
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Oncol. , 14 February 2023
Sec. Molecular and Cellular Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1145919
This article is part of the Research Topic Lipids, Lipid Oxidation, and Cancer: From Biology to Therapeutics View all 7 articles
 Nurmeilita Taher1*
Nurmeilita Taher1* Feny Mentang1
Feny Mentang1 Roike Iwan Montolalu1
Roike Iwan Montolalu1 William Ben Gunawan2
William Ben Gunawan2 Nurpudji Astuti Taslim3†
Nurpudji Astuti Taslim3† Nelly Mayulu4†
Nelly Mayulu4† Fahrul Nurkolis5†
Fahrul Nurkolis5†One of the most significant issues endangering human health today is cancer which has a direct correlation with mortality (1). Global cancer data indicate that there were 10.0 million cancer deaths (9.9 million excluding nonmelanoma skin cancer) and 19.3 million new cases globally in 2020 (2). Depending on the features and stage of the tumor, the treatment of cancer often entails a mix of treatments, such as surgery, radiotherapy, chemotherapy, and most recently, immunotherapy (3). As a result, the creation of medications targeting a particular cancer-related target in conjunction with a thorough comprehension of how pharmaceuticals interact with the biology of human tumors has emerged as the key to the present quest to cure cancer (4). Despite great efforts of research, effective cancer treatment is still lacking, implying the need for other sources of the cancer-treating agent such as natural sources. Natural sources – especially underutilized marine products – have promising potential as functional food or nutraceutical with anticancer properties (5, 6).
Seaweed – which is frequently consumed in East Asian nations – provides nutrients such as minerals, vitamins, soluble dietary fibers, and flavonoids that are thought to protect against illnesses brought on by a sedentary lifestyle (7). The notion of using seaweed as functional foods or nutraceuticals with nutritional advantages beyond their basic macronutrient content is now a focus of various studies and developments. Furthermore, several studies highlighted that dietary seaweed intake is a protective factor against cancer by decreasing the risk and mortality of cancers (8, 9). Due to the anticarcinogenic properties of its bioactive compounds, which include preventing or delaying the development of cancer in both in vitro and in vivo models as well as regulating tumor cell metabolism, cell proliferation, apoptosis, and the cell cycle, dietary seaweed consumption has recently attracted attention on a global scale (10–12).
Green seaweeds are good sources of monocarboxylic acids with a significant amount of hydrocarbon chain which are known as fatty acids (FA). They may generally be classified as either saturated or unsaturated and are produced by the cleavage of natural fats and oils like triacylglycerols or phospholipids (13). Moreover, the role of heterocyclic derivatives in improving the cytotoxicity activity of cancer drugs and therapies has been highlighted (13). There has been no publication of opinion papers discussing the combination of green seaweed’s fatty acids and heterocyclic derivatives as anticancer nutraceuticals. This opinion aims to interpret green seaweeds’ potential as anticancer nutraceuticals based on their fatty acids content and their potential application when incorporated into a fatty acid-heterocyclic hybrid (FAHH).
The marine, photosynthetic algae known as seaweed are abundant in all oceans and categorized into three primary classifications – Phaeophyceae (brown algae), Rhodophyta (red algae), and Chlorophyta (green algae) (14). Diverse settings, such as tidal or subtidal areas, or shallow coastal waters, are home to marine algae. Based on the existence of photosynthetic pigments, green algae are members of the Chlorophyta phylum of the Plantae kingdom (15). As stated above, seaweed has several health-benefiting potentials (16–18). Numerous sulfated and carboxylated polysaccharides, including alginate, ulvan, and fucoidan, as well as carotenoids, phenolic compounds, tocopherols, and peptides, are among the many bioactive substances found in seaweed (19).
Seaweeds have high carbohydrate content (31.8% to 59.1%) and ash content (12.4% to 29.9%) and a low total lipid content that varies from 0.60% to 7.87% (20–23). Different seaweed lipids have a wide range of fatty acid compositions, with saturates making up 51.9–67.4%, monoenes 22.0–32.9%, and polyunsaturated fatty acids (PUFA) 9.2–19.1% (22). Docosahexaenoic and Eicosapentaenoic acids are two examples of the omega-3 (n-3) fatty acids that make up most of the lipids in algae, while the two monounsaturated omega-6 (n-6) fatty acids that are most frequently found in algae are linoleic and arachidonic acids (24). Seaweeds also contained palmitic and myristic (fundamental saturated fatty acids) and phytanic acids (branched chain fatty acids) (20, 21). The fatty acid content of several green seaweeds were presented in Table 1.
