
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 01 August 2023
Sec. Cancer Epidemiology and Prevention
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1132816
 Sina Azadnajafabad1†
Sina Azadnajafabad1† Sahar Saeedi Moghaddam1,2†
Sahar Saeedi Moghaddam1,2† Esmaeil Mohammadi1,3
Esmaeil Mohammadi1,3 Negar Rezaei1,4
Negar Rezaei1,4 Mohammad-Mahdi Rashidi1
Mohammad-Mahdi Rashidi1 Nazila Rezaei1
Nazila Rezaei1 Ali H. Mokdad5,6
Ali H. Mokdad5,6 Mohsen Naghavi5,6
Mohsen Naghavi5,6 Christopher J. L. Murray5,6
Christopher J. L. Murray5,6 Bagher Larijani4
Bagher Larijani4 Farshad Farzadfar1,4* and GBD 2019 NAME Breast Cancer Collaborators
Farshad Farzadfar1,4* and GBD 2019 NAME Breast Cancer CollaboratorsBackground: Breast cancer (BC) is the most common cancer in women globally. The North Africa and Middle East (NAME) region is coping hard with the burden of BC. We aimed to present the latest epidemiology of BC and its risk factors in this region.
Methods: We retrieved the data on BC burden and risk factors from the Global Burden of Disease Study 2019 to describe BC status in the 21 countries of the NAME region from 1990 to 2019. We explored BC incidence, prevalence, deaths, disability-adjusted life years (DALYs), and attributable burden to seven risk factors of female BC, namely, alcohol use, diet high in red meat, low physical activity, smoking, secondhand smoke, high body mass index, and high fasting plasma glucose. Decomposition analysis on BC incidence trend was done to find out the contributing factors to this cancer’s growth.
Results: In 2019, there were 835,576 (95% uncertainty interval: 741,968 to 944,851) female and 10,938 (9,030 to 13,256) male prevalent cases of BC in the NAME region. This number leads to 35,405 (30,676 to 40,571) deaths among female patients and 809 (654 to 1,002) deaths in male patients this year. BC was responsible for 1,222,835 (1,053,073 to 1,411,009) DALYs among female patients in 2019, with a greater proportion (94.9%) of burden in years of life lost (YLLs). The major contributor to female BC incidence increase in the past three decades was found to be increase in age-specific incidence rates of BC (227.5%), compared to population growth (73.8%) and aging (81.8%). The behavioral risk factors were responsible for majority of attributable female BC burden (DALYs: 106,026 [66,614 to 144,247]). High fasting plasma glucose was found to be the risk factor with the largest effect (DALYs: 84,912 [17,377 to 192,838]) on female BC burden.
Conclusion: The increasing incidence and burden of BC in the NAME region is remarkable, especially when considering limited resources in the developing countries of this region. Proper policies like expanding screening programs and careful resource management are needed to effectively manage BC burden.
Breast cancer (BC) is the most prevalent neoplasm in female patients globally, with an increasing trend of incidence, almost in all regions (1, 2). BC is the leading cause of deaths due to cancer in female patients, and the mortality rate of this burdensome cancer has increased in most of the regions specifically in developing countries (3–5). In the most recent global cancer statistics updates (GLOBOCAN 2020), BC has overtaken lung cancer in first place to become the top cancer with the highest number of new cases (4, 6). BC contributes to about one-fourth of deaths due to malignancies in post-menopausal women, and female BC is the fifth leading cause of death due to cancer, following lung, colorectal, liver, and gastric cancers globally (4, 7). Although BC incidence is higher in developed countries, the relative mortality rate of this cancer is higher in developing countries (8). The increasing incidence of BC is suggested to happen due to improved BC screening tools like widespread use of mammography besides a substantial increase in exposure to various risk factors (4, 9, 10). So far, screening programs for BC in populations are designed and implemented to reduce BC death by early detection of malignancy and timely treatment interventions (11, 12).
The North Africa and Middle East (NAME) region is projected to have the greatest increase in all types of cancers in the next decades due to population aging and growth, compared to other World Health Organization (WHO) regions (13–15). In the period of 2005–2015, BC was the most common cancer in incident cases and the second leading cause of cancer deaths in this region (16). Among all cancers, BC has a significant importance when inspecting its epidemiology in the NAME region, due to various reasons. Cultural and socio-economic differences, the developing status of most of the countries located in this region, and multiple barriers to an efficient approach to handle the growing burden of BC are the leading issues that need to be considered in these countries (17–19). It is notable that most of the BC cases in this region are amenable to prevention and treatment; however, delayed diagnosis and treatment may explain the increase in burden of this cancer (20). On the other hand, management of BC in the NAME region faces several challenges, including younger age at presentation, aggressive behavior, lack of national breast screening programs, and lack of reliable data registries as well as socioeconomic factors; thus, these factors make it very challenging to apply the international guidelines for BC management in this region (10, 21, 22).
Besides the fragile status of female BC, male BC has a mysterious and poorly known epidemiology in the NAME region (23, 24). This poor knowledge is a global concern and is mainly due to scarcity of BC in men (25). BC in male patients is believed to be a distinct cancer from BC in female patients, due to different epidemiology, risk factors, prognosis, and profoundly varied molecular, biologic, and clinicopathologic features of this malignancy in men (25–28). Previous studies showed that male BC has a higher relative proportion of BC cases in the NAME region compared to the Western regions of world (24). Therefore, improvements in data shortages regarding male BC is necessary to pave the way for effective management and treatment of BC in men (29).
Epidemiologic data on the BC in the NAME region have many geographical variations with gaps in several areas (30, 31). In this paper, we present the major epidemiologic measures of BC and its risk factors in the NAME region and their trends during the 30-year period from 1990 to 2019 using the Global Burden of Disease (GBD) Study 2019. Additionally, epidemiology of risk factors of female BC in this region is presented in this study. A clear visualization of the BC status in this neglected region could help international, regional, and national health authorities to properly focus on this principal cancer and its huge burden in the NAME region. Therefore, we aimed to present the latest updated epidemiologic measures of BC and its responsible risk factors for the NAME region to provide the material for further actions and policies.
This study is a part of the GBD Study 2019 provided by the Institute for Health Metrics and Evaluation (IHME), conducted for the NAME region, whose main data are accessible through its portal (32). GBD 2019 provides data of global, regional, and national burden of 369 diseases and injuries and 87 risk factors in 204 countries and territories. Also, subnational data of 21 countries are available in the GBD 2019. Detailed methods of GBD studies are provided in previous publications (33, 34). NAME is one of the seven super-regions in this study that encompasses 21 countries, namely, Afghanistan, Algeria, Bahrain, Egypt, Iran (Islamic Republic of), Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Sudan, Syrian Arab Republic, Tunisia, Turkey, United Arab Emirates, and Yemen. IHME uses a hierarchical leveling of causes into three main categories of communicable, maternal, neonatal, and nutritional diseases (CMNNDs); non-communicable diseases (NCDs); and injuries, in which neoplasms belong to the NCDs category. BC as the investigated cause in this study is defined as cause B.1.14 in the GBD cause list. More details on the mapped International Classification of Diseases and Injuries (ICD) codes of BC incidence and mortality to this cause in GBD are available in the Supplementary Methods. Also, diseases’ risk factors are categorized into three main categories of environmental and occupational risks, behavioral risks, and metabolic risks and follows a hierarchy of risks in GBD risk assessments (34). The other data source used to evaluate the cancer screening, registry, and action plans among the countries of the region of focus in this investigation was the WHO Global Health Observatory (GHO) database for 2019 (35). Moreover, the universal health coverage (UHC) based on an index of effective coverage of health services provided by GBD 2019 study was the other dataset used to rank and discuss the overall healthcare services and BC management state among the countries of the NAME region in this study (36). This study is conducted according to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) statement (37).
