
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 14 March 2023
Sec. Thoracic Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1134151
This article is part of the Research Topic Molecular Genetic Testing and Emerging Targeted Therapies for Non-Small Cell Lung Cancer View all 7 articles
Large-cell neuroendocrine carcinoma (LCNEC) is a rare subtype of non-small-cell lung cancer associated with a poor prognosis. LCNEC is genetically heterogeneous, and studies have revealed distinct molecular subtypes of LCNEC, which may have therapeutic implications. Herein, we present a case of a patient with stage IV LCNEC harboring a KIF5B–RET fusion whose disease responded to the selective RET inhibitor selpercatinib both extra- and intra-cranially, highlighting the importance of comprehensive molecular testing in LCNEC for selection of optimal treatment.
RET fusions are oncogenic drivers found in 1%–2% of non-small cell lung cancer (NSCLC) (1). Patients with RET fusion-positive NSCLC are characterized by younger age and never-smoker status and frequently have adenocarcinoma histology (1). Several fusion partners have been identified, including KIF5B, CCDC6, and NCOA4 (2). RET fusions promote carcinogenesis by activating various downstream signaling pathways such as RAS/MAPK/ERK, PI3K/AKT, and JAK/STAT (2). There are two potent and selective RET tyrosine kinase inhibitors (TKIs) approved by the U.S. Food and Drug Administration: selpercatinib and pralsetinib. Several strategies and new target drugs are currently under investigation (3).
According to the 2021 World Health Organization (WHO) classification of lung tumors, neuroendocrine neoplasms encompass typical carcinoid and atypical carcinoid, as well as high-grade neuroendocrine carcinomas (NECs), including large cell neuroendocrine carcinoma (LCNEC) and small-cell lung cancer (4). LCNEC is a rare subtype of non-small cell lung cancer (NSCLC), accounting for about 3% of all lung malignancies. LCNEC is associated with poor prognosis, with median overall survival less than a year in patients with stage IV disease (5, 6). Although not common, targetable genomic alterations such as alterations to EGFR, BRAF, and ALK are also seen (7, 8).
Here, we describe a patient with stage IV pulmonary LCNEC harboring a KIF5B–RET fusion whose disease responded to selpercatinib, highlighting the importance of characterizing the molecular profile of pulmonary LCNEC for optimal treatment selection.
A 52-year-old never-smoking Asian female with no significant past medical history presented with back pain and increasing abdominal girth with firmness in the right upper quadrant. A computed tomography (CT) chest showed a spiculated mass in the right upper lobe measuring 2.1 cm, a left lower lobe nodule measuring 5 mm, extensive mediastinal and hilar adenopathy, diffuse hepatic metastases, and numerous osseous lesions in the thoracic and lumbar spine and in the left iliac bone. A brain magnetic resonance imaging (MRI) scan showed numerous sub-centimeter brain lesions.
Subsequently, she underwent a liver biopsy. Pathologic evaluation revealed a poorly differentiated carcinoma, growing in nests or trabecular patterns without gland formation or keratinization and morphologically resembling large-cell neuroendocrine carcinoma (Figure 1A). Immunohistochemistry (IHC) was positive for synaptophysin, chromogranin (Figure 1B), pankeratin (Figure 1C), calcitonin (Figure 1D), and CK7 with a high Ki-67 proliferative index of 80%. Other markers, including mammaglobin, GATA-3, CK20, CK903, TTF-1, Napsin A, mucicarmine, WT-1, p63, p16, p53, gastrin, CDX-2, and PAX8, were negative. No lesions were identified on thyroid ultrasound, ruling out the possibility of medullary thyroid carcinoma.
