- Department of Radiation Oncology, Miller School of Medicine, University of Miami, Miami, FL, United States
Background: Primary sinonasal mucosal melanoma (SNMM) is a rare, aggressive histology usually diagnosed at advanced stages and associated with poor prognosis. Evidence regarding etiology, diagnosis, and treatment mainly derives from case reports, retrospective series, and national databases. In the treatment of metastatic melanoma, anti-CTLA-4 and anti-PD-1 checkpoint blockade increased 5-year overall survival from ~10% (prior to 2011) to ~50% (between 2011 and 2016). In March of 2022, the FDA approved the use of relatlimab, a novel anti-LAG3 immune checkpoint inhibitor, for the treatment of melanoma.
Case presentation: A 67-year-old woman with locally advanced SNMM underwent debulking surgery, adjuvant RT, and first-line immunotherapy (ImT) with nivolumab but developed local progression. The patient started a second course of ImT with nivolumab and ipilimumab, but this was discontinued after two cycles due to an immune-related adverse event (irAE, hepatitis with elevated liver enzymes). Interval imaging identified visceral and osseous metastases including multiple lesions in the liver and in the lumbar spine. She went on to receive a third course of ImT with nivolumab and the novel agent relatlimab with concurrent stereotactic body radiation therapy (SBRT) to the largest liver tumor only, delivered in five 10-Gy fractions using MRI guidance. A PET/CT performed 3 months after SBRT demonstrated complete metabolic response (CMR) of all disease sites including non-irradiated liver lesions and spinal metastatic sites. After two cycles of the third course of ImT, the patient developed severe immune-related keratoconjunctivitis and ImT was discontinued.
Conclusion: This case report describes the first complete abscopal response (AR) in an SNMM histology and the first report of AR following liver SBRT with the use of relatlimab/nivolumab combination ImT for metastatic melanoma in the setting of both visceral and osseous lesions. This report suggests that the combination of SBRT with ImT potentiates the adaptive immune response and is a viable path for immune-mediated tumor rejection. The mechanisms behind this response are hypothesis-generating and remain an area of active research with exceedingly promising potential.
Introduction
Approximately 100,000 new melanoma cases (all types) were diagnosed in 2022 with nearly 8,000 estimated deaths based on American Cancer Society Statistics (1). Melanoma incidence is highest among white patients, increases with age, shows a slight female predominance, and has the largest racial survival gap among all invasive tumors, with 22% lower absolute survival for black patients (1).
Primary sinonasal mucosal melanoma (SNMM) is a rare and highly aggressive histologic subtype associated with poor prognosis. The 5-year overall survival (OS) rate is less than 25% with high post-treatment recurrence (50%–70%) (2). Evidence regarding etiology, diagnosis, and treatment outcomes is mainly derived from case reports, national cancer databases, and retrospective series (3).
Prior to 2011, chemotherapy was the mainstay of medical management for melanoma. Adjuvant treatment was limited to interferon-α2b for locoregional disease (4) and dacarbazine or high-dose interleukin-2 (IL-2) for metastatic disease (5). Interferon-α2b is associated with serious side effects (6); cytotoxic chemotherapy proved to have poor response rates, limited duration of response, and no survival benefit (7–9); and IL-2 improved OS in a small fraction of patients but with high rates of severe multiorgan toxicity (10, 11). At present, targeted agents and immunotherapy (ImT) have supplanted interferon, chemotherapy, and high-dose IL-2 as systemic treatments of choice in the adjuvant, unresectable, and metastatic settings (12, 13).
ImT has revolutionized the treatment paradigm for melanoma and many other cancers by improving treatment response and OS (1, 14–18). In metastatic melanoma, dual ImT with anti-programmed cell death protein 1 (PD-1) and anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) checkpoint inhibition has increased 5-year OS from 10% before 2011 to ~50% for patients diagnosed between 2011 and 2016 (19). In 2022, the United Stated (US) Food and Drug Administration (FDA) approved the use of the novel anti-lymphocyte activation gene 3 (LAG-3) agent relatlimab following results of a phase 1/2 trial and the phase 2/3 RELATIVITY-047 trial (20).