The cornerstone of data estimations for GBD studies is mortality estimation made on the cause of death (CoD) database containing information from different sources, which are mainly cancer registries in the cancer data estimation, like BC in this study (Supplementary Figures 1, 2). The full list of data sources used to estimate BC data for the NAME region and countries in this study is provided in the Supplementary Table 1. In brief, cancer registries, including both incidence and mortality data of cancers, are used to obtain mortality to incidence (MIR) ratios in the estimation process. The three indices of mortality, incidence, and MIR are then aggregated and estimated by several calculations to provide the most precise information on cancers (33, 38). Risk factor estimations are made by the comparative risk assessment (CRA) conceptual framework that provides a hierarchy of risks and causes contributing to health outcomes. The effect size estimation in GBD is done by the relative risk (RR) modeling for level of exposure to risk or cause for mortality or morbidity (34) (Supplementary Table 2). The full description of the data estimation process for BC and attributable burden to its risk factors are provided in the Supplementary Methods (section A) of this paper.
To investigate epidemiology and burden of BC in the NAME region, a series of variables including incidence, prevalence, deaths, MIR, years of life lost (YLLs), years lived with disability (YLDs), and disability-adjusted life years (DALYs) were explored. Also, the measures were compared in different socio-demographic index (SDI) quintiles of included countries. Various risk factors for BC approved by IHME were included in two main categories of behavioral risk factors comprising alcohol use, diet high in red meat, low physical activity, smoking, and secondhand smoke, and metabolic risk factors comprising high body mass index (BMI) and high fasting plasma glucose (FPG) for female BC, and alcohol use, secondhand smoke, and diet high in red meat for BC in male patients (34). Deaths, YLLs, YLDs, and DALYs were explored for the investigated burden attributable to BC risk factors in this study. MIR and YLL/YLD ratio were estimated using age-standardized rates and utilized to compare the BC statistics between countries and sexes. Higher MIR values indicate higher fatality of condition and higher YLL/YLD values indicate higher mortality rather than morbidity for BC. Furthermore, we used the SDI and Healthcare Access and Quality (HAQ) Index to present results of this study. SDI is a composite index of income per capita, educational attainment, and fertility rates of geographic areas provided by IHME to compare diseases and risks based on socioeconomic factors (33). The estimated SDI values for the regions and countries included in this study could be found in Supplementary Table 3. The HAQ Index is a proxy of personal healthcare access and quality developed by IHME and is calculated by using MIRs for cancers and risk-standardized mortality rates for non-cancer causes (39). We used the recent estimated all-ages HAQ Index values for both sexes for the countries of the NAME region using the results of GBD study 2019 to compare BC measures, whose index values are found in Supplementary Table 4.
Fourteen age groups (15–19, 20–24, …, 75–79, 80+) were used to investigate BC indices in this study. We chose this age range and groups based on the richness of estimated data of the GBD Study 2019 on the BC cause and the responsible risk factors. The number values were reported in all-ages scale and the rates were reported in age-standardized scale per 100,000 population. Age-standardized rates were estimated using the GBD age standard world population, updated to the 2019 standard population age structure (33). Percent changes of measures were calculated for the period 1990–2019. All estimations were made with a 95% uncertainty interval (UI) generated using the 25th and 975th ordered values in 1,000 draws of the posterior distribution. To evaluate the contribution of population growth, population aging (or age structure changes), and age-specific incidence rate changes on the BC incidence trend 1990–2019, we did a decomposition analysis on incidence in two scenarios. In the first scenario, we applied the age and sex structure and the age-specific rates of 1990 to the 2019 population. In the second scenario, we applied the age-specific rates of 1990 to the age and sex structure and population size of 2019. The difference between the total number of incident cases in 1990 and the first scenario was attributable to population growth. The difference between the second and first scenarios was attributable to population aging. The difference between the total number of incident cases in 2019 and the second scenario was attributable to age-specific rates changes (40). The full description of the steps of the decomposition analysis is found in Supplementary Methods (section B) of the Supplementary Material. Percentages of each of the three factors in a hypothetical assumption of two other factors being constant, and the overall change of BC incidence are provided for a better understanding of contributors to BC incidence growth through the past three decades. Data handling and visualizations in this study were done using STATA version 11 (STATA Corp., College Station, Texas, USA) and R 4.1.2 for Windows.
Various epidemiologic features of BC in the NAME region have different patterns in the last three decades (Figure 1, Table 1). Also, each country had distinct characteristics based on BC statistics (Supplementary Table 5). A brief notation of results is provided here and more are available in the appendices of this paper. The results provided in this manuscript focus on female and male patients by sex separately.
