- 1Sichuan Cancer Hospital & Institute Sichuan Cancer Center, School of Medicine University of Electronic Science and Technology, Chengdu, Sichuan, China
- 2Collaborative Innovation Centre of Regenerative Medicine and Medical BioResource Development and Application Co-constructed by the Province and Ministry, Guangxi Medical University, Nanning, Guangxi, China
- 3Department of Oncology & Cancer Institute, Sichuan Academy of Medical Sciences, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
- 4Department of Laboratory Medicine and Sichuan Provincial Key Laboratory for Human Disease Gene Study, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, Sichuan, China
Squamous cell lung carcinoma (LUSC) originates from squamous cells and has a high rate of metastasis and recurrence. The lack of effective genetic targets and specific therapies has resulted in a poor prognosis for patients with LUSC. Gastrointestinal metastasis of LUSC is a rare occurrence in clinical practice. Patients with gastrointestinal metastasis usually have worse overall survival and the process of diagnosis is more complicated than those with metastasis elsewhere. What’s more, there are no helpful guidelines for treating patients with a clinically confirmed diagnosis of gastrointestinal metastasis, which means the treatment method is limited. Here, we review the clinical features, diagnosis, and treatment of LUSC patients with gastrointestinal metastasis and report a rare case of LUSC accompanied by gastrointestinal metastasis. The patient was admitted to the hospital with coughing and hemoptysis. A tumor was found in his lung, and lesions were initially controlled with standard treatment. The patient’s tumor re-occurred again shortly for which treatment was lacking. Without effective treatment methods, the disease was difficult to control. Our learnings from the case demonstrate that LUSC metastasizes to secondary lymphoid organs of the gastrointestinal tract, usually with a poor prognosis.
Introduction
Squamous cell lung carcinoma (LUSC) is a prevalent type of non-small cell lung cancer (NSCLC), accounting for approximately 25% to 30% of all NSCLCs. Epidemiological investigations have shown that LUSC occurs more commonly in elderly men and is more strongly associated with smoking than any other type of NSCLC (1, 2). The early symptoms of LUSC are mild and easy to ignore. At the time of diagnosis, most patients are already in the advanced stage of the disease and often present with distal metastasis, which results in a poor prognosis of advanced LUSC with a 5-year survival rate of only 6% (3).
In NSCLC, metastases occur easily and are more often localized to the bone (34%), liver (20%), brain, adrenal glands, thoracic cavity, and lymph nodes (4, 5). Some rare sites of metastasis include the soft tissues (0-0.8%), bone marrow (0.16%), intestine (0.2%–1.8%), eye (0.1%–7%), thyroid (1.6%), tongue (0.2%–1.6%), pancreas, spleen, peritoneum, ovary, heart, breast, kidney, tonsil, and nasal cavity (6–19). Compared to other types of NSCLC, LUSC has a higher rate of gastrointestinal metastasis, which has been associated with a worse outcome (9, 20–22). Clinically diagnosing the gastrointestinal metastasis of LUSC mainly depends on imaging examination results (6). There are no helpful guidelines for treating patients with a clinically confirmed diagnosis of gastrointestinal metastasis of LUSC. Here, we review the clinical features, diagnosis, and treatment of LUSC patients with gastrointestinal metastasis and report a rare case of LUSC accompanied by gastrointestinal metastasis.
Case presentation
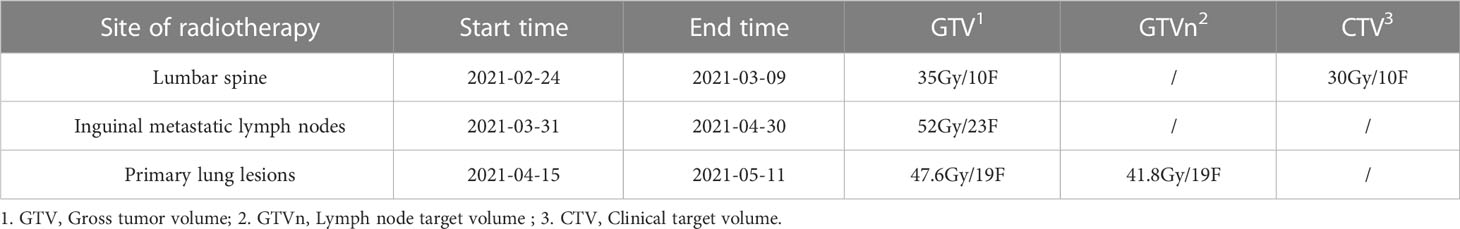
Our patient is a 50-year-old male who presented with lumbago, cough, and sputum accompanied by hemoptysis and was admitted to our outpatient clinic. Pathological biopsy confirmed squamous cell carcinoma of the left lower lung lobe with CK5/6 (+) and P63 (+). Similar pathological results confirmed cecum metastasis (Figure 1). FDG-PET CT identified an intensely avid lobe of left lung (LLL) mass (maximum standardized uptake value 10.6 (Max SUV), 32 mm×30 mm) extending to the cecum, top cranial skin, lumbar vertebra, inguinal groin, and left ventricle (Figures 2A, B). The nasopharyngeal roof and bilateral walls were symmetrically swollen, with increased metabolism and a tendency to inflammation (Figure 2A, 2021-02-05). The patient completed 3-cycle chemotherapies on 2021-03-03, 2021-03-26, and 2021-04-19, respectively. We administered the standard chemotherapy regimen for primary squamous lung cancer: paclitaxel combined with DDP. The primary lesion was markedly reduced in size after two cycles of chemotherapy. The oncological control was satisfied at this point (Figure 2C). At the same time, the patient received concurrent radiotherapy for the lumbar spine, inguinal metastatic lymph nodes, and primary lung lesions. The detailed radiotherapy regimens for each site were shown in Figure 3 and Table 1. After radiotherapy, his low back pain and cough symptoms were relieved. The patient was re-evaluated following radiotherapy and 3-cycle of chemotherapies. The imaging showed stabilized lesions of pulmonary origin, appendix, and inguinal metastases (Figures 2D, E). Unfortunately, after cycle 3 of treatment, the patient had progressive enlargement of the metastases at the top of the skull and new metastases in the nasal cavity (Figure 2F). We recommend that this patient receive chemotherapy combined with Carelizumab (anti-PD-1) immunotherapy on 2021-05-20. Unfortunately, the patients died four months later.
