
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 March 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1139940
This article is part of the Research Topic Real-World Data and Real-World Evidence in Lung Cancer View all 23 articles
 Saud Alhusaini1
Saud Alhusaini1 Tyler A. Lanman1
Tyler A. Lanman1 Ryan B. Ko2
Ryan B. Ko2 Kate E. Therkelsen1
Kate E. Therkelsen1 Rie Von Eyben2
Rie Von Eyben2 Maximilian Diehn2
Maximilian Diehn2 Scott G. Soltys2
Scott G. Soltys2 Erqi L. Pollom2
Erqi L. Pollom2 Alexander Chin2
Alexander Chin2 Lucas Vitzthum2
Lucas Vitzthum2 Heather A. Wakelee3
Heather A. Wakelee3 Sukhmani K. Padda3
Sukhmani K. Padda3 Kavitha Ramchandran3
Kavitha Ramchandran3 Billy W. Loo Jr.2
Billy W. Loo Jr.2 Joel W. Neal3
Joel W. Neal3 Seema Nagpal1*
Seema Nagpal1*Objective: The 2-year incidence of brain metastases (BrMs) in stage III non-small lung cell cancer (NSCLC) has been estimated to be around 30%. However, recent clinical trials have demonstrated considerably lower BrMs rates in this patient population. In this study, we aimed to review the real-world incidence, surveillance, and treatment patterns of BrMs in stage III NSCLC.
Materials and methods: Using a retrospective single-center study design, we identified patients with stage III NSCLC who received radiation with curative intent over a 10-year period. Outcome variables included BrMs incidence, overall survival (OS), and survival from date of BrMs. Additionally, we assessed patterns of BrMs surveillance in stage III NSCLC and treatment.
Results: We identified a total of 279 stage III NSCLC patients, of which 160 with adequate records were included in the final analyses [adenocarcinoma (n = 96), squamous cell carcinoma (n = 53), other histology subtype (n = 11)]. The median OS for the entire cohort was 41 months (95% CI, 28-53), while the median time from BrMs to death was 19 months (95% CI, 9-21). Twenty-three patients (14.4%) received planned surveillance brain MRIs at 6, 12, and 24 months after completion of treatment. The remaining 137 patients (85.6%) received brain MRIs at systemic recurrence (restaging) or when neurologically symptomatic. A total of 37 patients (23%) developed BrMs, with a 2-year cumulative BrMs incidence of 17% (95% CI, 11-23). A higher incidence of BrMs was identified in patients with adenocarcinoma relative to those with squamous cell carcinoma (p < 0.01). Similarly, a higher 2-year BrMs incidence was observed in patients who received planned surveillance brain MRI relative to those who did not, although statistical significance was not reached. Stereotactic radiosurgery (SRS) treated 29 of BrMs patients (78.4%) and was preferred over WBRT, which treated only 3 patients (8.1%).
Conclusions: At our center, BrMs incidence in stage III NSCLC patients was lower than historically reported but notably higher than the incidence described in recent clinical trials. Routine BrMs surveillance potentially allows earlier detection of asymptomatic BrMs. However, asymptomatic BrMs were mostly detected on restaging MRI at the time of recurrence.
Lung cancer is the leading cause of cancer death worldwide, accounting for 23% and 24% of all deaths by cancer in females and males respectiely (1). Non-small cell lung cancer (NSCLC) accounts for the majority of cases, constituting approximately 76% of all lung cancers (2). Patients with stage III NSCLC have no brain metastases (BrMs) at the time of diagnosis but are at high risk for developing BrMs during their disease course. Despite recent treatment advances, BrMs are a common manifestation of recurrence in stage III NSCLC and often associated with a decreased quality of life and poor prognosis (3–5). The incidence of BrMs in stage III NSCLC has historically been estimated to be around 30% at 2-years (4, 6). However, recent clinical trials have demonstrated considerably lower BrMs rates in enrolled stage III NSCLC patients. Specifically, the PACIFIC trial (PD-L1 inhibitor, Durvalumab vs. placebo for stage III NSCLC) reported a BrMs rate of 11.8% in the placebo arm and 6.3% in the treatment arm at a median follow-up period of 25.2 months (7). It is worth noting however that in this multi-center international clinical trial, fewer brain magnetic resonance imaging (MRI) may have possibly been performed relative to standard practice in US-based health care systems.
With recent advances in diagnostic imaging and rapid accessibility to brain MRI, the true incidence of BrMs in stage III NSCLC in a real-world (outside the strictly controlled clinical trials) setting remains unclear. Given that up to one third of stage III NSCLC patients often develop BrMs over their disease course, our local practice has evolved to performing surveillance brain MRI at 6, 12, and 24 months after completion of initial treatment. Nonetheless, the support for routine asymptomatic BrMs surveillance in this patient population remains to be demonstrated.
In this study, we aimed to determine the real-world incidence of BrMs in stage III NSCLC and analyze the surveillance and treatment patterns of BrMs in this patient population.
In this single-center retrospective study, we identified 279 patients who were seen at the Stanford Cancer Center from 2008-2018 and had a confirmed diagnosis of stage IIIA/B/C NSCLC (AJCC 8th edition staging criteria). All patients whose treatment plan included radiation with curative intent were included, regardless of treatment completion. The following information was collected: age at diagnosis of stage III NSCLC, diagnostic imaging findings [including computerized tomography (CT) and whole-body positron emission tomography (PET) scans, and brain MRI], pathology results, disease staging, radiation, and treatment plans [including whether prophylactic cranial irradiation (PCI) was utilized for BrMs prevention], and available follow-up visit information within the prior 6 months, or date of death. A total of 160 stage III NSCLC had complete data and were included in the final analyses of this study. Patients who completed treatment and surveillance elsewhere, did not have available follow-up data, or had a second primary cancer with high potential for brain metastatic disease (e.g., triple negative breast cancer), were excluded (see Figure 1).
