- 1Department of Thoracic Surgery, The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University, Huaian, China
- 2Department of Pathology, The Affiliated Huaian No. 1 People’s Hospital of Nanjing Medical University, Huaian, China
Background: Postoperative outcomes for patients suffering from resectable esophageal squamous cell carcinoma (ESCC) are related to sarcopenia. In patients with resectable ESCC, this study investigated the link between sarcopenia and postoperative pneumonia.
Methods: The McKewon procedure was the only one used to treat resectable ESCC patients from January 2018 to December 2021 in this retrospective analysis. Sarcopenia was assessed using skeletal muscles at L3 and planning CT scans. It was defined when PMI was below 6.36 cm2/m2 and 3.92 cm2/m2 for men and women, separately. Analyses of multivariate and univariate logistic regression were applied for identifying the risk factors for postoperative pneumonia.
Results: The study included 773 patients with resectable ESCC in total. Sarcopenia was an independent risk factor for postoperative pneumonia in individuals with resectable ESCC based on univariate and multivariate analysis (P < 0.05). The stratified analysis indicated that neither of the clinical outcomes in the logistic regression model were affected by gender, age, BMI, smoking, or pre-albumin (P for interaction > 0.006).
Conclusion: Following the McKewon procedure, patients with resectable ESCC who were sarcopenic had a higher postoperative pneumonia rate. To prevent the development of postoperative pneumonia during the perioperative period, it may be important to control the incidence of sarcopenia.
1 Introduction
Due to its complicated nature, poor prognosis, and high mortality, esophageal cancer places a significant social burden on society (1, 2). Incidence of esophageal cancer in China ranks sixth among malignant tumors, with the fourth highest mortality rate, accounting for more than half of all cases (3, 4). Esophageal squamous cell carcinoma (ESCC) is the major subtype of esophageal cancer, representing 95% of the diagnosed esophageal cancer (5). Treatment options for ESCC mainly include radiotherapy or chemotherapy or esophagectomy, with the goal of curative resection (6, 7). Surgery is still the primary treatment, especially for patients suffering from early-stage esophageal cancer (8, 9). The traditional esophagectomy is among the most invasive surgical procedure, and the current mainstream treatment types of minimally invasive esophagectomy (MIE) include McKeown procedure (cervical anastomosis) as well as Ivor Lewis procedure (intrathoracic anastomosis) with the advent of thoracoscopic/laparoscopic esophagectomy (TLE) (10, 11).
Although reported rates are close to 60% (12, 13), the rate of postoperative complications linked to MIE continues to be significant. Postoperative pneumonia is among the most frequent complications after MIE, which accounts for 25-30% of all cases (14, 15). Postoperative pneumonia is frequently associated with prolonged hospitalization and a poor long-term prognosis (16, 17). According to current research, malnutrition, diabetes mellitus, advanced age, increased intraoperative blood loss, thoracotomy, obesity, together with recurrent laryngeal nerve palsy (RLNP), these are all the risk factors for postoperative pneumonia in esophageal cancer patients (18–21). Improved predictors of post-operative pneumonia in individuals receiving MIE are required as these indicators have not been widely accepted as essential predictors of the condition.
Recently, it has been identified that loss of skeletal muscle mass, also known as “sarcopenia”, is a poor prognostic factor for various conditions and diseases (22–24). In cancer patients, sarcopenia is linked to more severe treatment toxicity and poorer survival (25, 26). Additionally, sarcopenia is a prominent predictor of complications following surgery for bladder, pancreatic, and colorectal cancers and liver transplantation (27–30). Sugimura et al. have reported that sarcopenia assessed through the use of the L3 skeletal muscle mass index (SMI) may be a predictor of pulmonary complications following esophagectomy (31). Besides, the psoas major muscle index (PMI) is now among the diagnostic standards for the diagnosis of sarcopenia recommended from the Asian Working Group on Sarcopenia (AWGS) together with European Working Group on Sarcopenia in the Older People (EWGSOP) (32, 33). Nevertheless, many researches have adopted SMI as a diagnostic standard for sarcopenia to assay the correlation between postoperative complications and sarcopenia, while PMI has been rarely utilized. In contrast to SMI, PMI features the advantages of clear boundaries, small measurement area, and single muscle measurement (34). As a result, the measurement is simpler and more precise. Simultaneously, there has been little research into the ability of sarcopenia based on PMI diagnosis to predict postoperative pneumonia in patients with ESCC treated with McKewon procedure alone.
As a result, the purpose of this work is to clarify, using the McKeown approach, the predictive value of sarcopenia based on PMI diagnosis for postoperative pneumonia following MIE.
2 Materials and methods
2.1 Study design and participants
A retrospective cohort study was implemented within the Department of Thoracic Surgery, Huai’an No. 1 People’s Hospital, Nanjing Medical University, Nanjing, China, from January 2018 to December 2021. In the current work, patients had resectable esophageal squamous cell carcinoma and underwent only a thoraco-laparoscopic esophagectomy, which is a minimally invasive procedure. Patients with either of the following conditions will be excluded/included from the trial. The exclusion criteria are listed below: (A) comorbidity with other malignancies; (B) preoperative CT imaging data not available; (C) patients receiving neoadjuvant therapy; (D) perioperative clinicopathological data not available.
Inclusion criteria are as below: (A) Patients receiving digestive tract endoscopy prior to surgery and were pathologically confirmed to have squamous cell carcinoma;
Patients confirmed as squamous cell carcinoma by digestive endoscopy before surgery; (B) CT imaging data of chest and abdomen scan within one week before operation in our hospital. All patients were clinically staged in accordance with the eighth edition of the TNM classification. The study followed the Declaration of Helsinki guidelines and was authorized through the Ethics Committee of Nanjing Medical University.
