- 1Department of Hematology and Institute of Hematology, West China Hospital, Sichuan University, Chengdu, China
- 2Stem Cell Transplantation and Cellular Therapy Division, Clinic Trial Center, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Hematology, West China Hospital/Shangjin Nanfu Hospital, Chengdu, China
Background: The prognosis of patients with peripheral T-cell (PTCL) or lymphoblastic T-cell lymphoma (T-LBL) remains poor under current conditioning regimens before receiving autologous stem cell transplantation (ASCT).
Methods: Patients with PTCL or T-LBL were enrolled to receive ASCT using the conditioning regimen of chidamide, cladribine, gemcitabine, and busulfan (ChiCGB). Positron emission tomography-computed tomography (PET/CT) was used to evaluate the response to ASCT. Overall survival (OS) and progression-free survival (PFS) were employed to assess the patient outcome, and adverse events were used to assess the regimen’s safety. The survival curve was estimated via the Kaplan-Meier method.
Results: Twenty-five PTCL and 11 T-LBL patients were recruited. The median time to neutrophile and platelet engraftments was 10 days (8–13 days) and 13 days (9–31 days), respectively. The 3-year PFS and OS were 81.3 ± 7.2% and 88.5 ± 5.4% for all patients; 92.0 ± 5.4% and 81.2 ± 8.8% for PTCL patients; and both 81.8 ± 11.6% for T-LBL patients, respectively. The 3-year PFS and OS were both 92.9 ± 4.9% for patients with complete response (CR) but 50.0 ± 17.7% and 75.0 ± 15.3% for patients with non-CR, respectively. Infection was the most common non-hematological toxicity, and all toxicities were mild and controllable.
Conclusions: ChiCGB was a potentially effective and well-tolerated conditioning regimen to improve the prognosis of patients with aggressive T-cell lymphoma. Future randomized controlled trials are needed to assess ChiCGB as a conditioning regimen for ASCT.
Introduction
Peripheral T-cell lymphoma (PTCL) and lymphoblastic T-cell lymphoma (T-LBL) are rare and aggressive subtypes of non-Hodgkin lymphoma that exhibit chemotherapy insensitivity and poor prognosis. Clinical data on the two subtypes have shown 5-year progression-free survival (PFS) of 13–29% and 5-year overall survival (OS) of 15–64% after conventional chemotherapy (1, 2). Autologous stem cell transplantation (ASCT) is an essential treatment for aggressive T-cell lymphoma. It is frequently used as standard-of-care for patients in first complete response (CR1), despite the advent of new drugs. Several retrospective and prospective studies have demonstrated that ASCT significantly prolongs the survival time of patients with aggressive T-cell lymphoma (3, 4). However, the prognosis for patients with aggressive T-cell lymphoma remains unfavorable compared to other lymphomas.
The conditioning regimen used in ASCT is critical to the effectiveness of treatment, and different conditioning regimens may result in different outcomes even for the same disease. For instance, patients with Hodgkin lymphoma receiving carmustine, etoposide, cytarabine, and melphalan (BEAM) as a conditioning regimen had a 5-year PFS of 66% and a 5-year OS of 79%. In contrast, those receiving busulfan, cyclophosphamide, and etoposide (BUCYVP16) had a 5-year PFS of 33% and a 5-year OS of 54% (5). Standard conditioning regimens for T-cell lymphomas include BEAM, total body irradiation (TBI) with cyclophosphamide, cyclophosphamide, carmustine, etoposide (CBV), BUCYVP16, and others. These regimens have resulted in PFS rates of 33%–53%, which is markedly lower than the survival rates of patients with B-cell lymphomas undergoing ASCT (6–8). Therefore, selecting a conditioning regimen according to the characteristics of tumor cells may improve the efficacy of ASCT and the prognosis of patients.
In our previous work, we demonstrated that cladribine, gemcitabine, and busulfan (CGB) had a synergistic effect on killing lymphoma cells, and a histone deacetylase inhibitor (HDACi), vorinostat, sensitized the lymphoma cells to the CGB regimen by causing changes in the chromatin structure (9). Another HDACi, chidamide, inhibits HDAC 1, 2, 3, and 10 and is an oral agent approved by the National Medical Products Administration (NMPA) of China for refractory/relapsed PTCL, also synergized with CGB in killing lymphoma cells. Based on this work, we previously conducted a Phase 2 clinical trial on the ChiCGB conditioning regimen in ASCT for non-Hodgkin’s lymphoma, which showed that the regimen was effective and safe (10). Herein, building on our previous work, we further subdivided the disease categories and designed a single-arm, Phase 2 clinical trial to investigate whether the conditioning regimen of chidamide with CGB (ChiCGB) could improve the clinical efficacy compared to historical other conditioning regimens for aggressive T-cell lymphomas.
Methods
Study design
Our study was a single-arm, prospective, Phase 2 clinical trial registered on the Clinical Trial Registry (clinicaltrials.gov, NCT03602131). The primary endpoint was PFS. Other endpoints included OS, overall response rate, CR rate, relapse rate, and non-hematological adverse events graded by the National Cancer Institute Common Toxicity Criteria (NCI-CTC, Version 4.0).
All patients were reinfused with peripheral hematopoietic stem cells. PFS was defined as the period from ASCT to disease progression, relapse, death from any cause, or the last follow-up; OS was defined as the period from ASCT to death from any cause or the last follow-up. Positron emission tomography-computed tomography (PET/CT) was used to evaluate disease status prior to ASCT and the response to ASCT every three months in the first year and every six months after one year. According to the Lugano classification in 2013 (11), those with a Deauville score of 3 or less were determined to be effective, while those with a Deauville score of 4 or more were considered ineffective or relapsed.
