- 1Department of Pharmacy, National Cancer Center Hospital East, Kashiwa, Japan
- 2Department of Clinical Pharmacology and Therapeutics, Kyoto University Hospital, Kyoto, Japan
- 3Department of Head and Neck Medical Oncology, National Cancer Center Hospital East, Kashiwa, Japan
- 4Department of Otorhinolaryngology-Head and Neck Surgery, Tokyo Medical University, Shinjuku, Japan
- 5Department of Head and Neck Medical Oncology, Miyagi Cancer Center, Natori, Japan
Background: In the phase 3 SELECT study, lenvatinib significantly improved prognostic outcomes vs. placebo in patients with radioiodine-refractory differentiated thyroid cancer (RR-DTC). However, toxicity of lenvatinib is sometimes considerable and requires frequent dose interruptions and modifications. Recently, planned drug holidays have been proposed as a means of avoiding severe adverse events (AEs).
Methods: We retrospectively reviewed medical records to compare the efficacy and safety of lenvatinib in RR-DTC patients who underwent planned drug holidays (planned holiday group) vs. those who received conventional daily oral administration (daily group).
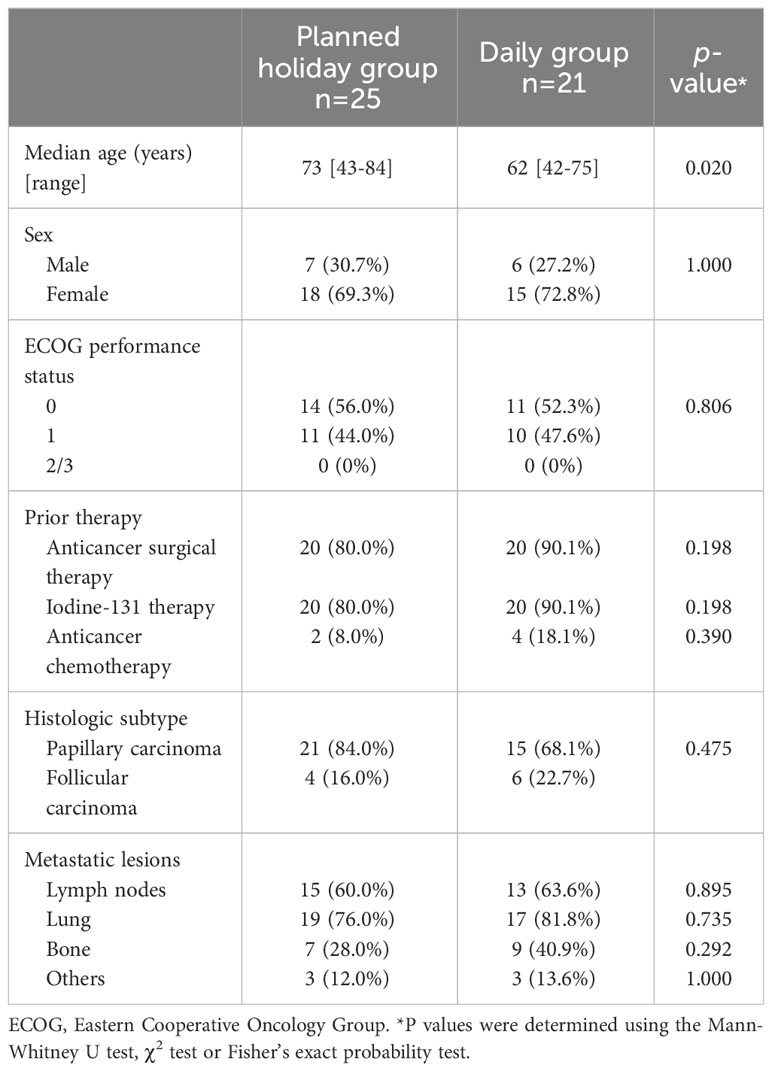
Results: The subjects were 25 patients in the planned holiday group and 21 in the daily group. Median age was 73 years (range 43-84) and 62 years (range 42-75), and histologic subtype of papillary/follicular was 21/4 cases and 15/6 cases, respectively. Time to treatment failure (TTF) and overall survival (OS) were significantly longer in the planned holiday group than the daily group (not reached [NR] vs. 14.9 months, hazard ratio [HR] 0.25, 95% confidence interval [Cl] 0.11-0.58, p<0.001; NR vs. 26.6 months, HR 0.20, 95% CI 0.073-0.58, p=0.001, respectively). Median progression-free survival (PFS) was NR in the planned holiday group vs. 15.1 months in the daily group (HR 0.31, 95% CI 0.14-0.68, p=0.002). Duration of the period with lenvatinib dose ≥10 mg was significantly longer in the planned holiday group (NR vs. 6.5 months, HR 0.22, 95% CI 0.10-0.49, p<0.001), and the frequency of drug interruption due to intolerable AEs was lower (68.0% vs. 95.2%, p=0.027).
Conclusion: Planned drug holidays for lenvatinib demonstrated significantly longer PFS, TTF, and OS than daily oral administration, and less intolerable toxicity leading to further unplanned treatment interruption. These benefits were apparently associated with a more extended period of lenvatinib administration at ≥10 mg. These findings might contribute to a favorable patient prognosis and safer toxicity profile.
1 Introduction
Thyroid cancer accounts for 1% of all cancer cases and 90% of malignant endocrine tumors. The prognosis of thyroid cancer is favorable, with a 10-year survival rate of 85%. Further, the incidence of distant metastasis is less than 5%, and 10-year survival in patients with metastasis is 25%–42% (1, 2). Currently, the initial treatment for differentiated thyroid cancer (DTC) is surgery, followed in some high-risk cases by radioactive iodine internal radiation iodine therapy (RAI) and TSH suppression using 131I (1). However, prognosis is poor in patients who are refractory to RAI (3). Lenvatinib is an oral multikinase inhibitor that inhibits vascular endothelial growth factor receptors (VEGFR) 1–3, fibroblast growth factor receptors (FGFR) 1–4, platelet-derived growth factor receptor a, and RET and KIT signaling pathways (4–6). In the SELECT trial, an international randomized phase 3 study in patients with RAI-refractory thyroid cancer, lenvatinib significantly prolonged median progression-free survival (PFS) to 18.3 months, compared to 3.6 months with a placebo, and improved overall response rate (ORR) (7). Accordingly, lenvatinib is now considered the standard therapy for thyroid cancer which is unresectable and refractory to treatment with radioactive iodine.
