- 1Department of Laboratory Medicine, the First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 2Branch of National Clinical Research Center for Laboratory Medicine, Nanjing, China
- 3NHC Contraceptives Adverse Reaction Surveillance Center, Jiangsu Health Development Research Center, Nanjing, China
Background: Tumor-specific protein 70 (SP70) was identified as a new biomarker associated with the proliferation and invasion of cancer cells. This study aimed to investigate the expression of SP70 in hepatocellular carcinoma (HCC) and assess its clinical value in the diagnosis and prediction of early HCC recurrence.
Methods: A total of 1049 subjects from the First Affiliated Hospital of Nanjing Medical University were recruited in this study. Serum SP70, alpha-fetoprotein (AFP) and prothrombin induced by vitamin K absence II (PIVKA-II) were measured. The diagnostic performance for HCC was obtained using the receiver operating characteristic (ROC) curve, and recurrence-free survival (RFS) was calculated using the Kaplan–Meier method. Univariate and multivariate analyses were performed to identify predictive factors of RFS.
Results: SP70 was highly expressed in HCC cells and HCC tissue. Serum SP70 levels in the HCC group were significantly higher than in the benign liver diseases group and healthy control group (P<0.001). SP70 combined with AFP showed the best diagnostic performance (AUC=0.909, 95%CI [confidence interval]=0.890–0.929). Kaplan–Meier analysis revealed that patients with high SP70 levels had shorter median RFS than those with low SP70 levels (P=0.003). In addition, high SP70 levels were significantly associated with shorter RFS (P=0.037) in the AFP-negative subgroup. Univariate and multivariate analyses confirmed that preoperative serum SP70 level, serum AFP, tumor diameter and microvascular invasion were independent prognostic factors of RFS.
Conclusion: SP70 is a promising biomarker in diagnosing HCC. High preoperative serum SP70 level is associated with an increased risk of early relapse and could be used as a valuable marker to predict early recurrence of HCC after resection.
Introduction
Liver cancer represents the sixth most common malignancy worldwide and the fourth leading cause of cancer-related death (1, 2). Hepatocellular carcinoma (HCC) accounts for 90% of all new liver cancer cases, and approximately half of cases are attributed to hepatitis B virus (HBV) infection. HCC constitutes a serious health problem, especially in China, where HBV is the most predominant risk factor. Currently, the diagnosis of HCC mainly depends on biopsy and imaging techniques, but a large portion of patients are diagnosed at an advanced stage. HCC has a dismal prognosis, with a 5-year overall survival rate of less than 15%, because of the high incidence of metastasis and recurrence after surgery and limited therapeutic options. Therefore, it is necessary to identify novel sensitive biomarkers for early diagnosis and prediction of HCC recurrence. Recent large-scale genomic studies have been performed to explore multiple mutations for diagnostic biomarkers and potential therapeutic targets (3–5), but there is still a long way to translate these findings into clinical practice.
Alpha-fetoprotein (AFP) has been the most utilized surveillance biomarker for HCC worldwide. However, its role has been controversial for decades due to the lack of sensitivity (6). In addition, prothrombin induced by vitamin K absence II (PIVKA-II), considered another biomarker of HCC unrelated to AFP, has been reported to exhibit a better diagnostic performance than AFP (7, 8). Nevertheless, the clinical utility of PIVKA-II remains uncertain due to substantial variations in its diagnostic efficiency across most studies and the lack of outcome prediction (9, 10).
We previously reported a new antigen with the relative molecular mass (Mr) of 70 kDa—tumor-specific protein 70 (TSP70 or SP70)—recognized by the monoclonal antibody (McAb) named NJ001. As a tumor derived antigen, SP70 is widely involved in the regulating the expression of numerous genes (GEO accession number: GSE59655), promoting cancer cell proliferation and metastasis. Serum SP70 levels were significantly correlated with tumor differentiation and lymph node metastasis and played an important role in cancer chemotherapy monitoring (11, 12). Moreover, SP70-targeted imaging was found to be able to markedly improve the detection of lung adenocarcinoma detection (13). Recent results showed that SP70 was also expressed in HCC. However, the significance of SP70 detection in HCC remains unclear. The aim of this study was to explore the clinical value of SP70 in the diagnosis and prediction of early HCC recurrence.
Material and methods
Study population
A total of 1049 subjects from the First Affiliated Hospital of Nanjing Medical University were enrolled in this study between October 2017 and March 2019, including 365 HCC patients, 310 patients with benign liver diseases (BLD) and 374 healthy volunteers. The BLD group included 101 patients with liver cirrhosis (LC), 100 with chronic hepatitis, 53 with hepatic hemangioma, 35 with hepatic cyst, 18 with hepatapostema and 3 with focal nodular hyperplasia. Among the HCC patients, 168 patients who underwent hepatectomy were followed up. Patients with HCCs were confirmed by pathology, and who had complete clinical data were included in the study. We excluded patients: 1) with other malignant tumors; 2) recurrent HCC; 3) who received preoperative anti-cancer treatment; and 4) who died during the perioperative period. All patients with benign liver diseases were followed up for 6 months after sampling to confirm non-HCC. The staging was assessed using the Barcelona Clinic Liver Cancer (BCLC) staging system.
Measurements of serum tumor markers
All blood samples were centrifuged at 3000 rpm for 10 minutes at room temperature for serum separation within 2 hours after collection. Serum SP70 was detected using an enzyme-linked immunosorbent assay (ELISA) kit (Code Biotech, Jiangsu, China). Serum AFP and PIVKA-II levels were measured on a Roche cobas e602 automated analyzer (Roche, Mannheim, Germany) and LUMIPULSE G1200 immunochemistry system (FujirebioInc, Tokyo, Japan), respectively.
Immunohistochemistry
To determine the expression of SP70 in liver tissues, we collected HCC and corresponding adjacent tissues. Paraffin-embedded samples were sectioned at 4 mm thickness, and SP70 expression was detected with immunohistochemistry (IHC) kit (Code Biotech). All the specimens were independently examined by two investigators.
Cell culture
Human HCC lines Hep3B, Huh7 and normal human hepatic cell L02 were purchased from the cell bank of the Chinese Academy of Sciences in Shanghai. DMEM (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (Gibco) was used as the culture medium for all HCC cell lines. The RPMI-1640 medium (Gibco) supplemented with 10% FBS (Gibco) was used for L02 cell culture. Cell lines were incubated in a humidified atmosphere containing 5% CO2 at 37°C.
Immunofluorescence analysis
Direct immunofluorescence was performed as follows. A dose of 100 μg NJ001 was labeled with Mix-n-StainTM CF® 488A Antibody Labeling Kit (Biotium, California, USA) for fluorescence imaging analysis and the final concentration of NJ001-488A was 250 μg/mL. Briefly, cells were grown to 80% confluence on coverslips and then fixed in 95% ethanol at room temperature. After washing with PBS, the cell membranes were broken in 0.25% Triton X-100 for 15 min, blocked with 3% BSA for 2 hours at room temperature, and then incubated with diluent (1:50) NJ001-488A for 2 h at 37°C. Finally, the slides were washed with PBS three times and sealed with Antifade Mounting Medium with DAPI (Beyotime Biotechnology) and visualized using a Nikon Eclipse Ti fluorescence microscope (Nikon, Melville, NY, USA).
Follow-up for recurrence
All HCC patients who underwent curative resection were followed up. Liver ultrasonography and tumor marker assays were performed every 1–2 months after surgery. Suspected tumor recurrence in the liver was confirmed using enhanced computed tomography (CT) or magnetic resonance imaging (MRI) or biopsy. Recurrence-free survival (RFS) was defined as the time from curative surgery to recurrence.
