- 1Department of Nuclear Medicine, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 2Nuclear Medicine and Molecular Imaging Key Laboratory of Sichuan Province, Luzhou, Sichuan, China
- 3Department of Ophthalmology, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, China
- 4Academician (expert) Workstation of Sichuan Province, Luzhou, Sichuan, China
Objective: Peptide receptor radionuclide therapy (PRRT) for advanced pheochromocytomas and paragangliomas (PPGLs) has received increasing attention. The purpose of this article is to evaluate the efficacy and safety of PRRT in patients with metastatic or inoperable PPGLs by meta-analysis.
Methods: A literature search was conducted in PubMed, Embase, Scopus, and Cochrane Library databases up to November 2022. All articles on PRRT for PPGLs were searched, and appropriate data were included for analysis. The measures evaluated included objective response rate (ORR), disease control rate (DCR), clinical response rate, biochemical response rate, progression-free survival (PFS), overall survival (OS), and adverse events. Statistical analysis was performed using Stata 16.0 and the R programming language, data were combined using a random-effects model, and the results were presented using forest plots.
Results: A total of 20 studies with 330 patients were included in the analysis. The results showed that ORR and DCR were 20.0% (95% CI: 12.0%-28.0%) and 90.0% (95% CI: 85.0%-95.0%), respectively. Clinical and biochemical responses were 74.9% (95% CI: 56.3%-90.2%) and 69.5% (95%CI: 40.2%-92.9%). Median PFS and median OS were 31.79 (95% CI:21.25-42.33) months and 74.30 (95% CI: 0.75-147.84) months, respectively. Any grade of hematotoxicity and nephrotoxicity occurred in 22.3% (95% CI:12.5%-33.5%) and 4.3% (95% CI:0.2%-11.4%) patients. Grade 3-4 hemotoxicity occurred in 4.3% (95% CI:0.2%-11.4%) and grade 3-4 nephrotoxicity in 4/212 patients. Additionally, Treatment was discontinued in 9.0% (95% CI: 0.5%-23.3%) patients and one patient died as a result of a toxicity.
Conclusion: Patients with metastatic or inoperable PPGLs can be effectively treated with PRRT, and it has a favorable safety profile.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO, identifier CRD42022359232.
1 Introduction
Pheochromocytomas (PCCs) originate from the adrenal medulla and paragangliomas (PGLs) originate from the paraganglia of the sympathetic or parasympathetic nerves located outside the adrenal glands, both of which are rare types of neuroendocrine tumors (1, 2). Metastatic pheochromocytomas and paragangliomas (MPPGLs) are defined as tumors found outside the adrenal medulla or paraganglion tissue (3). Approximately 10% of PCCs and 34% of PGLs present with metastases at initial diagnosis, which can occur many years after initial diagnosis (4, 5). PPGLs usually metastasize to lymph nodes, bones, the liver, and the lungs (6–8). The median 5-year overall survival of metastatic disease is only about 60%, and its prognosis is usually dismal (8). Treatment of MPPGLs is challenging, with most MPPGLs progressing slowly and patients with progressive MPPGLs requiring systemic therapy (8, 9). Systemic chemotherapy and 131I-MIBG therapy often have significant hematotoxicity (10, 11). Recent approval of 177Lu-DOTATATE by the US Food and Drug Administration (FDA) for the treatment of well-differentiated inoperable/metastatic gastrointestinal-pancreatic neuroendocrine tumors (GEP-NETs) and clinical trial of metastatic or inoperable PPGLs has increased awareness of Peptide receptor radionuclide therapy (PRRT) for the treatment of PPGLs expressing somatostatin analog receptor (SSTR). SSTR imaging is typically used to select PRRT candidates (12). The most commonly used radionuclides for PRRT are 177Lu and 90Y, which release β rays to cause cell damage and achieve the therapeutic effect. However, a novel therapy option is the use of radionuclides with high linear energy transfer (LET) α emission, like 225Ac and 213Bi, as there are still a lot of patients who do not respond to the available choices. Since 225Ac has a much longer half-life (240 hours) and emits a lot of particles, it is much more cytotoxic than the emitter 213Bi, which has a very short half-life (46 minutes) (13). Patients with NETs who do not respond to 177Lu-DOTATATE or who have completed the recommended number of treatment cycles have been demonstrated to have better outcomes with 225Ac-DOTATATE (14). There have been recent developments in the usage of PRRT for advanced PPGLs, particularly the use of 225Ac in PRRT to offer new choices (15, 16). We, therefore, conducted this meta-analysis to assess the efficacy and safety of PRRT in patients with advanced (metastatic or inoperable) PPGLs.
2 Materials and methods
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (17).
The registration number on the International Prospective Register of systematic reviews (PROSPERO) is: CRD42022359232.
2.1 Literature search strategy
Two authors (DS and HY) independently conducted a systematic literature search in PubMed, Embase, Scopus, and Cochrane Library databases. The time frame of the systematic literature search is from the establishment to November 2022. Search terms included, “Pheochromocytoma”, “Pheochromocytomas”, “Paraganglioma”, “Paragangliomas”, “peptide receptor radionuclide therapy”,”PRRT”,”177Lu”,”Lu-177”, “Lutetium-177”, “177Lutetium”,”90Y”,”Y-90”,”Yttrium-90”,”90Yttrium”,”225Ac”,”Ac-225”,”Actinium-225”,”225Actinium”. All original articles were searched and relevant data were included for analysis. The full text was searched if the article met the study criteria.
2.2 Study selection
We only selected studies that meet the following criteria: Participants (P) were no less than 5 people who had been diagnosed as inoperable or metastatic pheochromocytoma or paraganglioma. Interventions (I) were completed at least one cycle of PRRT with no restrictions on prior treatment; If data came from the same study group, the study with the highest number of patients will be included. The main outcome endpoint (O) was objective response rate (ORR) and disease control rate (DCR) are assessed by response evaluation criteria in solid tumors (RECIST) or Southwest Oncology Group (SWOG) criteria (18, 19). The type of study (S) included in the article was retrospective or prospective research. Exclusion criteria include: non-clinical studies; the number of patients <5; multiple treatments are carried out simultaneously; reviews, meta-analysis, case reports, letters to editors, conference abstracts, articles on biodistribution and dosimetry, articles reporting only adverse events; and articles for which the English text was not available.
