotherapy. Moreover, patients with low vitamin D values were characterized by higher relapse rates, suggesting that vitamin D deficiency is associated with disease and is more prone to relapse.
Numerous studies found a significant correlation between vitamin D value and overall and/or progression-free survival in cancers such as melanoma, colorectal, and breast (41–43). One possible explanation might be that vitamin D deficiency is associated with more aggressive elements in cancer, such as altered signaling pathways (1, 16, 23, 36), which may contribute to more unfavorable tumor phenotypes. However, a study evaluating whether vitamin D supplementation changes germ cell tumor patient survival has not yet been carried out; therefore, this question is still a challenge for researchers. While recent data from multi-analysis studies (72, 73) suggest a reduction in cancer mortality. Though various cancers were included in studies, drawing conclusions about their applicability to a precise type of malignancy from meta-analyses (72, 73) is questionable.
This study has several limitations. One of them could be the timeline of patient plasma sample storage, which for some patients is approximately 9 years. Another could be a small number of patients characterized by clinico-pathological findings, such as our cohort consisted mostly of patients in clinical stage III, whereas patients in clinical stage I were included in only 10%. Predominant (>70%) non-seminoma histology in our studied cohort is an additional study limitation that might have an impact on our results. Unlike seminoma, non-seminoma histology might be categorized as a poor-risk disease, and its adverse tumor and/or clinical features are associated with vitamin D deficiency; hence, hypovitaminosis prevalence and impact on PFS among the studied cohort might be affected by a significant contribution from non-seminoma histology predominance with higher clinical stage in our studied cohort. Confounding factors could play an important role in this study, such as patient sun exposure during the season. Most of our blood samples were collected during the winter season (December, January, February, and March) compared to the summer season (June, July, August, and September), which counts for 40% vs 28% among the studied cohort, respectively. Vitamin D level was also compared according to quarter of year, where highest level of vitamin D was observed during summer quarter. This general fact might be associated with higher sun exposure during summer. Thus, extensive, or low sun exposure depending on the season may affect vitamin D plasma values. The performance status of patients in advanced stages of disease must be considered as well. Patients with more advanced stages of disease tend to present with worsening symptoms such as fatigue, weakness, pain, and decreased social interaction (74, 75), which may have an impact on time spent outdoors.
In conclusion, this is the first report that proves an association between GCT patients and clinico-pathological findings; furthermore, we report a significant correlation between plasma vitamin D level and overall and/or progression-free survival in selected populations. This conclusion opens up the question of whether vitamin D supplementation proves advantageous in OS or PFS in a precisely selected population. Those questions deserve further evaluation in animal and interventional studies. Moreover, our observation suggests that a low vitamin D level is associated with relapse in GCT. This observation deserves further evaluation and should be applied and re-evaluated in a more selected population, such as a stage I germ cell tumor, which could provide additional insight into stratifying patients.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional review Board of National Cancer institute of Slovakia. The patients/participants provided their written informed consent to participate in this study.
Author contributions
PL, MM, and KK wrote the main manuscript text, analyzed, and discussed the data. KK, PC, and BV prepared samples and performed experiments. PL, KK, PC, and MM contributed to the interpretation of the results, revised, and edited the manuscript. MM made substantial contributions to the conception and design of the work, revised, and edited the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the Scientific Grant Agency of the Ministry of Education (Project No. VEGA 1/0349/21) and a grant by the Slovak Research and Development Agency (Project number APVV-20-0158).
Acknowledgments
We would like to acknowledge Pekova Z for providing administrative support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Raghavan D. Testicular cancer: maintaining the high cure rate. Oncol (Williston Park) (2003) 17:218–28.