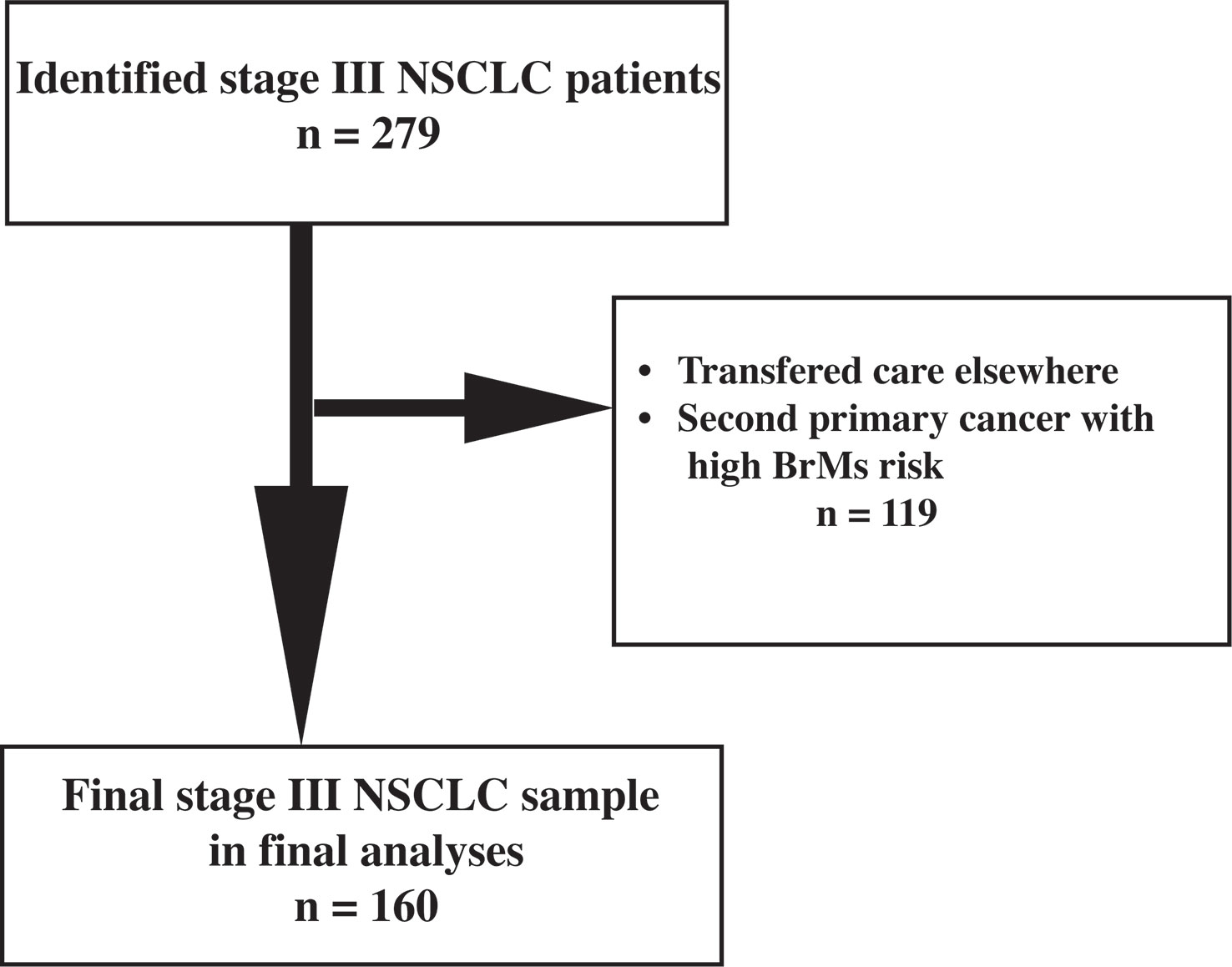
Figure 1 Flowchart indicating the selection process of the final study sample of stage III NSCLC. BrMs: brain metastases.
This retrospective study was approved by Stanford University IRB (reference: IRB-44962).
The following time to event outcomes were assessed and analyzed for the entire cohort as well as for each NSCLC histology subtype. The time to overall survival (OS) outcome was summarized using Kaplan-Meier curves. The time to OS was defined as the time from date of diagnosis until death from any cause, and patients who were still alive at the completion of analysis were censored at the date of the last follow up. The time to BrMs outcome was analyzed using competing risk methods and summarized using cumulative incidence curves. Time to BrMs was defined as the time from date of diagnosis until date of BrMs with death as a competing event. Patients who experienced neither BrMs nor death were censored at the date of last follow up. The cumulative incidence of BrMs was calculated over a 2-year period. The OS from date of BrMs was defined as the time from date of BrMs until death from any cause, and patients who were still alive were censored at the date of the last follow up. The “number needed to scan” to detect asymptomatic BrMs was calculated as the total number of patients needed to get surveillance brain MRI to detect asymptomatic BrMs for the entire cohort and for patients with adenocarcinoma subtype (the most common histopathology subtype).
Descriptive statistics were utilized to summarize the characteristics of the study cohort. Chi-square tests were applied to assess outcome differences between histology subtypes. All tests were two-sided with an alpha level of 0.05. All analyses were performed in SAS v9.4 (SAS Institute Inc, Cary, NC).