While melanoma patients show superior response to ImT regimens, treatment resistance remains common due to immune evasion, an emerging hallmark of cancer (21). There is ample preclinical and growing clinical evidence that radiotherapy (RT) as an adjunct to ImT can potentiate systemic disease response. This synergy is mainly due to RT’s ability to cause an immunogenic form of cell death that counteracts tumor immune escape mechanisms (22), and to the increasingly recognized phenomenon known as “abscopal response” (AR). The term “abscopal” (“ab”—away from, “scopus”—target) was coined in 1953 by R.H. Mole to refer to effects of ionizing radiation “at a distance from the irradiated volume but within the same organism” (23). In 2004, it was postulated for the first time that the immune system might be responsible for these “off-target” anti-tumor effects and subsequent preclinical work confirmed that AR is mediated by immunocytes (T cells). It was therefore theorized that combining immunotherapy and local radiotherapy (ImRT) could augment AR (24–26). Early case reports in melanoma trailblazed the possibility of such effects (27, 28).
Herein, we describe the case of a 67-year-old woman who presented with advanced primary SNMM, underwent debulking surgery, adjuvant RT, and an initial course of ImT, but progressed locally 2 months after RT. During her second course of ImT, she developed visceral and skeletal metastases. A third ImT course was initiated with dual anti-LAG-3 and anti-PD-1 with concurrent liver-directed stereotactic body radiation therapy (SBRT) targeting only the largest among several liver metastases. Two months after SBRT, positron emission tomography (PET)/computed tomography (CT) revealed complete metabolic response (CMR) of all intrahepatic and extrahepatic fluorodeoxyglucose (FDG)-avid disease, two cycles into the third ImT course.
Case description
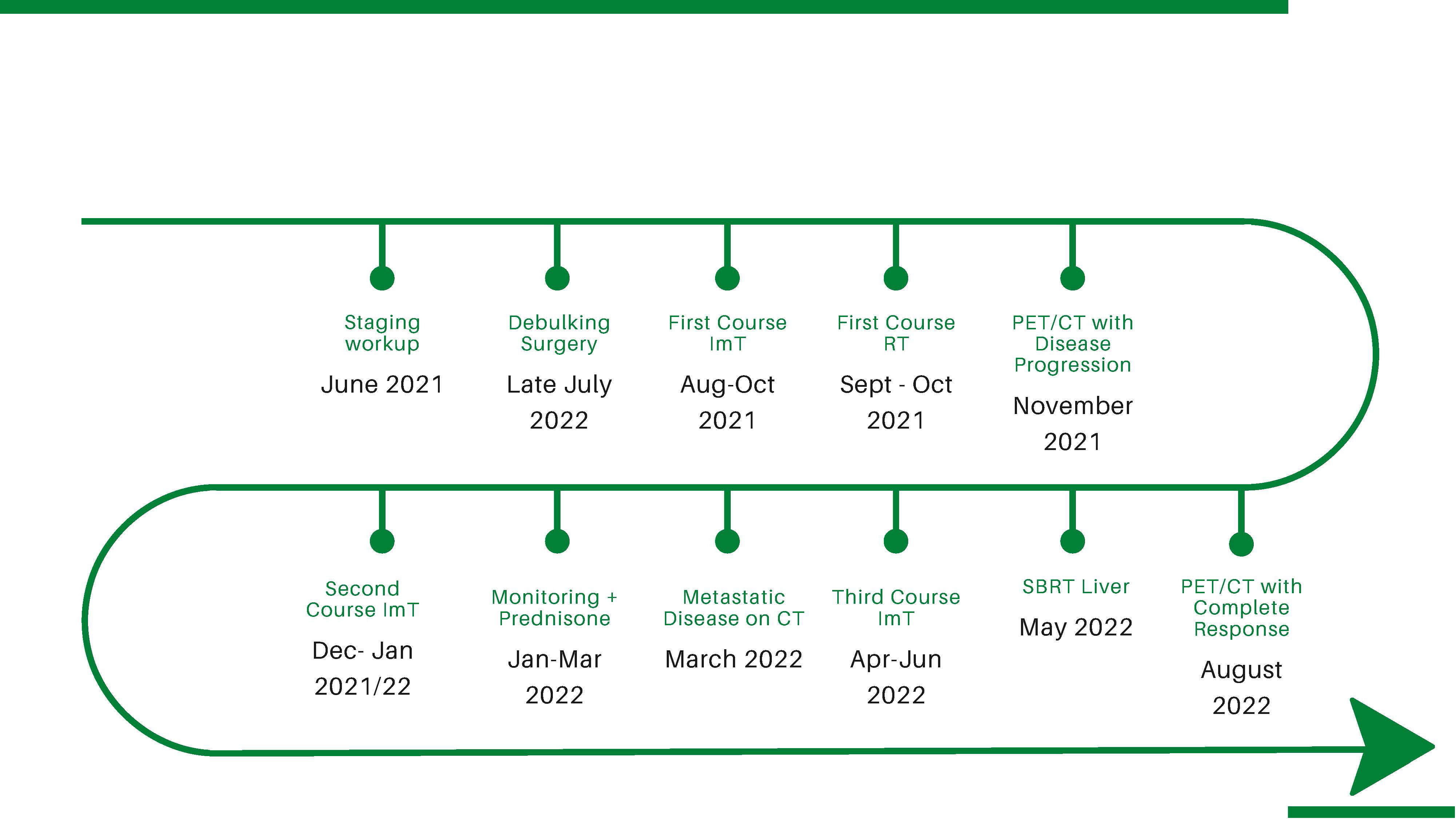
A 67-year-old woman with a history of rhinitis and coblation of the nasal turbinates presented to the emergency department for recurrent epistaxis and pain after a traumatic COVID-19 nasal swab test (see Figure 1 for timeline).
On fiberoptic nasopharyngoscopy, a dark polypoid lesion was visualized between the middle and inferior left turbinates. Biopsy was performed and final pathology reported a heavily pigmented malignant melanoma involving the nasal mucosa, positive for S100 protein. Maxillofacial CT scan without contrast showed an abnormal soft tissue density in the left nasal cavity extending into the proximal upper left maxillary sinus and obstructing the left ostiomeatal