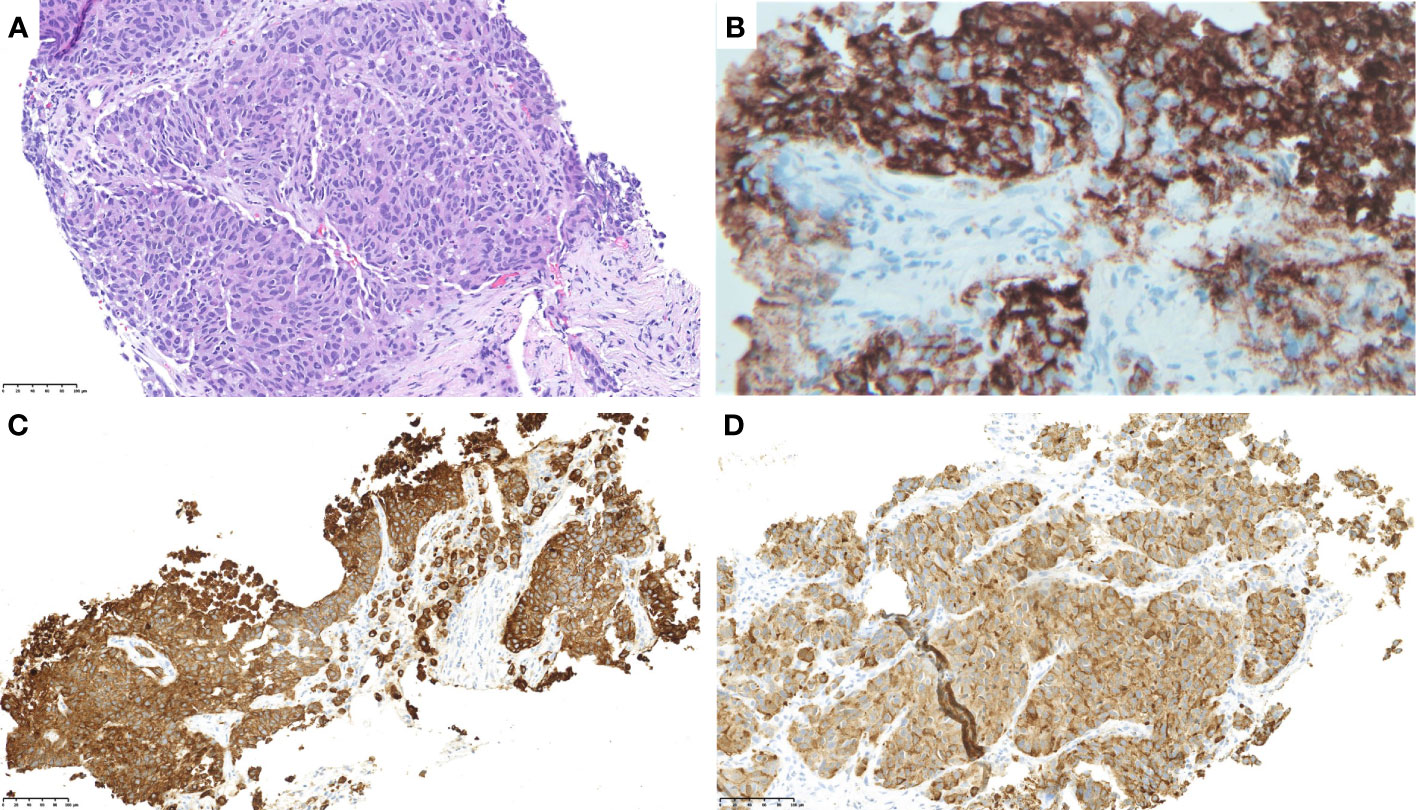
Figure 1 Pathology evaluation of a liver biopsy sample showed a poorly differentiated carcinoma, growing in nests or trabecular patterns without gland formation or keratinization and morphologically resembling large-cell neuroendocrine carcinoma. [(A); ×400 H&E]. The tumor was positive for chromogranin (B), pan-keratin (C), and calcitonin (D).
