
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 30 March 2023
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1143154
This article is part of the Research Topic Biomarkers, Functional Mechanisms, and Therapeutic Potentials in Gastrointestinal Cancers View all 40 articles
A correction has been applied to this article in:
Corrigendum: Single and combined use of the platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index in gastric cancer diagnosis
Introduction: The platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), and systemic immune-inflammation index (SII) are markers for systemic inflammatory responses and have been shown by numerous studies to correlate with the prognosis of gastric cancer (GC). However, the diagnostic value of these three markers in GC is unclear, and no research has examined them in combination. In this study, we investigated the value of the PLR, NLR, and SII individually or in combination for GC diagnosis and elucidated the connection of these three markers with GC patients’ clinicopathological features.
Methods: This retrospective study was conducted on 125 patients diagnosed with GC and 125 healthy individuals, whose peripheral blood samples were obtained for analysis. The preoperative PLR, NLR, and SII values were subsequently calculated.
Results: The results suggest that the PLR, NLR, and SII values of the GC group were considerably higher than those of the healthy group (all P ≤ 0.001); moreover, all three parameters were notably higher in early GC patients (stage I/II) than in the healthy population. The diagnostic value of each index for GC was analyzed using receiver operating characteristic (ROC) curve analysis and area under the curve (AUC) calculation. The diagnostic efficacy of the SII alone (AUC: 0.831; 95% confidence interval [CI], 0.777–0.885) was expressively better than those of the NLR (AUC: 0.821; 95% CI: 0.769–0.873, P = 0.017) and PLR (AUC: 0.783; 95% CI: 0.726–0.840; P = 0.020). The AUC value of the combination of the PLR, NLR, and SII (AUC: 0.843; 95% CI: 0.791–0.885) was significantly higher than that of the combination of the SII and NLR (0.837, 95% CI: 0.785–0.880, P≤0.05), PLR (P = 0.020), NLR (P = 0.017), or SII alone (P ≤ 0.001). The optimal cut-off values were determined for the PLR, NLR, and SII using ROC analysis (SII: 438.7; NLR: 2.1; PLR: 139.5). Additionally, the PLR, NLR, and SII values were all meaningfully connected with the tumor size, TNM stage, lymph node metastasis, and serosa invasion (all P ≤ 0.05). Elevated levels of the NLR and SII were linked to distant metastasis (all P ≤ 0.001).
Discussion: These data suggest that the preoperative PLR, NLR, and SII could thus be utilized as diagnostic markers for GC or even early GC. Among these three indicators, the SII had the best diagnostic efficacy for GC, and the combination of the three could further improve diagnostic efficiency.
A report published by the International Agency for Research on Cancer, the 2020 update on the global burden of cancer, showed that gastric cancer (GC) has the fifth highest incidence among all cancers with a mortality rate that ranks fourth in cancer-related deaths worldwide (1).In China, GC is the most common gastrointestinal tumor (2). The 5-year survival rate for early-stage GC after operation surpasses 97% (3). Unfortunately, because the early symptoms of GC are atypical, patients with GC are usually detected in the middle and late stages, with the 5-year survival rate being poor (4, 5). Therefore, diagnosing GC as early as possible is key to effective treatment and prognosis. Currently, endoscopy is the most common and effective method for diagnosing GC (6), but it is not suitable for mass screening of GC because of its invasive nature, low tolerability, and high cost. Moreover, the GC detection rate heavily depends on the endoscopist’s level of practice. Therefore, identifying a noninvasive, inexpensive, easily accessible, specific, and sensitive biological indicator is crucial for the early diagnosis of GC.
The immune inflammatory response can promote blood vessel growth, stimulate tumor cell proliferation, invasion, and metastasis, and decrease immunomodulatory responses (7), which are directly linked to the occurrence and development of tumors (8, 9). The platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), and systemic immune-inflammation index (SII) are biological indicators that can reflect the status of systemic immune inflammation and are easy to measure and calculate (10). According to previous studies, the PLR, NLR, and SII are linked to the tumor-node-metastasis (TNM) stage, lymph node metastasis, and the invasion depth of GC. They can potentially be used as indicators to evaluate prognosis in GC patients (10–12). The SII combines three types of inflammatory cells, platelets, neutrophils, and lymphocytes, to more comprehensively exhibit the balance between the inflammatory status and host immune response (13, 14). The SII has a significantly higher predictive power than the PLR and/or NLR for survival in GC patients (10, 15–17). Recent research has shown the value of the PLR and NLR in diagnosing GC (18, 19), but the diagnostic value of the SII is unclear. No studies have reported results of combining the PLR, NLR, and SII.
In this study, we investigated the value of the PLR, NLR, and SII, individually or in combination, in diagnosing GC. This work can offer new insight into the early diagnosis of GC.
