
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 06 April 2023
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1138357
This article is part of the Research Topic Optimizing the First-Line Treatment for Metastatic Colorectal Cancer View all 7 articles
 Pui Lam Yip1,2
Pui Lam Yip1,2 Wai Him Brian Fung3
Wai Him Brian Fung3 Francis Ann Shing Lee1
Francis Ann Shing Lee1 Chak Fei Lee4
Chak Fei Lee4 Natalie Sean Man Wong1
Natalie Sean Man Wong1 Shing Fung Lee1,2*
Shing Fung Lee1,2*Introduction: Capecitabine, irinotecan, and panitumumab (CAPIRI-P) is a controversial regimen for metastatic colorectal cancer, with concerns regarding the efficacy and toxicity. However, its toxicity profile has been improved with dose reduction, and concerns regarding efficacy have been extrapolated from other trials. This retrospective study reports the real-world effectiveness and safety of modified CAPIRI-P (mCAPIRI-P).
Material and methods: Advanced colorectal cancer patients receiving mCAPIPI-P in the first-line setting between July 2019 and December 2021 were analyzed. The progression-free survival on treatment (PFSOT) and overall survival (OS) were estimated using the Kaplan–Meier method, and the association with clinical and disease factors was analyzed using the Cox regression model. Serial changes in carcinoembryonic antigen (CEA) level and treatment toxicity were also evaluated.
Results: A total of 106 patients were included, of whom 97 (92%) and 31 (29%) had left-sided primary and unresectable liver-only disease, respectively. The median PFSOT and OS were 15.4 (95% CI 12.5–18.3) and 25.5 (95% CI 17.6–33.4) months, respectively. Sixteen (51.6%) and 10 (32.3%) liver-only disease patients underwent secondary liver treatment and R0 resection, respectively. In multivariable Cox regression, CEA responders (PFSOT: HR 0.53) and CEA normalization (PFSOT: HR 0.27; OS: HR 0.28) were independent favorable prognostic factors for PFSOT and OS. Grade ≥3 toxicity rate was 43%, mainly related to uncomplicated hematological toxicities.
Conclusion: The real-world data show that mCAPIRI-P is safe and effective as the first-line treatment regimen for RAS wild-type advanced colorectal cancer and warrants further study.
Combination chemotherapy with fluoropyrimidine and oxaliplatin or irinotecan is the standard backbone in the first-line treatment for metastatic colorectal cancer (1, 2). The addition of anti-epidermal growth factor receptor (EGFR) antibodies to doublet chemotherapy has improved the treatment outcome (3–5). Although many landmark trials have studied the combination of panitumumab with infusional 5-fluorouracil (5-FU) and oxaliplatin (5–8), its effectiveness when added to irinotecan and capecitabine (CAPIRI) has not been reported. Moreover, CAPIRI with an anti-EGFR antibody is a much debated regimen (1, 9), with concerns regarding its efficacy and toxicity. In our institute, a modified three-week capecitabine, irinotecan, and panitumumab (mCAPIRI-P) has been widely adopted due to the COVID-19 pandemic.
This retrospective cohort study reports the effectiveness and safety of mCAPIRI-P regimen on consecutive RAS wild-type (WT) advanced colorectal cancer patients and carcinoembryonic antigen (CEA) kinetics.
Patients receiving combination chemotherapy with capecitabine, irinotecan, and panitumumab (mCAPIRI-P) from July 2019 to December 2021 at Tuen Mun Hospital were evaluated. Patients were included if chemotherapy was administered for RAS WT unresectable locally advanced or metastatic colorectal cancer in the first-line setting. The patient was excluded if panitumumab was started later than the fifth cycle of chemotherapy and/or if the patient had no active disease, for example, upfront metastectomy was performed.
Patient demographics, chemotherapy dose and record, treatment outcomes and toxicity, and CEA levels were retrieved from electronic medical records and hospital records. In our institute, the mCAPIRI-P regimen comprised three-week cycles of oral 800 mg/m2 capecitabine twice daily on days 1–14, intravenous 200 mg/m2 irinotecan on day 1, and intravenous 9 mg/kg panitumumab on day 1. Patients were monitored for clinical symptoms, tumor markers, and radiological findings. For patients with liver-only disease, the management of liver metastasis was reviewed with hepatobiliary surgeons in regular multidisciplinary team meetings. Chemotherapy was continued until disease progression or in some patients, drug holidays. A drug holiday with subsequent resumption was offered at the physician’s discretion and patient preference.
Effectiveness of the chemotherapy was assessed by progression-free survival on treatment (PFSOT) and overall survival (OS). For survival endpoints, patients were censored at the last follow-up visit. PFSOT is the time from the date of the first cycle of chemotherapy to the date of documented disease progression during active treatment or death. Disease progression during drug holidays was not considered true progression; thus, it was not a PFSOT event. The patient would resume mCAPIRI-P until progression on treatment or another drug holiday. OS is the time interval from the date of the first cycle of chemotherapy until death from any cause. A subgroup of patients receiving conversion chemotherapy for liver-only disease were assessed for the treatment response in the liver by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (10), secondary liver treatment, and R0 resection (defined as no microscopic or macroscopic residual tumor). Disease progression was determined by the treating physician with supporting evidence from clinical symptoms, radiological findings, and/or biomarkers.
