- 1Johns Hopkins University School of Medicine, Baltimore, MD, United States
- 2Department of Radiotherapy and Oncology, University of KwaZulu-Natal, Durban, South Africa
- 3Department of Oncology, Inkosi Albert Luthuli Central Hospital, Durban, South Africa
- 4Department of Public Health, ICT University USA, Yaoundé, Cameroon
- 5NSIA-LUTH Cancer Center, Lagos University Teaching Hospital, Lagos, Nigeria
- 6Ocean Road Cancer Institute, Dar Es Salaam, Tanzania
- 7Oncology Center, University of Nigeria Teaching Hospital, Ituku Ozalla, Enugu, Nigeria
- 8Department of Clinical Oncology, Muhimbili University of Health and Allied Sciences, Dar Es Salaam, Tanzania
- 9College of Health Science, University of KwaZulu-Natal, Durban, South Africa
- 10Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, United States
Purpose: The purpose of this project was to examine the travel burdens for radiotherapy patients in Nigeria, Tanzania, and South Africa, and to assess the patient-related benefits of hypofractionated radiotherapy (HFRT) for breast and prostate cancer patients in these countries. The outcomes can inform the implementation of the recent Lancet Oncology Commission recommendations on increasing the adoption of HFRT in Sub-Saharan Africa (SSA) to enhance radiotherapy access in the region.
Methods: Data were extracted from electronic patient records at the NSIA-LUTH Cancer Center (NLCC) in Lagos, Nigeria and the Inkosi Albert Luthuli Central Hospital (IALCH) in Durban, South Africa, from written records at the University of Nigeria Teaching Hospital (UNTH) Oncology Center in Enugu, Nigeria, and from phone interviews at Ocean Road Cancer Institute (ORCI) in Dar Es Salaam, Tanzania. Google Maps was used to calculate the shortest driving distance between a patient’s home address and their respective radiotherapy center. QGIS was used to map the straight-line distances to each center. Descriptive statistics were used to compare transportation costs, time expenditures, and lost wages when using HFRT versus conventionally fractionated radiotherapy (CFRT) for breast and prostate cancer.
Results: Patients in Nigeria (n=390) traveled a median distance of 23.1 km to NLCC and 86.7 km to UNTH, patients in Tanzania (n=23) traveled a median distance of 537.0 km to ORCI, and patients in South Africa (n=412) traveled a median distance of 18.0 km to IALCH. Estimated transportation cost savings for breast cancer patients in Lagos and Enugu were 12,895 Naira and 7,369 Naira, respectively and for prostate cancer patients were 25,329 and 14,276 Naira, respectively. Prostate cancer patients in Tanzania saved a median of 137,765 Shillings in transportation costs and 80.0 hours (includes travel, treatment, and wait times). Mean transportation cost savings for patients in South Africa were 4,777 Rand for breast cancer and 9,486 Rand for prostate cancer.
Conclusion: Cancer patients in SSA travel considerable distances to access radiotherapy services. HFRT decreases patient-related costs and time expenditures, which may increase radiotherapy access and alleviate the growing burden of cancer in the region.
1 Introduction
Sub-Saharan Africa (SSA) is facing a growing cancer crisis. A recent Lancet Oncology Commission predicts that, if current trends continue, overall cancer deaths in SSA could increase to about 1 million deaths per year by 2030 (1). Cancers of the breast and prostate are the most common cancers among women and men, respectively, in SSA (2). It is estimated that in the year 2020, 206,710 people in SSA were diagnosed with either breast or prostate cancer and another 104,285 died from their disease (2). Despite the region’s projected increase in cancer incidence and mortality, access to radiotherapy services remains unacceptably low. There is a paucity of radiotherapy machines in SSA, limited trained radiation oncology staff, and long patient travel distances to radiotherapy centers (1). The majority of SSA countries have less than 1 radiotherapy machine per 1 million people, in stark contrast to high-income countries, which have 5 or more machines per 1 million people (3).
This inadequate access is complicated by the fact that external beam radiotherapy (EBRT) is the most appropriate treatment for men with intermediate-and high-risk prostate cancer and is considered the standard of care for breast cancer patients following breast-conserving surgery (4, 5). Hypofractionated radiotherapy (HFRT) is a form of EBRT that provides a potential solution to low radiotherapy access in resource-limited settings. HFRT increases the dose of radiation administered per treatment fraction, reducing the overall length of treatment. Randomized phase III trials have supported the clinical efficacy of HFRT for breast and prostate cancer when compared to conventionally fractionated radiotherapy (CFRT) (6–16). Additionally, the reduction in overall treatment duration and increases in national cost savings may improve the treatment capacity of radiotherapy centers, yielding increased access to RT (17). The Lancet Oncology Commission for SSA recommends increased adoption of HFRT to augment such access (1).
While the potential financial advantages of HFRT for African hospitals and governments have been reported (17), there is a paucity of research conducted on the patient-related benefits of adopting HFRT in SSA. Historically, compared to HFRT, conventional fractionation schedules are more time-consuming for patients. Conventional radiotherapy typically involves 25 fractions administered over 5 weeks for breast cancer (25-30 fractions in 5-6 weeks if including a boost) and 35 to 40 fractions over 7 to 8 weeks for prostate cancer. HFRT reduces the number of visits to the radiotherapy center to approximately 15 and 20 visits for breast and prostate cancer, respectively (10, 15). The longer treatment regimens associated with CFRT can increase financial hardship and pose an inconvenience for patients in SSA, leading to treatment nonadherence or abandonment. Through this study, we sought to close the literature gap and explore the benefits of HFRT from a patient-centered perspective. First, we examined the travel distances of cancer patients from their home address to their respective radiotherapy center in Nigeria, Tanzania, and South Africa. Second, we assessed the patient-related benefits of HFRT for breast and prostate cancer patients in these three countries.
2 Materials and methods
Following IRB approvals, primary data collection occurred across four sites between June – November 2022: two in Nigeria, one in Tanzania, and one in South Africa. Patient addresses were extracted from electronic records at the NSIA-LUTH Cancer Center (NLCC) in Lagos, Nigeria and from written records at the University of Nigeria Teaching Hospital (UNTH) Oncology Center in Enugu, Nigeria. Included patients from NLCC were all breast and prostate cancer patients who started receiving either HFRT or CFRT at the NLCC between February 1- July 27, 2022. Included patients from UNTH Oncology Center were all breast or prostate cancer patients who started receiving either HFRT or CFRT at the center between July 1, 2021 – August 4, 2022.
Patient addresses and transportation cost data were extracted from electronic records at the Inkosi Albert Luthuli Central Hospital (IALCH) in Durban, South Africa. Included patients from IALCH were all breast and prostate cancer patients who started receiving HFRT at the center between December 1, 2021 – June 29, 2022.