The patient received cisplatin (75 mg/m2 on day 1) and etoposide (100 mg/m2 on days 1–3), which was complicated by grade 4 neutropenia. Subsequently, molecular testing including whole exome sequencing and whole transcriptome sequencing (Caris Life Sciences, Phoenix, AZ) revealed KIF5B–RET fusion and NFE2L2 E82D without other genomic alterations such as mutations in TP53 and Rb1, and selpercatinib 160 mg twice a day (BID) was initiated on cycle 1, day 21. Ten days after initiation of selpercatinib, she received cycle 2, day 1 carboplatin (AUC 5 on day 1) and etoposide (100 mg/m2 on days 1–3). The decision to combine selpercatinib and chemotherapy was based on the limited knowledge about the efficacy of RET inhibitor therapy in LCNEC and the effectiveness of platinum-etoposide for LCNEC, as well as the encouraging initial results from clinical trials testing the combination of platinum doublet chemotherapy and targeted therapy for driver mutation-positive NSCLC, such as EGFR-mutant NSCLC. Cycle 2, day 1 chemotherapy was complicated by reactions including chest tightness and elevated blood pressure during etoposide administration. Chemotherapy was discontinued, and the patient continued selpercatinib at a lower dose of 120 mg BID. A month after initiation of selpercatinib, she developed grade 2 alanine aminotransferase (ALT; 227 units/L) elevation and grade 1 aspartate aminotransferase (AST; 83 units/L) elevation, leading to dose interruption for 10 days. After improvement in liver function tests, selpercatinib was restarted at 80 mg BID, which led to another dose interruption for 10 days due to elevations in grade 2 ALT (239 units/L) and grade 1 AST (95 units/L). Selpercatinib was resumed at 40 mg BID, which was titrated up over 6 months to 120 mg BID without episodes of transaminitis. Due to hypertension, an anti-hypertensive, amlodipine, was started about 5 months after the initiation of selpercatinib.
The patient achieved a partial response with shrinkage of the right upper lobe nodule (Figures 2A–D) and liver lesions (Figures 2E–H). Her brain lesions also responded to selpercatinib (Figures 2I–L, arrows), except for the development of tiny new brain lesions when the patient was on a low dose of selpercatinib 40 mg BID (Figure 2K, arrowhead), which have improved (Figure 2L, arrowhead) or remained stable with subsequent increases in dose of selpercatinib. At the time of this report, the patient is 1 year into treatment with selpercatinib and continues to derive clinical benefit from selpercatinib.
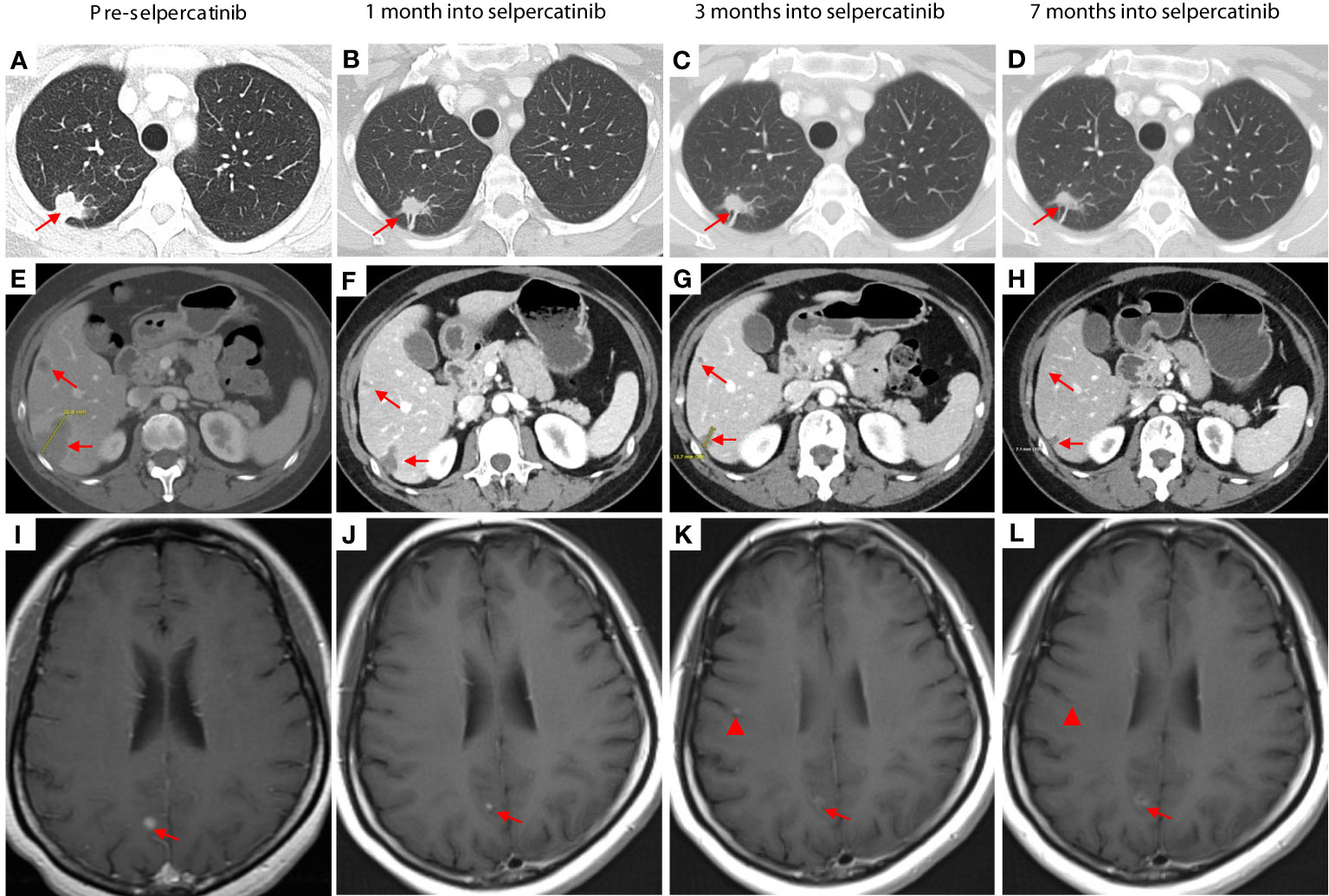
Figure 2 Pretreatment images were obtained 3 weeks prior to the initiation of chemotherapy. The images in the second column were taken 1 month after the start of selpercatinib treatment (3 weeks after the first day of the second cycle of chemotherapy). CT images show shrinkage of lesions in the right upper lobe (A–D) and in the liver (E–H). Brain lesions responded to selpercatinib [(I–L), arrows] except for the development of tiny new brain lesions when the patient was on a low dose of selpercatinib 40 mg twice daily [(K), arrowhead], which have improved [(L), arrowhead] or remained stable with subsequent increases in dose of selpercatinib. At 7 months, the patient was receiving a dose of 120 mg of selpercatinib in the morning and 80 mg in the afternoon.