3. Oldenburg J, Berney DM, Bokemeyer C, Climent MA, Daugaard G, Gietema JA, et al. Testicular seminoma and non-seminoma: ESMO-EURACAN clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33:362–75. doi: 10.1016/j.annonc.2022.01.002
4. Ataergin S, Ozet A, Arpaci F, Kilic S, Beyzadeoglu M, Komurcu S. Outcome of patients with stage II and III nonseminomatous germ cell tumors: results of a single center. Indian J Cancer (2007) 44:6–11. doi: 10.4103/0019-509x.31161
5. Msaouel P, Bilen MA, Zhang M, Campbell M, Wang J, Tu SM. Recent developments in the management of germ cell tumors. Curr Opin Oncol (2017) 29:172–8. doi: 10.1097/CCO.0000000000000361
6. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. international germ cell cancer collaborative group. J Clin Oncol (1997) 15:594–603. doi: 10.1200/JCO.1997.15.2.594
7. Chovanec M, Cierna Z, Miskovska V, Machalekova K, Svetlovska D, Kalavska K, et al. Prognostic role of programmed-death ligand 1 (PD-L1) expressing tumor infiltrating lymphocytes in testicular germ cell tumors. Oncotarget (2017) 8:21794–805. doi: 10.18632/oncotarget.15585
8. Wang S, Yang X, Yu Z, Du P, Cao Y, Ji Y, et al. The values of systemic immune-inflammation index and neutrophil-lymphocyte ratio in predicting testicular germ cell tumors: A retrospective clinical study. Front Oncol (2022) 12:893877. doi: 10.3389/fonc.2022.893877
9. Gil A, Plaza-Diaz J, Mesa MD. Vitamin d: Classic and novel actions. Ann Nutr Metab (2018) 72:87–95. doi: 10.1159/000486536
10. Krajewski W, Dziegala M, Kolodziej A, Dembowski J, Zdrojowy R. Vitamin d and urological cancers. Cent Eur J Urol (2016) 69:139–47. doi: 10.5173/ceju.2016.784
11. Ng K, Sargent DJ, Goldberg RM, Meyerhardt JA, Green EM, Pitot HC, et al. Vitamin d status in patients with stage IV colorectal cancer: findings from intergroup trial N9741. J Clin Oncol (2011) 29:1599–606. doi: 10.1200/JCO.2010.31.7255
12. DeLuca HF. The metabolism and functions of vitamin d. Adv Exp Med Biol (1986) 196:361–75. doi: 10.1007/978-1-4684-5101-6_24
13. Alvarez-Diaz S, Valle N, Garcia JM, Pena C, Freije JM, Quesada V, et al. Cystatin d is a candidate tumor suppressor gene induced by vitamin d in human colon cancer cells. J Clin Invest (2009) 119:2343–58. doi: 10.1172/jci37205
14. Pereira F, Barbachano A, Silva J, Bonilla F, Campbell MJ, Munoz A, et al. KDM6B/JMJD3 histone demethylase is induced by vitamin d and modulates its effects in colon cancer cells. Hum Mol Genet (2011) 20:4655–65. doi: 10.1093/hmg/ddr399
15. Bernardi RJ, Johnson CS, Modzelewski RA, Trump DL. Antiproliferative effects of 1alpha,25-dihydroxyvitamin D3 and vitamin d analogs on tumor-derived endothelial cells. Endocrinology (2002) 143:2508–14. doi: 10.1210/endo.143.7.8887
16. Mantell DJ, Owens PE, Bundred NJ, Mawer EB, Canfield AE. 1 alpha,25-dihydroxyvitamin D3 inhibits angiogenesis in vitro and in vivo. Circ Res (2000) 87:214–20. doi: 10.1161/01.res.87.3.214
17. Suares A, Tapia C, Gonzalez-Pardo V. VDR agonists down regulate PI3K/Akt/mTOR axis and trigger autophagy in kaposi's sarcoma cells. Heliyon (2019) 5:e02367. doi: 10.1016/j.heliyon.2019.e02367
18. Wang J, Lian H, Zhao Y, Kauss MA, Spindel S. Vitamin D3 induces autophagy of human myeloid leukemia cells. J Biol Chem (2008) 283:25596–605. doi: 10.1074/jbc.M801716200
19. McGlorthan L, Paucarmayta A, Casablanca Y, Maxwell GL, Syed V. Progesterone induces apoptosis by activation of caspase-8 and calcitriol via activation of caspase-9 pathways in ovarian and endometrial cancer cells in vitro. Apoptosis (2021) 26:184–94. doi: 10.1007/s10495-021-01657-1
20. Wu X, Hu W, Lu L, Zhao Y, Zhou Y, Xiao Z, et al. Repurposing vitamin d for treatment of human malignancies via targeting tumor microenvironment. Acta Pharm Sin B (2019) 9:203–19. doi: 10.1016/j.apsb.2018.09.002
21. Kato S. The function of vitamin d receptor in vitamin d action. J Biochem (2000) 127:717–22. doi: 10.1093/oxfordjournals.jbchem.a022662
22. Hutchinson PE, Pringle JH. Consideration of possible effects of vitamin d on established cancer, with reference to malignant melanoma. Pigment Cell Melanoma Res (2022) 35:408–24. doi: 10.1111/pcmr.13040
23. Bremmer F, Thelen P, Pottek T, Behnes CL, Radzun HJ, Schweyer S. Expression and function of the vitamin d receptor in malignant germ cell tumour of the testis. Anticancer Res (2012) 32:341–9.