We retrospectively examined 125 GC patients who were originally diagnosed at Taiyuan Central Hospital of Shanxi Medical University between May 2017 and March 2022. The 8th edition of the American Joint Committee on Cancer (AJCC)/TNM tumor classification system was used to classify the tumors (20). The enrollment criteria for patients were as follows: (a) diagnosis with the testing of tissue acquired during gastroscopy and verified by postoperative pathology; (b) no radiotherapy, chemotherapy, or immunotherapy performed prior to surgery; and (c) available pretreatment routine blood indicators. The exclusion criteria for candidates were as follows: (a) preoperative combination of serious infectious, autoimmune, or cardiovascular diseases; (b) treatment with antiplatelet medication or statins within 3 months prior to blood examination; (c) received anti-inflammatory and/or blood transfusion therapy within 1 month prior to blood examination; (d) recurrent GC; and (e) combined hematological system diseases and history of other systemic malignancies within 5 years. We enrolled 125 patients in the GC group based on these criteria, which comprised 95 men and 30 women (mean age: 61.91 ± 10.82 years; range: 26–82 years).
For the healthy control (HC) group, we chose 125 healthy subjects who visited the hospital for routine physical examination. They had no history of treatment with antiplatelet therapy, inflammatory disease, autoimmune disease, hematological disease, or cancer. The HC group had 87 men and 38 women (mean age: 59.86 ± 13.32 years; range: 35–89 years).
This study was approved by the Ethics Committee of Taiyuan Central Hospital of Shanxi Medical University (2022010). Because the protocol involved a retrospective study, informed consent was waived, and patient data were treated confidentially.
All participants had 2 mL of venous blood drawn from the cubital vein on the day of admission or early the next morning with an empty stomach for complete blood cell count measurement. The total platelet, absolute neutrophil, total white blood cell (WBC), and absolute lymphocyte counts were acquired using a blood cell analyzer (MaiRui BC6800 PLUS,Shenzhen, China). The SII was determined by the formula SII = (P × N)/L, where L, N, and P are the lymphocyte, neutrophil, and platelet counts, respectively. The absolute neutrophil and lymphocyte values were used to calculate the NLR values. The total number of platelets and absolute values of lymphocytes were used to calculate the PLR values.
Data were analyzed using MedCalc version 20.0 (MedCalc Software, Mariakerke,Belgium) and SPSS 25.0 (SPSS, Chicago, IL, USA) statistical software. Measures obeying normality are expressed as the mean ± standard deviation (SD). Non-normal values are expressed as the median and quartiles. Comparisons between groups were carried out using the Mann-Whitney U-test or t-test. The chi-square test was used to evaluate the categorical variables. The inflammatory indicator levels were compared between groups using the Kruskal-Wallis test. Intergroup comparisons were performed using Bonferroni correction. The diagnostic values of the PLR, NLR, and SII alone and in combination were compared by plotting the receiver operating characteristic (ROC) curve, and the optimal cut-off values were determined for the three inflammatory markers. Statistical significance was defined as P-values less than 0.05.
Statistical data, such as sex and age, and the levels of various serum biomarkers of the two groups of participants in this study are shown in Table 1. There were no statistical differences in sex or age between the GC and control groups. Compared with the control group, GC patients had significantly higher PLR, NLR, and SII values, higher WBC platelet and neutrophil counts, and a lower lymphocyte count, all of which were statistically significant (P < 0.05).
As shown in Table 2, the optimal cut-off values for the PLR, NLR, and SII were determined to be 139.5, 2.1, and 438.7, respectively, for GC diagnosis when the Youden index was taken as the maximum value. As shown in Table 2 and Figure 1, the area under the curve (AUC) value of the SII (AUC: 0.831; 95% confidence interval [CI]: 0.779–0.875) was considerably higher than those of the NLR (AUC: 0.821; 95% CI: 0.768–0.866; P = 0.016) and PLR (AUC: 0.783; 95% CI: 0.783; P = 0.016) when the PLR, NLR, or SII were applied alone to diagnose GC. In addition, the SII had the highest specificity and sensitivity values: 94.40% and 68.80%, respectively. When the three inflammatory indices were combined, the AUC increased to 0.843 (95% CI: 0.791–0.885). The AUC value of the combination of the PLR, NLR, and SII was significantly greater than that of the combination of the SII and NLR (0.837, 95% CI: 0.785–0.880, P<0.05), PLR (P = 0.020), NLR (P = 0.017), or SII alone (P < 0.001).
