- 1Department of Liver Surgery, West China Hospital, Sichuan University, Chengdu, China
- 2West China School of Medicine, Sichuan University, Chengdu, China
- 3Chinese Evidence-based Medicine Center, West China Hospital, Sichuan University, Chengdu, China
Background: The high recurrence rate of hepatocellular carcinoma (HCC) after surgery negatively affects the prognosis of patients. There is currently no widely accepted adjuvant therapy strategy for patients with HCC. A clinical study of effective adjuvant therapy is still needed.
Methods: In this prospective, single-arm, phase II clinical trial, an adjuvant regimen of donafenib plus tislelizumab combined with transarterial chemoembolization (TACE) will be used to treat enrolled HCC patients after surgery. Briefly, patients newly diagnosed with HCC by pathological examination who underwent curative resection and had a single tumor more than 5 cm in diameter with microvascular invasion as detected by pathological examination are eligible. The primary endpoint of the study is the recurrence-free survival (RFS) rate at 3 years, and secondary endpoints are the overall survival (OS) rate and the incidence of adverse events (AEs). The planned sample size, 32 patients, was calculated to permit the accumulation of sufficient RFS events in 3 years to achieve 90% power for the RFS primary endpoint.
Discussion: Vascular endothelial growth factor (VEGF) and programmed cell death protein 1 (PD-1)/programmed cell death ligand 1 (PD-L1) pathways regulate the relevant immunosuppressive mechanisms of HCC recurrence. Our trial will evaluate the clinical benefit of adding donafenib plus tislelizumab to TACE in patients with early-stage HCC and a high risk of recurrence.
Clinical trial registration: www.chictr.org.cn, identifier ChiCTR2200063003.
Introduction
Hepatocellular carcinoma (HCC) remains one of the most common and fatal cancers worldwide, especially in China, where the annual number of new HCC cases and deaths associated with HCC reached 431,000 and 412,000, respectively (1). Surgical resection is still recommended as the main therapeutic strategy for early-stage HCC patients, but the overall prognosis of HCC patients after radical resection is poor due to the high recurrence rate (approximately 70% at 5 years after surgical resection) (2, 3). It is reasonable to believe that a reduction in the postoperative recurrence rate could help to improve the overall prognosis of HCC.
Although the current clinical guidelines do not recommend any adjuvant therapy, clinical investigators initiated some trials of adjuvant therapy for HCC after radical resection. It has been reported that interferon (IFN)-α can reduce recurrence and prolong survival time (4), but the conclusion remains controversial (5). The results of two randomized controlled trials (RCTs) from Wang et al. and Wei et al. showed that postoperative adjuvant transarterial chemoembolization (PA-TACE) therapy has the effect of reducing recurrence risk and prolonging survival (6, 7). However, inconsistent results were found in some other studies (8). Other studies also suggested that conventional TACE (c-TACE) suffers from unstable drug loading and inadequate drug dosing (9, 10). A meta-analysis of eight RCTs showed that cytokine-induced killer (CIK) cellular therapy reduced the postoperative recurrence rate at 1 year and 3 years and improved the overall survival rate from 1 to 5 years but had no effect on the 5-year recurrence rate and the 6-year overall survival rate (11). Several retrospective studies have shown that adjuvant therapy with sorafenib after hepatectomy is effective in preventing recurrence (12, 13). However, the STORM trial, the largest clinical trial of adjuvant therapy in HCC to date, failed to draw a solid conclusion regarding the use of sorafenib for adjuvant therapy for HCC (14). These results indicated that further exploration of adjuvant therapy for HCC is needed.
The LANCE study initiated by Qin et al. showed that patients in the lenvatinib plus TACE group had a significantly longer median recurrence-free survival (RFS) than those in the TACE only group (17.0 months vs. 9.0 months, P=0.02) (15). This undoubtedly enhanced the confidence in the combination of local and systemic adjuvant therapy to prevent the postoperative recurrence of HCC. At present, several clinical trials of adjuvant therapy for HCC after resection are in process, including nivolumab (CheckMate-9DX) or pembrolizumab (KEYNOTE-937), EMERALD-2 study and IMbrave 050 study. The regimens of these trials were previously investigated for patients with advanced HCC, and survival benefits were obtained.