Despite these significant treatment benefits, the incidence of adverse events (AEs) in the SELECT trial was 97.3%, and 89.7% of patients experienced temporary interruption or dose reduction of lenvatinib (7). A subanalysis of the Japanese population also showed that all cases experienced AEs, and 93.3% of cases required temporary drug interruption or dose reductions to manage AEs due to lenvatinib (8). A systematic review reported that more than half of patients treated with lenvatinib experienced AEs such as proteinuria and fatigue, and 15%–25% of patients had grade > 3 AEs, including thrombocytopenia and hypertension (9). These findings indicate that continuation of lenvatinib requires proper management of adverse drug reactions. Another subgroup analysis of the SELECT study reported a negative correlation between treatment effect and duration of drug interruption due to lenvatinib-induced AEs. This study suggested that minimizing toxicity-induced continuous lenvatinib interruption might prolong PFS (10). Together, these findings indicate that improving treatment outcomes in lenvatinib therapy requires adequate management against AEs that may cause prolonged treatment interruption.
One general strategy in the management of intolerable AEs is rescheduling of the administration schedule. For example, the standard treatment schedule for sunitinib, regorafenib, and S-1 is four weeks of administration and two weeks of drug holiday. However, toxicities became more acceptable when the administration schedule was modified to two weeks on one-week off, without any sacrifice in anti-tumor effects (11–13). Regarding lenvatinib for hepatocellular carcinoma, weekend-off administration is reportedly helpful in maintaining therapeutic effect and improving overall survival (14). Moreover, planned drug holidays have been proposed to avoid severe AEs in thyroid cancer (15). The planned drug holiday is adjusted depending on the patient’s AE pattern, which means that the strategy is considered only for patients with intolerable AEs and is not constitutionally applied in patients who can tolerate daily administration. Thus, planned drug holidays appear a promising strategy for the continuation of anticancer treatment in patients intolerant of conventional schedules. However, the benefits of planned drug holidays have yet to be clarified.
Here, we report the potential impact of planned drug holiday administration on patient prognosis and safety in DTC patients, as well as compliance with lenvatinib.
2 Materials and methods
2.1 Patients
We retrospectively reviewed the medical records of recurrent or metastatic thyroid cancer patients treated with lenvatinib at the National Cancer Center Hospital East, Kashiwa, Japan, from May 2011 to December 2019 and compared the efficacy and safety of planned drug holiday administration with daily administration. Inclusion criteria were (1) pathologically proven papillary or follicular thyroid cancer, and (2) RAI refractory or RAI not indicated. Exclusion criteria were (1) histology of poorly differentiated cancer, medullary cancer, and anaplastic thyroid cancer, (2) indication for definitive treatment (surgery or radiotherapy), (3) every other day administration not defined as planned drug holiday administration and (4) daily protocol administration in a phase 2 or 3 clinical trial of a drug and subsequent planned drug holiday administration in daily practice after post-marketing in Japan. Written informed consent for Lenvatinib therapy was obtained from each patient. In the process, a potential modification of the treatment schedule and drug dose considering the adverse event was explained. The degree of proteinuria was assessed by the dipstick method in all cases throughout treatment (16), with grade 3 proteinuria defined as 3+ or above, as measured by the test in the current study. The study for summarizing their clinical information was approved by the Clinical Research and Ethical Review Board of the National Cancer Center East (task number: 2016-245).
2.2 Definition of planned drug holiday administration
The actual procedure of the drug holiday is demonstrated in Figure 1. We defined a planned drug holiday as an intentional drug interruption to avoid a repeat of treatment withdrawal which would eventually leads to tumor regrowth due to intolerable AEs. The schedule of the planned drug holiday was set as follows: if severe or intolerable AEs occurred at X days after the initiation of lenvatinib, administration in the next cycle should continue until day “X-1” (Figure 1A). Following initial introduction, the duration of drug interruption (duration of drug holiday) is determined according to recovery from the corresponding AEs, and the presence or absence of tumor progression during the treatment interruption. In patients whose AEs recovered within seven days after treatment cessation, a one-week drug holiday was applied. In patients whose tumor growth occurred Y days after treatment cessation and Y was less than one week, a drug holiday duration of “Y-1” days was applied. On the other hand, if a patient did not recover from the AE within one week after treatment cessation, the planned drug holiday could be extended to 14 days if disease progression did not occur (Figure 1B), and if an adverse event did not improve after 14 days off or day “Y-1”, dose reduction should be considered (15).