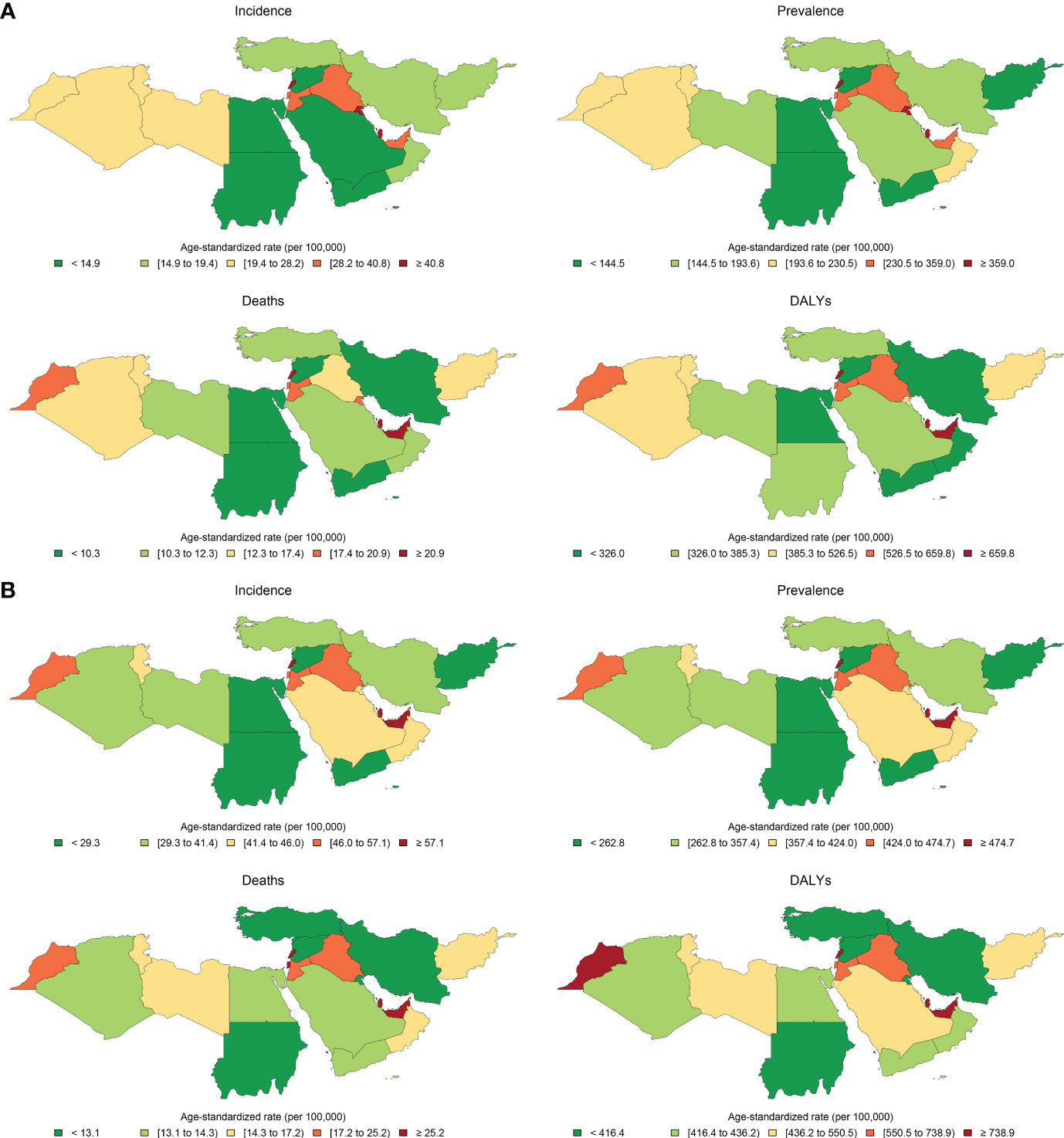
Figure 1 Age-standardized rates of incidence, prevalence, deaths, and disability-adjusted life years (DALYs) of breast cancer in female population in (A) 1990 and (B) 2019 in countries of the North Africa and Middle East region.
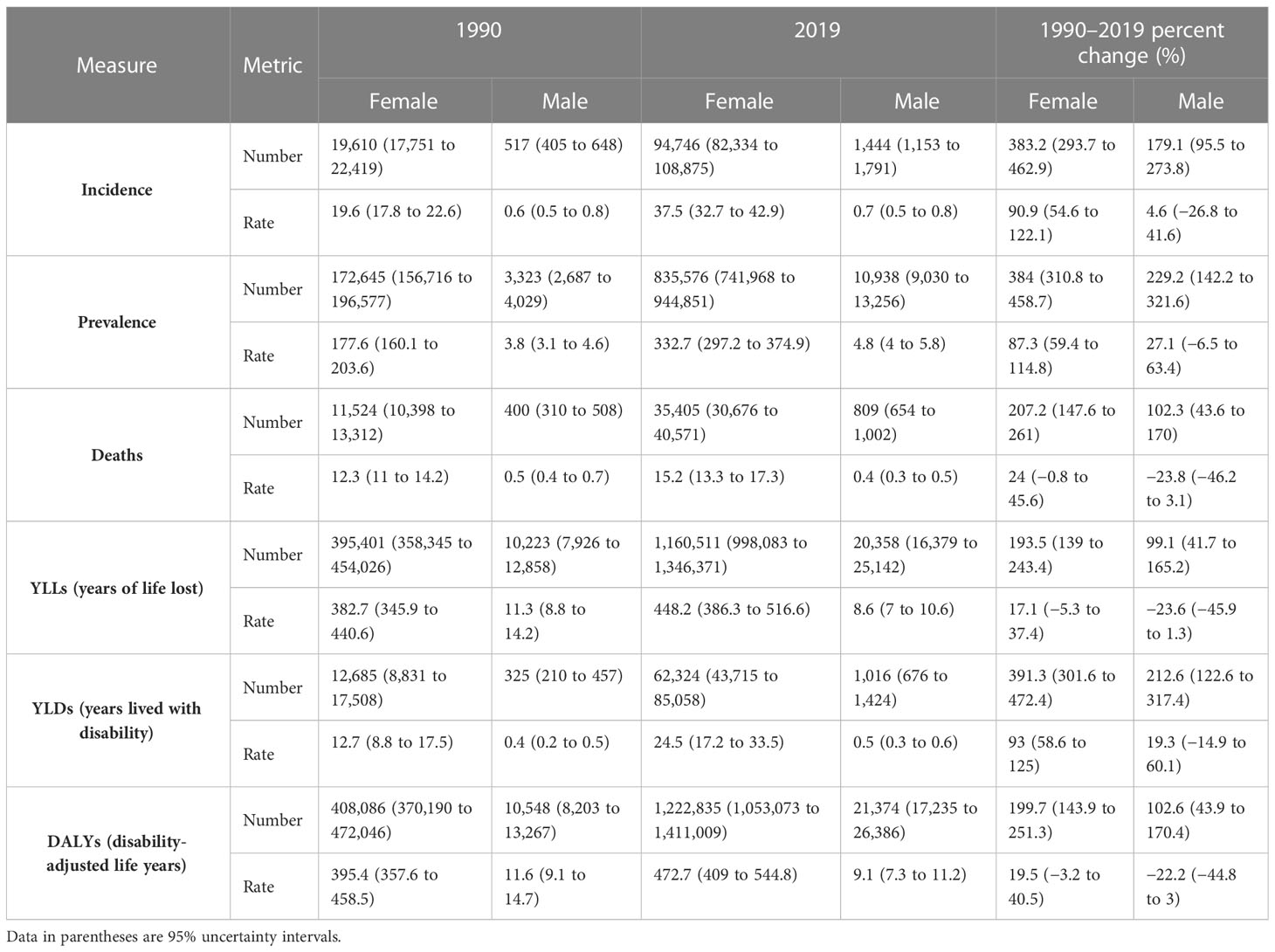
Table 1 Trend of epidemiologic indices of breast cancer in the North Africa and Middle East region, number for all ages and age-standardized rates, by sex, in 1990 and 2019 and percent of changes in the 1990–2019 period.
The all-ages number of BC among female patients of NAME region was estimated to be 19,610 (95% UI: 17,751 to 22,419) in 1990 and 94,746 (82,334 to 108,875) in 2019, reflecting a 383.2% (293.7 to 462.9) increase. The overall age-standardized rates of BC incidence per 100,000 among female patients in this region was estimated to be 37.5 (32.7 to 42.9) in 2019 compared to 19.6 (17.8 to 22.6) in 1990, indicating an almost two times (90.9% [54.6 to 122.1]) increase in the comparison of rates. Investigating the incidence rates among countries located in this region, we found Lebanon (122.5 [92.1 to 160.7]) to have the highest female BC rate and Afghanistan (22.3 [16.8 to 29.1]) to have the lowest rate. Age-standardized incidence rate was most prominently increased in Saudi Arabia (189.8% [79.0 to 358.9]), Lebanon (152.9% [76.0 to 255.6]), and Oman (131.5% [45.4 to 263.8]), in contrast to Kuwait (3.4% [−18.3 to 36.7]), Afghanistan (30.7% [−8.5 to 79.5]), and United Arab Emirates (41.0% [−6.9 to 112.1]) with the lowest rates of increase in terms of percent change. Considering age groups, BC is first detected in female patients in their 20s and accelerates almost consistently by advancing age (Supplementary Figure 3).