In recent years, the advent of immune checkpoint inhibitors (ICIs) has transformed therapeutic strategies for various solid tumors. However, the effect of ICI only therapy was unsatisfactory (16, 17). Not only is the effective response rate to monotherapy low (18, 19), but there is insufficient knowledge of predictive biomarkers of ICI treatment response such as programmed death ligand 1 (PD-L1) expression, tumor mutational burden (TMB), and microsatellite instability (MSI) status (20, 21). Conversely, some studies have shown that antiangiogenic therapy can enhance the antitumor sensitivity of programmed cell death protein 1 (PD-1)/PD-L1 inhibitors, so antiangiogenic therapy combined with immunotherapy can achieve synergistic antitumor effects (22–24). The results of two multicenter phase III studies (Imbrave150 (25) and ORIENT32 (26)) showed that overall survival (OS) and progression-free survival (PFS) in the combination therapy group were significantly better than those in the monotherapy group. A systematic review and meta-analysis of 56 RCTs by Viscardi et al. showed that early death rates were significantly lower in patients treated with ICI plus other therapies than in patients treated with ICI therapy alone (6.7% vs. 14.2%) (27). Recently, we reported a novel conversion therapy strategy of TACE combined with tyrosine kinase inhibitor (TKI) and PD-1 inhibitor for patients with unresectable HCC at the 2022 American Society of Clinical Oncology Gastrointestinal (ASCO-GI) Cancers Symposium (28). Our statistical results showed that the objective response rate (ORR) was as high as 84.2%, the disease control rate (DCR) was as high as 94.7%, and the conversion resection rate was as high as 50%. Grade 3 adverse events (AEs) occurred in 22 patients (22/38, 57.9%), and no grade 4/5 AEs occurred. We initiated this phase II trial of TACE plus TKI and PD-1 inhibitor with the inspiration of LANCE, IMbrave 050 and EMERALD-2. Donafenib (TKI) and tislelizumab (PD-1 inhibitor) were selected for this trial.
Donafenib is a new generation of small molecule multikinase inhibitors. An open-label, randomized, parallel-controlled, multicenter phase II/III clinical study of donafenib as first-line therapy for advanced HCC (ZGDH3) included a total of 668 patients with advanced HCC (29). The results showed that the OS with donafenib was significantly longer than that with sorafenib (12.1 months vs. 10.3 months; P=0.0245). In addition, the AE spectrum of the two groups was similar, and the sorafenib group showed better tolerability than the sorafenib group. Donafenib is also the only monotherapy drug to date that has been superior to sorafenib in OS in a first-line head-to-head study in advanced HCC.
Tislelizumab is a humanized IgG4 monoclonal antibody against immune checkpoint inhibitory receptors with high affinity and binding specificity for PD-1. In a global multicenter, phase IA/IB clinical study (NCT02407990), tislelizumab was used as a second-line drug in the therapy of advanced HCC, and the results showed that 6 patients (6/50) achieved partial response (PR), and the ORR reached 12.2% (30). In another global single-arm, multicenter, open-label, phase II study (NCT03419897), the ORR by independent review committee (ORR IRC) of tislelizumab as a second-line drug for advanced HCC even reached 12.9%, exhibiting patient safety and good tolerance (31).
The purpose of this study is to observe and evaluate the efficacy and safety of the adjuvant therapy regimen of donafenib and tislelizumab combined with TACE in patients with a high risk of HCC recurrence after resection. We named this phase II, prospective, single-arm clinical trial as “TIDE”.
