- 1Institute of Biomedical Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan
- 2Department of Medical Research, Chi-Mei Medical Center, Chiali, Tainan, Taiwan
- 3Department of Medical Research, Chi Mei Medical Center, Tainan, Taiwan
- 4Department of Anesthesiology, Tri-Service General Hospital and National Defense Medical Center, Taipei, Taiwan
- 5Department of Internal Medicine, Chi Mei Medical Center, Chiali, Tainan, Taiwan
- 6Department of Information Management, Southern Taiwan University of Science and Technology, Tainan, Taiwan
- 7Cancer Center, Taipei Municipal Wanfang Hospital, Taipei Medical University, Taipei, Taiwan
The global incidence of early-onset colorectal cancer (EO-CRC) is increasing. Although the mortality rate is relatively stable, some comorbidities have been associated with a higher mortality rate. This study estimated the mortality risk in patients with EO-CRC with various comorbidities using real-world data to identify the high-risk group using Cox proportional regression for overall and cancer-specific mortality. The incidence rate of EO-CRC significantly increased from 6.04 per 100,000 population in 2007 to 12.97 per 100,000 population in 2017. The five-year overall mortality rate was 101.50 per 1000 person year and the cancer-specific mortality rate was 94.12 per 1000 person year. Patients with cerebrovascular disease (CVD) had a higher mortality risk (hazard ratio (HR): 1.68; 95% confidence interval (CI): 1.25-2.28; p=0.0007). After subgroup analyses based on age, sex, clinical stage, and treatment type, patients with CVD had a higher overall mortality risk compared to non-CVD patients, except for patients undergoing surgery and chemotherapy. Patients with chronic kidney disease had a higher mortality risk in the early clinical stages (HR: 2.31; 95% CI: 1.08-4.96; p=0.0138). Patients who underwent radiotherapy had a higher overall mortality risk (HR: 1.38; 95% CI: 1.04-1.85; p=0.0285) than those without liver disease. Identifying specific comorbidity mortality risks in patients with EO-CRC allows for risk stratification when screening target groups and may lower disease mortality.
1 Introduction
Cancer is one of the leading causes of death worldwide, and colorectal cancer (CRC) was the second leading cause of cancer death in 2020 (1). The age-standardized mortality rate for colorectal cancer is approximately 8.9 per 100,000 population; however, patients with CRC aged under 50 years of age have a higher survival rate than those over 50 years of age (2). The mortality rate of early-onset colorectal cancer (EO-CRC) increased by approximately 1.3% per year from 2008 to 2017, except for patients aged 20-29 years, for whom it remained stable (3).
The incidence of CRC decreased in patients aged 50 years or older, though the incidence increased in young adults. In patients less than 50 years old, the incidences of localized, regional, and distant CRC increased. The incidence of CRC is predicted to increase by 90.0% by 2030 in patients aged 20-34 years (4). In 2021, the US Preventive Services Task Force reported that screening for CRC in adults aged 50-75 years has a substantial net benefit, and screening for CRC in adults aged 45-49 years has a moderate net benefit (5). Before 2021, patients aged 45-49 years did not undergo regular screening for CRC. The incidence of EO-CRC is also increasing in other countries, including France (6), Canada (7), Australia (8), Japan, South Korea, and Taiwan (9). The incidence of EO-CRC increased by 3.9% per year in Taiwan and 6.0% per year in South Korea (9).
In Taiwan, the increasing incidence of CRC is attributed to the implementation of population-based screening programs and westernized dietary lifestyle over the past three decades (10, 11). The Taiwanese government offered a nationwide screening program using a fecal immunochemical test for individuals aged 50 to 75 years (12), though the failure to screen patients younger than 50 years may have resulted in an increased rate of advanced CRC in young patients. Potential risk factors associated with EO-CRC include diet, a sedentary lifestyle, smoking, and alcohol consumption (7, 13–15). Few studies regarding the mortality risk factors in patients with EO-CRC that use real-world data have been reported; studies using real-world data typically report the patient’s age and clinical stage of the disease (16, 17). However, the comorbidities of patients with CRC may also affect the treatment, prognosis, and survival (18–20). This study aimed to understand the role of comorbidities in the mortality risk of patients with EO-CRC using population-based, real-world data.
2 Materials and methods
2.1 Data sources
Data from patients with CRC registered in the Taiwan Cancer Registry (TCR) and the National Health Insurance research database were used in this study. The TCR database was established in 1979 with a high-quality, precise diagnosis, and treatment items to track the cancer incidence and mortality rates in Taiwan. Long-form datasets for cancer in the colon and rectum, oral cavity and pharynx (except nasopharynx), liver, lung, breast, and cervix were added to the TCR in 2002, and prostate cancer was added in 2007 (21, 22).
To obtain data regarding comorbidities and medical history, administrative claims from Taiwan’s National Health Insurance program, which covers all inpatient and outpatient health services, were also used in this study. For research purposes, Taiwan’s Health and Welfare Data Science Center (HWDC) integrated the population database, which was linked from different health-related datasets, and managed the application to avoid violations of personal information protection.
2.2 Study population
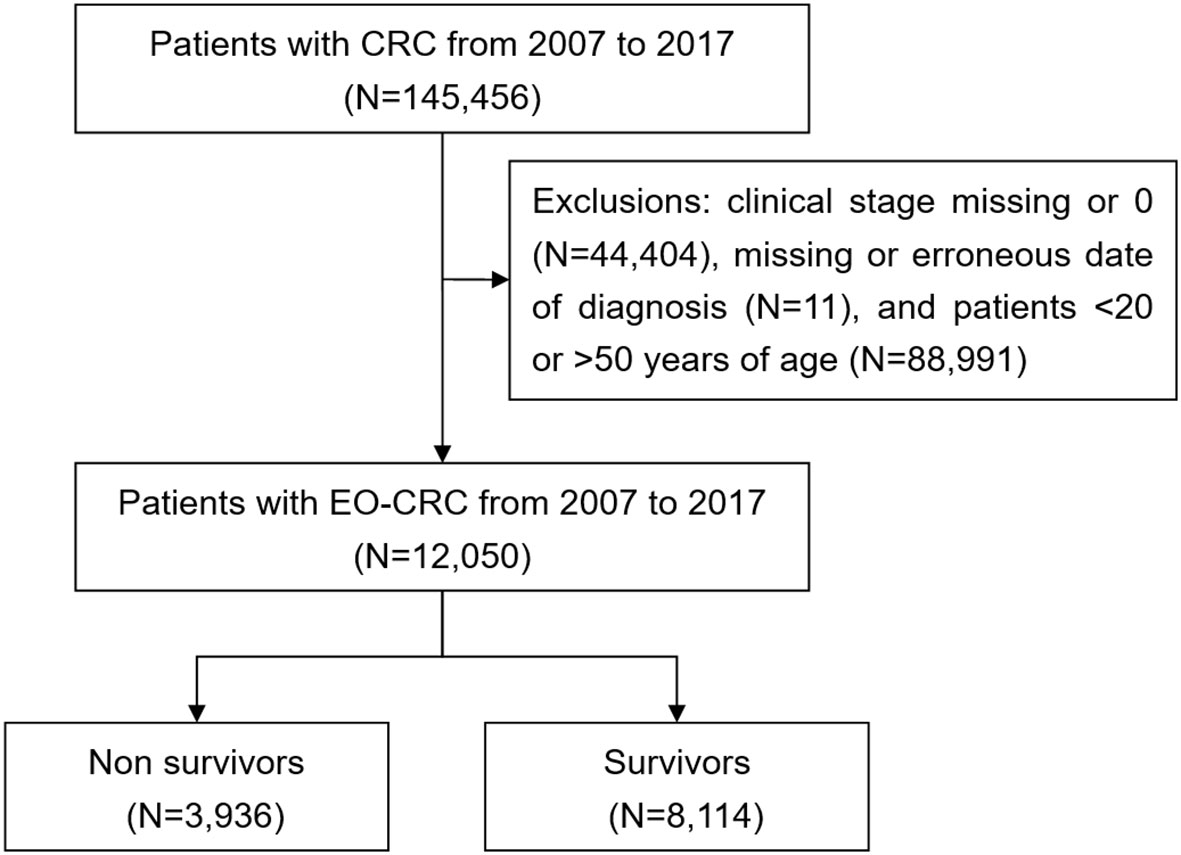
Patients with CRC were selected from the TCR using the International Classification of Diseases for Oncology, third edition (ICD-O-3) classification for CRC (ICD-O-3: C18-C20) from January 2007 to December 2017. All patients were followed up until December 31, 2017. The maximum follow-up period was five years after the date of CRC diagnosis. EO-CRC is defined as CRC in patients less than 50 years of age. Therefore, only patients less than 50 years old were included in this study. Patients with a history of cancer (before the diagnosis of CRC) and those with missing data were excluded from the study (Figure 1).