Patient population
After finishing planned chemotherapy, patients who met the following inclusion criteria were recruited for our study: (a) age ranged from 18 to 70 years old; (b) aggressive T-cell lymphomas including i) PTCLs other than ALK+ anaplastic large cell lymphoma (ALCL) in CR1, ii) T-LBL without BM involvement in CR1, iii) any chemo-sensitive relapsed PTCL in CR or partial response (PR); (c) adequate renal function, as defined by estimated serum creatinine clearance ≥ 50 ml/min and/or serum creatinine ≤ 1.8 mg/dL; (d) adequate hepatic function, as defined by serum glutamate oxaloacetate transaminase (SGOT) and/or serum glutamate pyruvate transaminase (SGPT) ≤ 3 times the upper limit of normal; serum bilirubin and alkaline phosphatase ≤ 2 times the upper limit of normal; (e) adequate pulmonary function with forced expiratory volume at one second (FEV1), forced vital capacity (FVC) and diffusing lung capacity for carbon monoxide (DLCO) ≥ 50% of expected after correction for hemoglobin; (f) adequate cardiac function with left ventricular ejection fraction ≥ 50% and no uncontrolled arrhythmias or symptomatic cardiac disease; and (g) negative Beta human chorionic gonadotropin (HCG) test in a woman with child-bearing potential, defined as not post-menopausal for 12 months or no previous surgical sterilization.
Of the patients who met the above criteria, those with the following conditions were excluded from the study: (a) central nervous system was involved; (b) relapse after stem cell transplantation; (c) active infection requiring parenteral antibiotics; (d) active hepatitis B or C(HBV DNA ≥ 10,000 copies/mL); (e) human immunodeficiency virus (HIV) infection, unless the current recipient of effective antiretroviral therapy with undetectable viral load and a normal cluster of differentiation 4 (CD4) counts; (f) evidence of either cirrhosis or Stage 3–4 liver fibrosis alongside chronic hepatitis C or positive hepatitis C serology; and (g) a corrected QT interval (QTc) longer than 500 ms.
Treatment plan
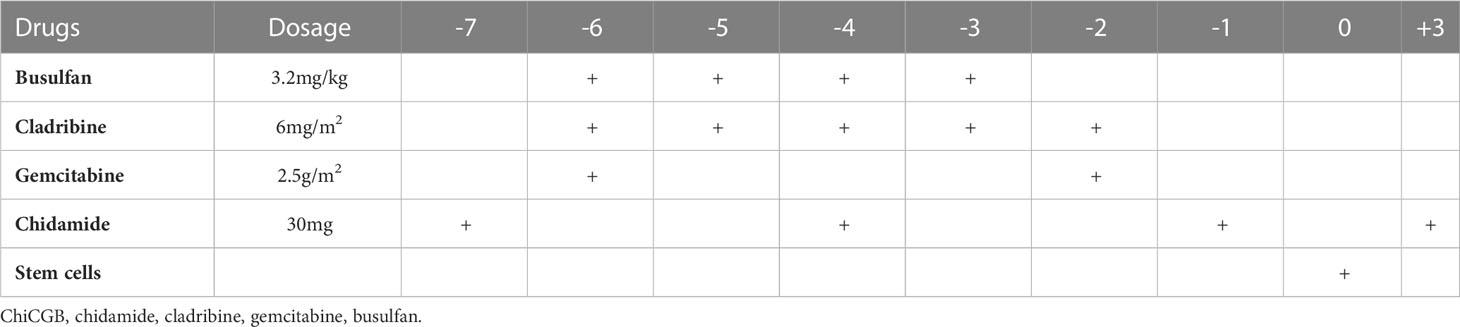
The conditioning regimen of ChiCGB started with a 30mg dose of chidamide taken orally on Day -7. Subsequently, chidamide was given at the same dose one hour before chemotherapy on Days -4, -1, and +3. Then, a 6 mg/m2 dose of cladribine was given from Day -6 to Day -2, while a 2.5g/m2 dose of gemcitabine was given on Day -6 and Day -2, with a 4-hour infusion interval between the two drugs. Meanwhile, a 3.2mg/kg dose of busulfan was administrated intravenously for more than 3 hours after the finish of gemcitabine once daily from Day -6 to Day -3. Finally, hematopoietic stem cells were reinfused on Day 0 (Table 1). All patients received the netupitant/palonosetron capsules to prevent chemotherapy-induced nausea and vomiting (CINV).
Statistical analysis
The sample size was estimated via the Weibull model. A previous study reported a PFS of 40% in aggressive T-cell lymphomas, while we estimated a PFS increase to 65% in our study (12). The sample size was calculated with 80% power and an overall 5% significance level using PASS 21.0 software (NCSS Statistical Software, Kaysville, Utah, USA). Considering the 10% drop-out rate during the follow-up period, the final calculated sample size required was 26 patients.
OS and PFS were employed to evaluate the prognosis of patients with T-cell lymphoma undergoing ASCT. The survival curve was estimated using the Kaplan-Meier method and compared via log-rank tests. Two-sided P values < 0.05 were considered significant. All statistical analyses were performed using GraphPad Prism 8.0 software (Graphpad Software, San Diego, CA, USA).
