
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 03 May 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1141833
This article is part of the Research Topic Advances in Molecular Biology Knowledge of Rectal Cancer and Forthcoming Role of Liquid Biopsy View all 10 articles
 Alexander Baraniskin1,2*
Alexander Baraniskin1,2* Hideo A. Baba3
Hideo A. Baba3 Dirk Theegarten3
Dirk Theegarten3 Thomas Mika2
Thomas Mika2 Roland Schroers2
Roland Schroers2 Susanne Klein-Scory2
Susanne Klein-Scory2In the context of colorectal cancer (CRC), circulating tumor DNA (ctDNA) is frequently used to monitor the minimal residual disease (MRD). ctDNA has become an excellent biomarker to predict which patients with CRC are likely to relapse due to the persistence of micrometastases. MRD diagnosis via analysis of ctDNA may allow much earlier detection of relapse compared with conventional diagnosis during follow-up. It should lead to an increased rate of curative-intended complete resection of an asymptomatic relapse. Besides, ctDNA can provide crucial information on whether and how intensively adjuvant or additive therapy should be administered. In the present case, analysis of ctDNA gave us a crucial hint to the use of more intensive diagnostics (MRI and Positron emission tomography–computed tomography PET-CT) which led to earlier detection of CRC relapse. Metastasis detected early are more likely to be completely resectable with curative intent.
Despite advances in diagnostic imaging, surgery, and chemotherapy, the 5-year mortality rate of colorectal cancer (CRC) remains high with nearly 40% (1). The key for successful CRC treatment is early detection, as the 5-year survival rate at stages I and II is above 80%, but after the development of distant metastases, it decreases to approximately 10% (2). The primary goal of curative CRC therapy is complete resection of the tumor tissues combined with adjuvant chemotherapy in advanced situations. Identifying patients with minimal residual disease (MRD), i.e. clinically hidden micrometastases remaining after initial therapy, and treating with additional or intensified therapy could potentially increase the rate of cured patients.
One limiting factor in MRD diagnostics of CRC is the low sensitivity of standard diagnostical tools including imaging (e.g., Magnetic Resonance Imaging (MRI), Computed Tomography (CT), or Positron emission tomography–computed tomography (PET/CT) and serum tumor markers, e.g. carcinoembryonic antigen (CEA) or cancer antigen 19-9 (CA 19-9) (3). Serial CEA analyses may recognize relapse with a sensitivity of 69% and specificity of 64% (4). Furthermore, detection of ctDNA after precise and complete surgical removal of the tumor can be used to manage the chemotherapy options for patients with CRC stage II and III (5, 6). Only patients with MRD should receive chemotherapy, while 13% of patients could be spared the unnecessary chemotherapy (6, 7). The reduction of the number of chemotherapies results in the same 83% rate of cure as the standard treatment management.
Stage IV CRC is associated with higher likelihood of relapse and poorer survival outcomes. Oligometastatic CRC is eligible for surgery with curative intent, but 60%–70% of patients will go on to relapse postresection (8).
Thus, there is an unmet need for the development of better tools to facilitate physician’s decision-making in identifying and stratifying resected patients by risk of relapse with still curative intent. Strikingly, patients with asymptomatic recurrences reveal a more than 5-fold higher 5-year overall survival compared to symptomatic recurrences (9). It is remarkable that nowadays, over 60% are still diagnosed with recurrence secondary to symptoms. Thus, early detection of higher risk of metastasis after tumor resection is crucial for improving clinical outcomes of patients.
In the past few years, blood-based liquid biopsies - especially the analysis of cell-free tumor DNA (ctDNA) - have received widespread attention due to increased sensitivity of modern polymerase chain reaction (PCR)-based technologies. The detection of plasma ctDNA is prognostic in CRC and has the potential to serve as a highly specific and low-invasive test for early prediction of disease recurrence in clinical routine may enable a locoregional approach (5, 6, 10).
In the here reported CRC case, we present the significance of liquid biopsy for MRD diagnostics in advanced CRC.