A total of 160 patients (93 male and 67 females) with stage III NSCLC were included in the final analyses of this study, see Figure 1. Baseline characteristics of the study cohort are described in Table 1. The median age at diagnosis of stage III NSCLC was 67 years (range, 34-90 years). Adenocarcinoma was the most common histopathology subtype and was identified in 96 patients (60%), with squamous cell carcinoma in 53 patients (33%). The remaining 11 patients (7%) had other histology subtypes, including large cell and neuro-endocrine tumor. Based on staging work-up, 90 patients (56%) had stage IIIA, 67 patients (42%) had stage IIIB, and 3 patients (2%) had stage IIIC.
The median OS from time of diagnosis for the entire cohort was 41 (95% CI, 28-53) months. In patients with adenocarcinoma, the median OS was 51 (95% CI, 36-68) months. Meanwhile, the median OS was 22 (95% CI, 14-43) months for those with squamous cell carcinoma and 21 (95% CI, 10-NR) months for those with other histology subtypes. The higher OS in patients with adenocarcinoma relative to patients with squamous cell carcinoma was statistically significant (p < 0.01; see Figure 2).
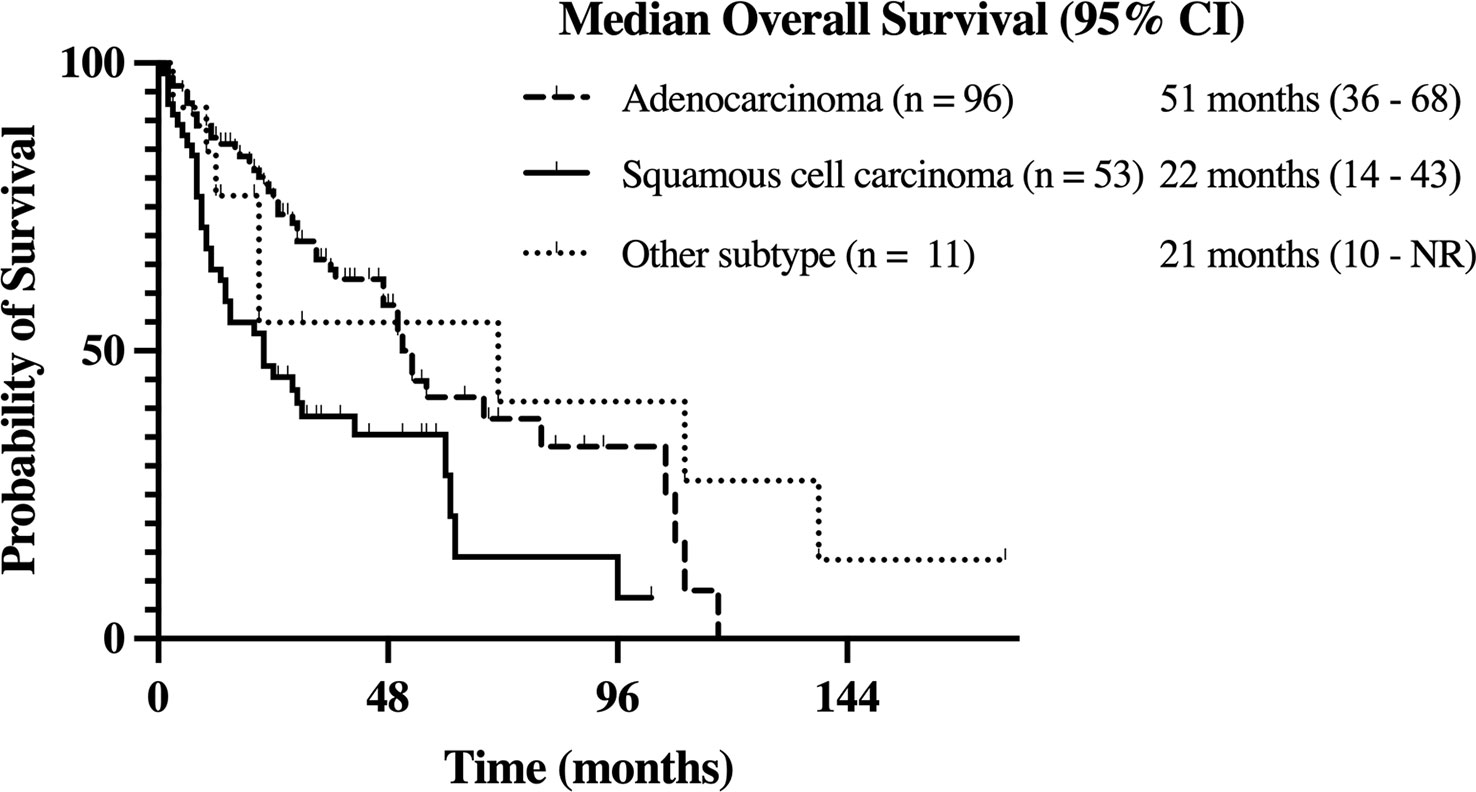
Figure 2 Kaplan-Meier estimate of overall survival (OS) in stage III NSCLC patients with adenocarcinoma, squamous cell carcinoma, and other history types.
Of the entire cohort, only two patients (1%) received PCI at an outside institution prior to establishing care at our cancer center. Neither of these two patients developed BrMs. Institutionally, we offer surveillance brain MRI at 6, 12, and 24 months after completion of treatment. Twenty-three patients (14.4%) received planned surveillance brain MRI to screen for asymptomatic BrMs. For the remaining patients (n = 137, 85.6%), in accordance with current guidelines (8, 9), no planned BrMs surveillance was carried out. Instead, brain MRI was only obtained at systemic recurrence for restaging or on development of neurological symptoms.
A total of 37 patients (23%) developed BrMs. Of those, 20 (54.1%) were asymptomatic. On initial BrMs identification, 18 patients (48.7%) had one brain metastasis. Meanwhile, 8 patients (21.6%) had 2-3 BrMs and 11 (29.7%) had >3 BrMs. The estimated 2-year cumulative incidence of BrMs was 17% (95% CI, 11-23) for the entire cohort, see Figure 3A.