CEA ≤ 5 ng/mL was considered normal. CEA response rate (11) is the percentage reduction of CEA from the initial value to the nadir after chemotherapy. CEA responders are patients having ≥75% CEA response rate (11).
Toxicities of chemotherapy were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v4 (12).
Descriptive statistics for demographics, follow-up duration, and characteristic prevalence were generated. Continuous variables were presented as medians with interquartile ranges (or means and standard deviations).
We estimated PFSOT and OS using the Kaplan–Meier method (13, 14). Univariable Cox regression was performed to evaluate the associations between variables (demographic factors, clinical characteristics, and CEA kinetics) and PFSOT and OS. Variables with a significant association (p<0.05) were selected and tested using multivariable Cox regression (15). Variables linked to CEA kinetics (namely baseline normal CEA, CEA normalization and CEA responder) were added individually to the Cox models. A two-sided p<0.05 was used to determine statistical significance. All statistical analyses were performed using IBM SPSS Statistics for Windows version 21 (IBM Corp.; Armonk, N.Y., USA).
The study protocol was approved by the Research Ethics Committee of the New Territories West Cluster, Hong Kong Hospital Authority (reference no. NTWC/REC/21037).
This study analyzed data from 106 consecutive patients. The mean age was 63 years, and the median follow-up period was 16 months (IQR 11–23 months). Among them, 97 (91.5%) had left-sided colorectal cancer, 97 (91.5%) were treated with palliative intent for metastatic disease, and 31 (29.3%) had unresectable liver-only disease. During analysis, 91.5% of patients completed at least one treatment course. 26 (24.5%) decided for drug holiday after the first treatment course. A median of nine cycles of chemotherapy was administered (Table 1).
During analysis, 68 (64.2%) patients had PFSOT events. Moreover, three patients documented progressive disease on drug holiday; among them, two decided to continue drug holiday and one died before resuming chemotherapy. The median PFSOT was 15.4 months (95% CI 12.5–18.3). The 1-year PFSOT was 61.9% (95% CI 52.5%–71.3%). Furthermore, 46 (43.4%) deaths occurred, and the median OS was 25.5 months (95% CI 17.6–33.4; Figure 1).
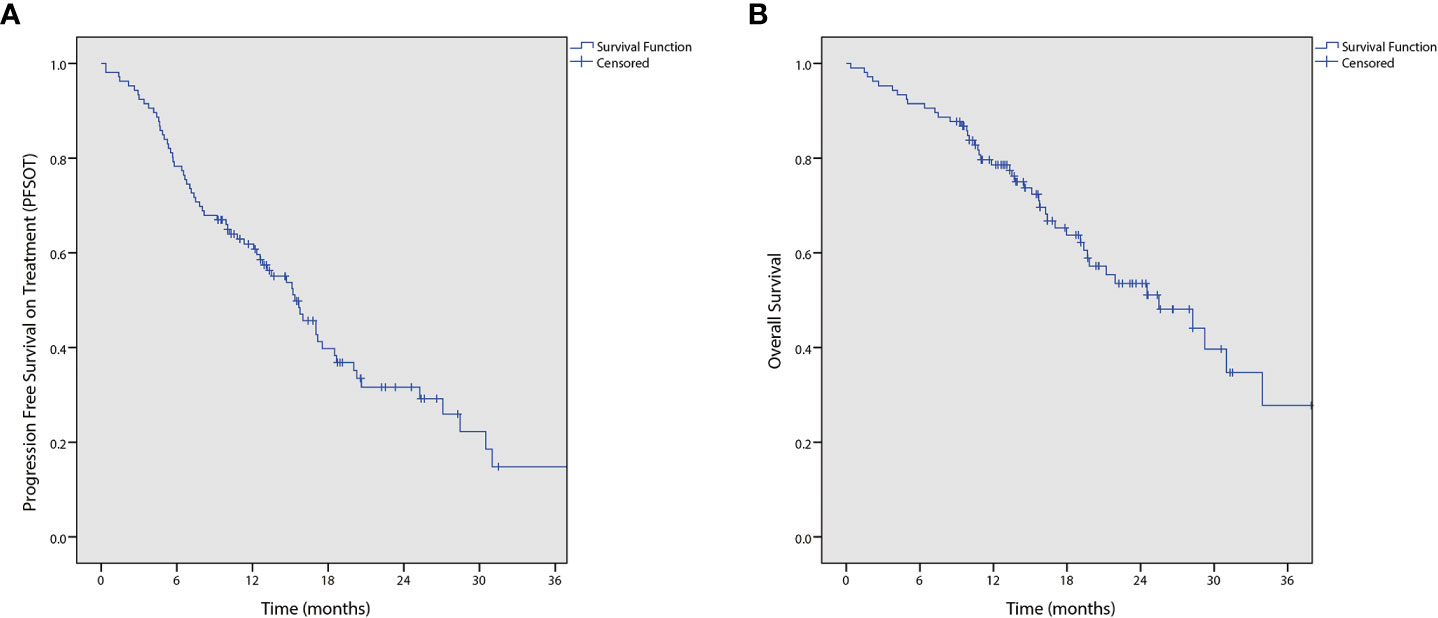
Figure 1 Kaplan-Meier curves of (A) progression free survival on treatment (PFSOT) and (B) overall survival (OS).