24. Aranow C. Vitamin d and the immune system. J Investig Med (2011) 59:881–6. doi: 10.2310/JIM.0b013e31821b8755
25. Morgan D, Berggren KL, Spiess CD, Smith HM, Tejwani A, Weir SJ, et al. Mitogen-activated protein kinase-activated protein kinase-2 (MK2) and its role in cell survival, inflammatory signaling, and migration in promoting cancer. Mol Carcinog (2022) 61:173–99. doi: 10.1002/mc.23348
26. Blomberg Jensen M, Jorgensen A, Nielsen JE, Steinmeyer A, Leffers H, Juul A, et al. Vitamin d metabolism and effects on pluripotency genes and cell differentiation in testicular germ cell tumors in vitro and in vivo. Neoplasia (2012) 14:952–63. doi: 10.1593/neo.121164
27. Gugatschka M, Kiesler K, Obermayer-Pietsch B, Groselj-Strele A, Griesbacher A, Friedrich G. Vitamin d status is associated with disease-free survival and overall survival time in patients with squamous cell carcinoma of the upper aerodigestive tract. Eur Arch Otorhinolaryngol (2011) 268:1201–4. doi: 10.1007/s00405-010-1481-y
28. Hu K, Callen DF, Li J, Zheng H. Circulating vitamin d and overall survival in breast cancer patients: A dose-response meta-analysis of cohort studies. Integr Cancer Ther (2018) 17:217–25. doi: 10.1177/1534735417712007
29. Zhou J, Ge X, Fan X, Wang J, Miao L, Hang D. Associations of vitamin d status with colorectal cancer risk and survival. Int J Cancer (2021) 149:606–14. doi: 10.1002/ijc.33580
30. Dev R, Del Fabbro E, Schwartz GG, Hui D, Palla SL, Gutierrez N, et al. Preliminary report: vitamin d deficiency in advanced cancer patients with symptoms of fatigue or anorexia. Oncologist (2011) 16:1637–41. doi: 10.1634/theoncologist.2011-0151
31. Stone CA, Kenny RA, Healy M, Walsh JB, Lawlor PG. Vitamin d depletion: of clinical significance in advanced cancer? Support Care Cancer (2011) 19:865–7. doi: 10.1007/s00520-011-1117-9
32. Bao Y, Li Y, Gong Y, Huang Q, Cai S, Peng J. Vitamin d status and survival in stage II-III colorectal cancer. Front Oncol (2020) 10:581597. doi: 10.3389/fonc.2020.581597
33. Borchmann S, Cirillo M, Goergen H, Meder L, Sasse S, Kreissl S, et al. Pretreatment vitamin d deficiency is associated with impaired progression-free and overall survival in Hodgkin lymphoma. J Clin Oncol (2019) 37:3528–37. doi: 10.1200/JCO.19.00985
34. Johansson H, Spadola G, Tosti G, Mandala M, Minisini AM, Queirolo P, et al. Vitamin d supplementation and disease-free survival in stage II melanoma: A randomized placebo controlled trial. Nutrients (2021) 13(6):1931. doi: 10.3390/nu13061931
35. Bajbouj K, Al-Ali A, Shafarin J, Sahnoon L, Sawan A, Shehada A, et al. Vitamin d exerts significant antitumor effects by suppressing vasculogenic mimicry in breast cancer cells. Front Oncol (2022) 12:918340. doi: 10.3389/fonc.2022.918340
36. Li J, Luco AL, Camirand A, St-Arnaud R, Kremer R. Vitamin d regulates CXCL12/CXCR4 and epithelial-to-Mesenchymal transition in a model of breast cancer metastasis to lung. Endocrinology (2021) 162(7):162. doi: 10.1210/endocr/bqab049
37. Tokar EJ, Webber MM. Cholecalciferol (vitamin D3) inhibits growth and invasion by up-regulating nuclear receptors and 25-hydroxylase (CYP27A1) in human prostate cancer cells. Clin Exp Metastasis (2005) 22:275–84. doi: 10.1007/s10585-005-8393-z
