
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 23 March 2023
Sec. Genitourinary Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1142022
This article is part of the Research TopicClinical and Translational Research in Prostate CancerView all 15 articles
Purpose: To compare the diagnostic performance of transperineal targeted biopsy (TB) or systematic biopsy (SB) alone based on combined TB+SB and radical prostatectomy (RP) specimen for detecting prostate cancer (PCa) according to the prostate imaging reporting and data system (PI-RADS) score.
Materials and methods: This study included 1077 men who underwent transperineal bi-parametric (bp) magnetic resonance imaging (MRI)–ultrasound (US) fusion TB+SB (bpMRI-US FTSB) between April 2019 and March 2022. To compare the performance of each modality (TB, SB, and combined TB+SB) with the RP specimen (as the standard) for detecting PCa and clinically significant PCa (csPCa), receiver operating characteristic (ROC) curves were plotted.
Results: PCa was detected in 581 of 1077 men (53.9%) using bpMRI-US FTSB. CsPCa was detected in 383 of 1077 men (35.6%), 17 of 285 (6.0%) with PI-RADS 0 to 2, 35 of 277 (12.6%) with PI-RADS 3, 134 of 274 (48.9%) with PI-RADS 4, and 197 of 241 (81.7%) with PI-RADS 5, respectively. The additional diagnostic value of TB vs. SB compared to combined TB+SB for diagnosing csPCa were 4.3% vs. 3.2% (p=0.844), 20.4% vs 5.1% (p<0.001), and 20.3% vs. 0.7% (p<0.001) with PI-RADS 3, 4, and 5, respectively. TB alone showed no significant difference in diagnostic performance for csPCa with combined TB+SB based on RP specimens in patients with PI-RADS 5 (p=0.732).
Conclusion: A need for addition of SB to TB in patients with PI-RADS 3 and 4 lesions, however, TB alone may be performed without affecting the management of patients with PI-RADS 5.
Prostate cancer (PCa) diagnosis relies on prostate-specific antigen (PSA) and prostate biopsy, and transrectal ultrasonography-guided systematic biopsy (TRUSB) has been considered the standard diagnostic pathway in men with a clinical suspicion of PCa (1).
However, TRUSB has led to missed diagnosis in >30% of patients with PCa and has poor discriminative power in diagnosing cancerous tissue (2, 3). In this regard, to improve the discriminative power and diagnostic accuracy of prostate biopsy, visualization of PCa through magnetic resonance imaging (MRI) has been attempted. Accordingly, the prostate imaging reporting and data system (PI-RADS) was developed to maximize the standardized utilization of MRI for detecting PCa, which led to increased usage of MRI as a guide for targeted biopsy (TB) (4). Studies have suggested that MRI-TB can provide additional value in diagnosis of PCa for clinically significant PCa (csPCa) categorized as International Society for Urological Pathology (ISUP) grade ≥2 (5). Additionally, MRI-TB based on PI-RADS significantly outperforms systematic biopsy (SB) for detection of csPCa with the probability of sparing the potential redundancy of SB (6–8).
However, MRI was missing PCa in 20% of index tumor and 79% of non-index tumor (9). Therefore, the performance of MRI-TB alone may be not good enough to omit systematic biopsy (SB) in every man with a clinical suspicion for PCa (10). TB is the standard pathway in most cancers, nevertheless the current guidelines for detecting PCa have recommended SB and additional TB with a suspicious lesion in MRI (11). However, SB may be associated with over-diagnose the clinically insignificant PCa and result in overtreatment and impose the risk of adverse events, complications, and comes with consequence of medical burden (12, 13). Notably, in PI-RADS 5, MRI-TB have shown good performance with high predictive rates for csPCa that suggests TB alone might also be valuable in diagnosing csPCa (14–16).
The purpose of this study was to compare the diagnostic performance of TB or SB alone according to the PI-RADS scores with combined TB+SB based on the standard transperineal bi-parametric magnetic resolution imaging-ultrasound fusion TB+SB (bpMRI-US FTSB) and radical prostatectomy (RP) specimen.
We analyzed the medical records of 1077 men, between April 2019 and March 2022, who were clinically suspected for PCa with an elevated prostate-specific antigen (PSA) level (≥ 4.0 ng/mL), and/or abnormal findings on digital rectal examination (DRE). All enrolled patients underwent bi-parametric MRI (bpMRI) prior to the prostate biopsy, and regions of interest (ROIs) on MRI were established according to the PI-RADS version 2.0. Subsequent transperineal bpMRI-US FTSB and RP were performed (Figure 1).