In total, 31 patients had unresectable liver-only disease (Table 2). Two (6.5%), 20 (64.5%), 3 (9.7%), and 4 (12.9%) achieved complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD) in the liver, respectively. The objective response rate was 71%. The two patients with CR were put on observation and were not offered liver treatment. Sixteen (51.6%) patients underwent secondary liver treatment. The R0 resection rate was 32.3%.
In total, 93 (87.7%) patients had baseline elevated CEA. Among them, CEA was normalized in 40 (43.0%) patients. The median time to nadir was 138.50 days (93.50–196.25). The median CEA response rate was 88.9% (67.3–96.9). Moreover, 62 (68.9%) patients were CEA responders with ≥75% CEA response rate.
Univariable analyses (Figure 2 and Tables 3, 4) reported that liver-only disease, CEA normalization, and CEA responder were both OS and PFSOT predictors. Additionally, Karnofsky performance status (KPS) and baseline normal CEA levels were OS predictors. However, multivariable analyses (Tables 3, 4) reported that CEA responders (PFSOT: HR 0.53, CI 0.31–0.92; OS: HR 0.53, CI 0.27–1.04) and CEA normalization (PFSOT: HR 0.27, CI 0.15–0.48; OS: HR 0.28, CI 0.13–0.58) remained independent predictors of better OS and PFSOT.
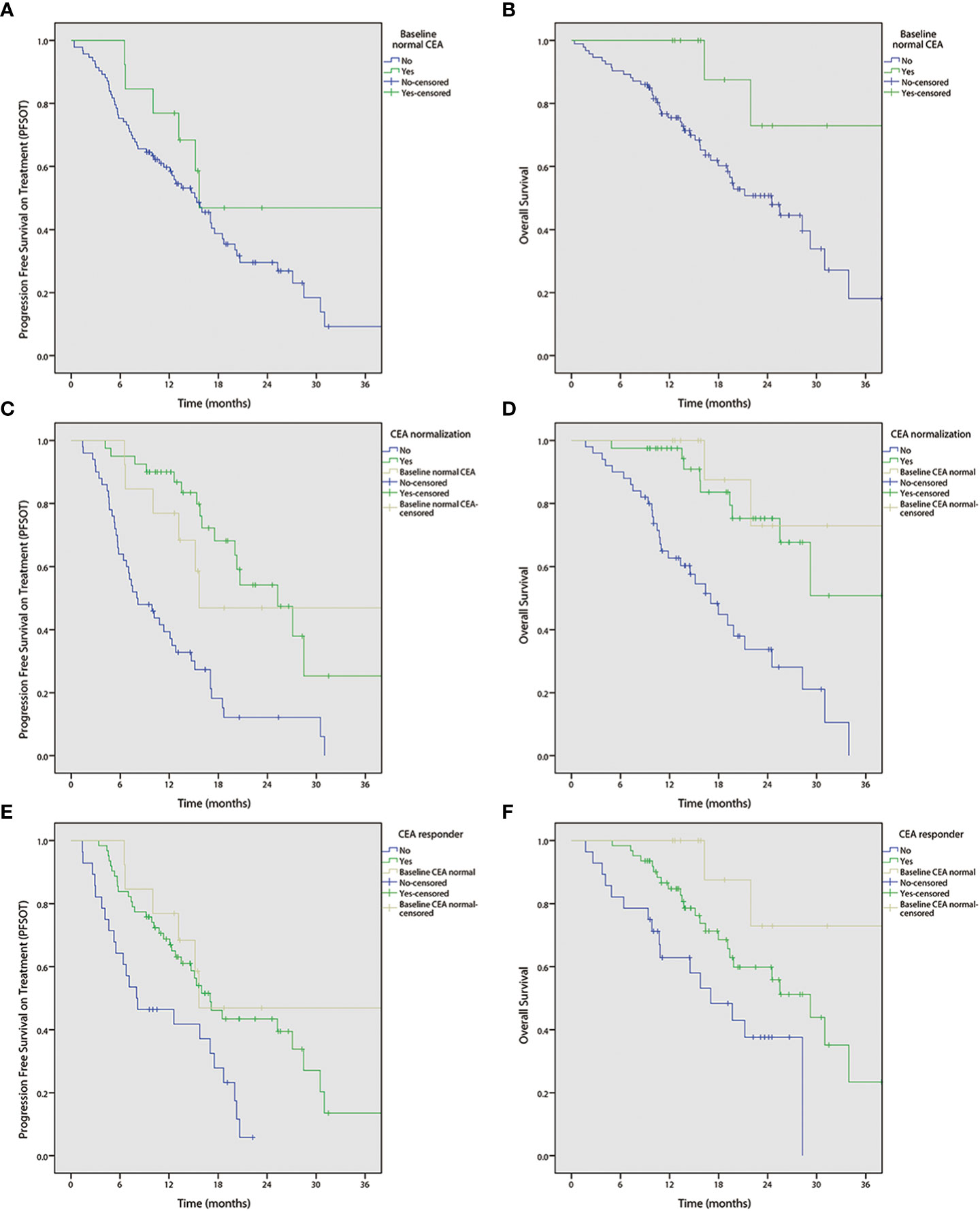
Figure 2 Kaplan-Meier curves of progression free survival on treatment (PFSOT) and overall survival (OS) by carcinoembryonic antigen (CEA) kinetics. (A) PFSOT by baseline normal CEA, (B) OS by baseline normal CEA. (C) PFSOT by CEA normalization. (D) OS by CEA normalization. (E) PFSOT by CEA responder. (F) OS by CEA responder.