38. Mego M, Vlkova B, Minarik G, Cierna Z, Karaba M, Benca J, et al. Vitamin d and circulating tumor cells in primary breast cancer. Front Oncol (2022) 12:950451. doi: 10.3389/fonc.2022.950451
39. Ooi LL, Zheng Y, Zhou H, Trivedi T, Conigrave AD, Seibel MJ, et al. Vitamin d deficiency promotes growth of MCF-7 human breast cancer in a rodent model of osteosclerotic bone metastasis. Bone (2010) 47:795–803. doi: 10.1016/j.bone.2010.07.012
40. Kalavska K, Sestakova Z, Mlcakova A, Gronesova P, Miskovska V, Rejlekova K, et al. Comprehensive assessment of selected immune cell subpopulations changes in chemotherapy-naive germ cell tumor patients. Front Oncol (2022) 12:858797. doi: 10.3389/fonc.2022.858797
41. Ben-Shoshan M, Amir S, Dang DT, Dang LH, Weisman Y, Mabjeesh NJ. 1alpha,25-dihydroxyvitamin D3 (Calcitriol) inhibits hypoxia-inducible factor-1/vascular endothelial growth factor pathway in human cancer cells. Mol Cancer Ther (2007) 6:1433–9. doi: 10.1158/1535-7163.MCT-06-0677
42. Fang S, Sui D, Wang Y, Liu H, Chiang YJ, Ross MI, et al. Association of vitamin d levels with outcome in patients with melanoma after adjustment for c-reactive protein. J Clin Oncol (2016) 34:1741–7. doi: 10.1200/JCO.2015.64.1357
43. Mohr SB, Gorham ED, Kim J, Hofflich H, Garland CF. Meta-analysis of vitamin d sufficiency for improving survival of patients with breast cancer. Anticancer Res (2014) 34:1163–6.
44. Paolino G, Moliterni E, Didona D, Garelli V, Corsetti P, Lopez T, et al. Clinicopathological features, vitamin d serological levels and prognosis in cutaneous melanoma of shield-sites: an update. Med Oncol (2015) 32:451. doi: 10.1007/s12032-014-0451-4
45. Thanasitthichai S, Chaiwerawattana A, Prasitthipayong A. Association of vitamin d level with clinicopathological features in breast cancer. Asian Pac J Cancer Prev (2015) 16:4881–3. doi: 10.7314/apjcp.2015.16.12.4881
46. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin d deficiency and insufficiency revisited. J Clin Endocrinol Metab (2012) 97:1153–8. doi: 10.1210/jc.2011-2601
47. Nappi L, Damiano V, Ottaviano M, Rescigno P, Condello C, Curci C, et al. Correlation between plasmatic levels of vitamin d and testicular cancer. J Clin Oncol (2015) 33:e15561–1. doi: 10.1200/jco.2015.33.15_suppl.e15561
48. Foresta C, Selice R, De Toni L, Di Mambro A, Carraro U, Plebani M, et al. Altered bone status in unilateral testicular cancer survivors: Role of CYP2R1 and its luteinizing hormone-dependency. J Endocrinol Invest (2013) 36:379–84. doi: 10.3275/8650
49. Schepisi G, De Padova S, Scarpi E, Lolli C, Gurioli G, Menna C, et al. Vitamin d status among long-term survivors of testicular cancer. Oncotarget (2017) 8:36780–6. doi: 10.18632/oncotarget.14167
50. Nappi L, Ottaviano M, Rescigno P, Fazli L, Gleave ME, Damiano V, et al. Long term deficiency of vitamin d in germ cell testicular cancer survivors. Oncotarget (2018) 9:21078–85. doi: 10.18632/oncotarget.24925