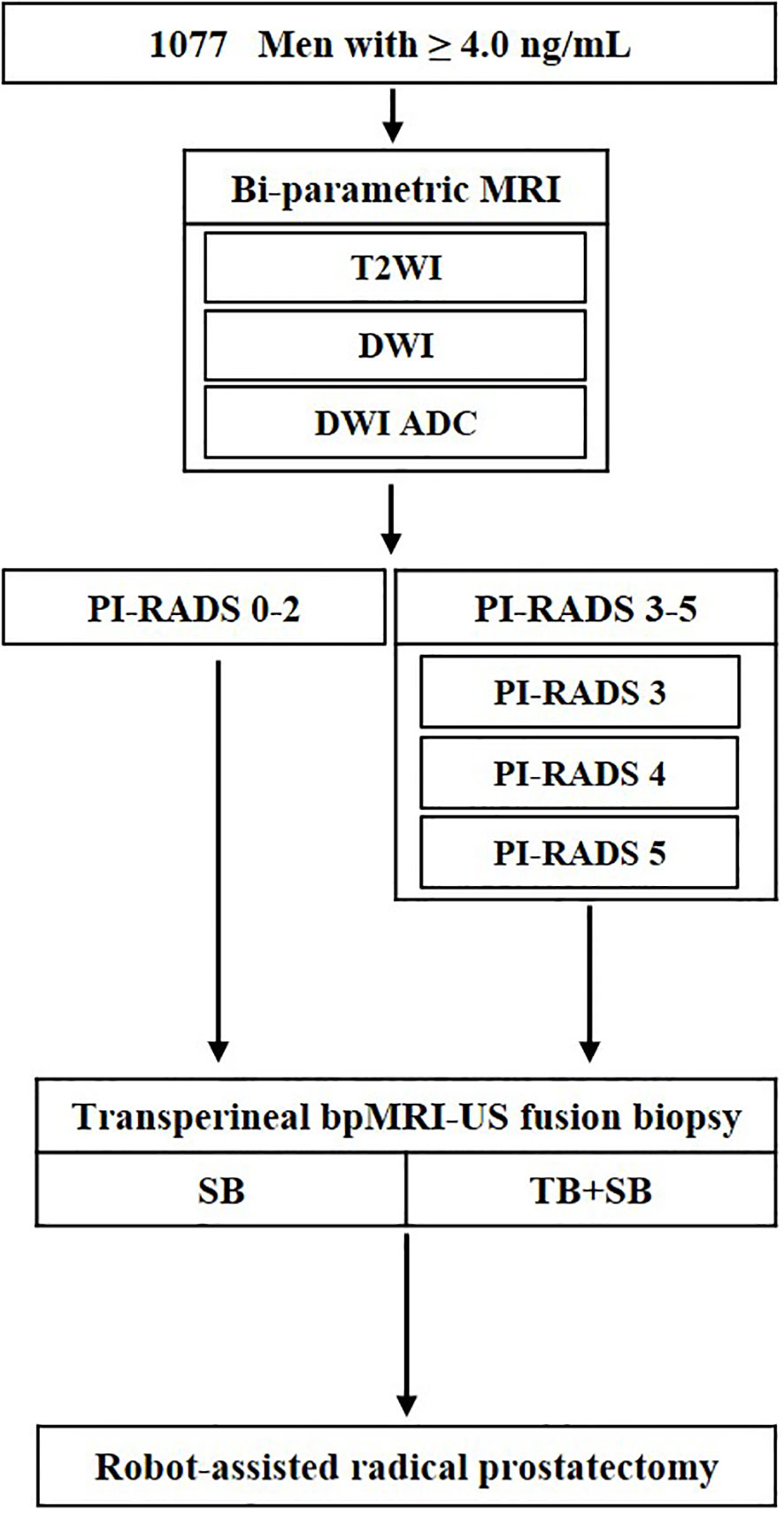
Figure 1 Flowchart of study design. ADC, apparent diffusion coefficient; T2WI, T2-weighted images; DWI, diffusion weighted images; TB, targeted biopsy; SB, systematic biopsy; US, ultrasound.
The bpMRI, contrast-free protocol, was performed using a 3.0-T scanner (Magnetom Skyra and Prisma, Siemens Healthineers, Erlangen, Germany or Achieva, Philips Healthcare, Best, Netherlands) with a multichannel phased-array external surface coil. T2-weighted images (T2WI) and diffusion-weighted images (DWI) were obtained, whereas dynamic contrast-enhanced (DCE) images were omitted. ROIs on the bpMRI were marked by three dedicated uroradiologists based on PI-RADS version 2.0 (Figure 2A).
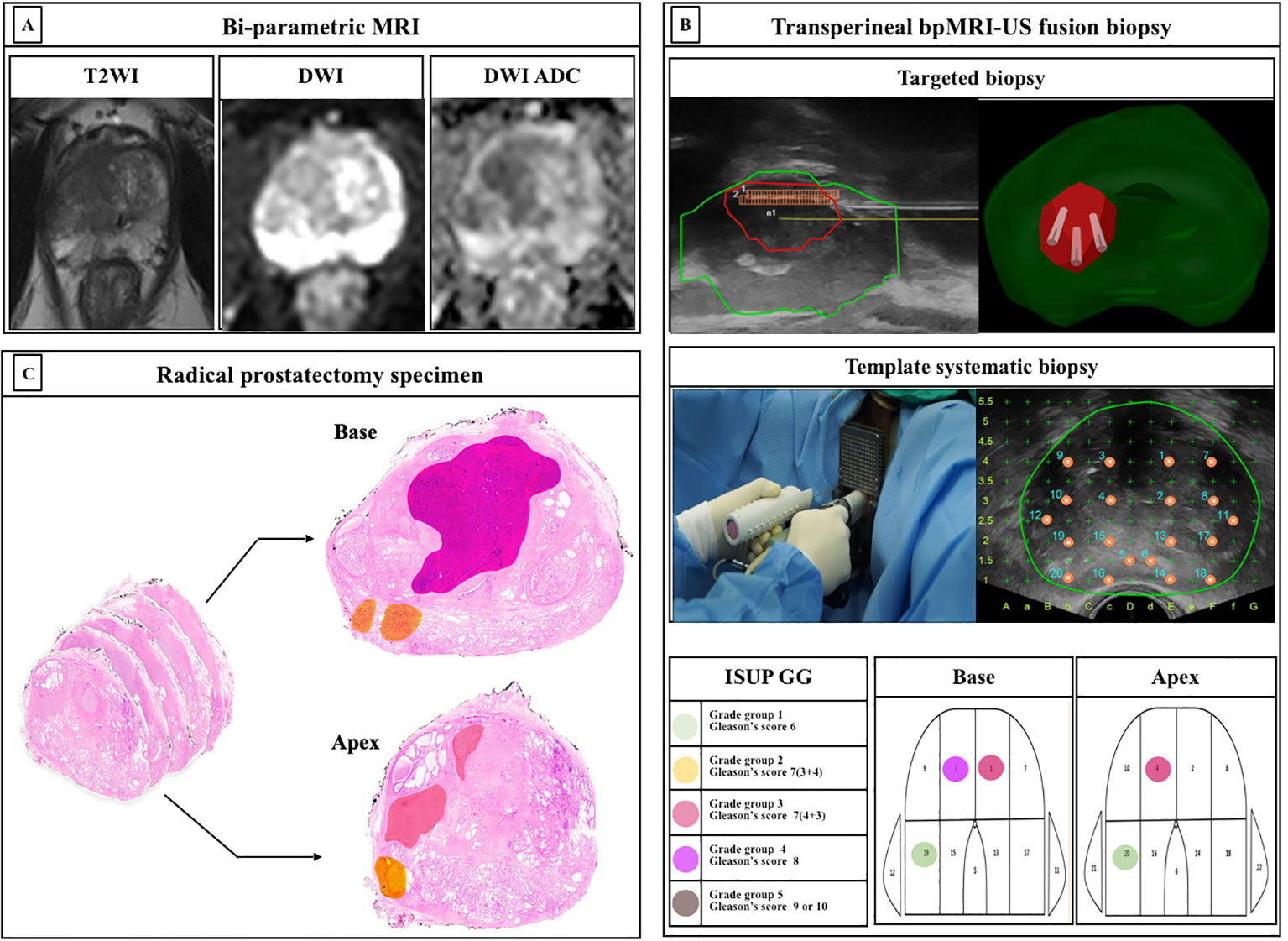
Figure 2 Protocols of study. (A) Bi-parametric magnetic resonance imaging (bpMRI) (B) Transperineal bpMRI-Ultrasound fusion targeted and systematic biopsy (C) Whole-mount radical prostatectomy specimen ISUP, International Society for Urological Pathology; GG, grade group; MRI, Magnetic resonance imaging; TB, targeted biopsy; SB, systematic biopsy.