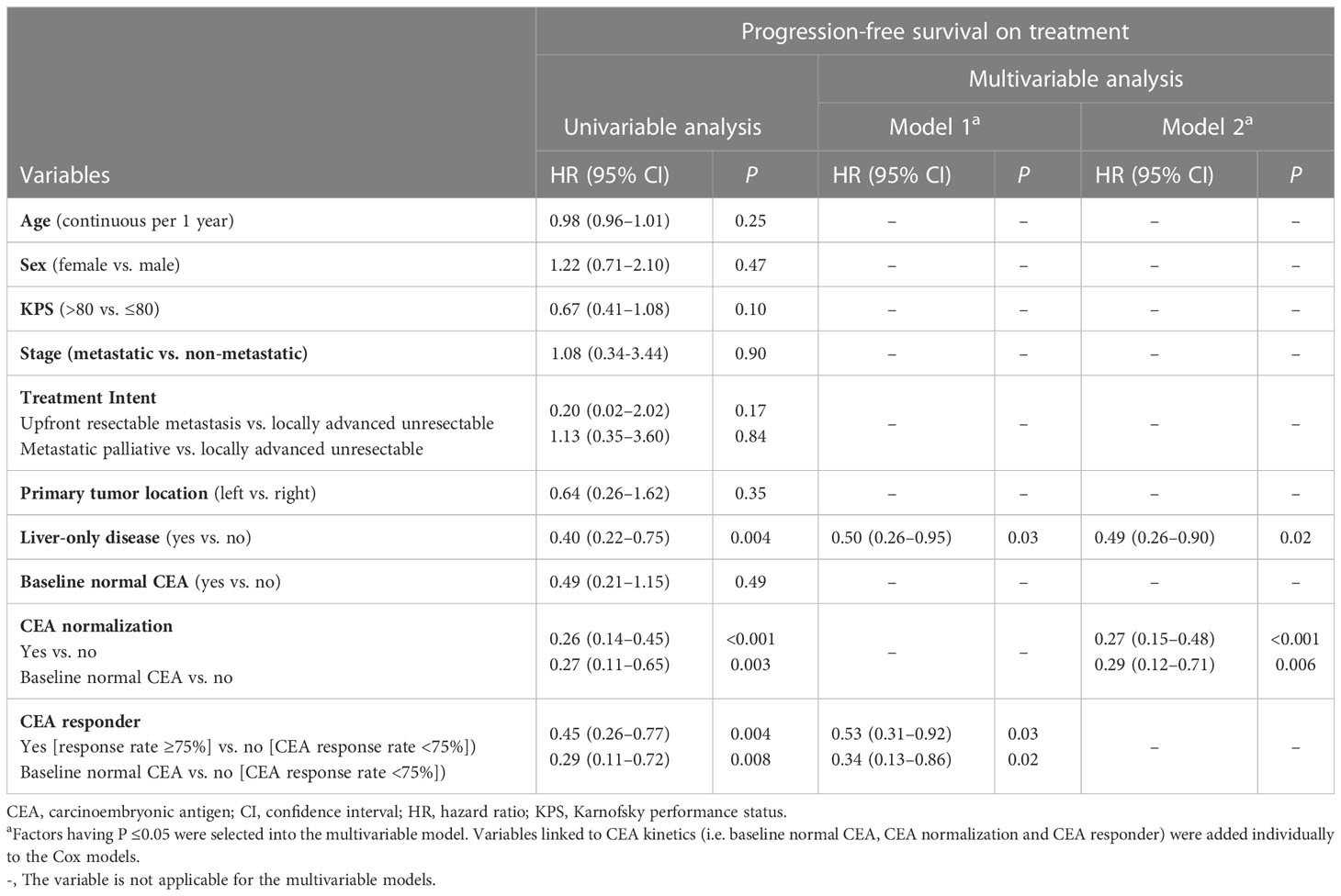
Table 3 Univariable and multivariable analyses of prognostic factors for progression-free survival on treatment, Hong Kong, 2019–2021 (N = 106).