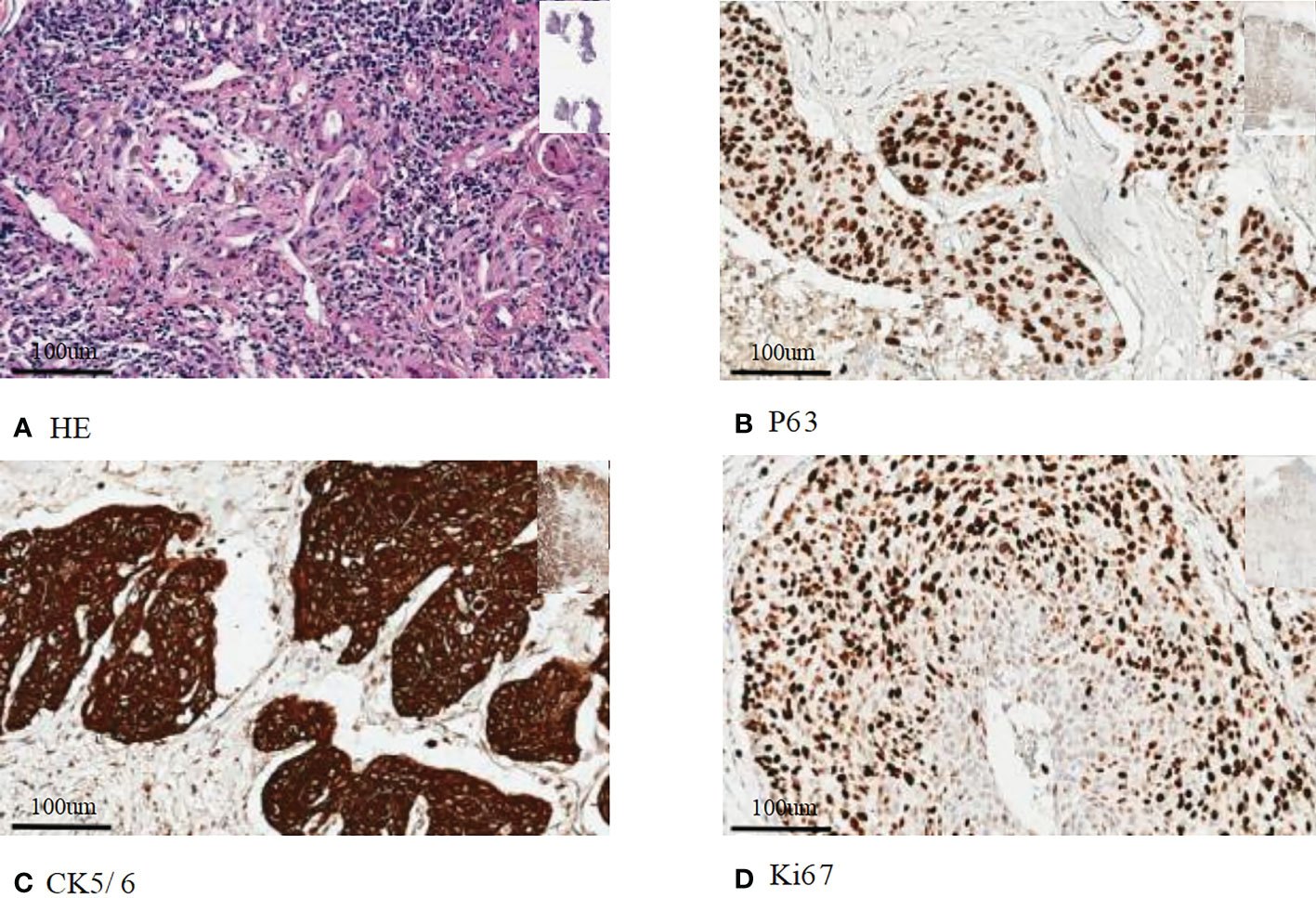
Figure 1 Immunohistochemical staining of histopathology: (A) Hematoxylin eosin staining of primary squamous cell carcinoma; (B) Immunohistochemical staining of P63; (C) Immunohistochemical staining of CK5/6; (D) Immunohistochemical staining of Ki67. Bar=100 um.