Statistical analysis
All statistical analyses were performed with SPSS for Windows, version 22 (SPSS Inc., Chicago, IL, USA). Data were expressed as median and interquartile range. Differences between categorical variables were assessed using the Pearson’s chi-squared test or Fisher exact test. Mann–Whitney and Kruskal–Wallis tests were used to assess differences between groups of nonparametrically distributed continuous variables. Bivariate correlations were evaluated using Spearman rank correlation coefficient. A P-value of <0.05 was considered significant. Diagnosis-related indicators obtained from receiver operating characteristics (ROC) analysis, including sensitivity, specificity and the area under the curve (AUC), were used to assess the performance of tumor markers. The Cox proportional hazards regression model was performed to identify the independent predictive factors of RFS. A cut-off of P<0.05 was used to select variables entered into the multivariate model from the univariate analysis. Kaplan-Meier analysis with the log-rank test was utilized to compare RFS of different risk groups, and P<0.05 was considered statistically significant.
Results
The expression of SP70 in liver cell lines and tissues
The results of indirect immunofluorescence showed that SP70 was highly expressed in Hep3B cells, mainly expressed in the nucleus of Huh7 cells and lowly expressed in L02 cells (Figure 1A). We detected SP70 in cell culture supernatant by ELISA and found that the levels of SP70 increased with the proliferation of HCC cells (Figure 1C). Immunohistochemistry analysis indicated that the expression of SP70 in HCC was higher than that in cancer-adjacent tissues and normal liver tissue (Figure 1B). Furthermore, Spearman correlation analysis showed that SP70 expression in HCC tissues and serum SP70 were positively correlated (r=0.402, P<0.05).
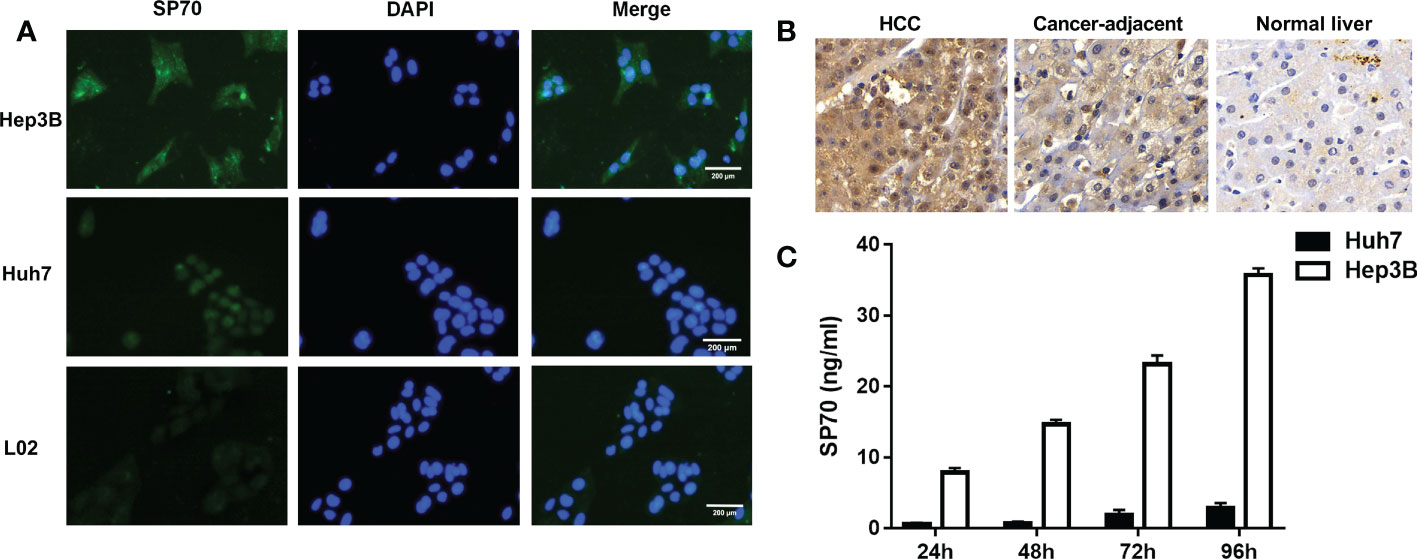
Figure 1 The expression of SP70 in HCC cells and tissues. (A) Immunofluorescence analysis showed that SP70 was highly expressed in Hep3B cells, mainly expressed in the nucleus of Huh7 cells and lowly expressed in L02 cells; (B) Immunohistochemistry staining showed that SP70 was highly expressed in HCC tissues (×400); (C) Levels of SP70 in culture supernatant increased further with the proliferation of HCC cells.
Patient characteristics
To evaluate the potential application of SP70 in HCC, we divided the 1049 study participants into three groups. The HCC group had significantly higher SP70 levels compared with the BLD group and healthy controls (HC) (all P<0.001). The median levels of SP70 were 11.4 ng/mL, 9.0 ng/mL and 6.0 ng/mL in the HCC, BLD and HC groups, respectively (Figure 2A). In addition, SP70 levels were significantly higher in the BLD group compared with the HC group (P<0.001, respectively).
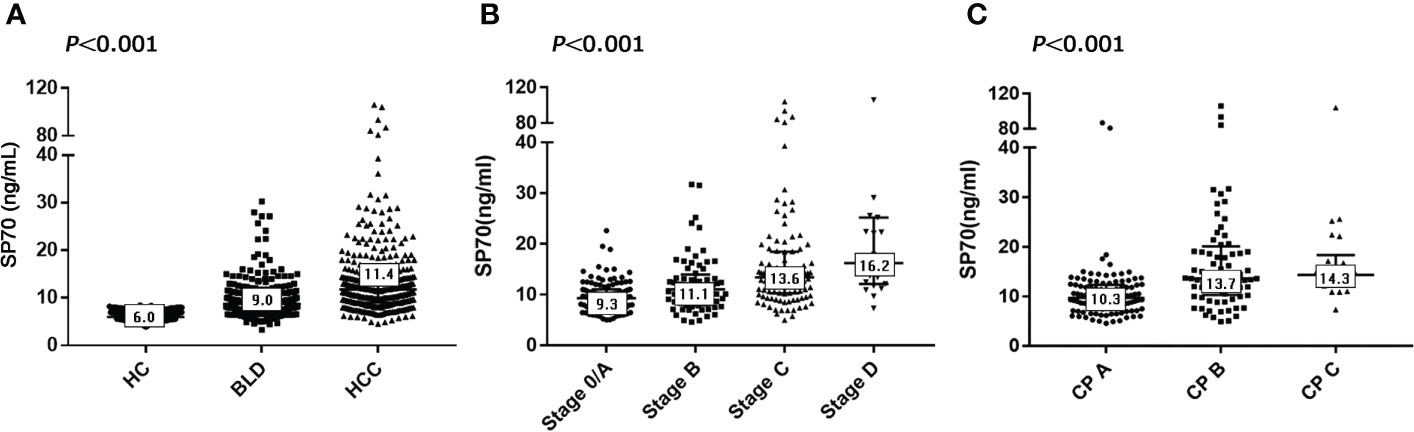
Figure 2 SP70 levels in different groups and associations with BCLC and CP stages. (A) Serum levels of SP70 in the HCC, BLD and HC groups; (B) SP70 associations with BCLC stage; (C) SP70 associations with CP stage. The median level of SP70 for each group has been marked in the appropriate box.