Methods
Study design
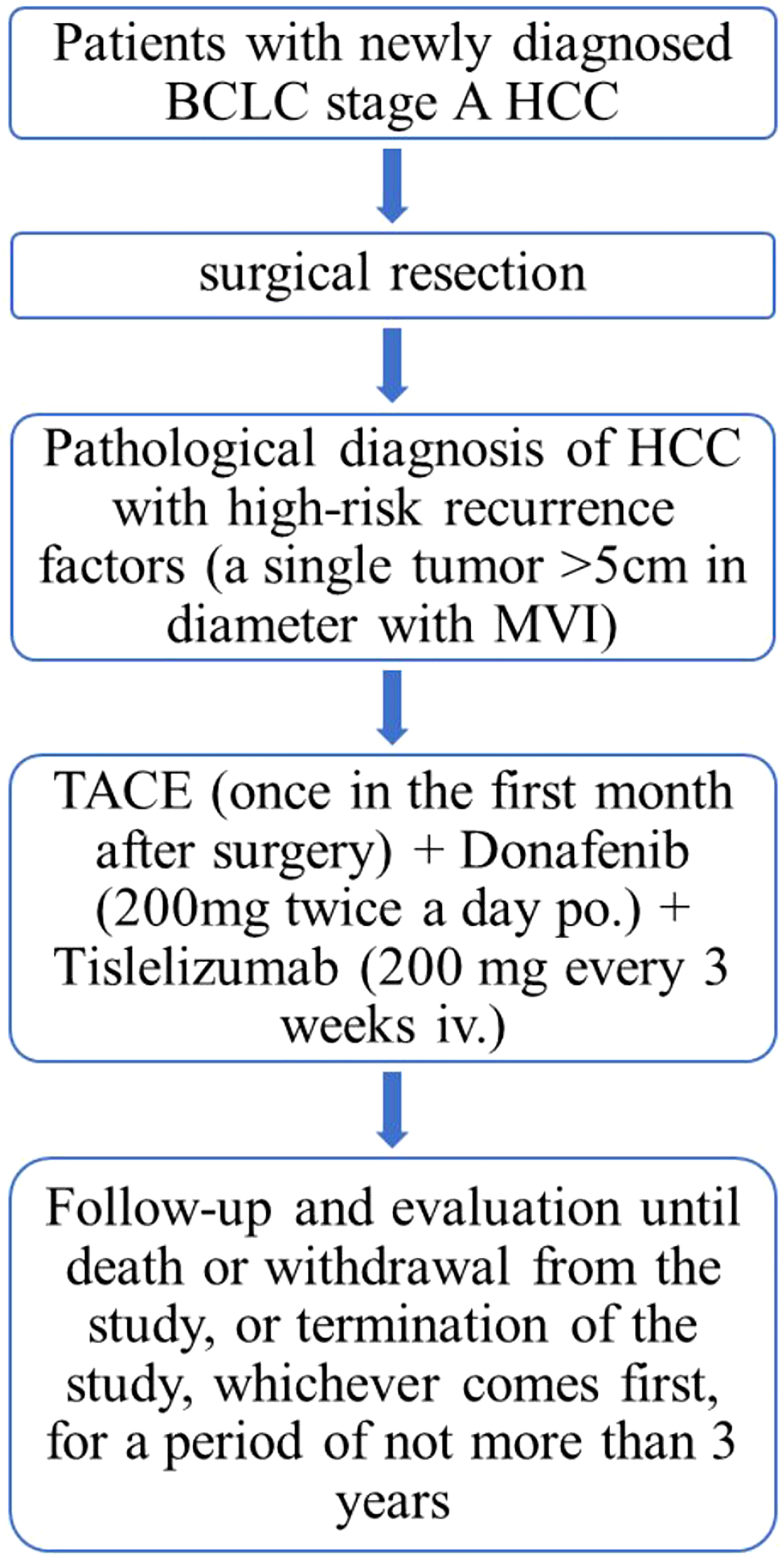
This study is a single-center, single-arm, prospective, phase II trial to evaluate the efficacy and safety of donafenib plus tislelizumab combined with TACE in adjuvant therapy after surgical resection to delay HCC recurrence (Figure 1). The study will begin enrollment in September 2022, and approximately 32 patients who have undergone surgical resection and are at high risk for HCC recurrence will receive the abovementioned adjuvant therapy and will be followed up.
Key eligibility criteria
Inclusion criteria
1) Voluntarily enrollment in the study and signed informed consent; 2) age 18~75 years old; 3) newly diagnosed HCC confirmed by pathological examination addressed by curative resection (R0 resection); 4) single tumor larger than 5 cm in diameter with microvascular invasion (MVI) and with or without satellite nodules; 5) no extrahepatic HCC; 6) complete recovery within 4 weeks after surgery; 7) Child Pugh A and ECOG performance status of 0 or 1; 8) adequate hematologic and vital organ function defined by laboratory test results; 9) for patients with hepatitis B virus (HBV) infection, HBV DNA <2000 IU/ml during screening, initiation of anti-HBV therapy at least 14 days prior to adjuvant therapy and willingness to continue anti-HBV. R0 resection was defined as follows: histological confirmation of surgical margin no residual tumor cells; decrease in alpha-fetoprotein (AFP) and des-gamma-carboxy-prothrombin (DCP) levels to normal levels at 1 month after surgery; and no evidence of residual lesions or recurrence on radiological evaluation at 1 month after surgery.