2.3 Quality evaluation
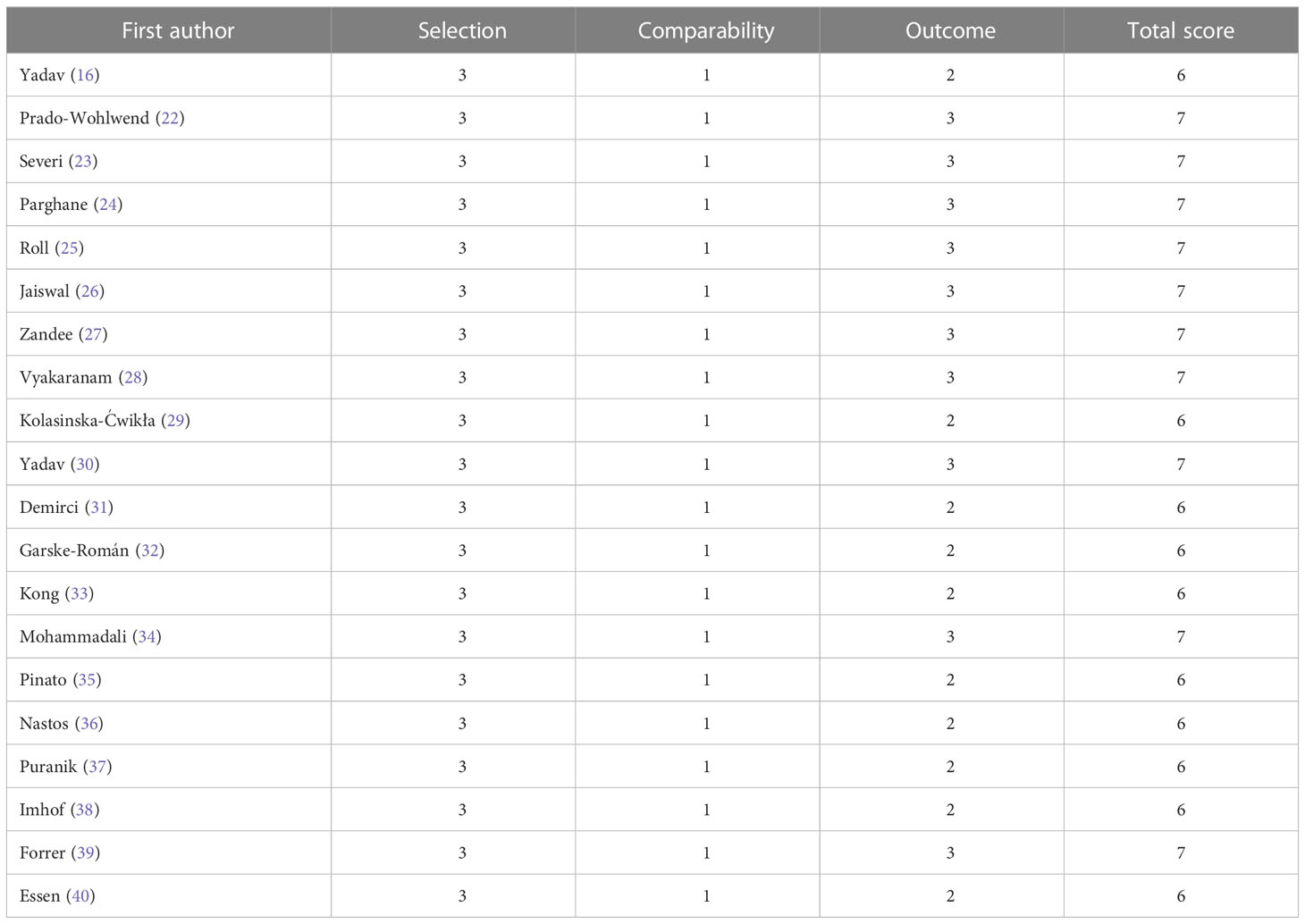
Two authors (DS and HY) separately evaluated the quality of each article included in the analysis, and any disagreements were resolved by consensus. The quality of cohort studies was assessed by the Newcastle-Ottawa Quality Assessment Scale used for cohort studies. The Newcastle-Ottawa Quality Assessment Scale contains three sections with a total of nine assessment elements, each of which can be rated as one star if the study is of high quality for the particular element, for a total score of 9 stars. High-quality studies were those that obtained 6 or more stars (20).
2.4 Data extraction
Two authors (DS and HY) independently reviewed all articles eventually included in the analysis and extracted pertinent data, and any disagreements were resolved by consensus. First author, year of publication, study type, patient demographics, PRRT treatment parameters, including radiopharmaceuticals, radiopharmaceutical activity, number of treatment cycles, duration of follow-up, and adverse events were all included in the basic data that was extracted for all studies. The ORR and DCR were the primary outcome endpoints. Clinical response rate, biochemical response rate, progression-free survival (PFS), overall survival (OS), and adverse events were secondary outcome endpoints.
ORR was defined as the percentage of complete response (CR) and partial response (PR). DCR was the proportion of CR, PR and stable disease (SD). Clinical response rate was the percentage of any improvement in tumor-related symptoms and/or decrease in relevant drug dosage after treatment. The biochemical response rate was the proportion of patients with any decline in tumor markers. PFS was the period, measured in months, between the first PRRT and the commencement of progression or death. OS was the period, measured in months, between the first PRRT and death from any cause. The median, mean, and 95% confidence intervals for PFS and OS were extracted from individual articles. Toxicity was defined according to the Common Terminology Criteria for Adverse Events Version 5.0 (CTCAE V5.0) (21).
2.5 Statistical analysis
Stata16.0 and the R programming language was used for meta-analysis. Because of the clinical heterogeneity in study designs, random-effects models were employed for the computations, and the findings of the meta-analysis were displayed as forest plots. Statistical heterogeneity between studies was assessed using the Cochran Q test and Higgins I2 statistics. Heterogeneity is deemed significant if the P < 0.1 or I2 ≥ 50%. Heterogeneity is regarded as insignificant, if the P ≥ 0.1 and the I2 < 50%.