We have previously reported a protocol for transperineal bpMRI-US FTSB (16). In brief, the elastic image registration type of the MRI-US fusion technique using a mechanical position encoder and robotic articulated arm system (Biojet, USA) was used and TB and SB were performed by urologists during the same session. Further, we considered suspicious lesions as ROI (PI-RADS ≥3) for TB, and 3-4 cores of TB and sequential 22-cores of SB were performed using a prostate mapping template (modified Barzell-template). The ROI for the TB was not intentionally avoided. Each core was labelled separately and subjected to histopathology. The number of biopsy cores was decided depending on the prostate size. The prostate biopsy results were reported by three uropathologists based on the International Society of Urological Pathology (ISUP) grade groups (GG). Clinically insignificant PCa was defined as an ISUP GG1. Clinically significant PCa was defined as > ISUP GG2 (Figure 2B).
Localized PCa with PI-RADS 3-5, sequentially underwent robot-assisted RP (RARP) using da Vinci Si, Xi, or SP system (Intuitive Surgical, Sunnyvale, CA, USA) by two surgeons. For histopathological examination, whole-mount histopathology slides were used, and each prostate was sectioned in the axial plane from the basal to the apex at approximately 4-5 mm intervals (Figure 2C).
The endpoint was to compare the impact of TB or SB alone according to PI-RADS scores, referring to the standard of combined TB+SB and RP specimens.
To quantify and compare the performance of each modality (TB, SB, and combined TB+SB) in detecting PCa and csPCa, receiver operating characteristic (ROC) curve analyses were performed considering combined TB+SB and RP specimens as standards. Accordingly, the results were summarized using Delong’s test as the areas under the ROC curves (AUCs) and 95% CI. All statistical analyses were performed using IBM SPSS version 26.0 (IBM Corp., Armonk, NY, USA). The level of statistical significance was considered P<0.05.
This study was conducted in accordance with the Declaration of Helsinki and current ethical guidelines. The study was reviewed and approved by the Ethics Committee and Institutional Review Board of Korea University Anam Hospital (IRB No. 2018AN0339).
In total, 1077 men were included in the analysis. The median (interquartile range (IQR)) age was 69.0 (62.0-75.0) years. The median (IQR) PSA and PSA density (PSAD) were 6.66 (4.57-11.57) ng/mL and 0.18 (0.11-0.35) ng/mL2. The demographics of the study population are reported in Table 1.
PCa (GG1) was detected in 581 of 1077 men (53.9%) by bpMRI-US FTSB. Accordingly, it was detected in 58 of 285 cases (35.6%) with PI-RADS 0-2, in 91 of 277 cases (32.9%) with PI-RADS 3, in 209 of 274 cases (76.3%) with PI-RADS 4, and in 220 of 241 cases (91.3%) with PI-RADS 5 (Figure 3A). Further, csPCa (≥ GG2) was detected in 383 of 1077 men (35.6%). Accordingly, it was detected in 17 of 285 men (6.0%) with PI-RADS 0-2, in 35 of 277 men (12.6%) with PI-RADS 3, in 134 of 274 men (48.9%) with PI-RADS 4, and in 197 of 241 men (81.7%) with PI-RADS 5 (Figure 3B). The distribution of ISUP grade groups is shown in Table 2. Patients with csPCa (GG2≥2) had higher median PSA, PSAD, and lower prostate volume than those with GG1 pathology; PSA(IQR) [66.0 vs. 72.0, p= 0.038], PSAD (0.14 vs. 0.35, p=0.011), and lower prostate volume (41.2 vs. 30.3, p=0.047) than those with GG1 pathology (Supplementary Table 1).