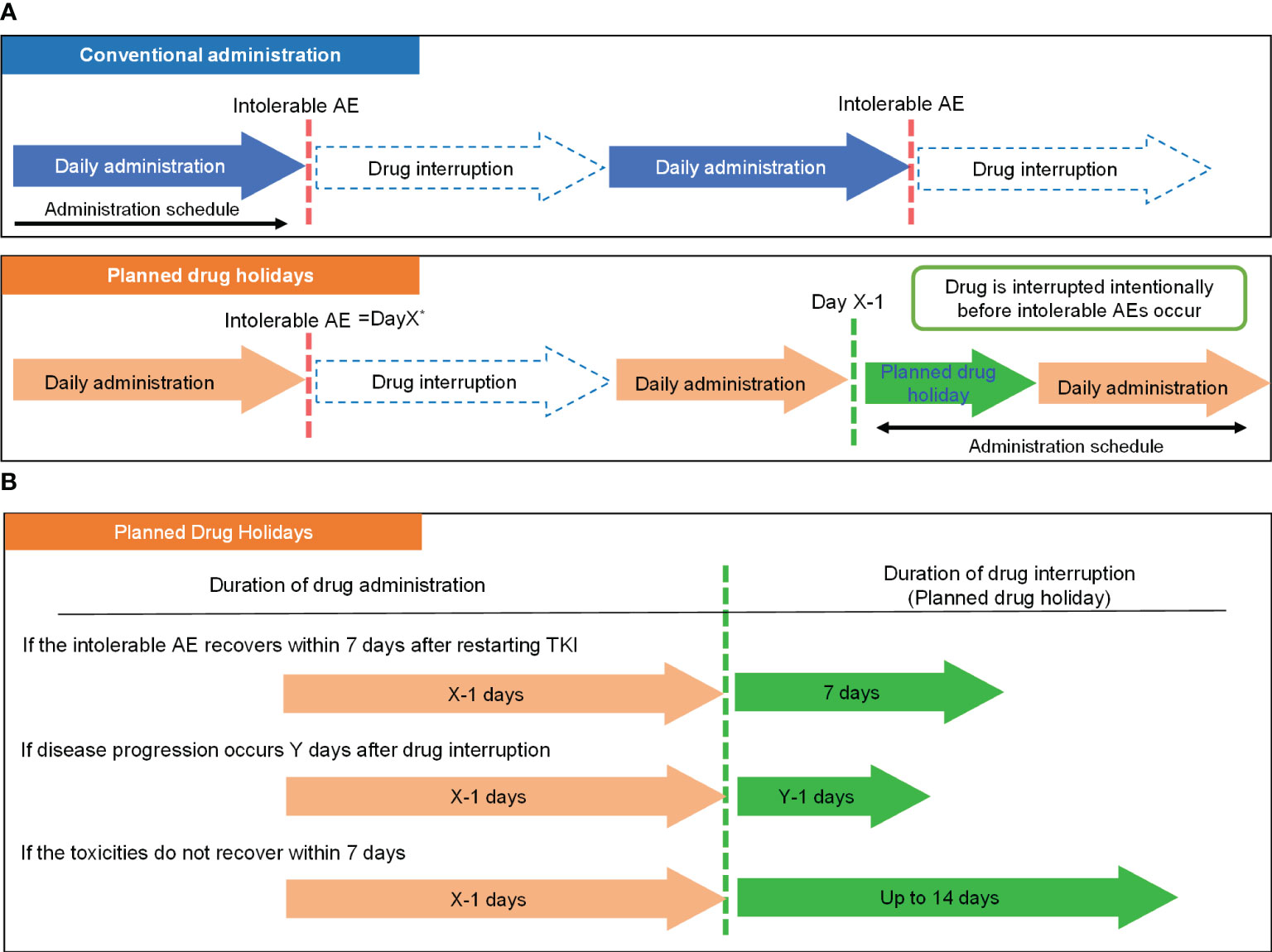
Figure 1 Concept of the planned drug holiday strategy. (A) Comparison of the basic strategy of conventional administration and planned drug holiday-based administration. (B) Determination of the duration of drug holiday by clinical situation. *Day X: Day on which intolerable AEs occurred. **Day Y: Day on which disease progression occurred. AE, Adverse Event; TKI, tyrosine kinase inhibitor.
2.3 Evaluation of efficacy and statistical analysis
Clinical response to treatment was evaluated radiographically using computerized tomography or magnetic resonance imaging approximately every eight weeks until disease progression or treatment discontinuation. Anti-tumor activity was confirmed according to the Response Evaluation Criteria in Solid Tumors (RECIST) v.1.1 (17) by review of imaging results. After completing treatment, disease progression, survival status, and any further anticancer treatment were documented until death or loss to follow-up. All disease progression was determined radiologically using computed tomography or magnetic resonance imaging. The event of PFS was defined as disease progression or death from any cause, while the event of time to treatment failure (TTF) was determined as lenvatinib discontinuation or death from any cause. Namely, if lenvatinib was continued when disease progression occurred, given concerns about rapid tumor regrowth, it was defined as an event of PFS but not TTF. The event of overall survival (OS) was determined as death from any cause. Evaluation of duration was limited to doses of ≥10 mg/day since 10 mg was determined as a minimum dose in the SELECT trial (7).
Bivariate analyses were employed to examine differences in background characteristics, with the t-test used for continuous variables and the chi-square test or Fisher’s exact probability test for categorical variables. AE grade was evaluated according to CTCAE version 4.0. PFS, TTF, OS, and duration of dosing of ≥10 mg were calculated by the Kaplan-Meier product-limit method. The log-rank test was used to compare the survival or treatment duration of the two groups. All data were analyzed using SPSS version 22.0 (SPSS Inc., Chicago, IL), and p-values of <0.05 were considered statistically significant.
3 Results
3.1 Patient and treatment characteristics
Seventy recurrent or metastatic thyroid cancer patients treated with lenvatinib were available for review. The following cases were excluded: those with every other day administration (n=1), daily administration with a phase 2 or 3 trial followed by planned holiday administration after the trial (n=10), and those with anaplastic thyroid cancer (n=8) or medullary thyroid cancer (n=5). Finally, 46 patients were included in the analysis, 25 in the planned holiday group and 21 in the daily group. The patients in the daily group were treated between September 2011 and December 2017, while those in the planned holiday group were treated between March 2016 and June 2019, and the AEs, which led to the introduction of the planned holiday, are listed in Supplementary Table 1. Twenty-one patients had papillary thyroid cancer (PTC) and 4 had follicular thyroid cancer (FTC) in the planned holiday group, versus 15 and 6 patients in the daily group, respectively. More than 80% of patients received surgery or iodine-131 therapy before lenvatinib. Demographic and baseline characteristics were balanced between treatment arms, except for slightly younger age in the daily group (Table 1).