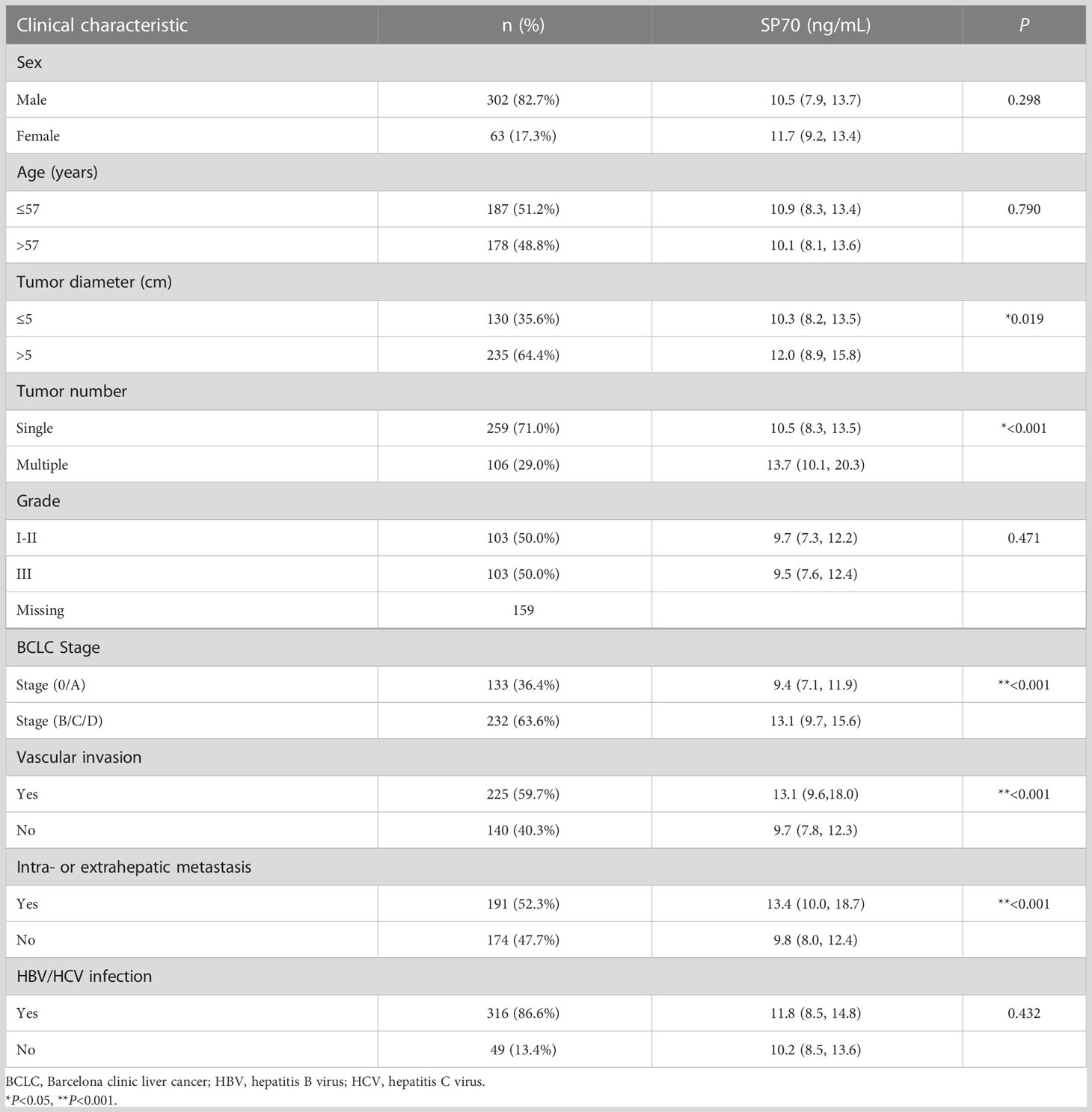
The characteristics of HCC patients and preoperative SP70 levels in subgroups are summarized in Table 1. Among the 365 HCC patients enrolled, 302 (82.7%) were men, and the median age was 57 years (range: 20–86). There were 4.8 times more men than women (17.3%). Chronic hepatitis B virus (HBV) infection was by far the most common underlying cause of liver diseases (n=299, 81.9%), far more than chronic hepatitis C virus (HCV) infection (n=14, 3.8%). Other factors included cryptogenic/other cirrhosis (n=13, 3.6%) and no underlying chronic liver disease (n=39, 10.7%). In the HCC group, 133 patients were diagnosed at BCLC stage 0/A (36.4%), 72 patients at stage B (19.7%), 137 patients at stage C (37.5%) and 23 patients at stage D (6.3%). Two-thirds of the patients could not be diagnosed early, causing about half of the patients to miss opportunities for radical resection and be prescribed other treatment options, including transarterial chemoembolization (n=120, 32.9%), ablation (n=24, 6.6%) and supportive care (n=49, 13.4%). Serum SP70 levels were significantly different in HCC patients grouped by tumor number, tumor diameter, BCLC stage, vascular invasion and intra- or extrahepatic metastasis (Table 1). Moreover, further analysis indicated that serum SP70 increased with advancing BCLC and Child-Pugh (CP) stage (Figures 2B, C).
The diagnostic value of SP70 in HCC
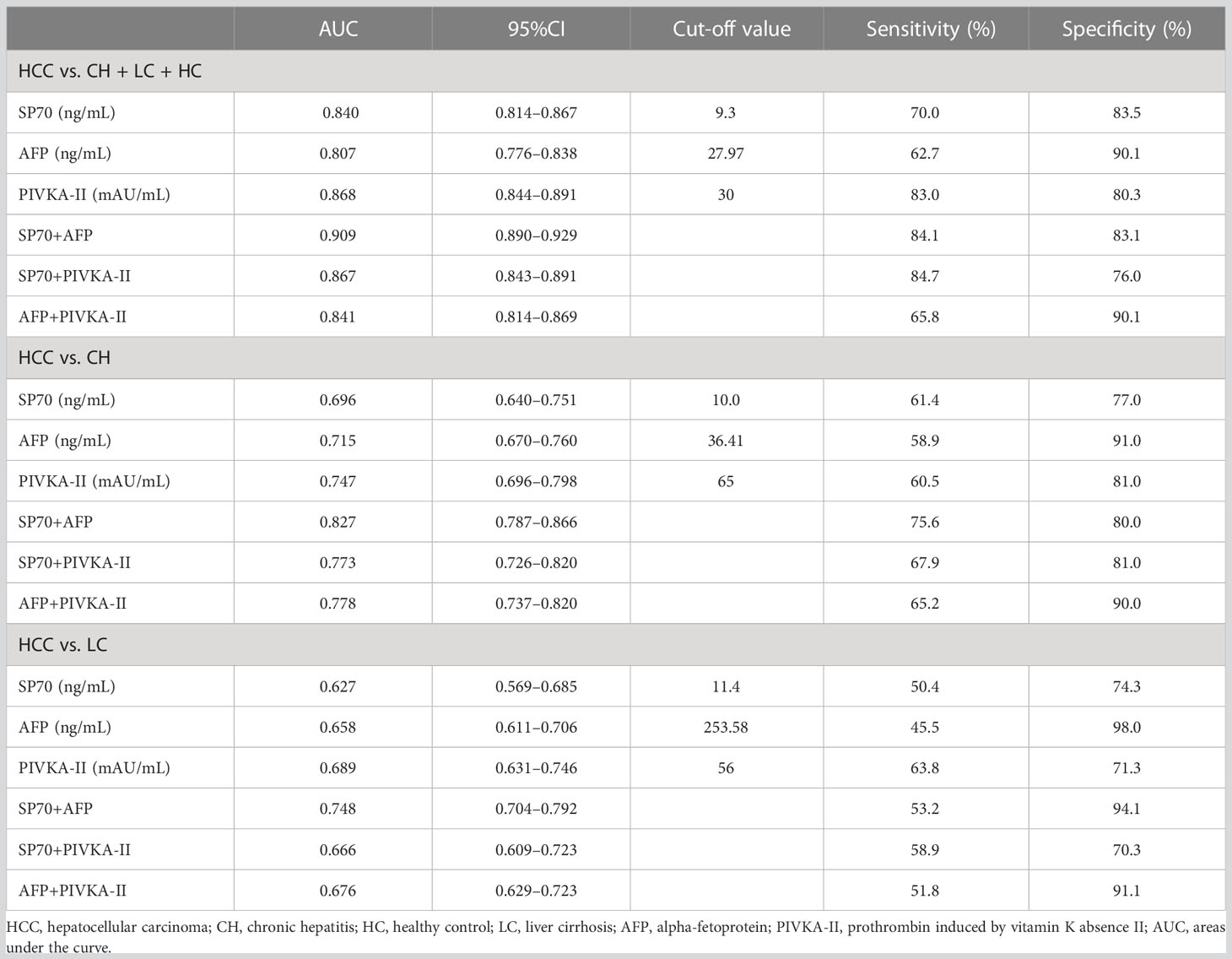
To evaluate the diagnostic performance of SP70, AFP and PIVKA-II in HCC, we performed ROC analysis (Figure 3). The findings revealed that SP70 could serve as a valuable marker for detecting HCC with a sensitivity of 70.0% and a specificity of 83.5% at a cut-off value of 9.3 ng/mL (AUC=0.840, 95% CI [confidence interval]=0.814–0.867). PIVKA-II (AUC=0.868, 95% CI 0.844–0.891) had a higher AUC value than AFP (AUC=0.807, 95% CI=0.776-0.838, P<0.001), and there was no significant difference between SP70 and PIVKA-II (P>0.05). Figure 