Result
Patient characteristics
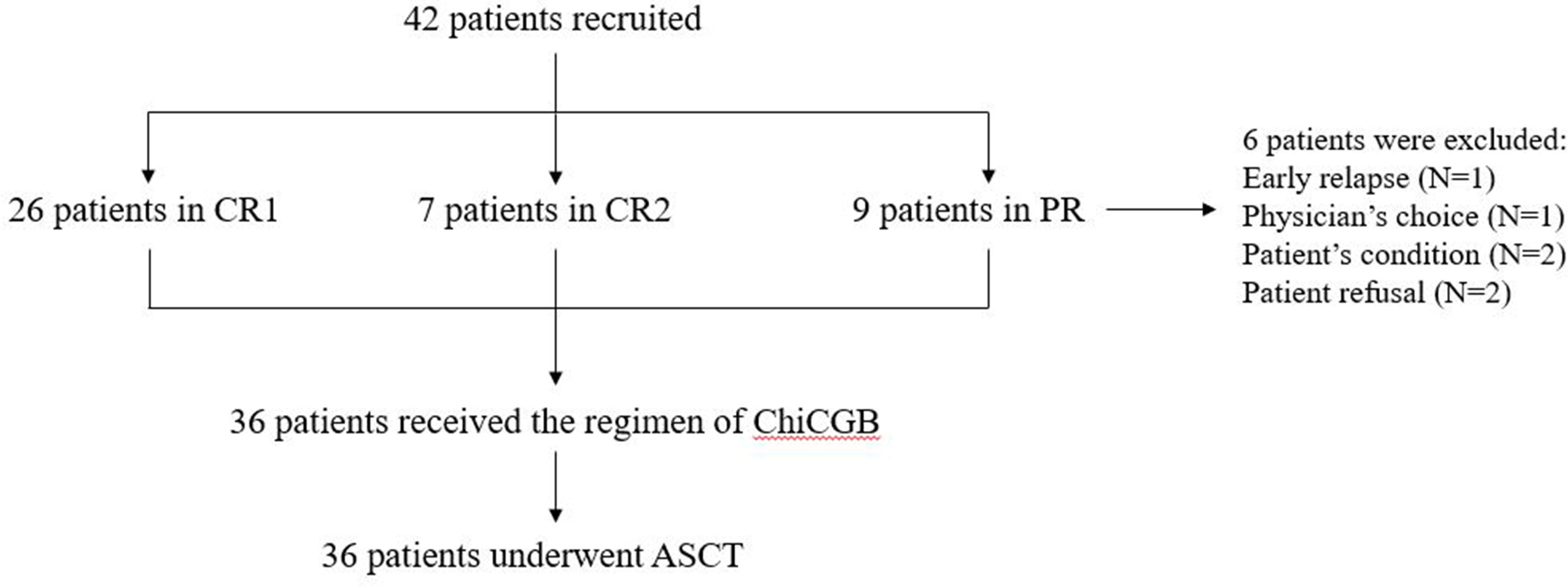
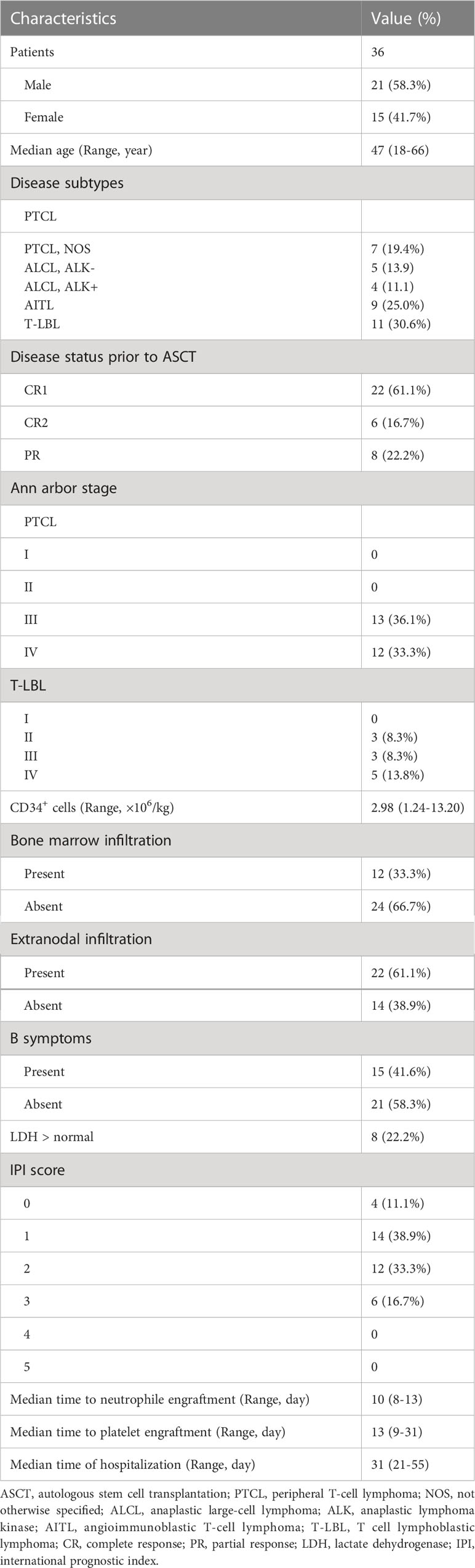
From April 2015 to April 2022, 42 patients with PTCL or T-LBL were recruited for our study after finishing the planned chemotherapy at West China Hospital. After inclusion in the clinical trial, 6 patients were excluded due to the patient’s refusal, early relapse, physician’s choice, and patient’s condition, and altogether 36 patients, with 10.2 months of median duration between diagnosis and ASCT, were eventually enrolled in our trials (Figure 1). Of these patients, 21 (58.3%) were male, and the median age was 47 years old (range: 18–66 years). Twenty-five patients (69.4%) were diagnosed with PTCL, and 11 (30.6%) with T-LBL. Among these patients, 22 (61.1%), including all T-LBL, achieved CR1, 6 (16.7%) achieved CR2, and 8 (22.2%) achieved PR prior to ASCT.
All hematopoietic stem cells for ASCT were mobilized and collected from peripheral blood within three months prior to ASCT. The median number of CD34+ cells in the graft was 2.98×106/kg (range: 1.24–13.20×106/kg). All patients were fully engrafted during hospitalization. The median time to neutrophile engraftment was 10 days (range: 8–13 days) while the median time to platelet engraftment was 13 days (range: 9–31 days). The remaining information is listed in Table 2.