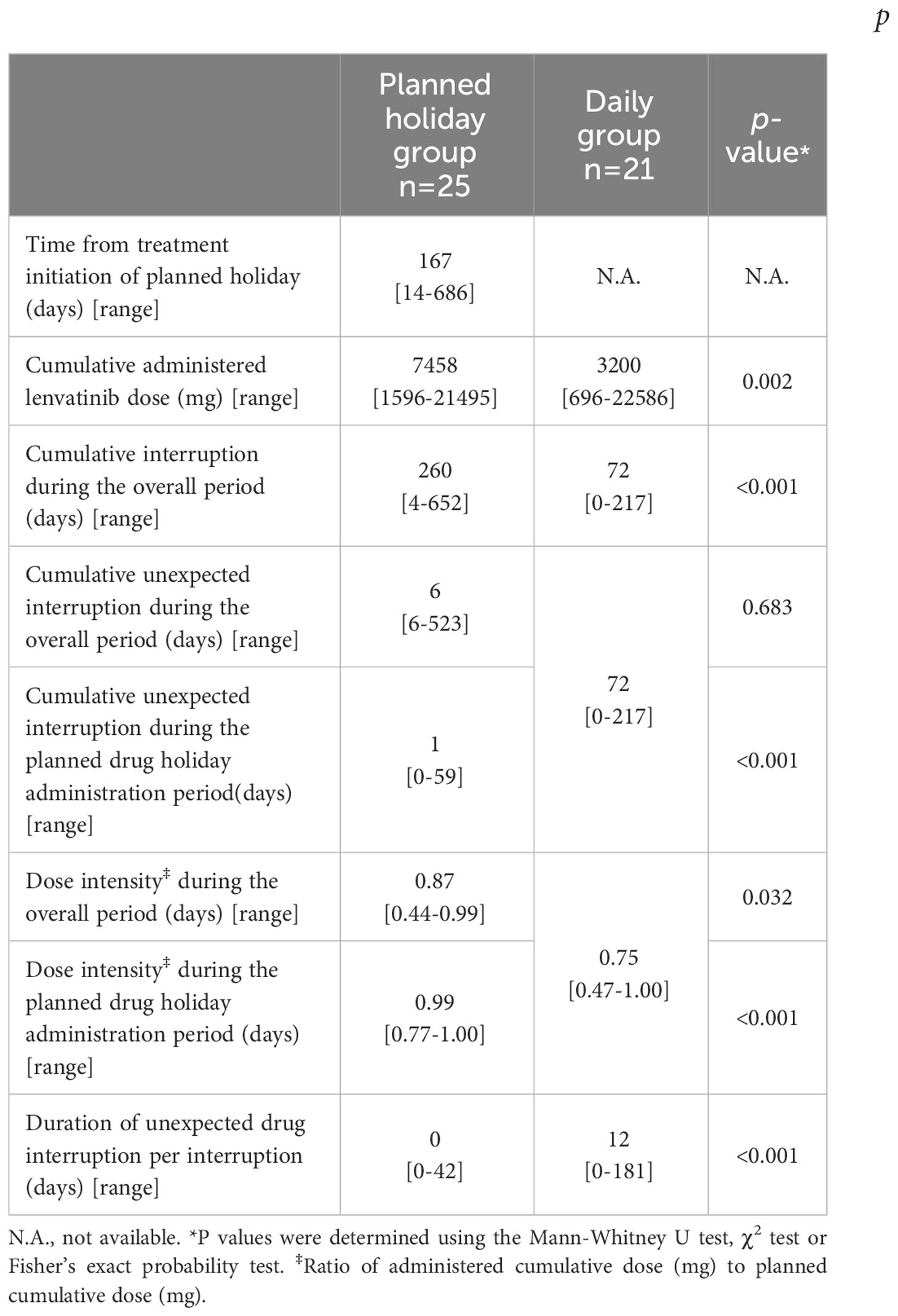
Details of treatment delivery by group are shown in Table 2. In the planned drug holiday group, median period from treatment initiation to the introduction of a planned drug holiday was 167 days (range 14-686) with the cumulative lenvatinib dose of 1746 mg (range 264-13412). The median lenvatinib dose at the point was 14 mg (range 10-24) daily, and the median administration and drug holiday period was eight days (range 4-21) and seven days (range 2-14), respectively. The total lenvatininb dose after the initiation of the planned drug holiday was 2641 mg (range 80-7000). During the study period, although the cumulative number of days of drug interruption was longer in the planned drug holiday group (260 days vs. 72 days, p<0.001), the eventual cumulative lenvatinib dose was substantially higher in that group than in the daily group (7458 vs. 3200, p=0.002).
3.2 Impact on clinical response, treatment duration, and survival
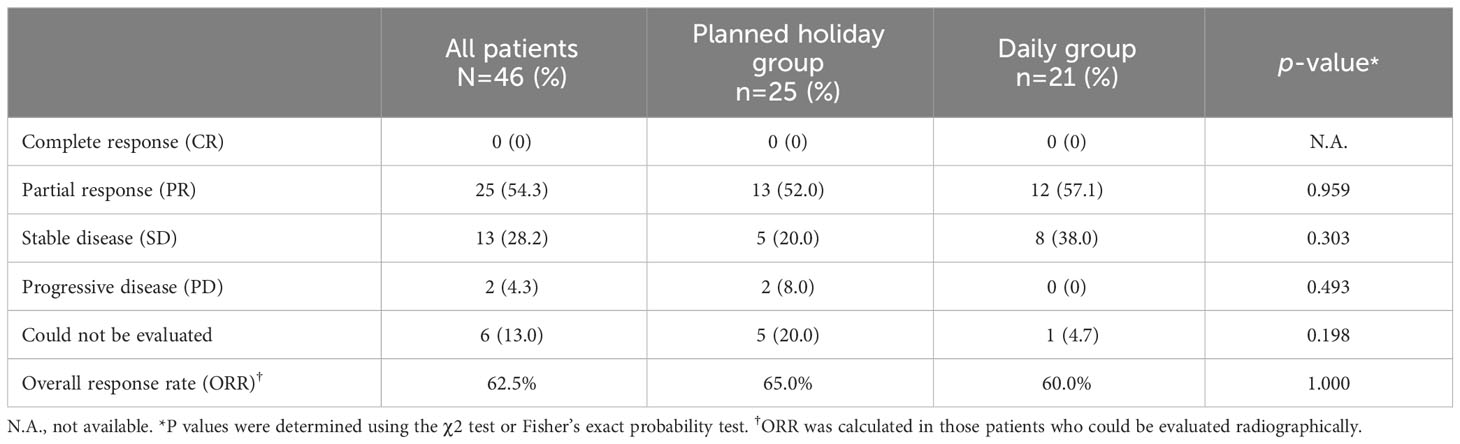
Overall response rate (ORR) was 62.5% in all patients and did not statistically differ between the planned drug holiday and daily groups (65.0% vs. 60.0%, p=1.000) (Table 3). TTF and PFS were significantly longer in the planned holiday group than the daily group (TTF: not reached [N.R.] vs. 14.9 months, hazard ratio [HR] 0.25, 95% confidence interval [Cl] 0.11-0.58, p<0.001. PFS: N.R. vs. 15.1 months, HR 0.31, 95% CI 0.14-0.68, p=0.002). Further, OS was significantly longer in the planned holiday group than in the daily group (N.R. vs. 26.6 months, HR, 0.20; 95% CI, 0.073-0.58; p=0.001). Duration of the period with lenvatinib dose ≥10 mg/day was significantly longer in the planned holiday group (N.R. vs. 6.5 months, HR 0.22; 95% CI 0.10-0.49, p<0.001) (Figure 2).