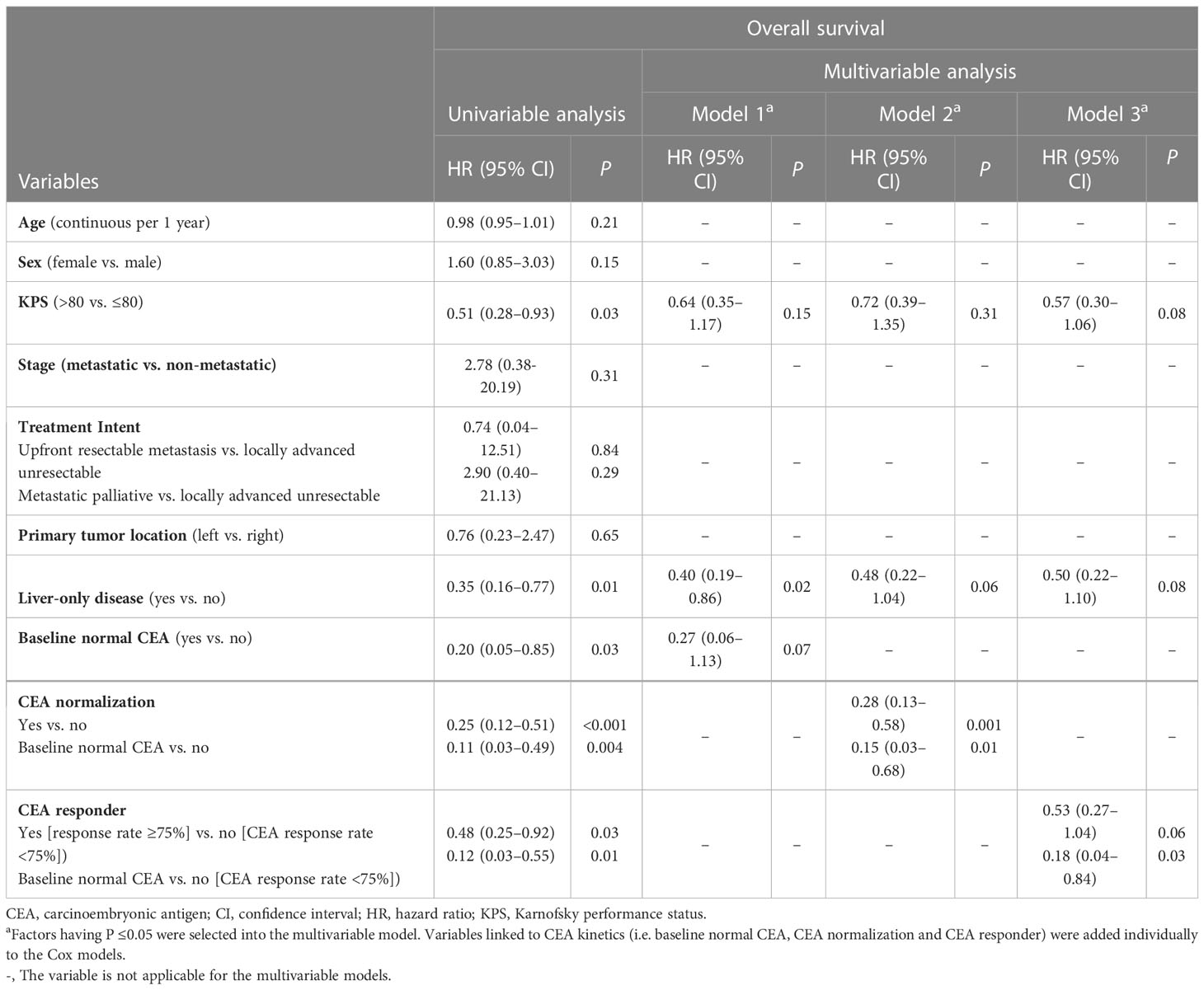
Table 4 Univariable and multivariable analyses of prognostic factors for overall survival, Hong Kong, 2019–2021 (N = 106).
Ten (9.4%), six (5.7%), and four (3.8%) patients required dose reduction to <70% of the intended dose of capecitabine, irinotecan, and panitumumab, respectively. Unplanned hospitalization occurred in 44 patients, of whom 15 (14.2%) and 19 (17.9%) were due to treatment toxicity and disease-related symptoms or complications, respectively. Five (4.7%) patients terminated the treatment due to toxicity (Table 1). Grade 3-4 toxicities were observed in 46 (43.4%) patients, of whom 32.1%, 16.0%, and 11.3% experienced uncomplicated neutropenia, uncomplicated leukopenia, and diarrhea, respectively. Febrile neutropenia occurred in 3.8%. No grade 5 toxicity was observed (Table 5).