3 Results
3.1 Literature search and screening
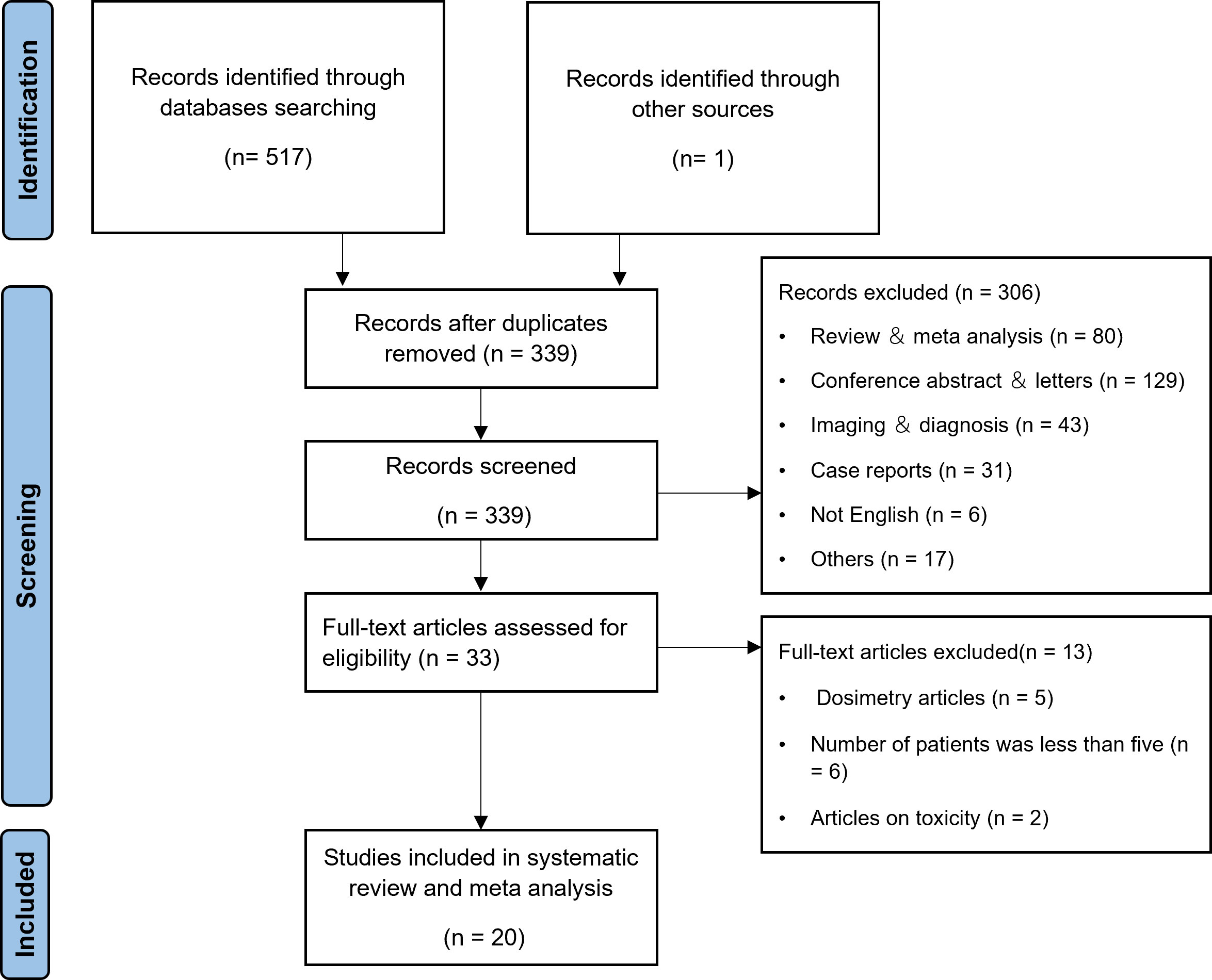
According to the search strategy, a total of 517 articles were retrieved, and 338 articles remained after excluding duplicate articles. 306 articles were excluded by title and abstract. After reading through the full text, 13 articles were excluded by the inclusion and exclusion criteria. One more article was found for inclusion throughout the full-text review process by manually searching all references. In the end, 20 articles (16, 22–40) were included in the meta-analysis, as shown in Figure 1.
3.2 Characteristics and quality of the studies
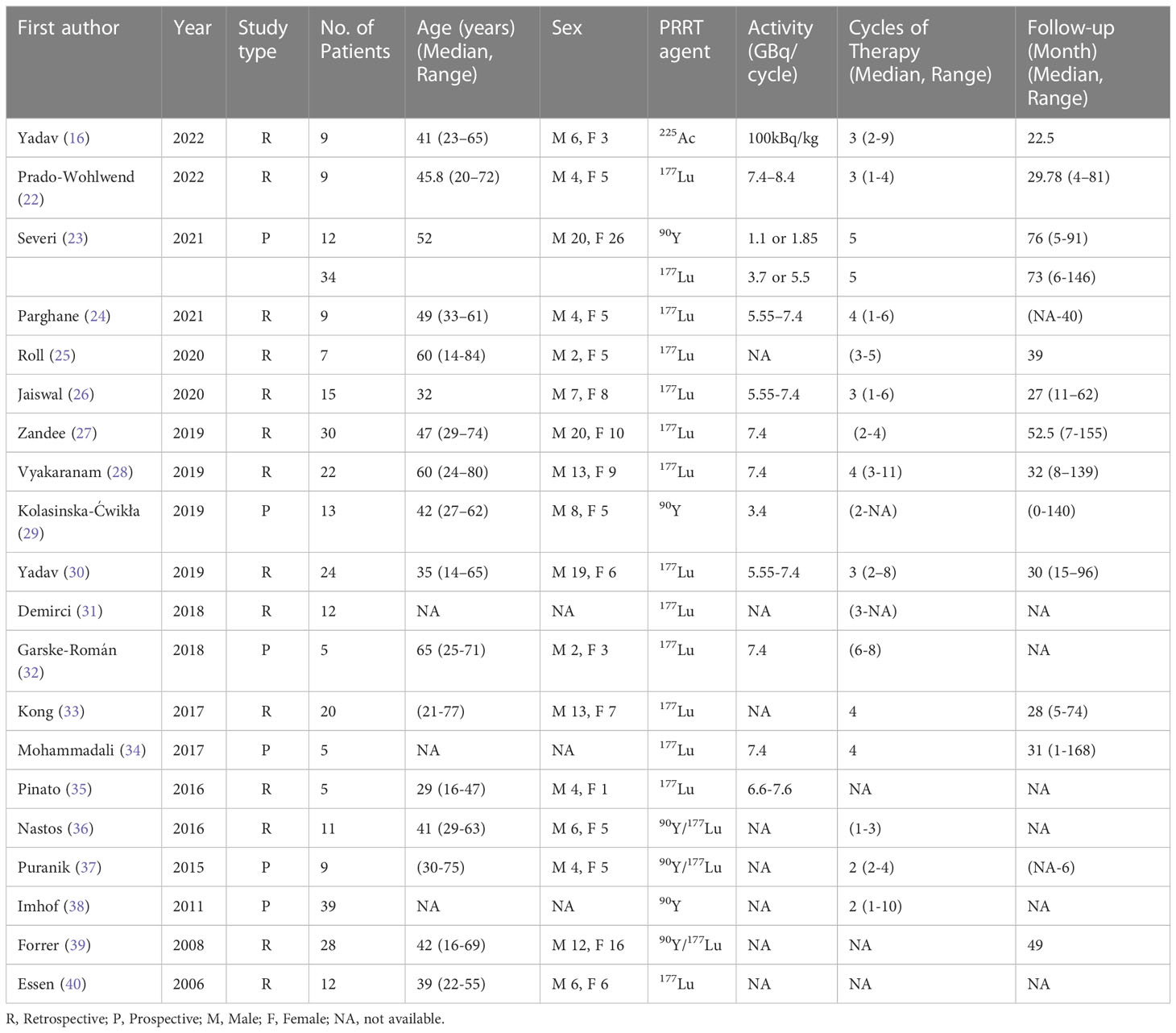
A total of 330 patients were included in the 20 articles that made up the meta-analysis published between 2006 and 2022. The detailed characteristics of the included studies are shown in Table 1. The included studies were all cohort studies and were assessed for quality using the Newcastle-Ottawa Quality Assessment Scale. All the included studies were of good quality. The evaluation of the included studies’ quality is shown in Table 2.