Investigating BC among male patients, the all-ages number of incident cases in 2019 were approximately 1,444 (1,153 to 1,791) with a 179.1% (95.5 to 273.8) increase compared to this measure in 1990 (517 [405 to 648]). However, it is important to mention that the age-standardized incidence rate of BC among male patients living in the NAME region changed slightly (4.6% [−26.8 to 41.6]) during the 30 years of this study (0.6 [0.5 to 0.8] per 100,000 in 1990 and 0.7 [0.5 to 0.8] in 2019). Comparing the recent incidence rates of male BC among countries of this region in 2019, we found Egypt (0.1 [0.06 to 0.15]) to have the lowest rate and Algeria (2.7 [1.7 to 3.9]) to have the highest rate in this regard. The trend of male BC incidence rates in terms of percent change in this period showed Bahrain (134.8% [27.0 to 306.4]), Iraq (92.2% [15.4 to 218.8]), and Syrian Arab Republic (60.6% [0.2 to 159.4]) having the highest increase, and on the other side, Algeria (−33.8% [−60.0 to 5.6]), Afghanistan (−27.0% [−53.9 to 26.1]), and Yemen (−20.1% [−51.1 to 46.3]) had the highest reductions in this measure.
Based on the decomposition analysis of incidence rate change of BC in female patients in the NAME region, it is evident that in 2019, a total of 75,136 more cases of BC has been found compared to 1990, representing a 383.2% overall increase, of which 73.8% was estimated to be attributable to population growth, 81.8% was attributable to population aging, and 227.5% was attributable to change of incidence rates of BC. The highest detected overall increase of incidence was in the United Arab Emirates, Qatar, and Saudi Arabia, respectively, and in all these three countries, the true increase of BC incidence was the prominent contributor in the decomposition analysis. Also, of the 179.1% increase of BC in male patients, 78.9% was attributable to population growth, 81.9% was attributable to population aging, and 18.3% was attributable to change of BC incidence rates (Table 2).

Table 2 Decomposition analysis of breast cancer incidence trend 1990–2019, by sex, in the North Africa and Middle East region and countries.
The all-ages prevalence of BC among female patients in the NAME region was 172,645 (156,716 to 196,577) in 1990 and 835,576 (741,968 to 944,851) in 2019, showing a 384% (310.8 to 458.7) increase. The age-standardized prevalence of female BC has increased steadily from 177.6 (160.1 to 203.6) per 100,000 female individuals in 1990 to 332.7 (297.2 to 374.9) in 2019 with an 87.3% (59.4 to 114.8) increase. Among countries of this region in 2019, Lebanon (1,083.5 [844.6 to 1,383.5]) ranked first in comparison of prevalence rates with a noticeable difference with the second-ranked country, Qatar (880.3 [701.3 to 1,098.3]), while Afghanistan (153.1 [121.8 to 192.8]) ranked the last in this measure. The rising prevalence of BC among female patients has been detected in all the nations of this region with no exception. Lebanon (161.6% [90.9 to 252.8]), Saudi Arabia (155.7% [77.4 to 267.6]), and Oman (113.3% [54.3 to 191.9]) had the highest increase in the BC prevalence rates among female patients, while Kuwait (12.5% [−6.9 to 39.7]), Afghanistan (22.5% [−6.9 to 58.9]), and United Arab Emirates (44.6% [5.0 to 96.5]) had the lowest changes in this regard.
The BC prevalence in male patients of this region estimated approximately 10,938 (9,030 to 13,256) cases in 2019 and 3,323 (2,687 to 4,029) in 1990, exhibiting a 229.2% (142.2 to 321.6) increase during the study period. However, the age-standardized rates of BC in male patients increased more slightly (27.1% [−6.5 to 63.4]) in NAME and reached 4.8 (4 to 5.8) cases per 100,000 in 2019. Algeria (18.1 [12.2 to 25.6]), Oman (18.1 [12.9 to 25.1]), and Lebanon (10.5 [7.4 to 14.9]) had the highest prevalence rates of BC in male patients in 2019 contrary to Egypt (0.9 [0.6 to 1.2]), Syrian Arab Republic (1.1 [0.9 to 1.5]), and Iran (2.8 [2.3 to 3.3]). The estimated change in BC prevalence rates among male patients during the study period was highest in Iraq (116.8% [39.2 to 236.7]), Bahrain (105.5% [32.4 to 214.5]), and Lebanon (75.0% [7.0 to 184.9]), while Afghanistan (−14.7% [−43.2 to 32.7]), Algeria (−9.1% [−42.6 to 41.0]), and Yemen (−5.5% [−38.3 to 54.1]) had a negative change in this regard.
Altogether, there were 35,405 (30,676 to 40,571) deaths among female patients in 2019 in the NAME region, accounting for 15.2 (13.3 to 17.4) age-standardized deaths in 100,000 individuals and 2.6% (2.4 to 2.8) of all-cause female patients’ deaths. In comparison to 1990, 11,524 (10,398 to 13,312) deaths are estimated for NAME, accounting for 12.3 (11.0 to 14.2) age-standardized deaths and 1.0% (0.1 to 1.1) of their total deaths. Both the percent change in all-age deaths number (207.2% [147.6 to 261]) and the death rates (24.0% [−0.8 to 45.6]) were increased in female patients with BC during the study period. Investigating countries showed Qatar (36.9 [28.9 to 45.8]), Lebanon (35.5 [27.2 to 46.4]), and United Arab Emirates (26.2 [20.0 to 33.6]) having the highest death rates in 2019, in contrast to Syrian Arab Republic (11.3 [8.1 to 15.5]), Iran (11.9 [10.8 to 13.1]), and Turkey (12.7 [10.1 to 15.7]). Exploring changes in age-standardized rates found Egypt (49.0% [−2.0 to 106.6]), Yemen (46.9% [−6.7 to 159.1]), and Libya (46.7% [−8.3 to 126.5]) with the highest growth, while Kuwait (−26.7% [−41.3 to −4.2]), Bahrain (−8.1% [−28.5 to 16.6]), and Jordan (−5.1% [−31.0 to 33.8]) had the best improvements in death rates (Figure 2).