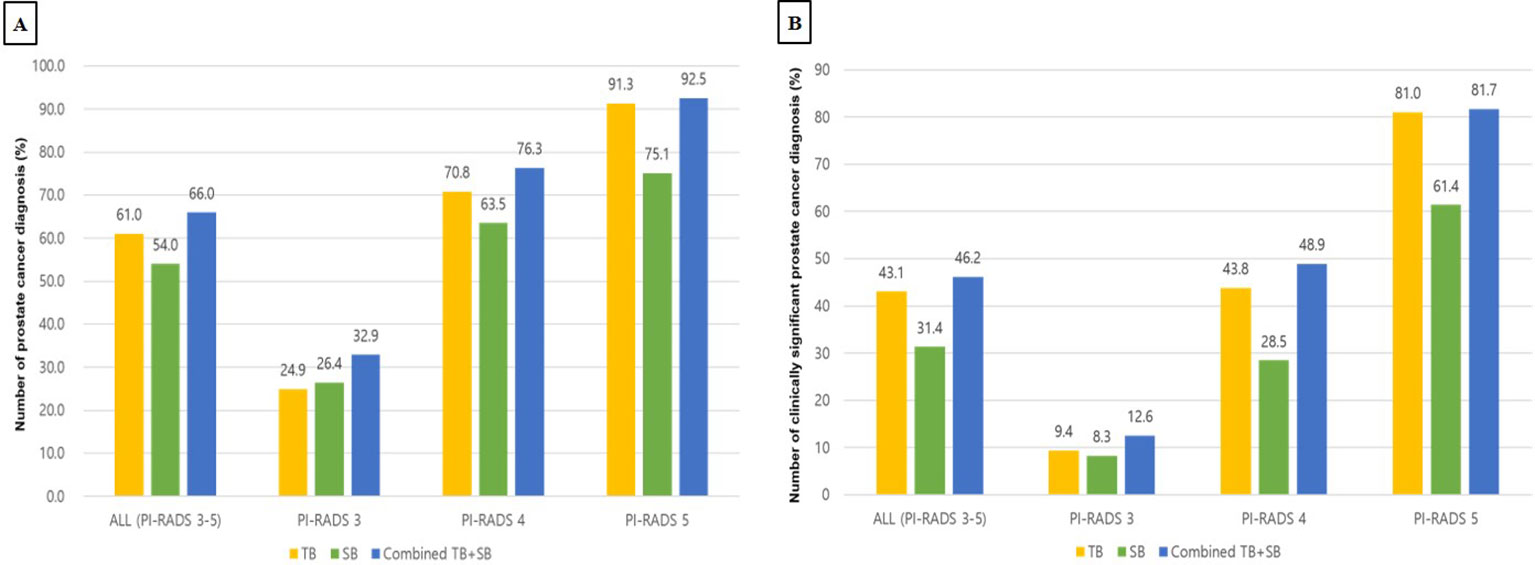
Figure 3 Diagnostic performance of TB, SB, TB+SB in patients with PI-RADS 3 to 5 (A) Detection rate for prostate cancer (B) Detection rate for clinically significant prostate cancer PI-RADS, prostate imaging-reporting and data systems; TB, targeted biopsy; SB, systematic biopsy.
In patients with PI-RADS 3 to 5, TB, SB, and Combined TB+SB were able to detect PCa in 61.0%, 54.0%, and 66.0% of cases, respectively. Accordingly, the diagnosis rate of TB, SB, and combined TB+SB for diagnosing PCa were 24.9%, 26.4%, and 32.9% in patients with PI-RADS 3, 70.8%, 63.5%, and 76.3% in patients with PI-RADS 4, and 91.3%, 75.1%, and 92.5% in patients with PI-RADS 5, respectively (Figure 3A). The additional diagnostic value for PCa detection of TB vs. SB compared to combined TB+SB were 12.0% vs. 5.0% (p<0.001) in patients with PI-RADS 3-5; PI-RADS 3: 6.5% vs. 8.0% (p=0.535), PI-RADS 4: 12.8% vs. 5.5% (p<0.001), and PI-RADS 5: 17.4% vs. 1.2% (p<0.0001), respectively (Table 3).
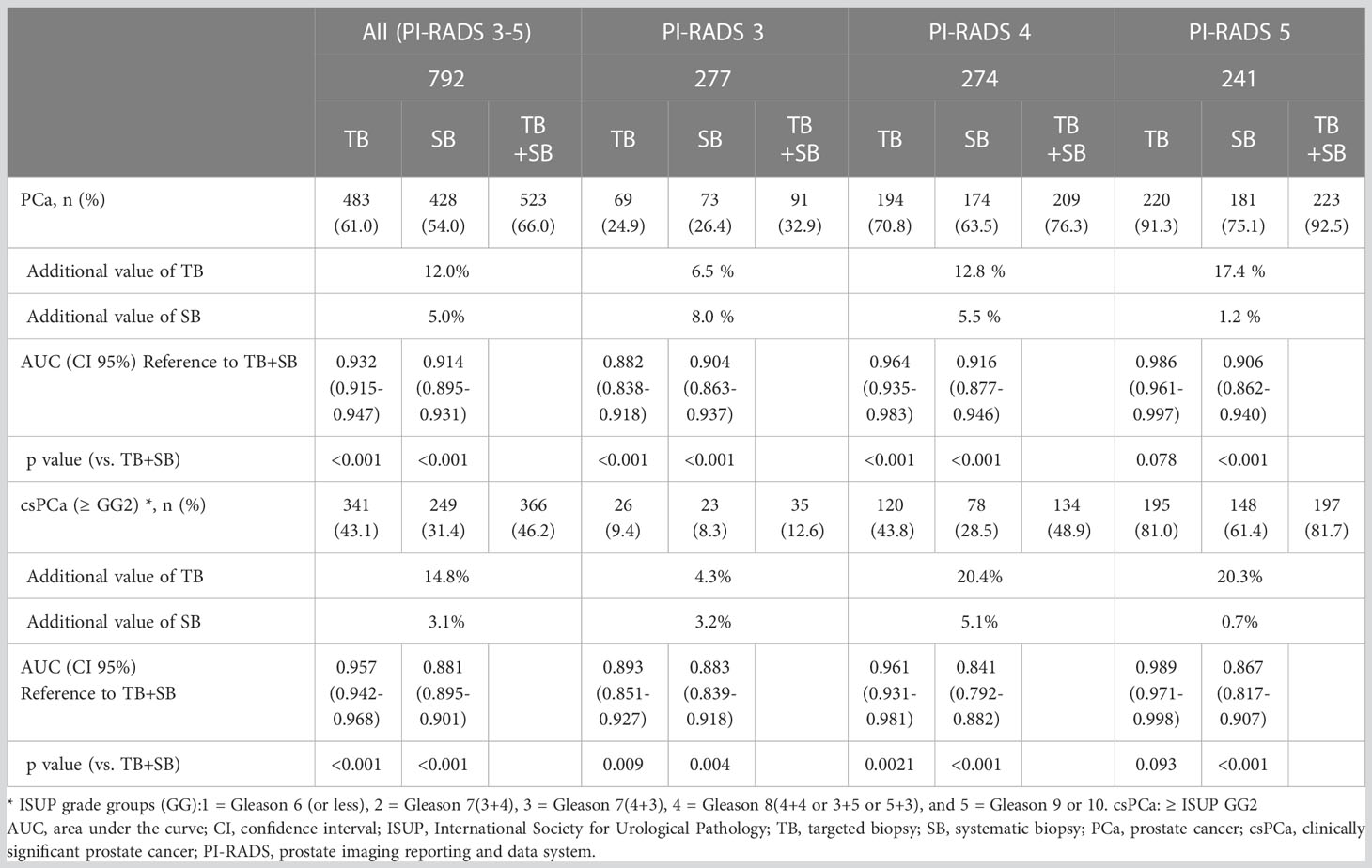
Table 3 Diagnostic performance of TB or SB alone according to PI-RADS compared to combined TB and SB.