A 47-year-old man without any significant pre-existing conditions was diagnosed in September 2019 with a rectum adenocarcinoma (upper rectal) with synchronous liver metastases (limited to right liver lobe) (see the magnet resonance imaging (MRI in Figure 1). The molecular diagnostics of tumor tissue revealed mutations in neuroblastoma RAS viral oncogene homolog (NRAS2 G12D c.35G>A) and in tumor protein p53 (TP53 C141Y c.422G>A). A neoadjuvant therapy with four cycles of FOLFOX4 (Folic acid, fluorouracil, and oxiliplatin 24h) and bevacizumab was administered and was well tolerated. In December 2019, the patient underwent exploratory laparoscopy and right hemihepatectomy. Five metastases were removed. By the tumor regression grading (TRG) of 2, the histological response to chemotherapy of hepatic colorectal metastases (HCRM) was grade 4 with 90% of necrosis of surface.
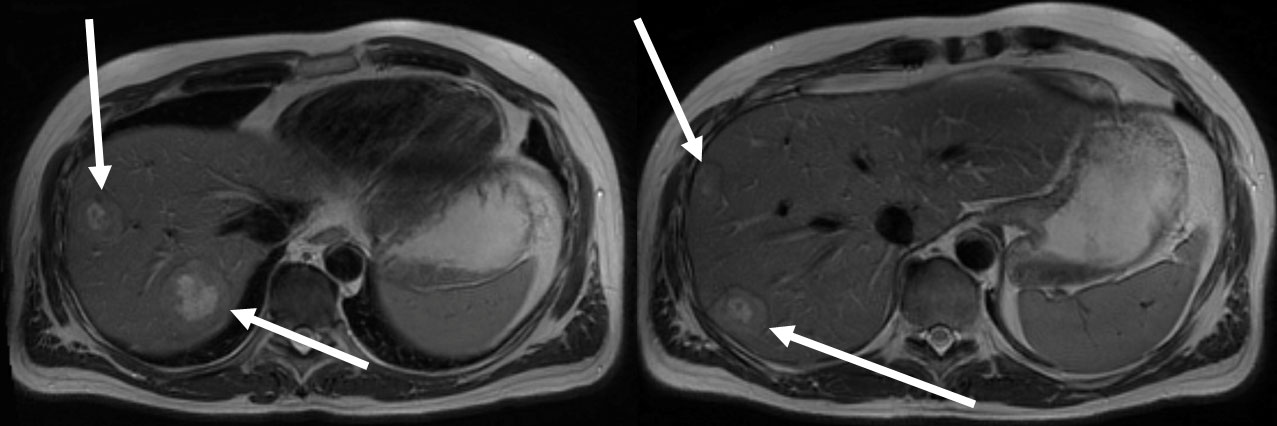
Figure 1 Multiple liver metastases limited to right liver lobe in MRI at diagnosis in September 2019 (white arrows).
One month later, laparoscopically assisted anterior rectum resection, sigmoid resection and descendorectostomy followed. The TNM stage was ypT2 ypN0(0/17) ypM1 (hep). Thereupon, additive therapy with eight cycles of FOLFOX4 was given and was well tolerated again.
Starting in September 2020, liquid biopsies to detect the known NRAS mutation were carried every six months. In July 2021, NRAS mutations were not yet detectable.
A routine examination for relapse in November 2021 included computed tomography of chest and abdomen, MRI of the liver, sigmoidoscopy and analyses of tumor markers CEA and CA19-9. The MRI showed an annular contrast medium enhancement at the resection margin in liver segment IV. To further classification of the contrast medium enhancement, subsequently conducted 2-Deoxy-2-[18F]fluoro-d-glucose emission tomography (FDG-PET-CT) showed diffusion restriction at the resection margin with albeit minimal FDG activity (white arrow; Standardized uptake value (SUV) max 4.3), which fell within the range of the liver parenchyma (SUV 1.9-4.8) (See PET-CT scan in Figure 2A). Overall, the examinations were assessed as not malignancy-suspect.