Patient outcomes
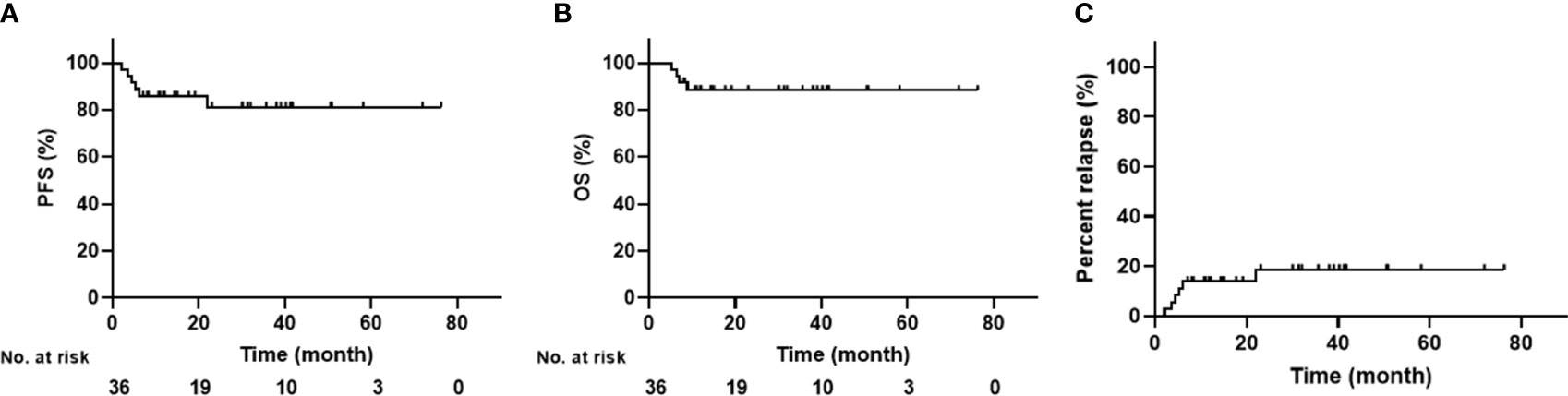
The last follow-up time ended on November 1, 2022, and the median follow-up period was 30 months (range: 7.1–76.3 months). Overall, the 3-year PFS was 81.3 ± 7.2% and the 3-year OS was 88.5 ± 5.4%; neither medians was reached (Figure 2). All but one patient responded well to the treatment and were in CR in the third month after ASCT. The outlier patient died 6.9 months after relapse. Three patients relapsed at 3.5, 5.3, and 6.1 months, and all died within two months (Figure 2). All four patients died from disease progression. Another two patients relapsed but have survived to date. However, one patient with angioimmunoblastic T-cell lymphoma (AITL) experienced recurrent lymphadenopathy in the neck at 28 months post-transplantation. The pathological finding showed that the heterogeneous lymphocytes were of B-cell origin and EBV-positive, and EBV-DNA was detected in a blood sample, indicating the diagnosis of EBV-positive diffuse large B cell lymphoma (DLBCL). No sign of a T-cell lymphoma relapse was found in this patient. To date, the remaining patients have survived without relapse.
In the subgroup analysis, disease status prior to ASCT, disease subtype, and IPI score were included. Figure 3 shows that the 3-year OS and PFS for the patients with CR were 92.9 ± 4.9%, while those with non-CR were 75.0 ± 15.3% and 50.0 ± 17.7%. A difference occurred between CR and non-CR prior to ASCT regarding the PFS and OS. Especially in PFS, the difference was statistically significant (P < 0.01). No differences were found between PTCL and T-LBL (3-year OS: 92.0 ± 5.4% vs. 81.8 ± 11.6%, P = 0.30; 3-year PFS: 81.2 ± 8.8% vs. 81.8 ± 11.6%, P = 0.91), between PTCL in CR1 and T-LBL in CR1 (3-year OS: 100.0% vs. 81.8 ± 11.6%, P=0.15; 3-year PFS: 100.0% vs. 81.8 ± 11.6%, P = 0.15), and between IPI score 0, 1, 2, and 3 (3-year OS: 89.4 ± 5.8% vs. 83.3 ± 15.2%, P=0.61; 3-year PFS: 84.7 ± 7.3% vs. 66.7 ± 19.2%, P = 0.14) (Figure 3).
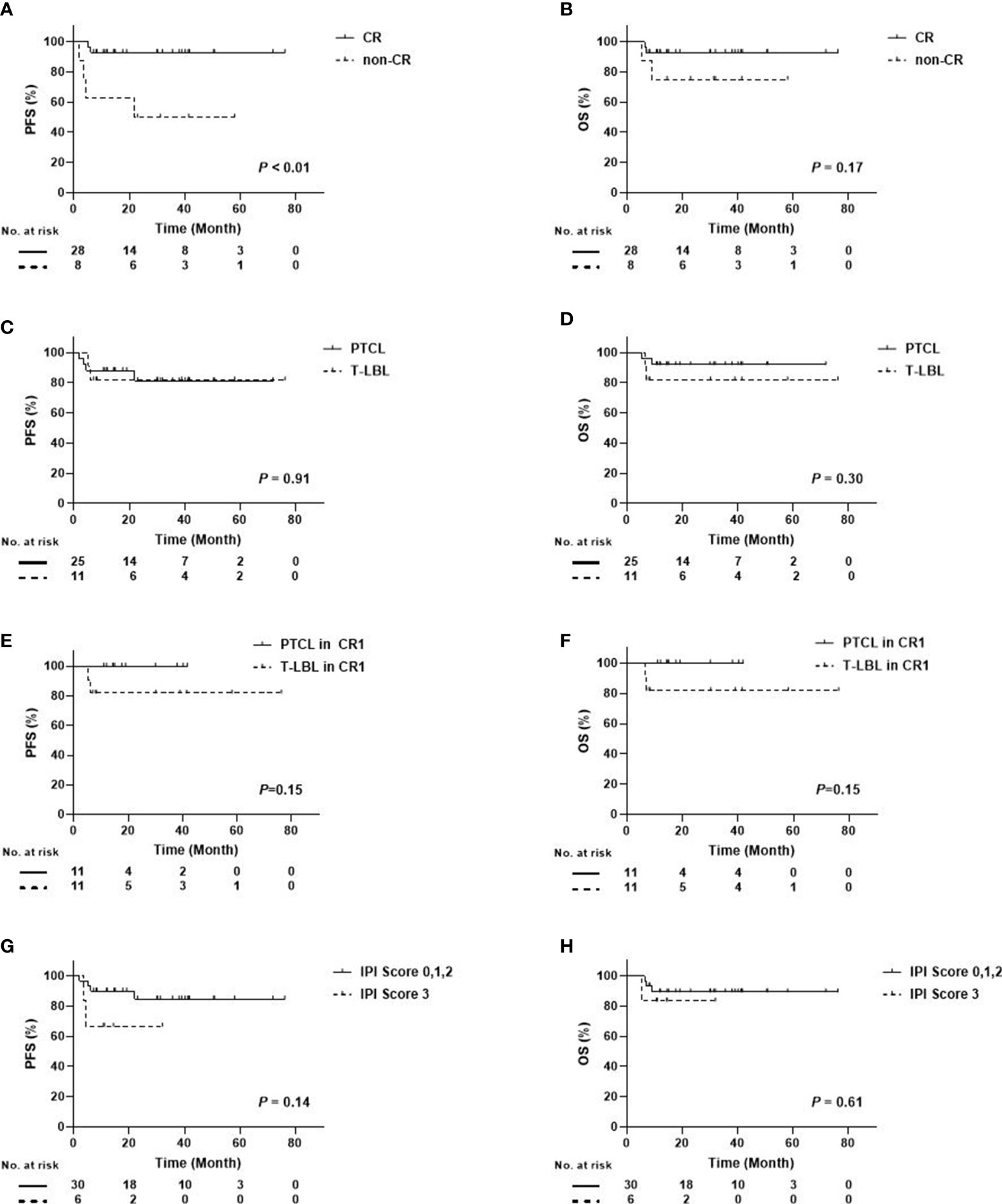
Figure 3 Survival Curves Between Different Subgroups in Patients with PTCL/T-LBL (A) PFS Between CR and Non-CR; (B) OS Between CR and Non-CR; (C) PFS Between PTCL and T-LBL; (D) OS Between PTCL and T-LBL; (E) PFS Between PTCL in CR1 and T-LBL in CR1; (F) OS Between PTCL in CR1 and T-LBL in CR1; (G) PFS Between IPI score 0, 1, 2, and 3; (H) OS Between IPI score 0, 1, 2, and 3).