Combined TB+SB showed superior diagnostic performance for TB or SB alone in patients with PI-RADS 3 and 4 (p <0.001). However, TB alone showed no significant difference in diagnostic performance for csPCa with combined TB+SB in patients with PI-RADS 5; PI-RADS 3: area under the curve (AUC) [95% confidence interval (CI)], 0.882 [0.838–0.918], p<0.001; PI-RADS 4: AUC, 0.964 [0.935–0.983], p<0.001; PI-RADS 5: AUC, 0.986 [0.961–0.997], p=0.078 (Table 3).
In patients with PI-RADS 3 to 5, csPCa (ISUP ≥GG2) was detected in 43.1%, 31.4%, and 46.2% cases via TB, SB, and combined TB+SB, respectively. Accordingly, the diagnosis rate of TB, SB, and combined TB+SB for diagnosing csPCa were 9.4%, 8.3%, and 12.6% in patients with PI-RADS 3, 43.8%, 28.5%, and 48.9% in patients with PI-RADS 4, and 81.0%, 61.4%, and 81.7% in patients with PI-RADS 5, respectively (Figure 3B). The additional diagnostic value for csPCa detection of TB vs. SB alone compared to combined TB+SB was 14.8% vs. 3.1%(p<0.001) in patients with PI-RADS 3-5; PI-RADS 3: 4.3% vs. 3.2% (p=0.844), PI-RADS 4: 20.4% vs. 5.1% (p<0.001), and PI-RADS 5: 20.3% vs. 0.7% (p<0.001), respectively (Table 3). Further, TB alone showed no significant difference in diagnostic performance for csPCa to combined TB+SB in patients with PI-RADS 5; PI-RADS 3: area under the curve (AUC) [95% confidence interval (CI)], 0.893 [0.851–0.927], p=0.0088; PI-RADS 4: AUC, 0.961 [0.931–0.981], p=0.002; PI-RADS 5: AUC, 0.989 [0.971–0.998], p=0.093 (Table 3).
The RARP was performed in 289 of 483 diagnosed with PCa with PI-RADS 3-5; 59 of 91 (64.8%) with PI-RADS 3, 122 of 209 (58.4%) with PI-RADS 4, and 108 of 220 (49.1%) with PI-RADS 5, respectively (Table 4).
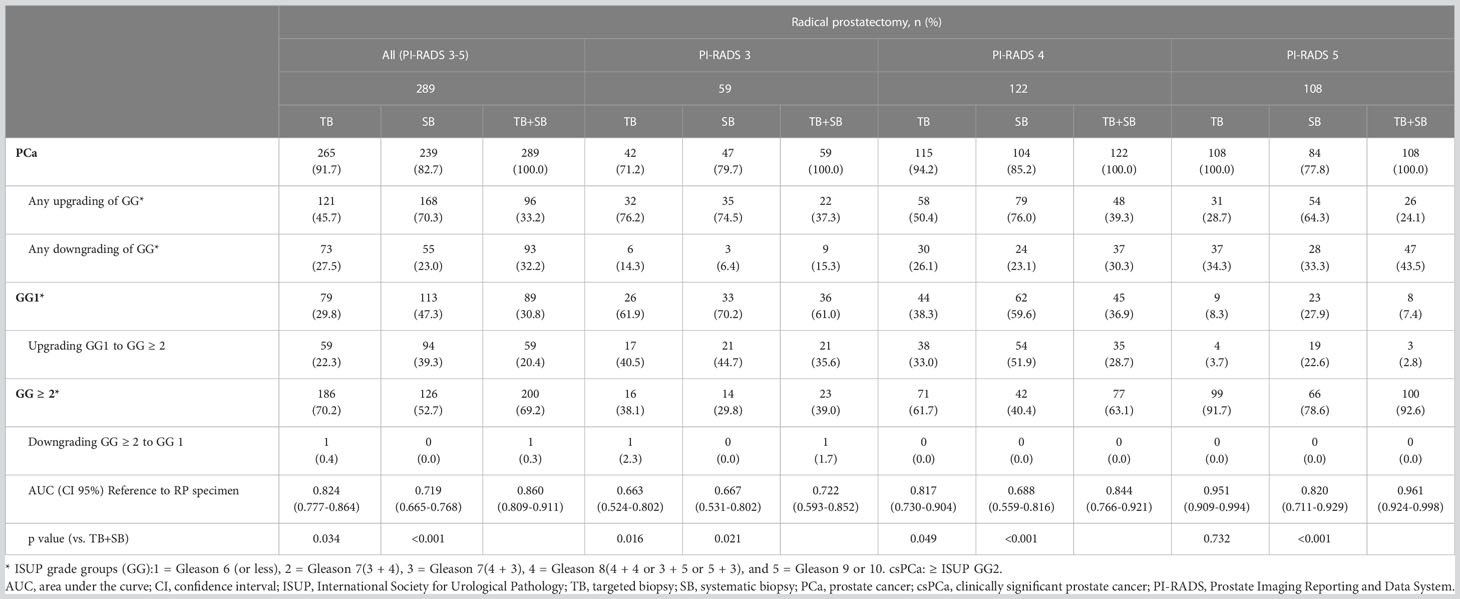
Table 4 Concordance of prostate cancer grade group on targeted, systematic, and combined targeted and systematic biopsy according to radical prostatectomy specimen by PI-RADS scores.