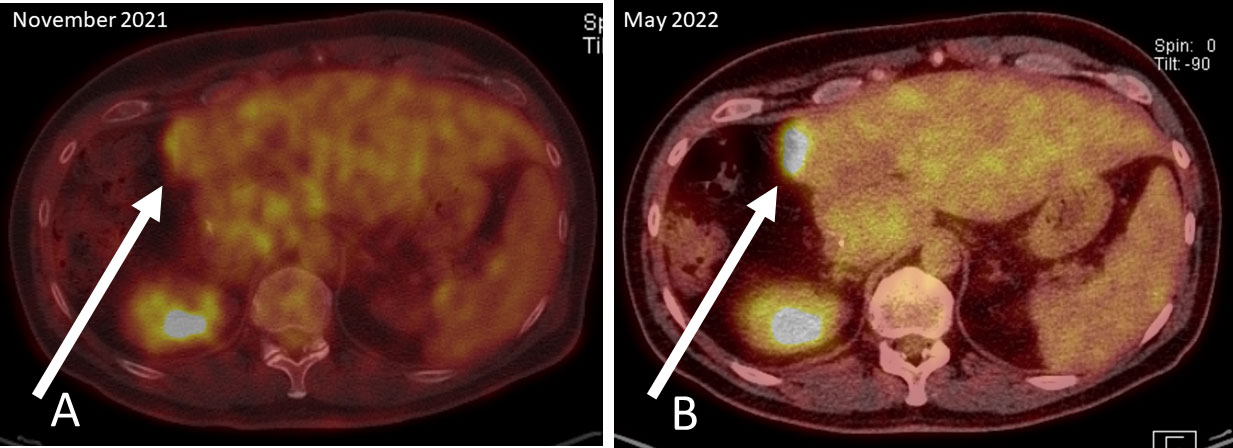
Figure 2 PET-CT scans: (A) November 2021; diffusion restriction at the resection margin with albeit minimal FDG activity (white arrow), (B) May 2022; clear increase of FDG activity in the resection margin in liver segment IV.
However, five weeks later (January 2022), both, the original NRAS and TP53 mutations were detectable with mutant allele fractions (MAF) of 0.37% and 0.9% respectively (Figure 3).
An intensified search for a relapse was undertaken since January 2022. CT scans, MRI of the liver and the tumor markers showed unchanged findings and an assessment that no metastasis exist. The next liquid biopsy analysis in March 2022 and April 2022 continued to show increasing MAF for both mutations. Based on liquid biopsy result, a premature PET-CT was performed in May 2022. This PET-CT revealed a clear increase of FDG activity in the resection margin in liver segment IV (See PET-CT scan in Figure 2B). The curative-intent complete resection of asymptomatic relapse followed. The tissue analysis of resected material confirmed the NRAS and TP53 mutations detected in liquid biopsy and measured the NRAS2 mutation with an allele frequency of 47.4% and the TP53 mutation with 68.4%. The metastasis tissue was graded as G2 with 50% necrotic parts. No hint for microsatellite instability was found. The postoperative liquid biopsy samples no longer contained ctDNA with NRAS or TP53 mutations. The tumor marker carcinoembryonic antigene CEA was not substantially elevated until March 2022 and decreased to normal after surgery in July 2022 (Figure 3). We have no signs of a recurrence so far (till February 2023). The patient is symptom-free, works full-time and leads a normal family life.
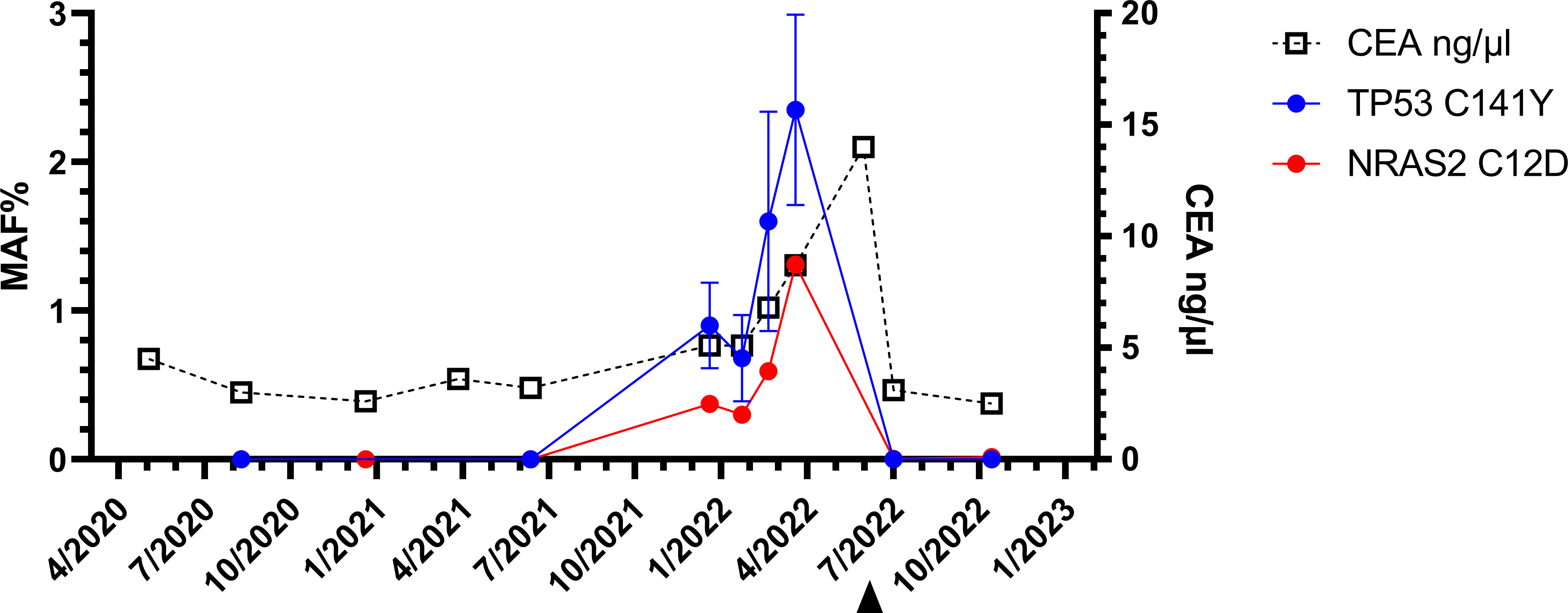
Figure 3 Longitudinal liquid biopsy results: The first three liquid biopsy analyses until the 15th month of the follow up were negative. The fourth sample revealed NRAS (red)- and TP53-(circles blue) mutations. Only after the resection (arrowhead), the ctDNA was no longer detectable. Three months after the reappearance of the NRAS2 C12D mutations, the CEA level also increased substantially.