Green seaweeds are member of algae which also known as Chlorophyta. They are less common than brown and red algae and exhibit greenish-yellow to dark green coloration (23). Over 900 types of green seaweed, over 1500 species of brown seaweed, and over 4000 species of red seaweed have been identified globally. While green and red seaweed only grows in tropical environments, brown seaweed may be found in temperate areas (33). In general, these three types of seaweed have different coloring pigments and compositions of the cell wall’s polysaccharides (34). The cell wall polysaccharides, which account for 38–54% of the dry algal matter in green seaweeds, are interesting bioactive compounds that can be used for various applications. Green algae contain water-soluble sulfated polysaccharides that have antioxidant and anticancer properties (35). On the other hand, the proximate and mineral compositions of green seaweed have been determined (19, 28, 36), and compared to brown seaweed, green seaweed has lower protein, lipid, and zinc content with higher carbohydrate content (19). A review by Peñalver et al., 2020 also pointed out that overall, green seaweed has lower protein, lipid, and ash content in comparison to red and brown seaweed (23). The major active compounds of green seaweed are flavonoid compounds, steroids, triterpenoids, saponins, alkaloids, and phenol hydroquinone, which are supplemented with the ability as antioxidants (28).
Elevated risk for several types of human cancer has been linked to having an excessive quantity of serum free fatty acid level. By controlling fatty acid metabolism, cancer cells can reroute metabolic pathways to fulfill energy needs (Figure 1). Energy, macromolecules for membrane synthesis, and lipid signals are all crucial components of reprogramming in fatty acid metabolism during the development of cancer (37). Given the significance of both fatty acid production and oxidation in cancer cells, it is possible to target fatty acid metabolism for anticancer therapy by using pharmacological inhibition to reduce cell division, growth, and transformation (38). Furthermore, promising methods to work in conjunction with immunotherapies have emerged, especially involving controlling fatty acids metabolism in immune cells, as primary immune cells require fatty acids metabolism to survive and perform at their best (39). The activation of fatty acid oxidation (which limit the availability of fatty acid) is linked with lower cancer cell proliferation and improved cancer outcomes (40, 41).
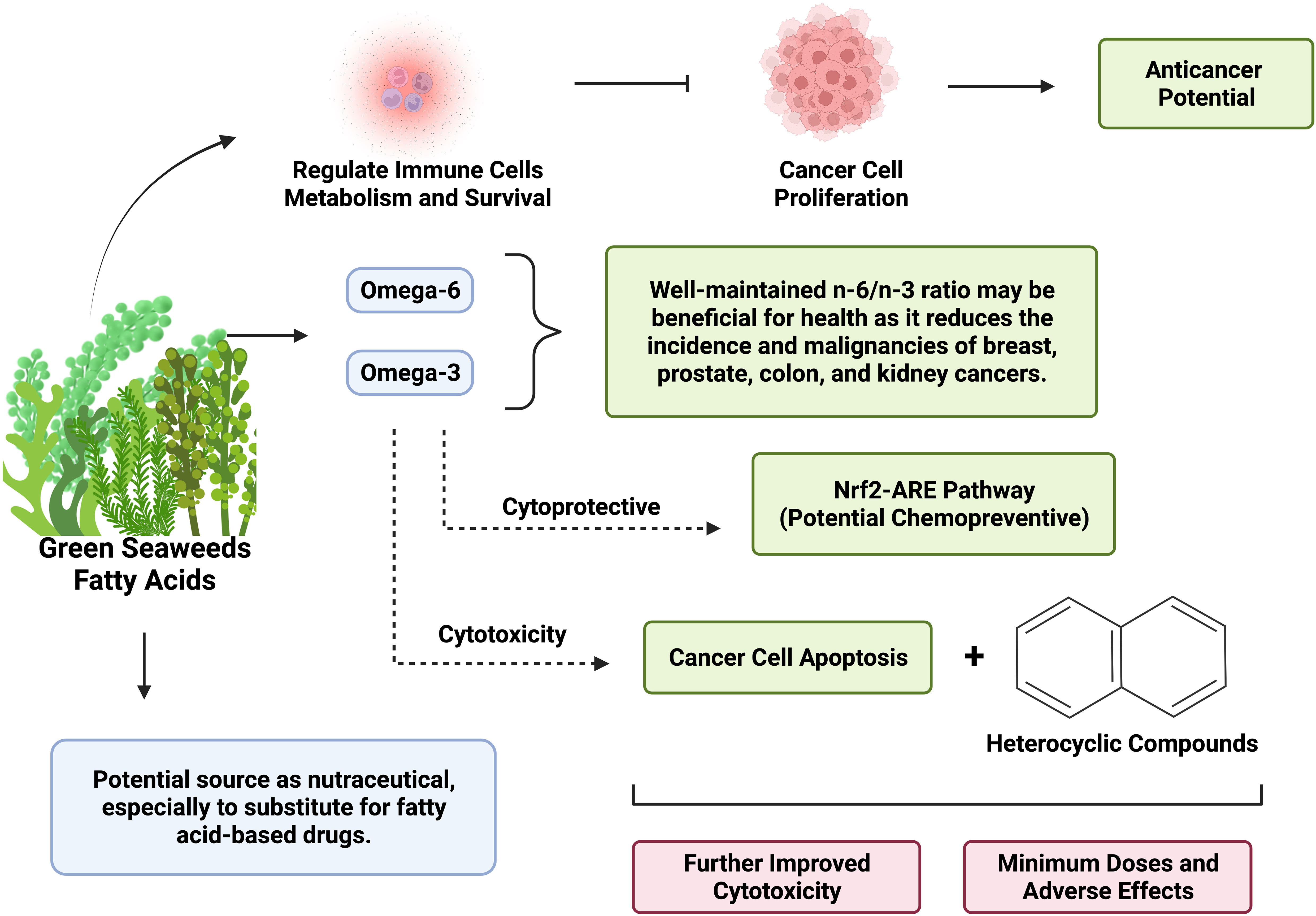