Phone interviews were conducted in June 2022 among prostate cancer patients who had received or were receiving HFRT at Ocean Road Cancer Institute (ORCI) in Dar es Salaam, Tanzania between January 6 – June 16, 2022. Interviews were conducted in Swahili and responses were translated to English. Phone interviews rather than electronic medical records were used to obtain data from ORCI due to the increased feasibility of gathering additional data on time expenditures and wage savings through direct patient interviews. Google Maps was used to calculate the shortest driving distance between a patient’s home address and their respective radiotherapy center. QGIS was used to map the straight-line distances to each center. Data collected from all four sites were used to analyze transportation cost savings. Transportation cost for a single fraction comprised of the transportation cost to and from the patient’s respective radiotherapy center. Transportation costs for patients in Nigeria were estimated based on the round-trip bus fare between a patient’s accommodation in Lagos or Enugu and NLCC or UNTH (1,271 Naira and 706 Naira, respectively, as of July 2022). This assumption was made based on the fact that most patients remained in either Lagos or Enugu for the duration of their treatment period. Data collected from ORCI were also used to analyze time expenditure savings and wage savings. Time expenditure for a single fraction comprised of the sum of the transportation time to and from ORCI, the patient’s waiting time at the radiotherapy center, and the treatment time for one fraction of HFRT. Descriptive statistics were used to compare transportation costs, time expenditures, and lost wages when using HFRT versus CFRT for breast and prostate cancer. Statistics were calculated based solely on patients with complete data. All patient-related cost and time savings were estimated on the basis of what the transportation costs, time expenditures, and lost wages would have been had the patients received CFRT (defined as 25 visits for breast cancer and 40 visits for prostate cancer) instead of HFRT (15 visits for breast cancer and 20 visits for prostate cancer). Calculations were based on a CFRT regimen of 25 visits for breast cancer and 40 visits for prostate cancer, and a HFRT regimen of 15 visits for breast cancer and 20 visits for prostate cancer since these are the fractions that were implemented across the cancer centers in this study. Additionally, while some studies have established the clinical efficacy of as few as 5 fractions for breast cancer (16), these shorter treatment regimens require investments in high technology equipment which is not feasible in many SSA countries, and calculations using these fractions may not be as generalizable to the region as a whole.
All transportation- and wage-related cost data were contextualized within each country’s monthly adjusted net national income (MANNI) per capita, as reported by 2020 data from the World Bank. These values are 143 USD, 76 USD, and 387 USD for Nigeria, Tanzania, and South Africa, respectively.
3 Results
3.1 Patient characteristics
Table 1 displays the characteristics of the interviewed HFRT prostate cancer patients at ORCI. Of the 30 patients who were eligible for interview, data were collected from 23 patients. 4 patients were unable to be contacted, 2 patients had not yet started treatment, and 1 patient elected to opt out of treatment.
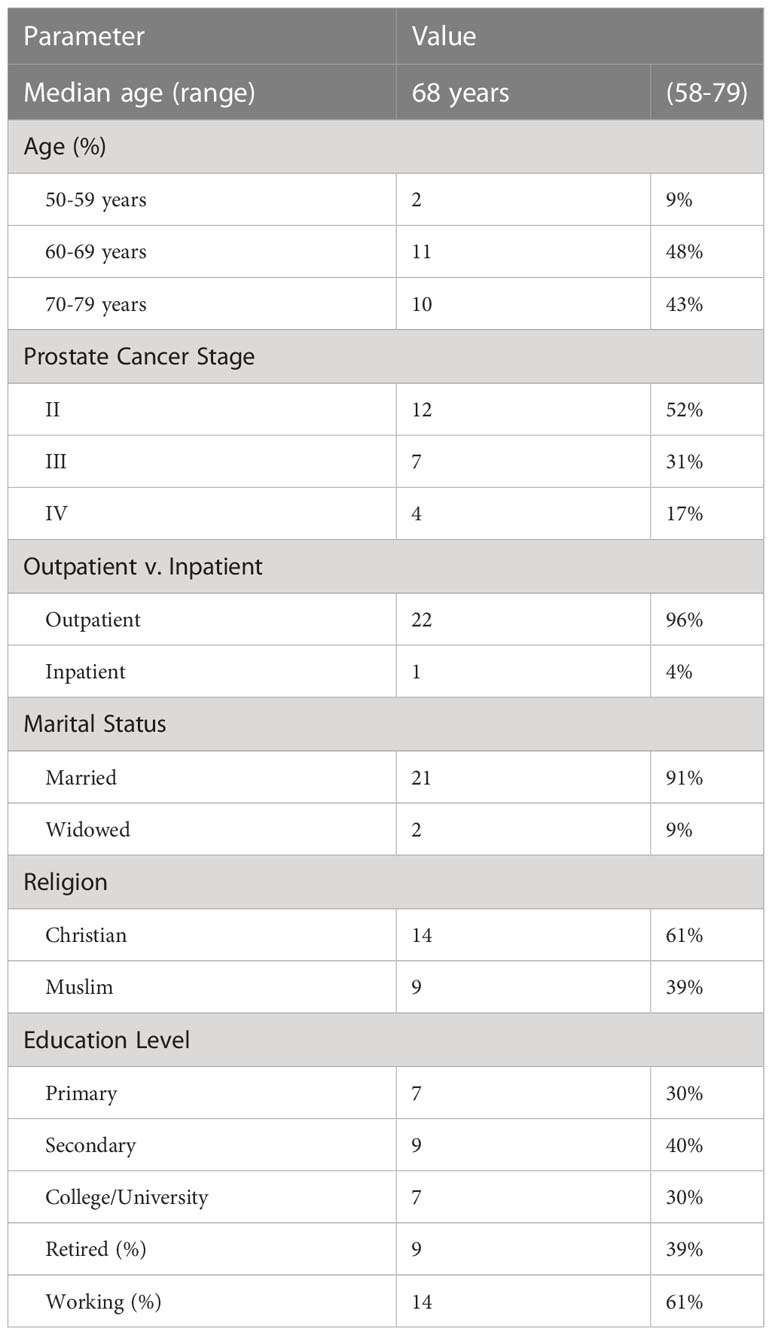
Table 1 Characteristics of interviewed patients (n=23) at Ocean Road Cancer Institute (ORCI) in Dar Es Salaam, Tanzania.
The mean age of breast cancer patients at NLCC, UNTH, and IALCH was 48, 46, and 59 years respectively, and the mean age of prostate cancer patients was 67, 68, and 70 years, respectively.
3.2 Travel distances
Data were collected from 180 breast and prostate cancer patients who traveled to NLCC, 211 breast and prostate cancer patients who traveled to UNTH, 23 prostate cancer patients who traveled to ORCI, and 412 breast and prostate cancer patients who traveled to IALCH. Patients in Nigeria traveled a median distance of 23.1 km (IQR=109.2 km) and 86.7 km (IQR=87.3 km) to NLCC and UNTH, respectively (Figures 1, 2). Patients in Tanzania traveled a median of 537.0 km (IQR=587.5 km) to ORCI (Figure 3). Patients in South Africa traveled a median of 18.0 km (IQR=15.0 km) to IALCH (Figure 4). These findings are summarized in Table 2. The QGIS maps in Figures 1–4 were made using straight-line distances rather than the road-network distances that were calculated with Google Maps. Therefore, these figures depict shorter-than-actual travel distances.