complex. On metastatic workup, PET/CT revealed an FDG-avid right middle lobe lung nodule. The patient underwent bronchoscopy with fine needle aspiration (FNA) of the nodule. Pathology reported typical carcinoid, a finding managed separately and irrelevant to this discussion. After negative metastatic workup, the patient underwent mapping biopsies and nasal/sinus endoscopic tumor debulking. During resection, the tumor was found to extend along the nasal floor from the inferior meatus to the level of the inferior nasal septum, with pigmented mucosal changes involving the lateral aspect of the nasal vestibule, the superior aspect of the nasal septum extending onto the skull base, and the area of attachment of the middle turbinate. Pathology of the fragmented resection specimen confirmed SNMM. PD-L1 immunohistochemistry returned as high expression, intensity 3+, and tumor proportion score (TPS) ≥ 50%. The consensus recommendation at the multidisciplinary tumor board was to initiate ImT (nivolumab) and RT, to be followed by ImT (nivolumab) alone. The patient received nivolumab monotherapy (480 mg on a q4 week schedule) with concurrent intensity modulated (IM) RT to a total dose of 60 Gy in 20 fractions targeting the left nasal cavity. The patient tolerated ImT/RT well and went on to receive three cycles of adjuvant nivolumab as scheduled. However, nasal endoscopy and interval MRI identified local disease progression. Anti-CTLA-4 (Ipilimumab) was added to the ImT regimen with a plan for four cycles. After Cycle 1, the patient developed elevated liver enzymes (AST 289 U/L, ALT 301 U/L, and alkaline phosphatase 156 U/L), attributed to ImT-induced autoimmune hepatitis. ImT was discontinued and the patient was treated with intravenous solumedrol 150 mg followed by an extended course of prednisone (total duration, 30 days). Interval PET/CT showed no evidence of metastatic disease and the patient was followed closely in the clinic with radiographic surveillance. A repeat maxillofacial CT showed an increase in the size of the nodular enhancing soft tissue mass in the left posterior nasal cavity, and CT abdomen with contrast revealed a new 3.3-cm hypoenhancing liver lesion in segment 5, consistent with metastasis. CT biopsy of the liver lesion confirmed metastatic malignant melanoma. PET/CT showed rapid progression of numerous metastatic lesions within liver segments 2, 5, and 8, with the segment 5 lesion growing from 3 to 10 cm over 2 months. PET/CT also identified new FDG-avid lesions in thoracic, lumbar, and sacral vertebrae (T9, T12, L3, and S1) highly suspicious for spinal metastases.