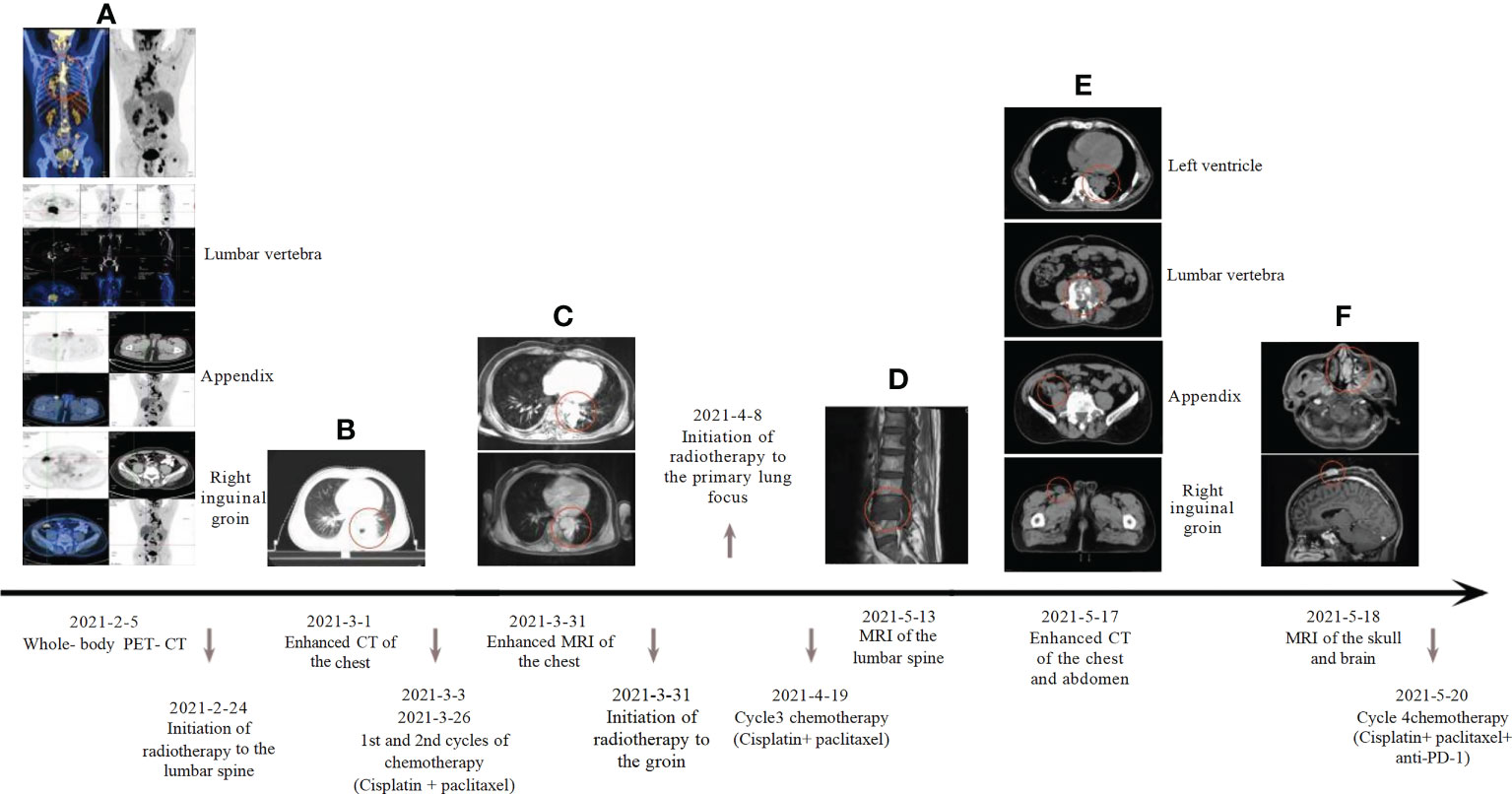
Figure 2 Systemic treatment strategies: (A) Whole-body PET-CT: maximum standardized uptake value: lung tumor 10.6, cecum 11.2, lumbar vertebra 15.7, inguinal groin 10.7, left ventricle 9.0, Cranial top skin 18.6; (B) Baseline computed tomography-enhanced CT of the chest; (C) The patient’s chest enhancement MRI was reassessed two weeks after chemotherapy; (D) MRI of lumbar spine metastases; (E) Review of chest, abdomen and pelvic enhancement CT; (F) MRI of the head suggests nasal and scalp neoplasm.