2.3 Measurements
The patients’ baseline characteristics, including age, sex, clinical stage, treatment type, Charlson’s comorbidity index (CCI) score, and comorbidities, were recorded. The patients were classified into three age groups: <30 years, 30-39 years, and 40-49 years. The CCI score was calculated as previously described (23, 24). The patients were divided into three disease severity groups based on CCI score: 0, 1-2, and ≥3. Comorbidities, including congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary disease, liver disease, chronic kidney disease, diabetes mellitus, hyperlipidemia, and hypertension, were identified using the International Classification of Diseases, Ninth Revision, Clinical Modification or the International Classification of Diseases, Tenth Revision, Clinical Modification diagnostic codes (Table S1). The comorbidities were included in the study if they were noted during hospitalization or three or more outpatient visits within one year before the cancer diagnosis.
2.4 Outcomes
The primary outcome of this study was overall mortality, which was identified as death for any reason. In addition, cancer-specific mortality was used to estimate the risk of mortality due to cancer. Mortality was defined using Taiwan’s cause-of-death database.
As different comorbidities may affect the mortality risk, the overall and cancer-specific mortality analyses of patients based on age, sex, clinical stage, and treatment type were estimated for each comorbidity.
2.5 Statistical analysis
The incidence of EO-CRC was estimated as the number of patients aged 20-49 years with CRC divided by the total population of 20-49-year-olds that year. The trend of the mortality rate was defined as the number of patients with EO-CRC who died within one year divided by the total number of patients with EO-CRC that year. Categorical variables, including age group, sex, clinical stage, treatment type, CCI score classification, and comorbidities, are presented as numbers and percentages. Pearson’s chi-square test was used to compare the differences in the categorical variables between patients with EO-CRC who died within five years of the diagnosis and those who survived. Continuous variables, such as age, CCI score, and time to follow-up are presented as mean ± standard deviation. Student’s t-test was used to compare the differences in the continuous variables between patients who died within five years of the diagnosis and those who survived. The Cox proportional regression model was used to estimate the overall and cancer-specific relative risk of mortality. A stratified analysis of age, sex, clinical stage, and treatment type was conducted. All p values are two-tailed, and statistical significance was set at p<0.05. All statistical analyses were conducted using the SAS software (version 9.4; SAS Institute, Inc., Cary, NC, USA).
3 Results
The incidence of EO-CRC significantly increased from 6.04 per 100,000 persons in 2007 to 12.97 per 100,000 persons in 2017, and the mortality rate of enrolled EO-CRC patients decreased from 14.05% in 2007 to 8.25% in 2017 (Figure 2).
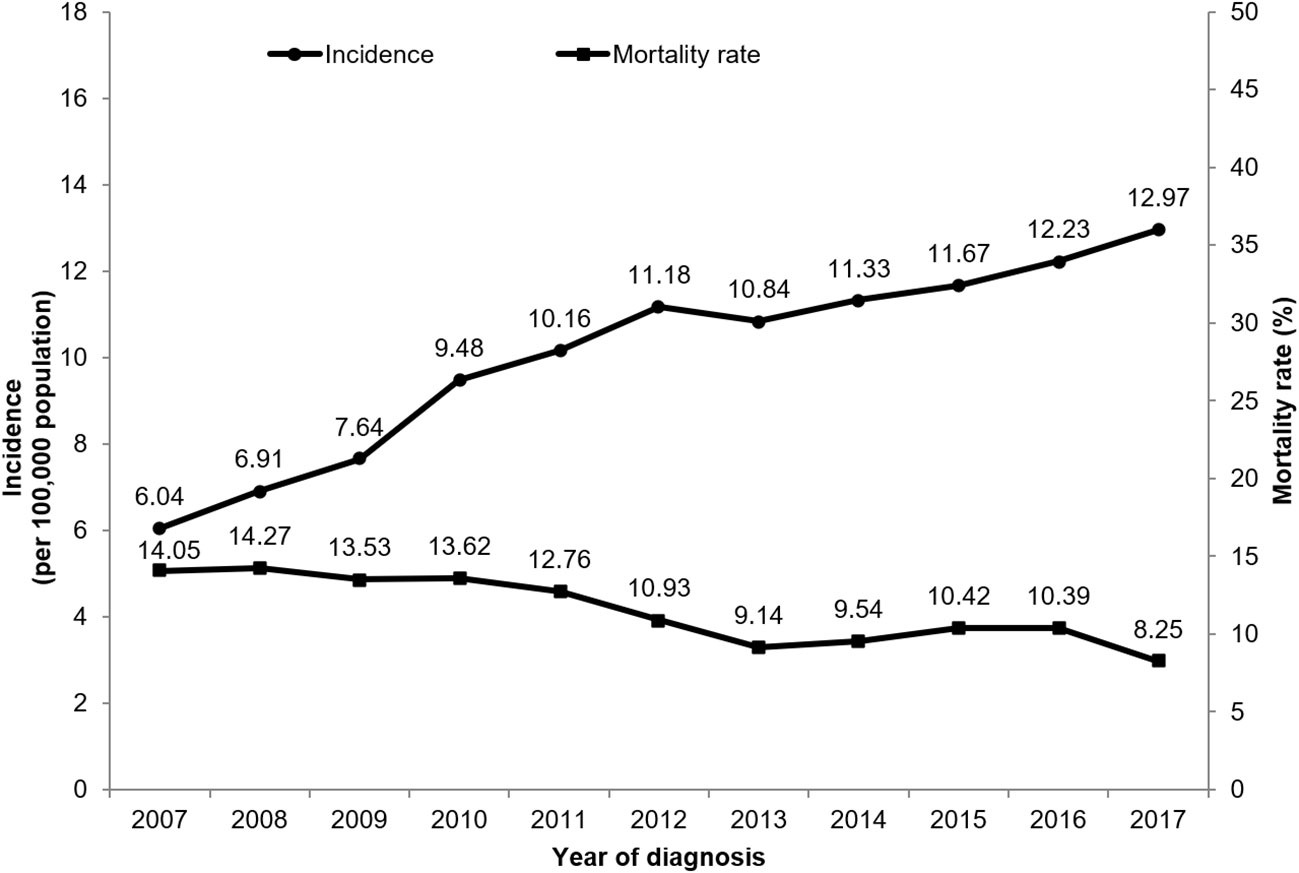
Figure 2 The incidence rate of EO-CRC and the one-year mortality rate of enrolled EO-CRC patients from 2007 to 2017.