3.3 Pooled analysis of efficacy
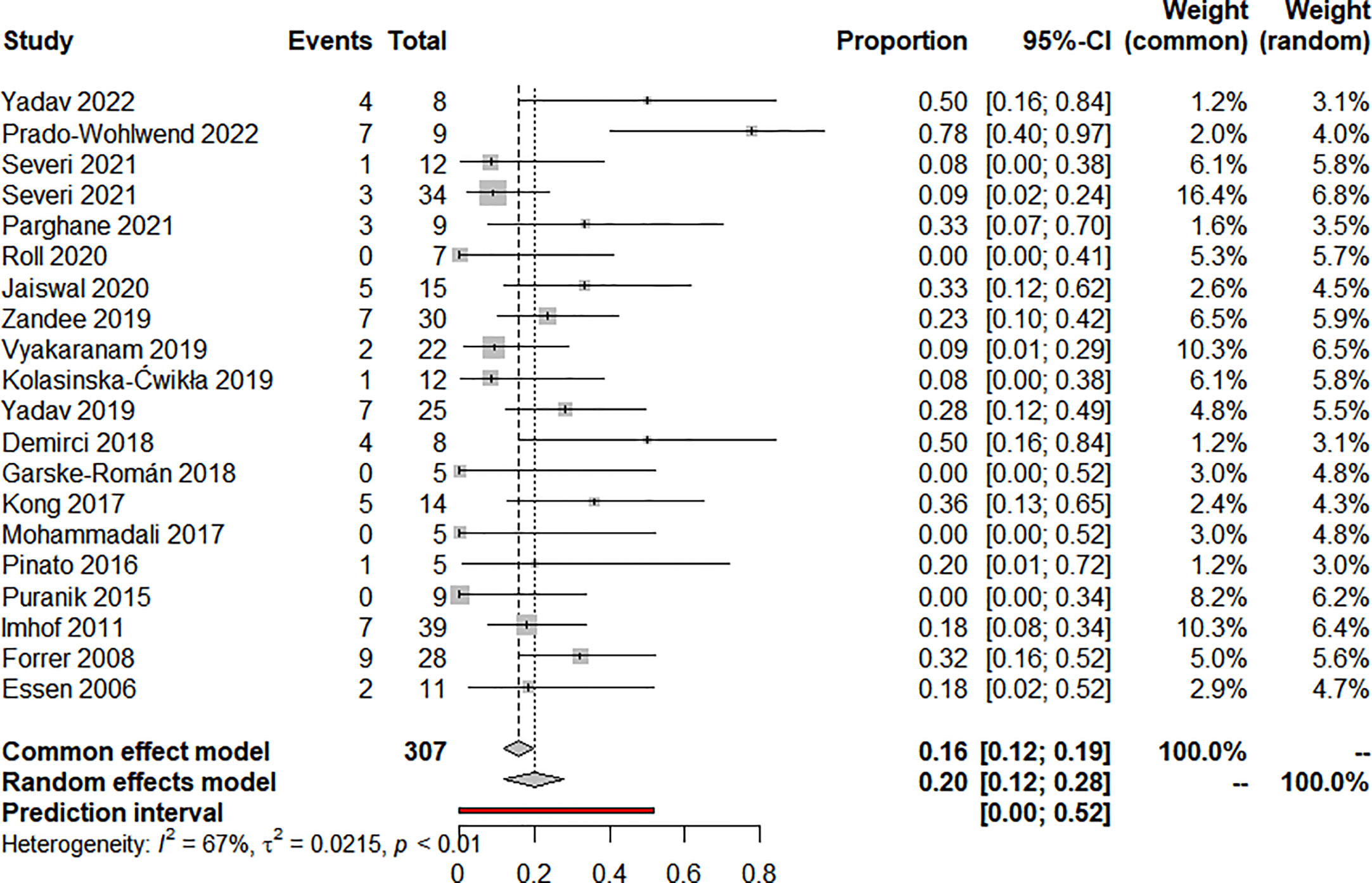
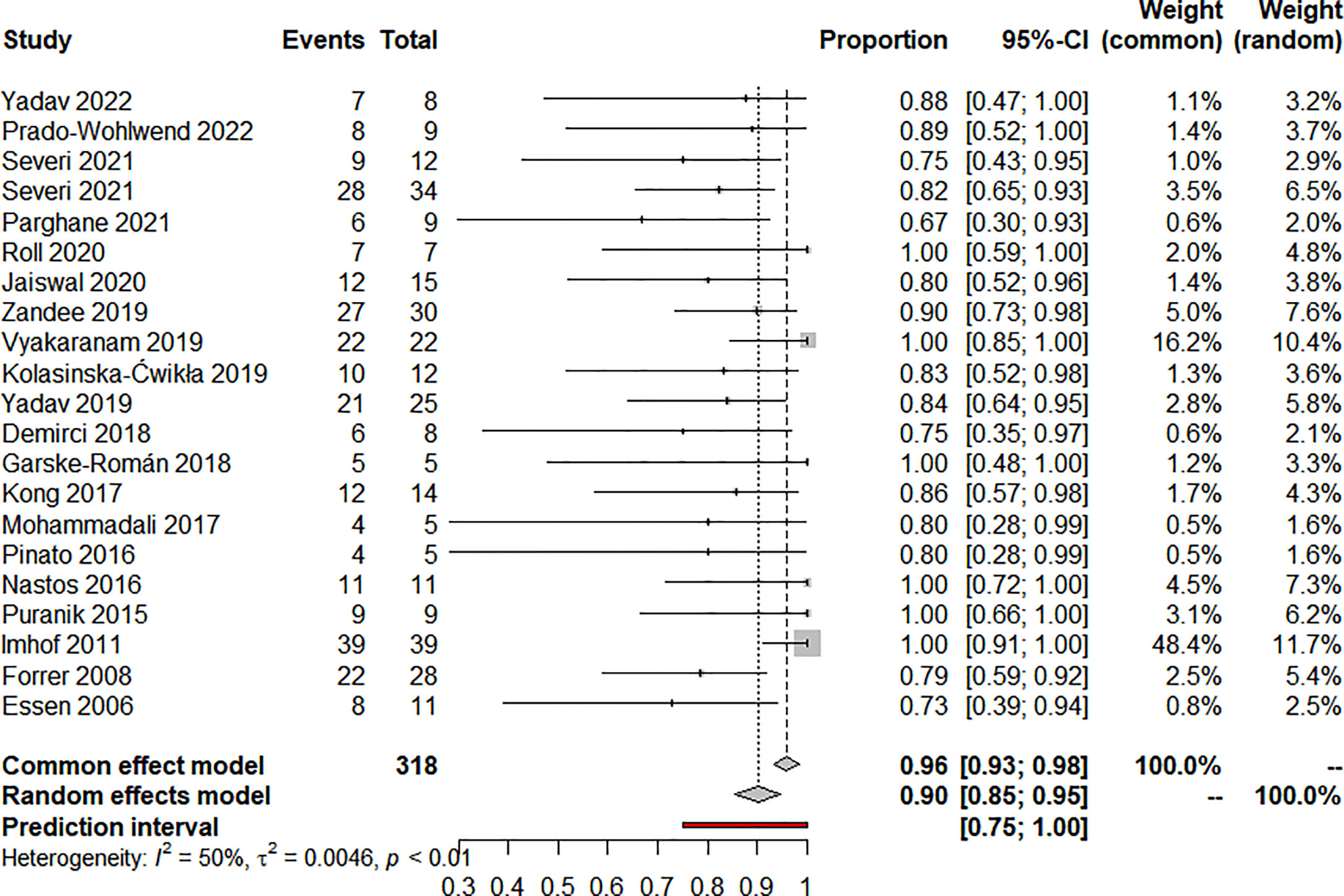
Nineteen articles reported on ORR and 20 articles reported on DCR, eighteen used RECIST, and two used the SWOG criteria. The pooled analysis showed that the pooled ORR and DCR were 20.0% (95% CI: 12.0%-28.0%) (Figure 2) and 90.0% (95% CI: 85.0%-95.0%) (Figure 3), respectively. Clinical and biochemical responses were examined in 11 and 8 articles, respectively, with pooled proportions of 74.9% (95% CI: 56.3%-90.2%) and 69.5% (95%CI: 40.2%-92.9%). Thirteen articles reported median PFS, two of which did not reach median PFS at follow-up, and two articles fully reported the median PFS and 95% confidence interval, with a pooled estimate of 31.79 (95% CI: 21.25-42.33) months. Besides, 11 articles reported median OS, two of which did not reach median OS at follow-up, and four articles fully reported the median OS and 95% confidence interval, with a pooled estimate of 74.30 (95% CI: 0.75-147.84) months. All of the included studies’ efficacy results are shown in Table 3.