In this case report, we describe a patient with stage IV pulmonary LCNEC harboring a KIF5B–RET fusion whose disease responded to selpercatinib both extra- and intra-cranially, highlighting the importance of comprehensive molecular testing in LCNEC for selection of optimal treatment. To the best of our knowledge, this is one of the first reports demonstrating the presence of RET fusions and the activity of RET inhibitor therapy in pulmonary LCNEC.
LCNEC is associated with a high mutation burden and alterations in various molecular pathways such as cell cycle signaling, RAS/MAPK, and PI3K/AKT/mTOR pathways (9). Studies investigating the molecular characteristics of pulmonary LCNEC have revealed two major subtypes (10, 11): small-cell lung cancer (SCLC)-like LCNEC characterized by co-alterations in TP53 and RB1, and NSCLC-like LCNEC characterized by harboring NSCLC-type mutations. Targetable genomic alterations such as EGFR mutations, KRAS G12C mutations, BRAF V600E mutations, and ALK fusions have been identified in LCNEC (8–12), though at lower rates compared with lung adenocarcinoma. Response to matching targeted therapy has been reported for EGFR mutations (13, 14), BRAF V600E mutation (15), and ALK fusions (16, 17), suggesting the role of targeted therapy in driver mutation-positive LCNEC. These findings emphasize the importance of performing comprehensive molecular profiling for patients with LCNEC in order to select the most effective treatment options, in accordance with evidence-based guidelines such as the CAP/IASLC/AMP molecular testing guideline for lung cancer (18).
RET fusions have not been well described in pulmonary LCNEC, likely because of the rarity of the genomic alteration and the fact that only a handful of studies utilized genomic technologies involving RNA analysis. In a study of 52 pulmonary LCNECs where reverse transcription-polymerase chain reaction (RT-PCR) was used for analysis, only one patient was found to have a RET fusion (12). The patient received first-line treatment with carboplatin and pemetrexed, with progression-free survival (PFS) of 3.3 months and overall survival (OS) of 34 months. No further details about the treatment course are available in the report. There are two case reports describing the activity of selpercatinib against RET-fusion-driven high-grade neuroendocrine carcinoma of thoracic origin (19) and atypical lung carcinoid (20), suggesting that, across the spectrum of pulmonary neuroendocrine neoplasms, RET fusions are actionable alterations and RET-targeting therapy is a therapeutic option.