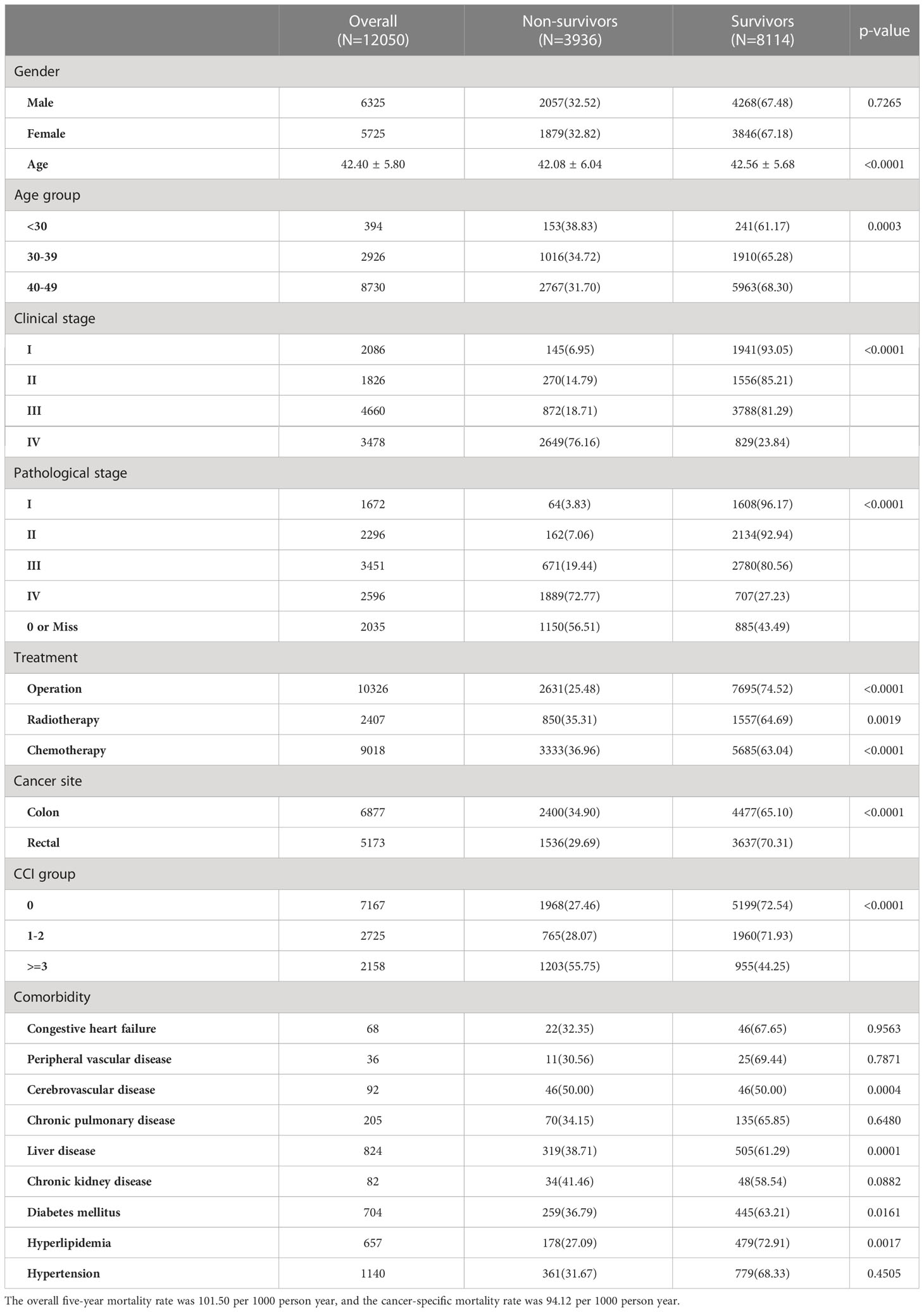
A total of 12,050 patients with EO-CRC, including 6,325 (52.49%) men and 5,725 (47.51%) women, were included in this study (Table 1). The mean patient age was 42.40 ± 5.80 years, and 72.45% of patients were 40-49 years of age. Approximately two-thirds of the patients (67.54%) were diagnosed with late-stage EO-CRC (clinical stages III or IV). The overall five-year mortality rate was 32.66%, and the cancer-specific mortality rate was 30.29%.
Patients < 30 years of age had a higher non-survivor rate (38.83%) than patients aged 30-39 years (34.72%) and those aged 40-49 years (31.70%; p=0.0003). Patients with clinical stage IV EO-CRC had the highest non-survivor rate (p<0.0001). Patients who underwent treatment also show a significant difference between survivors and non-survivors compared with those who did not undergo treatment. Patients with a CCI score ≥ 3 had a significantly higher non-survivor rate (55.75%) than other CCI groups (p<.0001). Patients with a history of cerebrovascular disease, liver disease, diabetes mellitus, or hyperlipidemia had significantly higher percentages of non-survivors than patients without these comorbidities.