After progression through two courses of ImT, the patient was presented again at tumor board and recommended to restart ImT with Opdualag (dual anti-PD-1 nivolumab combined with anti-LAG-3 relatlimab), and was referred to radiation oncology for concurrent stereotactic ablative radiotherapy (SBRT) of the dominant hepatic lesion. The patient received the first cycle of Opdualag with a concomitant course of SBRT, delivering 50 Gy in five fractions to the segment 5 liver tumor. SBRT was performed on a hybrid magnetic resonance/linear accelerator (MR/Linac) platform using MR-guided online adaptive radiotherapy (MRgOART) (29) (Figure 2).

Figure 2 Example of the dose distribution on MRI from the SBRT course, composed of five 10-Gy fractions delivered over consecutive days. The transverse, sagittal, and coronal views demonstrate the isodose levels from 50 Gy (prescription) to 25 Gy.
After the second cycle of Opdualag, the patient developed severe keratoconjunctivitis with corneal ulcerations. The patient had a history of ImT-induced hepatitis, and systemic therapy was discontinued out of concern for another severe ImT-related adverse event (irAE). Interval PET/CT 3 months after SBRT showed a CMR with no suspicious hypermetabolic activity within the surgical cavity and complete resolution of all FDG-avid lesions in the liver (including unirradiated ones) and axial spine where hypermetabolism had previously been identified (Figure 3).
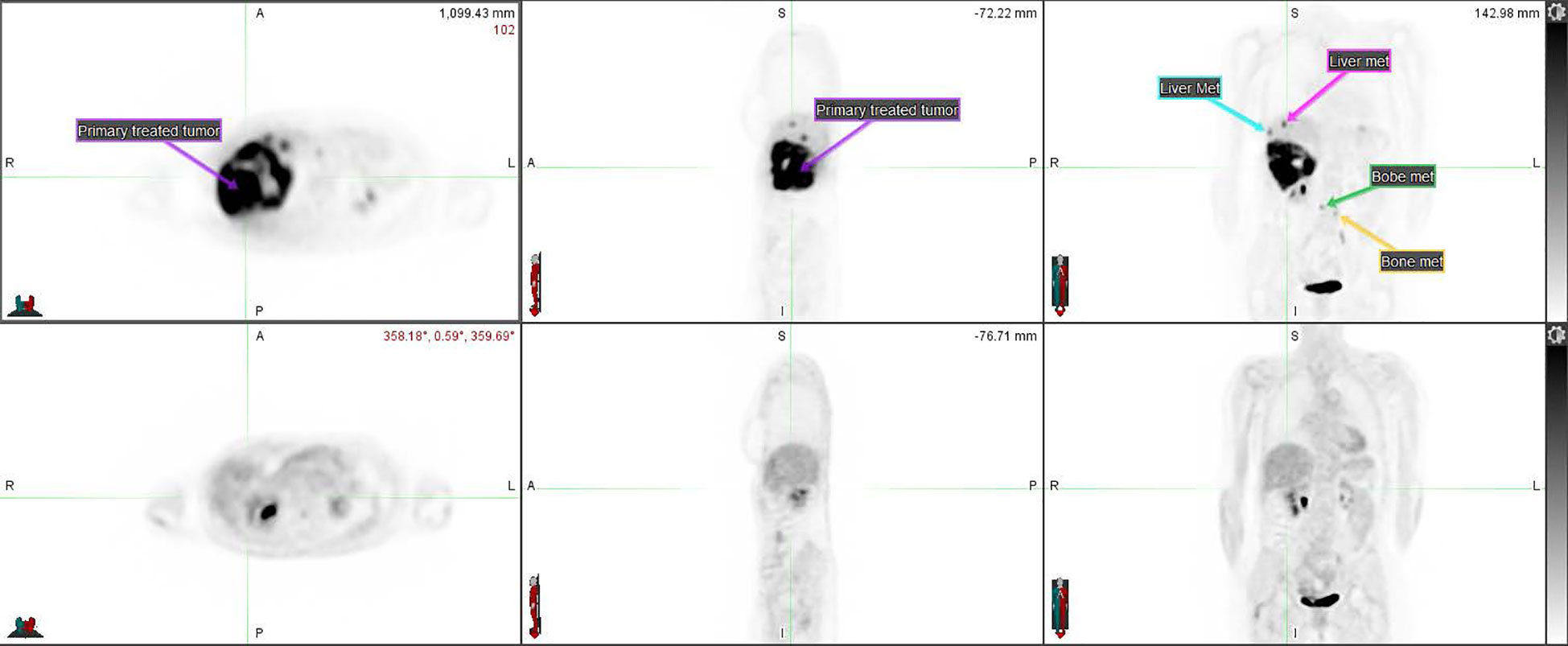
Figure 3 PET CT comparison of the disseminated disease before (top row) and after (bottom row) the SBRT course combined with ImT (nivolumab/relatlimab). The primary tumor treated with SBRT (compare to Figure 2) as well as some of the metastases are annotated on the pre-treatment imaging.