3B shows the performance of SP70, AFP and PIVKA-II to distinguish between HCC from CH. The AUC values of SP70, AFP and PIVKA-II were 0.696 (95% CI=0.640–0.751), 0.715 (95% CI=0.670–0.760) and 0.747 (95% CI=0.696–0.798), respectively. However, there was no statistical difference among the AUC values of three markers (P>0.05). When distinguishing HCC from LC, the diagnostic performance of three markers decreased further. The AUC values were 0.627 (95% CI=0.569–0.685), 0.658 (95% CI=0.611–0.706) and 0.689 (95% CI=0.631–0.746) for SP70, AFP and PIVKA-II, respectively (Table 2). Similarly, no significant difference was observed among the AUC values of three markers (P>0.05).
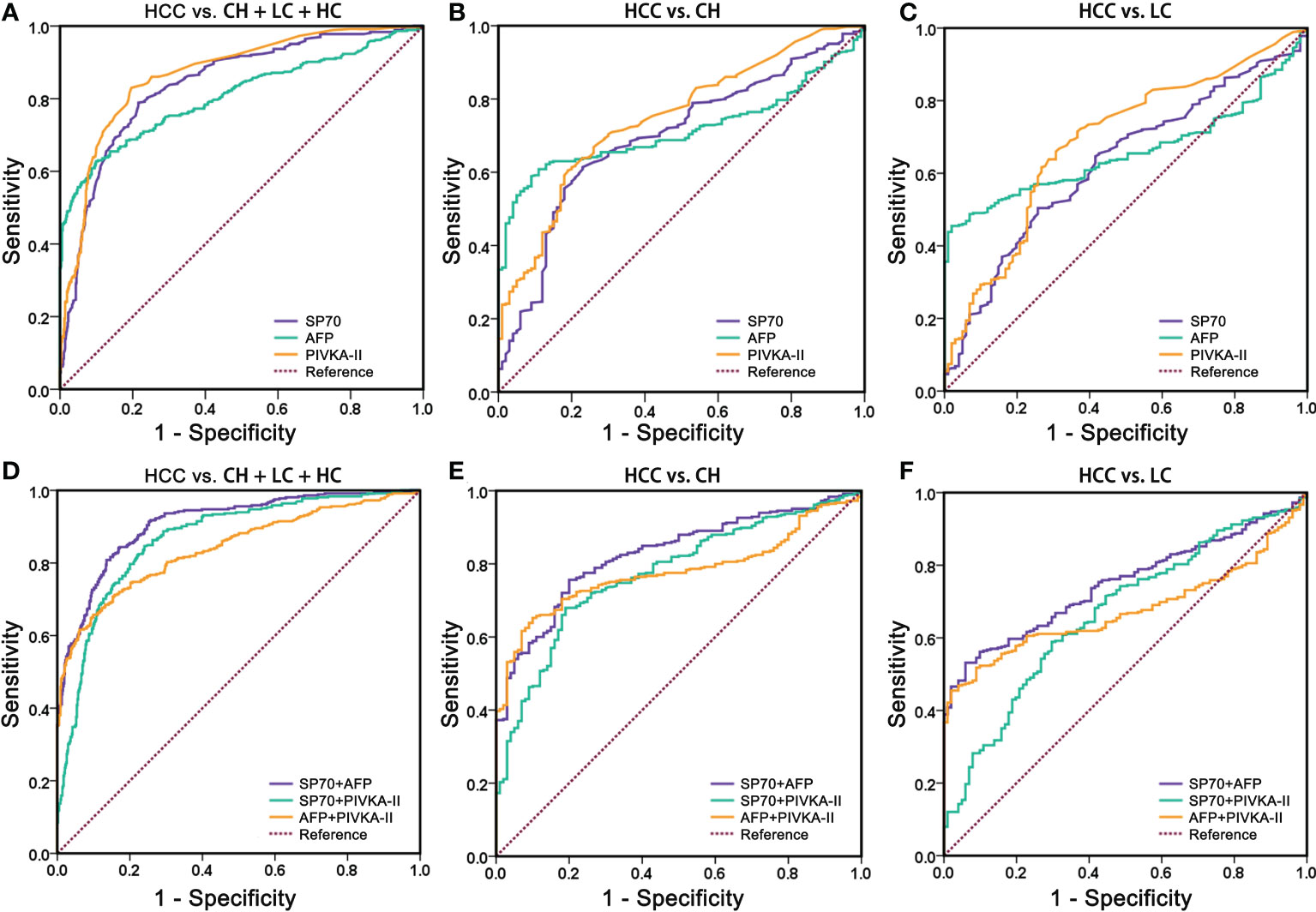
Figure 3 Comparison of receiver operating characteristics curves of SP70, AFP, PIVKA-II and three different multi-marker combinations. (A, D) HCC vs. BLD + HC; (B, E) HCC vs. BLD; (C, F) HCC vs. LC. BLD, benign liver diseases; HC, healthy control; LC, liver cirrhosis.
We combined different markers to enhance their diagnostic performance. Comparisons of different two-marker combinations are shown in Table 2. Overall, the combination of SP70 and AFP showed the best diagnostic performance, which had a significantly better AUC (AUC=0.909, 95% CI=0.890–0.929) compared to a combination of SP70 and PIVKA-II (AUC=0.867, 95% CI=0.843–0.891, P<0.001), combination of AFP and PIVKA-II (AUC=0.841, 95% CI=0.814–0.869, P<0.001) and PIVKA-II (P=0.002) alone. When distinguishing HCC from CH, an AUC value of 0.827 (95% CI=0.787–0.866) could be obtained by combining SP70 and AFP, which was significantly higher than a combination of AFP and PIVKA-II (AUC=0.778, 95% CI=0.737–0.820, P=0.015), combination of SP70 and PIVKA-II (AUC=0.773, 95% CI=0.726–0.820, P=0.002) and PIVKA-II (P=0.009) alone. Likewise, the combination of SP70 and AFP showed a better diagnostic value in differentiating HCC from LC (AUC=0.748, 95% CI=0.704–0.792) than the other two combinations (P<0.001).