Of note, it was observed that the patient developed small new brain lesions when the dose of selpercatinib was decreased due to toxicities. With increased doses of selpercatinib, stabilization and improvement in the lesions were observed. An important clinical question is whether increasing the dose of targeted therapy in cases of central nervous system (CNS) progression is effective. This has been studied in certain driver mutation-positive NSCLCs. For example, in patients with EGFR-mutant NSCLC who were experiencing CNS progression while taking osimertinib at 80 mg per day, increasing the dose to 160 mg resulted in modest improvement with CNS control lasting about 3 to 6 months (21). Further studies are necessary to determine the possible advantages of increasing the dose of RET inhibitor therapy for patients with RET-fusion-driven NSCLC who are experiencing progression in the CNS.
Identification of actionable genomic alterations via molecular profiling can play an important role in the care of patients with pulmonary LCNEC. RET-TKI therapy is a viable therapeutic option for RET fusion-driven pulmonary LCNEC.
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
AA: data curation, formal analysis, investigation, visualization, writing—original draft, and writing—review and editing. JZ: data curation and writing—review and editing. MO: data curation, visualization, and writing—review and editing. CK: conceptualization, data curation, investigation, supervision, visualization, writing—original draft, and writing—review and editing.
CK served as a consultant or advisory board member for Novartis, Janssen, AstraZeneca, Sanofi, PierianDx, Diffuse Pharmaceuticals, Mirati, Jazz Pharmaceuticals, Arcus Biosciences, Daiichi Sankyo, and Eisai, and received research funding to institution from AstraZeneca, Bristol-Myers Squibb, Novartis, Genentech, Janssen, Regeneron, Debiopharm, Karyopharm, and Daiichi Sankyo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Wang R, Hu H, Pan Y, Yuan L, Ye T, Li C, et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol (2012) 30:4352–9. doi: 10.1200/JCO.2012.44.1477
2. Choudhury NJ, Drilon A. Decade in review: A new era for RET-rearranged lung cancers. Transl Lung Cancer Res (2020) 9:2571–80. doi: 10.21037/tlcr-20-346
3. Fancelli S, Caliman E, Mazzoni F, Brugia M, Castiglione F, Voltolini L, et al. Chasing the target: New phenomena of resistance to novel selective RET inhibitors in lung cancer. Updated evidence Future perspectives. Cancers (2021) 13:1091. doi: 10.3390/cancers13051091
4. Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol (2022) 17:362–87. doi: 10.1016/j.jtho.2021.11.003
5. Derks JL, Hendriks LE, Buikhuisen WA, Groen HJM, Thunnissen E, Suylen RJV, et al. Clinical features of large cell neuroendocrine carcinoma: A population-based overview. Eur Respir J (2016) 47:615–24. doi: 10.1183/13993003.00618-2015
6. Naidoo J, Santos-Zabala ML, Iyriboz T, Woo KM, Sima CS, Fiore JJ, et al. Large Cell neuroendocrine carcinoma of the lung: Clinico-pathologic features, treatment, and outcomes. Clin Lung Cancer (2016) 17:e121–9. doi: 10.1016/j.cllc.2016.01.003
7. Lantuejoul S, Fernandez-Cuesta L, Damiola F, Girard N, McLeer A, et al. New molecular classification of large cell neuroendocrine carcinoma and small cell lung carcinoma with potential therapeutic impacts. Transl Lung Cancer Res (2020) 9:2233–44. doi: 10.21037/tlcr-20-269
8. Kim C, McGrath JE, Xiu J, Nagasaka M, Ma PC, Nieva JJ, et al. Genomic and immunologic characterization of large-cell neuroendocrine carcinoma of the lung. J Clin Oncol (2021) 39:8535–5. doi: 10.1200/JCO.2021.39.15_suppl.8535