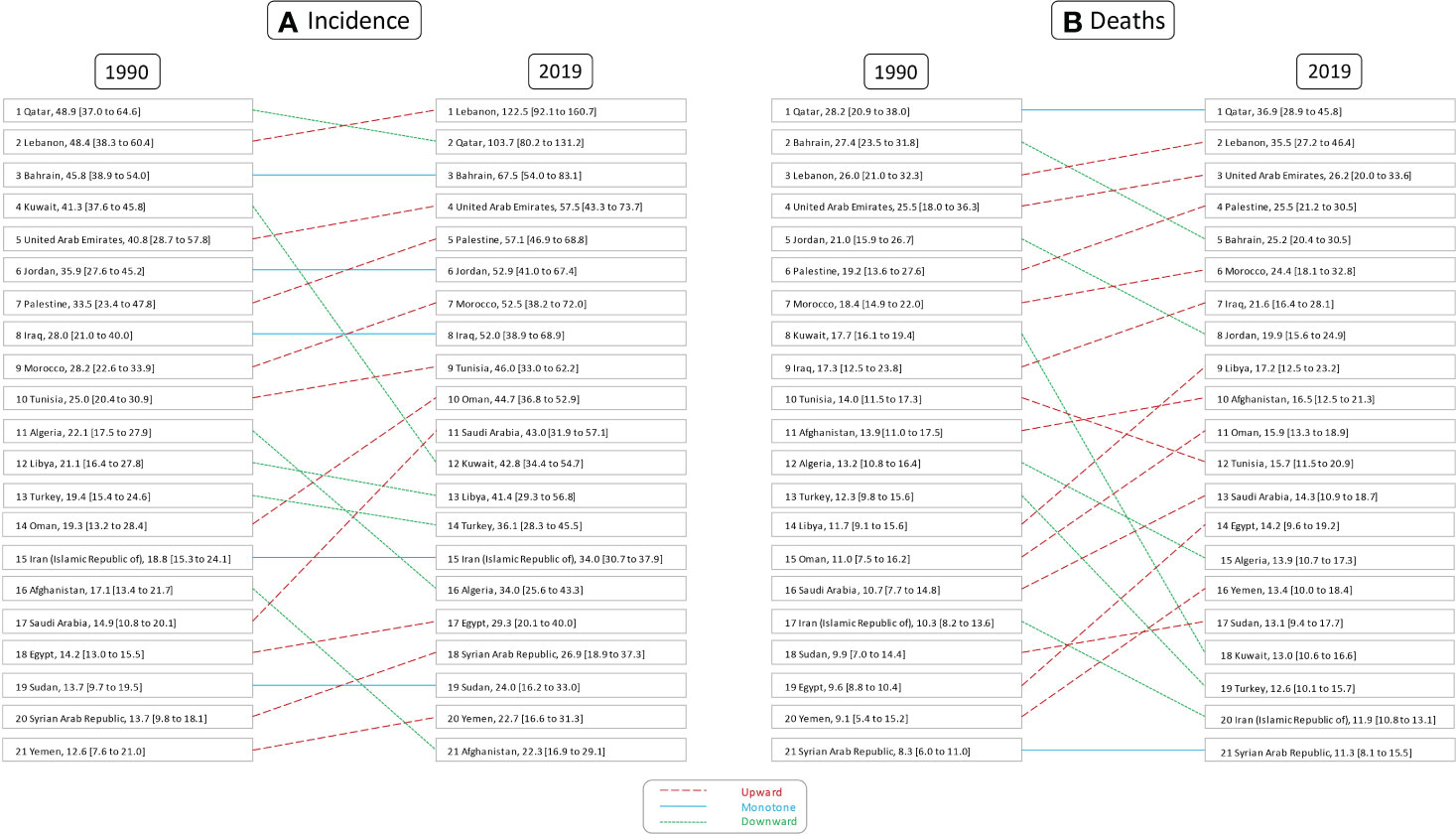
Figure 2 Rank of age-standardized rates of female breast cancer (A) incidence and (B) deaths in countries of the North Africa and Middle East region, in 1990 and 2019.
In 2019 in the NAME region, approximately 809 (654 to 1,002) deaths were estimated to happen due to BC in male patients, which is double that of 1990 (400 [310 to 508]). Although the percent change in all-age deaths number was positive (102.3% [43.6 to 170]) in the study period, the death rates had a negative (−23.8% [−46.2 to 3.1]) change in male patients with BC. Investigating death rates among countries of this region, we found Algeria (1.7 [1.1 to 2.5]), Oman (1.4 [1.0 to 2.1]), and Lebanon (0.7 [0.5 to 1.1]) to have the highest deaths, as compared to Egypt (0.06 [0.04 to 0.09]), Syrian Arab Republic (0.07 [0.05 to 0.1]), and Iran (0.16 [0.13 to 0.17]) with the lowest death rates in male patients with BC. Percent changes in death rates due to BC in male patients showed Bahrain (63.1% [−12.3 to 176.1]), Iraq (41.1% [−15.0 to 127.2]), and Palestine (31.0% [−18.4 to 125.6]) having the greatest increases, in contrast to Algeria (−52.2% [−70.9 to −19.2]), Jordan (−36.8% [−64.9 to 5.2]), and Afghanistan (−32.4% [−57.5 to 15.7]) with most improvements in this regard.
The 2019 GBD estimates revealed that BC was responsible for 1,222,835 (1,053,073 to 1,411,009) DALYs among female patients, which was composed of 1,160,511 (998,083 to 1,346,371) YLLs and 62,324 (43,715 to 85,058) YLDs. The all-ages number of DALYs, YLLs, and YLDs due to BC in female patients had a 199.7% (143.9 to 251.3), 193.5% (139 to 243.4), and 391.3% (301.6 to 472.4) increase during the study period. Among countries located in this region, Lebanon (1,067 [809 to 1,407]), Qatar (856 [663 to 1,075]), and Morocco (843 [612 to 1,158]) had the highest BC burden in terms of age-standardized DALY rates in 2019, while Syrian Arab Republic (334 [237 to 472]), Kuwait (359 [290 to 462]), and Iran (369 [337 to 404]) had the lowest rates (Figure 3).