2.2 Information on clinical parameters
Retrospectively obtained clinical parameters from medical records included demographics, co-morbidities, medication information, and laboratory data. The calculation of BMI is weight (kg) divided by height squared (m2). Normal weight is measured by a 18.5 kg/m2 BMI, it is then measured by a BMI between 18.5 and 24 kg/m2, and overweight (with a BMI of 24 kg/m2) (35). The cut-off point for stratifying prealbumin was the identification of low prealbumin by prealbumin <160 mg/L (36). Diagnostic criteria for the diagnosis of postoperative pneumonia within thirty days of surgery should conform to the following three criteria in the meantime: (A) at least two chest radiographs and at least one diagnosis of pneumonia; (B) at least meet the following one, age ≥70 years, peripheral blood WBC count <4 x 109/L or >12 x 109/L, and fever (the temperature of body >38°C) with altered consciousness; (C) at least two of the following, such as the emergence of purulent sputum or sputum properties change, or increased respiratory secretions, or need to increase the number of sputum, or dyspnea.
2.3 Determination of sarcopenia according to PMI index
Prior to surgery, abdominal computed tomography (CT) scans from ESCC patients were applied for calculating the skeletal muscle mass. The software Philips Vue PACS (Philips Electronics UK LTD, Farnborough, UK) was used to retrospectively analyze CT images in our institution. The muscles were measured in the HU range between -29 and +150 HU, and any necessary manual corrections to the tissue boundaries were made. PMI was estimated after summing the right and left psoas muscle areas in the L3 segment and normalizing it to the height of the patient. (Bilateral psoas area/height2). In accordance with the new diagnostic standard recommended for low skeletal muscle mass on CT imaging in Asian adults, we applied the gender-specific PMI thresholds for sarcopenia of 3.92 cm2/m2 and 6.36 cm2/m2 for women and men, separately (37).
2.4 Statistical analysis
Baseline pathological and clinical variables were expressed as range and median for consecutive variables and as proportion and frequency for categorical variables. Pathological and clinical variables were examined via employing chi-square tests. The relation between postoperative pneumonia and sarcopenia was examined after the use of models of univariate and multivariate logistic regression analysis. Three