CT chest, abdomen, and pelvis 3 months after SBRT demonstrated a decrease in the size of all liver lesions, with the segment 5 lesion measuring 2 cm in maximum diameter down from 10 cm. Six months after SBRT, the patient’s treatment response persisted despite discontinuation of all therapy for 4 months.
Discussion
To our knowledge, this is the first description of CMR of metastatic melanoma following liver SBRT combined with relatlimab/nivolumab, notable in the setting of both visceral and osseous lesions. Furthermore, this is the first report to describe AR of SNMM, a rare (incidence rate 0.05 per 100,000) and aggressive histology.
Currently, there are no therapeutic standards specific to management of SNMM. As in most malignant melanoma, surgical resection and adjuvant radiotherapy ± systemic therapy constitute definitive treatment (3). Surgical excision is the mainstay of care, with positive or close surgical margins as risk factors for poor outcome (2, 30). Clear resection margins are nearly impossible to obtain due to the morbidity associated with aggressive sinonasal surgery. Adjuvant RT is commonly added in efforts to improve local control; however, evidence of benefit is mixed. A meta-analysis of 1,392 SNMM patients reported improved OS for patients treated with adjuvant RT (31), while a National Cancer Database (NCDB) series of 1,874 SNMMs showed no significant difference in OS. The latter series did find an association between ImT and improved OS but only in the metastatic setting (2).
The current case report describes an approach to treating SNMM with liver metastases, using SBRT to target intrahepatic disease concurrent with dual ImT, harnessing the synergism of these treatment modalities to potentiate systemic response. This combination holds the potential to impact disease control, OS, and even cure.