3.4 Pooled analysis of toxicity
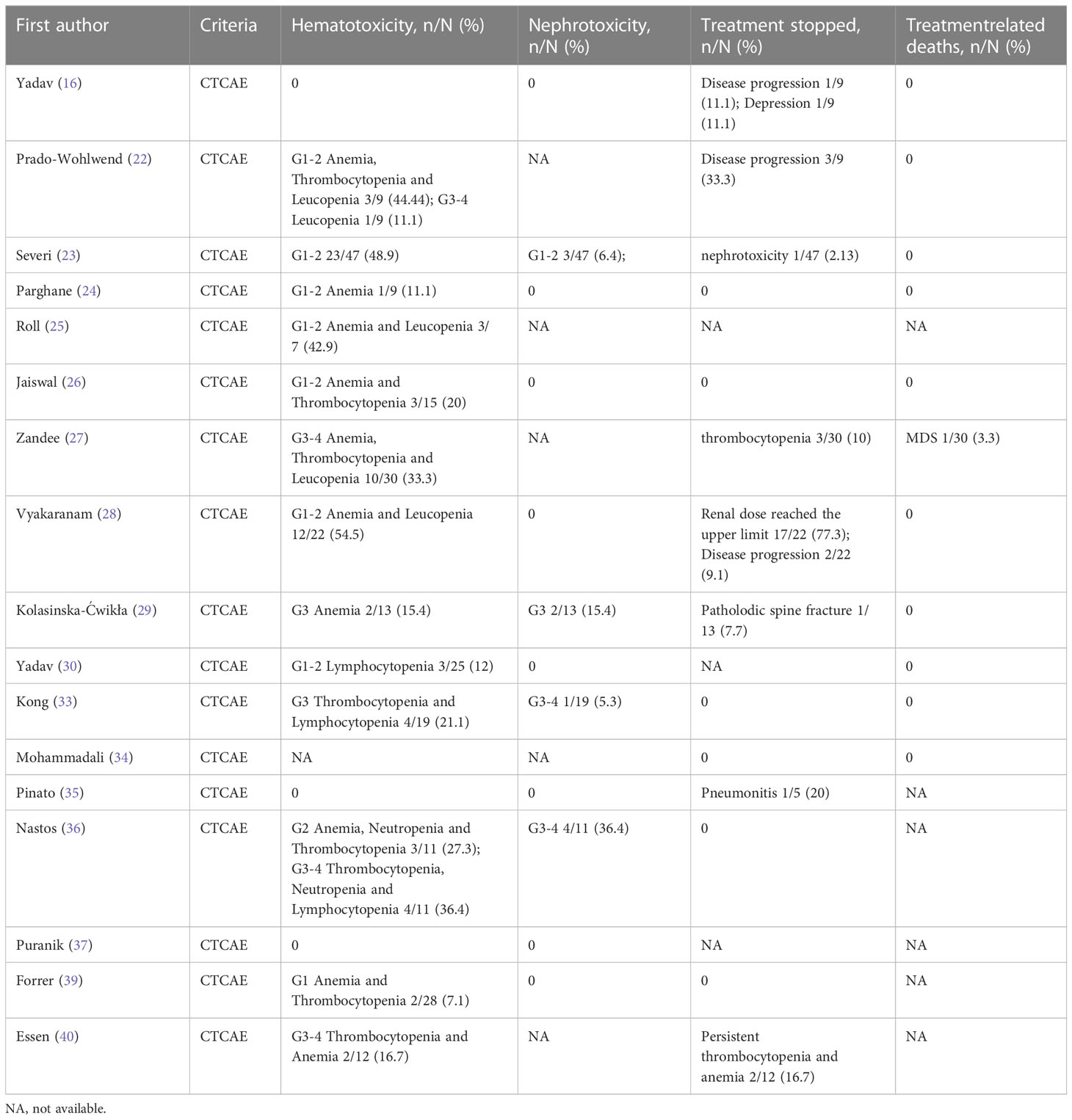
A total of 17 articles reported data on toxicity. Analysis of 270 patients from 16 articles showed that 22.3% (95% CI: 12.5%-33.5%) of patients developed any grade of hematotoxicity and 4.3% (95% CI: 0.2%-11.4%) of patients developed grade 3-4 hematotoxicity. Analysis of 212 patients from 12 articles showed that nephrotoxicity of any grade occurred in 1.9% (95% CI: 0.0%-6.2%) of patients, and grade 3-4 in 4 out of 212 patients. The pooled estimate for treatment discontinuation was 9.0% (95% CI: 0.5%-23.3%), with most patients due to disease progression or upper renal dose limits. Three patients discontinued treatment due to recurrent thrombocytopenia, while one patient discontinued treatment due to nephrotoxicity after completing the third PRRT cycle (cumulative dose was 11.1GBq). Additionally, one patient had myelodysplastic syndrome (MDS) after receiving a cumulative dose of 44.4GBq and passed away 4.5 years later from complications related to MDS. Detailed data on toxicity are shown in Table 4.
3.5 Heterogeneity
There was clinical heterogeneity between included studies in terms of study design and PRRT treatment parameters. Statistical heterogeneity was significant in the combined effects of ORR (I2 = 67%, P<0.01), DCR ((I2 = 50%, P<0.01), clinical response rate (I2 = 79.5%, P<0.01) and biochemical response rate (I2 = 88.3%, P<0.01). Similarly, statistical heterogeneity was significant in the combined effects of median OS (I2 = 99.8%, P<0.01), hematotoxicity at any level (I2 = 71.5%, P<0.01) and treatment discontinuation (I2 = 85.6%, P<0.01). median PFS (I2 = 0.0%, P=0.824) and nephrotoxicity (I2 = 37.2%, P=0.093) were not statistically significantly heterogeneous in the combined effects.