51. Dieckmann KP, Andura O, Pichlmeier U, Otte KM, Isbarn H, Wulfing C. Revised manuscript R2, clean version are serum levels of 25-hydroxy vitamin d reduced following orchiectomy in testicular cancer patients? Basic Clin Androl (2021) 31:14. doi: 10.1186/s12610-021-00132-w
52. Schepisi G, Gianni C, Bleve S, De Padova S, Menna C, Lolli C, et al. Vitamin d deficiency in testicular cancer survivors: A systematic review. Int J Mol Sci (2021) 22(10):5145. doi: 10.3390/ijms22105145
53. Shahriari M, Kerr PE, Slade K, Grant-Kels JE. Vitamin d and the skin. Clin Dermatol (2010) 28:663–8. doi: 10.1016/j.clindermatol.2010.03.030
54. Kimlin MG. Geographic location and vitamin d synthesis. Mol Aspects Med (2008) 29:453–61. doi: 10.1016/j.mam.2008.08.005
55. Benedik E. Sources of vitamin d for humans. Int J Vitam Nutr Res (2022) 92:118–25. doi: 10.1024/0300-9831/a000733
56. Sebekova K, Krivosikova Z, Gajdos M, Podracka L. Vitamin d status in apparently healthy medication-free slovaks: Association to blood pressure, body mass index, self-reported smoking status and physical activity. Bratisl Lek Listy (2016) 117:702–9. doi: 10.4149/BLL_2016_135
57. Zittermann A. The estimated benefits of vitamin d for Germany. Mol Nutr Food Res (2010) 54:1164–71. doi: 10.1002/mnfr.200900494
58. Yang P, Hu Y, Zhou Q. The CXCL12-CXCR4 signaling axis plays a key role in cancer metastasis and is a potential target for developing novel therapeutics against metastatic cancer. Curr Med Chem (2020) 27:5543–61. doi: 10.2174/0929867326666191113113110
59. Salmaggi A, Maderna E, Calatozzolo C, Gaviani P, Canazza A, Milanesi I, et al. CXCL12, CXCR4 and CXCR7 expression in brain metastases. Cancer Biol Ther (2009) 8:1608–14. doi: 10.4161/cbt.8.17.9202
60. Gilbert DC, Chandler I, McIntyre A, Goddard NC, Gabe R, Huddart RA, et al. Clinical and biological significance of CXCL12 and CXCR4 expression in adult testes and germ cell tumours of adults and adolescents. J Pathol (2009) 217:94–102. doi: 10.1002/path.2436
61. Larriba MJ, Gonzalez-Sancho JM, Barbachano A, Niell N, Ferrer-Mayorga G, Munoz A. Vitamin d is a multilevel repressor of wnt/b-catenin signaling in cancer cells. Cancers (Basel) (2013) 5:1242–60. doi: 10.3390/cancers5041242
62. Chovanec M, Cierna Z, Miskovska V, Machalekova K, Kalavska K, Rejlekova K, et al. Betacatenin is a marker of poor clinical characteristics and suppressed immune infiltration in testicular germ cell tumors. BMC Cancer (2018) 18:1062. doi: 10.1186/s12885-018-4929-x
63. Schmidtova S, Kalavska K, Liskova V, Plava J, Miklikova S, Kucerova L, et al. Targeting of deregulated wnt/beta-catenin signaling by PRI-724 and LGK974 inhibitors in germ cell tumor cell lines. Int J Mol Sci (2021) 22(8):4263. doi: 10.3390/ijms22084263
64. Kumar B, Koul S, Petersen J, Khandrika L, Hwa JS, Meacham RB, et al. p38 mitogen-activated protein kinase-driven MAPKAPK2 regulates invasion of bladder cancer by modulation of MMP-2 and MMP-9 activity. Cancer Res (2010) 70:832–41. doi: 10.1158/0008-5472.CAN-09-2918
65. Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene (2006) 25:2987–98. doi: 10.1038/sj.onc.1209337
66. Jorgensen A, Blomberg Jensen M, Nielsen JE, Juul A, Rajpert-De Meyts E. Influence of vitamin d on cisplatin sensitivity in testicular germ cell cancer-derived cell lines and in a NTera2 xenograft model. J Steroid Biochem Mol Biol (2013) 136:238–46. doi: 10.1016/j.jsbmb.2012.10.008