Non-hematological toxicity associated with ChiCGB
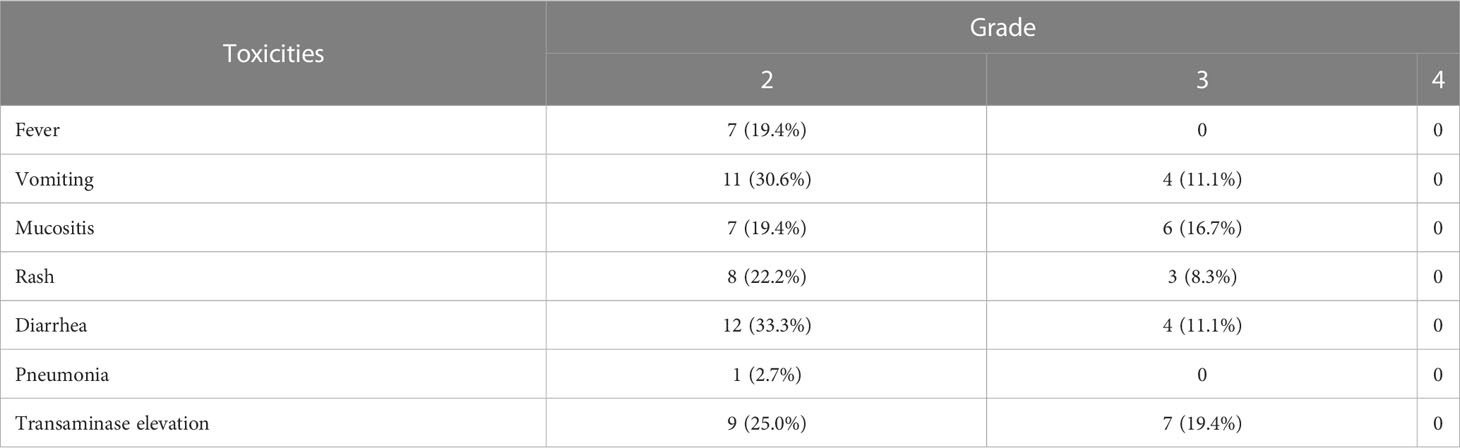
The most common non-hematological adverse event caused by the new conditioning regimen of ChiCGB was infection (58.3%), including in the respiratory and gastrointestinal tract. The main manifestation of infection was fever and the incidence of neutropenic fever was 36.1%. Infection symptoms could be significantly improved after empirical anti-infection treatment. Furthermore, Epstein-Barr virus (EBV) reactivation and post-transplant lymphoproliferative disorders (PTLD) were not observed in all patients. Based on the NCI-CTC (Version 4.0), other toxicities of Grades 2 to 4 are displayed in Table 3. No Grade 4 or 5 toxicities were reported for any of the adverse events. In addition, some adverse events, (e.g., constipation, cardiac general, and central nervous system) were not listed in the table due to Grade 1. All adverse events were controlled rapidly after appropriate corresponding treatments. No non-relapse mortality (NRM) occurred.
Discussion
The results of this study suggest that ChiCGB represents a promising conditioning regimen for ASCT in patients with aggressive T-cell lymphoma. Compared with previously reported data, the outcomes in patients receiving ChiCGB were inspiring.