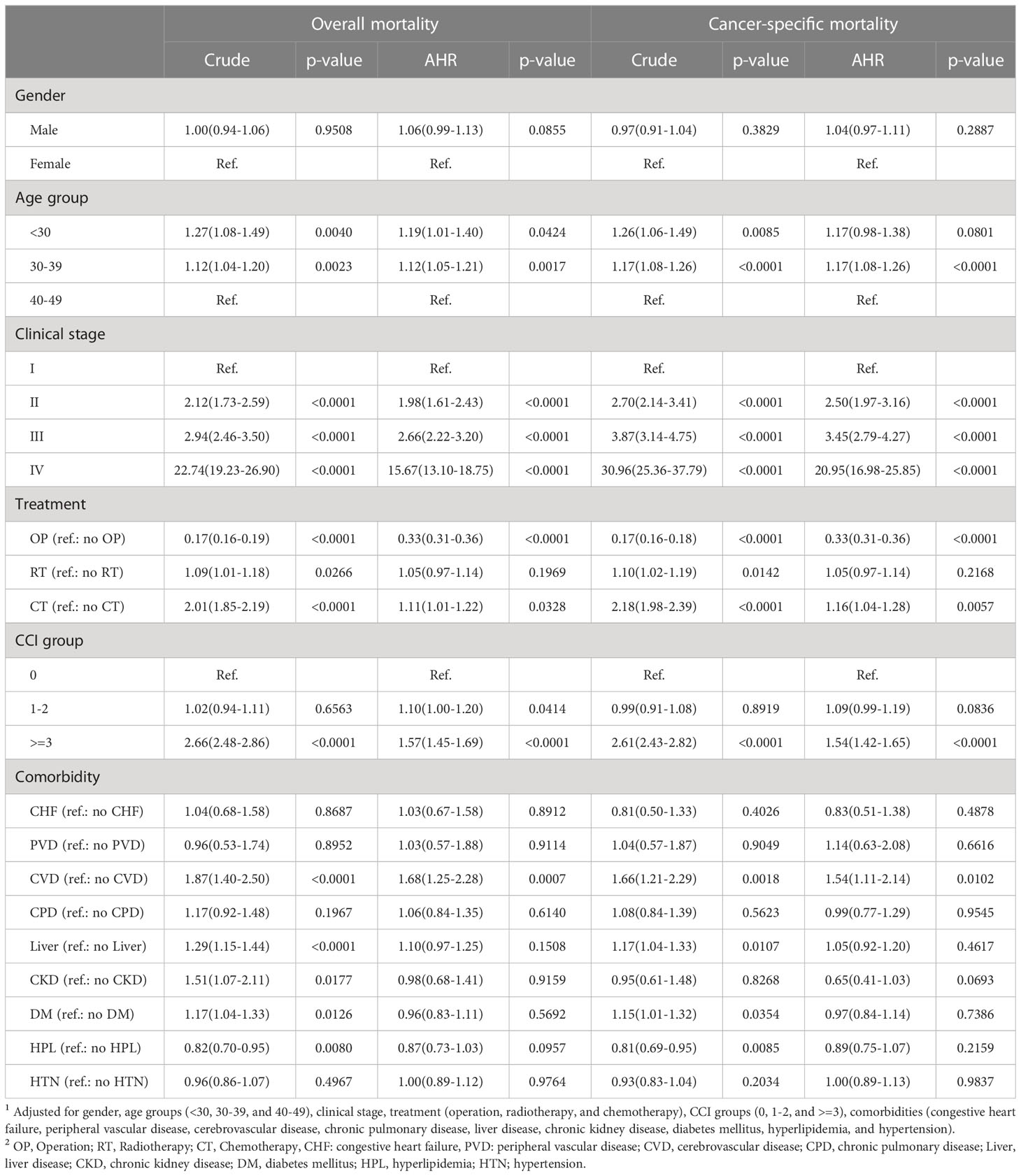
Patients aged <30 years (hazard ratio (HR): 1.19; 95% confidence interval (CI): 1.01-1.40; p=0.0424) and those aged 30-39 years (HR: 1.12; 95% CI: 1.05-1.21; p=0.0017) had a significantly higher overall mortality risk than those aged 40-49 years. Patients with clinical stage IV had a significantly higher mortality risk than those with clinical stage I EO-CRC (HR: 15.67; 95% CI: 13.10-19.75; p<0.0001). Patients who underwent surgical treatment for EO-CRC had a lower mortality risk than those who did not (HR: 0.33; 95% CI: 0.31-0.36; p<0.0001). Patients who underwent chemotherapy had a higher mortality risk than those who did not (HR: 1.11; 95% CI: 1.01-1.22; p=0.0038). Patients with higher CCI scores (CCI score ≥3) had a higher mortality risk than those without comorbidities. Patients with the cerebrovascular disease had a higher mortality risk than those without when adjusted for confounding factors (HR: 1.68; 95% CI: 1.25-2.28, p=0.0007). The cancer-specific mortality risks are similar to those of overall mortality. Patients aged <40 years, those with clinical stage II, III, or IV EO-CRC, those who underwent chemotherapy, those with a CCI score ≥3, and those with cerebrovascular disease all had a higher risk of cancer-specific mortality. Patients who underwent surgical treatment for EO-CRC had a significantly lower mortality risk (Table 2).
Both male and female patients with cerebrovascular disease had a significantly higher overall mortality risk compared with those without (males: HR: 1.61; 95% CI: 1.11-2.34; p=0.0128; females: HR: 1.69; 95% CI: 1.00-2.86; p=0.0485) (Table 3). Patients aged 40-49 years with cerebrovascular disease had a significantly higher risk of mortality than patients aged 40-49 years without the cerebrovascular disease (HR: 1.63; 95% CI: 1.17-2.27; p=0.0037). Patients with cerebrovascular disease and early clinical stage EO-CRC had a higher risk of overall mortality than patients without (HR: 3.58; 95% CI: 1.84-6.96; p=0.0002), as did patients with cerebrovascular disease and late clinical-stage EO-CRC (HR: 1.49; 95% CI: 1.06-2.09; p=0.0221). Patients with chronic kidney disease and early clinical stage EO-CRC had a higher mortality risk than patients without chronic kidney disease (HR: 2.31; 95% CI: 1.08-4.96; p=0.0138). However, patients with hyperlipidemia and early clinical stage EO-CRC had a lower mortality risk than patients without hyperlipidemia (HR: 0.44; 95% CI: 0.26-0.76; p=0.0027). Patients who underwent radiotherapy had a higher overall mortality risk when paired with cerebrovascular disease CVD (HR: 2.90; 95% CI: 1.41-5.96; p=0.0037) or liver disease (HR: 1.38; 95% CI: 1.04-1.85; p=0.0285) than those without these comorbidities.