4 Discussion
Currently, PRRT on NETs is being tested in different countries, showing encouraging results. However, as most trials were small samples, single-institutional, and about mixed-type NETs, there are few systematic reviews of PRRT for PPGLs. This meta-analysis evaluated the efficacy and safety of PRRT in patients with advanced PPGLs from studies published to date. Most patients with advanced PPGLs were controlled after PRRT, and the overall DCR was 90.0%, with good clinical and biochemical response rates and prolonged survival. In the studies, most patients received other treatments (surgery, radiotherapy, chemotherapy, 131I-MIBG) before PRRT, and the results showed that PRRT still has some efficacy in patients whose disease progressed after these treatments. Interestingly, in the study of Yadav et al. (16), 225Ac-DOTATATE therapy was a salvage treatment option in patient refractory to 177Lu-PRRT. Their study provides a framework for a new promising aspect of treatment of advanced-stage PGLs. Moreover, the preliminary results of this pilot study provide a blueprint and encourage to conduct future prospective, two-armed randomized control studies on the head-to-head comparison between beta-particle-based 177Lu-DOTATATE and alpha-particle-based 225Ac-DOTATATE treatment in PGLs.
SSTR targeted imaging is the basis of PRRT by documenting adequate SSTR expression, which is the target of therapy. The Krenning score was used to grade the affinity of radiotracer in 111In-Pentetreotide imaging (40) and was later modified for SSTR PET. In principle, most patients with no or low affinity on SSTR PET should not be considered for PRRT. The expression of SSTR in lesions should not be used as an indication for PRRT, whether visually or based on standardized uptake value (SUV), and the clinical context must always be considered when selecting treatment, while taking advantage of the unique advantages of systemic evaluation of SSTR PET.
Large differences in survival in patients with pheochromocytoma and paraganglioma are related to several variables, including genetic status, size of the primary tumor, biochemical phenotype, and presence of metastatic disease at initial diagnosis (41, 42). Because current treatment of metastatic disease is rarely curative, systemic therapy is also an option for inoperable or metastatic disease that is progressive or has refractory symptoms. The most common chemotherapy regimen for systemic treatment is dacarbazine, cyclophosphamide and vinblastine, which may be chosen for rapidly developing diseases (11). Sunitinib is one example of a tyrosine kinase inhibitor that targets the vascular endothelial growth factor pathway (43). External radiation is typically effective for local management of diseases with localized symptoms (44). HSA 131I-MIBG and PRRT are options for less urgent treatments. Currently, the US FDA has only approved HSA 131I-MIBG for the treatment of pheochromocytoma and paraganglioma.
Regarding the adverse events (toxicity) of treatment, the most common adverse effect observed in all studies was hematotoxicity, which was mild in most patients. Grade 3-4 hematotoxicity in most patients was thought to be associated with prior chemotherapy (22, 29, 36), and PRRT was rarely discontinued. Besides, few patients developed nephrotoxicity after treatment. In addition, a hormonal crise caused by radionuclide therapy is a real problem with PPGLs, such as hypotension and myocardial ischemia in one patient with metastatic PCC one day after 177Lu-DOTATATE therapy (45). In the study of Makis et al. (46), two patients were also described with acute catecholamine crises within hours and 3 days after treatment, respectively. Of course, it is necessary to pay close attention to fluctuations in blood pressure control after each treatment. Pulmonary toxicity and severe pain associated with reactive tumor swelling have also been reported (35). Current studies showed that PRRT could be tolerated by most patients, but there was fewer data on long-term adverse events after treatment.
The current empirical treatment regimen for 177Lu-DOTATATE is 4 cycles of 7.4 GBq each, administered every 2 months, to avoid exceeding dose limits in critical organs. There is growing evidence to support patient-based individualized PRRT therapy, which can not only optimize the absorbed dose of tumor, but also limit radiation dose to critical organs. It has been suggested that dosimetry-based optimization methods should be added to the registry in addition to fixed treatment regimens to enable clinical dosimetry (47). Individualized PRRT therapy using renal dosimetry appears to be feasible and safe, and could result in an increased number of treatment cycles for most patients (48). Individualized PRRT based on renal absorbed dose results in an average 1.48-fold increase in cumulative maximum tumor absorbed dose over empirical PRRT, which may lead to increased response to treatment in clinical situations (49). In addition, somatostatin receptor PET imaging can predict tumor absorption dose (50). Pretreatment 68Ga-DOTATATE PET renal uptake can be used to predict 177lu-PRRT SPECT-derived mean renal absorbed dose with an average accuracy of within 18% (51). As the majority of patients treated with PRRT are those with advanced tumors, individualized therapy may prolong survival in relatively healthy conditions.
Our meta-analysis has some limitations as well. The included data had a high inherent bias risk since they lacked outcomes from randomized controlled trials. Clinical heterogeneity was still present despite the random-effects model’s ability to explain statistical heterogeneity. Besides, the majority of articles don’t use consistent standards for evaluating clinical and biochemical responses. Additionally, few studies estimate survival and offer information on long-term adverse effects. Nonetheless, our analysis results show good consistency compared with previous studies, indicating that PRRT has a good therapeutic effect in advanced PPGLs.