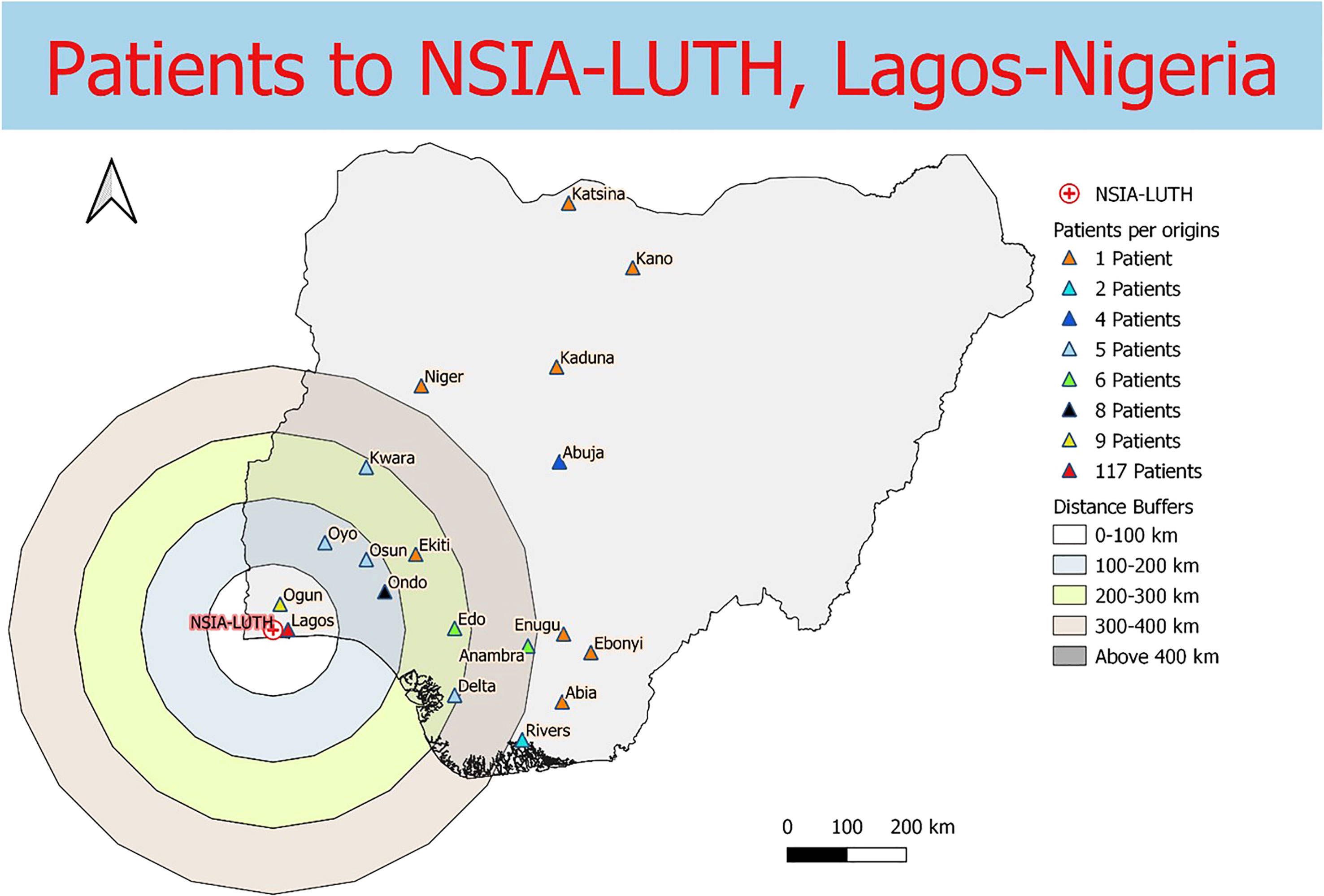
Figure 1 Straight-line distances between the home addresses of radiotherapy patients (n=180) and NSIA-LUTH Cancer Center in Lagos, Nigeria.
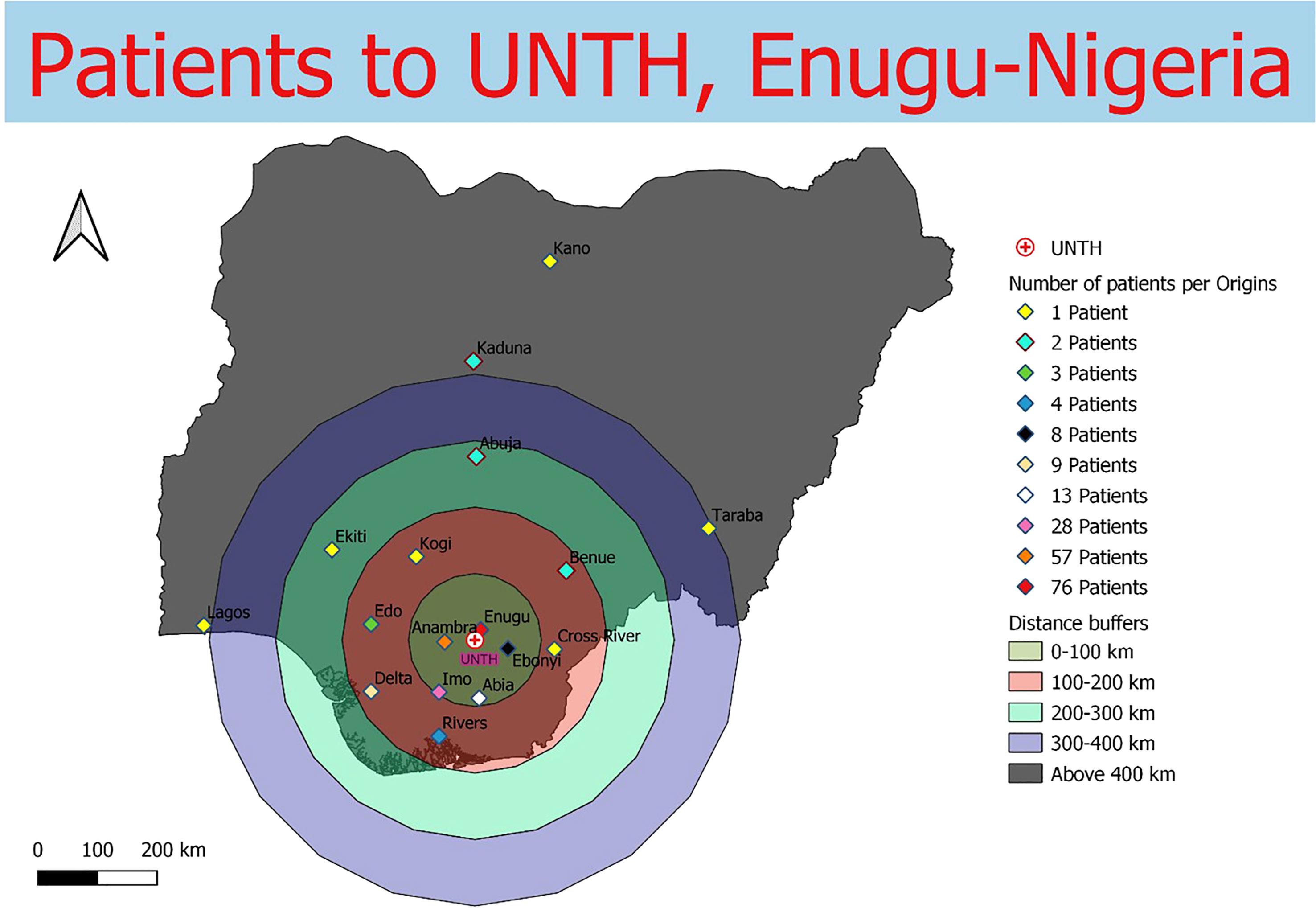
Figure 2 Straight-line distances between the home addresses of radiotherapy patients (n=211) and UNTH Oncology Center in Enugu, Nigeria.