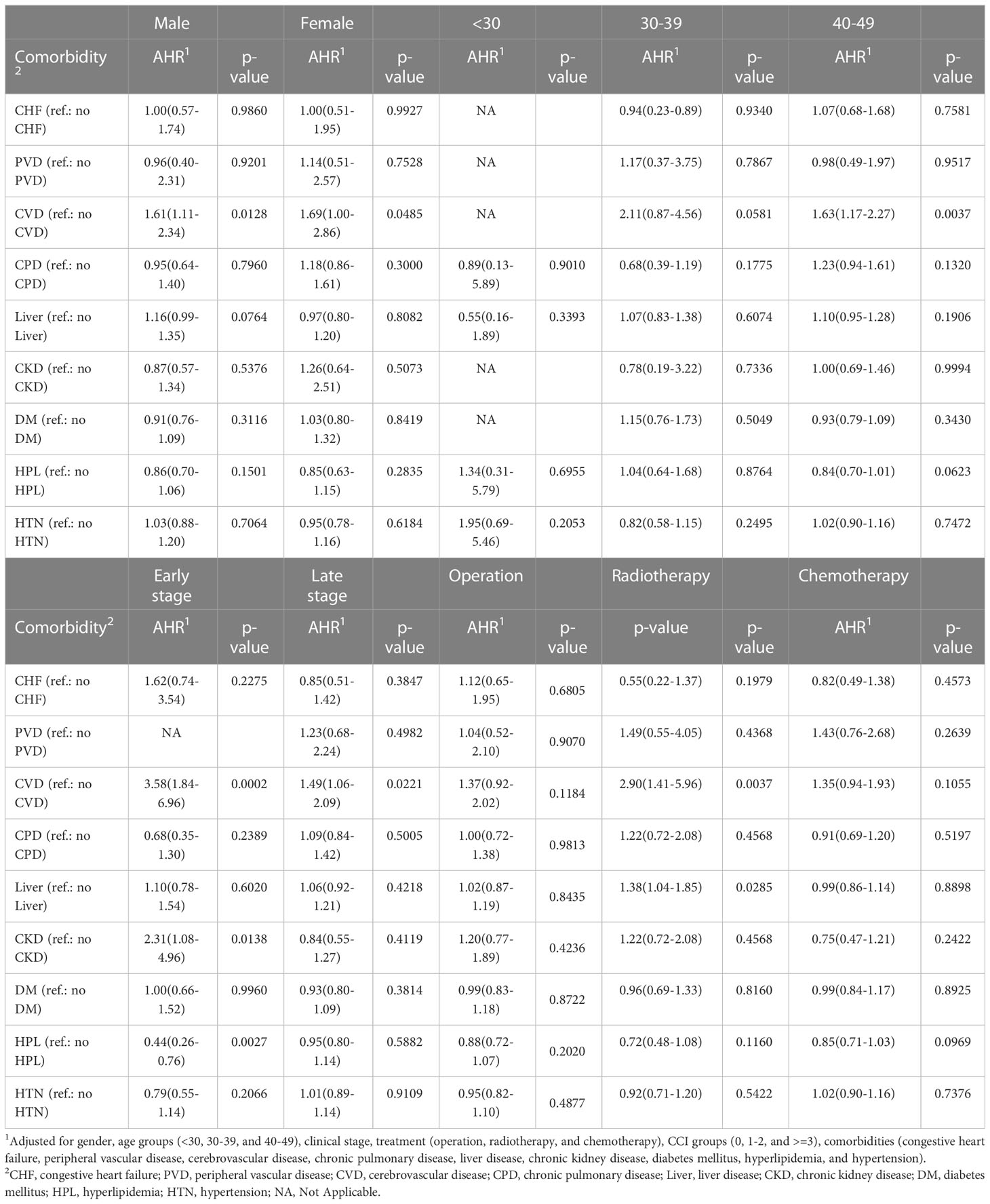
Table 3 The subgroup analysis of 5-year overall mortality risk among EO-CRC patients of different sex, age, clinical stages, and treatment types.
Male patients with cerebrovascular disease had a higher cancer-specific mortality risk than those without the cerebrovascular disease (HR: 1.59; 95% CI: 1.07-2.37; p=0.0206) (Table 4). Male patients with chronic kidney disease had a lower cancer-specific mortality risk than those without chronic kidney disease (HR: 0.54; 95% CI: 0.31-0.96; p=0.0357). Among patients <30 years of age, those with hypertension had a higher cancer-specific mortality risk than those without hypertension (HR: 2.53; 95% CI: 1.01-6.32; p=0.0471). Among patients aged 30-39 years, those with cerebrovascular disease had a higher cancer-specific mortality risk than those without the cerebrovascular disease (HR: 2.20; 95% CI: 1.02-4.76; p=0.0451). Patients with hyperlipidemia and early clinical stage EO-CRC had a lower mortality risk than those without hyperlipidemia (HR: 0.47; 95% CI: 0.26-0.85; p=0.0121). Patients with cerebrovascular disease and early clinical stage EO-CRC had a higher cancer-specific mortality risk than those without the cerebrovascular disease (HR: 3.30; 95% CI: 1.50-7.27; p=0.0031). Patients with chronic kidney disease and late clinical-stage EO-CRC had a lower risk of mortality than those without chronic kidney disease (HR: 0.54; 95% CI: 0.32-0.92; p=0.0226). Among patients undergoing radiotherapy, those with cerebrovascular disease had a higher cancer-specific mortality risk than those without the cerebrovascular disease (HR: 2.73; 95% CI: 1.27-5.89; p=0.0102). Among patients undergoing chemotherapy, those with chronic kidney disease had a lower cancer-specific mortality risk than those without chronic kidney disease (HR: 0.52; 95% CI: 0.29-0.93; p=0.0281). In addition, the association between different treatment types and CCI is presented in Supplemental Table 2.
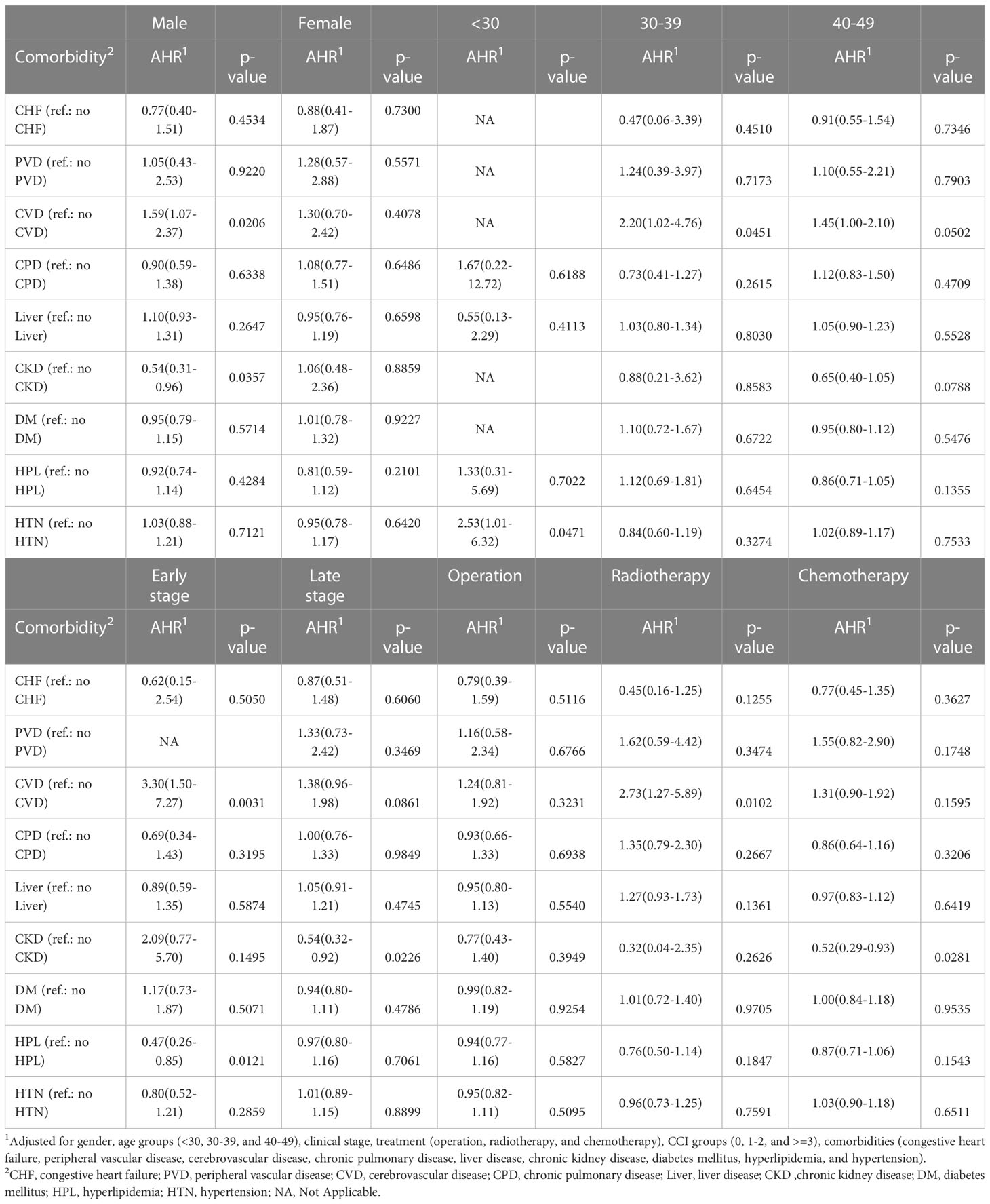
Table 4 The subgroup analysis of 5-year cancer-specific mortality risk among EO-CRC patients of different sex, age, clinical stages, and treatment types.