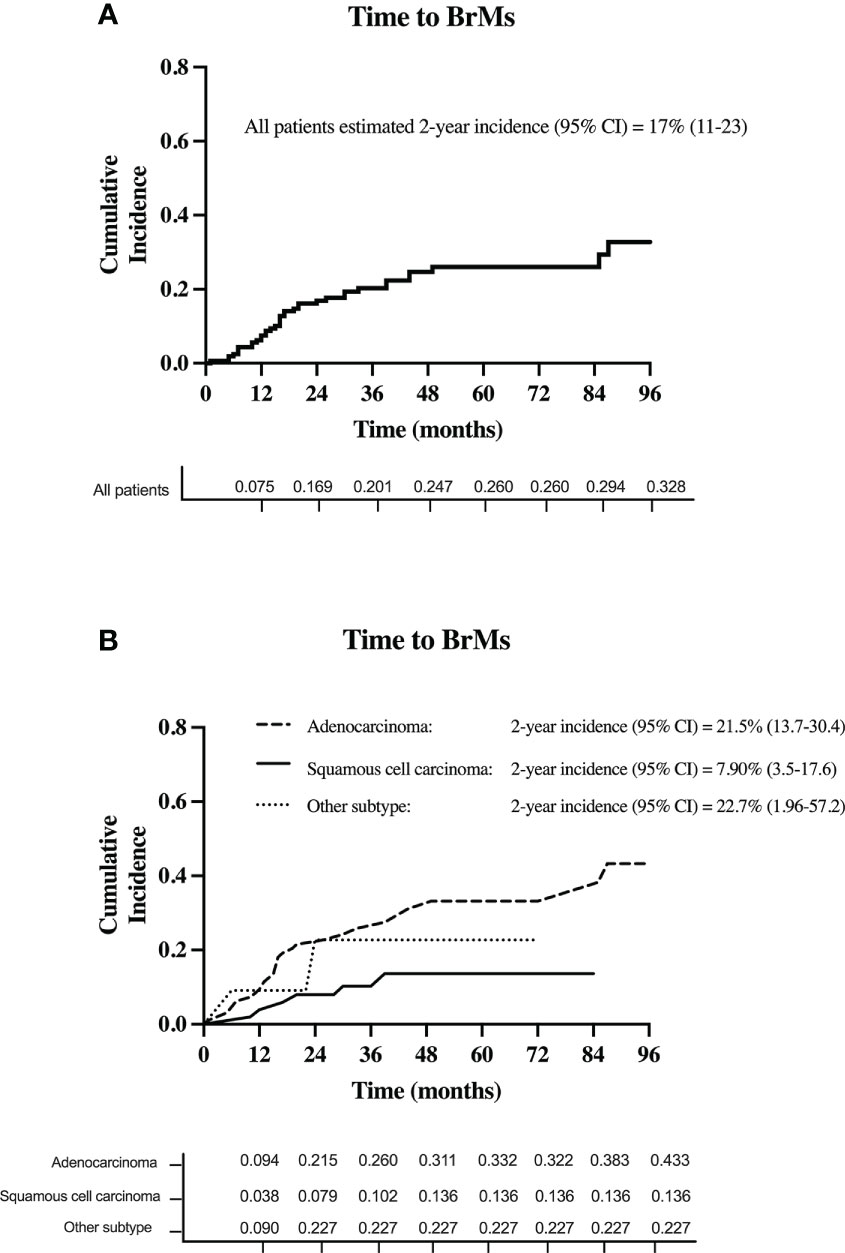
Figure 3 The cumulative incidence of BrMs in (A) all patients with stage III NSCLC and (B) for each stage III NSCLC histology subtype.
Based on histology subtype, 29 patients with adenocarcinoma (total n = 96; 30.2%) developed BrMs. Of the 53 patients with squamous cell carcinoma, 6 (11.3%) developed BrMs. Meanwhile, in the 11 patients with other histology subtypes, BrMs was identified in 2 (18%) patients. The higher incidence of BrMs in patients with adenocarcinoma [2-year BrMs incidence was 21.5% (13.7-30.4)], compared to squamous cell carcinoma [2-year BrMs incidence was 7.9% (3.5-17.6)], was statistically significant (p < 0.01), see Figure 3B.
The median time from BrMs development to death was 19 months (95% CI, 9-21) for all patients. No significant differences were noted in time from BrMs to death between NSCLC histology subtypes (see Table 1).
In patients with BrMs (n = 37), stereotactic radiosurgery (SRS) was utilized to treat 29 patients (78.4%) and whole brain radiation therapy (WBRT) was used to treat 3 patients (8.1%). The remaining 5 patients (13.5%) were treated with systemic therapies alone, without any form of radiation therapy, and were clinically monitored.
Among patients who received no BrMs surveillance (n = 137), 31 developed BrMs (22.6%), 17 of whom were asymptomatic. Meanwhile, among the 23 patients who received surveillance brain MRI, 6 developed BrMs (26.1%), of whom 3 were asymptomatic (see Table 2). There was no statistically significant difference in the incidence of BrMs between patients who received BrMs surveillance and those who did not. However, a trend of higher 2-year incidence of BrMs was noted in those received BrMs surveillance [2-year BrMs incidence was 28.5% (11.1-48.9)], compared to patients who did not [2-year BrMs incidence was 15.0% (9.6-21.7)], indicating earlier detection of BrMs (see Table 2).