5 Conclusion
Peptide receptor radionuclide therapy has achieved a significant role in patients with advanced pheochromocytomas or paragangliomas. To further assess its efficacy and safety, prospective, multi-armed, multi-center randomized controlled studies are required.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
DS and HY wrote this manuscript together. DS, HY and CQ collected relevant information. YC revised the manuscript finally and provided some critical suggestions. All authors listed have read and approved this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, et al. Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab (2014) 99(6):1915–42. doi: 10.1210/jc.2014-1498
2. Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet (2005) 366(9486):665–75. doi: 10.1016/S0140-6736(05)67139-5
3. Lam AK. Update on adrenal tumours in 2017 world health organization (WHO) of endocrine tumours. Endocr Pathol (2017) 28(3):213–27. doi: 10.1007/s12022-017-9484-5
4. Angelousi A, Peppa M, Chrisoulidou A, Alexandraki K, Berthon A, Faucz FR, et al. Malignant Pheochromocytomas/Paragangliomas and ectopic hormonal secretion: a case series and review of the literature. Cancers (Basel) (2019) 11(5):724. doi: 10.3390/cancers11050724
5. Scholz T, Eisenhofer G, Pacak K, Dralle H, Lehnert H. Clinical review: current treatment of malignant pheochromocytoma. J Clin Endocrinol Metab (2007) 92(4):1217–25. doi: 10.1210/jc.2006-1544
6. Eisenhofer G, Bornstein SR, Brouwers FM, Cheung NK, Dahia PL, de Krijger RR, et al. Malignant pheochromocytoma: current status and initiatives for future progress. Endocr Relat Cancer (2004) 11(3):423–36. doi: 10.1677/erc.1.00829
7. Ayala-Ramirez M, Feng L, Johnson MM, Ejaz S, Habra MA, Rich T, et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: primary tumor size and primary tumor location as prognostic indicators. J Clin Endocrinol Metab (2011) 96(3):717–25. doi: 10.1210/jc.2010-1946
8. Jimenez C, Rohren E, Habra MA, Rich T, Jimenez P, Ayala-Ramirez M, et al. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr Oncol Rep (2013) 15(4):356–71. doi: 10.1007/s11912-013-0320-x
9. Hescot S, Leboulleux S, Amar L, Vezzosi D, Borget I, Bournaud-Salinas C, et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J Clin Endocrinol Metab (2013) 98(10):4006–12. doi: 10.1210/jc.2013-1907
10. van Hulsteijn LT, Niemeijer ND, Dekkers OM, Corssmit EP. 131I-MIBG therapy for malignant paraganglioma and phaeochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf) (2014) 80(4):487–501. doi: 10.1111/cen.12341
11. Niemeijer ND, Alblas G, van Hulsteijn LT, Dekkers OM, Corssmit EP. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: systematic review and meta-analysis. Clin Endocrinol (Oxf) (2014) 81(5):642–51. doi: 10.1111/cen.12542
12. Janssen I, Blanchet EM, Adams K, Chen CC, Millo CM, Herscovitch P, et al. Superiority of 68Ga-DOTATATE PET/CT to other functional imaging modalities in the localization of SDHB-associated metastatic pheochromocytoma and paraganglioma. Clin Cancer Res (2015) 21(17):3888–95. doi: 10.1158/1078-0432.CCR-14-2751
13. Song H, Hobbs RF, Vajravelu R, Huso DL, Esaias C, Apostolidis C, et al. Radioimmunotherapy of breast cancer metastases with alpha-particle emitter 225Ac: comparing efficacy with 213Bi and 90Y. Cancer Res (2009) 69(23):8941–8. doi: 10.1158/0008-5472.CAN-09-1828
14. Ballal S, Yadav MP, Bal C, Sahoo RK, Tripathi M. Broadening horizons with 225Ac-DOTATATE targeted alpha therapy for gastroenteropancreatic neuroendocrine tumour patients stable or refractory to 177Lu-DOTATATE PRRT: first clinical experience on the efficacy and safety. Eur J Nucl Med Mol Imaging (2020) 47(4):934–46. doi: 10.1007/s00259-019-04567-2
15. Peng D, Liu H, Huang L, Cao J, Chen Y. 225Ac-DOTATATE therapy in a case of metastatic pheochromocytoma. Eur J Nucl Med Mol Imaging (2022) 49(10):3596–7. doi: 10.1007/s00259-022-05826-5
16. Yadav MP, Ballal S, Sahoo RK, Bal C. Efficacy and safety of 225Ac-DOTATATE targeted alpha therapy in metastatic paragangliomas: a pilot study. Eur J Nucl Med Mol Imaging. (2022) 49(5):1595–606. doi: 10.1007/s00259-021-05632-5
17. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoSMed (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
18. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
19. Green S, Weiss GR. Southwest oncology group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs (1992) 10(4):239–53. doi: 10.1007/BF00944177
20. Wells G, Shea B, O'Connell D. The Newcastle-Ottawa scale (NOS) for assessing the quality of non randomised studies in meta-analyses. Ottawa: The Ottawa Health Research Institute (2011).
21. Freites-Martinez A, Santana N, Arias-Santiago S, Viera A. Using the common terminology criteria for adverse events (CTCAE - version 5.0) to evaluate the severity of adverse events of anticancer therapies Actas Dermosifiliogr (Engl Ed) (2021) 112(1):90–2. doi: 10.1016/j.ad.2019.05.009
22. Prado-Wohlwend S, Del Olmo-García MI, Bello-Arques P, Merino-Torres JF. 177Lu-DOTA-TATE and 131I-MIBG phenotypic imaging-based therapy in Metastatic/Inoperable pheochromocytomas and paragangliomas: comparative results in a single center. Front Endocrinol (Lausanne) (2022) 13:778322. doi: 10.3389/fendo.2022.778322
23. Severi S, Bongiovanni A, Ferrara M, Nicolini S, Di Mauro F, Sansovini M, et al. Peptide receptor radionuclide therapy in patients with metastatic progressive pheochromocytoma and paraganglioma: long-term toxicity, efficacy and prognostic biomarker data of phase II clinical trials. ESMO Open (2021) 6(4):100171. doi: 10.1016/j.esmoop.2021.100171
24. Parghane RV, Talole S, Basu S. 131I-MIBG negative progressive symptomatic metastatic paraganglioma: response and outcome with 177Lu-DOTATATE peptide receptor radionuclide therapy. Ann Nucl Med (2021) 35(1):92–101. doi: 10.1007/s12149-020-01541-z
25. Roll W, Müther M, Sporns PB, Zinnhardt B, Suero Molina E, Seifert R. Somatostatin receptor-targeted radioligand therapy in head and neck paraganglioma. World Neurosurg (2020) 143:e391–9. doi: 10.1016/j.wneu.2020.07.165
26. Jaiswal SK, Sarathi V, Memon SS, Garg R, Malhotra G, Verma P, et al. 177Lu-DOTATATE therapy in metastatic/inoperable pheochromocytoma-paraganglioma. Endocr Connect. (2020) 9(9):864–73. doi: 10.1530/EC-20-0292
27. Zandee WT, Feelders RA, Smit Duijzentkunst DA, Hofland J, Metselaar RM, Oldenburg RA, et al. Treatment of inoperable or metastatic paragangliomas and pheochromocytomas with peptide receptor radionuclide therapy using 177Lu-DOTATATE. Eur J Endocrinol (2019) 181(1):45–53. doi: 10.1530/EJE-18-0901