SP70 as a predictor for early recurrence after surgery
A total of 168 cases were followed up after hepatectomy. None of the patients received postoperative adjuvant treatment. Twelve were lost to follow-up, giving a follow-up rate was 92.9%. During a median of 11 months of follow-up, 71 patients (45.5%) cases experienced HCC recurrence, including 64 (90.1%) with intrahepatic recurrence and 7 (9.2%) with extrahepatic metastasis. The 1- and 2-year recurrence-free survival rates were 62.3% and 33.2%, respectively. According to ROC analysis, the cut-off values of preoperative serum SP70, AFP and PIVKA-II for identifying early recurrence were 10.4 ng/mL, 20.94 ng/mL and 406 mAU/mL respectively. The clinical and biological features of HCC patients who underwent resection are available in Table 3. Kaplan–Meier analysis revealed that patients with high SP70 levels displayed worse median recurrence-free survival than those with low SP70 levels (P=0.003), Figure 4A. Furthermore, preoperative AFP was negative in 41.7% (n=65) of all patients followed up. Moreover, among the 71 patients with recurrence, 23 (32.4%) had a negative preoperative AFP. High SP70 levels were associated with significantly worse RFS in the AFP-negative subgroup (P=0.037), Figure 4B.
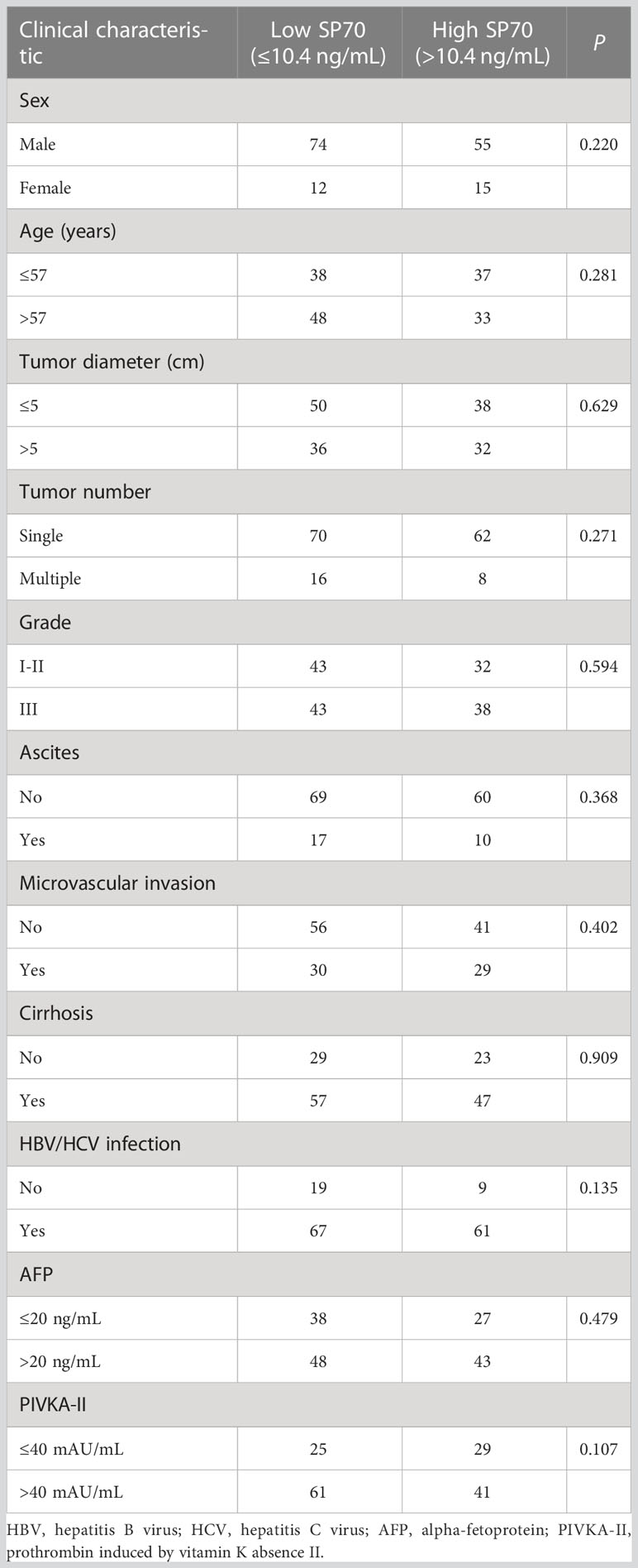
Table 3 Clinical and biological features of HCC patients undergoing resection according to the preoperative serum level of SP70.
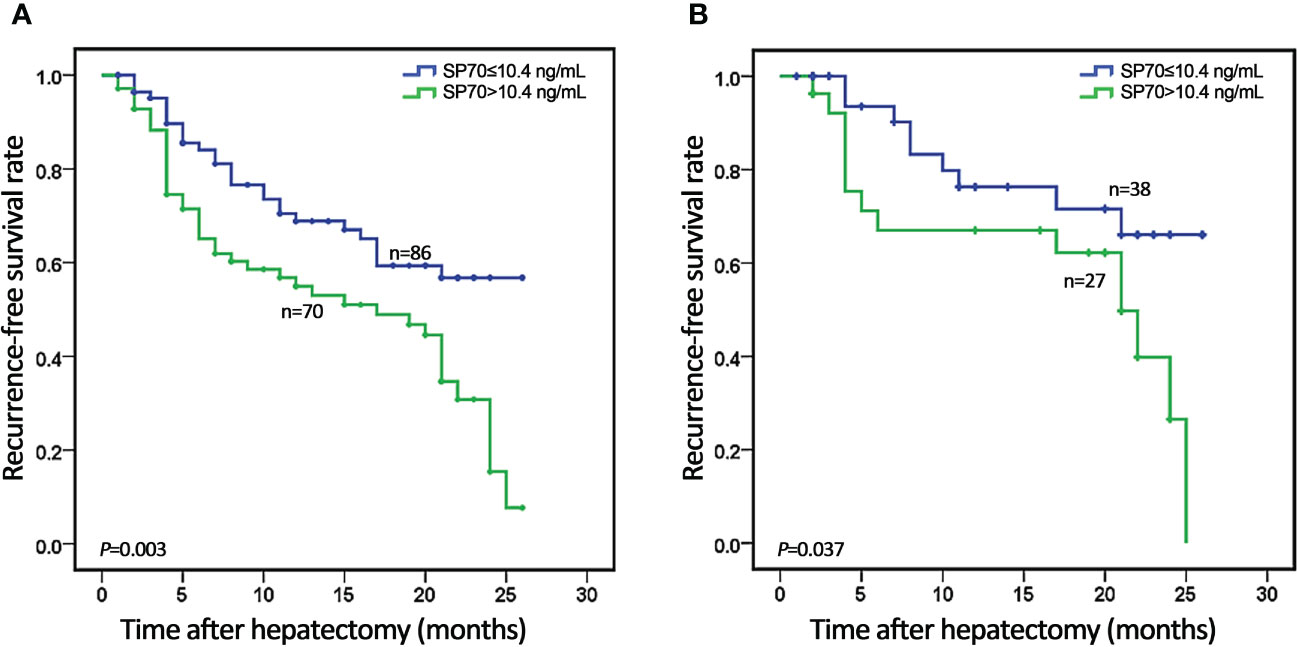
Figure 4 Kaplan–Meier analysis of recurrence-free survival (RFS) stratified by serum SP70 level. (A) Association of SP70 level with RFS in all 156 HCC patients; (B) Association of SP70 level with RFS in the AFP-negative subgroup.