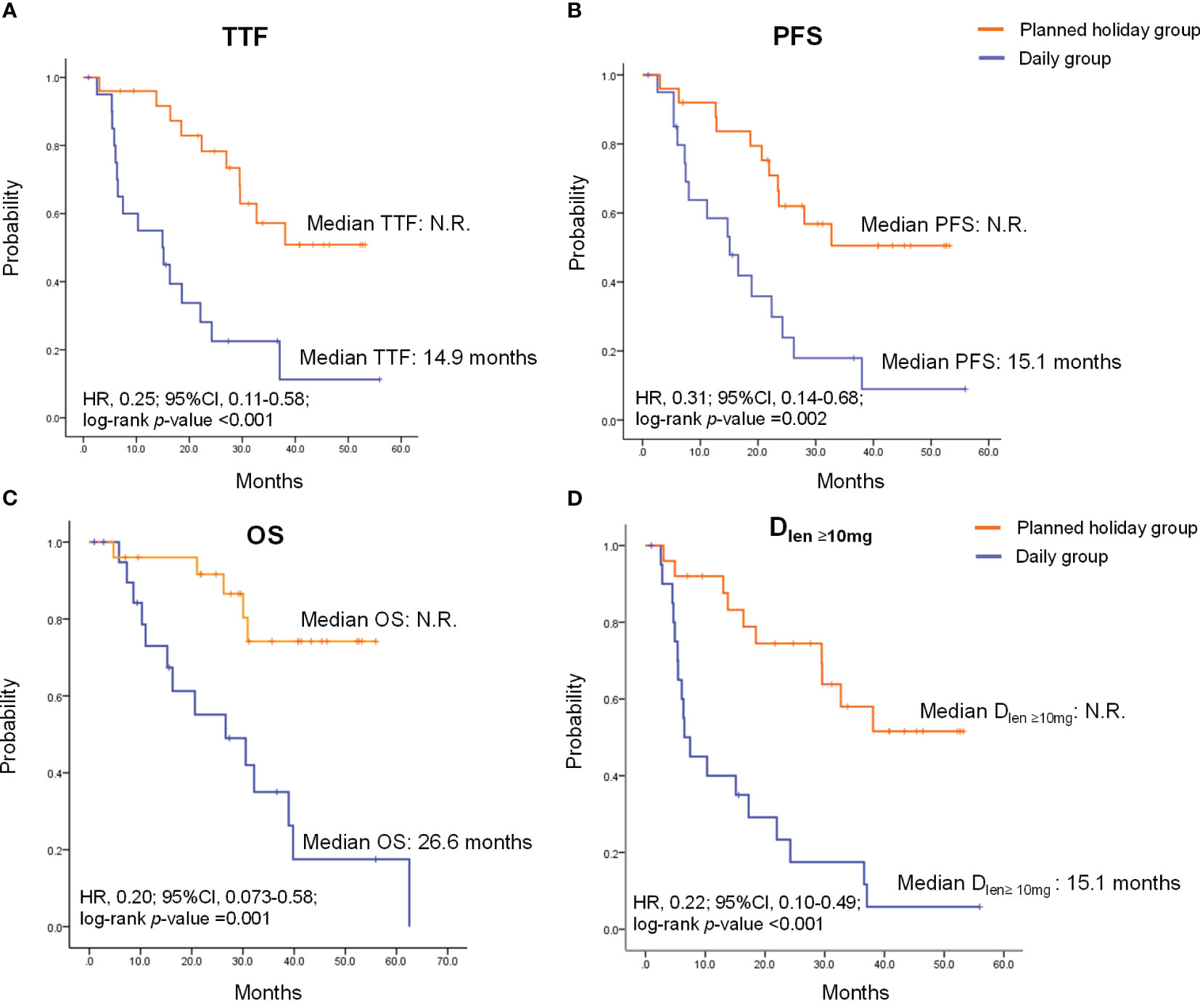
Figure 2 Time to treatment failure (TTF), progression-free survival (PFS), overall survival (OS) and duration of the period with lenvatinib dose ≥10 mg/day (Dlen ≥10mg) in patients treated with lenvatinib. (A) TTF. (B) PFS. (C) OS (D) Dlen ≥10mg. HR, hazard ratio; CI, confidence interval; N.R., not reached.