Accordingly, TB alone and combined TB+SB showed 45.7% and 33.2% of any upgrading in RP specimens with PI-RADS 3-5; 76.2% and 37.3% with PI-RADS 3, 50.4% and 39.3% with PI-RADS 4, 28.7% and 24.1% with PI-RADS 5, respectively; and upgrading of GG1 to GG ≥ 2 occurred in 59 of 265 (22.3%) and 59 of 289 (20.4%) cases with PI-RADS 3-5; 17 of 42 (40.5%) and 21of 59 (35.6%) with PI-RADS 3, 38 of 115 (33.0%) and 35 of 122 (28.7%) with PI-RADS 4, and 4 of 108 (3.7%) and 3 of 108 (2.8%) with PI-RADS 5, respectively. Further, downgrading of GG ≥ 2 to GG1 occurred in only one in 289 (0.3%) (Table 4).
The combined TB+SB showed superior diagnostic performance compared to TB alone for diagnosing csPCa when compared to the standard of RP specimen; TB alone vs TB+SB, AUC (95% CI); PI-RADS 3-5: 0.824 (0.777-0.864) vs. 0.860 (0.809-0.911), p=0.034; PI-RADS 3: 0.663 (0.524-0.802) vs. 0.722 (0.593-0.852), p=0.016; PI-RADS 4: 0.817 (0.730-0.904) vs. 0.844 (0.766-0.921), p=0.049. TB alone showed no significant difference in diagnostic performance for csPCa to combined TB+SB in patients with PI-RADS 5; TB alone vs. combined TB+SB, AUC (95% CI), 0.951(0.909-0.994) vs. 0.961(0.924-0.998), p=0.732 (Table 4).
In recent years with significant improvements in the accuracy of MRI after implementation of the PI-RADS, the use of prebiopsy MRI for PCa diagnosis has increased (4, 6, 17). Furthermore, numerous studies have demonstrated that MRI-TB could offer improved diagnostic value for csPCa with pooled sensitivity and specificity of 0.80 (95%CI: 0.69-0.87) and 0.94 (95%CI: 0.90-0.97) (5). However, addition of TB to SB increases the number of csPCa (≥ ISUP GG2) by 6.7-7.6%, while added value of SB to TB is 4.3-5.2% for csPCa (5, 14, 18). Further, MRI was missing PCa in 20% of index tumor and 79% of non-index tumor (9). Therefore, due to the additional diagnostic value of SB and the risk of missing csPCa with TB alone, combined TB + SB has been suggested for dignosing PCa (5, 10, 11).
However, it should be noted that obtaining more prostate cores accompanies with a greater risk of complications, such as prostatitis, sepsis events, visits to the emergency room, rectal bleeding, hematuria, and pain (7, 19, 20). MRI-TB alone with fewer core biopsies per patient might lead to fewer complications. The net benefit of adding SB to TB for prostate biopsy optimization according to PI-RADS score should be weighed against accuracy for csPCa detection and additional burden such as overdiagnosis of indolent PCa, resulting in overtreatment and complications from increased numbers of biopsies. For predicting csPCa, several predictors and their combination such as clinical parameters including PSAD and PI-RADS score have been suggested (21). In addition, for risk assessment to determine the need for biopsy, risk calculators (RCs) have been suggested, thereby may be reducing the number of unnecessary biopsies (22).
Notably, MRI-TB showed good performance and was highly predictive for diagnosing csPCa in cases with PI-RADS 5 (77-85%) (7, 14, 16). In a study comparing the concordances between PI-RADS and histologic reports of the RP specimen, the PI-RADS≥3 was further associated with csPCa in 92.4% of cases, with 100% association in cases with a PI-RADS 5 score (23). High performance of MRI-TB and low additional diagnostic value (2-4%) of SB for detection of csPCa in patients with PI-RADS 5 that suggests the probability of sparing the potential redundancy of SB in PI-RADS 5 (12, 24, 25).
For MRI-TB, mpMRI have shown a high sensitivity and negative predictive value (NPV) of 93.0% and 89.0% for csPCa (6). However, it is time-consuming (~ 40 min) to acquire T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI), and dynamic contrast-enhanced (DCE) imaging requires intravenous administration of contrast media.
Several studies have demonstrated comparable diagnostic performance of bpMRI (contrast-free protocol) to mpMRI (26). In a systematic review and meta-analysis of the diagnostic accuracy of bpMRI and mpMRI for PCa detection, pooled sensitivity and specificity did not show significant difference and the AUCs were similar; 0.87 and 0.90 for mpMRI and bpMRI (27). In this regard, bpMRI is more rapid (~15 min) due to exclusion of DCE, and safer from potential side effects of contrast media than mpMRI while retaining a sufficient diagnostic value (16).