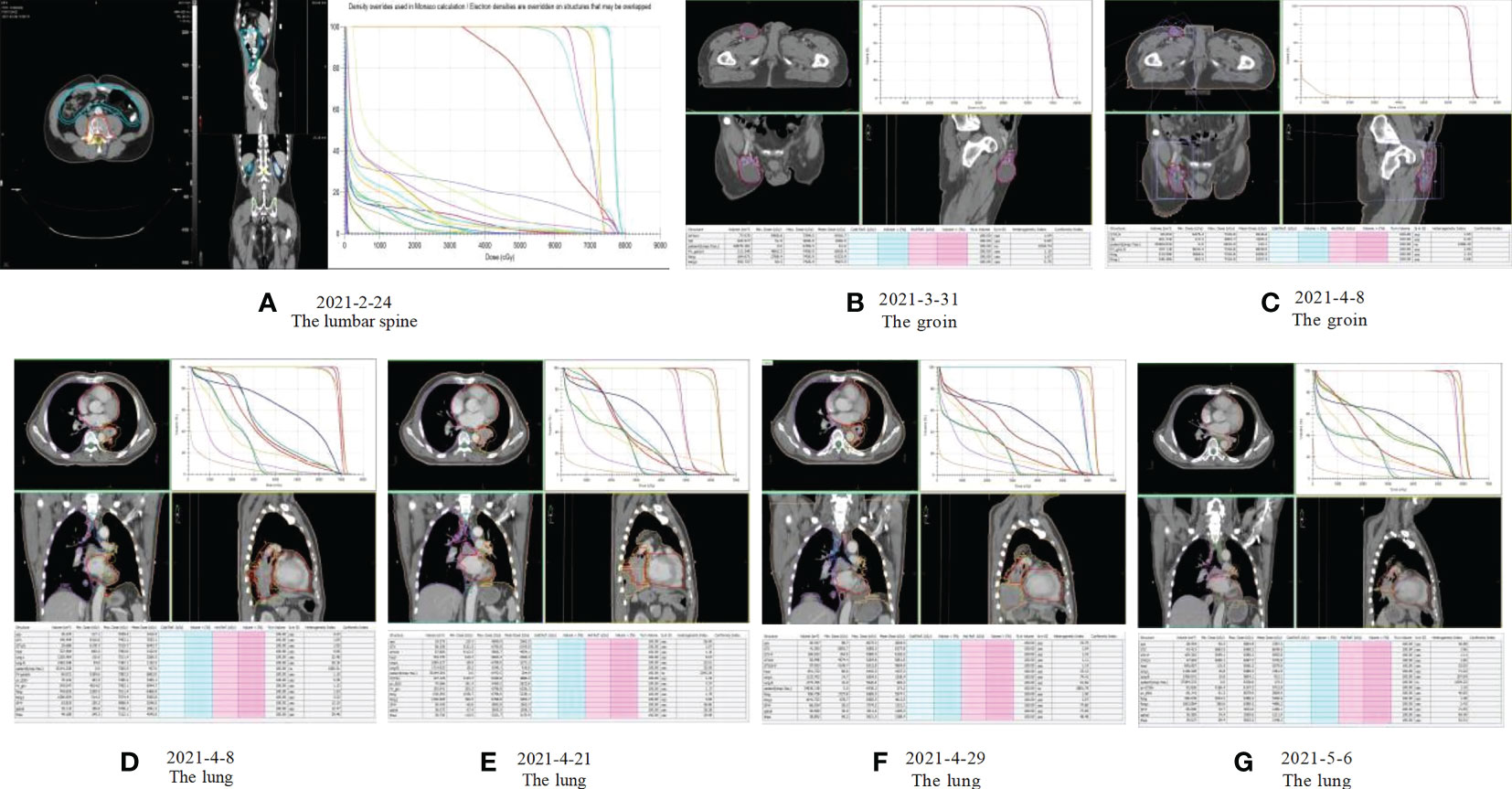
Figure 3 Radiotherapy plan: (A) Radiotherapy plan for the lumbar spine: Gross tumor volume (GTV L4-5) 35Gy/10F, Clinical target volume (CTV) 30Gy/10F; (B, C) Radiotherapy plan for inguinal metastatic lymph nodes: Gross tumor volume (GTVp) 52Gy/23F; (D-G) Radiotherapy plan for primary lung lesions: Gross tumor volume (GTVp) 47.6Gy/19F, Lymph node target volume (GTVn) 41.8Gy/19F.
Discussion
Cecum metastases from squamous lung cancer are extremely rare. This case reports a rare case of squamous lung cancer metastasis to the appendix, scalp, and bone. Early examination confirmed the presence of cecum metastasis without clinical symptoms in this case. The vast majority of patients with LUSC have no clinical manifestations (23). LUSC usually requires ancillary testing and pathological biopsy to confirm (24–26). This patient’s aggressive evaluation at the onset of symptoms was consistent with our care principles. Unfortunately, the patient had multiple metastases and no indication for surgery at the diagnosis. Therefore, we could only use radiation therapy to control the disease.