Figure 1 Big picture of the anticancer potential of fatty acid-heterocyclic hybrid of green seaweeds as nutraceuticals. Created with BioRender.com premium license by Fahrul Nurkolis.
Some of the trending fatty acids are the omega groups (n-3, n-6, and n-9). Through selective cytotoxicity, Omega-3 polyunsaturated fatty acids (n-3 PUFAs; Ω3 fatty acids) can cause cancer cells to undergo apoptosis (42). Subsequently, n-3 PUFAs could make tumor cells more susceptible to conventional treatments, which might boost their effectiveness. Omega-9 (n-9) has also been studied for its antiproliferative activity against cancer by suppressing the migration and stimulation of tumor cells (43). If the ratio of n-6 to n-3 is taken into account in functional foods and nutraceuticals, these PUFAs may be advantageous for human health (31). An earlier investigation found a reduction in the incidence of breast, prostate, colon, and kidney malignancies with an n-6/n-3 fatty acid ratio lower than 2/1 to 4/1 (44). In this sense, green seaweeds were proven to prevent cancer due to their n-6/n-3 fatty acid ratio being 0.5 to 1.1 (22). Ulva lactuca, a green alga, produces unsaturated fatty acid components that are cytoprotective Nrf2-ARE pathway activators, demonstrating their potential as chemopreventive dietary unsaturated fatty acids (45).
Heterocycles are defined as “cyclic compounds possessing ring members atoms of at least two other elements” (46). Heterocycles are essentially made of elements other than carbon, with oxygen, nitrogen, and sulfur being the most common substituents. Heterocycles have become parts of cancer chemotherapy drugs with interesting cytotoxicity profiles, especially nitrogen-containing heterocyclic compounds such as pyrrole, purine, pyrrolidine, pyridine, imidazole, pyrimidines, pyrazole, indole, quinoline, oxadiazole, azole, benzimidazole (47, 48). Using a fatty acid-heterocyclic hybrid brings many beneficial influences on cancer therapies. For therapy agents that have cytotoxicity activity, FAHH will improve their therapeutic efficacy, preventing high-dose drugs which can lead to toxicity (13). The activation of fatty acids has significant roles to play in carcinogenesis and cancer development (49), such as cancer immunotherapy efficacy boosters (50). This activation may be influenced by the addition of heterocyclic compounds (e.g. through condensation reactions) which alter the systems and structures of fatty acids (13). More interestingly, FAHH – as a hybrid molecule – may also have additional antimicrobial, antifungal, and antituberculosis activities aside from anticancer (51).