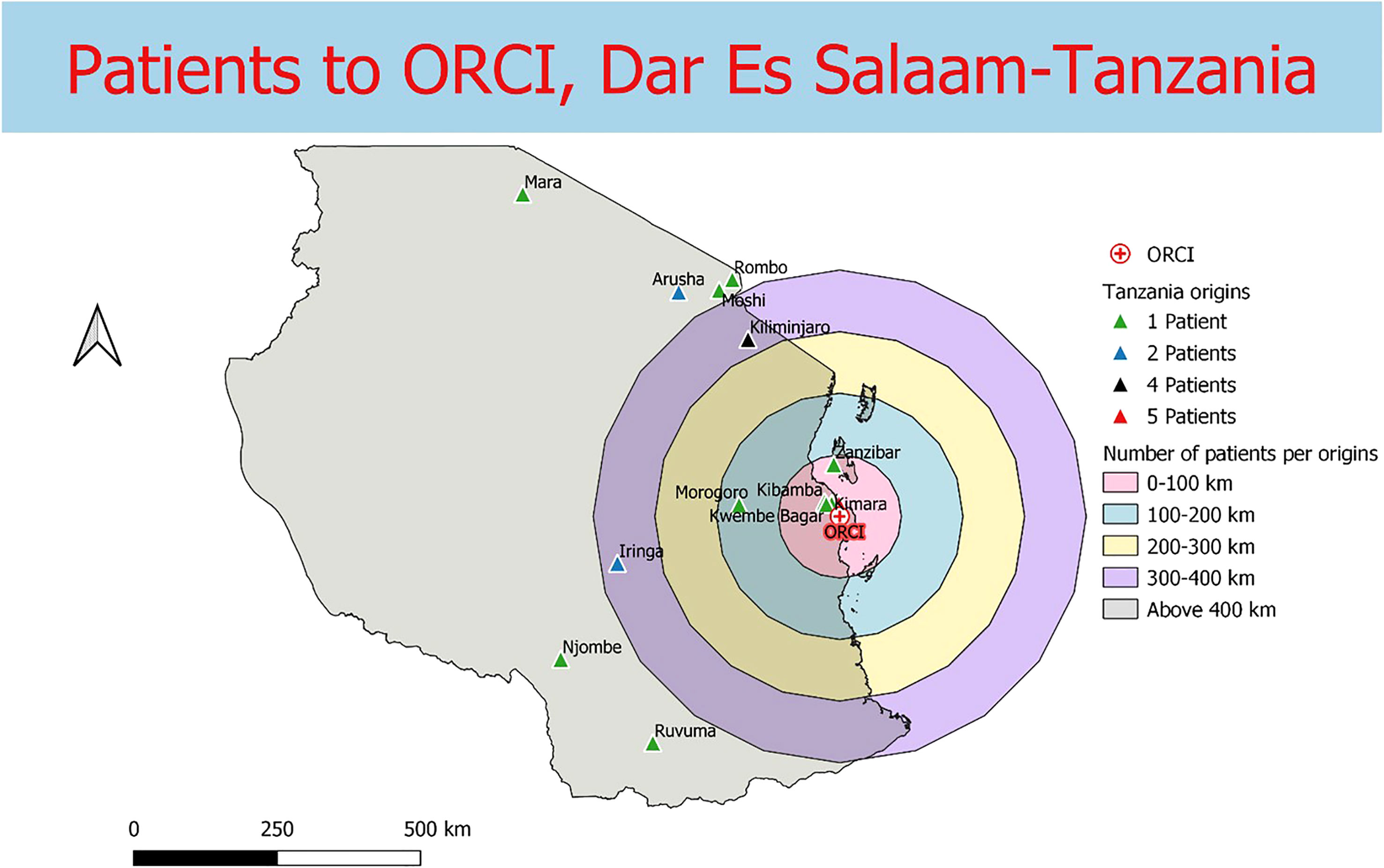
Figure 3 Straight-line distances between the home addresses of radiotherapy patients (n=23) and Ocean Road Cancer Institute (ORCI) in Dar Es Salaam, Tanzania.
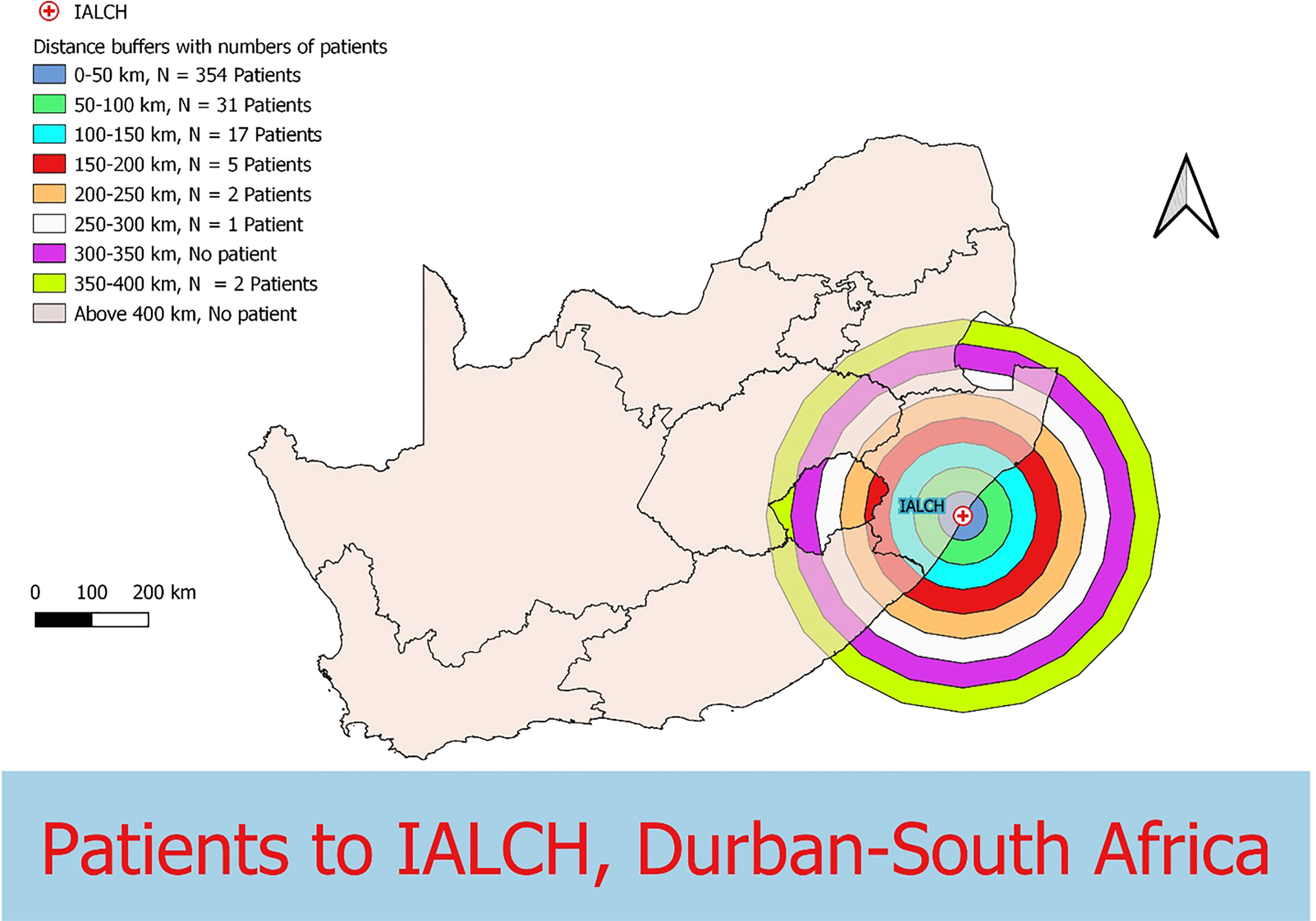
Figure 4 Straight-line distances between the home addresses of radiotherapy patients (n=412) and Inkosi Albert Luthuli Central Hospital (IALCH) in Durban, South Africa.