The results of the univariate and multivariate analysis for RFS are shown in Table 4. In univariate analysis, RFS was significantly associated with tumor diameter (HR [hazard ratio]=2.512, 95% CI=1.562–4.040, P<0.001), tumor number (HR=1.863, 95% CI=1.033–3.361, P=0.039), microvascular invasion (HR=2.477, 95% CI=1.551–3.956, P<0.001), serum AFP (HR=2.115, 95% CI=1.269–3.526, P=0.004) and serum SP70 (HR=2.008, 95% CI=1.247–3.233, P=0.004). Multivariate analysis demonstrated that tumor diameter >5 cm (HR=1.952, 95% CI=1.182–3.222, P=0.009), microvascular invasion (HR=1.787, 95% CI=1.085–2.944, P=0.023), preoperative high-level SP70 (HR=1.927, 95% CI=1.177–3.156, P=0.009) and high-level AFP (HR=1.766, 95% CI=1.043–2.991, P=0.034) were independent prognostic factors of RFS.
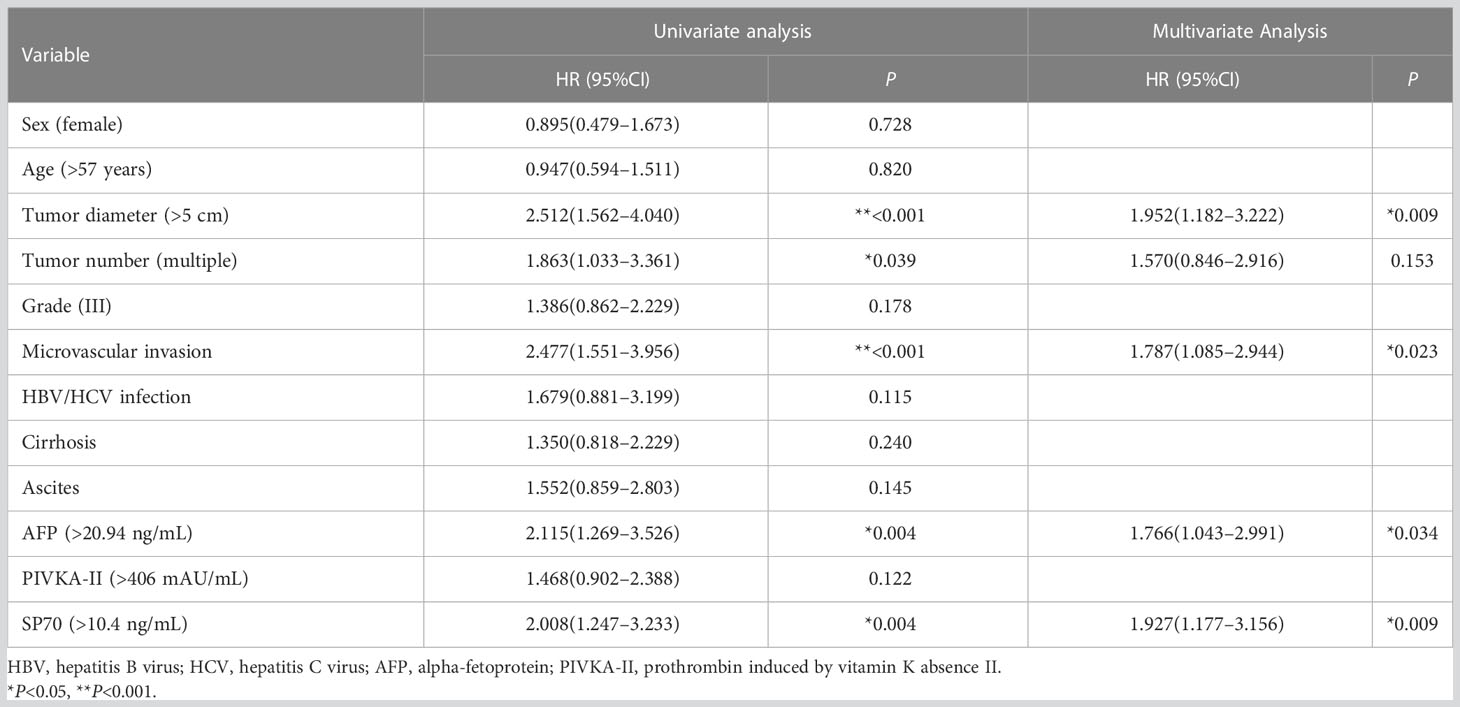
Table 4 Univariate and multivariate analysis of risk factors predicting recurrence-free survival after radical hepatectomy in HCC patients.
Discussion
Despite being one of the most common malignancies with poor survival globally, HCC is limited in diagnosis and treatment due to its atypical early symptoms and high recurrence rate. Although guidelines from the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommend surveillance to be primarily imaging-based (14, 15), it is usually difficult to distinguish malignant from benign hepatic lesions when the size of the lesion is small (less than 2 cm) (16). Pahwa et al. (17) reported that roughly 20% of presumed HCC nodules were inaccurately characterized, and many small HCC nodules remained undiagnosed using established imaging criteria. In addition, pathological biopsy, the golden standard for diagnosis of HCC, is limited because it is traumatic and accurate localization of small lesions and tumor heterogeneity is challenging. A negative biopsy result does not rule out HCC (18). Given these considerations, biomarkers are still important in improving HCC detection as their assessments are convenient, cost-effective, available and repeatable.
In this study, we first reported the role of SP70 in the diagnosis and prognosis of HCC. As a new potential biomarker, SP70 has been proved to be valuable in lung cancer and gastric cancer detection in previous studies (11, 19), while its expression and clinical value in HCC have not been studied. Our results showed the SP70 protein was mainly localized on the membrane and in the cytoplasm of HCC cells, and its expression in HCC tissues was stronger than in adjacent tissues and normal liver tissues. The HCC group had higher serum SP70 levels than the BLD and HC groups. Moreover, serum SP70 levels tended to increase with higher BCLC and CP stages, indicating SP70 may be vital for driving progression in HCC. Hepatocarcinogenesis is a slow, multistep process evolving from hepatitis, cirrhosis, and dysplasia to HCC. About 80% of all HCCs have a cirrhosis background (20). HBV drives the hepatocyte transformation, HCC development and progression through direct or indirect mechanisms, including chronic inflammation, DNA damage, chromosomal instability, epigenetic modification and early neovascularization (21). Of the 81 patients who provided tissues for immunohistochemistry, 76 (93.8%) had backgrounds of HBV-related cirrhosis accompanied by different levels of SP70 expression in adjacent samples. We speculated that the expression of SP70 reflected the malignant potential of cells. However, the clinical contribution of SP70 in differentiating benign and premalignant lesions needs further study.
For the diagnosis of HCC, AFP has high specificity but insufficient sensitivity. When using a cut-off level of 20 ng/mL, its sensitivities and specificities were 41%–65% and 80%–94%, respectively (22). AFP needs to be combined with imaging or other tumor markers for HCC screening. A large multicenter phase II study showed that the sensitivity of PIVKA-II in the diagnosis of early HCC was only 56% (9), and another case-control study demonstrated that PIVKA-II was more efficient than AFP with a sensitivity of 77% (10). Some researchers have advised combining AFP and PIVKA-II to improve diagnostic accuracy (23, 24). Lens culinaris agglutinin-reactive fraction of AFP (AFP-L3) is another recommended indicator for screening of HCC (25, 26). Unfortunately, AFP-L3 detection is not able to be provided in our lab. In the past decade, some circulating markers for diagnosing HCC have been reported, such as micro-RNA and circulating cell-free DNA in plasma, showing good diagnostic performance (27, 28). However, most detection methods require a long response time or high cost and have not been systematized and standardized, limiting their application in practice.