67. Lafin JT, Bagrodia A, Woldu S, Amatruda JF. New insights into germ cell tumor genomics. Andrology (2019) 7:507–15. doi: 10.1111/andr.12616
68. Shen H, Shih J, Hollern DP, Wang L, Bowlby R, Tickoo SK, et al. Integrated molecular characterization of testicular germ cell tumors. Cell Rep (2018) 23:3392–406. doi: 10.1016/j.celrep.2018.05.039
69. Young JC, Kerr G, Micati D, Nielsen JE, Rajpert-De Meyts E, Abud HE, et al. WNT signalling in the normal human adult testis and in male germ cell neoplasms. Hum Reprod (2020) 35:1991–2003. doi: 10.1093/humrep/deaa150
70. Huang Z, Zhang Y, Li H, Zhou Y, Zhang Q, Chen R, et al. Vitamin d promotes the cisplatin sensitivity of oral squamous cell carcinoma by inhibiting LCN2-modulated NF-kappaB pathway activation through RPS3. Cell Death Dis (2019) 10:936. doi: 10.1038/s41419-019-2177-x
71. Gan X, Chen B, Shen Z, Liu Y, Li H, Xie X, et al. High GPX1 expression promotes esophageal squamous cell carcinoma invasion, migration, proliferation and cisplatin-resistance but can be reduced by vitamin d. Int J Clin Exp Med (2014) 7:2530–40.
72. Manson JE, Bassuk SS, Buring JE, Group VR. Principal results of the VITamin d and OmegA-3 TriaL (VITAL) and updated meta-analyses of relevant vitamin d trials. J Steroid Biochem Mol Biol (2020) 198:105522. doi: 10.1016/j.jsbmb.2019.105522
73. Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, et al. Association between vitamin d supplementation and mortality: systematic review and meta-analysis. BMJ (2019) 366:l4673. doi: 10.1136/bmj.l4673
74. Bruera E, Yennurajalingam S. Challenge of managing cancer-related fatigue. J Clin Oncol (2010) 28:3671–2. doi: 10.1200/JCO.2010.29.8984
Keywords: cholecalciferol, overall survival, progression-free survival, prognostic biomarker, germ cell tumor
Citation: Lesko P, Vlkova B, Kalavska K, De Angelis V, Novotna V, Obertova J, Orszaghova Z, Palacka P, Rejlekova K, Sycova-Mila Z, Kollarik B, Aziri R, Pindak D, Mardiak J, Chovanec M, Celec P and Mego M (2023) Prognostic role of plasma vitamin D and its association with disease characteristics in germ cell tumours. Front. Oncol. 13:1149432. doi: 10.3389/fonc.2023.1149432
Received: 21 January 2023; Accepted: 23 March 2023;
Published: 11 April 2023.
Edited by:
Giovanni Rosti, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Giuseppe Schepisi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyMargaret Ottaviano, G. Pascale National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2023 Lesko, Vlkova, Kalavska, De Angelis, Novotna, Obertova, Orszaghova, Palacka, Rejlekova, Sycova-Mila, Kollarik, Aziri, Pindak, Mardiak, Chovanec, Celec and Mego. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Lesko, bGVza29wZXRlcjlAZ21haWwuY29t
 Peter Lesko
Peter Lesko Barbora Vlkova2
Barbora Vlkova2 Katarina Kalavska
Katarina Kalavska Zuzana Orszaghova
Zuzana Orszaghova Patrik Palacka
Patrik Palacka Katarina Rejlekova
Katarina Rejlekova Michal Chovanec
Michal Chovanec Peter Celec
Peter Celec Michal Mego
Michal Mego