We report the clinical course of a patient in follow-up after resection of rectum carcinoma and hepatic metastases. Longitudinal liquid biopsies revealed MRD with detection of NRAS and TP53 mutations 4 month earlier as detected by PET-CT. Only by intensifying the search for disease recurrence on the basis of ctDNA detection, the location of CRC relapse could be unraveled.
MRD-positivity in liquid biopsy displays without ifs and buts an overwhelming suspicion of a persistent disease. Several studies have demonstrated that patients with detectable ctDNA postsurgery will finally develop a relapse (4, 5, 11). In patients diagnosed with resected stage I-III CRC, MRD-positivity at the end-of-treatment (surgery with/without adjuvant chemotherapy) was accompanied by an over 40 times higher probability of disease recurrence as compared to MRD-negative patients (4, 11). In a study by Tie et al., patients who underwent curative intended resection of colorectal cancer liver metastases were further analyzed. MRD-positivity at the end-of-treatment was associated with a 5-year RFS (recurrence free survival) of only 0% and a 14.9 times higher recurrence probability. In line with our clinical report, longitudinal ctDNA analyses identified disease recurrence up to 16.5 months and in mean 8.7 months earlier than standard radiologic imaging (4).
In our clinical report, the knowledge of the MRD-positivity influenced the intensity and manner of follow up. Based on this finding, the decision to carry out the PET-CT examination was made. This approach may have enabled the curative-intent complete resection of the relapse.
Molecular analysis of the tissue samples was routinely performed by next generation sequencing (Institute of Pathology, University Hospital Essen, Germany). Two mutations were found at a 60% cellularity with a proportion of 68.4% MAF of TP53 C141Y c.422G>A mutation and 47.4% MAF% of NRAS2 G12D c.35G>A mutation.
Blood samples were obtained in cell free DNA collection tubes (Cell-Free DNA BCT; Streck™) and sent to our lab (Ruhr University Bochum, Knappschaftskrankenhaus). Plasma was isolated latest three days after blood collection.
The ctDNA was isolated from 3 ml plasma using the circAMP circulating nucleic acid isolation procedure (Qiagen™, Hilden, Germany) as described earlier (12). NRAS2-12 mutation detections were performed using the IVD certified all RAS mutation kit with ONCOBEAM technology (Sysmex Inostics, Hamburg, Germany).