3.3 Impact on adverse events
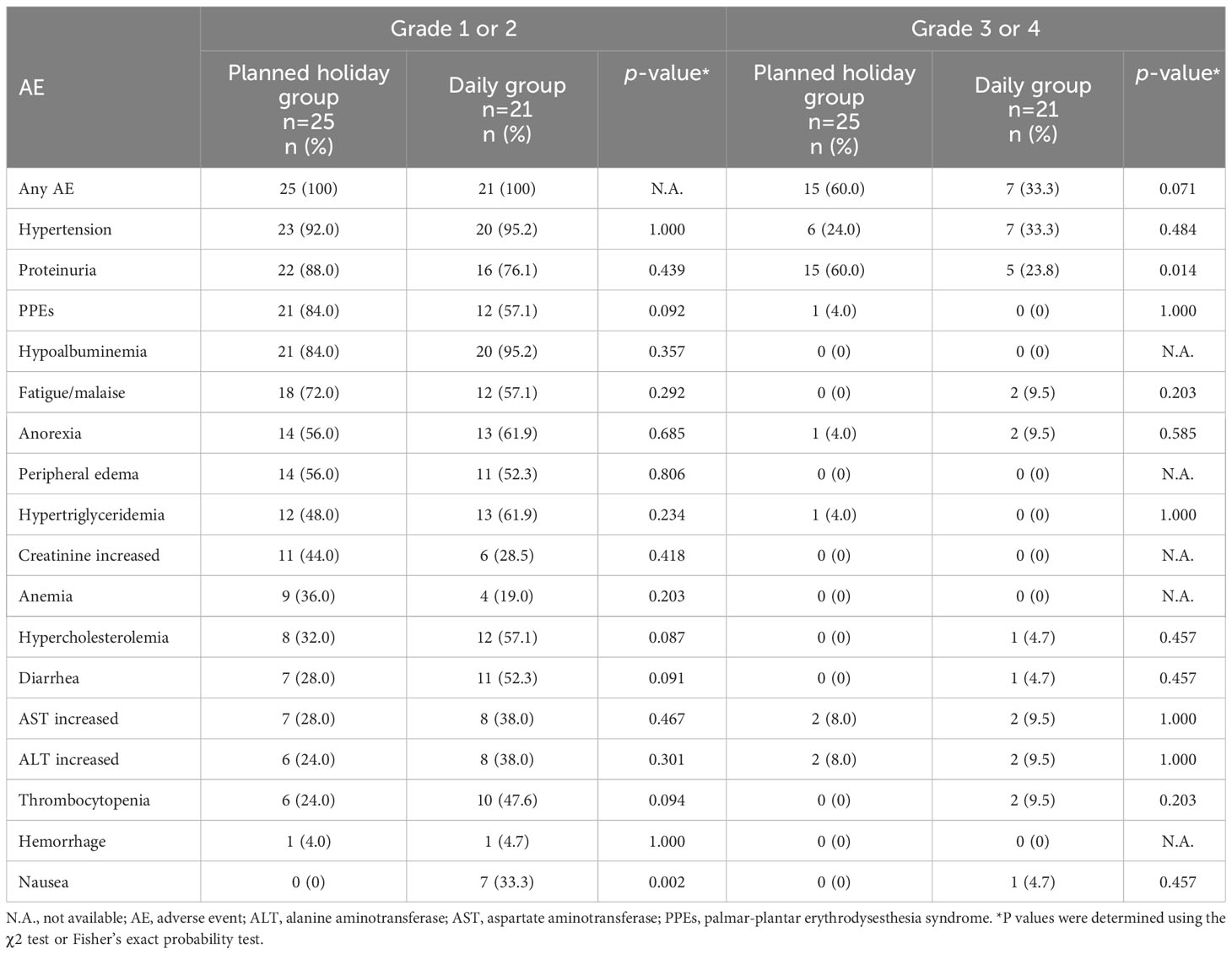
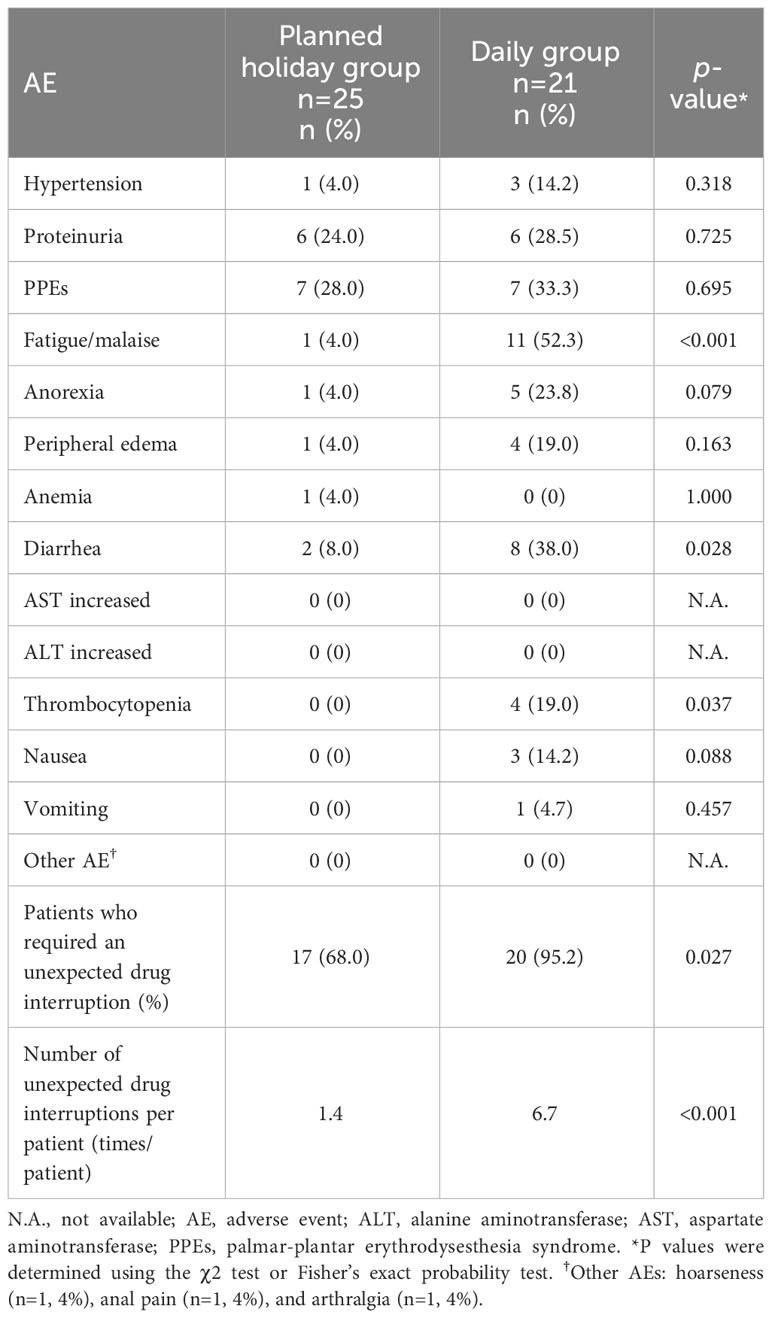
The observed AEs, and reasons for unplanned drug interruption and the introduction of planned drug holidays are presented in Tables 4 and 5, respectively. Significant differences in the incidence of AEs between the planned drug holiday and daily group were as follows: grade 1 or 2 nausea was frequent in the daily group (0% vs. 33.3%, p=0.002), while grade 3 proteinuria was more frequent in the planned drug holiday group (60.0% vs. 23.8%, p<0.01) (Table 4). Fewer patients required unplanned drug interruption due to AEs in the planned holiday group (68.0% vs. 95.0%; p=0.027) (Table 5). During the whole treatment period, unplanned drug interruptions per patient was significantly lower in the planned holiday group (1.4 times/patient vs. 6.7 times/patient, p<0.001). Among AEs which caused unplanned drug interruption, fatigue/malaise (4.0% vs. 52.3%, p<0.001), diarrhea (8.0% vs. 38.0%, p=0.028), and thrombocytopenia (0% vs. 19.0%, p=0.037) were statistically less frequent in the planned holiday group (Table 5).