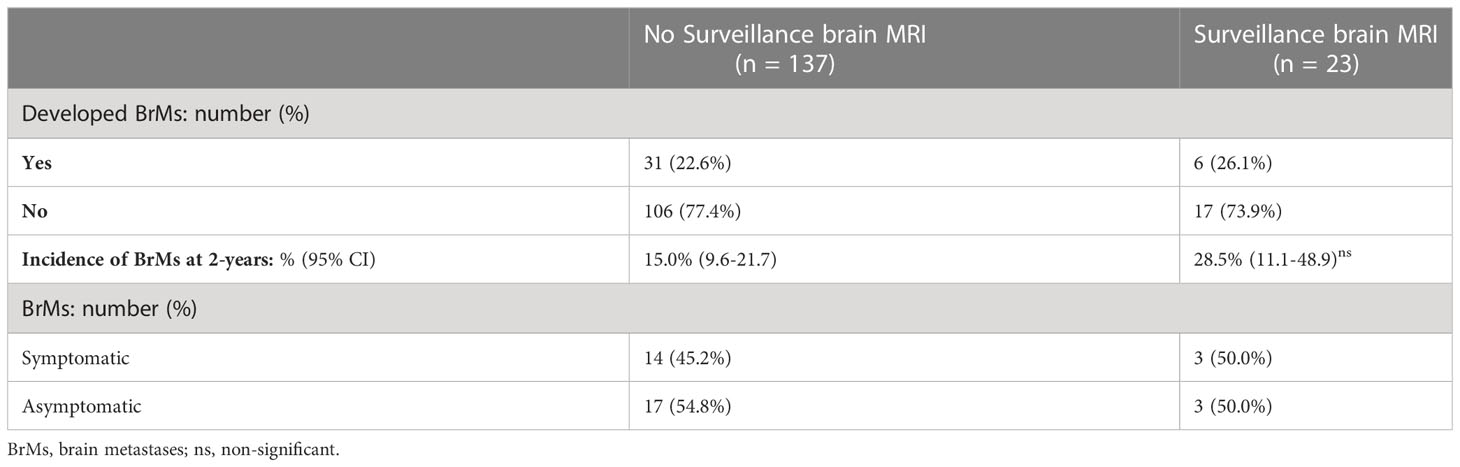
Table 2 Comparisons of stage III NSCLC patients who received surveillance brain MRI and those who did not.
Based on the total number of patients who did not develop BrMs (n = 123), the number of patients needed to receive surveillance brain MRI scans to detect 1 asymptomatic BrMs is 7 for all histology subtypes.
Focusing on adenocarcinoma patients (the most common histology subtype in stage III NSCLC, n = 96), 79 (82.3%) received no BrMs surveillance. Meanwhile, 17 (17.7%) patients received surveillance brain MRI according to our local practice. Among adenocarcinoma patients who received no BrMs surveillance (n = 79), 25 developed BrMs (31.6%), 13 of whom were asymptomatic. In the 17 adenocarcinoma patients who received surveillance brain MRI, 4 developed BrMs (23.5%), of whom 1 was asymptomatic (Table 3). Based on the total number of adenocarcinoma patients who did not develop BrMs (n = 67), the number of adenocarcinoma patients needed to receive surveillance brain MRI scans to detect 1 asymptomatic BrMs is 5.
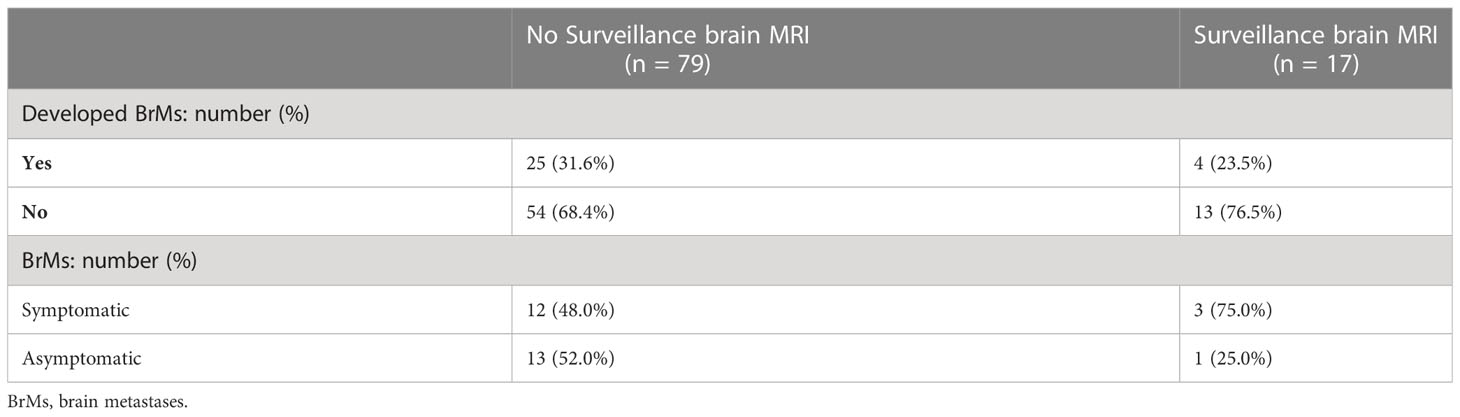
Table 3 Comparisons of stage III NSCLC patients with adenocarcinoma subtype who received surveillance brain MRI and those who did not.
In this single-center study, we assessed the real-world incidence of BrMs in stage III NSCLC in the modern era of cancer staging and rapid access to advanced diagnostic imaging, including whole body PET/CT scans and brain MRI. The estimated 2-year incidence of BrMs in stage III NSCLC patients treated at our center was 17%. This estimated BrMs incidence is lower than historically described (4). Yet, it is remarkably higher than that reported in more recent clinical trials, including the PACIFIC trial (7). This new evidence of BrMs incidence in stage III NSCLC highlights the considerable difference between outcomes and surveillance practices in the real-world setting versus clinical trials settings.