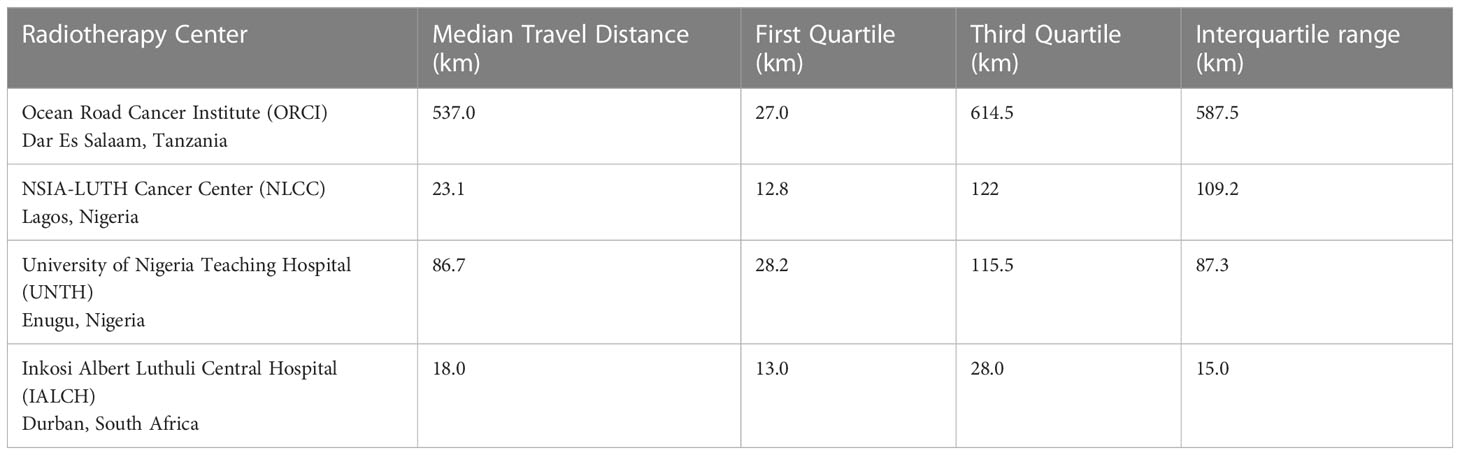
Table 2 Median travel distances of patients to four different radiotherapy centers across Sub-Saharan Africa.
3.3 Transportation cost and time expenditure savings
Estimated transportation cost savings for breast cancer patients in Lagos and Enugu were 12,895 Naira (20% of MANNI per capita) and 7,368 Naira (11% of MANNI per capita), respectively and for prostate cancer patients were 25,329 (38% of MANNI per capita) and 14,276 Naira (22% of MANNI per capita), respectively (Tables 3, 4). 22of the 23 ORCI respondents were outpatients, and data from these 22 prostate cancer patients were used to analyze transportation cost and time expenditure savings. Adopting hypofractionation saved ORCI patients a median of 137,765 shillings (78% of MANNI per capita, IQR= 203,145 shillings) in transportation costs and 80.0 hours (IQR=30.0 hours) over the course of their radiotherapy treatment (Figures 5, 6). Mean transportation cost savings for IALCH patients were 4,771 rand for breast cancer (72% of MANNI per capita) and 9,474 rand for prostate cancer (143% of MANNI per capita) (Table 5).
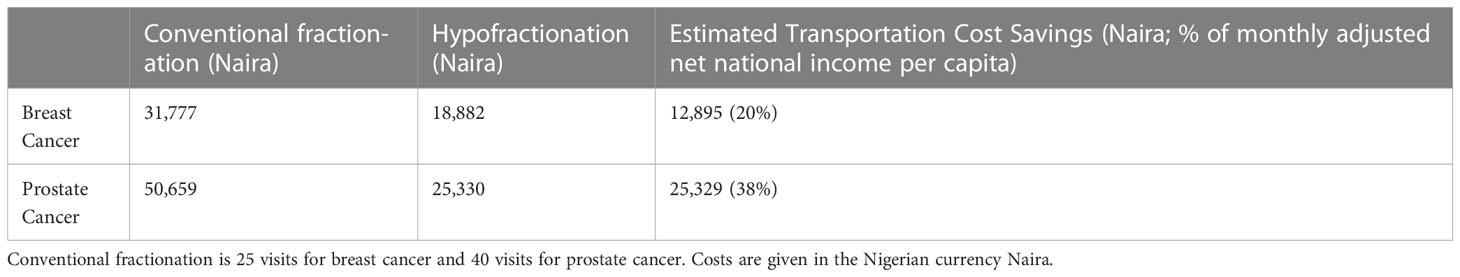
Table 3 Comparison of estimated transportation costs for breast and prostate cancer patients at NSIA-LUTH Cancer Center (NLCC) in Lagos, Nigeria. Hypofractionation is 15 visits for breast cancer and 20 visits for prostate cancer.
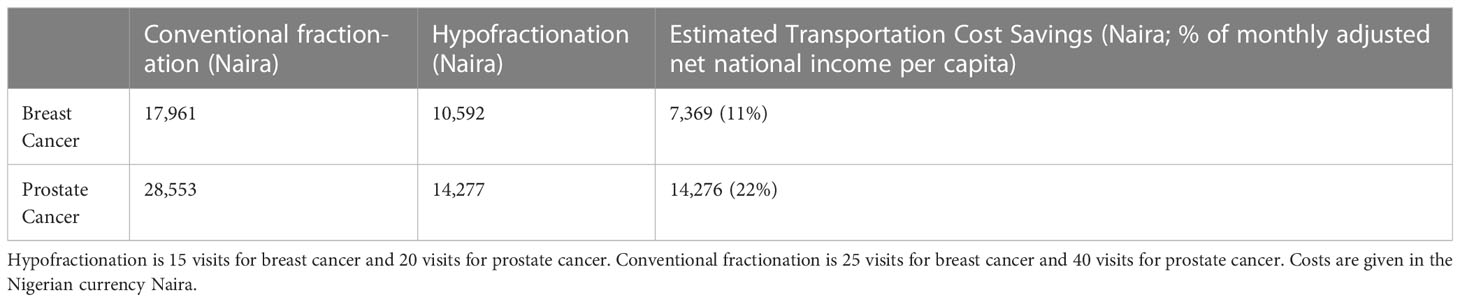
Table 4 Comparison of estimated transportation costs for breast and prostate cancer patients at University of Nigeria Teaching Hospital (UNTH) Oncology Center in Enugu, Nigeria.