The main idea of green seaweeds as nutraceutical with anticancer potential in terms of fatty acid-heterocyclic hybrid was presented in Figure 1. Taking into account the various health-benefiting potentials of green seaweeds, these algae can be continuously developed into functional foods and nutraceuticals, such as through fortification and incorporation of green seaweeds into new food or existing ones. In general, green seaweeds have alkaloids, phenols, flavonoids, quinine, and polysaccharides that exhibit anticancer activity and provide antioxidant-associated health benefits (5). On the other hand, food fermentation has been reported capable to increase the bioactive compounds and antioxidants of food, further improving the health benefits contained in the food product (52). Combined with the fatty acids profile of green seaweeds, greater anticancer potentials can be expected from green seaweed-based food. Furthermore, there is a growing interest in the application of FAHH as an anticancer agent. This trend highlights the hidden “treasure” of marine products especially since fatty acids are well-contained in green seaweeds and heterocyclic compounds can also be found in marine products (53, 54). The development and incorporation of fatty acid-heterocyclic hybrid through micro- and nanoencapsulation technologies will be crucial in the discovery of novel nutraceuticals, accompanied by greater value of stability and bioaccessibility.
Green seaweed has fatty acid content that makes up most of its fat content. Fatty acids have displayed a significant influence on the prevention and management of cancer. Recent evidence and insight regarding the incorporation of heterocyclic compounds into fatty acids are proposed to increase the anticancer cytotoxicity and efficacy of cancer therapeutic agents, along with other additional health-benefiting properties. Therefore, green seaweeds showcase their potential as anticancer nutraceuticals, and deeper research on this topic is crucial and greatly encouraged.
NT, FM, RM, WG, NT, NM, and FN: Contributed to the conceptualization with the design of the critical opinion study, drafted the manuscript, edited-revised it, and approved the final version of the submitted manuscript. All authors contributed to the article and approved the submitted version.
We offer a great thank you to the Chairman of the Indonesian Association of Clinical Nutrition Physicians, Professor NT, MD., MPH., PhD., Sp.GK(K), and the President of the Federation of Asian Nutrition Societies (FANS), Professor Hardinsyah, Ph.D. for reviewing and providing suggestions, as well as input on the draft of this opinion article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dianatinasab M, Mohammadianpanah M, Daneshi N, Zare-bandamiri M, Rezaeianzadeh A, Fararouei M. Socioeconomic factors, health behavior, and late-stage diagnosis of breast cancer: Considering the impact of delay in diagnosis. Clin Breast Cancer (2018) 18:239–45. doi: 10.1016/j.clbc.2017.09.005
2. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
3. Sun W. Recent advances in cancer immunotherapy. J Hematol Oncol (2017) 10:96. doi: 10.1186/s13045-017-0460-9
4. Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther (2018) 3:7. doi: 10.1038/s41392-017-0004-3
5. Permatasari HK, Bulain S, Amar N, Azizah MR, Muslim FZ, Daud VPA, et al. Anticancer properties of caulerpa racemosa: A review study. Nutr Clin y Diet Hosp (2022) 42:110–21. doi: 10.12873/423permatasari
6. Salindeho N, Nurkolis F, Gunawan WB, Handoko MN, Samtiya M, Muliadi RD. Anticancer and anticholesterol attributes of sea cucumbers: An opinion in terms of functional food applications. Front Nutr (2022) 9:986986. doi: 10.3389/fnut.2022.986986
7. Murai U, Yamagishi K, Kishida R, Iso H. Impact of seaweed intake on health. Eur J Clin Nutr (2021) 75:877–89. doi: 10.1038/s41430-020-00739-8