4 Discussion
The incidence of EO-CRC in Taiwan increased from 2007 to 2017. In our current study, we found that patients with CVD had a higher mortality risk (HR: 1.68; 95% CI: 1.25-2.28; p=0.0007). After subgroup analyses based on age, sex, clinical stage, and treatment type, patients with CVD still had a higher overall mortality risk than those without CVD, except for patients undergoing surgery and chemotherapy. Patients with chronic kidney disease had a higher mortality risk in the early clinical stages (HR: 2.31; 95% C.I.: 1.08-4.96; p=0.0138). Male patients, those with early cancer stage, those undergoing radiotherapy, and those aged 30-39 years or 40-49 years had higher mortality risks than others.
While the exact causes of the increased incidence of EO-CRC are unclear, several possible explanations for the increased incidence of EO-CRC exist. As diet and lifestyle play an important role in the development of CRC, individuals who consume high amounts of red meat, processed meat, and foods high in calories, fat, sugar, and processed carbohydrates become overweight or obese, which is also associated with an increased risk of CRC (25). Shih et al. (26) found that 23.92% of female school children between 11-12 years of age were overweight (25 < BMI ≤ 30), and 9.83% were obese (BMI >30), while 27.08% of boys aged 11-12 years were overweight and 20.35% were obese. Obesity is a risk factor for CRC and has been proven. It has been controversial whether body mass index was a prognostic role in determining the prognosis of patients suffering from CRC. Fanipakdel et al. retrospectively identified 920 patients with non-metastatic CRC and found no statistically significant difference between BMI and mortality (27). Chang et al. conducted three nutrition and health surveys in Taiwan from 1993–1996, 2005–2008, and 2013–2014 (28). The results of these surveys indicated that 80% of the population in Taiwan was <50 years of age and that the prevalence of morbid obesity (BMI ≥35 kg/m (2)) and obesity (BMI ≥27 kg/m (2)) both increased from the first study period to the last. Patients with morbid obesity tended to have lower levels of physical activity in these surveys. Furthermore, patients with morbid obesity consumed more red meat, processed meat products, and sugar-sweetened beverages and lower amounts of fresh fruits, nuts, and dairy products than individuals with normal weight. In Taiwan, the prevalence of obesity increased from 11.8% in 1993-1996 to 17.9% in 2005-2008 and 22.0% in 2013–2014. The prevalence of obesity indicates that the diet of the Taiwanese population has transitioned to one that includes more processed foods high in fats and sugar and calorie-dense foods and beverages (29). These changes lead to obesity and nutrition-related diet-related diseases (30). The rapid growth of international grocery chains and fast-food restaurants leads to food consumption patterns that are similar to those of a Western diet in Asian countries (31).
The underappreciation of the increasing risk of CRC among young adults may also contribute to the increased incidence of EO-CRC as nonspecific symptoms, such as changes in bowel habits, rectal bleeding with bright red blood, blood in the stool, cramping or abdominal pain, and weight loss may be overlooked. Young adults have lower rates of healthcare system utilization than older adults. In 2012, 27% of young American adults (aged 19-34 years) did not have health insurance (32). In 2017, 84.6% of the uninsured population in the United States was 19-64 years old, including one-fourth who were 26-34 years old and one-fifth who were 34-44 years old (33). Taiwan launched a single-payer National Health Insurance program on March 1, 1995, with 99.9% of Taiwan’s population enrolled (34). There are fewer uninsured young adults in Taiwan. Barriers to optimal health care for young adults have been reported (35). Young adults have poor health literacy, little knowledge regarding how to access the medical system and identify providers in the community, and tend to feel embarrassed during doctor visits. In addition, disease presentations in young adults may vary from those in older patients. Physicians who treat diseases that commonly affect older adults, such as cancer, may be less aware of the disease processes in young adults. Studies regarding EO-CRC and oncology training for physicians have emphasized the importance of understanding tumor biology, revealing that the manifestation, epidemiology, and biology of many types of cancer are different in young and old patients (36).
The age of regular CRC screening may also contribute to the increased incidence of EO-CRC. A systematic review confirmed that fecal occult blood test screening improves the survival rate of CRC (37). In 2021, the US cancer screening recommendations for CRC suggested screening adults aged 45-49 years (38) based on the increased incidence of CRC, disease burden, and the assumption that this screening age would have more benefits than screening patients >50 years of age (39). Although most organizations screen adults with an average risk of CRC at 50 years of age, young adults with an increased risk of CRC must undergo more frequent or earlier screening. In our study, EO-CRC patients aged 30-39 years had a higher overall mortality risk than those aged 40-49 years. In addition, cerebrovascular disease played a significant role in prognosis. We recommend screening young adults with cerebrovascular disease for CRC.
Comorbidities may positively or negatively affect the prognoses of patients with CRC (40). For example, patients may schedule regular checkups and necessary follow-up visits with health providers due to comorbidities, at which CRC may be diagnosed at an earlier stage. In contrast, signs and symptoms of CRC may be incorrectly regarded as symptoms of pre-existing comorbidities and may delay diagnosis (41). After the diagnosis of CRC, the timing of treatments, treatment course, and clinical outcomes may be affected by comorbidities. Our results also indicated that EO-CRC patients with a CCI score of more than 2 had higher overall and cancer-specific mortality risk than those with a CCI score of 0. Fowler et al. (42) determined the prevalence of comorbidities in patients with CRC via a survey study and found that one-fourth of patients with CRC had one comorbidity, while 17.7-27.6% of patients had more than one comorbidity. The three most prevalent comorbidities in patients with CRC were hypertension, chronic obstructive pulmonary disease, and diabetes. In this study, the three most common comorbidities were a cerebrovascular disease, chronic kidney disease, and liver disease. The differences in comorbidities may be because 80% of the patients with CRC in the previous study were >60 years of age, while the patients in this study were < 50 years old. Erichsen et al. (43) found that the presence of comorbidities increases the mortality risk in elderly patients with CRC, especially in the first year after diagnosis. In this study, EO-CRC patients with a CCI score ≥ 3 had a higher mortality risk than those without comorbidities. After further analysis, the mortality risk of patients with cerebrovascular disease and chronic kidney disease was increased. Therefore, further research is needed to understand the roles of cerebrovascular disease and chronic kidney disease in patients with EO-CRC.