models were employed: model 1, adjusted for age and sex; model 2, adjusted for hypertension, sex, age, smoking together with diabetes; and model 3, adjusted for hypertension, sex, age, smoking, diabetes grade, tumor location, N-stage, as well as T-stage. For identifying interactions and modifications, likelihood ratio tests and stratified logistic regression models were applied in subgroups of hypertension, age, gender, smoking, BMI, diabetes, grade, tumor location, prealbumin, N stage and T stage. All of the statistical analyses were implemented via applying the Free Statistics software version 1.7.1 along with the software package R (http://www.R-project.org, The R Foundation). When P < 0.05, the statistical differences were deemed significant.
3 Results
3.1 Baseline characteristics
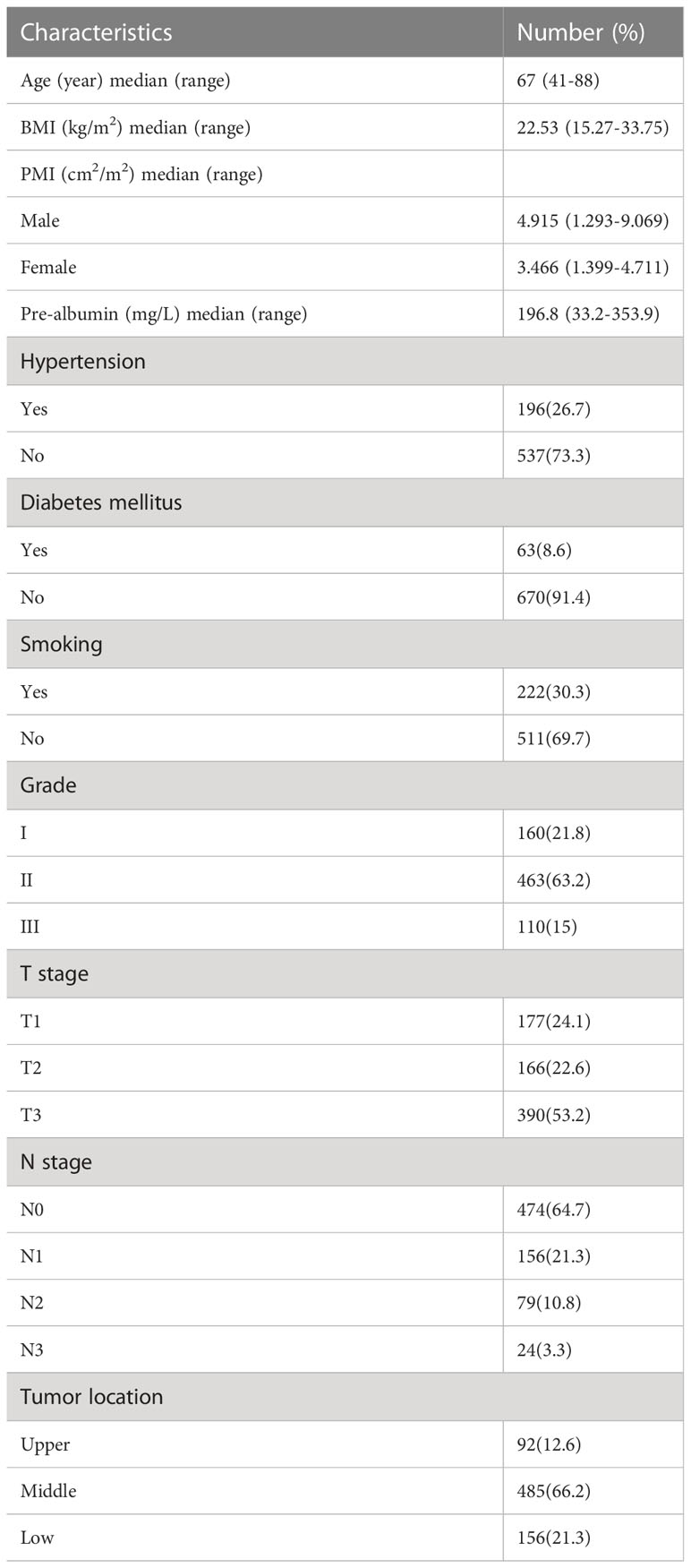
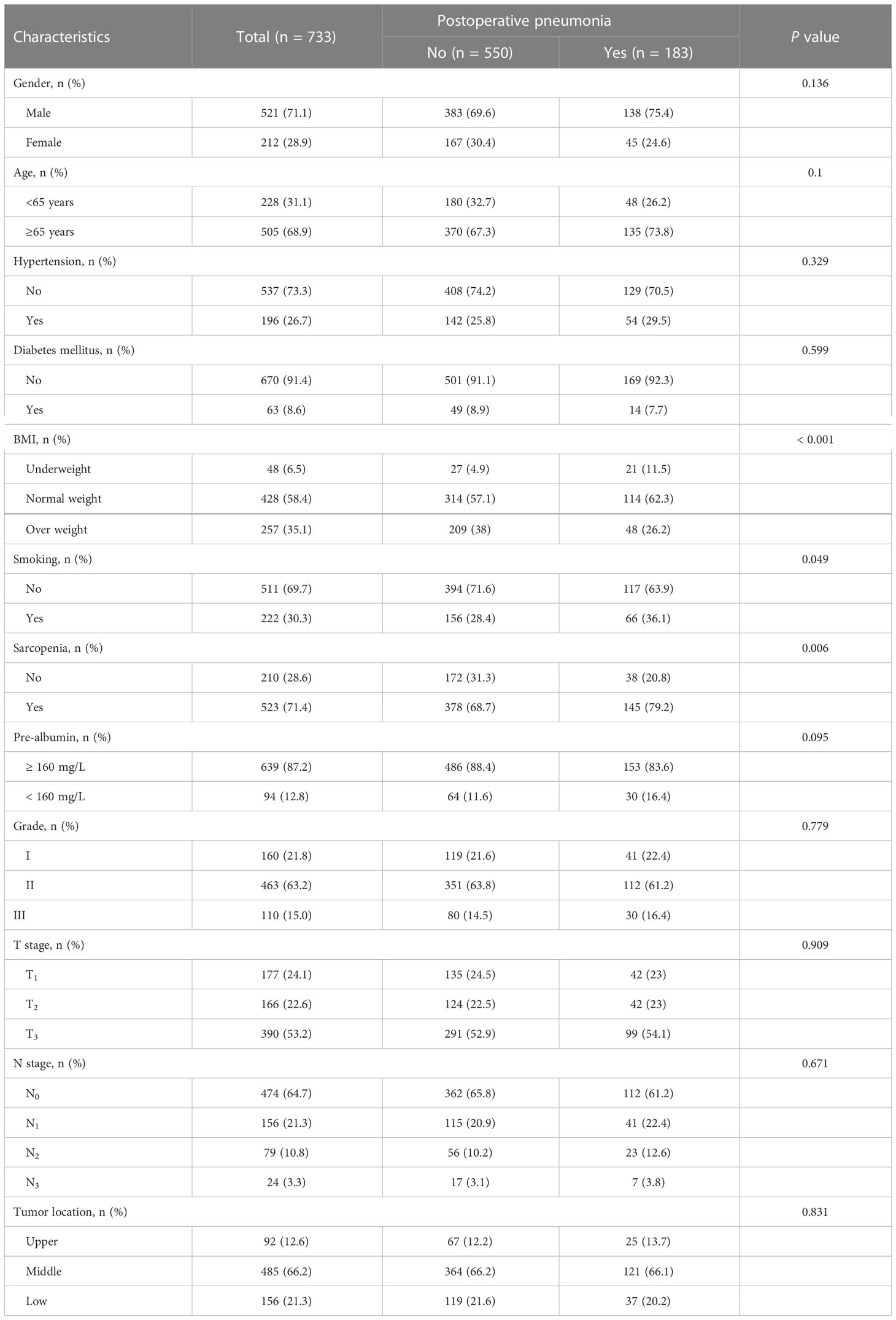
Table 1 presents the pathological and clinical characteristics of 773 participants enrolled in the work. Population-specific characteristics by postoperative pneumonia are presented in Table 2. 521 males and 212 females made up this cohort; 138 of the males had postoperative pneumonia. Of the females, 45 also had postoperative pneumonia. 505 participants were over the age of 65, while 228 participants were under that age. The overall cohort contained 523 patients with sarcopenia in accordance with the suggested sarcopenia diagnostic criteria, 145 of whom had pneumonia postoperatively. Postoperative pneumonia was used as a grouping variable, and BMI, smoking and sarcopenia were significantly different between groups. In comparison to the postoperative and non-postoperative pneumonia groups, the sarcopenia had more positive results (79.2% vs. 68.7%) in the postoperative pneumonia group.
3.2 Univariate and multivariate analyses of postoperative pneumonia
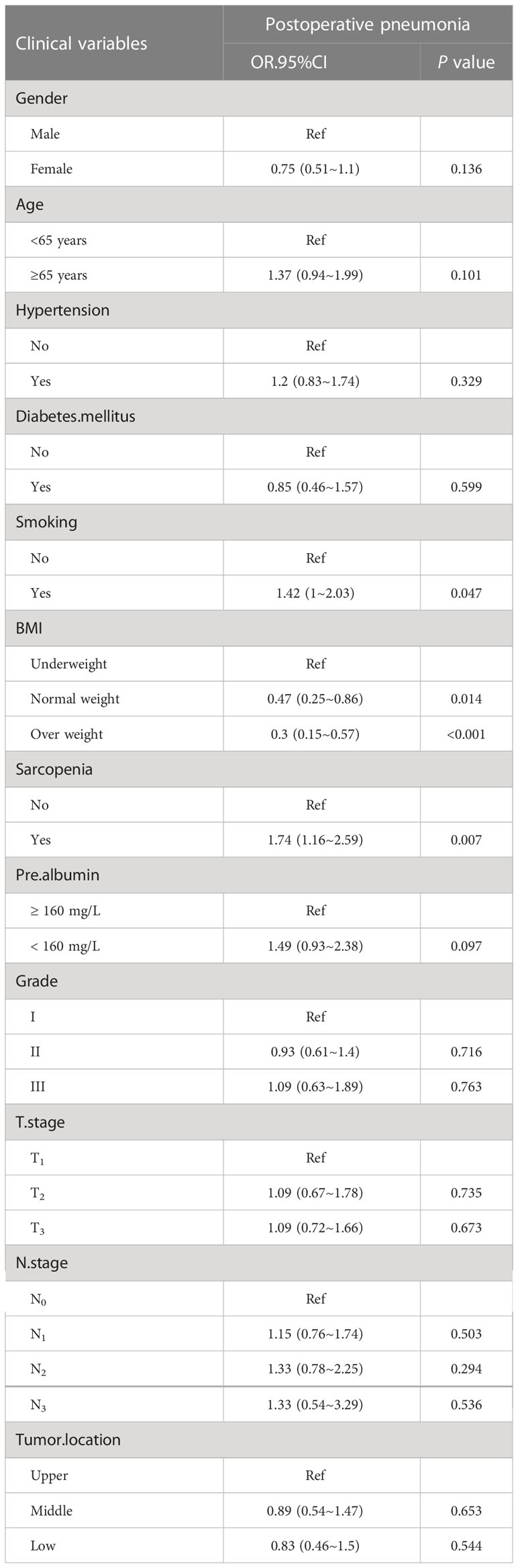
Among all clinicopathological factors analyzed, smoking, both sarcopenia and BMI were remarkably related to postoperative pneumonia (Table 3). After adjustment for various confounding factors, sarcopenia has a positive correlation with the postoperative pneumonia in three models. In three models, odds ratios (ORs) for sarcopenia were persistently significant. Of note, the adjusted OR for postoperative pneumonia was 1.53 (95% CI: 1.01-2.33) (Table 4), when sarcopenia was assessed as the categorical variable in the all-variable adjusted model (model 3).