4 Discussion
This study suggests that planned drug holiday administration of lenvatinib has significant benefits in survival and safety profile in patients with thyroid cancer. This strategy improved patient prognosis and had a better toxicity profile, probably through the avoidance of undesirable drug withdrawal and the relatively long duration of period with a higher (≥10 mg) dose of lenvatinib per single day through prevention of the occurrence of severe AEs.
Severe AEs often require prolonged drug withdrawal and frequent dose modification. In this situation, the planned drug holiday strategy enables oncologists to adjust the lenvatinib schedule based on the status of patient AEs. Interruption before the development of severe AEs can help avoid extended drug withdrawal and continue treatment (15). Similarly, administration with a scheduled drug holiday was reportedly associated with fewer toxicities and improved survival benefits in patients treated with VEGFR tyrosine kinase inhibitors such as sunitinib for renal cell cancer and lenvatinib for hepatocellular cancer (11, 14). These findings have led to speculation that a shorter treatment cycle may lead to a lower incidence of AEs, and associated longer drug exposure. Accordingly, the duration of unplanned drug interruption was shorter with fewer grade 3 or 4 AEs, except for proteinuria, which is more likely to occur with prolonged drug use (18), and ultimately to longer treatment duration in the planned holiday group than the daily group. Furthermore, we believe that our strategy, which modifies the treatment schedule according to the individual actual occurrence of AEs, would be more minute on maximizing therapeutic efficacy by maintaining the period of lenvatinib administration with safe, compared with the fixed or mandatory drug holidays strategies (i.e., two-weeks-on/one-week-off, weekends-off) (11, 14).
One concern with drug holidays relates to tumor growth during the planned drug interruption. The duration of our planned drug holiday was therefore determined with regard to not only AE status but also tumor progression; if disease progression occurred Y days after drug interruption, day “Y-1” was suggested as the planned drug holiday duration, with the result that the median drug holiday duration was 7 days. Considering that Yamazaki et al. reported a median time from lenvatinib cessation to tumor progression of nine days in eight thyroid cancer patients who experienced rapid tumor progression (flare phenomenon) after discontinuation of Lenvatinib (19), this length of treatment interruption (seven days) appears reasonable.
A further question is the inverse relationship between drug interruption and PFS observed in the SELECT study, in which a duration of lenvatinib interruption of more than 10% of the overall treatment period was associated with a significantly shorter PFS than an interruption of less than 10%. In contrast to that finding, despite a longer cumulative duration of dose interruption in the drug holiday, PFS was significantly longer than in the daily group. We believe that this difference can be explained by the longer duration of a relatively high dose (herein >10mg/day) of lenvatinib in the drug holiday group. Supporting this assumption, the previous reports indicated the importance of a daily dose of lenvatinib through their experience of “rechallenge” with a higher dose of lenvatinib after progression at a lower dose. Considering that they usually adopted drug holidays when rechallenge, our strategy shares the exact same basis as these earlier reports while representing a more preemptive strategy that can contribute to a better overall safety profile. Although our single-center retrospective design is a likely limitation of our study, we believe that this strategy is worth prospective evaluation, in which an ethical review board-approved standardized drug holiday strategy should be applied and tested.
5 Conclusion
In thyroid cancer patients, the planned holiday demonstrated significantly longer PFS, TTF, and OS and less severe toxicity than daily administration, presumably due to safer lenvatinib administration with minimum treatment interruption as well as maintenance of the duration of a higher dose of daily lenvatinib.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Clinical Research and Ethical Review Board of the National Cancer Center East (task number: 2016-245). The patients/participants provided their written informed consent to participate in this study.
Author contributions
CM, TE, YU, SS and MT participated in the study concept and design, interpreted the data, and drafted the manuscript. KI, TF, TE and SO extracted, managed and analyzed the data. All authors provided critical revisions and approved the final manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Acknowledgments
We thank our colleagues who participated in this study and are not included in the list of authors, namely Toshikatsu Kawasaki, Hayato Kamata, Asumi Kaneko (National Cancer Center Hospital East, Kashiwa, Japan). The content of this manuscript has been presented in a mini-oral session (MO8-5) at the Japanese Society of Medical Oncology Annual Meeting, 2021, Matsuyama C, Ueda Y, Suzuki S, et al. Ann Oncol. 2021; 32(suppl 4): S300, https:doi.org/10.1016/j.annonc.2021.05.580.