Due to increasing life expectancy of cancer patients and advances in diagnostic imaging, the incidence of BrMs in all cancer patients (in particular asymptomatic BrMs) is generally increasing (4). Interestingly, based on more recent evidence, the incidence of BrMs in stage III NSCLC has however been declining over time (7). This has been attributed to advances in NSCLC treatments and availability of newer and targeted therapies that has better CNS penetration, such as next-generation tyrosine kinase inhibitors (TKIs), small molecules like pemetrexed, and checkpoint inhibitors (10, 11). More importantly, better access to neuroimaging in recent years has allowed earlier detection of asymptomatic BrMs at initial disease staging, minimizing the possibility of patients being classified as having stage III NSCLC at the time of diagnosis.
Although our sample was likely enriched for targetable mutations (such as EGFR and ALK rearrangements), it remains a close representation of the real-world stage III NSCLC patient population treated at specialized cancer centers, with adenocarcinoma and squamous cell carcinoma histology subtypes comprising 60% and 33% of our cohort respectively. The incidence of BrMs was higher in patients with adenocarcinoma relative to other histology subtypes, with 30% of our patients with adenocarcinoma subtype developing BrMs. This is in alignment with the evidence of higher BrMs rates in adenocarcinoma subtype, especially in those harboring targetable mutations, such as EGFR and ALK rearrangements (12–16). Likewise, the significantly higher OS we observed in our patients with adenocarcinoma compared to squamous cell carcinoma histology subtype is consistent with recent literature (12, 17).
The clinical practice of BrMs surveillance in stage III NSCLC remains controversial. Surveillance brain MRIs are not explicitly recommended and current stage III NSCLC guidelines, including the 2020 National Comprehensive Cancer Network (NCCN) guidelines (9), recommend obtaining brain MRI to detect asymptomatic BrMs only at the time of initial diagnosis or systemic recurrence. It is unclear however how closely physicians adhere to these guidelines in the real-world setting (18). According to our local practice, 14.4% of (n = 23) of our stage III NSCLC patients received planned surveillance brain MRIs to screen for asymptomatic BrMs at 6, 12, and 24 months after completion of treatment. Most patients (85.6%) however were monitored clinically and only received surveillance brain MRI at the time of systemic recurrence as part of their re-staging process. The frequency of asymptomatic BrMs among patients who received BrMs surveillance was 13%, which was nearly equivalent to the frequency of asymptomatic BrMs among patients who did not receive surveillance brain MRI (12.4%). Although this is not surprising, a trend of earlier BrMs detection was noted in those who received BrMs surveillance. Interestingly, 50% of the patients who developed BrMs while undergoing BrMs surveillance required an additional brain MRI due to developing new neurological symptoms, indicating that regular surveillance was not entirely successful in detecting asymptomatic BrMs early. Overall, we estimated that 7 patients with stage III NSCLC “needed to be scanned” to detect one asymptomatic BrMs. Based on these observation, routine BrMs surveillance is likely to be challenged by its lack of superiority to clinical monitoring and potential cost-ineffectiveness, especially in less resourced areas and low-income countries. However, with ready access to, and growing affordability of, brain MRI, it can be argued that early BrMs detection is very pertinent to treatment planning and delaying BrMs-related complications. The benefit of BrMs surveillance might thus be justifiable in selected stage III NSCLC patients, such as patients with adenocarcinoma, those at high-risk of recurrence (including those harboring targetable mutations such as EGFR and ALK), and those with programmed cell death ligand 1 (PD-L1) expression on less than 1% of tumor cells (19, 20).
Recently, our group reviewed the landmark trials that investigated PCI in stage III NSCLC (10). Despite the fact that all, but one (21), of these trials were able to demonstrate a significant reduction in the incidence of BrMs in stage III NSCLC with PCI (22–28), they consistently demonstrated lack of significant benefit on OS. In the 2019 update to the Radiation Therapy Oncology Group (RTOG) 0214 phase 3 randomized clinical trial (RCT), the largest RTC of PCI in stage III NSCLC, the 5-year incidence of BrMs was 28.3% in the observation group compared to 16.7% in the PCI group (HR 0.43, p=0.003) (27). Despite this seemingly promising result, the findings unfortunately did not translate to an improvement in OS (neither 5-year, 10-year, nor median OS was significantly different between the two groups) (27). Interestingly, compared to our study, the 2-year incidence of BrMs in the NRG Oncology-RTOG 0214 Phase 3 RCT was 24.3% in the observation group (relative to 10.9% in the PCI group) (27). The higher 2-year BrMs incidence in the trial’s observation group likely reflects the study accrual period between 2002 and 2007 and does not appear entirely consistent with contemporary evidence of lower BrMs incidence in stage III NSCLC or the findings of the current study (7). In our cohort, only 2 patients (1%) received PCI at other institutions prior to establishing care at our center. Neurological complications associated with WBRT are well documented and range from long-term cognitive dysfunction, gait and motor disturbance, and urinary incontinence (29, 30). These detrimental neurological complications and the lack of significant benefit on survival outcomes argue against PCI application in stage III NSCLC.
This study has several limitations. Firstly, this is a retrospective single center study and thus the results are subject to bias. Compared to clinical trials settings, however, we believe this retrospective study was less limited by the strict inclusion/exclusion criteria often applied in clinical trials and thus had more potential to estimate a closer real-world incidence of BrMs. For example, patients participating in the PACIFIC trial successfully completed chemoradiation without disease progression prior to enrollment, whereas patients in this cohort were included if their treatment plan had curative intention, regardless of completion. Secondly, molecular testing (such as EGFR/ALK mutations status) was not available for all patients in our cohort and thus this relevant information was not incorporated in our analyses. Future studies are strongly encouraged to include the results of targetable mutations testing when assessing the association between stage III NSCLC BrMs predisposition and clinical outcomes. Thirdly, we considered the fact that the American Joint Committee on Cancer (AJCC) Classification of NSCLC underwent edition changes during our study period which may affect classification of stage III NSCLC patients. Although, we do not believe this had largely impacted our data as most of the study period comprised the 7th edition classification system. Lastly, given that our center is a major academic center, there is a possibility that most of the patients who continued long-term follow-up represent a population of patients that are often doing clinically better than those who were lost to follow-up.