The assay to detect the mutation TP53 C141Y c.422G>A p.C141Y, NM_002524.4 COSM43708 was produced by Bio-RAD (Hercules, California, USA). The context sequence was given in supplemented file (Supplementary material). The ddPCR was performed as described in (12).
To validate the ddPCR-based assay to detect the TP53 C141C mutation, genomic DNA from the patient´s liver metastasis was used (Supplementary material).
The tumor marker carcinoembryonic antigene was measured by routine procedure in clinical labs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
AB and SK-S contributed equally to the writing of the manuscript and designed the figures. AB was involved in the treatment of the patient. AB, DT, HB, RS, SK-S, and TM reviewed and approved the final version of this work. All authors contributed to the article.
The work was supported by financial funding from the Ruhr University Bochum, FoRUM 19-2 6805. The ONCOBEAM measurements were funded by Sysmex Inostics GmbH, Deutschland.
We thank the patient for the consent to publish the information about the illness. We would like to thank Bachmann Sabine, Eilert-Micus Christina, Matthias Becker and Ben Khalifa Urszula for excellent technical assistance in liquid biopsy analyses.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1141833/full#supplementary-material
1. Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis Colon Rectum (2018) 61:1016–25. doi: 10.1097/DCR.0000000000001158
2. McQuade RM, Stojanovska V, Bornstein JC, Nurgali K. Colorectal cancer chemotherapy: the evolution of treatment and new approaches. Curr Med Chem (2017) 24:1537–57. doi: 10.2174/0929867324666170111152436
3. Su B-B, Shi H, Wan J. Role of serum carcinoembryonic antigen in the detection of colorectal cancer before and after surgical resection. World J Gastroenterol (2012) 18(17):2121–6. doi: 10.3748/wjg.v18.i17.2121
4. Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol (2019) 5:1124–31. doi: 10.1001/jamaoncol.2019.0528
5. Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med (2016) 8(346):346ra92. doi: 10.1126/scitranslmed.aaf6219
6. Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol (2019) 5:1710–7. doi: 10.1001/jamaoncol.2019.3616
7. Tie J, Cohen JD, Lahouel K, Lo SN, Wang Y, Kosmider S, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med (2022) 386(24):2261–72. doi: 10.1056/NEJMoa2200075
8. Matsuoka T, Yashiro M. Precision medicine for gastrointestinal cancer: recent progress and future perspective. World J Gastrointest Oncol (2020) 12:1–20. doi: 10.4251/wjgo.v12.i1.1
9. Duineveld LA, van Asselt KM, Bemelman WA, Smits AB, Tanis PJ, van Weert HC, et al. Symptomatic and asymptomatic colon cancer recurrence: a multicenter cohort study. Ann Fam Med (2016) 14(3):215–20. doi: 10.1370/afm.1919
10. Wang Y, Li L, Cohen JD, Kinde I, Ptak J, Popoli M, et al. Prognostic potential of circulating tumor DNA measurement in postoperative surveillance of nonmetastatic colorectal cancer. JAMA Oncol (2019) 5(8):1118–23. doi: 10.1001/jamaoncol.2019.0512
11. Tarazona N, Henriksen TV, Carbonell-Asins JA, Reinert T, Sharma S, Roda D, et al. Circulating tumor DNA to detect minimal residual disease, response to adjuvant therapy, and identify patients at high risk of recurrence in patients with stage I-III CRC. JCO (2020) 38:4009. doi: 10.1200/JCO.2020.38.15_suppl.4009
Keywords: liquid biopsy, ddPCR, colorectal cancer, follow-up, Ras, tumor promotor P53 TP53, mutation detection, PET-CT
Citation: Baraniskin A, Baba HA, Theegarten D, Mika T, Schroers R and Klein-Scory S (2023) Liquid biopsy can cure early colorectal cancer recurrence – Case Report. Front. Oncol. 13:1141833. doi: 10.3389/fonc.2023.1141833
Received: 10 January 2023; Accepted: 18 April 2023;
Published: 03 May 2023.
Edited by:
Alessandro Passardi, (IRCCS), ItalyReviewed by:
Manuela Gariboldi, Fondazione IRCCS Istituto Nazionale Tumori, ItalyCopyright © 2023 Baraniskin, Baba, Theegarten, Mika, Schroers and Klein-Scory. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Baraniskin, QWxleGFuZGVyLkJhcmFuaXNraW5AdmFsZW8ta2xpbmlrZW4uZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.