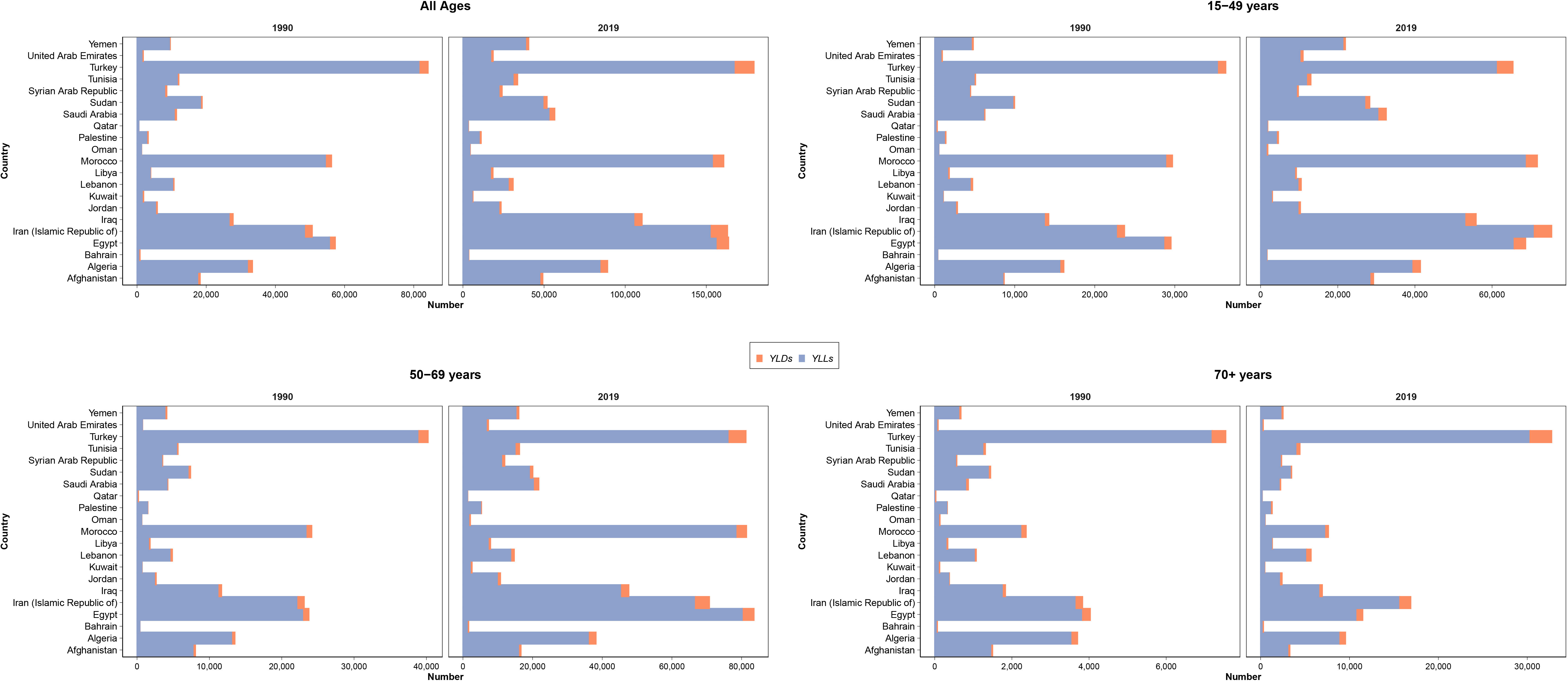
Figure 3 Distribution of years lived with disability (YLDs) and years of life lost (YLLs) due to female breast cancer in all ages, 15–49, 50–69, and 70+ age categories, in countries of the North Africa and Middle East region.
The BC in male patients of this region caused 21,374 (17,235 to 26,386) DALYs in 2019, which was an aggregation of 20,358 (16,379 to 25,142) YLLs and 1,016 (676 to 1,424) YLDs. The all-ages DALYs, YLLs, and YLDs of BC in male patients had a 102.6% (43.9 to 170.4), 99.1% (41.7 to 165.2), and 212.6% (122.6 to 317.4) increase, respectively. Although the age-standardized rates of DALYs (−22.2% [−44.8 to 3]) and YLLs (−23.6% [−45.9 to 1.3]) had a decreasing pattern during the study period, the YLD rates (19.3% [−14.9 to 60.1]) were increasing. DALY rates due to BC in male patients in 2019 were highest in Algeria (34.6 [22.5 to 48.9]), Oman (26.3 [17.7 to 37.0]), and United Arab Emirates (17.1 [8.1 to 30.7]), in contrast to Egypt (1.7 [1.0 to 2.4]), Syrian Arab Republic (1.9 [1.3 to 2.6]), and Iran (4.0 [3.5 to 4.5]).
MIR in female patients with BC was estimated to be 0.41 in 2019 and 0.63 in 1990 in the NAME region, reflecting a 35.05% decrease in this index. The countries with the highest MIR in female patients with BC in 2019 were Afghanistan (0.74), Yemen (0.59), and Sudan (0.55) compared to Lebanon (0.29), Kuwait (0.30), and Saudi Arabia (0.33) with the lowest estimated MIRs. This index was estimated to be approximately 0.84 in 1990 and 0.61 in 2019 among male patients with BC in this region, which showed a 27.2% decrease. Male patients with BC in Afghanistan (0.89), Yemen (0.79), and Sudan (0.76) had the worst state regarding MIR in 2019, while individuals in Kuwait (0.48), Lebanon (0.49), and Iran (0.50) had the lowest MIR. Dividing the MIR values of male to female patients in the NAME region showed that in both 1990 (1.3) and 2019 (1.5), the prognosis of BC is much poorer in male patients compared to female patients and this cancer takes more lives in the affected male patients.
The YLL/YLD ratio for BC in female patients was estimated to be 30.1 in 1990 and 18.3 in 2019, with a 39.3% decrease in this index. The countries with the highest age-standardized YLL/YLD ratio for BC in female patients in 2019 were Afghanistan (39.6), Yemen (30.5), and Sudan (26.7), and the countries with the lowest values for this index were Kuwait (11.2), Qatar (12.0), and Lebanon (12.2). This index was calculated for BC in male patients to be approximately 29.6 in 1990 and 18.9 in 2019, which had a 36% decrease. Among the countries of this region, Afghanistan (35.6), Yemen (28.3), and Sudan (26.4) earned the highest YLL/YLD ratios in 2019 against Qatar (12.0), Kuwait (12.1), and Lebanon (13.5). Comparison of this ratio between sexes showed almost a similar pattern in both 1990 (male/female ratio: 0.98) and 2109 (male/female ratio: 1.04). Both of the examined ratios had a similar decreasing pattern in the region and included countries (Figure 4).