In this retrospective single-center study, the 2-year incidence of BrMs in stage III NSCLC was 17%. This BrMs incidence is lower than historically reported, but higher than that reported in recent clinical trials. Although, we found surveillance brain MRI not superior to clinical monitoring in detecting asymptomatic BrMs, a trend of earlier BrMs detection was noted. Therefore, surveillance brain MRI may still be appropriate in selected subgroups of stage III NSCLC patients, such as patients with adenocarcinoma and those with high risk of recurrence. At our institution, PCI is not performed for stage III NSCLC and there is a clear preference to deliver SRS over WBRT to treat BrMs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
SA, TL, RK, KT, BL, JN, SN: Conceptualization, methodology, writing, investigation, editing. RE, MD, SS, EP, AC, LV, HW, SP, KR: Writing, visualization, diting. All authors contributed to the article and approved the submitted version.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author SS: Speaker Honoraria, Zap Surgical, Inc and consultant at Accuray, Inc. EP: Board, Vysioneer. HW: President of International Association for the Study of Lung Cancer IASLC. Executive committee member of ECOG-ACRIN. Advisory Board, AstraZeneca, Janssen, Daiichi Sankyo, Blueprint, Mirati, Merck, Genentech/Roche. BL: Co-founder and board member of TibaRay. Research support from Varian Medical Systems. JN: Consulting or Advisory Role: AstraZeneca, Genentech/Roche, Exelixis, Jounce Therapeutics, Takeda Pharmaceuticals, Eli Lilly and Company, Calithera Biosciences, Amgen, Iovance Biotherapeutics, Blueprint Pharmaceuticals, Regeneron Pharmaceuticals, Natera, Sanofi/Regeneron, D2G Oncology, Surface Oncology, Turning Point Therapeutics. SN: Consulting Role, Mirati, SeaGen, Biocept.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author SS received research funding from Novocure, Inc. EP received research funding from Genentech. HW received research funding from ACEA Biosciences, Arrys Therapeutics, AstraZeneca/Medimmune, BMS, Clovis Oncology, Genentech/Roche, Merck, Novartis, SeaGen, Xcovery, Helsinn. BL received research funding from Varian Medical Systems. JN received research funding from Genentech/Roche, Merck, Novartis, Boehringer Ingelheim, Exelixis, Nektar Therapeutics, Takeda Pharmaceuticals, Adaptimmune, GSK, Janssen, AbbVie. SN received research funding from Novocure, PharmAbcine, Berg Health, Agios, Pyramid Biosciences, ABM Therapuetics.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin (2019) 69:7–34. doi: 10.3322/caac.21551
2. Howlader N, Forjaz G, Mooradian MH, Meza R, Kong YC, Cronin KA, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med (2020) 383:640–9. doi: 10.1056/NEJMoa1916623
3. Ali A, Goffin JR, Arnold A, Ellis PM. Survival of patients with non-small-cell lung cancer after a diagnosis of brain metastases. Curr Oncol (2013) 20:e300–306. doi: 10.3747/co.20.1481
4. Nayak L, Lee EQ, Wen PY7. Epidemiology of brain metastases. Curr Oncol Rep (2012) 14:48–54. doi: 10.1007/s11912-011-0203-y
5. Penel N, Brichet A, Prevost B, Duhamel A, Assaker R, Dubois F, et al. Pronostic factors of synchronous brain metastases from lung cancer. Lung Cancer (2001) 33:143–54. doi: 10.1016/S0169-5002(01)00202-1
6. Mamon HJ, Yeap BY, Janne PA, Reblando J, Shrager S, Jaklitsch MT, et al. High risk of brain metastases in surgically staged IIIA non–small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J Clin Oncol (2005) 23:1530–7. doi: 10.1200/JCO.2005.04.123
7. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med (2018) 379:2342–50. doi: 10.1056/NEJMoa1809697
8. Postmus PE, Kerr KM, Oudkrek M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28:iv1–iv21. doi: 10.1093/annonc/mdx222
10. Moghavem N, Wakelee HA, Nagpal S. Case closed: another prophylactic cranial irradiation trial for stage 3 non-small cell lung cancer fails to improve overall survival. Ann Transl Med (2018) (Plymouth Meeting, PA: NCCN) 6:S118. doi: 10.21037/atm.2018.12.24
11. Thomas NJ, Myall NJ, Sun F, Patil T, Mushtaq R, Yu , et al. Brain metastases in EGFR- and ALK-positive NSCLC: Outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J Thorac Oncol (2022) 17:116–29. doi: 10.1016/j.jtho.2021.08.009
12. Lee JS, Hong JH, Sun DS, Won HS, Kim YH, Ahn MS, et al. The impact of systemic treatment on brain metastasis in patients with non-small-cell lung cancer: A retrospective nationwide population-based cohort study. Sci Rep (2019) 9:18689. doi: 10.1038/s41598-019-55150-6
13. Naresh G, Malik PS, Khurana S, Pushpam D, Sharma V, Yadav M, et al. Assessment of brain metastasis at diagnosis in non-small-cell lung cancer: a prospective observational study from north India. JCO Glob Oncol (2021) 7:593–601. doi: 10.1200/GO.20.00629