Exclusion criteria
1) Previous liver transplantation or presence on the waiting list for liver transplantation; 2) previous antitumor therapy, including local therapy, targeted therapy, immunotherapy, systemic chemotherapy, etc.; 3) any level of macrovascular invasion, including portal vein, hepatic vein, or vena cava invasion; 4) known fibrolamellar HCC, sarcomatoid HCC, or mixed hepatocellular-cholangiocellular tumor; 5) human immunodeficiency virus (HIV) infection autoimmune diseases; 6) known history of severe allergy to any monoclonal antibody or research drug excipients; 7) participation in other clinical studies, or the first dose is less than 30 days from the end of the previous clinical study drug therapy; 8) pregnant or breastfeeding status; 9) presence of other factors that may affect the results of the study or cause the study to be terminated halfway according to the judgment of the investigator; these include alcoholism, drug addiction, other serious diseases (including mental diseases) requiring combined therapy, serious abnormal laboratory tests, and family or social factors that affect drug safety.
Dropout case criteria
1) The investigator may decide to withdraw a patient from the study; 2) patients who have allergic reactions or serious AEs should cease participation in the trial according to the judgment of the investigator; 3) those who suffer from other complications during the trial and should not continue the trial; 4) patients with poor compliance may be excluded; 5) subjects can withdraw on their own.
Study procedures
Eligible subjects will receive one cycle of TACE one month after surgery. Two weeks after TACE, the patient will be given tislelizumab (200 mg every 3 weeks iv.) and donafenib (200 mg twice a day po.) This regimen will be given for a period of 1 year unless disease recurrence or unacceptable toxicity occurs.
Subjects must undergo imaging examinations, including computed tomography (CT) of the chest, abdomen, and pelvis, within 28 days before the first dose. If there are relevant clinical indications, other body part scans and/or (18)F-fluorodeoxyglucose-positron emission tomography (FDG-PET)/CT imaging can be performed. Tumor assessments will be performed every 12 weeks (± 1 week) after the initiation of study therapy by investigators on contrast-enhanced CT or contrast-enhanced magnetic resonance imaging (MRI) per RECIST v1.1 or mRECIST, respectively (the number of assessments may increase based on clinical needs as determined by the investigators). Relevant safety assessments will be carried out 28 days ( ± 7 days) after the last medication and once every 24 weeks ( ± 7 days) after therapy, either by telephone or face-to-face visits, until the subject dies or withdraws from the study, or the study is terminated, whichever occurs first, for a duration of up to 3 years.
A separate case report form (CRF) will be created for each subject, and follow-up, registration, form filling and data maintenance will be performed by dedicated researchers.
Sample size calculation
In a previous study on the effect of MVI on recurrence and prognosis after radical resection in 1517 patients with HCC, the 3-year RFS rate was less than 30% (32). We assumed that adjuvant therapy could increase the 3-year RFS rate to 50%. Using an alpha risk of 0.05 and a power of 80%, a sample size of 29 patients was needed, and considering a dropout rate of 10%, 32 patients needed to be enrolled.
Study end points
The primary endpoint of the study will be 3-year RFS, defined as the time from surgery to the first documented disease recurrence. The secondary key endpoint is OS, which is defined as the time from randomization to death from any cause. Other secondary endpoints include the incidence of AEs, considering the number, severity, duration and outcome of AEs during therapy according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v5.0 scale.
Statistics
All analyses will be performed on an intention-to-treat (ITT) basis. The Kaplan−Meier method will be used to determine RFS and OS. Hazard ratios (HRs) and their 95% confidence intervals (CIs) will be estimated using Cox proportional hazards models. Statistical significance will be defined when the P value is less than 0.05. A safety monitoring subcommittee will be established to monitor patient safety and study progress. The safety analysis set will include all treated patients.
Discussion
HCC is still an intractable disease. The high recurrence rate after surgical resection and the unsatisfactory overall prognosis have become major challenges for surgeons. Effective adjuvant therapy strategies are urgently needed to improve the OS and RFS of HCC patients. Currently, adjuvant therapy for HCC is still in the exploratory stage, and strategies for postoperative use of immunotherapy, targeted drugs, immunomodulators, hepatic arterial infusion chemotherapy (HAIC), and TACE alone or in combination are being actively carried out.