8. Kim J, Lee J, Oh JH, Chang HJ, Sohn DK, Shin A, et al. Associations among dietary seaweed intake, c-MYC rs6983267 polymorphism, and risk of colorectal cancer in a Korean population: a case–control study. Eur J Nutr (2020) 59:1963–74. doi: 10.1007/s00394-019-02046-w
9. Teas J, Vena S, Cone DL, Irhimeh M. The consumption of seaweed as a protective factor in the etiology of breast cancer: Proof of principle. J Appl Phycol (2013) 25:771–9. doi: 10.1007/s10811-012-9931-0
10. Ruan B-F, Ge W-W, Lin M-X, Li Q-S. A review of the components of seaweeds as potential candidates in cancer therapy. Anticancer Agents Med Chem (2018) 18:354–66. doi: 10.2174/1871520617666171106130325
11. Gutiérrez-Rodríguez AG, Juárez-Portilla C, Olivares-Bañuelos T, Zepeda RC. Anticancer activity of seaweeds. Drug Discovery Today (2018) 23:434–47. doi: 10.1016/j.drudis.2017.10.019
12. Permatasari HK, Wewengkang DS, Tertiana NI, Muslim FZ, Yusuf M, Baliulina SO, et al. Anti-cancer properties of caulerpa racemosa by altering expression of bcl-2, BAX, cleaved caspase 3 and apoptosis in HeLa cancer cell culture. Front Oncol (2022) 12:964816. doi: 10.3389/fonc.2022.964816
13. Jóźwiak M, Filipowska A, Fiorino F, Struga M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur J Pharmacol (2020) 871:172937. doi: 10.1016/j.ejphar.2020.172937
14. Shannon E, Abu-Ghannam N. Seaweeds as nutraceuticals for health and nutrition. Phycologia (2019) 58:563–77. doi: 10.1080/00318884.2019.1640533
15. Kalasariya HS, Yadav VK, Yadav KK, Tirth V, Algahtani A, Islam S, et al. Seaweed-based molecules and their potential biological. Molecules (2021) 26:1–22. doi: 10.3390/molecules26175313
16. Permatasari HK, Nurkolis F, Hardinsyah H, Taslim NA, Sabrina N, Ibrahim FM, et al. Metabolomic assay, computational screening, and pharmacological evaluation of caulerpa racemosa as an anti-obesity with anti-aging by altering lipid profile and peroxisome proliferator-activated receptor-γ coactivator 1-α levels. Front Nutr (2022) 9:939073. doi: 10.3389/fnut.2022.939073
17. Manoppo JIC, Nurkolis F, Pramono A, Ardiaria M, Murbawani EA, Yusuf M, et al. Amelioration of obesity-related metabolic disorders via supplementation of caulerpa lentillifera in rats fed with a high-fat and high-cholesterol diet. Front Nutr (2022) 9:1010867. doi: 10.3389/fnut.2022.1010867
18. Nurkolis F, Mario Yusuf V, Yusuf M, Jati Kusuma R, Gunawan WB, Wiratama Hendra I, et al. Metabolomic profiling, in vitro antioxidant and cytotoxicity properties of Caulerpa racemosa: Functional food of the future from algae. North Carolina (US): Research Square (2022). doi: 10.21203%2Frs.3.rs-2158307%2Fv1
19. Azizi MN, Loh TC, Foo HL, Akit H, Izuddin WI, Shazali N, et al. Chemical compositions of brown and green seaweed, and effects on nutrient digestibility in broiler chickens. Animals (2021) 11:1–11. doi: 10.3390/ani11072147
20. El Maghraby DM, Fakhry EM. Lipid content and fatty acid composition of Mediterranean macro-algae as dynamic factors for biodiesel production. Oceanologia (2015) 57:86–92. doi: 10.1016/j.oceano.2014.08.001
21. Rodrigues D, Freitas AC, Pereira L, Rocha-Santos TAP, Vasconcelos MW, Roriz M, et al. Chemical composition of red, brown and green macroalgae from buarcos bay in central West coast of Portugal. Food Chem (2015) 183:197–207. doi: 10.1016/j.foodchem.2015.03.057
22. Rohani-Ghadikolaei K, Abdulalian E, Ng WK. Evaluation of the proximate, fatty acid and mineral composition of representative green, brown and red seaweeds from the Persian gulf of Iran as potential food and feed resources. J Food Sci Technol (2012) 49:774–80. doi: 10.1007/s13197-010-0220-0
23. Peñalver R, Lorenzo JM, Ros G, Amarowicz R, Pateiro M, Nieto G. Seaweeds as a functional ingredient for a healthy diet. Mar Drugs (2020) 18:1–27. doi: 10.3390/md18060301
24. Belattmania Z, Engelen AH, Pereira H, Serrão EA, Custódio L, Varela JC, et al. Fatty acid composition and nutraceutical perspectives of brown seaweeds from the Atlantic coast of Morocco. Int Food Res J (2018) 25:1520–7.