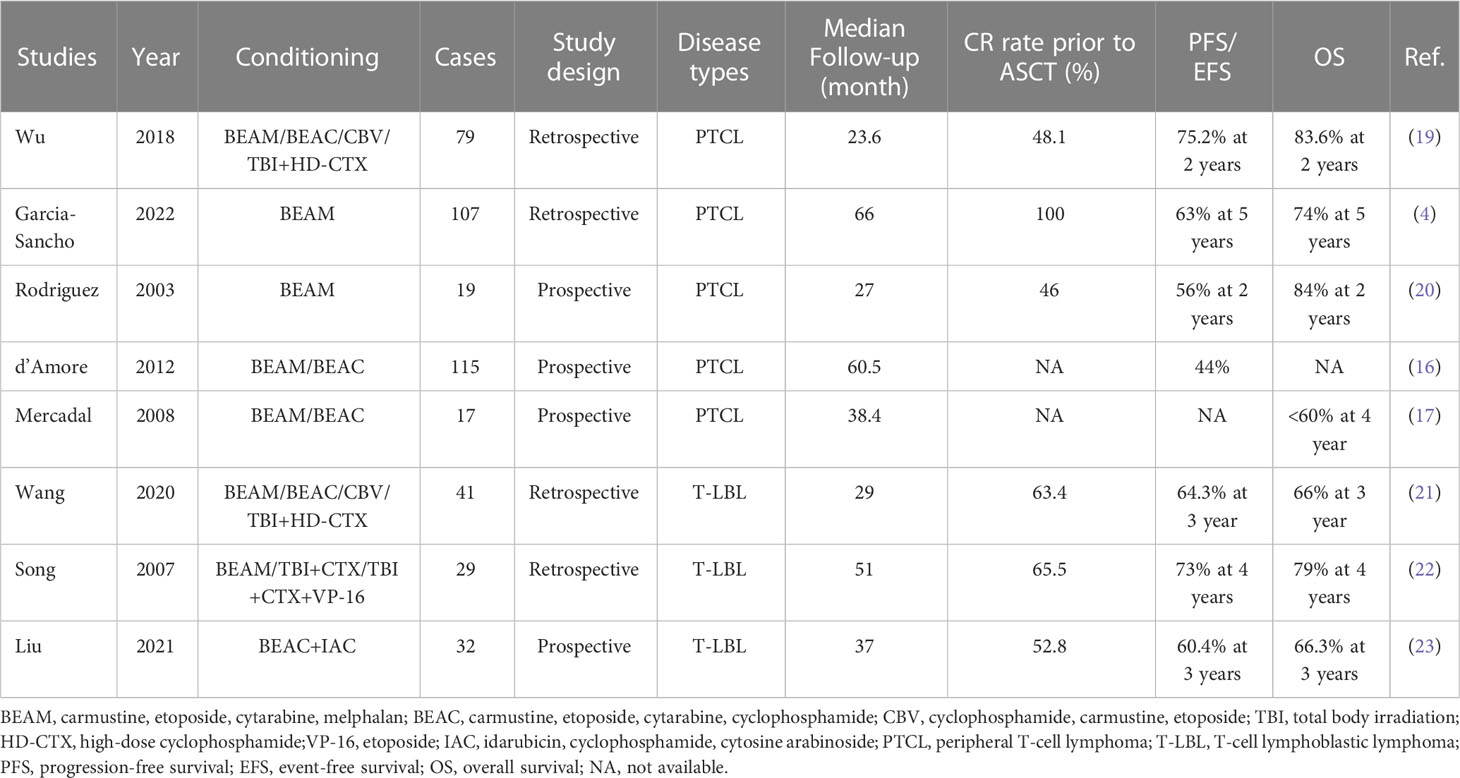
Although various conditioning regimens have been employed in ASCT for lymphomas, BEAM has been consistently favored as the preferred regimen (13). A large retrospective study confirmed that BEAM was the most effective regimen prior to ASCT (14). Clinical trials using BEAM or BEAM-like conditioning regimens in PTCLs have reported PFS rates of 30%–53% and OS rates of 39%–73% (15–18). In a retrospective study of GELTAMO/FIL, the PFS and OS rates were 63% and 74% in 103 patients with a median follow-up of 65.5 months (4). Other retrospective studies have reported varying outcomes with BEAM or BEAM-like regimens in PFS or event-free survival (EFS) ranging from 45% to 63% and OS ranging from 49% to 68% (Table 4) (20, 24–28). Notably, in an individual study, the survival rate of ASCT with BEAM as the conditioning regimen was found to be lower than that of conventional chemotherapy (24). In contrast, the new conditioning regimen of ChiCGB in this study yielded an encouraging PFS and OS of 81.2% and 92.0%, respectively, in all PTCL patients. For those in CR prior to ASCT, no relapses were reported at the last follow-up. We believe that the benefit of ChiCGB overcomes the negative impact of T-cell lymphoma on the prognosis compared with other types of lymphoma. For patients with T-LBL, previous literature has reported PFS rates of 46.9%–69% and OS rates of 58.3%–76% after undergoing ASCT (Table 4) (21–23). In this study, 11 T-LBL patients undergoing single ASCT after ChiCGB achieved both a PFS and an OS of 81.8% at three years, which was not inferior to the outcomes of tandem ASCT, although the sample size of this study was relatively small.
The synergistic effect of the chidamide, cladribine, gemcitabine, and busulfan combination was confirmed in previous research (9), which found that HDACi combined with CGB synergistically inhibited lymphoma cells in a certain order of medication administration. HDACi increased the sensitivity of genomic DNA to busulfan crosslinking via loosening and opening DNA. Cladribine and gemcitabine affected DNA synthesis and repair, leading to lymphoma cell apoptosis. All of these details serve as the theoretical and experimental basis for the effective conditioning regimen of ChiCGB.
Among the non-hematological toxicities, infection was the most frequently occurring non-hematological adverse event in patients receiving BEAM or BEAM-like regimens (29, 30). Other toxicities included mucositis, vomiting, and non-infective pulmonary complications (31, 32). n contrast, ChiCGB caused less severe toxicity, and all adverse events reported in this study were assessed as only Grade 3 or below. Compared with BEAM-like regimens, ChiCGB did not increase toxicity during the follow-up period. However, one patient with AITL developed EBV-positive DLBCL 28 months after ASCT, which is consistent with previous literature reporting that DLBCL secondary to AITL was often associated with EBV infection (33, 34). Furthermore, Zettl et al. discussed the onset pattern for these cases (35). The researchers thought that, in the setting of PTCL, B cells infected with EBV underwent atypical proliferation; together with the associated immune damage, that proliferation led to the development of B-cell lymphoma. On the other hand, one patient undergoing ASCT with another conditioning regimen also experienced DLBCL (36). The second tumor was therefore considered ChiCGB-independent. Overall, ChiCGB in our study was a safe and controlled conditioning regimen in T-cell lymphomas.
Nevertheless, our clinical trial had some limitations. First, as our study was a non-controlled trial where all included patients received ChiCGB as a conditioning regimen, no patients receiving other conditioning regimens were used as controls. The trial results were compared with other conditioning regimens in different periods. Second, although most patients participated in follow-ups for more than two years, the small sample size affected the statistical power, making us cautious in drawing conclusions. Third, a higher proportion of enrolled patients had IPI scores of 0, 1, 2, and 3, while IPI scores of 4 and 5 were absent, suggesting that patients with IPI scores of 3-5 were recruited to further strengthen the results.
Conclusion
The results of the Phase 2 clinical trial suggested that ChiCGB was a potentially effective and well-tolerated conditioning regimen to improve the prognosis of patients with aggressive T-cell lymphoma. A randomized controlled clinical trial with a larger sample size on ChiCGB is needed for further investigation in the future.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of West China Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
JJ and ZL designed the study. PK, JL, XC, TD, TN, TL, ZL, and JJ recruited patients. QW, CZ, and CC took care of patients. QZ and HZ collected and analyzed the data, and wrote the paper. All authors contributed to the article and approved the submitted version.