In the current study, we compared the impact of TB, SB, and combined TB+SB according to the PI-RADS score. Accordingly, the SB had only additional diagnostic values of 1.2% and 0.7% for detection of PCa and csPCa in patients with PI-RADS 5. Further, TB alone showed no significant difference of diagnostic performance with combined TB+SB for csPCa. Similarly, in a study conducted on 112 patients with PI-RADS 5 on MRI and subsequentially 78 of RP, TB alone could diagnose PCa with very high probability (97%) in patients with PSAD >0.15ng/ml2 (12). Accordingly, if SB was omitted, none of the PCa cases and only 4% of csPCa cases would be missed. Thus, the authors suggest that SB might be omitted for cases with PI-RADS 5 and PSAD >0.15ng/ml2.
Since the upgrading grade group of RP specimens from prostate biopsy has been reported, the omission of SB may lead to misclassification of PCa; TB (30.9%) and TB+SB (14.4%) of the upgraded grade group (10). These inconsistencies between biopsy and specimen of prostate, upgrading and misclassification of PCa, are the inherent limitations of prostate needle biopsy (28). Nevertheless, in this study, upgrading from GG1 to ≥ GG2, which has a potential risk of changing subsequent clinical management, showed difference in only one patient; TB alone vs. combined TB+SB, 4 of 108 (3.7%) vs. 3 of 108 (2.8%). Similarly, in another study, MRI-TB alone in PI-RADS 5 cases had meager upgrade rate (3.4%) (29). Further, addition of SB to TB in PI-RADS 5 cases altered only 3.1% of the highest grade group of PCa patients, all of whom had already been categorized as GG≥2 based on TB, and SB did not change subsequent clinical management (24). Current study supports the need for SB in patients with PI-RADS 3 and 4 lesions. However, minimal additional diagnostic values of SB and comparable diagnostic performance of MRI-TB suggest that SB potentially can be omitted in patients with PI-RADS 5.
The limitations of this study are its retrospective nature and accompanying bias. The other limitation is that this study was performed in a single tertiary center with transperineal prostate biopsy and bpMRI, and transrectal prostate biopsy with mpMRI, which is the common practice, was not considered. This may raise concerns toward extrapolating a general trend from our results. Nevertheless, this study can support that performing TB alone in patients with PI-RADS 5 lesions, might mitigate the medical burden by SB omission.
The current study suggests a need for addition of SB to TB in patients with PI-RADS 3 and 4 lesions, and TB alone may be performed for diagnosing csPCa in patients with PI-RADS 5, without changing the subsequent clinical management.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethics Committee and the Institutional Review Board of KUMC (IRB No. 2018AN0339).
TIN: protocol/project development, data acquisition, data analysis and interpretation, drafting of the manuscript. JSS: Protocol/project development, supervision. SHK: Protocol/project development, Data acquisition, supervision. JC: Protocol/project development, supervision. SGK: Protocol/project development, Data acquisition, supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1142022/full#supplementary-material
ADC, apparent diffusion coefficient; AUC, area under the ROC curve; bpMRI, bi-parametric MRI; bpMRI–US FTSB, bi-parametric MRI-US fusion (transperineal) targeted and systematic biopsy; csPCa, clinically significant prostate cancer; DCE, dynamic contrast enhanced; DWI, diffusion weighted images; FTSB, (transperineal) fusion targeted and systematic biopsy; GA, general anesthesia; MRI, magnetic resonance imaging; mpMRI, multi-parametric MRI; US, ultrasound; PCa, prostate cancer; PI-RADS, prostate Imaging Reporting and Data Systems; ROC, receiver-operating characteristic; ROI, regions of interest; SB, (template) systematic biopsy; TRUS, transrectal ultrasound; TRUSB, transrectal ultrasound guided systematic biopsy; T2WI, T2-weighted images; TB, targeted biopsy; US, ultrasound.
1. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol (2017) 71:618. doi: 10.1016/j.eururo.2016.08.003
2. Das CJ, Razik A, Sharma S, Verma S. Prostate biopsy: when and how to perform. Clin Radiol (2019) 74:853. doi: 10.1016/j.crad.2019.03.016
3. Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol (2012) 61:1019. doi: 10.1016/j.eururo.2012.01.050
4. Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, et al. PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol (2016) 69:16. doi: 10.1016/j.eururo.2015.08.052
5. Drost FH, Osses DF, Nieboer D, Steyerberg EW, Bangma CH, Roobol MJ, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev (2019) 4:Cd012663. doi: 10.1002/14651858.CD012663.pub2
6. Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet (2017) 389:815. doi: 10.1016/S0140-6736(16)32401-1
7. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med (2018) 378:1767. doi: 10.1056/NEJMoa1801993
8. Siddiqui MM, Rais-Bahrami S, Turkbey B, George AK, Rothwax J, Shakir N, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA (2015) 313:390. doi: 10.1001/jama.2014.17942
9. Le JD, Tan N, Shkolyar E, Lu DY, Kwan L, Marks LS, et al. Multifocality and prostate cancer detection by multiparametric magnetic resonance imaging: correlation with whole-mount histopathology. Eur Urol (2015) 67:569. doi: 10.1016/j.eururo.2014.08.079
10. Ahdoot M, Wilbur AR, Reese SE, Lebastchi AH, Mehralivand S, Gomella PT, et al. MRI-Targeted, systematic, and combined biopsy for prostate cancer diagnosis. N Engl J Med (2020) 382:917. doi: 10.1056/NEJMoa1910038
11. Mottet N, van den Bergh RCN, Briers E, Van den Broeck T, Cumberbatch MG, De Santis M, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer-2020 update. part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol (2021) 79:243. doi: 10.1016/j.eururo.2020.09.042