25. Gosch BJ, Magnusson M, Paul NA, de Nys R. Total lipid and fatty acid composition of seaweeds for the selection of species for oil-based biofuel and bioproducts. GCB Bioenergy (2012) 4:919–30. doi: 10.1111/j.1757-1707.2012.01175.x
26. Premarathna AD, Tuvikene R, Fernando PHP, Adhikari R, Perera MCN, Ranahewa TH, et al. Comparative analysis of proximate compositions, mineral and functional chemical groups of 15 different seaweed species. Sci Rep (2022) 12:19610. doi: 10.1038/s41598-022-23609-8
27. Mata L, Magnusson M, Paul NA, de Nys R. The intensive land-based production of the green seaweeds derbesia tenuissima and ulva ohnoi: Biomass and bioproducts. J Appl Phycol (2016) 28:365–75. doi: 10.1007/s10811-015-0561-1
28. Nufus C, Nurjanah AA. Karakteristik rumput laut hijau dari perairan kepulauan seribu dan sekotong nusa tenggara barat sebagai antioksidan. J Pengolah Has Perikan Indones (2017) 20:620–32.
29. Cardoso C, Ripol A, Afonso C, Freire M, Varela J, Quental-Ferreira H, et al. Fatty acid profiles of the main lipid classes of green seaweeds from fish pond aquaculture. Food Sci Nutr (2017) 5:1186–94. doi: 10.1002/fsn3.511
30. Nurkolis F, Taslim NA, Qhabibi FR, Kang S, Moon M, Choi J, et al. Ulvophyte green algae Caulerpa lentillifera: Metabolites profile and antioxidant, anticancer, anti-obesity, and In vitro cytotoxicity properties. Molecules (2023) 28:1365. doi: 10.3390/molecules28031365
31. Kendel M, Wielgosz-Collin G, Bertrand S, Roussakis C, Bourgougnon NB, Bedoux G. Lipid composition, fatty acids and sterols in the seaweeds ulva armoricana, and solieria chordalis from brittany (France): An analysis from nutritional, chemotaxonomic, and antiproliferative activity perspectives. Mar Drugs (2015) 13:5606–28. doi: 10.3390/md13095606
32. Magdugo RP, Terme N, Lang M, Pliego-Cortés H, Marty C, Hurtado AQ, et al. An analysis of the nutritional and health values of caulerpa racemosa (Forsskål) and ulva fasciata (Delile)–two chlorophyta collected from the Philippines. Molecules (2020) 25:2901. doi: 10.3390/molecules25122901
33. Olasehinde TA, Olaniran AO, Okoh AI. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of alzheimer’s disease. Mar Drugs (2019) 17:1–18. doi: 10.3390/md17110609
34. Hong IK, Jeon H, Lee SB. Comparison of red, brown and green seaweeds on enzymatic saccharification process. J Ind Eng Chem (2014) 20:2687–91. doi: 10.1016/j.jiec.2013.10.056
35. Cho M, You S. Sulfated polysaccharides from green seaweeds. Kim S-K, editor. Berlin, Heidelberg: Springer (2015) 941–53. Springer Handb. Mar. Biotechnol. doi: 10.1007/978-3-642-53971-8_40
36. Olsson J, Toth GB, Albers E. Biochemical composition of red, green and brown seaweeds on the Swedish west coast. J Appl Phycol (2020) 32:3305–17. doi: 10.1007/s10811-020-02145-w
37. Chen Y, Li P. Fatty acid metabolism and cancer development. Sci Bull (2016) 61:1473–9. doi: 10.1007/s11434-016-1129-4
38. Jin Z, Chai YD, Hu S. Fatty acid metabolism and cancer. Adv Exp Med Biol (2021) 1280:231–41. doi: 10.1007/978-3-030-51652-9_16
39. Luo Y, Wang H, Liu B, Wei J. Fatty acid metabolism and cancer immunotherapy. Curr Oncol Rep (2022) 24:659–70. doi: 10.1007/s11912-022-01223-1
40. Aiderus A, Black MA, Dunbier AK. Fatty acid oxidation is associated with proliferation and prognosis in breast and other cancers. BMC Cancer (2018) 18:1–15. doi: 10.1186/s12885-018-4626-9