At present, it is believed that the high recurrence rate of HCC after resection is mainly related to the existence of microdisseminated foci or multisite tumors before surgery, especially in patients with high risk factors for recurrence (33). PA-TACE can supplement the treatment of occult residual lesions that cannot be detected before or during surgery, reducing the recurrence of HCC after radical hepatectomy, and it is well tolerated (6). Therefore, TACE is often recommended as an adjuvant therapy strategy for HCC patients with indicators of a high recurrence risk after surgical resection in China. Unfortunately, the results of TACE in HCC therapy remain unsatisfactory, and hypoxia secondary to TACE is thought to play a key role (34). Some studies suggest that hypoxia leads to the overexpression of hypoxia inducible factor-1α (HIF-1α), resulting in the upregulation of VEGF, which may induce tumor revascularization and local recurrence (35, 36). Therefore, combining TACE with a VEGF inhibitor can inhibit tumor vascular remodeling and tumor (re)proliferation. Moreover, TACE has been shown to enhance the immune response to ischemia-induced immunogenic cell death (ICD) and induce tumor-associated antigen-specific responses (37, 38). TACE has been reported to increase the number of CD4+ and CD8+ T cells in peripheral blood mononuclear cells (PBMCs) of patients, decreasing the number of regulatory T cells (Tregs) at the same time, but this immune response tends to be short-lived and may not provide long-term antitumor effects (39). TACE combined with ICI can further enhance the development of tumor-specific memory T cells and maintain the antitumor response of patients (39).
The tumor microenvironment (TME) in the liver is currently thought to play a key role in determining the prognosis of HCC. Angiogenesis and immune escape are considered hallmarks of cancer and are interdependent processes that often coexist and together contribute to tumorigenesis and progression (40). The expression levels of VEGF and PD-L1 are upregulated in most tumors and mediate tumor angiogenesis and immune escape (41, 42). Tumor angiogenesis is mainly regulated by VEGF, while VEGF and its receptors are considered to constitute one of the most effective signaling pathways during angiogenesis (43). Apart from its angiogenic roles, VEGF also mediates immunosuppression in the TME by driving tumor-associated immunosuppression factors, such as inducing vascular abnormalities, reducing tumor antigen presentation, inhibiting the proliferation and cytotoxic function of effector T cells, and increasing Tregs and myeloid-derived suppressor cells (MDSCs) (44). Moreover, PD-L1 is highly expressed on the surface of tumor cells, which combine with PD-1 to inhibit the function of effector T cells, reduce the proliferation of PD-1-positive cells, inhibit the secretion of cytokines, and induce apoptosis (45). Signaling through the PD-1/PD-L1 axis restricts T-cell interactions with dendritic cells (DCs) (46) and converts T helper (TH) cells with immunosurveillance functions to negative immunoregulatory Treg cells (47). Thus, inhibition of VEGF-induced signaling not only inhibits angiogenesis but also stimulates immune responses, whereas anti-PD-1 antibodies can cause antibody-dependent cell-mediated cytotoxicity and promote tumor vascular normalization (48, 49).
The limitations of this study come mainly from its single-arm design. First, the subjects in the experimental group and the external control group were difficult to compare because they were not from the same subject pool. Furthermore, the lack of a parallel control resulted in low internal validity.
In summary, TACE causes tumor hypoxia and necrosis, which has a positive regulatory effect on the immune microenvironment; immunotherapy enhances the antitumor immune response of T cells; antiangiogenic therapy normalizes the tumor vascular structure, promoting the tumor microenvironment from immunosuppressed to immunopermissive. Therefore, the combination of the three therapies has a potential synergistic effect.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The ethics committee on Biomedical Research of West China Hospital of Sichuan University approved this study (IRB No. 2022-691). All participants will sign written informed consent prior to participation in any study activities.
Author contributions
WP and CL proposed the study. WQ, WP, XQ, ZQ, and TW performed the research. WQ and WP wrote the first draft, and CL and TW reviewed the paper. All authors contributed to the interpretation of the study. All authors contributed to the article and approved the submitted version.