Conflict of interest
MT reports grants and personal fees from Eisai during the conduct of the study; and grants and personal fees from Ono Pharmaceutical, BMS, Bayer, MSD, Eli Lilly, GSK, Pfizer, AstraZeneca, Rakuten Medical and Merck Biopharma, grants from Novartis, and personal fees from Boehringer Ingelheim and Genmab outside the submitted work. SO received honoraria from Ono Pharmaceutical, Bristol-Myers Squibb, MSD, Merck Biopharma and Meiji Sika Pharma. YU received honoraria from Bristol-Myers Squibb.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1139659/full#supplementary-material
References
1. Chougnet C, Brassard M, Leboulleux S, Baudin E, Schlumberger M. Molecular targeted therapies for patients with refractory thyroid cancer. Clin Oncol (R Coll Radiol) (2010) 22(6):448–55. doi: 10.1016/j.clon.2010.04.008
2. Durante C, Haddy N, Baudin E, Leboulleux S, Hartl D, Travagli JP, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab (2006) 91(8):2892–9. doi: 10.1210/jc.2005-2838
3. Pacini F, Ito Y, Luster M, Pitoia F, Robinson B, Wirth L. Radioactive iodine-refractory differentiated thyroid cancer: unmet needs and future directions. Expert Rev Endocrinol Metab (2012) 7(5):541–54. doi: 10.1586/eem.12.36
4. Matsui J, Funahashi Y, Uenaka T, Watanabe T, Tsuruoka A, Asada M. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res (2008) 14(17):5459–65. doi: 10.1158/1078-0432.CCR-07-5270
5. Matsui J, Yamamoto Y, Funahashi Y, Tsuruoka A, Watanabe T, Wakabayashi T, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer (2008) 122(3):664–71. doi: 10.1002/ijc.23131
6. Okamoto K, Kodama K, Takase K, Sugi NH, Yamamoto Y, Iwata M, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett (2013) 340(1):97–103. doi: 10.1016/j.canlet.2013.07.007
7. Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New Engl J Med (2015) 372(7):621–30. doi: 10.1056/NEJMoa1406470
8. Kiyota N, Schlumberger M, Muro K, Ando Y, Takahashi S, Kawai Y, et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in radioiodine-refractory differentiated thyroid cancer. Cancer Sci (2015) 106(12):1714–21. doi: 10.1111/cas.12826
9. Zhu C, Ma X, Hu Y, Guo L, Chen B, Shen K, et al. Safety and efficacy profile of lenvatinib in cancer therapy: a systematic review and meta-analysis. Oncotarget (2016) 7(28):44545–57. doi: 10.18632/oncotarget.10019
10. Tahara M, Brose MS, Wirth LJ, Suzuki T, Miyagishi H, Fujino K, et al. Impact of dose interruption on the efficacy of lenvatinib in a phase 3 study in patients with radioiodine-refractory differentiated thyroid cancer. Eur J Cancer (Oxford Engl 1990) (2019) 106:61–8. doi: 10.1016/j.ejca.2018.10.002
11. Makino K, Yoda K, Tomoishi J, Kume H. Efficacy and tolerability of a low-dose, 2-week administration of sunitinib followed by a week rest (2/1 schedule) for metastatic renal cell carcinoma: a single center experience of six cases. BMC Res Notes (2014) 7:872. doi: 10.1186/1756-0500-7-872
12. Yamatsuji T, Fujiwara Y, Matsumoto H, Hato S, Namikawa T, Hanazaki K, et al. Feasibility of oral administration of S-1 as adjuvant chemotherapy in gastric cancer: 4-week S-1 administration followed by 2-week rest vs. 2-week administration followed by 1-week rest. Mol Clin Oncol (2015) 3(3):527–32. doi: 10.3892/mco.2015.500
13. Petrioli R, Chirra M, Messuti L, Fiaschi AI, Savelli V, Martellucci I, et al. Efficacy and safety of regorafenib with 2/1 schedule for patients ≥ 75 years with metastatic colorectal cancer (mCRC) after failure of 2 lines of chemotherapy. Clin Colorectal Cancer (2018) 17(4):307–12. doi: 10.1016/j.clcc.2018.02.005
14. Iwamoto H, Suzuki H, Shimose S, Niizeki T, Nakano M, Shirono T, et al. Weekends-off lenvatinib for unresectable hepatocellular carcinoma improves therapeutic response and tolerability toward adverse events. Cancers (Basel) (2020) 12(4):1010. doi: 10.3390/cancers12041010
15. Tahara M. Management of recurrent or metastatic thyroid cancer. ESMO Open (2018) 3(Suppl 1):e000359. doi: 10.1136/esmoopen-2018-000359
16. Iseki K, Ikemiya Y, Iseki C, Takishita S. Proteinuria and the risk of developing end-stage renal disease. Kidney Int (2003) 63(4):1468–74. doi: 10.1046/j.1523-1755.2003.00868.x
17. Eisenhauer EA, Therasse P, Bogaerts J, LH S, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford Engl 1990) (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
18. Iwasaki H, Yamazaki H, Takasaki H, Suganuma N, Sakai R, Nakayama H, et al. Renal dysfunction in patients with radioactive iodine-refractory thyroid cancer treated with tyrosine kinase inhibitors: A retrospective study. Med (Baltimore) (2019) 98(42):e17588–e. doi: 10.1097/MD.0000000000017588
Keywords: lenvatinib, thyroid cancer, oral anticancer agent, VEGF tyrosine kinase inhibitor, adverse events, planned drug holidays
Citation: Matsuyama C, Enokida T, Ueda Y, Suzuki S, Fujisawa T, Ito K, Okano S and Tahara M (2023) Planned drug holidays during treatment with lenvatinib for radioiodine-refractory differentiated thyroid cancer: a retrospective study. Front. Oncol. 13:1139659. doi: 10.3389/fonc.2023.1139659
Received: 07 January 2023; Accepted: 26 September 2023;
Published: 11 October 2023.
Edited by:
Barbara Maria Jarzab, Maria Skłodowska-Curie National Research Institute of Oncology, PolandReviewed by:
Jolanta Anna Krajewska, Maria Skłodowska-Curie National Research Institute of Oncology, PolandShunji Takahashi, Japanese Foundation For Cancer Research, Japan
Copyright © 2023 Matsuyama, Enokida, Ueda, Suzuki, Fujisawa, Ito, Okano and Tahara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Makoto Tahara, bWF0YWhhcmFAZWFzdC5uY2MuZ28uanA=
†These authors have contributed equally to this work and share first authorship
 Chihiro Matsuyama
Chihiro Matsuyama Tomohiro Enokida
Tomohiro Enokida Yuri Ueda
Yuri Ueda Shinya Suzuki
Shinya Suzuki Takao Fujisawa
Takao Fujisawa Kazue Ito3,5
Kazue Ito3,5 Susumu Okano
Susumu Okano Makoto Tahara
Makoto Tahara