41. Currie E, Schulze A, Zechner R, Walther TC, Farese RV. Cellular fatty acid metabolism and cancer. Cell Metab (2013) 18:153–61. doi: 10.1016/j.cmet.2013.05.017
42. D’Eliseo D, Velotti F. Omega-3 fatty acids and cancer cell cytotoxicity: Implications for multi-targeted cancer therapy. J Clin Med (2016) 5:15. doi: 10.3390/jcm5020015
43. Farag MA, Gad MZ. Omega-9 fatty acids: Potential roles in inflammation and cancer management. J Genet Eng Biotechnol (2022) 20:43. doi: 10.1186/s43141-022-00329-0
44. Zárate R, Jaber-Vazdekis N, Tejera N, Pérez JA, Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin Transl Med (2017) 6:25. doi: 10.1186/s40169-017-0153-6
45. Wang R, Paul VJ, Luesch H. Seaweed extracts and unsaturated fatty acid constituents from the green alga ulva lactuca as activators of the cytoprotective Nrf2-ARE pathway. Free Radic Biol Med (2013) 57:141–53. doi: 10.1016/j.freeradbiomed.2012.12.019
46. Martins P, Jesus J, Santos S, Raposo LR, Roma-Rodrigues C, Baptista PV, et al. Heterocyclic anticancer compounds: Recent advances and the paradigm shift towards the use of nanomedicine’s tool box. Molecules (2015) 20:16852–91. doi: 10.3390/molecules200916852
47. Lang DK, Kaur R, Arora R, Saini B, Arora S. Nitrogen-containing heterocycles as anticancer agents: An overview. Anticancer Agents Med Chem (2020) 20:2150–68. doi: 10.2174/1871520620666200705214917
48. Kidwai M, Venktaramanan R, Mohan R, Sapra P. Cancer chemotherapy and heterocyclic compounds. Curr Med Chem (2012) 9:1209–28. doi: 10.2174/0929867023370059
49. Tang Y, Zhou J, Hooi SC, Jiang YM, Lu GD. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-coa synthetases (review). Oncol Lett (2018) 16:1390–6. doi: 10.3892/ol.2018.8843
50. Westheim AJF, Stoffels LM, Dubois LJ, van Bergenhenegouwen J, van Helvoort A, Langen RCJ, et al. Fatty acids as a tool to boost cancer immunotherapy efficacy. Front Nutr (2022) 9:868436. doi: 10.3389/fnut.2022.868436
51. Venepally V, Reddy Jala RC. An insight into the biological activities of heterocyclic–fatty acid hybrid molecules. Eur J Med Chem (2017) 141:113–37. doi: 10.1016/j.ejmech.2017.09.069
52. Permatasari HK, Nurkolis F, Gunawan WB, Yusuf VM, Yusuf M, Kusuma RJ, et al. Modulation of gut microbiota and markers of metabolic syndrome in mice on cholesterol and fat enriched diet by butterfly pea flower kombucha. Curr Res Food Sci (2022) 5:1251–65. doi: 10.1016/j.crfs.2022.08.005
53. Cha SH, Hwang Y, Heo SJ, Jun HS. Indole-4-carboxaldehyde isolated from seaweed, sargassum thunbergii, attenuates methylglyoxal-induced hepatic inflammation. Mar Drugs (2019) 17:486. doi: 10.3390/md17090486
Keywords: green seaweed, fatty acid, heterocyclic derivatives, cancer, nutraceutical, functional food
Citation: Taher N, Mentang F, Montolalu RI, Gunawan WB, Taslim NA, Mayulu N and Nurkolis F (2023) Green seaweeds fatty acids and heterocyclic derivatives against cancer: Opinion on future nutraceutical application. Front. Oncol. 13:1145919. doi: 10.3389/fonc.2023.1145919
Received: 16 January 2023; Accepted: 31 January 2023;
Published: 14 February 2023.
Edited by:
Marc Poirot, INSERM U1037 Centre de Recherche en Cancérologie de Toulouse, FranceReviewed by:
Mauro Finicelli, National Research Council (CNR), ItalyCopyright © 2023 Taher, Mentang, Montolalu, Gunawan, Taslim, Mayulu and Nurkolis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nurmeilita Taher, bi50YWhlckB1bnNyYXQuYWMuaWQ=
†These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.