Funding
This study is funded by Sichuan Cancer Society Clinical Research Fund Project (Grant No. SCS-LCXM-2022-001), the Key R&D Projects of Sichuan Science and Technology Department (Grant No. 2022YFS0377), and the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (Grant No. 2020HXFH010). The funders have no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
WP reports receiving grant support from Zelgen and lecture fees from Bayer, Merk, Roche, Hengrui, SciClone. TW reports receiving grant support from AstraZeneca, Zelgen, Merk, Roche, Eisai and Innovent, advisory board fees and lecture fees from Bayer, Merk, Roche, Hengrui. CL, receiving grant support from Eisai, Merk, and advisor board fees from Bayer, Merk, Roche.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
HCC, hepatocellular carcinoma; TACE, transarterial chemoembolization; HAIC, hepatic arterial infusion chemotherapy; RFS, recurrence-free survival; OS, overall survival; PFS, progression-free survival; RCT, randomized controlled trial; IFN-α, interferon-α; CIK, cytokine-induced killer; ICI, immune checkpoint inhibitor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; AE, adverse event; ORR, objective response rate; PR, partial response; DCR, disease control rate; HBV, hepatitis B virus; HIV, human immunodeficiency virus; MVI, microvascular invasion; CT, computed tomography; FDG-PET (18):F-fluorodeoxyglucose-positron emission tomography; MRI, magnetic resonance imaging; RECIST, Response Evaluation Criteria in Solid Tumors; mRECIST, modified Response Evaluation Criteria in Solid Tumors; CRF, case report form; NCI-CTCAE, National Cancer Institute Common Terminology Criteria for Adverse Events; ITT, intention-to-treat; HR, hazard ratio; CI, confidence interval; HIF-1α, hypoxia inducible factor-1α; TME, tumor microenvironment; ICD, immunogenic cell death; PBMC, peripheral blood mononuclear cell; Treg, regulatory T cell; MDSC, myeloid-derived suppressor cell; DC, dendritic cell; TH, T helper.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American association for the study of liver diseases. Hepatology (2018) 68:723–50. doi: 10.1002/hep.29913
3. European Association for the Study of the Liver. Electronic address eee, European association for the study of the l. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol (2018) 69:182–236. doi: 10.1016/j.jhep.2018.03.019
4. Chen LT, Chen MF, Li LA, Lee PH, Jeng LB, Lin DY, et al. Long-term results of a randomized, observation-controlled, phase III trial of adjuvant interferon Alfa-2b in hepatocellular carcinoma after curative resection. Ann Surg (2012) 255:8–17. doi: 10.1097/SLA.0b013e3182363ff9
5. Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology (2006) 44:1543–54. doi: 10.1002/hep.21415
6. Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, et al. Adjuvant transarterial chemoembolization for HBV-related hepatocellular carcinoma after resection: a randomized controlled study. Clin Cancer Res (2018) 24:2074–81. doi: 10.1158/1078-0432.Ccr-17-2899
7. Wei W, Jian PE, Li SH, Guo ZX, Zhang YF, Ling YH, et al. Adjuvant transcatheter arterial chemoembolization after curative resection for hepatocellular carcinoma patients with solitary tumor and microvascular invasion: a randomized clinical trial of efficacy and safety. Cancer Commun (London England) (2018) 38:61. doi: 10.1186/s40880-018-0331-y
8. Jiang JH, Guo Z, Lu HF, Wang XB, Yang HJ, Yang FQ, et al. Adjuvant transarterial chemoembolization after curative resection of hepatocellular carcinoma: propensity score analysis. World J Gastroenterol (2015) 21:4627–34. doi: 10.3748/wjg.v21.i15.4627
9. Li Z, Cheng H, Mao J, Liu G. Conversion therapy of intermediate and advanced hepatocellular carcinoma using superstable homogeneous iodinated formulation technology. Sci China Life Sci (2022) 65:2114–7. doi: 10.1007/s11427-022-2142-3
10. Cheng H, Yang X, Liu G. Superstable homogeneous iodinated formulation technology: revolutionizing transcatheter arterial chemoembolization. Sci Bull (2020) 65:1685–7. doi: 10.1016/j.scib.2020.06.029