Acknowledgments
All authors thank Prof. Liqun Zou and Prof. Ming Jiang of the Oncology department West China Hospital for their contributions to this clinical trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A systematic review and meta-analysis of front-line anthracycline-based chemotherapy regimens for peripheral T-cell lymphoma. ISRN Hematol (2011) 2011:623924. doi: 10.5402/2011/623924
2. Savage KJ, Chhanabhai M, Gascoyne RD, Connors JM. Characterization of peripheral T-cell lymphomas in a single north American institution by the WHO classification. Ann Oncol (2004) 15(10):1467–75. doi: 10.1093/annonc/mdh392
3. Foley NC, Mehta-Shah N. Management of peripheral T-cell lymphomas and the role of transplant. Curr Oncol Rep (2022) 24(11):1489–99. doi: 10.1007/s11912-022-01310-3
4. García-Sancho AM, Bellei M, López-Parra M, Gritti G, Cortés M, Novelli S, et al. Autologous stem-cell transplantation as consolidation of first-line chemotherapy in patients with peripheral T-cell lymphoma: a multicenter GELTAMO/FIL study. Haematologica (2022) 107(11):2675–84. doi: 10.3324/haematol.2021.279426
5. Singer S, Dean R, Zhao Q, Sharma N, Abounader D, Elder P, et al. BEAM versus BUCYVP16 conditioning before autologous hematopoietic stem cell transplant in patients with Hodgkin lymphoma. Biol Blood Marrow Transpl (2019) 25(6):1107–15. doi: 10.1016/j.bbmt.2019.01.032
6. Yhim HY, Kim JS, Mun YC, Moon JH, Chae YS, Park Y, et al. Clinical outcomes and prognostic factors of up-front autologous stem cell transplantation in patients with extranodal natural Killer/T cell lymphoma. Biol Blood Marrow Transpl (2015) 21(9):1597–604. doi: 10.1016/j.bbmt.2015.05.003
7. Fox CP, Boumendil A, Schmitz N, Finel H, Luan JJ, Sucak G, et al. High-dose therapy and autologous stem cell transplantation for extra-nodal NK/T lymphoma in patients from the Western hemisphere: A study from the European society for blood and marrow transplantation. Leuk Lymphoma (2015) 56(12):3295–300. doi: 10.3109/10428194.2015.1037764
8. Singer S, Sharma N, Dean R, Zhao Q, Abounader D, Elder P, et al. BEAM or BUCYVP16-conditioning regimen for autologous stem-cell transplantation in non-hodgkin's lymphomas. Bone Marrow Transpl (2019) 54(10):1553–61. doi: 10.1038/s41409-019-0463-y
9. Ji J, Valdez BC, Li Y, Liu Y, Teo EC, Nieto Y, et al. Cladribine, gemcitabine, busulfan, and SAHA combination as a potential pretransplant conditioning regimen for lymphomas: A preclinical study. Exp Hematol (2016) 44(6):458–65. doi: 10.1016/j.exphem.2016.03.001
10. Ji J, Liu Z, Kuang P, Dong T, Chen X, Li J, et al. A new conditioning regimen with chidamide, cladribine, gemcitabine and busulfan significantly improve the outcome of high-risk or relapsed/refractory non-hodgkin's lymphomas. Int J Cancer (2021) 149(12):2075–82. doi: 10.1002/ijc.33761
11. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the lugano classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
12. Al-Mansour Z, Li H, Cook JR, Constine LS, Couban S, Stewart DA, et al. Autologous transplantation as consolidation for high risk aggressive T-cell non-Hodgkin lymphoma: a SWOG 9704 intergroup trial subgroup analysis. Leuk Lymphoma (2019) 60(8):1934–41. doi: 10.1080/10428194.2018.1563691
13. Isidori A, Christofides A, Visani G. Novel regimens prior to autologous stem cell transplantation for the management of adults with relapsed/refractory non-Hodgkin lymphoma and Hodgkin lymphoma: Alternatives to BEAM conditioning. Leuk Lymphoma (2016) 57(11):2499–509. doi: 10.1080/10428194.2016.1185785
14. Chen YB, Lane AA, Logan B, Zhu X, Akpek G, Aljurf M, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transpl (2015) 21(6):1046–53. doi: 10.1016/j.bbmt.2015.02.005
15. Rodríguez J, Conde E, Gutiérrez A, Arranz R, León A, Marín J, et al. Frontline autologous stem cell transplantation in high-risk peripheral T-cell lymphoma: a prospective study from the gel-tamo study group. Eur J Haematol (2007) 79(1):32–8. doi: 10.1111/j.1600-0609.2007.00856.x
16. d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol (2012) 30(25):3093–9. doi: 10.1200/JCO.2011.40.2719
17. Mercadal S, Briones J, Xicoy B, Pedro C, Escoda L, Estany C, et al. Intensive chemotherapy (high-dose CHOP/ESHAP regimen) followed by autologous stem-cell transplantation in previously untreated patients with peripheral T-cell lymphoma. Ann Oncol (2008) 19(5):958–63. doi: 10.1093/annonc/mdn022
18. Tournilhac O, Truemper L, Ziepert M, Bouabdallah K, Schmitz N. First-line therapy of T-cell lymphoma: Allogeneic or autologous transplantation for consolidation-final results of the AATT study. Clin Adv Hematol Oncol (2019) 17(8):18–9. doi: 10.1002/hon.64_2629
19. Wu M, Wang X, Xie Y, Liu W, Zhang C, Ping L, et al. Outcome and prospective factor analysis of high-dose therapy combined with autologous peripheral blood stem cell transplantation in patients with peripheral T-cell lymphomas. Int J Med Sci (2018) 15(9):867–74. doi: 10.7150/ijms.23067
20. Rodríguez J, Conde E, Gutiérrez A, Arranz R, León A, Marín J, et al. The results of consolidation with autologous stem-cell transplantation in patients with peripheral T-cell lymphoma (PTCL) in first complete remission: The Spanish lymphoma and autologous transplantation group experience. Ann Oncol (2007) 18(4):652–7. doi: 10.1093/annonc/mdl466