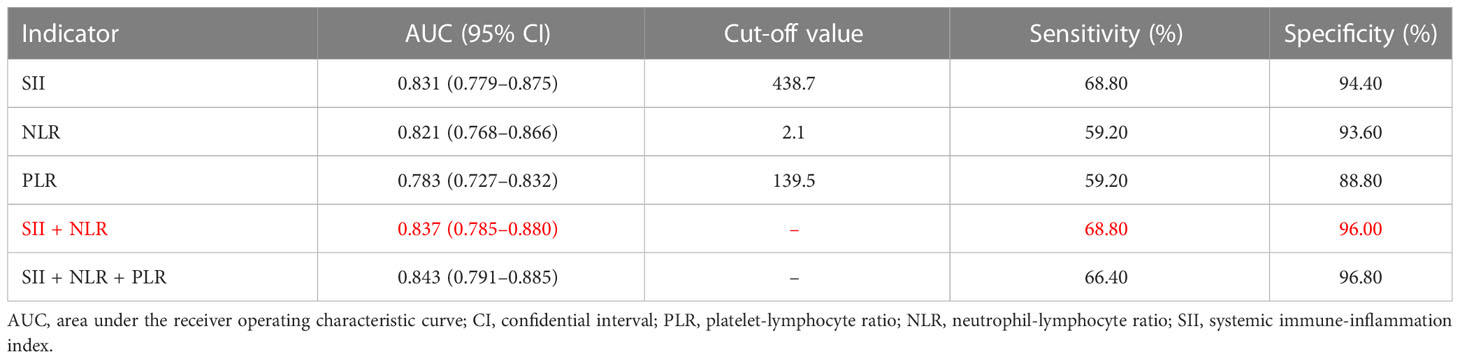
Table 2 Diagnostic efficiency of the platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index individually and in combination for gastric cancer patients.
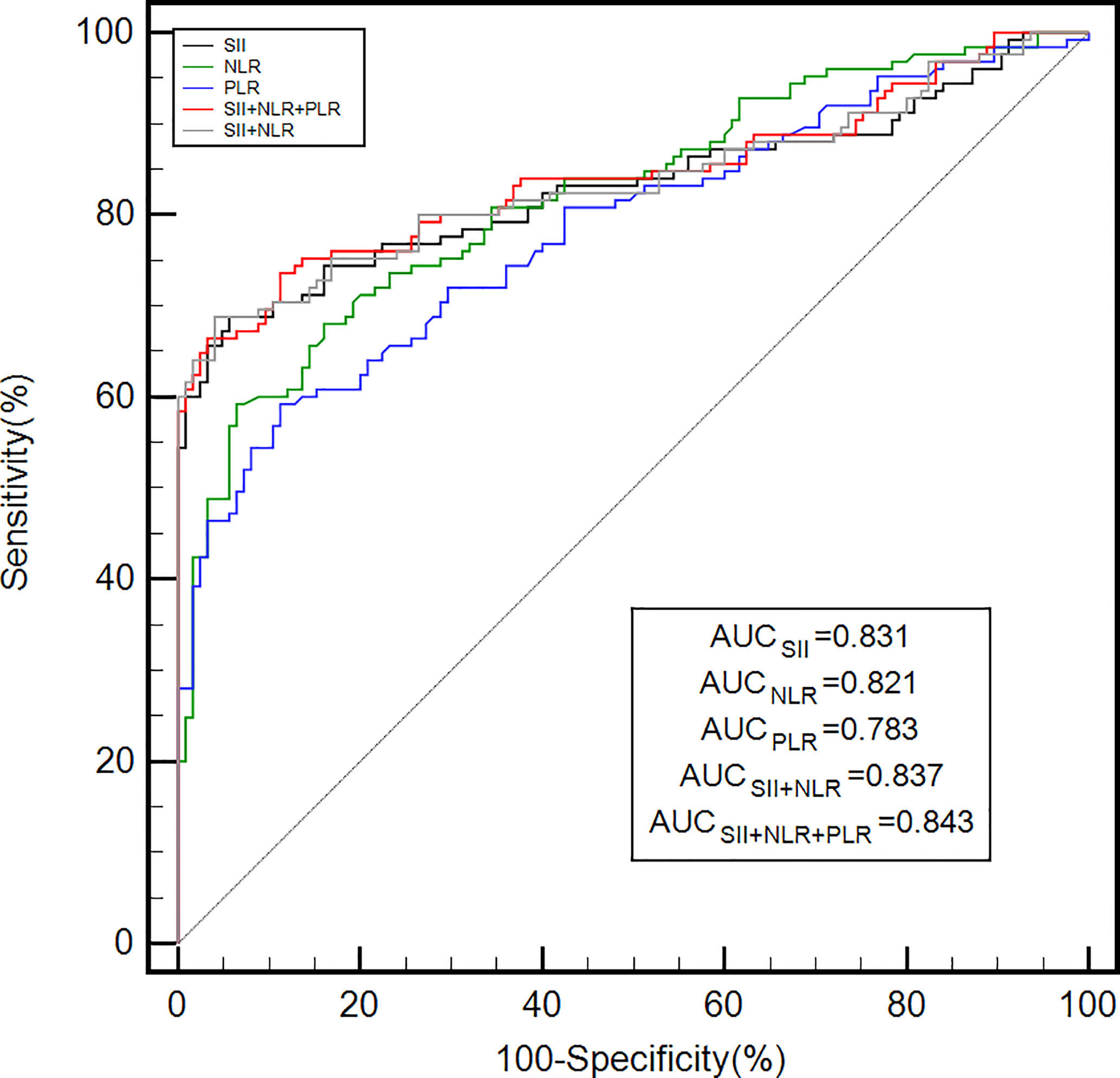
Figure 1 The diagnostic efficiency of the PLR, NLR, and SII alone and in combination for gastric cancer was analyzed by ROC curves. ROC, receiver operating characteristic; AUC, area under the ROC curve; PLR, platelet-lymphocyte ratio; NLR, neutrophil-lymphocyte ratio; SII, systemic immune-inflammation index.