Cases of AR in patients with melanoma have been reported since 1975 (28). This phenomenon presents most often in patients treated with RT while undergoing immune checkpoint blockade (32). The host immune system is clearly the mediator of such non-targeted effects; it is well known that canonical immune responses to viral infection can be exploited to elicit anti-tumor CD8 T-cell responses (33), as seen in case reports describing an abscopal effect with RT after infectious diseases (like COVID-19) (34) and standard vaccination (like pneumococcal polysaccharide vaccine) (35), or in the setting of autoimmune disease (36). All of these scenarios attest to the synergistic effect that RT triggers in the immune system by releasing a large number of tumor-related antigens, which, in turn, enhance anti-tumor response mediated by CD8+ T cells (37). In addition, RT can promote antigen presentation by upregulating tumor MHC I expression and stimulating activation and maturation of dendritic cells (38). These mechanisms provide the rationale for combining local radiation with checkpoint inhibition in an effort to potentiate systemic response. There are multiple clinical and preclinical studies evaluating combinations of RT and ImT; however, there is no consensus on the ideal sequence (39) or optimal radiation dose regimen for eliciting non-targeted effects (40–42). Some preclinical investigations support radiation doses between 8 and 10 Gy per fraction (41) to achieve synergy with ImT, while others assert that the probability of stimulating AR approaches 50% with ultrahigh-dose single fraction RT, when a biologically effective dose (BED) of at least 60 Gy is delivered to the tumor using a standard alpha/beta ratio estimate of 10 (42).
The patient described in this case report developed a severe irAE after receiving two cycles of relatlimab/nivolumab, suggesting that profound tumor response and autoimmune toxicity may be linked. There is a significant tension between the desire to avoid immunosuppressive drugs in order to potentiate anti-tumor effects and the risks associated with progression of autoimmune toxicity. This provokes the challenging clinical question of whether and when to initiate immunosuppression to treat autoimmune toxicity in the setting of AR.
Conclusion
While abscopal effects have been reported in immunogenic or “hot tumors” like melanoma, this case is the first of its kind to describe CMR in metastatic SNMM to the liver and axial spine. It is also the first to demonstrate this effect with the addition of an anti-LAG 3 immune checkpoint inhibitor. This report suggests that the combination of SBRT with ImT stimulates the adaptive immune response and is a viable path for immune-mediated tumor rejection. The mechanisms behind this response are hypothesis-generating and remain an area of active research with exceedingly promising potential.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by University of Miami IRB. The patients/participants provided their written informed consent to participate in this study.
Author contributions
DC prepared the manuscript. IM participated in the design of the study and the subsequent analyses and manuscript editing. BS conceptualized the idea and edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin Jan (2022) 72(1):7–33. doi: 10.3322/caac.21708
2. Ganti A, Raman A, Shay A, Kuhar HN, Auger SR, Patel T, et al. Treatment modalities in sinonasal mucosal melanoma: a national cancer database analysis. Laryngoscope (2020) 130(2):275–82. doi: 10.1002/lary.27995
3. Salari B, Foreman RK, Emerick KS, Lawrence DP, Duncan LM. Sinonasal mucosal melanoma: an update and review of the literature. Am J Dermatopathol (2022) 44(6):424–32. doi: 10.1097/DAD.0000000000002157
4. Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst (2010) 102(7):493–501. doi: 10.1093/jnci/djq009
5. Kaufman HL, Kirkwood JM, Hodi FS, Agarwala S, Amatruda T, Bines SD, et al. The society for immunotherapy of cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nat Rev Clin Oncol (2013) 10(10):588–98. doi: 10.1038/nrclinonc.2013.153
6. Hauschild A, Gogas H, Tarhini A, Middleton MR, Testori A, Dreno B, et al. Practical guidelines for the management of interferon-alpha-2b side effects in patients receiving adjuvant treatment for melanoma: expert opinion. Cancer (2008) 112(5):982–94. doi: 10.1002/cncr.23251
7. Avril MF, Aamdal S, Grob JJ, Hauschild A, Mohr P, Bonerandi JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol (2004) 22(6):1118–25. doi: 10.1200/JCO.2004.04.165
8. Hill GJ 2nd, Krementz ET, Hill HZ. Dimethyl triazeno imidazole carboxamide and combination therapy for melanoma. IV. late results after complete response to chemotherapy (Central oncology group protocols 7130, 7131, and 7131A). Cancer (1984) 53(6):1299–305.