Considering the limited accuracy of single indicator, it is often necessary to combine other markers to improve the diagnostic performance. In this study, combining SP70 with AFP or PIVKA-II significantly improved the performance compared with using SP70 alone. This may be attributed to the special expression mechanism of SP70 unrelated to the other two indicators. However, when used for detecting early HCC, the combinations did not display satisfactory results as expected. Additionally, the heterogeneity of assay methods in evaluating the performance of markers should not be ignored. Serum AFP was detected by electrochemiluminescence (ECL), and PIVKA-II was measured by chemiluminescence (CL). Due to their advantages of having a wide linear range, high sensitivity, rapid analysis with a low background and high automatization, ECL and CL have been broadly applied in biomolecule detection (29). In contrast, the ELISA method for serum SP70 detection had obvious defects with a lower linear range, sensitivity and efficiency. The improved analytical sensitivity could improve the detection of lower levels of SP70 and make HCC early diagnosis practical after updating the assay into ECL or CL.
The poor survival of HCC is mainly attributed to the high risk of recurrence. Close to 70% of patients relapse after surgery (30). Two types of recurrence have mostly been described in the literature: 1) early recurrence, caused by metastasis from the original tumor (≤2 years); and 2) late recurrence, due to de novo tumors arising in the cirrhotic liver (>2 years) (31, 32). The mechanism of recurrence has not been completely elucidated. Molecular characterization of HCC using genomics, transcriptomics and proteomics has developed our understanding of the mechanism recurrence, but most findings come from retrospective studies without validation (33). In this study, serum SP70 level, serum AFP level, tumor diameter and microvascular invasion (MVI) were independently correlated with RFS. MVI is an established histoprognostic factor that can only be assessed on surgical specimens. Nevertheless, SP70 can complement this limitation as a surrogate biomarker. To date, most studies have considered that continuous increase in AFP levels is a risk factor for HCC development. However, there has been no agreement on the use of AFP for surveillance and the best cut-off value for clinical practice (34–37), which may be due to a considerable number of patients with negative AFP. Whether preoperative AFP can be used to predict early recurrence of HCC is still controversial. In a large multicenter study, Farinati et al. (38) assessed the prognostic reliability of AFP and indicated that AFP correlated with overall survival in transplanted patients, patients undergoing locoregional treatments or untreated patients, but not in those undergoing resection. In addition, Calderaro et al. (39) showed that high levels of serum AFP (>300 ng/ml) were not significantly associated with the risk of early tumor recurrence. In our study, serum AFP was an independent factor for predicting early recurrence. Nevertheless, it was still negative in 43.5% of AFP-negative HCC patients at the time of recurrence. Our result showed that a high level of SP70 was associated with significantly worse RFS in the AFP-negative subgroup, indicating that SP70 can be used as a sensitive predictor for early recurrence, especially for AFP-negative patients. In addition, SP70 is involved in regulating gene expression related to migration and invasion of tumor cells. SP70 blocked could upregulate the expression of tissue inhibitor of metalloproteinase 3 (TIMP3) (40). The expression and involvement of TIMP3 in regulating the growth, migration and invasion of HCC and other tumors have been determined in several studies (41–43). As a tumor-derived antigen, SP70 induces immunosuppression in a tumor microenvironment by augmenting Tregs differentiation, potentially mediating tumor immune escape and anti-tumor immune collapse, according to our ongoing research. Based on the above evidence, we propose that SP70 may play a critical role in early metastasis of HCC and can be a sensitive indicator in detecting early recurrence after resection.
This study is the first to demonstrate the diagnostic and prognostic role of SP70 in HCC, affirming its clinical predictive value and application prospect. Undeniably, our study has some limitations. This is a single-center study, that may lead to potential bias in specimen and data collection. Another limitation is that data on AFP-L3 detection is not provided in the comparison of markers. Finally, data on the characteristics of SP70 in patients with unresectable HCC and its assessment of overall survival were not provided. The follow-up period needs to be extended in further studies.
In conclusion, SP70 is highly expressed on the membrane and in the cytoplasm of HCC cells. SP70 could be used as a valuable biomarker for HCC, and a combination of SP70 with AFP enhances diagnostic efficiency compared to individual biomarkers and a combination of AFP and PIVKA-II. Furthermore, SP70 is significantly and independently associated with RFS and could be a sensitive indicator in predicting early recurrence of HCC.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of the First Affiliated Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Study design, guidance in writing of the manuscript, and review of the final manuscript: S-YP. performance of experiments: HS, Y-XJ. collection of the data: JW, W-XC, C-RG, YM and JX. analysis and interpretation of data: LW and HS. statistical analysis: LW and YM. writing of the manuscript: LW. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by The National Natural Science Foundation of China (81672100, 82272401), Key Laboratory for Laboratory Medicine of Jiangsu Province of China (ZDXKB2016005) and Jiangsu Provincial Health Commission Planning (H201609).
Acknowledgments
We are grateful to Prof. Qinghe Meng from MD Anderson Cancer Center for his valuable suggestions on improving this manuscript, and we acknowledge the technical support from the National Key Clinical Department of Laboratory Medicine, Nanjing, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
3. Schulze K, Imbeaud S, Letouze E, Alexandrov LB, Calderaro J, Rebouissou S, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet (2015) 47:505–11. doi: 10.1038/ng.3252
4. Totoki Y, Tatsuno K, Covington KR, Ueda H, Creighton CJ, Kato M, et al. Trans-ancestry mutational landscape of hepatocellular carcinoma genomes. Nat Genet (2014) 46:1267–73. doi: 10.1038/ng.3126
5. Cancer Genome Atlas Research Network. Comprehensive and integrative genomic characterization of hepatocellular carcinoma. Cell (2017) 169:1327–41.e23. doi: 10.1016/j.cell.2017.05.046
6. Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol (2020) 72:250–61. doi: 10.1016/j.jhep.2019.08.025
7. Yang Y, Li G, Lu Z, Liu Y, Kong J, Liu J. Progression of prothrombin induced by vitamin K absence-II in hepatocellular carcinoma. Front Oncol (2021) 11:726213. doi: 10.3389/fonc.2021.726213
8. Caviglia GP, Ciruolo M, Abate ML, Carucci P, Rolle E, Rosso C, et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist II and glypican-3 for the detection and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers (Basel) (2020) 12:3218. doi: 10.3390/cancers12113218
9. Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, et al. Srivastava s and Schwartz m, alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology (2009) 137:110–18. doi: 10.1053/j.gastro.2009.04.005
10. Poté N, Cauchy F, Albuquerque M, Voitot H, Belghiti J, Castera L, et al. Performance of PIVKA-II for early hepatocellular carcinoma diagnosis and prediction of microvascular invasion. J Hepatol (2015) 62:848–54. doi: 10.1016/j.jhep.2014.11.005
11. Liu J, Zhang W, Gu M, Ji Y, Yang L, Cheng X, et al. Serum SP70 is a sensitive predictor of chemotherapy response in patients with advanced nonsmall cell lung cancer. Cancer Med (2018) 7:2925–33. doi: 10.1002/cam4.1555
12. Pan S, Wang F, Huang P, Xu T, Zhang L, Xu J, et al. The study on newly developed McAb NJ001 specific to non-small cell lung cancer and its biological characteristics. PloS One (2012) 7:e33009. doi: 10.1371/journal.pone.0033009
13. Xu J, Zhang S, Zhang W, Xie E, Gu M, Wang Y, et al. SP70-targeted imaging for the early detection of lung adenocarcinoma. Sci Rep (2020) 10:2509. doi: 10.1038/s41598-020-59439-9
14. European Association for the Study of the Liver. EASL clinical practice guidelines: Management of hepatocellular carcinoma. J Hepatol (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
15. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (2018) 67:358–80. doi: 10.1002/hep.29086
16. Ou Q, Mu H, Zhou C, Zheng Z, Geng J. Liver diseases. In: Pan S, Tang J, editors. Clinical molecular diagnostics. Singapore: Springer (2021). p. 463–92.