There were three study groups (Table 3): the HC group (n = 125, 87 men and 38 women; mean age: 59.86 ± 13.32 years; range: 35–89 years), early-stage (I/II) group (n = 64, 49 men and 15 women; mean age: 61.83 ± 10.34 years; range: 26–81 years), and progressive-stage (III/IV) group (n = 61, 46 men and 15 women; mean age: 62.00 ± 10.31 years; range: 35–82 years). The three groups did not significantly differ in age or sex. We performed an analysis based on TNM staging (Table 3 and Figure 2), which indicated that the PLR, NLR, and SII values were significantly higher in early-stage (I/II) and progressive-stage (III/IV) GC patients than in healthy controls. All three inflammatory indices were notably higher in progressive-stage GC patients than in early-stage GC patients, and the differences were statistically significant (all P < 0.05).
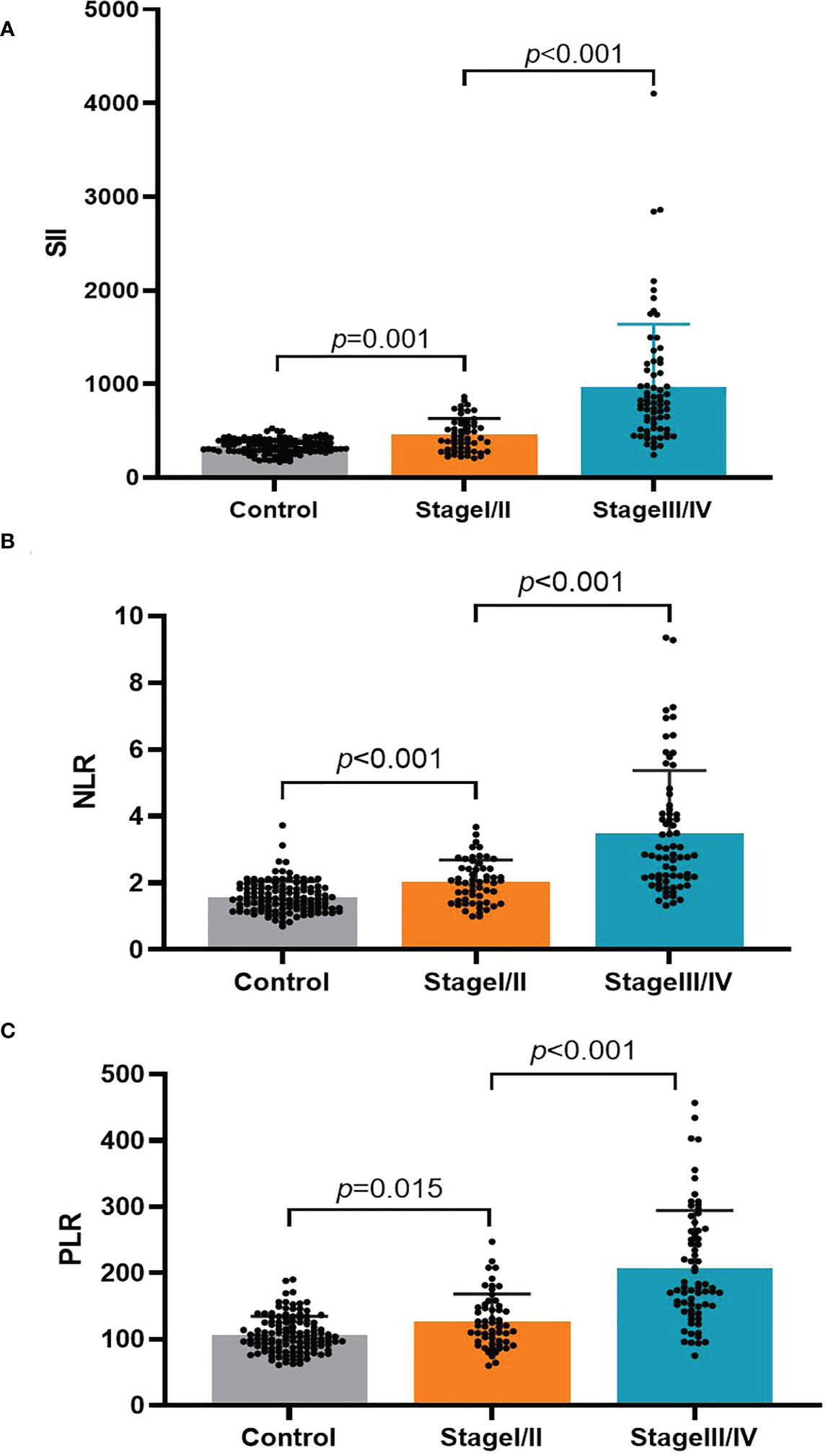
Figure 2 The association of the platelet-lymphocyte ratio (PLR), neutrophil-lymphocyte ratio (NLR), and systemic immune-inflammation index (SII) with the TNM stage in gastric cancer patients. (A) SII; (B) NLR; (C) PLR.