To the best of our knowledge, this is the first report on the effectiveness and safety of mCAPIRI-P in a three-week schedule. Due to the availability of public funding for panitumumab and the COVID-19 pandemic, mCAPIRI-P was offered to all left-sided RAS WT and selected right-sided RAS WT colorectal cancer patients as a first-line treatment choice. During the study period, only a small proportion of highly selected patients received triplet chemotherapy. Our consecutive data are representative of the mCAPIRI-P treatment outcomes in a real-world setting.
PFSOT was studied because intermittent therapy has been widely adopted in local practice. The median PFSOT (15.4 months) and 1-year PFSOT (61.9%) are similar to those reported in other landmark trials including PARADIGM (7) (12.9 months), PRIME (5) (10 months), and CRYSTAL (3) (9.9 months). It is also comparable to the results of IMPROVE (16) trial, which also studied PFSOT (median PFSOT 17.6 months, 1-year PFSOT 61.3%; Supplementary Table 1). The OS data of this study were not mature during the analysis. Furthermore, mCAPIRI-P was also proved to be an efficacious regimen in the conversion setting for liver-only disease. The secondary liver treatment rate (51.6%) and R0 resection rate of this study were similar to those of other major reports, including CELIM (17), Ye et al. (18), TRIPLETE (8), and VOLFI (6) (Supplementary Table 1).
mCAPIRI-P use raised no additional safety concerns in terms of toxicity. the rate of grade ≥3 diarrhea was 11.3% which is similar to that found in other trials with 5-FU (19) or oxaliplatin (5) backbones. Uncomplicated grade≥3 leukopenia and neutropenia rates were higher than those reported in other irinotecan-based trials (19, 20). This is probably because the interim blood cell count was frequent, such as at the second week. Nonetheless, the rate of febrile neutropenia (3.8%) remained similar to other landmark trials (5, 20). Notably, the incidence of severe skin/nail toxicity (<4%) was low, which could be attributed to the routine use of empirical oral doxycycline, topical steroid, and emulsifying lotion, as suggested in the STEPP (21) and JSTEPP (22). Pharmacogenetic tests are not routinely performed for proactive dose reduction. Some patients who experienced significant toxicities were later found to have dihydropyrimidine dehydrogenase (DPD) deficiency or UGT1A1 polymorphism.
mCAPIRI-P is not widely used. First, although CAPIRI combination demonstrated tolerable toxicity in European trials (23, 24), it was suggested to be too toxic in other trials (25, 26). Thus, the National Comprehensive Cancer Network (NCCN) guideline does not recommend CAPIRI (1). However, the toxicity profile was much improved after dose reduction (a.k.a. modified CAPIRI (mCAPIRI)), as demonstrated in the phase III AXEPT (20) and other trials (27, 28). Second, the European Society for Medical Oncology (9) and NCCN guidelines (1) do not recommend using anti-EGFR antibodies in combination with capecitabine-based regimens. This recommendation was based on MRC-COIN (4) indicating that cetuximab in combination with capecitabine and oxaliplatin had worse outcomes than 5-FU-based chemotherapy in a subgroup analysis, which, according to the authors, could be related to the increased toxicity with subsequent reduction in drug dose and exposure. Such toxicity was again improved after capecitabine dose reduction. Notably, there is no direct evidence suggesting inferior efficacy or toxicity of capecitabine and anti-EGFR antibodies using an irinotecan backbone. Indeed, the use of CAPIRI and anti-EGFR antibodies has been supported in previous phase II studies (23, 29). Third, panitumumab has been licensed as a biweekly regimen (30) instead of the three-week regimen in this study. The three-week regimen was supported in a phase I study (31), which indicated that three-week 9 mg/kg panitumumab and biweekly 6 mg/kg panitumumab have similar exposure and safety profiles. In addition, capecitabine and panitumumab administration every three weeks demonstrated efficacy and safety in a geriatric population in the Panel study (32).
Despite these controversies, three-week mCAPIRI-P has several advantages including convenient oral administration, less frequent clinic attendance, and the absence of oxaliplatin-associated disturbing peripheral neuropathy. It is conceivable that randomized studies would unlikely be open to compare this chemotherapy regimen with other regimens. This report demonstrates that mCAPIRI-P is both safe and effective. The results are of particular importance, with the expected wide adoption of doublet chemotherapy with anti-EGFR antibody in the first-line setting, following the latest evidence from the PARADIGM (7) and TRIPLETE (8) trials.
In addition, with prolonged survival in colorectal cancer patients, the interest in studying CEA as a surrogate marker to reflect treatment response has been increasing (33–35). CEA kinetics analysis was consistent with the findings of a previous report (11) on the prognostic value of CEA response rate. Furthermore, multivariable analyses showed that patients with elevated baseline CEA levels achieved CEA normalization and/or were CEA responders had better PFSOT and OS. The report on CEA kinetics in patients receiving mCAPIRI-P will further supplement the overall body of evidence.