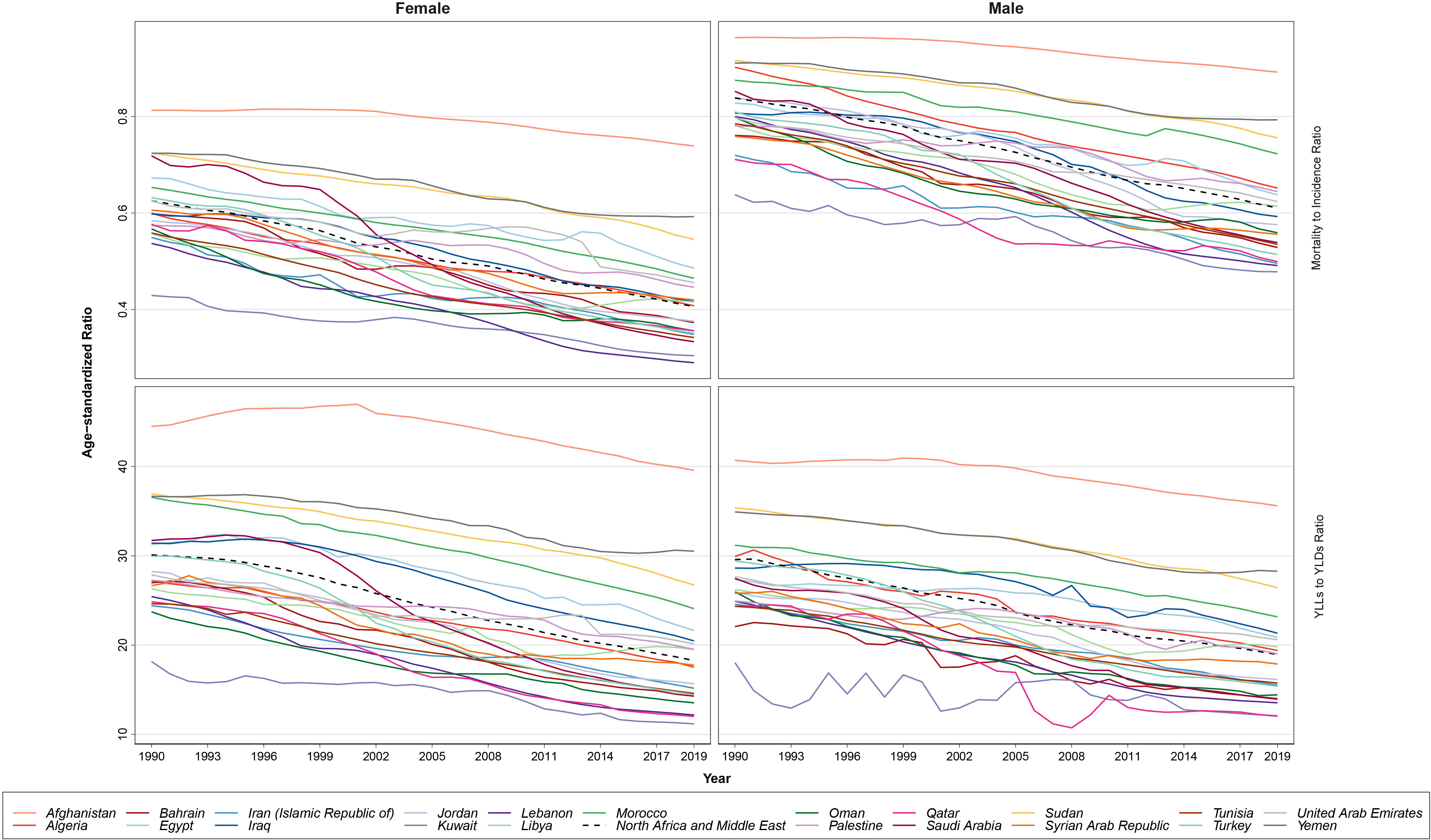
Figure 4 The patterns of the mortality to incidence ratio (MIR) and years of life lost (YLLs)/years lived with disability (YLDs) ratio trends based on age-standardized rates by sex, during the study period.
Investigating BC incidence and prevalence rates among female patients in 2019, almost all nations with a higher SDI and HAQ Index showed higher incidence and prevalence rates (Lebanon, Qatar, Bahrain) and the countries with the lowest rates belonged to lower SDI categories and were those with lower HAQ Index values (Afghanistan, Yemen, Sudan). Also, the top three countries in death rates of BC in female patients had middle to higher SDI (Qatar, Lebanon, and United Arab Emirates) and Qatar and Lebanon also had the highest HAQ Index in the region following Kuwait. However, the pattern of BC DALYs in this comparison had no clear pattern. Regarding MIR and YLL/YLD of BC in female patients in 2019, countries with higher ratios all had the lowest SDI and HAQ Index (Afghanistan, Yemen, and Sudan) and countries with a lower MIR (Lebanon, Kuwait, Saudi Arabia) and YLL/YLD (Kuwait, Qatar, Lebanon) nearly all had high SDI and HAQ Index. Exploring the abovementioned measures for BC in male patients of this region in 2019, we found the pattern only for MIR and YLL/YLD (higher in low SDI and HAQ Index countries—Afghanistan, Yemen, and Sudan—and lower in countries with better SDI and HAQ Index) (Figure 5).
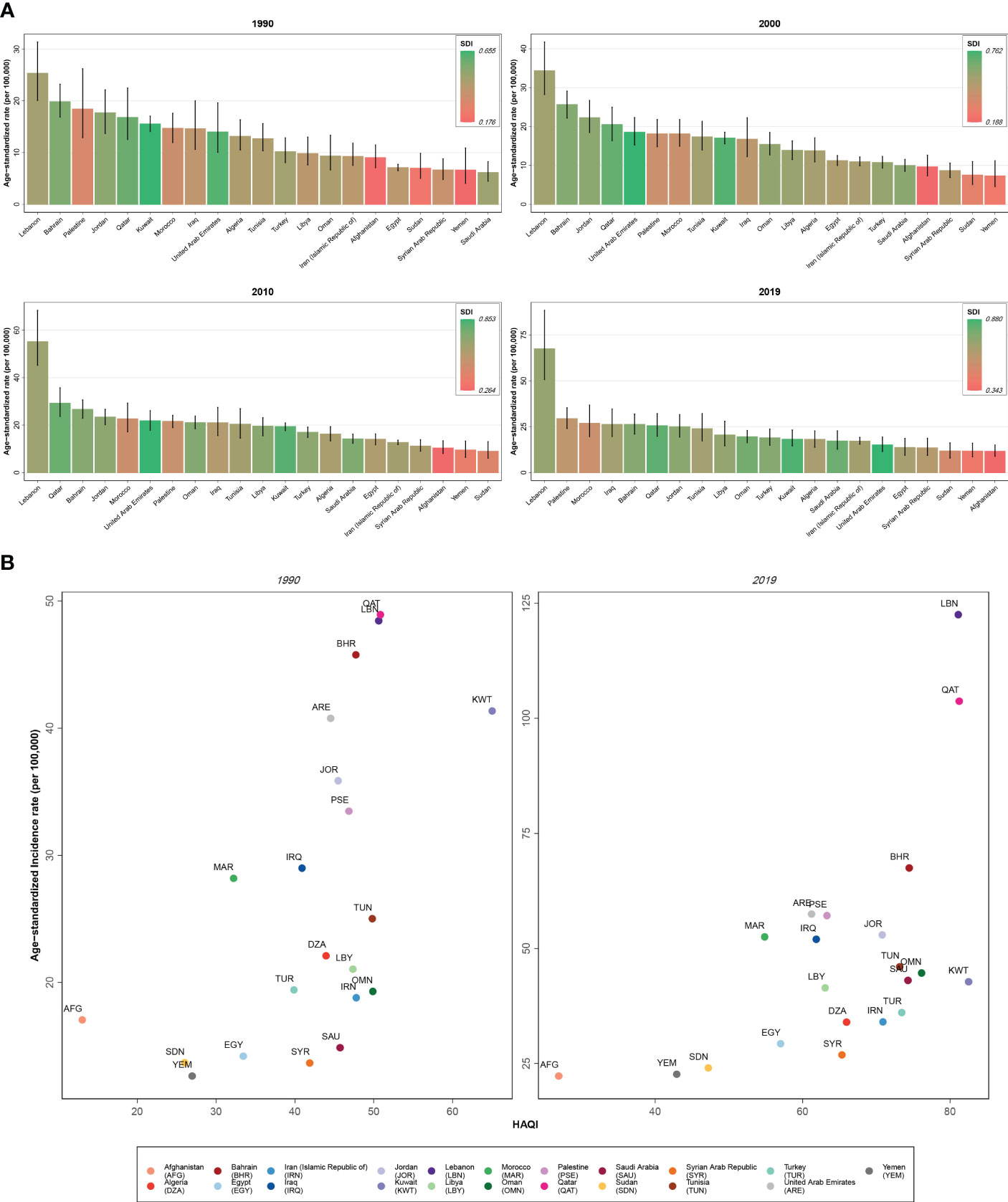
Figure 5 (A) Age-standardized rate of incidence of female breast cancer among countries of the North Africa and Middle East region compared by the socio-demographic index (SDI). (B) Age-standardized rate of incidence of female breast cancer among countries of the North Africa and Middle East region compared by the Healthcare Access and Quality (HAQ) Index.