Relative to the optimal cut-off values, we divided the three inflammatory indices into high- and low-value groups. As displayed in Table 4, the PLR, NLR, and SII values were significantly linked with the tumor size, tumor infiltration depth, and lymph node metastasis (all P < 0.05). Elevated SII and NLR values were accompanied by distant metastasis (both P < 0.001), while the PLR was not linked to distant metastasis (P = 0.163). No meaningful correlations existed (P > 0.05) between the three indicators and GC patients’ clinicopathological features, such as the degree of tumor differentiation, tumor location, and the presence of lymphatic vessel or nerve infiltration.
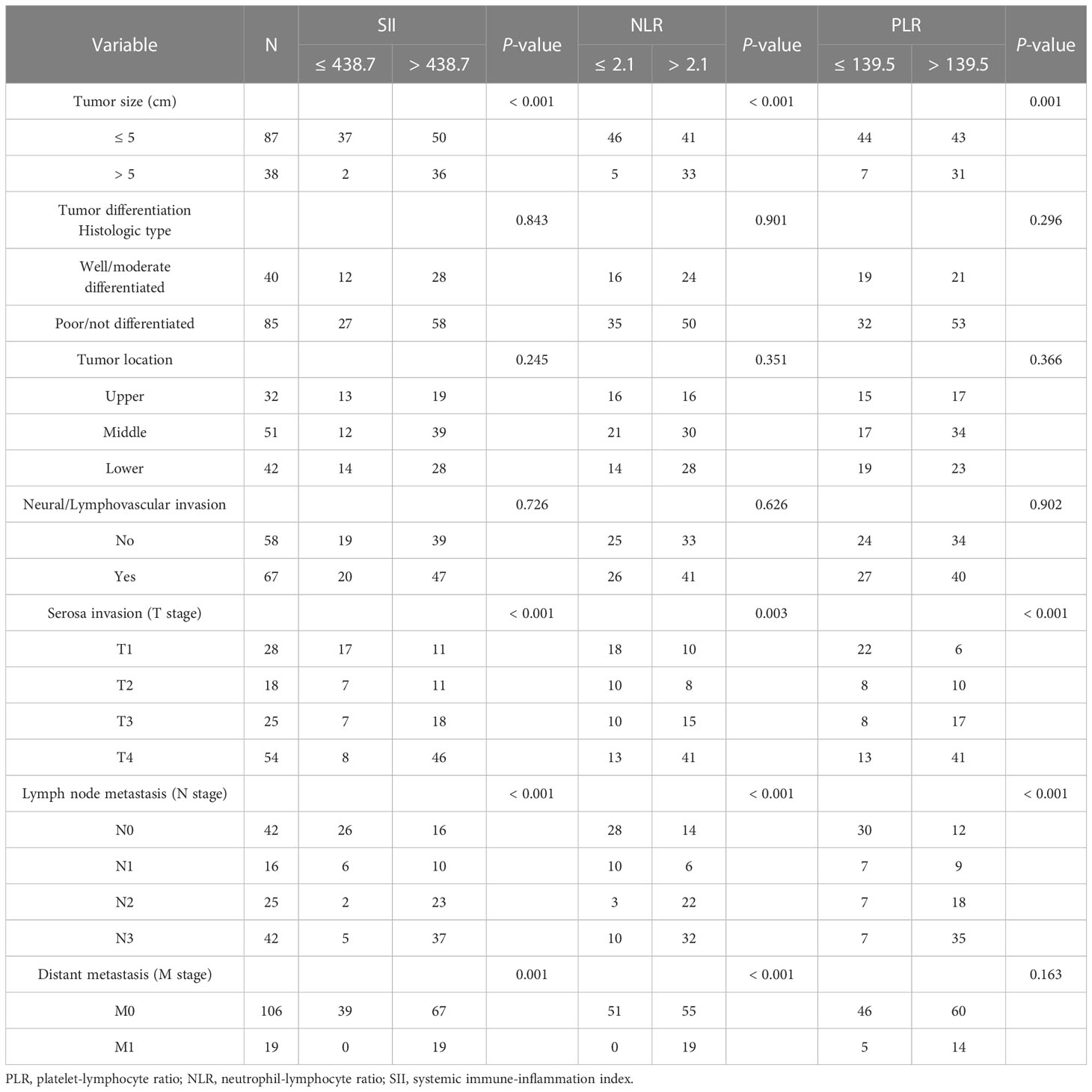
Table 4 Correlations between gastric cancer patient clinicopathological features and preoperative platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index.