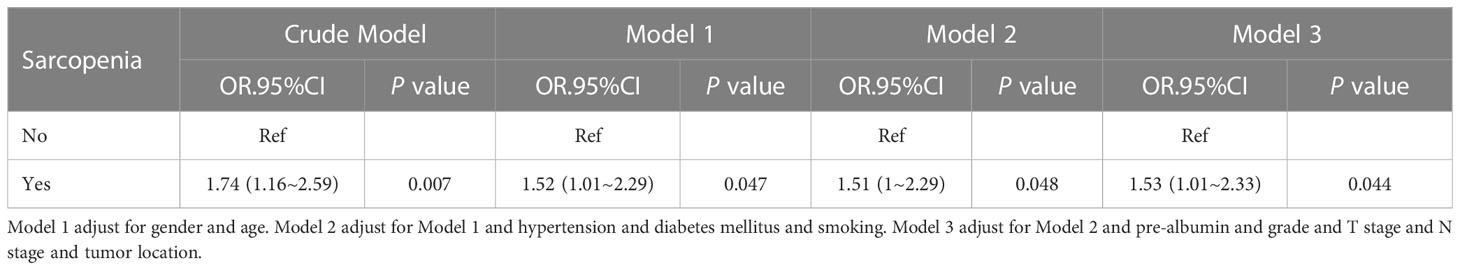
Table 4 Multivariable-adjust ORs and 95%CI of the sarcopenia associated with postoperative pneumonia.
3.3 Subgroup analyses by adjusted potential effect confounders
For assessing the impact of sarcopenia on pneumonia in various subgroups, subgroup analyses were carried out. The correlation between postoperative pneumonia and sarcopenia was coordinated in subgroups as below: age (≤65 years versus >65 years; P = 0.852 for interaction), gender (male versus female; P = 0.485 for interaction), prealbumin (≥160 mg/L versus <160 mg/L; P=0.921 for the interaction) and BMI (underweight versus normal weight/overweight; P = 0.785 for interaction), as shown in Figure 1.
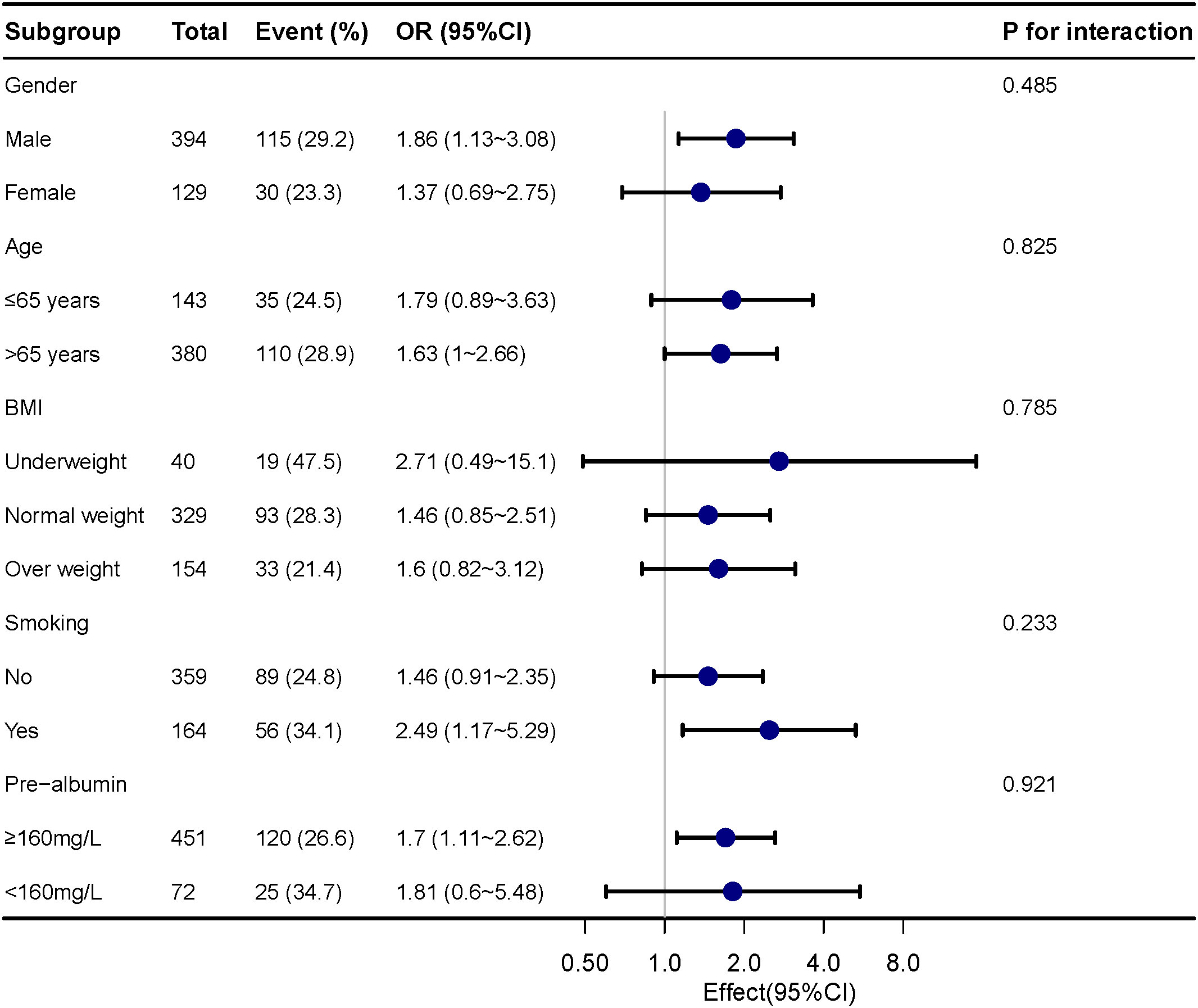
Figure 1 Subgroup analyses of the sarcopenia and postoperative pneumonia, stratified by gender, age, BMI, smoking and pre-albumin.