9. Middleton MR, Grob JJ, Aaronson N, Fierlbeck G, Tilgen W, Seiter S, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol (2000) 18(1):158–66. doi: 10.1200/JCO.2000.18.1.158
10. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol (1999) 17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105
11. Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, et al. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2. JAMA (1994) 271(12):907–13. doi: 10.1001/jama.1994.03510360033032
12. Nguyen K, Hignett E, Khachemoune A. Current and emerging treatment options for metastatic melanoma: a focused review. Dermatol Online J (2020) 26(7). doi: 10.5070/D3267049551
13. Skudalski L, Waldman R, Kerr PE, Grant-Kels JM. Melanoma: an update on systemic therapies. J Am Acad Dermatol (2022) 86(3):515–24. doi: 10.1016/j.jaad.2021.09.075
14. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol (2020) 17(8):807–21. doi: 10.1038/s41423-020-0488-6
15. Cercek A, Lumish M, Sinopoli J, Weiss J, Shia J, Lamendola-Essel M, et al. PD-1 blockade in mismatch repair-deficient, locally advanced rectal cancer. N Engl J Med (2022) 386(25):2363–76. doi: 10.1056/NEJMoa2201445
16. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares L, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-Small-Cell lung cancer. J Clin Oncol (2022) 40(12):1301–11. doi: 10.1200/JCO.21.01308
17. Mittendorf EA, Zhang H, Barrios CH, Saji S, Jung KH, Hegg R, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet (2020) 396(10257):1090–100. doi: 10.1016/S0140-6736(20)31953-X
18. Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol (2022) 23(5):660–70. doi: 10.1038/s41590-022-01141-1
19. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, et al. Long-term outcomes with nivolumab plus ipilimumab or nivolumab alone versus ipilimumab in patients with advanced melanoma. J Clin Oncol (2022) 40(2):127–37. doi: 10.1200/JCO.21.02229
20. Tawbi HA, Schadendorf D, Lipson EJ, Ascierto PA, Matamala L, Castillo Gutierrez E, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med (2022) 386(1):24–34. doi: 10.1056/NEJMoa2109970
21. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
22. Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol (2015) 16(13):e498–509. doi: 10.1016/S1470-2045(15)00007-8
23. Mole RH. Whole body irradiation; radiobiology or medicine? Br J Radiol (1953) 26(305):234–41. doi: 10.1259/0007-1285-26-305-234
24. Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys (2004) 58(3):862–70. doi: 10.1016/j.ijrobp.2003.09.012
25. Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J Radiat Oncol Biol Phys (2012) 84(4):879–80. doi: 10.1016/j.ijrobp.2012.06.020
26. Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol (2015) 1(9):1325–32. doi: 10.1001/jamaoncol.2015.2756
27. Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys (2013) 85(2):293–5. doi: 10.1016/j.ijrobp.2012.03.017
28. Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol (1975) 48(574):863–6. doi: 10.1259/0007-1285-48-574-863
29. Keall PJ, Brighi C, Glide-Hurst C, Liney G, Liu PZY, Lydiard S, et al. Integrated MRI-guided radiotherapy - opportunities and challenges. Nat Rev Clin Oncol (2022) 19(7):458–70. doi: 10.1038/s41571-022-00631-3