Previous studies have indicated that tumor cells can enter the peripheral blood in the early stages of different types of cancer (21). Therefore, circulating tumor cell (CTC) counts in the peripheral blood can possibly be used to diagnose cancer early, understand tumor progression, and assess prognosis (22, 23). However, evaluating CTCs has a limited value in the early diagnosis of various types of cancer because of their rarity and the high cost and complexity associated with the technique (24). A sustained inflammatory response can stimulate tumor growth, invasion, and metastasis (25), and the risk of developing cancer can be reduced with the use of non-steroidal anti-inflammatory drugs (26, 27). Inflammatory immune cells are a crucial component of the tumor microenvironment (7). Reactive oxygen species (ROS) secreted by neutrophils can result in DNA damage, and incomplete or inaccurate repair of genes may lead to carcinogenesis (28). In addition, neutrophils synthesize and secrete oncostatin M and vascular endothelial growth factor (VEGF) to initiate angiogenesis and stimulate tumor growth, thereby furthering the invasion and metastasis of the tumor (29). Lymphocyte activity can reduce tumor cell proliferation and migration rates and plays a crucial part in tumor immunosurveillance (25). The immune response is suppressed following reduced lymphocyte levels, which is directly linked to the occurrence of GC (30). Platelet levels are typically high in cancer patients (31), and activated platelets can promote tumor growth and blood vessel formation through the release of VEGF-integrin (32). Previous studies have shown GC patients have significantly higher neutrophil and platelet counts and significantly lower lymphocyte counts than healthy populations (18, 19). An elevated SII, NLR, or PLR value represents higher levels of inflammation and dysregulation of the immune-inflammation balance in the body, which may be closely related to cancer formation and progression (13, 19). Therefore, the PLR, NLR, and SII may be potential biological indicators for the early diagnosis of GC, as they can indicate the level of inflammation in the body.
In our study, the preoperative SII, NLR, and PLR values were considerably higher in the GC group than in the control group. Compared with the values in the healthy population, the PLR, NLR, and SII were notably elevated in early-stage (stage I/II) GC. This suggests that the preoperative PLR, NLR, and SII could potentially be accurate diagnostic indicators for GC, even at early stages. While evaluating the diagnostic value of the NLR and PLR, Fang et al. (18) learned that these factors were considerably higher in GC patients than in the healthy population, with these differences being more pronounced in the early tumor stages. Similar results were found in colon cancer, where Peng et al. (33) indicated that both the NLR and PLR were considerably higher in early-stage (stage I/II) colon cancer patients than in the healthy population. By plotting ROC curves, we found that applying the SII, NLR, or PLR alone had a better diagnostic value for GC. In addition, the SII had the highest AUC, sensitivity, and specificity values, indicating that the SII individually had the best diagnostic efficacy among the three inflammatory indices examined. This may be because the SII combines three inflammatory cell types, neutrophils, lymphocytes, and platelets, thus allowing for a more integrated and comprehensive representation of the level of inflammation in the body. We subsequently analyzed the diagnostic value of the combination of the PLR, NLR, and SII, which resulted in a higher AUC value than those with all three individually and the combination of SII and NLR. This suggests that the combination of the PLR, NLR, and SII is the most effective for GC diagnosis and may be an important tool that can complement existing diagnostic methods. No previous research has examined the value of the SII in diagnosing GC, nor has any study reported the combined application of the PLR, NLR, and SII for this purpose. Our study verifies the importance of the preoperative SII in GC diagnosis and shows that using the SII, NLR, and PLR in combination has a higher diagnostic value. Our work provides a safer, noninvasive, economical, and simple biological indicator for early GC screening and diagnosis, which can benefit people at high risk of GC.
Furthermore, we found here that the PLR, NLR, and SII differed significantly with the tumor size, invasion depth, lymph node metastasis, and TNM stage, while the SII and NLR were also linked to distant tumor metastasis. These three indices were not correlated with the degree of tumor differentiation and location or the presence/absence of lymphovascular or nerve infiltration. This study reveals the relationship of the SII, NLR, and PLR with GC clinicopathological features, suggesting that inflammation-related indicators may be potential markers of disease progression in GC patients. They may be valuable as a complement to TNM staging for evaluating GC patient survival before surgery and when selecting preoperative treatment options. In a study of 412 GC patients, Hirahara et al. (10) found that patients with higher SII levels had larger tumors, deeper infiltration, increased lymph node metastasis, more advanced TNM stages, and a worse prognosis, consistent with the results of previous studies (10, 11, 15, 16). Similarly, higher NLR and PLR values were associated with more advanced TNM stages in GC patients (19) and shorter postoperative survival time (12, 34, 35). However, there have been few studies on the relationships between the SII and the clinical features of GC, and the conclusions remain controversial. Additional prospective, large-sample, multicenter studies are required to explore this topic in the future.