The major limitation of this study is the retrospective study design. Non-laboratory toxicities can be potentially under-recorded. In addition, B-Raf was not routinely checked in the cohort. Finally, a long follow-up period is required to precisely estimate the survival data.
To conclude, mCAPIRI-P is an effective first-line treatment regimen for RAS wild-type advanced colorectal cancer in a real-world setting. It is generally safe with tolerable toxicity. Further studies are required to confirm these results.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by New Territories West Cluster, Hospital Authority, Hong Kong. The ethics committee waived the requirement of written informed consent for participation.
PY, SL, and FL contributed to conception and design of the study. PY organized the database. PY performed the statistical analysis. PY and SL wrote the first draft of the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1138357/full#supplementary-material
1. National Comprehensive Cancer Network (NCCN). Colon cancer. (2022). Available at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
2. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up☆. Ann Oncol (2014) 25:iii1–9. doi: 10.1093/annonc/mdu260
3. Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol (2011) 29(15):2011–9. doi: 10.1200/JCO.2010.33.5091
4. Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet (London England). (2011) 377(9783):2103–14. doi: 10.1016/S0140-6736(11)60613-2
5. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol (2014) 25(7):1346–55. doi: 10.1093/annonc/mdu141
6. Modest DP, Martens UM, Riera-Knorrenschild J, Greeve J, Florschütz A, Wessendorf S, et al. FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: The randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol (2019) 37(35):3401–11. doi: 10.1200/JCO.19.01340
7. Yoshino T, Watanabe J, Shitara K, Yasui H, Ohori H, Shiozawa M, et al. Panitumumab (PAN) plus mFOLFOX6 versus bevacizumab (BEV) plus mFOLFOX6 as first-line treatment in patients with RAS wild-type (WT) metastatic colorectal cancer (mCRC): Results from the phase 3 PARADIGM trial. J Clin Oncol (2022) 40(17_suppl):LBA1–LBA. doi: 10.1200/JCO.2022.40.17_suppl.LBA1
8. Rossini D, Antoniotti C, Lonardi S, Pietrantonio F, Moretto R, Antonuzzo L, et al. Upfront modified fluorouracil, leucovorin, oxaliplatin, and irinotecan plus panitumumab versus fluorouracil, leucovorin, and oxaliplatin plus panitumumab for patients with RAS/BRAF wild-type metastatic colorectal cancer: The phase III TRIPLETE study by GONO. J Clin Oncol (2022) 40(25):2878–88. doi: 10.1200/JCO.22.00839
9. Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up☆. Ann Oncol. 34(1):10–32. doi: 10.1016/j.annonc.2022.10.003
10. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer (Oxford England: 1990) (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
11. Michl M, Stintzing S, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, et al. CEA response is associated with tumor response and survival in patients with KRAS exon 2 wild-type and extended RAS wild-type metastatic colorectal cancer receiving first-line FOLFIRI plus cetuximab or bevacizumab (FIRE-3 trial). Ann Oncol (2016) 27(8):1565–72. doi: 10.1093/annonc/mdw222
12. U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES NIoH, National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0. U.S. Department of Health and Human Services, United States of America (2009).
13. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc (1958) 53(282):457–81. doi: 10.1080/01621459.1958.10501452
14. Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep (1966) 50(3):163–70.
15. Cox DR. Regression models and life-tables. J R Stat Soc Ser B (Methodological). (1972) 34(2):187–220. doi: 10.1111/j.2517-6161.1972.tb00899.x
16. Avallone A, Giuliani F, Nasti G, Montesarchio V, Santabarbara G, Leo S, et al. Randomized intermittent or continuous panitumumab plus FOLFIRI (FOLFIRI/PANI) for first-line treatment of patients (pts) with RAS/BRAF wild-type (wt) metastatic colorectal cancer (mCRC): The IMPROVE study. J Clin Oncol (2022) 40(16_suppl):3503. doi: 10.1200/JCO.2022.40.16_suppl.3503
17. Folprecht G, Gruenberger T, Bechstein WO, Raab HR, Lordick F, Hartmann JT, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol (2010) 11(1):38–47. doi: 10.1016/S1470-2045(09)70330-4
18. Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai SY, et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J Clin Oncol (2013) 31(16):1931–8. doi: 10.1200/JCO.2012.44.8308
19. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol (2010) 28(31):4706–13. doi: 10.1200/JCO.2009.27.6055