4 Discussion
Regarding postoperative pneumonia, this work assessed the value of the prognosis of sarcopenia in ESCC patients undergoing minimally invasive McKeown esophagectomy. In the current work, we found that postoperative pneumonia in ESCC patients had an independent risk factor for sarcopenia. Additionally, sarcopenia had a consistent predictive value for postoperative pneumonia across gender, age, BMI, smoking, and pre-albumin subgroups.
To our knowledge, this is the first research to examine the correlation between the prevalence of postoperative pneumonia and sarcopenia in ESCC patients only undergoing MIE-McKeown surgery. As we all know, surgery for ESCC in complex and entails many challenges. As science and technology advance, minimally invasive surgery has gradually become the standard surgical approach globally (38). However, patients with ESCC still experience up to 38% of postoperative pulmonary complications from surgery (39). The primary causes of postoperative pneumonia in patients suffering from ESCC after esophagectomy are thought to be malnutrition and surgical stress (40, 41).
Recently, studies have started to evaluate the correlation between sarcopenia and postoperative complications in various tumors. For example, Tolga Olmez et al. suggested that sarcopenia may lead to longer Length of intensive care unit stay in colon cancer (42). Sarcopenia may cause worse overall survival in renal cell carcinoma (43). Additionally, for some malignant tumors with a long-term prognosis, sarcopenia may be a predictor of OS or DFS (44–46). The number of patients suffering from sarcopenia is growing because of the disease-specificity of patients with upper gastrointestinal cancers, which are difficult to administer orally (47). In correspondence, over 70% of the patients in our work satisfied the criteria for sarcopenia. Several prior researches depicted sarcopenia diagnosed according to L3-SMI can lead to specific adverse events in ESCC patients after surgery (48). Therefore, the increased incidence of postoperative pneumonia and expectoration disorder among patients were probably due to deterioration of strength in the respiratory and swallowing muscles (49, 50). In addition to the SMI standard, the sarcopenia diagnosed by PMI based on CT scanning has gradually gained attention in recent years. SMI was calculated from the entire skeletal muscle area in cross-section of the third lumbar vertebral body, while PMI was measured from the sum of the right and left lumbar muscle areas next to the third lumbar vertebral body, with better accuracy and lower systematic error (37). Nevertheless, few researches have examined the association between PMI-based sarcopenia as the diagnostic criteria and postoperative pneumonia in ESCC patients. In our study, sarcopenia has been identified as a risk factor for post-operative pneumonia, simultaneously remained stable in the subgroup analysis results.
After surgery for gastrointestinal cancer, weight loss and skeletal muscle wasting are inevitable, especially in the first 10 days (51). If the patient has been diagnosed with preoperative sarcopenia before surgery and have to accept surgical treatment during this period, it will often lead to many postoperative complications (52). From the point of view of postoperative pneumonia, it is essential to ameliorate sarcopenia prior to esophagectomy. Moreover, over time, interventions of exercise therapy and nutritional support prior to surgery have become possible (53). Sarcopenia-related postoperative pneumonia is thought to have a secondary systemic inflammatory response in the muscles brought on by hypoactivity, malnutrition, and surgical stress (54). The underlying mechanisms of sarcopenia-induced postoperative pneumonia is still unclear. However, the current pathogenesis of sarcopenia involves the following aspects: Decline in exercise capacity associated with age is a primary factor in the loss of muscle strength along with mass in older adults (55). Another important reason is the deterioration of neuro-muscular function. Normal function of the motor neurons is critical to the viability of muscle fibers (56). Moreover, the alpha motor neuron loss is a critical factor in sarcopenia pathogenesis. It was discovered that the motor neurons were markedly reduced in older adults after the age of 70, while the loss of alpha motor neurons amounted to 50% (57). Furthermore, some related studies have suggested that insulin, estrogen, androgen, growth hormone and glucocorticoid changes were also involved in the pathogenesis of sarcopenia (58, 59). For example, fat in the body and muscle cells increases, which is associated with insulin resistance in sarcopenia (60). Currently, experiments have confirmed that protein anabolism is significantly reduced after aging muscle cells receive insulin (61). It was reported that pro-inflammatory cytokines were also implicated in the pathogenesis of elderly sarcopenia. The CRP, TNF-α and IL-6 levels were found to be linked to muscle strength and mass (62). In the last several years, it has been suggested that immunohistochemical markers CD34 and CD10 may be able to act as markers in basal cells, though this remains to be confirmed by further histopathology development (63–65). Muscle biopsy shows that muscle cell apoptosis in the elderly is significantly higher than in young people, which indicated that myocyte apoptosis may be related to mitochondrial dysfunction and muscle mass loss (66). Studies have confirmed that type II muscle fibers mainly involved in sarcopenia are more likely to die through apoptotic pathways (67). And, it was also reported that aging, oxidative stress, low growth factors, and complete immobilization can induce caspase-dependent or -independent apoptotic signaling pathways (68).
This study presents several limitations. First, it was a retrospective cohort study that was implemented at a single institution, which might have introduced a potential selection bias. Second, all cases in this research were all squamous cell carcinomas. Therefore, the enrolled population studied has potential selection bias. The strength of this work is that we have the maximum sample size for the analysis of the association of postoperative pneumonia in ESCC patients currently studied, with only McKinsey surgery treatment.