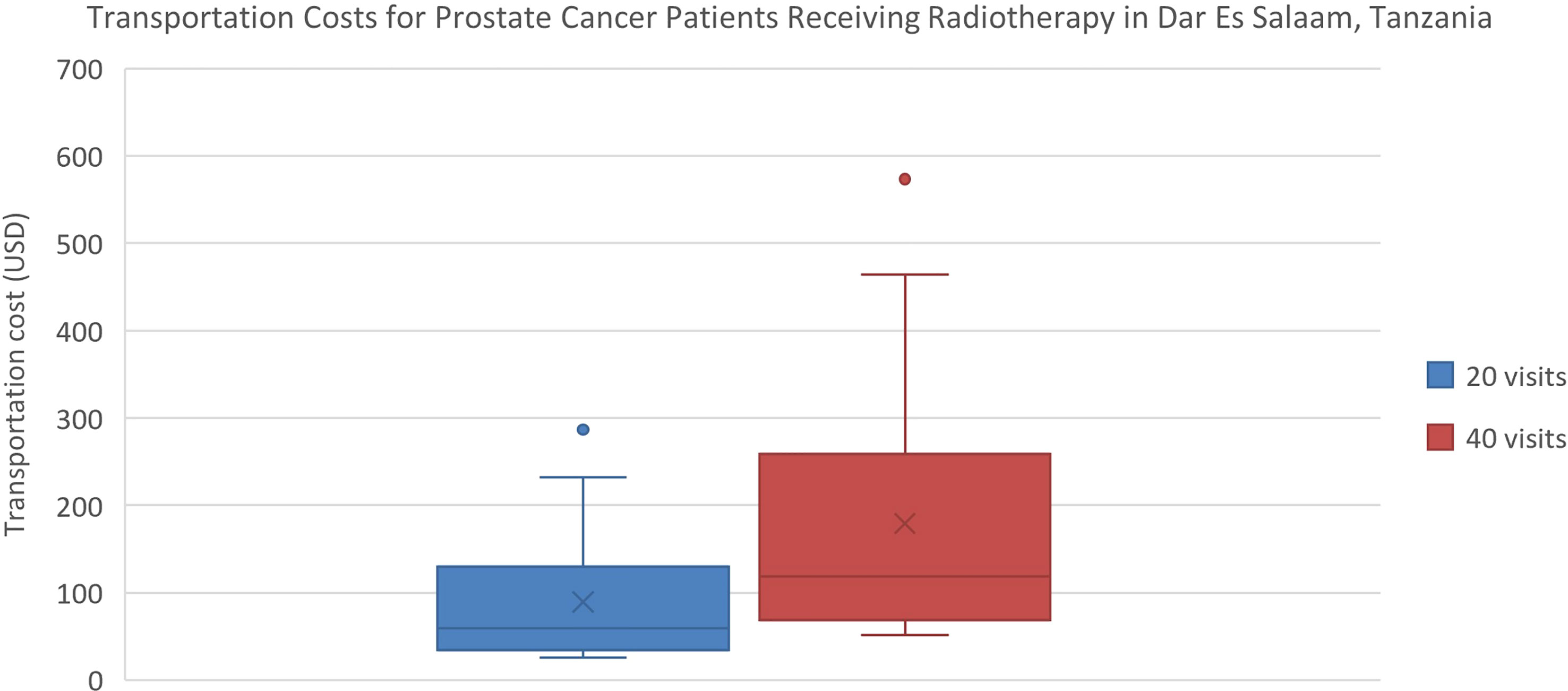
Figure 5 Comparison of transportation costs for prostate cancer patients at ORCI in Dar Es Salaam, Tanzania. 20 visits represents the HFRT course, and 40 visits represents the CFRT course. Error bars depict the interquartile range.
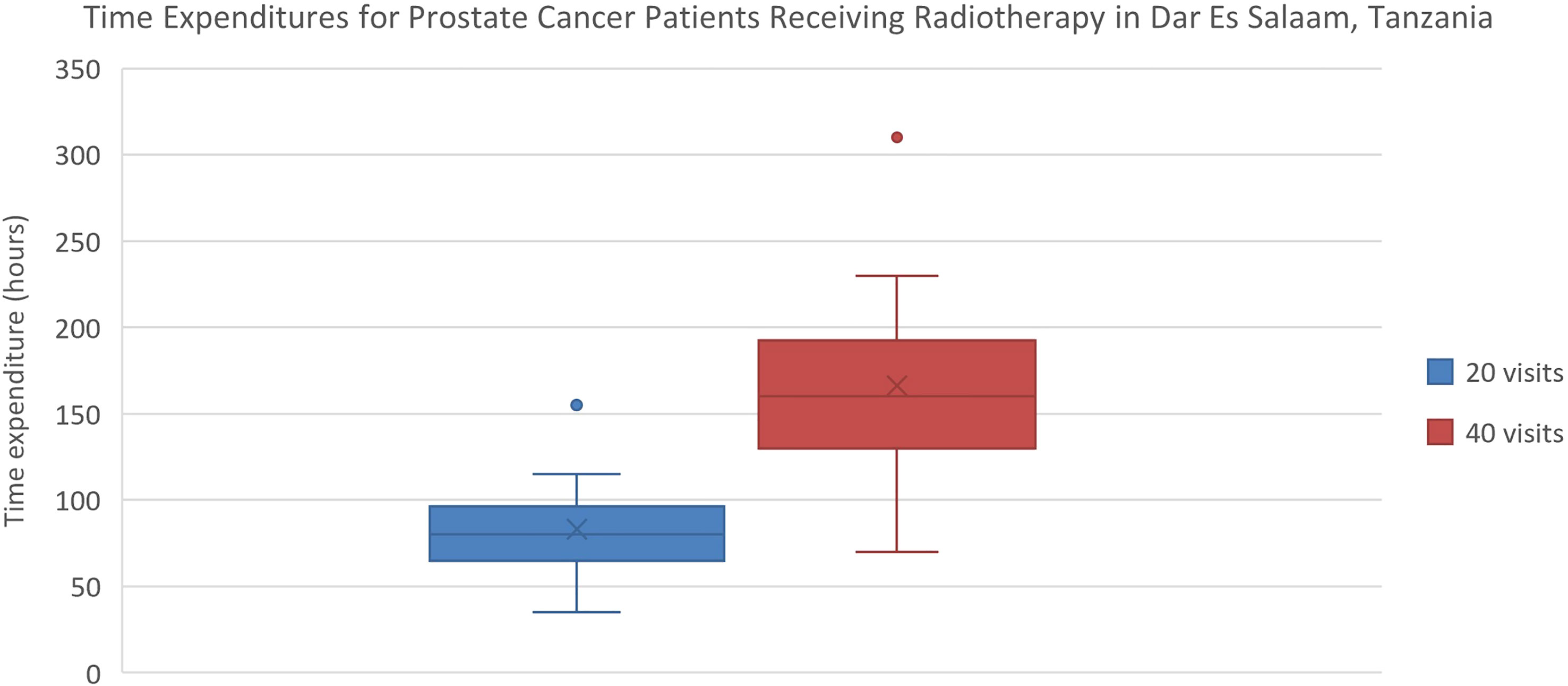
Figure 6 Comparison of time expenditures for prostate cancer patients at ORCI in Dar Es Salaam, Tanzania. 20 visits represents the HFRT course, and 40 visits represents the CFRT course. Error bars depict the interquartile range.
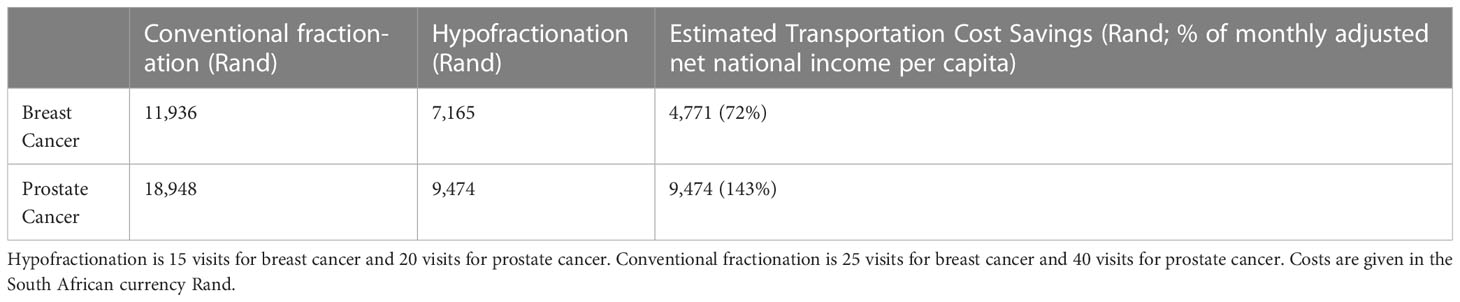
Table 5 Comparison of transportation costs for breast and prostate cancer patients at Inkosi Albert Luthuli Central Hospital (IALCH) in Durban, South Africa.