14. Shi AA, Digumarthy SR, Temel JS, Halpern EF, Kuester LB, Aquino SL. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non–small cell lung cancer? J Thorac Oncol (2006) 1:205–10. doi: 10.1016/S1556-0864(15)31569-0
15. Shin DY, Na II, Kim CH, Park S, Baek H, Yang SH. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol (2014) 9:195–9. doi: 10.1097/JTO.0000000000000069
16. Zhang I, Zaorsky NG, Palmer JD, Mehra R, Lu B. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol (2015) 16:e510–21. doi: 10.1016/S1470-2045(15)00013-3
17. D’Angelo SP, Janjigian YY, Ahye N, Riely GJ, Chaft JE, Sima CS, et al. Distinct clinical course of EGFR -mutant resected lung cancers: results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol (2012) 7:1815–22. doi: 10.1097/JTO.0b013e31826bb7b2
18. Cui W, Milner-Watts C, Saith S, Bhosle J, Minchom AR, Davidson M, et al. 180P incidence of brain metastases (BM) in newly diagnosed stage IV NSCLC during COVID-19. J Thorac Oncol (2021) 16:S795. doi: 10.1016/S1556-0864(21)02022-0
19. Faivre-Finn C, Vicente D, Kurata T, Planchard D, Paz-Ares L, Vansteenkiste JF, et al. Four-year survival with durvalumab after chemoradiotherapy in stage III NSCLC–an update from the PACIFIC trial. J Thorac Oncol (2021) 16:860–7. doi: 10.1016/j.jtho.2020.12.015
20. Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: a review. J Clin Oncol (2020) 38:2926–36. doi: 10.1200/JCO.19.03123
21. Russell AH, Pajak TE, Selim HM, Paradelo JC, Murray K, Bansal P, et al. Prophylactic cranial irradiation for lung cancer patients at high risk for development of cerebral metastasis: results of a prospective randomized trial conducted by the radiation therapy oncology group. Int J Radiat Oncol Biol Phys (1991) 21:637–43. doi: 10.1016/0360-3016(91)90681-S
22. Cox JD, Stanley K, Petrovich Z, Paig C, Yesner R. Cranial irradiation in cancer of the lung of all cell types. JAMA (1981) 245:469–72. doi: 10.1001/jama.1981.03310300023013
23. De Ruysscher D, Dingemans AMC, Praag J, Belderbos J, Tissing-Tan C, Herder J, et al. Prophylactic cranial irradiation versus observation in radically treated stage III non-small-cell lung cancer: a randomized phase III NVALT-11/DLCRG-02 study. J Clin Oncol (2018) 36:2366–77. doi: 10.1200/JCO.2017.77.5817
24. Gore EM, Bae K, Wong SJ, Sun A, Bonner JA, Schild SE, et al. Phase III comparison of prophylactic cranial irradiation versus observation in patients with locally advanced non-small-cell lung cancer: primary analysis of radiation therapy oncology group study RTOG 0214. J Clin Oncol (2011) 29:272–8. doi: 10.1200/JCO.2010.29.1609
25. Li N, Zeng ZF, Wang SY, Ou W, Ye X, Li J, et al. Randomized phase III trial of prophylactic cranial irradiation versus observation in patients with fully resected stage IIIA-N2 nonsmall-cell lung cancer and high risk of cerebral metastases after adjuvant chemotherapy. Ann Oncol (2015) 26:504–9. doi: 10.1093/annonc/mdu567
26. Pöttgen C, Eberhardt W, Grannass A, Korfee S, Stuben G, Teschler H, et al. Prophylactic cranial irradiation in operable stage IIIA non-small cell lung cancer treated with neoadjuvant chemoradiotherapy: results from a German multicenter randomized trial. J Clin Oncol (2007) 25:4987–92. doi: 10.1200/JCO.2007.12.5468
27. Sun A, Hu C, Wong SJ, Gore E, Videtic G, Dutta S, et al. Prophylactic cranial irradiation vs observation in patients with locally advanced non-small cell lung cancer: a long-term update of the NRG Oncology/RTOG 0214 phase 3 randomized clinical trial. JAMA Oncol (2019) 5:847–55. doi: 10.1001/jamaoncol.2018.7220
28. Umsawasdi T, Valdivieso M, Chen TT, Barkely HT Jr, Booser DJ, Chiuten DF, et al. Role of elective brain irradiation during combined chemoradiotherapy for limited disease non-small cell lung cancer. J Neurooncol (1984) 2:253–9. doi: 10.1007/BF00253278
29. Mehanna R, Jimenez-Shahed J, Itin I. Three cases of levodopa-resistant parkinsonism after radiation therapy. Am J Case Rep (2016) 17:916–20. doi: 10.12659/AJCR.900537
Keywords: non-small cell lung cancer, brain metastases, incidence, surveillance, MRI brain
Citation: Alhusaini S, Lanman TA, Ko RB, Therkelsen KE, Eyben RV, Diehn M, Soltys SG, Pollom EL, Chin A, Vitzthum L, Wakelee HA, Padda SK, Ramchandran K, Loo BW Jr., Neal JW and Nagpal S (2023) Real-world risk of brain metastases in stage III non-small cell lung cancer in the era of PET and MRI staging. Front. Oncol. 13:1139940. doi: 10.3389/fonc.2023.1139940
Received: 08 January 2023; Accepted: 15 March 2023;
Published: 24 March 2023.
Edited by:
Jiangning Song, Monash University, AustraliaReviewed by:
Leonidas Papastavrou, Athens Medical Center, GreeceCopyright © 2023 Alhusaini, Lanman, Ko, Therkelsen, Eyben, Diehn, Soltys, Pollom, Chin, Vitzthum, Wakelee, Padda, Ramchandran, Loo, Neal and Nagpal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seema Nagpal, c25hZ3BhbEBzdGFuZm9yZC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.