In this study, we gave the patient the standard chemotherapy regimen for primary squamous lung cancer: paclitaxel combined with cisplatin. After several cycles of chemotherapy and radiotherapy, the disease was controlled. However, the lesion soon became uncontrolled again and worsened. This phenomenon suggests that patients with squamous cell carcinoma of the lung that metastasizes to the appendix have a poorer prognosis with standard radiotherapy regimens (24, 27). More processing methods and optimization techniques still need to be further explored.
Clinical features of LUSC with gastrointestinal metastasis
Metastasis to the gastrointestinal tract secondary to lung cancer is considerably frequent in necropsy series (14%) (28), but is seldom recognized clinically. It is a rare disease, with a low incidence of 0.2% to 1.8% in clinical studies (8, 9, 29). The common sites are the small intestine and colon (24). Therefore, the appearance of cecum metastasis is very unusual. Detection of gastrointestinal tract abnormalities is usually incidental during the diagnosis of primary lung cancer; occasionally, these abnormalities are detected even earlier than lung cancer. Diagnosis usually can be made using computed tomography (CT), magnetic resonance imaging (MRI), bone scans, pathological biopsies, and endoscopic and immunochemistry studies. Most patients are found to have gastrointestinal metastasis on imaging at the time of diagnosis but with few clinical symptoms in the intestinal tract. Some patients with gastrointestinal metastasis of LUSC have intestinal symptoms, mostly in the form of abdominal pain, gastrointestinal bleeding, perforation, obstruction, constipation, and other symptoms (30–35). In Table 2, we summarize previously reported cases of gastrointestinal tract metastasis from primary LUSC with intestinal symptoms.
Diagnosis of LUSC with gastrointestinal metastasis
LUSC is prevalent in clinical practice, and its clinicopathological features are remarkably clear. Squamous cells are “scaly structures” that occur along the trachea and bronchi. Pathologists categorize squamous cells by “keratin pearls” under a microscope. LUSC immunophenotypes consistently express P63 and are negative for TTF1 (36). Other squamous immunomarkers include CK5/6 or P40. Several clinical studies have shown that squamous cell lung cancer is more likely to metastasize to the gastrointestinal tract than other lung cancers (37, 38). Therefore, whether the histological types are associated with gastrointestinal metastases remains unknown. The correct diagnosis of pulmonary tumors is crucial for treatment decisions. Clinically, the origin of the tissue type is often identified by immunohistochemistry (25). Information on the expression of immunohistochemical markers facilitates histopathological diagnostics. In most patients with LUSC, the expression of immune markers in metastatic sites was consistent with that in the corresponding primary tumors. Immunoprotein staining is consistent between the tumor’s gastrointestinal and pulmonary origin, and positive staining for CK-14 and CK-18 suggests squamous cell carcinoma and adenocarcinoma (26). Gastrointestinal metastasis from LUSC is rare and unique in clinical practice and is sometimes mistaken for primary digestive tract tumors (24, 30). Thus, some ancillary examinations help provide the basis for the diagnosis.
Laboratory examination, endoscopy, gastroenterography, CT, and positron emission tomography (PET)-CT may aid in diagnosing LUSC in patients with gastrointestinal metastases (24). CT is the mainstay for noninvasive diagnosis and staging of many gastrointestinal tumors, its positive signs can be recognized in gastrointestinal metastases patients, including localized gastrointestinal wall thickening, the presence of a mass in the gastrointestinal cavity, intussusception, and perforation (23). Abdominal pain is the most common clinical symptom of these patients. However, the symptoms are not entirely consistent with the severity of illness in some clinical settings. Most patients are no clinical symptoms. Consequently, most gastrointestinal metastases are associated with intestinal perforation, intractable gastrointestinal bleeding, intestinal obstruction, and other serious complications, thereby leading to accidental death. Endoscopic biopsy provides an opportunity for diagnosing unexplained metastases and for treatment, especially in patients with gastrointestinal bleeding. Endoscopy is more accurate than CT and MRI because it can depict small lesions that other imaging modalities cannot. In addition, PET-CT has good sensitivity and specificity for detecting metastatic tumors and is commonly used in diagnosing metastatic tumors of the gastrointestinal tract (39–41). However, pathology remains a critical factor for the final diagnosis of LUSC with intestinal metastasis. Simultaneously, the pathology of primary lung cancer should be compared with that of metastatic lesions in the gastrointestinal tract, which is crucial for patients.
Treatment of LUSC with gastrointestinal metastasis
Although the treatment patterns are rapidly changing, treatment options for first-line therapy of advanced LUSC remain limited compared to those for other types of lung cancer. Most patients with advanced LUSC have good survival after radiotherapy and chemotherapy. Platinum-based chemotherapy regimens have been shown to improve survival and enhance patient quality of life. However, rare cases with gastrointestinal metastasis have a poor prognosis with a median overall survival of only 4–8 weeks (24, 27, 30). Thus, LUSC patients with gastrointestinal metastasis should undergo early aggressive surgical treatment or local ablative therapy (26). Both of these are suitable for patients with good performance status. Resection of isolated gastrointestinal metastasis has been shown to improve the survival of patients with LUSC. Compared to surgical treatment (lobectomy for primary pulmonary tumors and lymphadenectomy and endarterectomy), local ablative therapy is less invasive, more beneficial, and recommended for patients.