Most previous research has examined the role of the PLR, NLR, and SII in evaluating the survival of GC patients, while few have explored the value of the PLR and NLR in diagnosing GC. Nevertheless, the value of the SII in diagnosing GC remains unclear. Our study comprehensively analyzed the value of the PLR, NLR, and SII in GC diagnosis. To our knowledge, this is the first study to examine the diagnostic value of combining the PLR, NLR, and SII in GC. However, our study has a few limitations, as it was a single-center study with Chinese participants. As a result, more investigations are required to verify whether the findings can be generalized to other countries and ethnicities. Second, because this study was carried out retrospectively, there may have been a selection bias. Finally, this study only compared patients with GC and healthy individuals. The PLR, NLR, and SII values in individuals with benign gastric disease are unknown, and further research is required to investigate these in the future.
In summary, the PLR, NLR, and SII have important diagnostic value in GC, including early-stage GC. The SII has the highest efficacy for GC diagnosis when applied individually, while combining all three parameters had the greatest diagnostic value.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by ethics committee of the Taiyuan Central Hospital of Shanxi Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JZ and ZL were responsible for statistics and data analysis. SD, GL, and HY were responsible for collecting and entering the data. JZ and LZ wrote and revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Key Research and Development Project of Shanxi Province (201903D321030).
We thank the Department of Gastroenterology, Department of Pathology, and Medical Examination Center of Taiyuan Central Hospital of Shanxi Medical University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AUC, Area under the curve; CI, Confidence interval; CTC, Circulating tumor cell; GC, Gastric cancer; HC, Healthy control; NLR, Neutrophil-lymphocyte ratio; PLR, Platelet-lymphocyte ratio; ROC, Receiver operating characteristic; ROS, Reactive oxygen species; SD, Standard deviation; SII, Systemic immune-inflammation index; SRC, Signet-ring cell; VEGF, Vascular endothelial growth factor; WBC, White blood cell.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
3. Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, et al. Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: A non-inferiority-Matched cohort study. Am J Gastroenterol (2016) 111:240–9. doi: 10.1038/ajg.2015.427
4. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
5. Jiang L, Yang KH, Guan QL, Chen Y, Zhao P, Tian JH. Survival benefit of neoadjuvant chemotherapy for resectable cancer of the gastric and gastroesophageal junction: a meta-analysis. J Clin Gastroenterol (2015) 49:387–94. doi: 10.1097/MCG.0000000000000212
6. Mori Y, Arita T, Shimoda K, Yasuda K, Yoshida T, Kitano S. Effect of periodic endoscopy for gastric cancer on early detection and improvement of survival. Gastric Cancer (2001) 4:132–6. doi: 10.1007/pl00011735
7. Qian S, Golubnitschaja O, Zhan X. Chronic inflammation: key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J (2019) 10:365–81. doi: 10.1007/s13167-019-00194-x
8. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis (2009) 30:1073–81. doi: 10.1093/carcin/bgp127
9. Liu D, Jin J, Zhang L, Li L, Song J, Li W. The neutrophil to lymphocyte ratio may predict benefit from chemotherapy in lung cancer. Cell Physiol Biochem (2018) 46:1595–605. doi: 10.1159/000489207
10. Hirahara N, Matsubara T, Fujii Y, Kaji S, Kawabata Y, Hyakudomi R, et al. Comparison of the prognostic value of immunoinflammation-based biomarkers in patients with gastric cancer. Oncotarget (2020) 11:2625–35. doi: 10.18632/oncotarget.27653
11. Wang PX, Wang HJ, Liu JH, Qiu GL, Lu J, Fan L, et al. A nomogram combining plasma fibrinogen and systemic immune−inflammation index predicts survival in patients with resectable gastric cancer. Sci Rep (2021) 11:10301. doi: 10.1038/s41598-021-89648-9
12. Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Med (Baltimore (2018) 97:e0144. doi: 10.1097/MD.0000000000010144
13. Hu B, Yang XR, Xu Y, Sun YF, Sun C, Guo W. Systemic immune-inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res (2014) 20:6212–22. doi: 10.1158/1078-0432.CCR-14-0442
14. Wang Y, Li Y, Chen P, Xu W, Wu Y, Che G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: a meta-analysis. Ann Transl Med (2019) 7:433. doi: 10.21037/atm.2019.08.116
15. Shi H, Jiang Y, Cao H, Zhu H, Chen B, Ji W. Nomogram based on systemic immune-inflammation index to predict overall survival in gastric cancer patients. Dis Markers (2018) 2018:1787424. doi: 10.1155/2018/1787424