3.4 Wage savings
All non-retired ORCI patients (61%) reported temporary absenteeism from work to receive treatment. Of these, 12 patients were wage-earning, and 75% of wage-earning patients (largely peasant farmers and business owners) did not receive sick leave compensation and incurred lost wages due to treatment. By undergoing HFRT instead of CFRT, these patients saved a median of 100,405 shillings (57% of monthly NNI per capita, IQR= 51,370 shillings) in lost wages.
3.5 Reported barriers and challenges
43% of ORCI patients reported challenges getting to the clinic for their daily treatment fractions. The most common barrier cited was a lack of empty seating on public transportation buses and resultant delays in getting to the radiotherapy center. Additionally, 61% of patients reported experiencing a negative financial impact due to the aforementioned patient-related expenses incurred during their treatment course.
4 Discussion
Adopting HFRT instead of CFRT in SSA reduces the transportation costs, time expenditures, and lost wages incurred by patients. When contextualized within the monthly adjusted net national income (MANNI) per capita for each country, the transportation cost and wage-related savings represent sizable portions of a typical patient’s monthly income and are significant economic savings from a patient-centered perspective. Since approximately 40% of SSA’s population lives below the poverty line, transportation and wage-related cost savings are important considerations for expanding RT access to the region’s most vulnerable communities (18). Additionally, many of the ORCI patients, particularly those who were not working prior to their course of HFRT, relied on their family members for financial support to offset costs associated with their treatment. Therefore, the financial impact of cancer treatment is felt by many individuals beyond the patient, and cost savings associated with HFRT benefit family units as a whole.
While salary data was solely collected from ORCI patients in Tanzania, the GDP per capita can be used to calculate a rough estimation of the wage savings for HFRT patients in Nigeria and South Africa. According to 2021 data from the World Bank, the GDP per capita for Nigeria and South Africa is 173.75 USD/month and 582.85 USD/month, respectively (19). Assuming that patients report temporary absenteeism from work for the duration of their treatment and do not receive sick leave compensation, estimated wage savings for breast cancer patients are 86.88 USD in Nigeria and 291.43 USD in South Africa, and for prostate cancer patients are 173.75 USD in Nigeria and 582.85 USD in South Africa.
Furthermore, our project illustrates the far distances that many patients have to travel to access radiotherapy treatment in SSA due to the scarce distribution of treatment centers in the region. Compared to patients in Tanzania, the calculated travel distances for patients in Nigeria and South Africa are shorter, likely due to the larger sample sizes and the increased number of radiotherapy centers in these two countries. However, the shorter travel distances for patients in Nigeria and South Africa do not capture the full travel burden associated with attending radiotherapy treatment, as traffic in large SSA cities, such as Lagos, can extend travel times for patients tremendously (20, 21). Potential transportation time savings associated with traffic congestion are reflected in our results, which, along with our findings on reductions in clinic wait times, are especially relevant for increasing patient convenience and treatment adherence in resource-limited settings. Additionally, the large IQR values obtained for NLCC and ORCI in particular (238.5 km and 587.5 km, respectively) reveal the wide range of distances that patients are travelling to obtain radiotherapy.
Inadequate access to radiotherapy machines has been cited as a major contributing factor to the disproportionately high – and increasing – cancer mortality rates in SSA (22). While all three countries fall short of the recommendations set forth by the IAEA of one radiation therapy unit per 200,000 persons, patients in South Africa have significantly better access to RT compared to patients in Nigeria and Tanzania (23, 24). South Africa has 1 RT machine per 608,000 persons, which is far more than Nigeria’s 1 machine per 29.4 million persons and Tanzania’s 1 machine per 11.9 million persons (24). These disparities in RT access are reflected in our findings, as patients in South Africa travelled the shortest median distance to their radiotherapy center.
Despite the cost and convenience benefits associated with adoption of HFRT, many of the interviewed patients reported facing barriers in getting to the clinic due to public transportation challenges and experiencing economic hardship as a result of patient-related treatment expenses. These challenges would have been exacerbated if the patients had adopted a conventionally fractionated regimen due to the additional visits to the radiotherapy center. Documentation of these challenges provides an incentive for exploration of treatments such as ultrahypofractionated radiation therapy in SSA to further reduce the number of visits to the radiotherapy center and improve existing patient burdens (25).
There are some limitations to this study. First, the small sample size of patients interviewed at ORCI limited the amount of data that could be extracted to calculate patient-related benefits. ORCI began offering HFRT for prostate cancer in January 2022, and when the interviews were conducted in June 2022, only 30 patients had met eligibility requirements for this study. Thus, fewer patients could be recruited from ORCI compared to the other centers. Additionally, the patient-related costs analyzed in this study do not include the cost of radiotherapy fractions. There are some patient-related costs, such as food costs and accommodation costs that were unaccounted for. This likely means that total patient-related expenses for the treatment course were underestimated. However, the vast majority of interviewed patients stayed with their family member(s) while in Dar es Salaam, so accommodation expenses were less relevant for this study. The calculated transportation cost savings for patients in Nigeria were estimated with the assumption that patients used a public transport bus to travel to and from the radiotherapy center each day. However, it is possible that these values could be over or under estimated if patients used a personal vehicle for transportation instead. Furthermore, our analyses did not address all aspects of the patient-related benefits of HFRT, such as potential psychosocial benefits. Also, as our project was limited to three countries across SSA, further research in this area could benefit from involving additional SSA countries and could be extended within the context of other common cancers within the region, such as cervical cancer. Lastly, the different methodologies used to collect data in the three countries does limit the extent to which the results may be generalizable. While transportation cost data was acquired directly from patient interviews in Tanzania, they were acquired more indirectly in South Africa from electronic medical records and were estimated in Nigeria. These differing methodologies highlight the lack of a cohesive medical record system throughout SSA, however, the necessary approximations in this study were reasonably calculated within an Afro-centric context. Additionally, while travel distances data was collected from both CFRT and HFRT patients in Nigeria and South Africa, data was collected solely from HFRT patients in Tanzania, potentially limiting the generalizability of these results to radiotherapy patients as a whole in SSA. However, it is worth noting that countries within SSA are diverse in transportation and medical infrastructure and while our analysis attempts to capture this significant regional variability in radiotherapy patient travel burden, it is not all-encompassing.
Despite these limitations, our project provides valuable analyses and perspectives on travel distances and the patient-related benefits of HFRT over CFRT for radiotherapy patients in SSA. Factors such as travel burdens, transportation costs, and lost wages are important barriers to consider for all patients seeking radiotherapy and are especially pertinent for patients in low- and middle-income countries. HFRT is better equipped to address these challenges in a patient-centered manner and its widespread adoption holds potential to alleviate the increasing cancer burden in SSA as recommended by the recent Lancet Oncology Commission (1).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Office of Research Integrity University of Massachusetts Lowell IRB#: 22-087. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization and design: SP, EO, AM, MV, WN. Project administration and supervision: AOJ, NL, MN, TAN, CO, MV, WN. Investigation: SP, EO, SJ, HM, GN, AM, MV. Data interpretation: SP, EO, BBB. Writing-original draft: SP. Writing-reviewing and editing: all authors. All authors contributed to the article and approved the submitted version.
Funding
Research reported in this publication was partially supported by the National Institutes of Health under Award Number R01CA239042. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health.