In summary, our findings indicate that sarcopenia is markedly related to postoperative pneumonia in resectable ESCC patients. More interest should be given to the perioperative nutritional status in patients with a preoperative diagnosis of “sarcopenia”. Further researches are required to verify these results.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The study was performed the Declaration of Helsinki guidelines and was approved by the Ethics Committee of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JZ, DT, and ZX designed the study. QW and ZZ collected the data. YZ and YC analyzed the data. ZX, QW, DT, and JZ interpreted the result. ZX wrote the first draft of the manuscript. ZZ contributed to the refinement of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
Innovation Talent Fund of The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University (YCQR201815).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg (2018) 41(3):210–5. doi: 10.1016/j.asjsur.2016.10.005
2. Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol (2015) 21(26):7933–43. doi: 10.3748/wjg.v21.i26.7933
3. He F, Wang J, Liu L, Qin X, Wan Z, Li W, et al. Esophageal cancer: trends in incidence and mortality in China from 2005 to 2015. Cancer Med (2021) 10(5):1839–47. doi: 10.1002/cam4.3647
4. Liu CQ, Ma YL, Qin Q, Wang PH, Luo Y, Xu PF, et al. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer (2023) 14(1):3–11. doi: 10.1111/1759-7714.14745
5. He S, Xu J, Liu X, Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sin B (2021) 11(11):3379–92. doi: 10.1016/j.apsb.2021.03.008
6. Bollschweiler E, Plum P, Mönig SP, Hölscher AH. Current and future treatment options for esophageal cancer in the elderly. Expert Opin Pharmacother (2017) 18(10):1001–10. doi: 10.1080/14656566.2017.1334764
7. Mwachiro M, White R. Management of esophageal cancer treatment in resource-limited settings. Thorac Surg Clin (2022) 32(3):397–404. doi: 10.1016/j.thorsurg.2022.04.007
8. Borggreve AS, Kingma BF, Domrachev SA, Koshkin MA, Ruurda JP, van Hillegersberg R, et al. Surgical treatment of esophageal cancer in the era of multimodality management. Ann N Y Acad Sci (2018) 1434(1):192–209. doi: 10.1111/nyas.13677
9. Mönig S, Chevallay M, Niclauss N, Zilli T, Fang W, Bansal A, et al. Early esophageal cancer: the significance of surgery, endoscopy, and chemoradiation. Ann N Y Acad Sci (2018) 1434(1):115–23. doi: 10.1111/nyas.13955
10. Bograd AJ, Molena D. Minimally invasive esophagectomy. Curr Probl Surg (2021) 58(10):100984. doi: 10.1016/j.cpsurg.2021.100984
11. Yu F, Zhang Y, Xu H, Li K, Gheng J, Lin C, et al. Comparison of McKeown minimally invasive esophagectomy vs sweet esophagectomy for esophageal squamous cell carcinoma: a retrospective study. Front Oncol (2022) 12:1009315. doi: 10.3389/fonc.2022.1009315
12. Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med (2019) 380(2):152–62. doi: 10.1056/NEJMoa1805101
13. van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer: a randomized controlled trial. Ann Surg (2019) 269(4):621–30. doi: 10.1097/SLA.0000000000003031
14. Mboumi IW, Reddy S, Lidor AO. Complications after esophagectomy. Surg Clin North Am (2019) 99(3):501–10. doi: 10.1016/j.suc.2019.02.011
15. Kurita D, Sakurai T, Utsunomiya D, Kubo K, Fujii Y, Kanematsu K, et al. Predictive ability of the five-time chair stand test for postoperative pneumonia after minimally invasive esophagectomy for esophageal cancer. Ann Surg Oncol (2022) 29(12):7462–70. doi: 10.1245/s10434-022-12002-4
16. Maruyama S, Okamura A, Ishizuka N, Kanie Y, Sakamoto K, Fujiwara D, et al. Airflow limitation predicts postoperative pneumonia after esophagectomy. World J Surg (2021) 45(8):2492–500. doi: 10.1007/s00268-021-06148-7
17. Takeuchi M, Kawakubo H, Mayanagi S, Yoshida K, Fukuda K, Nakamura R, et al. Postoperative pneumonia is associated with long-term oncologic outcomes of definitive chemoradiotherapy followed by salvage esophagectomy for esophageal cancer. J Gastrointest Surg (2018) 22(11):1881–9. doi: 10.1007/s11605-018-3857-z
18. Takahashi K, Nishikawa K, Tanishima Y, Ishikawa Y, Kurogochi T, Yuda M, et al. Risk stratification of postoperative pneumonia in patients undergoing subtotal esophagectomy for esophageal cancer. Anticancer Res (2022) 42(6):3023–8. doi: 10.21873/anticanres.15787
19. Kamada T, Ohdaira H, Ito E, Takahashi J, Nakashima K, Nakaseko Y, et al. Association between masseter muscle sarcopenia and postoperative pneumonia in patients with esophageal cancer. Sci Rep (2022) 12(1):16374. doi: 10.1038/s41598-022-20967-1
20. Baba H, Tokai R, Hirano K, Watanabe T, Shibuya K, Hashimoto I, et al. Risk factors for postoperative pneumonia after general and digestive surgery: a retrospective single-center study. Surg Today (2020) 50(5):460–8. doi: 10.1007/s00595-019-01911-9
21. Bakhos CT, Fabian T, Oyasiji TO, Gautam S, Gangadharan SP, Kent MS, et al. Impact of the surgical technique on pulmonary morbidity after esophagectomy. Ann Thorac Surg (2012) 93(1):221–6. doi: 10.1016/j.athoracsur.2011.07.030
22. Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PloS One (2017) 12(10):e0186990. doi: 10.1371/journal.pone.0186990
23. Curcio F, Testa G, Liguori I, Papillo M, Flocco V, Panicara V, et al. Sarcopenia and heart failure. Nutrients (2020) 12(1):211. doi: 10.3390/nu12010211