11. Wang J, Shen T, Wang Q, Zhang T, Li L, Wang Y, et al. The long-term efficacy of cytokine-induced killer cellular therapy for hepatocellular carcinoma: a meta-analysis. Immunotherapy (2019) 11:1325–35. doi: 10.2217/imt-2019-0079
12. Wang SN, Chuang SC, Lee KT. Efficacy of sorafenib as adjuvant therapy to prevent early recurrence of hepatocellular carcinoma after curative surgery: a pilot study. Hepatol Res (2014) 44:523–31. doi: 10.1111/hepr.12159
13. Zhang XP, Chai ZT, Gao YZ, Chen ZH, Wang K, Shi J, et al. Postoperative adjuvant sorafenib improves survival outcomes in hepatocellular carcinoma patients with microvascular invasion after R0 liver resection: a propensity score matching analysis. HPB (Oxford) (2019) 21:1687–96. doi: 10.1016/j.hpb.2019.04.014
14. Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol (2015) 16:1344–54. doi: 10.1016/s1470-2045(15)00198-9
15. Chen J, Lu L, Wen TF, Lu CD, Zeng YY, Xiang BD, et al. 945P adjuvant lenvatinib in combination with TACE for hepatocellular carcinoma patients with high risk of postoperative relapse (LANCE): updated results from a multi-center prospective cohort study. Ann Oncol (2021) 32:S824–5. doi: 10.1016/j.annonc.2021.08.165
16. Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol (2020) 38:193–202. doi: 10.1200/jco.19.01307
17. Yao X, Wang L, Gao J. Overshadowed prospect of programmed cell death protein-1 (PD-1) inhibitor as monotherapy for patients with advanced hepatocellular carcinoma. Biosci Trends (2019) 13:282–3. doi: 10.5582/bst.2019.01161
18. Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol (2022) 19:151–72. doi: 10.1038/s41571-021-00573-2
19. Cheng H, Fan X, Ye E, Chen H, Yang J, Ke L, et al. Dual tumor microenvironment remodeling by glucose-contained radical copolymer for MRI-guided photoimmunotherapy. Advanced materials (Deerfield Beach Fla.) (2022) 34:e2107674. doi: 10.1002/adma.202107674
20. Rizzo A, Cusmai A, Gadaleta-Caldarola G, Palmiotti G. Which role for predictors of response to immune checkpoint inhibitors in hepatocellular carcinoma? expert rev. Gastroenterol Hepatol (2022) 16:333–9. doi: 10.1080/17474124.2022.2064273
21. Rizzo A, Ricci AD, Di Federico A, Frega G, Palloni A, Tavolari S, et al. Predictive biomarkers for checkpoint inhibitor-based immunotherapy in hepatocellular carcinoma: where do we stand? Front Oncol (2021) 11:803133. doi: 10.3389/fonc.2021.803133
22. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. Semin Cancer Biol (2018) 52:117–24. doi: 10.1016/j.semcancer.2017.12.002
23. Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol (2018) 15:325–40. doi: 10.1038/nrclinonc.2018.29
24. Wang J, Wu R, Sun JY, Lei F, Tan H, Lu X. An overview: management of patients with advanced hepatocellular carcinoma. Biosci Trends (2022) 16:405–25. doi: 10.5582/bst.2022.01109
25. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. New Engl J Med (2020) 382:1894–905. doi: 10.1056/NEJMoa1915745
26. Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2-3 study. Lancet Oncol (2021) 22:977–90. doi: 10.1016/s1470-2045(21)00252-7
27. Viscardi G, Tralongo AC, Massari F, Lambertini M, Mollica V, Rizzo A, et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer (2022) 177:175–85. doi: 10.1016/j.ejca.2022.09.031
28. Zhang X, Zhu X, Liu C, Lu W, Li Q, Chen W, et al. The safety and efficacy of transarterial chemoembolization (TACE) + lenvatinib + programmed cell death protein 1 (PD-1) antibody of advanced unresectable hepatocellular carcinoma. J Clin Oncol (2022) 40:453–3. doi: 10.1200/JCO.2022.40.4_suppl.453
29. Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J Clin Oncol (2021) 39:3002–11. doi: 10.1200/jco.21.00163
30. Desai J, Deva S, Lee JS, Lin CC, Yen CJ, Chao Y, et al. Phase IA/IB study of single-agent tislelizumab, an investigational anti-PD-1 antibody J Immunother Cancer (2020) 8:e000453. doi: 10.1136/jitc-2019-000453