9. Derks JL, Leblay N, Lantuejoul S, Dingemans AMC, Speel EJM, Fernandez-Cuesta L. New insights into the molecular characteristics of pulmonary carcinoids and large cell neuroendocrine carcinomas, and the impact on their clinical management. J Thorac Oncol (2018) 13:752–66. doi: 10.1016/j.jtho.2018.02.002
10. Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, et al. Next-generation sequencing of pulmonary Large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin Cancer Res (2016) 22:3618–29. doi: 10.1158/1078-0432.CCR-15-2946
11. George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun (2018) 9:1048. doi: 10.1038/s41467-018-03099-x
12. Lou G, Yu X, Song Z. Molecular profiling and survival of completely resected primary pulmonary neuroendocrine carcinoma. Clin Lung Cancer (2017) 18:e197–201. doi: 10.1016/j.cllc.2016.11.014
13. De Pas TM, Giovannini M, Manzotti M, Trifirò G, Toffalorio F, Catania C, et al. Large-Cell neuroendocrine carcinoma of the lung harboring EGFR mutation and responding to gefitinib. J Clin Oncol (2011) 29:e819–822. doi: 10.1200/JCO.2011.36.2251
14. Wang Y, Shen YH, Ma S, Zhou J. A marked response to icotinib in a patient with large cell neuroendocrine carcinoma harboring an EGFR mutation: A case report. Oncol Lett (2015) 10:1575–8. doi: 10.3892/ol.2015.3405
15. Chae YK, Tamragouri KB, Chung J, Lin X, Miller V, Ali SM, et al. Large-Cell neuroendocrine carcinoma of the lung: A focused analysis of BRAF alterations and case report of a BRAF non-V600-Mutated tumor responding to targeted therapy. JCO Precis Oncol (2018) 2:1–12. doi: 10.1200/PO.17.00150
16. Lim CA, Banyi N, Tucker T, Ionescu DN, Melosky B. A case of ALK-rearranged combined lung adenocarcinoma and neuroendocrine carcinoma with diffuse bone metastasis and partial response to alectinib. Curr Oncol (2022) 29:848–52. doi: 10.3390/curroncol29020072
17. Leblanc A, Owen S, Fiset PO, Corrador ALG, Isenberg J, Bouganim N. Metastatic Large-cell neuroendocrine lung carcinoma with ALK fusion oncogene with partial response to alectinib. JCO Precis Oncol (2021) 5:802–7. doi: 10.1200/PO.20.00348
18. Lindeman NI, Cagle PT, Aisner DL, Arcila ME, Beasley MB, Bernicker EH, et al. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the college of American pathologists, the international association for the study of lung cancer, and the association for molecular pathology. Arch Pathol Lab Med (2018) 142:321–46. doi: 10.5858/arpa.2017-0388-CP
19. Subbiah V, Shen T, Tetzlaff M, Weissferdt A, Byers LA, Cascone T, et al. Patient-driven discovery and post-clinical validation of NTRK3 fusion as an acquired resistance mechanism to selpercatinib in RET fusion-positive lung cancer. Ann Oncol (2021) 32:817–9. doi: 10.1016/j.annonc.2021.02.010
20. Kander EM, Shah MH, Zhou Y, Goyal A, Palmer JD, Owen DH, et al. Response to the selective RET inhibitor selpercatinib (LOXO-292) in a patient with RET fusion-positive atypical lung carcinoid. Clin Lung Cancer (2021) 22:e442–5. doi: 10.1016/j.cllc.2020.06.011
Keywords: RET fusion, large cell neuroendocrine carcinoma, selpercatinib, lung cancer (LC), targeted
Citation: Arora A, Zaemes J, Ozdemirli M and Kim C (2023) Response to selpercatinib in a patient with RET fusion-positive pulmonary large-cell neuroendocrine carcinoma: A case report. Front. Oncol. 13:1134151. doi: 10.3389/fonc.2023.1134151
Received: 30 December 2022; Accepted: 20 February 2023;
Published: 14 March 2023.
Edited by:
Carlos Gil Ferreira, Instituto Oncoclínicas, BrazilReviewed by:
Lorenzo Antonuzzo, University of Florence, ItalyCopyright © 2023 Arora, Zaemes, Ozdemirli and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chul Kim, Y2h1bC5raW1AZ3VuZXQuZ2VvcmdldG93bi5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.