24. Abe S, Kawai K, Nozawa H, Sasaki K, Murono K, Emoto S, et al. Preoperative sarcopenia is a poor prognostic factor in lower rectal cancer patients undergoing neoadjuvant chemoradiotherapy: a retrospective study. Int J Clin Oncol (2022) 27(1):141–53. doi: 10.1007/s10147-021-02062-z
25. Aprile G, Basile D, Giaretta R, Schiavo G, La Verde N, Corradi E, et al. The clinical value of nutritional care before and during active cancer treatment. Nutrients (2021) 13(4):1196. doi: 10.3390/nu13041196
26. Chowdhry SM, Chowdhry VK. Cancer cachexia and treatment toxicity. Curr Opin Support Palliat Care (2019) 13(4):292–7. doi: 10.1097/SPC.0000000000000450
27. Hsu CS, Kao JH. Sarcopenia and chronic liver diseases. Expert Rev Gastroenterol Hepatol (2018) 12(12):1229–44. doi: 10.1080/17474124.2018.1534586
28. Nakanishi R, Oki E, Sasaki S, Hirose K, Jogo T, Edahiro K, et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg Today (2018) 48(2):151–7. doi: 10.1007/s00595-017-1564-0
29. Choi MH, Yoon SB. Sarcopenia in pancreatic cancer: effect on patient outcomes. World J Gastrointest Oncol (2022) 14(12):2302–12. doi: 10.4251/wjgo.v14.i12.2302
30. Hansen TTD, Omland LH, von Heymann A, Johansen C, Clausen MB, Suetta C, et al. Development of sarcopenia in patients with bladder cancer: a systematic review. Semin Oncol Nurs (2021) 37(1):151108. doi: 10.1016/j.soncn.2020.151108
31. Sugimura K, Miyata H, Kanemura T, Takeoka T, Shinnno N, Yamamoto K, et al. Impact of preoperative skeletal muscle mass and physical performance on short-term and long-term postoperative outcomes in patients with esophageal cancer after esophagectomy. Ann Gastroenterol Surg (2022) 6(5):623–32. doi: 10.1002/ags3.12560
32. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing (2019) 48(1):16–31. doi: 10.1093/ageing/afy169
33. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian Working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc (2020) 21(3):300–307.e302. doi: 10.1016/j.jamda.2019.12.012
34. Zhang JX, Yan HT, Ding Y, Liu J, Liu S, Zu QQ, et al. Low psoas-muscle index is associated with decreased survival in hepatocellular carcinoma treated with transarterial chemoembolization. Ann Med (2022) 54(1):1562–9. doi: 10.1080/07853890.2022.2081872
35. Caballero B. Humans against obesity: who will win? Adv Nutr (2019) 10(suppl_1):S4–s9. doi: 10.1093/advances/nmy055
36. Zhou YQ, Zhang XM, Chen ZQ, Wang JL, Qian YB, Xu RY. The prevalence of hypophosphatemia and refeeding-related hypophosphatemia in hospitalized patients requiring parental nutrition: a retrospective study. Support Care Cancer (2022) 30(8):6995–7003. doi: 10.1007/s00520-022-07141-z
37. Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Hammad A, Tamai Y, et al. Proposal for new diagnostic criteria for low skeletal muscle mass based on computed tomography imaging in Asian adults. Nutrition (2016) 32(11-12):1200–5. doi: 10.1016/j.nut.2016.04.003
38. Waters JK, Reznik SI. Update on management of squamous cell esophageal cancer. Curr Oncol Rep (2022) 24(3):375–85. doi: 10.1007/s11912-021-01153-4
39. Baba Y, Yoshida N, Shigaki H, Iwatsuki M, Miyamoto Y, Sakamoto Y, et al. Prognostic impact of postoperative complications in 502 patients with surgically resected esophageal squamous cell carcinoma: a retrospective single-institution study. Ann Surg (2016) 264(2):305–11. doi: 10.1097/SLA.0000000000001510
40. Nambara M, Miki Y, Tamura T, Yoshii M, Toyokawa T, Tanaka H, et al. The optimal definition of sarcopenia for predicting postoperative pneumonia after esophagectomy in patients with esophageal cancer. World J Surg (2021) 45(10):3108–18. doi: 10.1007/s00268-021-06223-z
41. Tangoku A, Yoshino S, Abe T, Hayashi H, Satou T, Ueno T, et al. Mediastinoscope-assisted transhiatal esophagectomy for esophageal cancer. Surg Endosc (2004) 18(3):383–9. doi: 10.1007/s00464-003-8181-2
42. Olmez T, Karakose E, Bozkurt H, Pence HH, Gulmez S, Aray E, et al. Sarcopenia is associated with increased severe postoperative complications after colon cancer surgery. Arch Med Sci (2021) 17(2):361–7. doi: 10.5114/aoms.2019.88621
43. Hu X, Liao DW, Yang ZQ, Yang WX, Xiong SC, Li X. Sarcopenia predicts prognosis of patients with renal cell carcinoma: a systematic review and meta-analysis. Int Braz J Urol (2020) 46(5):705–15. doi: 10.1590/s1677-5538.ibju.2019.0636
44. Yamahara K, Mizukoshi A, Lee K, Ikegami S. Sarcopenia with inflammation as a predictor of survival in patients with head and neck cancer. Auris Nasus Larynx (2021) 48(5):1013–22. doi: 10.1016/j.anl.2021.03.021
45. McBee MP, Woodhouse C, Trout AT, Geller JI, Smith EA, Zhang B, et al. Skeletal muscle mass as a marker to predict outcomes in children and young adults with cancer. Abdom Radiol (NY) (2022) 47(1):452–9. doi: 10.1007/s00261-021-03301-7
46. Deng HY, Hou L, Zha P, Huang KL, Peng L. Sarcopenia is an independent unfavorable prognostic factor of non-small cell lung cancer after surgical resection: a comprehensive systematic review and meta-analysis. Eur J Surg Oncol (2019) 45(5):728–35. doi: 10.1016/j.ejso.2018.09.026
47. Poisson J, Martinez-Tapia C, Heitz D, Geiss R, Albrand G, Falandry C, et al. Prevalence and prognostic impact of cachexia among older patients with cancer: a nationwide cross-sectional survey (NutriAgeCancer). J Cachexia Sarcopenia Muscle (2021) 12(6):1477–88. doi: 10.1002/jcsm.12776