20. Xu RH, Muro K, Morita S, Iwasa S, Han SW, Wang W, et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol (2018) 19(5):660–71. doi: 10.1016/S1470-2045(18)30140-2
21. Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol (2010) 28(8):1351–7. doi: 10.1200/JCO.2008.21.7828
22. Kobayashi Y, Komatsu Y, Yuki S, Fukushima H, Sasaki T, Iwanaga I, et al. Randomized controlled trial on the skin toxicity of panitumumab in Japanese patients with metastatic colorectal cancer: HGCSG1001 study; J-STEPP. Future Oncol (London England). (2015) 11(4):617–27. doi: 10.2217/fon.14.251
23. Moosmann N, von Weikersthal LF, Vehling-Kaiser U, Stauch M, Hass HG, Dietzfelbinger H, et al. Cetuximab plus capecitabine and irinotecan compared with cetuximab plus capecitabine and oxaliplatin as first-line treatment for patients with metastatic colorectal cancer: AIO KRK-0104–a randomized trial of the German AIO CRC study group. J Clin Oncol (2011) 29(8):1050–8. doi: 10.1200/JCO.2010.31.1936
24. Pectasides D, Papaxoinis G, Kalogeras KT, Eleftheraki AG, Xanthakis I, Makatsoris T, et al. XELIRI-bevacizumab versus FOLFIRI-bevacizumab as first-line treatment in patients with metastatic colorectal cancer: A Hellenic cooperative oncology group phase III trial with collateral biomarker analysis. BMC cancer. (2012) 12:271. doi: 10.1186/1471-2407-12-271
25. Köhne CH, De Greve J, Hartmann JT, Lang I, Vergauwe P, Becker K, et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann Oncol (2008) 19(5):920–6. doi: 10.1093/annonc/mdm544
26. Fuchs CS, Marshall J, Mitchell E, Wierzbicki R, Ganju V, Jeffery M, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: Results from the BICC-c study. J Clin Oncol (2007) 25(30):4779–86. doi: 10.1200/JCO.2007.11.3357
27. Schmiegel W, Reinacher-Schick A, Arnold D, Kubicka S, Freier W, Dietrich G, et al. Capecitabine/irinotecan or capecitabine/oxaliplatin in combination with bevacizumab is effective and safe as first-line therapy for metastatic colorectal cancer: A randomized phase II study of the AIO colorectal study group. Ann Oncol (2013) 24(6):1580–7. doi: 10.1093/annonc/mdt028
28. Hamamoto Y, Yamaguchi T, Nishina T, Yamazaki K, Ura T, Nakajima T, et al. A phase I/II study of XELIRI plus bevacizumab as second-line chemotherapy for Japanese patients with metastatic colorectal cancer (BIX study). oncolo (2014) 19(11):1131–2. doi: 10.1634/theoncologist.2014-0159
29. Cartwright T, Kuefler P, Cohn A, Hyman W, Berger M, Richards D, et al. Results of a phase II trial of cetuximab plus capecitabine/irinotecan as first-line therapy for patients with advanced and/or metastatic colorectal cancer. Clin colorectal cancer. (2008) 7(6):390–7. doi: 10.3816/CCC.2008.n.052
30. PRESCRIBING INFORMATION- VECTIBIX: Federal drug administration . Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125147s080lbl.pdf.
31. Weiner LM, Belldegrun AS, Crawford J, Tolcher AW, Lockbaum P, Arends RH, et al. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res (2008) 14(2):502–8. doi: 10.1158/1078-0432.CCR-07-1509
32. Méndez Méndez JC, Salgado Fernández M, de la Cámara Gómez J, Pellón Augusto ML, Covela Rua M, Quintero Aldana G, et al. First-line panitumumab plus capecitabine for the treatment of older patients with wild-type RAS metastatic colorectal cancer. the phase II, PANEL study. J geriatric Oncol (2020) 11(8):1263–7. doi: 10.1016/j.jgo.2020.06.003
33. Colloca GA, Venturino A, Guarneri D. Carcinoembryonic antigen-related tumor kinetics after eight weeks of chemotherapy is independently associated with overall survival in patients with metastatic colorectal cancer. Clin colorectal cancer. (2020) 19(4):e200–e7. doi: 10.1016/j.clcc.2020.05.001
34. Gulhati P, Yin J, Pederson L, Schmoll HJ, Hoff P, Douillard JY, et al. Threshold change in CEA as a predictor of non-progression to first-line systemic therapy in metastatic colorectal cancer patients with elevated CEA. J Natl Cancer Institute. (2020) 112(11):1127–36. doi: 10.1093/jnci/djaa020
Keywords: colorectal neoplasms, drug therapy, anti-EGFR, survival, toxicity
Citation: Yip PL, Fung WHB, Lee FAS, Lee CF, Wong NSM and Lee SF (2023) Effectiveness and safety of capecitabine, irinotecan and panitumumab in advanced colorectal cancer. Front. Oncol. 13:1138357. doi: 10.3389/fonc.2023.1138357
Received: 10 January 2023; Accepted: 06 March 2023;
Published: 06 April 2023.
Edited by:
Angelica Petrillo, Centro Sanitario Locale Napoli 1 Centro, ItalyReviewed by:
Dimitra Grapsa, National and Kapodistrian University of Athens, GreeceCopyright © 2023 Yip, Fung, Lee, Lee, Wong and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shing Fung Lee, bGVlc2ZAbnVocy5lZHUuc2c=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.