Distinguishing squamous cell carcinoma from adenocarcinoma is vital for drug selection. Unlike lung adenocarcinoma, squamous cell lung cancer lacks effective targets, including mutations and alterations, for which the approved targeted treatments are rare in LUSC (42–47). Consequently, it is critical that the use of new treatment modalities be taken into account to ensure that patients with LUSC receive the most appropriate treatment and have better outcomes. Given the approval for targeted therapies and immunotherapies for advanced NSCLC and the extension toward personalization of advanced lung cancer treatment, these methods can also be applied to patients with LUSC with gastrointestinal metastases to achieve better outcomes.
Conclusions
Treatment of advanced LUSC remains challenging because of specific tumor characteristics. These characteristics result in fewer treatment options and shorter overall survival. Herein, we report the treatment process of a rare case of squamous lung cancer with metastases to the cecum, scalp, and bone. Cecum metastasis is rare in LUSC. This case demonstrates the poor prognosis of squamous lung cancer metastasizing to secondary lymphoid organs of the gastrointestinal tract. Therefore, patients with squamous cell carcinoma of the lung who develop gastrointestinal metastases are advised to prolong their survival through surgical resection or local ablative therapy once detected.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
HW, QL, FFL designed, discussed, wrote, and submitted this manuscript. YQL, KX, QY access to literature. All authors contributed to the article and approved the submitted version.
Funding
Supported by the School of Medicine University of Electronic Science and Technology, project Number: ZYGX2021YGCX004.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn P, et al. Clinicopathologic features of advanced squamous NSCLC. J Thorac Oncol (2016) 11:1411–22. doi: 10.1016/j.jtho.2016.05.024
2. Huber R. Is lung cancer in never-smokers a different disease?–back to the figures. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer (2007) 2:787–8. doi: 10.1097/JTO.0b013e318153f3c5
3. Siegel R, Miller K, Fuchs H, Jemal A. Cancer statistics, 2021. CA: Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
4. Eberhardt WE, Mitchell A, Crowley J, Kondo H, Kim YT, Turrisi A 3rd, et al. The IASLC lung cancer staging project: Proposals for the revision of the m descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol (2015) 10:1515–22. doi: 10.1097/JTO.0000000000000673
5. Riihimaki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, et al. Metastatic sites and survival in lung cancer. Lung Cancer (2014) 86:78–84. doi: 10.1016/j.lungcan.2014.07.020
6. Niu FY, Zhou Q, Yang JJ, Zhong WZ, Chen ZH, Deng W, et al. Distribution and prognosis of uncommon metastases from non-small cell lung cancer. BMC Cancer (2016) 16:149. doi: 10.1186/s12885-016-2169-5
7. Lin L, Wang X, Tang C, Liang J. Clinical characteristics and prognosis of gastrointestinal metastases in solid tumor patients: A retrospective study and review of literatures. Anal Cell Pathol (Amst) (2019) 2019:4508756. doi: 10.1155/2019/4508756
8. Lee PC, Lo C, Lin MT, Liang JT, Lin BR. Role of surgical intervention in managing gastrointestinal metastases from lung cancer. World J Gastroenterol (2011) 17:4314–20. doi: 10.3748/wjg.v17.i38.4314
9. Kim SY, Ha HK, Park SW, Kang J, Kim KW, Lee SS, et al. Gastrointestinal metastasis from primary lung cancer: CT findings and clinicopathologic features. AJR Am J Roentgenol (2009) 193:W197–201. doi: 10.2214/AJR.08.1907
10. Guo Y, Wang X, Xiao J, Xu Y, Cai Y, Sun C, et al. Lung squamous cell carcinoma with solitary ocular metastasis and its successful treatment with thoracic surgery and chemotherapy: an interesting and rare case report. BMC Cancer (2018) 18:1004. doi: 10.1186/s12885-018-4944-y
11. Gelsomino F, Lamberti G, Ambrosini V, Sperandi F, Agosti R, Morganti AG, et al. Metachronous solitary metastasis to the thyroid gland from squamous cell carcinoma of the lung: a case report and literature review. Tumori (2017) 103:e12–5. doi: 10.5301/TJ.5000605
12. Kurt M, Bulut N, Aksoy S, Kosemehmetoglu K, Kars A. Anterior tongue metastasis from lung cancer. South Med J (2006) 99:784–5. doi: 10.1097/01.smj.0000224129.90177.2f
13. Perisano C, Spinelli MS, Graci C, Scaramuzzo L, Marzetti E, Barone C, et al. Soft tissue metastases in lung cancer: a review of the literature. Eur Rev Med Pharmacol Sci (2012) 16:1908–14.