16. Zhu Z, Cong X, Li R, Yin X, Li C, Xue Y. Preoperative systemic immune-inflammation index (sii) for predicting the survival of patients with stage I-III gastric cancer with a signet-ring cell (SRC) component. BioMed Res Int (2020) 2020:5038217. doi: 10.1155/2020/5038217
17. He K, Si L, Pan X, Sun L, Wang Y, Lu J, et al. Preoperative systemic immune-inflammation index (SII) as a superior predictor of long-term survival outcome in patients with stage I-II gastric cancer after radical surgery. Front Oncol (2022) 12:829689. doi: 10.3389/fonc.2022.829689
18. Fang T, Wang Y, Yin X, Zhai Z, Zhang Y, Yang Y, et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J Immunol Res (2020) 2020:9146042. doi: 10.1155/2020/9146042
19. Wu Y, Jiang M, Qin Y, Lin F, Lai M. Single and combined use of neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and carcinoembryonic antigen in diagnosing gastric cancer. Clin Chim Acta (2018) 481:20–4. doi: 10.1016/j.cca.2018.02.027
20. In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T. Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the national cancer database. Ann Surg Oncol (2017) 24:3683–91. doi: 10.1245/s10434-017-6078-x
21. Dasgupta A, Lim AR, Ghajar CM. Circulating and disseminated tumor cells: harbingers or initiators of metastasis? Mol Oncol (2017) 11:40–61. doi: 10.1002/1878-0261.12022
22. Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, et al. Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis. Mol Cancer (2019) 18:64. doi: 10.1186/s12943-019-0976-4
23. Ferreira MM, Ramani VC, Jeffrey SS. Circulating tumor cell technologies. Mol Oncol (2016) 10:374–94. doi: 10.1016/j.molonc.2016.01.007
24. Mong J, Tan MH. Size-based enrichment technologies for non-cancerous tumor-derived cells in blood. Trends Biotechnol (2018) 36:511–22. doi: 10.1016/j.tibtech.2018.02.010
25. Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol (2010) 28:4045–51. doi: 10.1200/JCO.2010.27.9992
26. Burr NE, Hull MA, Subramanian V. Does aspirin or non-aspirin non-steroidal anti-inflammatory drug use prevent colorectal cancer in inflammatory bowel disease? World J Gastroenterol (2016) 22:3679–86. doi: 10.3748/wjg.v22.i13.3679
27. Shebl FM, Hsing AW, Park Y, Hollenbeck AR, Chu LW, Meyer TE, et al. Non-steroidal anti-inflammatory drugs use is associated with reduced risk of inflammation-associated cancers: NIH-AARP study. PloS One (2014) 9:e114633. doi: 10.1371/journal.pone.0114633
28. Weitzman SA, Gordon LI. Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood (1990) 76:655–63. doi: 10.1182/blood.V76.4.655.655
29. Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin m: potential implications for tumor progression. Cancer Res (2005) 65:8896–904. doi: 10.1158/0008-5472.CAN-05-1734
30. Lee WJ, Chang KJ, Lee CS, Chen KM. Selective depression of T-lymphocyte subsets in gastric cancer patients: an implication of immunotherapy. J Surg Oncol (1994) 55:165–9. doi: 10.1002/jso.2930550307
31. Xin-Ji Z, Yong-Gang L, Xiao-Jun S, Xiao-Wu C, Dong Z, Da-Jian Z. The prognostic role of neutrophils to lymphocytes ratio and platelet count in gastric cancer: A meta-analysis. Int J Surg (2015) 21:84–91. doi: 10.1016/j.ijsu.2015.07.681
32. Jiang L, Luan Y, Miao X, Sun C, Li K, Huang Z, et al. Platelet releasate promotes breast cancer growth and angiogenesis via VEGF-integrin cooperative signalling. Br J Cancer (2017) 117:695–703. doi: 10.1038/bjc.2017.214
33. Peng HX, Yang L, He BS, Pan YQ, Ying HQ, Sun HL, et al. Combination of preoperative NLR, PLR and CEA could increase the diagnostic efficacy for I-III stage CRC. J Clin Lab Anal (2017) 31:e22075. doi: 10.1002/jcla.22075
34. Lian L, Xia YY, Zhou C, Shen XM, Li XL, Han SG, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer biomark (2015) 15:899–907. doi: 10.3233/CBM-150534
Keywords: gastric cancer, diagnosis, systemic immune-inflammation index, neutrophil-lymphocyte ratio, platelet-lymphocyte ratio
Citation: Zhang J, Zhang L, Duan S, Li Z, Li G and Yu H (2023) Single and combined use of the platelet-lymphocyte ratio, neutrophil-lymphocyte ratio, and systemic immune-inflammation index in gastric cancer diagnosis. Front. Oncol. 13:1143154. doi: 10.3389/fonc.2023.1143154
Received: 12 January 2023; Accepted: 16 March 2023;
Published: 30 March 2023.
Edited by:
Dongshi Chen, University of Southern California, United StatesReviewed by:
Yating Xiao, University of Chinese Academy of Sciences, ChinaCopyright © 2023 Zhang, Zhang, Duan, Li, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, emh1YW5nemh1YW5nMDYyMkAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.