Acknowledgments
Special thanks to Mariza Vorster for providing guidance and mentorship with data collection at Inkosi Albert Luthuli Central Hospital in Durban, South Africa.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ngwa W, Addai BW, Adewole I, Ainsworth V, Alaro J, Alatise OI, et al. Cancer in sub-Saharan Africa: a lancet oncology commission. Lancet Oncol (2022) 23(6):e251–312. doi: 10.1016/S1470-2045(21)00720-8
2. World Health Organization Organization International Agency for Research on Cancer. Global cancer observatory. Sub-Saharan Africa hub. In: Globocan (2020). Availble at: https://gco.iarc.fr/today/data/factsheets/populations/971-sub-saharan-africa-hub-fact-sheets.pdf.
3. IAEA. Directory of radiotherapy centers (2022). Available at: https://dirac.iaea.org/Query/Countries.
4. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (2022). Available at: https://www.nccn.org/guidelines/category.
5. Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet (2011) 378(9804):1707–16. doi: 10.1016/S0140-6736(11)61629-2
6. Pollack A, Walker G, Horwitz EM, Price R, Feigenberg S, Konski AA, et al. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol (2013) 31(31):3860–8. doi: 10.1200/JCO.2013.51.1972
7. Hoffman KE, Voong KR, Pugh TJ, Skinner H, Levy LB, Takiar V, et al. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: results from a randomized trial. Int J Radiat. Oncol Biol Phys (2014) 88(5):1074–84. doi: 10.1016/j.ijrobp.2014.01.015
8. Lee WR, Dignam JJ, Amin MB, Bruner DW, Low D, Swanson GP, et al. Randomized phase III noninferiority study comparing two radiotherapy fractionation schedules in patients with low-risk prostate cancer. J Clin Oncol (2016) 34(20):2325–32. doi: 10.1200/JCO.2016.67.0448
9. Aluwini S, Pos F, Schimmel E, Krol S, van der Toorn PP, De Jager H, et al. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol (2016) 17(4):464–74. doi: 10.1016/S1470-2045(15)00567-7
10. Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol (2016) 13(1):43–54. doi: 10.1016/S1470-2045(11)70293-5
11. Arcangeli G, Saracino B, Arcangeli S, Gomellini S, Petrongari MG, Sanguineti G, et al. Moderate hypofractionation in high-risk, organ-confined prostate cancer: final results of a phase III randomized trial. J Clin Oncol (2017) 35(17):1891–7. doi: 10.1200/JCO.2016.70.4189
12. Catton CN, Lukka H, Gu CS, Martin JM, Supiot S, Chung PWM, et al. Randomized trial of a hypofractionated radiation regimen for the treatment of localized prostate cancer. J Clin Oncol (2017) 35(17):1884–90. doi: 10.1200/JCO.2016.71.7397
13. Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trial a of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol (2008) 14(11):1086–94. doi: 10.1016/S1470-2045(13)70386-3
14. START Trialists' Group, Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trial b of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet (2008) 371(9618):1098–107. doi: 10.1016/S0140-6736(08)60348-7
15. Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J, Barrett-Lee PJ, et al. The UK standardisation of breast radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol (2013) 14(11):1086–94. doi: 10.1016/S1470-2045(13)70386-3
16. Murray BA, Haviland JS, Wheatley DA, Sydenham MA, Alhasso A, Bloomfield DJ, et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (fast-forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet (2020) 395(10237):1613–26. doi: 10.1016/S0140-6736(20)30932-6
17. Irabor OC, Swanson W, Shaukat F, Wirtz J, Mallum AA, Ngoma T, et al. Can the adoption of hypofractionation guidelines expand global radiotherapy access? An analysis for breast and prostate radiotherapy. JCO Global Oncol (2020) 6:667–78. doi: 10.1200/JGO.19.00261
18. Schoch M, Christoph L. The number of poor people continues to rise in Sub-Saharan Africa, despite a slow decline in the poverty rate. In: World bank blogs. World Bank (2020). Available at: https://blogs.worldbank.org/opendata/number-poor-people-continues-rise-sub-saharan-africa-despite-slow-decline-poverty-rate.
19. World Bank Open Data. Data Internet (2021). Available at: https://data.worldbank.org.
20. Banke-Thomas A, Wong KLM, Ayomoh FI, Giwa-Ayedun RO, Benova L. “In cities, it’s not far, but it takes long”: comparing estimated and replicated travel times to reach life-saving obstetric care in Lagos, Nigeria. BMJ Global Health (2021) 6(1):e004318. doi: 10.1136/bmjgh-2020-004318
21. Banke-Thomas A, Balogun M, Wright O, Ajayi B, Abejirinde IOO, Olaniran A, et al. Reaching health facilities in situations of emergency: qualitative study capturing experiences of pregnant women in africa’s largest megacity. Reprod Health (2020) 17(1):145. doi: 10.1186/s12978-020-00996-7
22. Atun R, Jaffray DA, Barton MB, Bray F, Baumann M, Vikram B, et al. Expanding global access to radiotherapy. Lancet Oncol (2015) 16(10):1153–86. doi: 10.1016/S1470-2045(15)00222-3
23. International Atomic Energy Agency Directory of Radiotherapy Centres (DIRAC). . Available at: http://www-naweb.iaea.org/nahu/dirac.
24. Ige TA, Jenkins A, Burt G, Angal-Kalinin D, McIntosh P, Coleman CN, et al. Surveying the challenges to improve linear accelerator-based radiation therapy in Africa: A unique collaborative platform of all 28 African countries offering such treatment. Clin Oncol (2021) 33(12):e521–9. doi: 10.1016/j.clon.2021.05.008
25. Lehrer EJ, Kishan AU, James BY, Trifiletti DM, Showalter TN, Ellis R, et al. Ultrahypofractionated versus hypofractionated and conventionally fractionated radiation therapy for localized prostate cancer: A systematic review and meta-analysis of phase III randomized trials. Radiother Oncol (2020) 148:235–42. doi: 10.1016/j.radonc.2020.04.037
Keywords: hypofractionated radiotherapy, sub-Saharan Africa, prostate cancer, breast cancer, travel distance, cost savings, time savings
Citation: Patel S, Olatunji E, Mallum A, Benjika BB, Joseph AO, Joseph S, Lasebikan N, Mahuna H, Ngoma M, Ngoma TA, Nnko G, Onwualu C, Vorster M and Ngwa W (2023) Expanding radiotherapy access in Sub-Saharan Africa: an analysis of travel burdens and patient-related benefits of hypofractionation. Front. Oncol. 13:1136357. doi: 10.3389/fonc.2023.1136357
Received: 03 January 2023; Accepted: 20 March 2023;
Published: 18 April 2023.
Edited by:
Laurent Quero, Université Paris Cité, FranceReviewed by:
Matthew Katz, Lowell General Hospital, United StatesColin Robert Muirhead, Independent researcher, Newcastle upon Tyne, United Kingdom
Copyright © 2023 Patel, Olatunji, Mallum, Benjika, Joseph, Joseph, Lasebikan, Mahuna, Ngoma, Ngoma, Nnko, Onwualu, Vorster and Ngwa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saloni Patel, c3BhdGUxOTBAamhtaS5lZHU=
†These authors share first authorship
‡Senior author
 Saloni Patel
Saloni Patel Elizabeth Olatunji1†
Elizabeth Olatunji1† Abba Mallum
Abba Mallum Binsila Bernard Benjika
Binsila Bernard Benjika Shomari Joseph
Shomari Joseph Nwamaka Lasebikan
Nwamaka Lasebikan Godwin Nnko
Godwin Nnko Chinelo Onwualu
Chinelo Onwualu