17. Pahwa A, Beckett K, Channual S, Tan N, Lu DS, Raman SS. Efficacy of the American association for the study of liver disease and Barcelona criteria for the diagnosis of hepatocellular carcinoma. Abdom Imaging (2014) 39:753–60. doi: 10.1007/s00261-014-0118-9
18. Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut (2014) 63:844–55. doi: 10.1136/gutjnl-2013-306627
19. Luo R, Huang W, Chen L, Liu Y, Xu L, Zhang X, et al. SP70 is a potential biomarker to identify gastric fundic gland neoplasms. World J Surg Oncol (2022) 20:132. doi: 10.1186/s12957-022-02564-8
20. Libbrecht L, Desmet V, Roskams T. Preneoplastic lesions in human hepatocarcinogenesis. Liver Int (2005) 25:16–27. doi: 10.1111/j.1478-3231.2005.01016.x
21. Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol (2016) 64:S84–S101. doi: 10.1016/j.jhep.2016.02.021
22. Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis c. A systematic review and critical analysis. Ann Intern Med (2003) 139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012
23. Chen H, Zhang Y, Li S, Li N, Chen Y, Zhang B, et al. Direct comparison of five serum biomarkers in early diagnosis of hepatocellular carcinoma. Cancer Manag Res (2018) 10:1947–58. doi: 10.2147/CMAR.S167036
24. Park SJ, Jang JY, Jeong SW, Cho YK, Lee SH, Kim SG, et al. Usefulness of AFP, AFP-L3, and PIVKA-II, and their combinations in diagnosing hepatocellular carcinoma. Med (Baltimore) (2017) 96:e5811. doi: 10.1097/MD.0000000000005811
25. Zhang Z, Zhang Y, Wang Y, Xu L, Xu W. Alpha-fetoprotein-L3 and golgi protein 73 may serve as candidate biomarkers for diagnosing alpha-fetoprotein-negative hepatocellular carcinoma. Onco Targets Ther (2015) 9:123–29. doi: 10.2147/OTT.S90732
26. Tayob N, Kanwal F, Alsarraj A, Hernaez R, El-Serag HB. The performance of AFP, AFP-3, DCP as biomarkers for detection of hepatocellular carcinoma (HCC): A phase 3 biomarker study in the united states. Clin Gastroenterol Hepatol (2023) 21:415–23.e4. doi: 10.1016/j.cgh.2022.01.047
27. Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, et al. Plasma microRNA panel to diagnose hepatitis b virus-related hepatocellular carcinoma. J Clin Oncol (2011) 29:4781–88. doi: 10.1200/JCO.2011.38.2697
28. Cai J, Chen L, Zhang Z, Zhang X, Lu X, Liu W, et al. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut (2019) 68:2195–205. doi: 10.1136/gutjnl-2019-318882
29. Yao J, Li L, Li P, Yang M. Quantum dots: from fluorescence to chemiluminescence, bioluminescence, electrochemiluminescence, and electrochemistry. Nanoscale (2017) 9:13364–83. doi: 10.1039/c7nr05233b
30. Lee IC, Huang JY, Chen TC, Yen CH, Chiu NC, Hwang HE, et al. Evolutionary learning-derived clinical-radiomic models for predicting early recurrence of hepatocellular carcinoma after resection. Liver Cancer (2021) 10:572–82. doi: 10.1159/000518728
31. Bruix J, Reig M, Sherman M. Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology (2016) 150:835–53. doi: 10.1053/j.gastro.2015.12.041
32. Marasco G, Colecchia A, Colli A, Ravaioli F, Casazza G, Bacchi Reggiani ML, et al. Role of liver and spleen stiffness in predicting the recurrence of hepatocellular carcinoma after resection. J Hepatol (2019) 70:440–48. doi: 10.1016/j.jhep.2018.10.022
33. Erstad DJ, Fuchs BC, Tanabe KK. Molecular signatures in hepatocellular carcinoma: A step toward rationally designed cancer therapy. Cancer (2018) 124:3084–104. doi: 10.1002/cncr.31257
34. Tang J, Ou J, Xu C, Yi C, Xue F, Xu L, et al. EVI5 is a novel independent prognostic predictor in hepatocellular carcinoma after radical hepatectomy. Oncol Rep (2017) 38:2251–58. doi: 10.3892/or.2017.5862
35. Ahn SM, Haq F, Park I, Nault JC, Zucman-Rossi J, Yu E. The clinical implications of G1-G6 transcriptomic signature and 5-gene score in Korean patients with hepatocellular carcinoma. BMC Cancer (2018) 18:571. doi: 10.1186/s12885-018-4192-1
36. Gu JX, Zhang X, Miao RC, Xiang XH, Fu YN, Zhang JY, et al. Six-long non-coding RNA signature predicts recurrence-free survival in hepatocellular carcinoma. World J Gastroenterol (2019) 25:220–32. doi: 10.3748/wjg.v25.i2.220
37. Giannini EG, Marenco S, Borgonovo G, Savarino V, Farinati F, Del Poggio P, et al. Alpha-fetoprotein has no prognostic role in small hepatocellular carcinoma identified during surveillance in compensated cirrhosis. Hepatology (2012) 56:1371–79. doi: 10.1002/hep.25814
38. Farinati F, Marino D, De Giorgio M, Baldan A, Cantarini M, Cursaro C, et al. Diagnostic and prognostic role of alpha-fetoprotein in hepatocellular carcinoma: both or neither? Am J Gastroenterol (2006) 101:524–32. doi: 10.1111/j.1572-0241.2006.00443.x
39. Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol (2019) 70:58–65. doi: 10.1016/j.jhep.2018.09.003
40. Gu C, Luo Y, Zhang S, Xu J, Zhang J, Ju H, et al. MAb NJ001 inhibits lung adenocarcinoma invasiveness by directly regulating TIMP-3 promoter activity via FOXP1 binding sites. Thorac Cancer (2020) 11:2630–38. doi: 10.1111/1759-7714.13593
41. Han M, Gao H, Ju P, Gao MQ, Yuan YP, Chen XH, et al. Hispidulin inhibits hepatocellular carcinoma growth and metastasis through AMPK and ERK signaling mediated activation of PPAR gamma. BioMed Pharmacother (2018) 103:272–83. doi: 10.1016/j.biopha.2018.04.014
42. Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, et al. TGF beta-mediated up regulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene (2010) 29:1787–97. doi: 10.1038/onc.2009.468
Keywords: hepatocellular carcinoma, SP70, early recurrence, diagnostic performance, recurrence-free survival
Citation: Wang L, Shi H, Wei J, Chen W-X, Jin Y-X, Gu C-R, Mu Y, Xu J and Pan S-Y (2023) SP70 is a novel biomarker of hepatocellular carcinoma. Front. Oncol. 13:1149397. doi: 10.3389/fonc.2023.1149397
Received: 21 January 2023; Accepted: 28 March 2023;
Published: 06 April 2023.
Edited by:
Takahiro Kodama, Osaka University, JapanReviewed by:
Satoru Hagiwara, Kindai University Faculy of Medicine, JapanHao Xing, Second Military Medical University, China
Copyright © 2023 Wang, Shi, Wei, Chen, Jin, Gu, Mu, Xu and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shi-Yang Pan, c3lwYW5AbmptdS5lZHUuY24=
 Lin Wang
Lin Wang Hui Shi1,3
Hui Shi1,3 Wen-Xiu Chen
Wen-Xiu Chen Yue-Xinzi Jin
Yue-Xinzi Jin Jian Xu
Jian Xu Shi-Yang Pan
Shi-Yang Pan