14. Plaza JA, Perez-Montiel D, Mayerson J, Morrison C, Suster S. Metastases to soft tissue: a review of 118 cases over a 30-year period. Cancer (2008) 112:193–203. doi: 10.1002/cncr.23151
15. Surov A, Hainz M, Holzhausen HJ, Arnold D, Katzer M, Schmidt J, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol (2010) 20:649–58. doi: 10.1007/s00330-009-1577-1
16. Kataoka K, Osaka E, Shimizu T, Okamura Y, Yoshida Y, Tokuhashi Y. Lung squamous cell carcinoma with brachial soft tissue metastasis responsive to gefitinib: Report of a rare case. Thorac Cancer (2016) 7:676–80. doi: 10.1111/1759-7714.12366
17. Minomo S, Tokoro A, Utsumi T, Ishihara M, Akira M, Atagi S. A case of long-term survival after multimodal local treatments of intramedullary spinal cord metastasis of squamous cell lung cancer. J Thorac Dis (2016) 8:E681–683. doi: 10.21037/jtd.2016.06.58
18. Chattopadhyay S, Aich RK, Sengupta A, Kumari P. Squamous cell carcinoma of lung metastasizinig in breast. J Cancer Res Ther (2012) 8:630–2. doi: 10.4103/0973-1482.106582
19. Hu JB, Jin M, Chen EG, Sun XN. Lung squamous cell carcinoma metastasizing to the nasopharynx following bronchoscopy intervention therapies: a case report. World J Surg Oncol (2014) 12:68. doi: 10.1186/1477-7819-12-68
20. Nemoto M, Prasoon P, Ichikawa H, Hanyu T, Kano Y, Muneoka Y, et al. Primary lung squamous cell carcinoma and its association with gastric metastasis: A case report and literature review. Thorac Cancer (2020) 11:1708–11. doi: 10.1111/1759-7714.13410
21. Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang TH, Sheu CC, et al. Gastro-intestinal metastasis of primary lung carcinoma: clinical presentations and outcome. Lung Cancer (2006) 54:319–23. doi: 10.1016/j.lungcan.2006.08.007
22. Azar I, Koutroumpakis E, Patel R, Mehdi S. Squamous cell lung carcinoma presenting as melena: a case report and review of the literature. Rare Tumors (2017) 9:7164. doi: 10.4081/rt.2017.7164
23. Li X, Li S, Ma Z, Zhao S, Wang X, Wen D. Multiple gastrointestinal metastases of squamous-cell lung cancer: a case report. Medicine (2018) 97:e11027. doi: 10.1097/MD.0000000000011027
24. Hu Y, Feit N, Huang Y, Xu W, Zheng S, Li X. Gastrointestinal metastasis of primary lung cancer: an analysis of 366 cases. Oncol Lett (2018) 15:9766–76. doi: 10.3892/ol.2018.8575
25. Vidarsdottir H, Tran L, Nodin B, Jirstrom K, Planck M, Jonsson P, et al. Immunohistochemical profiles in primary lung cancers and epithelial pulmonary metastases. Hum Pathol (2019) 84:221–30. doi: 10.1016/j.humpath.2018.10.009
26. Shih-Chun C, Shih-Chiang H, Chun-Yi T, Shan-Yu W, Keng-Hao L, Jun-Te H, et al. Non-small cell lung cancer with gastric metastasis and repeated gastrointestinal bleeding: a rare case report and literature review. Thorac Cancer (2021) 12:560–3. doi: 10.1111/1759-7714.13815
27. Taira N, Kawabata T, Gabe A, Furugen T, Ichi T, Kushi K, et al. Analysis of gastrointestinal metastasis of primary lung cancer: clinical characteristics and prognosis. Oncol Lett (2017) 14:2399–404. doi: 10.3892/ol.2017.6382
28. Antler AS, Ough Y, Pitchumoni CS, Davidian M, Thelmo W. Gastrointestinal metastases from malignant tumors of the lung. Cancer (1982) 49:170–2. doi: 10.1002/1097-0142(19820101)49:1<170::AID-CNCR2820490134>3.0.CO;2-A