30. Shuman AG, Light E, Olsen SH, Pynnonen MA, Taylor JM, Johnson TM, et al. Mucosal melanoma of the head and neck: predictors of prognosis. Arch Otolaryngol Head Neck Surg (2011) 137(4):331–7. doi: 10.1001/archoto.2011.46
31. Hu R, Yang BB. Surgery alone versus post-operative radiotherapy for sinonasal malignant melanoma: a meta-analysis. J Laryngol Otol (2018) 132(12):1051–60. doi: 10.1017/S0022215118002189
32. D'Andrea MA, Reddy GK. Systemic antitumor effects and abscopal responses in melanoma patients receiving radiation therapy. Oncology (2020) 98(4):202–15. doi: 10.1159/000505487
33. Rodriguez-Ruiz ME, Vanpouille-Box C, Melero I, Formenti SC, Demaria S. Immunological mechanisms responsible for radiation-induced abscopal effect. Trends Immunol (2018) 39(8):644–55. doi: 10.1016/j.it.2018.06.001
34. Herrscher H, Sauer B, Truntzer P, Robert C. Abscopal antitumor effect in a patient with melanoma and coronavirus disease 2019. Eur J Cancer (2021) 149:91–3. doi: 10.1016/j.ejca.2021.03.003
35. Huang YS, Li Z, Xiao ZF, Li D, Liu WY. Case report: radiotherapy plus pneumococcal conjugate vaccine stimulates abscopal immune response in a patient with ALK+ NSCLC. Front Immunol (2022) 13:950252. doi: 10.3389/fimmu.2022.950252
36. Beyls C, Haustermans K, Deroose CM, Pans S, Vanbeckevoort D, Verslype C, et al. Could autoimmune disease contribute to the abscopal effect in metastatic hepatocellular carcinoma? Hepatology (2020) 72(3):1152–4. doi: 10.1002/hep.31186
37. Gupta A, Probst HC, Vuong V, Landshammer A, Muth S, Yagita H, et al. Radiotherapy promotes tumor-specific effector CD8+ T cells via dendritic cell activation. J Immunol (2012) 189(2):558–66. doi: 10.4049/jimmunol.1200563
38. Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med (2006) 203(5):1259–71. doi: 10.1084/jem.20052494
39. Aliru ML, Schoenhals JE, Venkatesulu BP, Anderson CC, Barsoumian HB, Younes AI, et al. Radiation therapy and immunotherapy: what is the optimal timing or sequencing? Immunotherapy-Uk (2018) 10(4):299–316. doi: 10.2217/imt-2017-0082
40. Janopaul-Naylor JR, Shen Y, Qian DC, Buchwald ZS. The abscopal effect: a review of pre-clinical and clinical advances. Int J Mol Sci (2021) 22(20). doi: 10.3390/ijms222011061
41. Buchwald ZS, Wynne J, Nasti TH, Zhu S, Mourad WF, Yan W, et al. Radiation, immune checkpoint blockade and the abscopal effect: a critical review on timing, dose and fractionation. Front Oncol (2018) 8:612. doi: 10.3389/fonc.2018.00612
Keywords: radiotherapy, abscopal, liver, effect, SBRT, immunotherapy
Citation: Cerbon D, Moya-Brown K, Mihaylov IB and Spieler B (2023) Abscopal effect observed in visceral and osseous metastases after liver SBRT in combination with nivolumab and relatlimab for sinonasal mucosal melanoma—a case report. Front. Oncol. 13:1143335. doi: 10.3389/fonc.2023.1143335
Received: 12 January 2023; Accepted: 04 April 2023;
Published: 27 April 2023.
Edited by:
Lina Zhao, Fourth Military Medical University, ChinaReviewed by:
Antonino Romeo, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyUpendra Hegde, University of Connecticut, United States
Copyright © 2023 Cerbon, Moya-Brown, Mihaylov and Spieler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin Spieler, YnhzNzM3QG1lZC5taWFtaS5lZHU=
 Danielle Cerbon
Danielle Cerbon Karen Moya-Brown
Karen Moya-Brown Ivaylo B. Mihaylov
Ivaylo B. Mihaylov Benjamin Spieler
Benjamin Spieler