48. Onishi S, Tajika M, Tanaka T, Yamada K, Abe T, Higaki E, et al. Prognostic impact of sarcopenic obesity after neoadjuvant chemotherapy followed by surgery in elderly patients with esophageal squamous cell carcinoma. J Clin Med (2020) 9(9):2974. doi: 10.3390/jcm9092974
49. Okazaki T, Ebihara S, Mori T, Izumi S, Ebihara T. Association between sarcopenia and pneumonia in older people. Geriatr Gerontol Int (2020) 20(1):7–13. doi: 10.1111/ggi.13839
50. Komatsu R, Okazaki T, Ebihara S, Kobayashi M, Tsukita Y, Nihei M, et al. Aspiration pneumonia induces muscle atrophy in the respiratory, skeletal, and swallowing systems. J Cachexia Sarcopenia Muscle (2018) 9(4):643–53. doi: 10.1002/jcsm.12297
51. Yoon SL, Kim JA, Kelly DL, Lyon D, George TJ Jr. Predicting unintentional weight loss in patients with gastrointestinal cancer. J Cachexia Sarcopenia Muscle (2019) 10(3):526–35. doi: 10.1002/jcsm.12398
52. Chen F, Chi J, Liu Y, Fan L, Hu K. Impact of preoperative sarcopenia on postoperative complications and prognosis of gastric cancer resection: a meta-analysis of cohort studies. Arch Gerontol Geriatr (2022) 98:104534. doi: 10.1016/j.archger.2021.104534
53. Wobith M, Weimann A. Oral nutritional supplements and enteral nutrition in patients with gastrointestinal surgery. Nutrients (2021) 13(8):2655. doi: 10.3390/nu13082655
54. Fedele D, De Francesco A, Riso S, Collo A. Obesity, malnutrition, and trace element deficiency in the coronavirus disease (COVID-19) pandemic: an overview. Nutrition (2021) 81:111016. doi: 10.1016/j.nut.2020.111016
55. Wilkinson DJ, Piasecki M, Atherton PJ. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res Rev (2018) 47:123–32. doi: 10.1016/j.arr.2018.07.005
56. Tintignac LA, Brenner HR, Rüegg MA. Mechanisms regulating neuromuscular junction development and function and causes of muscle wasting. Physiol Rev (2015) 95(3):809–52. doi: 10.1152/physrev.00033.2014
57. Krenovsky JP, Bötzel K, Ceballos-Baumann A, Fietzek UM, Schoser B, Maetzler W, et al. Interrelation between sarcopenia and the number of motor neurons in patients with parkinsonian syndromes. Gerontology (2020) 66(4):409–15. doi: 10.1159/000505590
58. Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol (2016) 229(2):R67–81. doi: 10.1530/JOE-15-0533
59. Ikeda K, Horie-Inoue K, Inoue S. Functions of estrogen and estrogen receptor signaling on skeletal muscle. J Steroid Biochem Mol Biol (2019) 191:105375. doi: 10.1016/j.jsbmb.2019.105375
60. Hong SH, Choi KM. Sarcopenic obesity, insulin resistance, and their implications in cardiovascular and metabolic consequences. Int J Mol Sci (2020) 21(2):494. doi: 10.3390/ijms21020494
61. Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y. Sarcopenia. Joint Bone Spine (2019) 86(3):309–14. doi: 10.1016/j.jbspin.2018.08.001
62. Bano G, Trevisan C, Carraro S, Solmi M, Luchini C, Stubbs B, et al. Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas (2017) 96:10–5. doi: 10.1016/j.maturitas.2016.11.006
63. Sengul D, Sengul I, Astarci MH, Ustun H, Mocan G. CD10 for the distinct differential diagnosis of basal cell carcinoma and benign tumours of cutaneous appendages originating from hair follicle. Pol J Pathol (2010) 61(3):140–6.
64. Sengul D, Sengul I, Astarci MH, Ustun H, Mocan G. Differential diagnosis of basal cell carcinoma and benign tumors of cutaneous appendages originating from hair follicles by using CD34. Asian Pac J Cancer Prev (2010) 11(6):1615–9.
65. Sengul I, Sengul D, Astarci MH, Ustun H. Histopathological characteristics may not be useful in the differential diagnosis between basal cell carcinoma and benign tumors of cutaneous appandages originating from hair follicle. Int J Hematol Oncol (2011) 32(1):223–9. doi: 10.4999/uhod.10044
66. Xu Z, Fu T, Guo Q, Zhou D, Sun W, Zhou Z, et al. Disuse-associated loss of the protease LONP1 in muscle impairs mitochondrial function and causes reduced skeletal muscle mass and strength. Nat Commun (2022) 13(1):894. doi: 10.1038/s41467-022-28557-5
67. Leeuwenburgh C. Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci (2003) 58(11):999–1001. doi: 10.1093/gerona/58.11.M999
Keywords: esophageal squamous cell carcinoma, postoperative pneumonia, sarcopenia, McKewon procedure, complications
Citation: Xu Z, Wang Q, Zhang Z, Zhu Y, Chen Y, Tang D and Zhao J (2023) Association between preoperative diagnosis of sarcopenia and postoperative pneumonia in resectable esophageal squamous cell carcinoma patients: a retrospective cohort study. Front. Oncol. 13:1144516. doi: 10.3389/fonc.2023.1144516
Received: 18 January 2023; Accepted: 02 May 2023;
Published: 18 May 2023.
Edited by:
Savvas Lampridis, Hammersmith Hospital, United KingdomReviewed by:
Mikhail Danilov, A.S.Loginov Moscow Clinical Scientific Centre, RussiaIlker Sengul, Giresun University, Türkiye
Copyright © 2023 Xu, Wang, Zhang, Zhu, Chen, Tang and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Derong Tang, RGVyb25nVGFuZzE5ODJAMTI2LmNvbQ==; Jianqiang Zhao, c2hlbmdsZWU2ODcxQHNpbmEuY29t
 Zhiyun Xu
Zhiyun Xu Qianwei Wang
Qianwei Wang Zhenzhong Zhang
Zhenzhong Zhang Yaning Zhu
Yaning Zhu Yunyun Chen
Yunyun Chen Derong Tang
Derong Tang Jianqiang Zhao
Jianqiang Zhao