29. Lau CP, Leung WK. Caecal metastasis from a primary small-cell lung carcinoma. Hong Kong Med J (2008) 14:152–3.
30. Cedres S, Mulet-Margalef N, Montero MA, Martinez P, Martinez A, Felip E. Rectal metastases from squamous cell carcinoma: a case report and review of the literature. Case Rep Med (2012) 2012:947524. doi: 10.1155/2012/947524
31. Moazzam N, Mir A, Potti A. Pancreatic metastasis and extrahepatic biliary obstruction in squamous cell lung carcinoma. Med Oncol (2002) 19:273–6. doi: 10.1385/MO:19:4:273
32. Miyazaki J, Hirota S, Abe T. Metastasis of lung cancer to the gastrointestinal tract, presenting with a volcano-like ulcerated mass. Dig Endosc (2015) 27:397–8. doi: 10.1111/den.12412
33. Kadowaki T, Hamada H, Yokoyama A, Ito R, Ishimaru S, Ohnishi H, et al. Hemoperitoneum secondary to spontaneous rupture of hepatic metastasis from lung cancer. Intern Med (2005) 44:290–3. doi: 10.2169/internalmedicine.44.290
34. Meneses Grasa Z, Coll Salinas A, Macias Cerrolaza JA, Aguayo Albasini JL, Campillo Soto A, Guillen Paredes MP. Intestinal obstruction by metastasis in mesentery from squamous cell lung carcinoma. Rev Esp Enferm Dig (2009) 101:817–8. doi: 10.4321/s1130-01082009001100014
35. Shiraishi T, Araki M, Sumida Y, Fujita T, Hashimoto S, Nishimuta M, et al. Acute perforating appendicitis caused by metastatic squamous cell carcinoma from the lung: a case report. Int J Surg Case Rep (2020) 77:279–83. doi: 10.1016/j.ijscr.2020.10.116
36. Umakanthan S, Chalapathi Rao AV, Mohammed W. Role of immunohistochemistry markers in neoplastic lung lesions. J Cancer Res Ther (2021) 17:1382–8. doi: 10.4103/jcrt.JCRT_187_19
37. Sakai H, Egi H, Hinoi T, Tokunaga M, Kawaguchi Y, Shinomura M, et al. Primary lung cancer presenting with metastasis to the colon: a case report. World J Surg Oncol (2012) 10:127. doi: 10.1186/1477-7819-10-127
38. Habesoglu MA, Oguzulgen KI, Ozturk C, Akyurek N, Memis L. A case of bronchogenic carcinoma presenting with acute abdomen. Tuberk Toraks (2005) 53:280–3.
39. Zhou X, Chen R, Huang G, Liu J. Potential clinical value of PET/CT in predicting occult nodal metastasis in T1-T2N0M0 lung cancer patients staged by PET/CT. Oncotarget (2017) 8:82437–45. doi: 10.18632/oncotarget.19535
40. Wu Q, Luo W, Zhao Y, Xu F, Zhou Q. The utility of 18F-FDG PET/CT for the diagnosis of adrenal metastasis in lung cancer: a PRISMA-compliant meta-analysis. Nucl Med Commun (2017) 38:1117–24. doi: 10.1097/MNM.0000000000000757
41. Suzuki H, Kato K, Nishio M, Tamaki T, Fujimoto Y, Hiramatsu M, et al. FDG-PET/CT predicts survival and lung metastasis of hypopharyngeal cancer in a multi-institutional retrospective study. Ann Nucl Med (2017) 31:514–20. doi: 10.1007/s12149-017-1176-1
42. König K, Peifer M, Fassunke J, Ihle MA, Künstlinger H, Heydt C, et al. Implementation of amplicon parallel sequencing leads to improvement of diagnosis and therapy of lung cancer patients. J Thorac Oncol (2015) 10:1049–57. doi: 10.1097/JTO.0000000000000570
43. Caliò A, Nottegar A, Gilioli E, Bria E, Pilotto S, Peretti U, et al. ALK/EML4 fusion gene may be found in pure squamous carcinoma of the lung. J Thorac Oncol (2014) 9:729–32. doi: 10.1097/JTO.0000000000000109
44. Miyamae Y, Shimizu K, Hirato J, Araki T, Tanaka K, Ogawa H, et al. Significance of epidermal growth factor receptor gene mutations in squamous cell lung carcinoma. Oncol Rep (2011) 25:921–8. doi: 10.3892/or.2011.1182
45. Pan Y, Wang R, Ye T, Li C, Hu H, Yu Y, et al. Comprehensive analysis of oncogenic mutations in lung squamous cell carcinoma with minor glandular component. Chest (2014) 145:473–9. doi: 10.1378/chest.12-2679
46. Wang J, Shen Q, Shi Q, Yu B, Wang X, Cheng K, et al. Detection of ALK protein expression in lung squamous cell carcinomas by immunohistochemistry. J Exp Clin Cancer Res (2014) 33:109. doi: 10.1186/s13046-014-0109-2
Keywords: squamous cell lung carcinoma (LUSC), gastrointestinal metastasis, rare cecum metastasis, surgical resection, neoadjuvant chemoradiotherapy
Citation: Li F, Liu Y, Xu K, Yao Q, Li Q and Wu H (2023) Squamous cell lung carcinoma with gastrointestinal metastasis: a case report and review of literature. Front. Oncol. 13:1138871. doi: 10.3389/fonc.2023.1138871
Received: 06 January 2023; Accepted: 24 March 2023;
Published: 21 April 2023.
Edited by:
Yongfeng Yu, Shanghai Jiao Tong University, ChinaReviewed by:
Cristian Rapicetta, IRCCS Local Health Authority of Reggio Emilia, ItalySong Xu, Tianjin Medical University General Hospital, China
Copyright © 2023 Li, Liu, Xu, Yao, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Wu, d3Vob25nemFvQDEyNi5jb20=; Qiang Li, bGlxaWFuZzE5MDdAdmlwLnNpbmEuY29t
†These authors have contributed equally to this work
 Feifei Li1,2,3,4†
Feifei Li1,2,3,4† Quan Yao
Quan Yao Hong Wu
Hong Wu