12. Tafuri A, Iwata A, Shakir A, Iwata T, Gupta C, Sali A, et al. Systematic biopsy of the prostate can be omitted in men with PI-RADS™ 5 and prostate specific antigen density greater than 15. J Urol (2021) 206:289. doi: 10.1097/JU.0000000000001766
13. Weiner AB, Manjunath A, Kirsh GM, Scott JA, Concepcion RD, Verniero J, et al. The cost of prostate biopsies and their complications: a summary of data on all medicare fee-for-service patients over 2 years. Urol Pract (2020) 7:145. doi: 10.1097/UPJ.0000000000000072
14. Rouvière O, Puech P, Renard-Penna R, Claudon M, Roy C, Mège-Lechevallier F, et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol (2019) 20:100. doi: 10.1016/S1470-2045(18)30569-2
15. Filson CP, Natarajan S, Margolis DJ, Huang J, Lieu P, Dorey FJ, et al. Prostate cancer detection with magnetic resonance-ultrasound fusion biopsy: The role of systematic and targeted biopsies. Cancer (2016) 122:884. doi: 10.1002/cncr.29874
16. Noh TI, Tae JH, Kim HK, Shim JS, Kang SG, Sung DJ, et al. Diagnostic accuracy and value of magnetic resonance imaging-ultrasound fusion transperineal targeted and template systematic prostate biopsy based on bi-parametric magnetic resonance imaging. Cancer Res Treat (2020) 52:714. doi: 10.4143/crt.2019.716
17. Oberlin DT, Casalino DD, Miller FH, Meeks JJ. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom Radiol (NY) (2017) 42:1255. doi: 10.1007/s00261-016-0975-5
18. van der Leest M, Cornel E, Israël B, Hendriks R, Padhani AR, Hoogenboom M, et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: A Large prospective multicenter clinical study. Eur Urol (2019) 75:570. doi: 10.1016/j.eururo.2018.11.023
19. Klotz L, Chin J, Black PC, Finelli A, Anidjar M, Bladou F, et al. Comparison of multiparametric magnetic resonance imaging-targeted biopsy with systematic transrectal ultrasonography biopsy for biopsy-naive men at risk for prostate cancer: A phase 3 randomized clinical trial. JAMA Oncol (2021) 7:534. doi: 10.1001/jamaoncol.2020.7589
20. Ghani KR, Dundas D, Patel U. Bleeding after transrectal ultrasonography-guided prostate biopsy: a study of 7-day morbidity after a six-, eight-and 12-core biopsy protocol. BJU Int (2004) 94:1014. doi: 10.1111/j.1464-410X.2004.05096.x
21. Massanova M, Vere R, Robertson S, Crocetto F, Barone B, Dutto L, et al. Clinical and prostate multiparametric magnetic resonance imaging findings as predictors of general and clinically significant prostate cancer risk: A retrospective single-center study. Curr Urol (2023). doi: 10.1097/cu9.0000000000000173
22. Alberts AR, Roobol MJ, Verbeek JFM, Schoots IG, Chiu PK, Osses DF, et al. Prediction of high-grade prostate cancer following multiparametric magnetic resonance imaging: Improving the Rotterdam European randomized study of screening for prostate cancer risk calculators. Eur Urol (2019) 75:310–8. doi: 10.1016/j.eururo.2018.07.031
23. Rapisarda S, Bada M, Crocetto F, Barone B, Arcaniolo D, Polara A, et al. The role of multiparametric resonance and biopsy in prostate cancer detection: comparison with definitive histological report after laparoscopic/robotic radical prostatectomy. Abdom Radiol (NY) (2020) 45:4178–84. doi: 10.1007/s00261-020-02798-8
24. Drobish JN, Bevill MD, Tracy CR, Sexton SM, Rajput M, Metz CM, et al. Do patients with a PI-RADS 5 lesion identified on magnetic resonance imaging require systematic biopsy in addition to targeted biopsy? Urol Oncol (2021) 39:235 e1. doi: 10.1016/j.urolonc.2020.12.015
25. Kilic M, Vural M, Coskun B, Acar Ö, Saglican Y, Akpek, et al. Accuracy of sampling PI-RADS 4-5 index lesions alone by MRI-guided in-bore biopsy in biopsy-naive patients undergoing radical prostatectomy. Eur Urol Focus (2020) 6:249. doi: 10.1016/j.euf.2019.04.010
26. Bass EJ, Pantovic A, Connor M, Gabe R, Padhani AR, Rockall A, et al. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis (2021) 24:596. doi: 10.1038/s41391-020-00298-w
27. Alabousi M, Salameh JP, Gusenbauer K, Samoilov L, Jafri A, Yu H, et al. Biparametric vs multiparametric prostate magnetic resonance imaging for the detection of prostate cancer in treatment-naïve patients: a diagnostic test accuracy systematic review and meta-analysis. BJU Int (2019) 124:209. doi: 10.1111/bju.14759
28. Noh TI, Shim JS, Kang SG, Cheon J, Lee JG, Lee JH, et al. Concordance between biparametric MRI, transperineal targeted plus systematic MRI-ultrasound fusion prostate biopsy, and radical prostatectomy pathology. Sci Rep (2022) 12:6964. doi: 10.1038/s41598-022-10672-4
29. Arabi A, Deebajah M, Yaguchi G, Pantelic M, Williamson S, Gupta N, et al. Systematic biopsy does not contribute to disease upgrading in patients undergoing targeted biopsy for PI-RADS 5 lesions identified on magnetic resonance imaging in the course of active surveillance for prostate cancer. Urology (2019) 134:168. doi: 10.1016/j.urology.2019.08.035
Keywords: magnetic resonance imaging, transperineal biopsy, prostate cancer, PI-RADS, target biopsy
Citation: Noh TI, Shim JS, Kang SH, Cheon J and Kang SG (2023) Diagnostic performance of transperineal prostate targeted biopsy alone according to the PI-RADS score based on bi-parametric magnetic resonance imaging. Front. Oncol. 13:1142022. doi: 10.3389/fonc.2023.1142022
Received: 11 January 2023; Accepted: 10 March 2023;
Published: 23 March 2023.
Edited by:
Ran Xu, Second Xiangya Hospital, Central South University, ChinaReviewed by:
Biagio Barone, University of Naples Federico II, ItalyCopyright © 2023 Noh, Shim, Kang, Cheon and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Gu Kang, a2thbmdzdW5nN0Brb3JlYS5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.