31. Ren Z, Li Z, Zhang T, Fang W, Hu S, Pan H, et al. P-25 tislelizumab monotherapy for patients with previously treated advanced hepatocellular carcinoma (HCC): RATIONALE-208 Chinese subpopulation. Ann Oncol (2022) 33: S255. doi: 10.1016/j.annonc.2022.04.116
32. Chen ZH, Zhang XP, Feng JK, Li LQ, Zhang F, Hu YR, et al. Actual long-term survival in hepatocellular carcinoma patients with microvascular invasion: a multicenter study from China. Hepatol Int (2021) 15:642–50. doi: 10.1007/s12072-021-10174-x
33. Wu JC, Huang YH, Chau GY, Su CW, Lai CR, Lee PC, et al. Risk factors for early and late recurrence in hepatitis b-related hepatocellular carcinoma. J Hepatol (2009) 51:890–7. doi: 10.1016/j.jhep.2009.07.009
34. Lin WH, Yeh SH, Yeh KH, Chen KW, Cheng YW, Su TH, et al. Hypoxia-activated cytotoxic agent tirapazamine enhances hepatic artery ligation-induced killing of liver tumor in HBx transgenic mice. Proc Natl Acad Sci U S A (2016) 113:11937–42. doi: 10.1073/pnas.1613466113
35. Ader I, Brizuela L, Bouquerel P, Malavaud B, Cuvillier O. Sphingosine kinase 1: a new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Res (2008) 68:8635–42. doi: 10.1158/0008-5472.CAN-08-0917
36. Gai X, Zhou P, Xu M, Liu Z, Zheng X, Liu Q. Hyperactivation of IL-6/STAT3 pathway leaded to the poor prognosis of post-TACE HCCs by HIF-1alpha/SNAI1 axis-induced epithelial to mesenchymal transition. J Cancer (2020) 11:570–82. doi: 10.7150/jca.35631
37. Greten TF, Mauda-Havakuk M, Heinrich B, Korangy F, Wood BJ. Combined locoregional-immunotherapy for liver cancer. J Hepatol (2019) 70:999–1007. doi: 10.1016/j.jhep.2019.01.027
38. Greten TF, Lai CW, Li G, Staveley-O’Carroll KF. Targeted and immune-based therapies for hepatocellular carcinoma. Gastroenterology (2019) 156:510–24. doi: 10.1053/j.gastro.2018.09.051
39. Liao J, Xiao J, Zhou Y, Liu Z, Wang C. Effect of transcatheter arterial chemoembolization on cellular immune function and regulatory T cells in patients with hepatocellular carcinoma. Mol Med Rep (2015) 12:6065–71. doi: 10.3892/mmr.2015.4171
40. Khan KA, Kerbel RS. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol (2018) 15:310–24. doi: 10.1038/nrclinonc.2018.9
41. Morse MA, Sun W, Kim R, He AR, Abada PB, Mynderse M, et al. The role of angiogenesis in hepatocellular carcinoma. Clin Cancer Res (2019) 25:912–20. doi: 10.1158/1078-0432.Ccr-18-1254
42. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest (2015) 125:3384–91. doi: 10.1172/jci80011
43. Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature (1993) 362:841–4. doi: 10.1038/362841a0
44. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med (2013) 19:1423–37. doi: 10.1038/nm.3394
45. Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med (2002) 8:793–800. doi: 10.1038/nm730
46. Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol (2009) 10:1185–92. doi: 10.1038/ni.1790
47. Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med (2011) 3:111ra120. doi: 10.1126/scitranslmed.3003130
48. Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature (2017) 544:250–4. doi: 10.1038/nature21724
Keywords: hepatocellular carcinoma, adjuvant therapy, VEGF, PD-1, TACE
Citation: Qi W, Peng W, Qi X, Qiu Z, Wen T and Li C (2023) TIDE: adjuvant tislelizumab plus donafenib combined with transarterial chemoembolization for high-risk hepatocellular carcinoma after surgery: protocol for a prospective, single-arm, phase II trial. Front. Oncol. 13:1138570. doi: 10.3389/fonc.2023.1138570
Received: 05 January 2023; Accepted: 03 April 2023;
Published: 17 April 2023.
Edited by:
John Gibbs, Hackensack Meridian Health, United StatesReviewed by:
Zhang Wen, Guangxi Medical University, ChinaHongwei Cheng, Xiamen University, China
Angela Dalia Ricci, “Saverio de Bellis” Research Hospital, Italy
Copyright © 2023 Qi, Peng, Qi, Qiu, Wen and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chuan Li, bGljaHVhbkBzY3UuZWR1LmNu
†These authors have contributed equally to this work
 Weili Qi
Weili Qi Wei Peng1,3†
Wei Peng1,3† Xin Qi
Xin Qi Zhancheng Qiu
Zhancheng Qiu Tianfu Wen
Tianfu Wen Chuan Li
Chuan Li