The incidence of EO-CRC has been reported in several countries, including the USA (44). In Australia and China, the incidence of EO-CRC has increased, which may be attributed to the adoption of Western lifestyles and dietary habits (45). More studies are needed to monitor the incidence of EO-CRC and further evaluate the screening procedures. Furthermore, the underlying causes of EO-CRC should be investigated so that young adults at high risk for CRC who would benefit from early screening can be identified. Physicians must also be aware of the increased incidence of EO-CRC and the appropriate management strategies for comorbidities.
The main strength of this study is the results from the longitudinal population database, which could present the full picture of mortality risk from comorbidities in patients with EO-CRC. However, several limitations of the current study need to be acknowledged. First, our data did not offer information on the socioeconomic status and lifestyle behaviors of the patients, which may affect the estimated mortality risk. Second, misclassification bias might have occurred as the claims data may have differences in the definition of the comorbidities based on the diagnostic codes from the international classification of diseases system. However, the misclassification bias of TCR and the National Health Insurance research database has been validated by a previous study (46). Another potential limitation of registry data may be the lack of actual assessment of patients due to patients not continuing treatments; therefore, the main cause of death or the presence of recurrence may be unclear. However, the national cancer registry of Taiwan has been in operation for over 40 years and the completeness of the TCR is approximately 98.4% (22). Since the TCR is linked to the cause-of-death database and the National Health Insurance research database, we were able to follow these patients almost completely, from being diagnosed with cancer via treatments to death. This limitation can be reduced from linked data. Finally, due to the limited administrative registry databases, information on lifestyles, dietary information, family history of CRC, functional status, physical activity, and CRC screening that may be independently associated with risk of mortality has not been comprehensively recorded.
In conclusion, identifying specific risk factors in patients with EO-CRC allows for the risk stratification of a specific target screening group. More studies are needed to determine if the increased incidence of EO-CRC can be reduced and to identify the associations between comorbidities and EO-CRC. The appropriate management of comorbidities is also important in lowering the incidence of EO-CRC.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The data sources are the Taiwan Nation Health Insurance Database and Taiwan Cancer Registry. The data are available with the permission from Taiwan Health and Welfare Data Science Centre (https://dep.mohw.gov.tw/DOS/np-2497-113.html, accessed on 06 Jun 2022). Restrictions apply to the availability of these data, which were used under license for this study.
Ethics statement
The studies involving human participants were reviewed and approved by Research Ethics Committee of Chi Mei Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization, S-CY, Y-LS and K-ML and C-HH. Methodology, S-CY, K-ML and C-HH. Formal analysis, Y-CW. Writing—original draft preparation, S-CY, Y-LS and K-ML. Writing—review and editing, Y-LS, K-ML and C-HH. Visualization, Y-CW. Funding acquisition, C-HH and J-JW. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Ministry of Health and Welfare, Taiwan using health and welfare surcharge of tobacco products, grant number MOHW110-TDU-B-212-144020 and Chi Mei Medical Center, grant number CMFHR11186.
Acknowledgments
We are grateful to the Health Data Science Center, National Cheng Kung University Hospital for providing administrative and technical support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1139925/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Cheng E, Blackburn HN, Ng K, Spiegelman D, Irwin ML, Ma X, et al. Analysis of survival among adults with early-onset colorectal cancer in the national cancer database. JAMA Netw Open (2021) 4:e2112539. doi: 10.1001/jamanetworkopen.2021.12539
3. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin (2020) 70:145–64. doi: 10.3322/caac.21601
4. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the united states, 1975-2010. JAMA Surg (2015) 150:17–22. doi: 10.1001/jamasurg.2014.1756
5. US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, et al. Screening for colorectal cancer: Us preventive services task force recommendation statement. JAMA (2021) 325:1965–77. doi: 10.1001/jama.2021.6238
6. Chauvenet M, Cottet V, Lepage C, Jooste V, Faivre J, Bouvier AM. Trends in colorectal cancer incidence: A period and birth-cohort analysis in a well-defined french population. BMC Cancer (2011) 11:1–6. doi: 10.1186/1471-2407-11-282
7. Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969-2010. Cancer Epidemiol (2016) 42:90–100. doi: 10.1016/j.canep.2016.03.009
8. Young JP, Win AK, Rosty C, Flight I, Roder D, Young GP, et al. Rising incidence of early-onset colorectal cancer in australia over two decades: Report and review. J Gastroenterol Hepatol (2015) 30:6–13. doi: 10.1111/jgh.12792
9. Sung JJY, Chiu HM, Jung KW, Jun JK, Sekiguchi M, Matsuda T, et al. Increasing trend in young-onset colorectal cancer in asia: More cancers in men and more rectal cancers. Am J Gastroenterol (2019) 114:322–9. doi: 10.14309/ajg.0000000000000133