21. Wang P, Li CX, Zhang Y, Chen J, Chen XC, Yang D, et al. [Autologous hematopoietic stem cell transplantation treatment for T cell lymphoblastic lymphoma]. Zhonghua xue ye xue za zhi = Zhonghua xueyexue zazhi (2020) 41(3):198–203. doi: 10.3760/cma.j.issn.0253-2727.2020.03.003
22. Song KW, Barnett MJ, Gascoyne RD, Chhanabhai M, Forrest DL, Hogge DE, et al. Primary therapy for adults with T-cell lymphoblastic lymphoma with hematopoietic stem-cell transplantation results in favorable outcomes. Ann Oncol (2007) 18(3):535–40. doi: 10.1093/annonc/mdl426
23. Liu Y, Rao J, Li J, Wen Q, Wang S, Lou S, et al. Tandem autologous hematopoietic stem cell transplantation for treatment of adult T-cell lymphoblastic lymphoma: a multiple center prospective study in China. Haematologica (2021) 106(1):163–72. doi: 10.3324/haematol.2019.226985
24. Fossard G, Broussais F, Coelho I, Bailly S, Nicolas-Virelizier E, Toussaint E, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: An analysis of patients from LYSA centers. Ann Oncol (2018) 29(3):715–23. doi: 10.1093/annonc/mdx787
25. Mehta N, Maragulia JC, Moskowitz A, Hamlin PA, Lunning MA, Moskowitz CH, et al. A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clin Lymphoma Myeloma Leuk (2013) 13(6):664–70. doi: 10.1016/j.clml.2013.07.005
26. Numata A, Miyamoto T, Ohno Y, Kamimura T, Kamezaki K, Tanimoto T, et al. Long-term outcomes of autologous PBSCT for peripheral T-cell lymphoma: retrospective analysis of the experience of the Fukuoka BMT group. Bone Marrow Transpl (2010) 45(2):311–6. doi: 10.1038/bmt.2009.165
27. Feyler S, Prince HM, Pearce R, Towlson K, Nivison-Smith I, Schey S, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transpl (2007) 40(5):443–50. doi: 10.1038/sj.bmt.1705752
28. Mounier N, Gisselbrecht C, Brière J, Haioun C, Feugier P, Offner F, et al. All aggressive lymphoma subtypes do not share similar outcome after front-line autotransplantation: A matched-control analysis by the groupe d'Etude des lymphomes de l'Adulte (GELA). Ann Oncol (2004) 15(12):1790–7. doi: 10.1093/annonc/mdh471
29. Yeral M, Aytan P, Gungor B, Boga C, Unal A, Koc Y, et al. A comparison of the BEAM and MITO/MEL conditioning regimens for autologous hematopoietic stem cell transplantation in Hodgkin lymphoma: An analysis of efficiency and treatment-related toxicity. Clin Lymphoma Myeloma Leuk (2020) 20(10):652–60. doi: 10.1016/j.clml.2020.05.009
30. Dahi PB, Lee J, Devlin SM, Ruiz J, Maloy M, Rondon-Clavo C, et al. Toxicities of high-dose chemotherapy and autologous hematopoietic cell transplantation in older patients with lymphoma. Blood Adv (2021) 5(12):2608–18. doi: 10.1182/bloodadvances.2020004167
31. Jo JC, Kang BW, Jang G, Sym SJ, Lee SS, Koo JE, et al. BEAC or BEAM high-dose chemotherapy followed by autologous stem cell transplantation in non-hodgkin's lymphoma patients: comparative analysis of efficacy and toxicity. Ann Hematol (2008) 87(1):43–8. doi: 10.1007/s00277-007-0360-0
32. Salar A, Sierra J, Gandarillas M, Caballero MD, Marín J, Lahuerta JJ, et al. Autologous stem cell transplantation for clinically aggressive non-hodgkin's lymphoma: the role of preparative regimens. Bone Marrow Transpl (2001) 27(4):405–12. doi: 10.1038/sj.bmt.1702795
33. Maegawa RO, Hussain S, Danila DC, Teruya-Feldstein J, Maki RG, O'Connor OA. Angioimmunoblastic T-cell lymphoma with an evolving Epstein Barr virus-positive diffuse large b-cell lymphoma with unusual clinical and pathologic findings. Leuk Lymphoma (2007) 48(10):2071–4. doi: 10.1080/10428190701593628
34. Skugor ND, Perić Z, Vrhovac R, Radić-Kristo D, Kardum-Skelin I, Jaksić B. Diffuse large b-cell lymphoma in patient after treatment of angioimmunoblastic T-cell lymphoma. Coll Antropol (2010) 34(1):241–5.
35. Zettl A, Lee SS, Rüdiger T, Starostik P, Marino M, Kirchner T, et al. Epstein-Barr Virus-associated b-cell lymphoproliferative disorders in angloimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol (2002) 117(3):368–79. doi: 10.1309/6UTX-GVC0-12ND-JJEU
Keywords: chidamide, cladribine, gemcitabine, busulfan, ASCT - autologous stem cell transplantation, T-cell lymphoma
Citation: Zeng Q, Zhang H, Kuang P, Li J, Chen X, Dong T, Wu Q, Zhang C, Chen C, Niu T, Liu T, Liu Z and Ji J (2023) A novel conditioning regimen of chidamide, cladribine, gemcitabine, and busulfan in the autologous stem cell transplantation of aggressive T-cell lymphoma. Front. Oncol. 13:1143556. doi: 10.3389/fonc.2023.1143556
Received: 13 January 2023; Accepted: 27 February 2023;
Published: 09 March 2023.
Edited by:
Liren Qian, Fifth Medical Center of the PLA General Hospital, ChinaReviewed by:
Xiaoxia Hu, Ruijin Hospital, ChinaLi Xiaoyang, Shanghai Jiao Tong University, China
Liu Hui, Beijing Hospital, China
Copyright © 2023 Zeng, Zhang, Kuang, Li, Chen, Dong, Wu, Zhang, Chen, Niu, Liu, Liu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhigang Liu, aHhkb2N0b3JsaXVAMTI2LmNvbQ==; Jie Ji, amllamlAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Qiang Zeng
Qiang Zeng Hang Zhang
Hang Zhang Pu Kuang1
Pu Kuang1 Ting Niu
Ting Niu Ting Liu
Ting Liu Jie Ji
Jie Ji