28. Vyakaranam AR, Crona J, Norlén O, Granberg D, Garske-Román U, Sandström M, et al. Favorable outcome in patients with pheochromocytoma and paraganglioma treated with 177Lu-DOTATATE. Cancers (Basel) (2019) 11(7):909. doi: 10.3390/cancers11070909
29. Kolasinska-Ćwikła A, Pęczkowska M, Ćwikła JB, Michałowska I, Pałucki JM, Bodei L, et al. A clinical efficacy of PRRT in patients with advanced, nonresectable, paraganglioma-pheochromocytoma, related to SDHx gene mutation. J Clin Med (2019) 8(7):952. doi: 10.3390/jcm8070952
30. Yadav MP, Ballal S, Bal C. Concomitant 177Lu-DOTATATE and capecitabine therapy in malignant paragangliomas. EJNMMI Res (2019) 9(1):13. doi: 10.1186/s13550-019-0484-y
31. Demirci E, Kabasakal L, Toklu T, Ocak M, Şahin OE, Alan-Selcuk N, et al. 177Lu-DOTATATE therapy in patients with neuroendocrine tumours including high-grade (WHO G3) neuroendocrine tumours: response to treatment and long-term survival update. Nucl Med Commun (2018) 39(8):789–96. doi: 10.1097/MNM.0000000000000874
32. Garske-Román U, Sandström M, Fröss Baron K, Lundin L, Hellman P, Welin S, et al. Prospective observational study of 177Lu-DOTA-octreotate therapy in 200 patients with advanced metastasized neuroendocrine tumours (NETs): feasibility and impact of a dosimetry-guided study protocol on outcome and toxicity. Eur J Nucl Med Mol Imaging (2018) 45(6):970–88. doi: 10.1007/s00259-018-3945-z
33. Kong G, Grozinsky-Glasberg S, Hofman MS, Callahan J, Meirovitz A, Maimon O, et al. Efficacy of peptide receptor radionuclide therapy for functional metastatic paraganglioma and pheochromocytoma. J Clin Endocrinol Metab (2017) 102(9):3278–87. doi: 10.1210/jc.2017-00816
34. Hamiditabar M, Ali M, Roys J, Wolin EM, O'Dorisio TM, Ranganathan D, et al. Peptide receptor radionuclide therapy with 177Lu-octreotate in patients with somatostatin receptor expressing neuroendocrine tumors: six years' assessment. Clin Nucl Med (2017) 42(6):436–43. doi: 10.1097/RLU.0000000000001629
35. Pinato DJ, Black JR, Ramaswami R, Tan TM, Adjogatse D, Sharma R. Peptide receptor radionuclide therapy for metastatic paragangliomas. Med Oncol (2016) 33(5):47. doi: 10.1007/s12032-016-0737-9
36. Nastos K, Cheung VTF, Toumpanakis C, Navalkissoor S, Quigley AM, Caplin M, et al. Peptide receptor radionuclide treatment and 131I-MIBG in the management of patients with metastatic/progressive phaeochromocytomas and paragangliomas. J Surg Oncol (2017) 115(4):425–34. doi: 10.1002/jso.24553
37. Puranik AD, Kulkarni HR, Singh A, Baum RP. Peptide receptor radionuclide therapy with 90Y/177Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumours). Eur J Nucl Med Mol Imaging (2015) 42(8):1223–30. doi: 10.1007/s00259-015-3029-2
38. Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue 90Y-DOTA-TOC in metastasized neuroendocrine cancers. J Clin Oncol (2011) 29(17):2416–23. doi: 10.1200/JCO.2010.33.7873
39. Forrer F, Riedweg I, Maecke HR, Mueller-Brand J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q J Nucl Med Mol Imaging (2008) 52(4):334–40.
40. van Essen M, Krenning EP, Kooij PP, Bakker WH, Feelders RA, de Herder WW, et al. Effects of therapy with [177Lu-DOTA0, Tyr3] octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J Nucl Med (2006) 47(10):1599–606.
41. Hescot S, Curras-Freixes M, Deutschbein T, van Berkel A, Vezzosi D, Amar L, et al. Prognosis of malignant pheochromocytoma and paraganglioma (MAPP-prono study): a European network for the study of adrenal tumors retrospective study. J Clin Endocrinol Metab (2019) 104(6):2367–74. doi: 10.1210/jc.2018-01968
42. Crona J, Lamarca A, Ghosal S, Welin S, Skogseid B, Pacak K. Genotype-phenotype correlations in pheochromocytoma and paraganglioma: a systematic review and individual patient meta-analysis. Endocr Relat Cancer (2019) 26(5):539–50. doi: 10.1530/ERC-19-0024
43. O'Kane GM, Ezzat S, Joshua AM, Bourdeau I, Leibowitz-Amit R, Olney HJ, et al. A phase 2 trial of sunitinib in patients with progressive paraganglioma or pheochromocytoma: the SNIPP trial. Br J Cancer (2019) 120(12):1113–9. doi: 10.1038/s41416-019-0474-x
44. Vogel J, Atanacio AS, Prodanov T, Turkbey BI, Adams K, Martucci V, et al. External beam radiation therapy in treatment of malignant pheochromocytoma and paraganglioma. Front Oncol (2014) 4:166. doi: 10.3389/fonc.2014.00166
45. de Keizer B, van Aken MO, Feelders RA, de Herder WW, Kam BL, van Essen M, et al. Hormonal crises following receptor radionuclide therapy with the radiolabeled somatostatin analogue [177Lu-DOTA0,Tyr3]octreotate. Eur J Nucl Med Mol Imaging (2008) 35(4):749–55. doi: 10.1007/s00259-007-0691-z
46. Makis W, McCann K, McEwan AJ. The challenges of treating paraganglioma patients with 177Lu-DOTATATE PRRT: catecholamine crises, tumor lysis syndrome and the need for modification of treatment protocols. Nucl Med Mol Imaging (2015) 49(3):223–30. doi: 10.1007/s13139-015-0332-6
47. Chiesa C, Sjogreen Gleisner K, Flux G, Gear J, Walrand S, Bacher K, et al. The conflict between treatment optimization and registration of radiopharmaceuticals with fixed activity posology in oncological nuclear medicine therapy. Eur J Nucl Med Mol Imaging (2017) 44(11):1783–6. doi: 10.1007/s00259-017-3707-3
48. Sundlöv A, Sjögreen-Gleisner K, Svensson J, Ljungberg M, Olsson T, Bernhardt P, et al. Individualised 177Lu-DOTATATE treatment of neuroendocrine tumours based on kidney dosimetry. Eur J Nucl Med Mol Imaging. (2017) 44(9):1480–9. doi: 10.1007/s00259-017-3678-4
49. Del Prete M, Buteau FA, Beauregard JM. Personalized 177Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: a simulation study. Eur J Nucl Med Mol Imaging (2017) 44(9):1490–500. doi: 10.1007/s00259-017-3688-2
50. Ezziddin S, Lohmar J, Yong-Hing CJ, Sabet A, Ahmadzadehfar H, Kukuk G, et al. Does the pretherapeutic tumor SUV in 68Ga DOTATOC PET predict the absorbed dose of 177Lu octreotate? Clin Nucl Med (2012) 37(6):e141–7. doi: 10.1097/RLU.0b013e31823926e5
Keywords: pheochromocytomas, paragangliomas, PPGLs, PRRT, meta-analysis
Citation: Su D, Yang H, Qiu C and Chen Y (2023) Peptide receptor radionuclide therapy in advanced Pheochromocytomas and Paragangliomas: a systematic review and meta-analysis. Front. Oncol. 13:1141648. doi: 10.3389/fonc.2023.1141648
Received: 10 January 2023; Accepted: 20 June 2023;
Published: 06 July 2023.
Edited by:
Nadia Gisella Di Muzio, Vita-Salute San Raffaele University, ItalyReviewed by:
Murat Fani Bozkurt, Hacettepe University, TürkiyeAndrei Fodor, IRCCS San Raffaele Scientific Institute, Italy
Copyright © 2023 Su, Yang, Qiu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yue Chen, Y2hlbnl1ZTU1MjNAMTI2LmNvbQ==
†These authors contributed equally to this work and share first authorship
 Dan Su
Dan Su Hongyu Yang1,2†
Hongyu Yang1,2† Yue Chen
Yue Chen