10. Welfare M. 2020 cause of death statistics . Available at: https://www.mohw.gov.tw/cp-5256-63399-2.html.
11. Onyoh EF, Hsu W-F, Chang L-C, Lee Y-C, Wu M-S, Chiu H-M. The rise of colorectal cancer in Asia: epidemiology, screening, and management. Curr Gastroenterol Rep (2019) 21:1–10. doi: 10.1007/s11894-019-0703-8
12. Wang YW, Chen HH, Wu MS, Chiu HM. Taiwanese Nationwide colorectal cancer screening program current status and future challenge of population-based organized colorectal cancer screening: Lesson from the first decade of taiwanese program. J Formos Med Assoc (2018) 117:358–64. doi: 10.1016/j.jfma.2017.09.010
13. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut (2019) 68:2179–85. doi: 10.1136/gutjnl-2019-319511
14. Nguyen LH, Liu PH, Zheng X, Keum N, Zong X, Li X, et al. Sedentary behaviors, tv viewing time, and risk of young-onset colorectal cancer. JNCI Cancer Spectr (2018) 2:pky073. doi: 10.1093/jncics/pky073
15. Hofseth LJ, Hebert JR, Chanda A, Chen H, Love BL, Pena MM, et al. Early-onset colorectal cancer: Initial clues and current views. Nat Rev Gastroenterol Hepatol (2020) 17:352–64. doi: 10.1038/s41575-019-0253-4
16. Holleczek B, Rossi S, Domenic A, Innos K, Minicozzi P, Francisci S, et al. On-going improvement and persistent differences in the survival for patients with colon and rectum cancer across europe 1999–2007–results from the eurocare-5 study. Eur J Cancer (2015) 51:2158–68. doi: 10.1016/j.ejca.2015.07.024
17. Petrelli F, Tomasello G, Borgonovo K, Ghidini M, Turati L, Dallera P, et al. Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol (2017) 3:211–9. doi: 10.1001/jamaoncol.2016.4227
18. Morishima T, Matsumoto Y, Koeda N, Shimada H, Maruhama T, Matsuki D, et al. Impact of comorbidities on survival in gastric, colorectal, and lung cancer patients. J Epidemiol (2018) 29:110–5. doi: 10.2188/jea.JE20170241
19. Hines RB, Shanmugam C, Waterbor JW, McGwin G Jr, Funkhouser E, Coffey CS, et al. Effect of comorbidity and body mass index on the survival of african-american and caucasian patients with colon cancer. Cancer (2009) 115:5798–806. doi: 10.1002/cncr.24598
20. Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol (2000) 53:1258–67. doi: 10.1016/s0895-4356(00)00256-0
21. Chiang CJ, You SL, Chen CJ, Yang YW, Lo WC, Lai MS. Quality assessment and improvement of nationwide cancer registration system in taiwan: A review. Jpn J Clin Oncol (2015) 45:291–6. doi: 10.1093/jjco/hyu211
22. Chiang CJ, Wang YW, Lee WC. Taiwan’s nationwide cancer registry system of 40 years: Past, present, and future. J Formosan Med Assoc (2019) 118:856–8. doi: 10.1016/j.jfma.2019.01.012
23. Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in icd-9-cm and icd-10 administrative data. Med Care (2005) 43:1130–9. doi: 10.1097/01.mlr.0000182534.19832.83
24. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol (2011) 173:676–82. doi: 10.1093/aje/kwq433
25. Pietrzyk Ł. Food properties and dietary habits in colorectal cancer prevention and development. Int J Food Prop (2017) 20:2323–43. doi: 10.1080/10942912.2016.1236813
26. Shih SF, Liu CH, Liao LL, Osborne RH. Health literacy and the determinants of obesity: A population-based survey of sixth grade school children in taiwan. BMC Pub Health (2016) 16:280. doi: 10.1186/s12889-016-2879-2
27. Fanipakdel A, Hosseini S, Javadinia SA, Jeddi FA, Vasei M. The prognostic role of body mass index in survival of non-metastatic postoperative patients with colorectal cancer. Int J Cancer Manage (2021) 14:1–6. doi: 10.5812/ijcm.110257
28. Chang HC, Yang HC, Chang HY, Yeh CJ, Chen HH, Huang KC, et al. Morbid obesity in taiwan: Prevalence, trends, associated social demographics, and lifestyle factors. PloS One (2017) 12:e0169577. doi: 10.1371/journal.pone.0169577
29. Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev (2012) 70:3–21. doi: 10.1111/j.1753-4887.2011.00456.x
30. Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Pub Health Nutr (2004) 7:123–46. doi: 10.1079/phn2003585
31. Pingali P. Westernization of asian diets and the transformation of food systems: Implications for research and policy. Food Pol (2007) 32:281–98. doi: 10.1016/j.foodpol.2006.08.001
32. Bureau U.S.C. Infographic profiles young adults without health insurance . Available at: https://www.census.gov/newsroom/archives/2013-pr/cb13-tps100.html.
33. Bureau U.S.C. Most uninsured were working-age adults . Available at: https://www.census.gov/library/stories/2018/09/who-are-the-uninsured.html.
34. Wu T-Y, Majeed A, Kuo KN. An overview of the healthcare system in Taiwan. London J Prim Care (2010) 3:115–9. doi: 10.1080/17571472.2010.11493315
35. Bonnie RJ, Stroud C, Breiner H. Committee on Improving the Health, Safety, and Well-Being of Young Adults; Board on Children, Youth, and Families; Institute of Medicine; Investing in the Health and Well-Being of Young Adults. Washington (DC): National Academies Press (US).
36. Hayes-Lattin B, Mathews-Bradshaw B, Siegel S. Adolescent and young adult oncology training for health professionals: A position statement. J Clin Oncol (2010) 28:4858–61. doi: 10.1200/JCO.2010.30.5508
37. Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): An update. Am J Gastroenterol (2008) 103:1541–9. doi: 10.1111/j.1572-0241.2008.01875.x
38. Barsouk A, Saginala K, Aluru JS, Rawla P, Barsouk A. Us cancer screening recommendations: Developments and the impact of covid-19. Med Sci (2022) 10:1–10. doi: 10.3390/medsci10010016
39. Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the american cancer society. CA Cancer J Clin (2018) 68:250–81. doi: 10.3322/caac.21457
40. Van Leersum N, Janssen-Heijnen M, Wouters M, Rutten H, Coebergh JW, Tollenaar R, et al. Increasing prevalence of comorbidity in patients with colorectal cancer in the south of the netherlands 1995–2010. Int J Cancer (2013) 132:2157–63. doi: 10.1002/ijc.27871
41. Mounce LT, Price S, Valderas JM, Hamilton W. Comorbid conditions delay diagnosis of colorectal cancer: A cohort study using electronic primary care records. Br J Cancer (2017) 116:1536–43. doi: 10.1038/bjc.2017.127
42. Fowler H, Belot A, Ellis L, Maringe C, Luque-Fernandez MA, Njagi EN, et al. Comorbidity prevalence among cancer patients: A population-based cohort study of four cancers. BMC Cancer (2020) 20:2. doi: 10.1186/s12885-019-6472-9
43. Erichsen R, Horváth-Puhó E, Iversen LH, Lash TL, Sørensen HT. Does comorbidity interact with colorectal cancer to increase mortality? a nationwide population-based cohort study. Br J Cancer (2013) 109:2005–13. doi: 10.1038/bjc.2013.541
44. Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the united states, 1974-2013. J Natl Cancer Inst (2017) 109:1–6. doi: 10.1093/jnci/djw322
45. Deng Y. Rectal cancer in asian vs. Western countries: Why the variation in incidence? Curr Treat Opt Oncol (2017) 18:64. doi: 10.1007/s11864-017-0500-2
Keywords: early-onset colorectal cancer, mortality risk, comorbidities, Taiwan cancer registry, real-world database (RWD)
Citation: Yu S-C, Shiue Y-L, Wu Y-C, Wang J-J, Liao K-M and Ho C-H (2023) The mortality risk in patients with early onset colorectal cancer: the role of comorbidities. Front. Oncol. 13:1139925. doi: 10.3389/fonc.2023.1139925
Received: 08 January 2023; Accepted: 29 March 2023;
Published: 14 April 2023.
Edited by:
Alireza Sadjadi, Tehran University of Medical Sciences, IranReviewed by:
Seyed Alireza Javadinia, Sabzevar University of Medical Sciences, IranMesnad Alyabsi, King Abdullah International Medical Research Center (KAIMRC), Saudi Arabia
Copyright © 2023 Yu, Shiue, Wu, Wang, Liao and Ho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yow-Ling Shiue, c2hpcmxleUBpbXN0Lm5zeXN1LmVkdS50dw==; Kuang-Ming Liao, YWJjODg3MEB5YWhvby5jb20udHc=; Chung-Han Ho, aG8uYy5oYW5rQGdtYWlsLmNvbQ==
 Shou-Chun Yu1,2
Shou-Chun Yu1,2 Yow-Ling Shiue
Yow-Ling Shiue Yu-Cih Wu
Yu-Cih Wu Kuang-Ming Liao
Kuang-Ming Liao Chung-Han Ho
Chung-Han Ho