The all-ages deaths attributable to the risk factors of female BC were estimated to be 6,507 (3,576 to 10,469) in 2019 in the NAME region with a 290.3% (198.8 to 387.9) increase compared to 1990 (1,667 [959 to 249]). In total, 171,616 (80,804 to 297,481) DALYs were attributable to all seven BC risk factors in 2019 among female patients of the NAME region, with behavioral risk factors (firsthand and secondhand smoking, alcohol use, low physical activity, and diet high in red meat) being responsible for 106,026 (66,614 to 144,247) DALYs, followed by metabolic risk factors (high FPG and high BMI) being responsible for 72,729 (−22,214 to 192,180) DALYs. In 1990, 46,992 (24,371 to 72,765) DALYs were attributable to all these risk factors, indicating a 265.2% (169.9 to 361.8) change. High FPG was the risk factor with the largest effect (84,912 [17,377 to 192,838] DALYs), followed by secondhand smoking (41,012 [9,770 to 72,205]) and diet high in red meat (24,129 [5,414 to 34,461]). The protective effects of high BMI in relation to BC burden in DALYs (−7,656 [−66,973 to 36,339]) was found. The negative estimated DALYs were mainly due to a high value of YLLs in the premenopausal women (Table 3). The age-standardized DALY rates attributed to all BC risk factors in female patients were found to be the highest in Qatar (270.8 [137.2 to 443.3]), Lebanon (250.3 [150.3 to 390.9]), and United Arab Emirates (193.2 [100.3 to 320.5]), while being smallest in Iran (55.5 [27.5 to 91.0]), Syrian Arab Republic (55.8 [27.7 to 97.7]), and Yemen (57.7 [29.5 to 99.4]), in 2019. The countries with the highest growth of attributable DALY rates to BC risk factors in female patients during the study period were Egypt (116.0% [31.2 to 232.8]), Oman (102.0% [24.1 to 231.6]), and Libya (89.4% [5.9 to 206.0]), while Kuwait had a decreasing pattern (−14.5% [−34.2 to 15.5]), and Bahrain (2.4% [−28.1 to 38.4]) and Turkey (3.0% [−30.8 to 44.6]) were countries with the lowest growth in this regard (Figure 6, Supplementary Tables 6, 7).
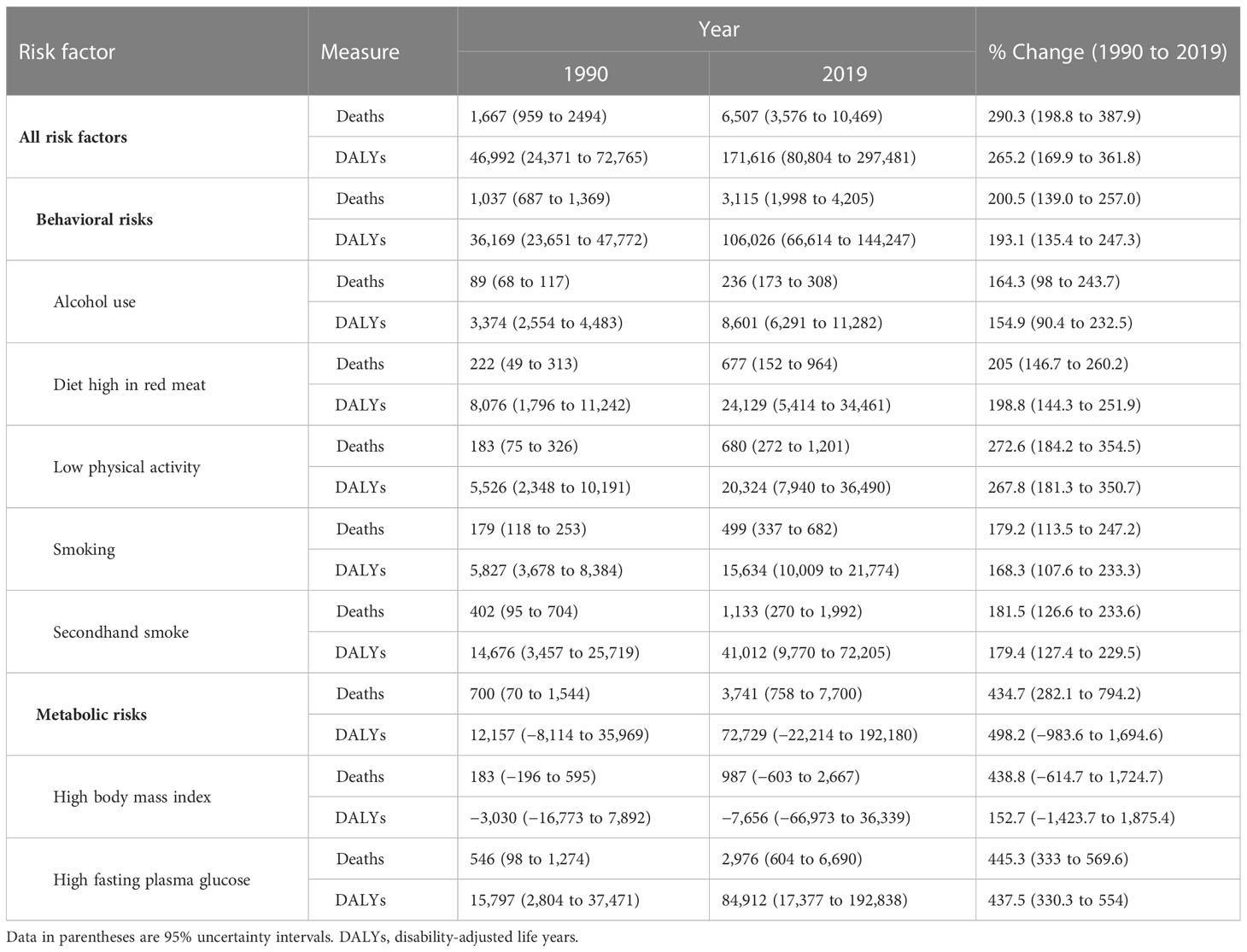
Table 3 The all-ages numbers of deaths and disability-adjusted life years attributed to various breast cancer risk factors for female population, in the North Africa and Middle East region in 1990 and 2019 and the percentages of change in this period.
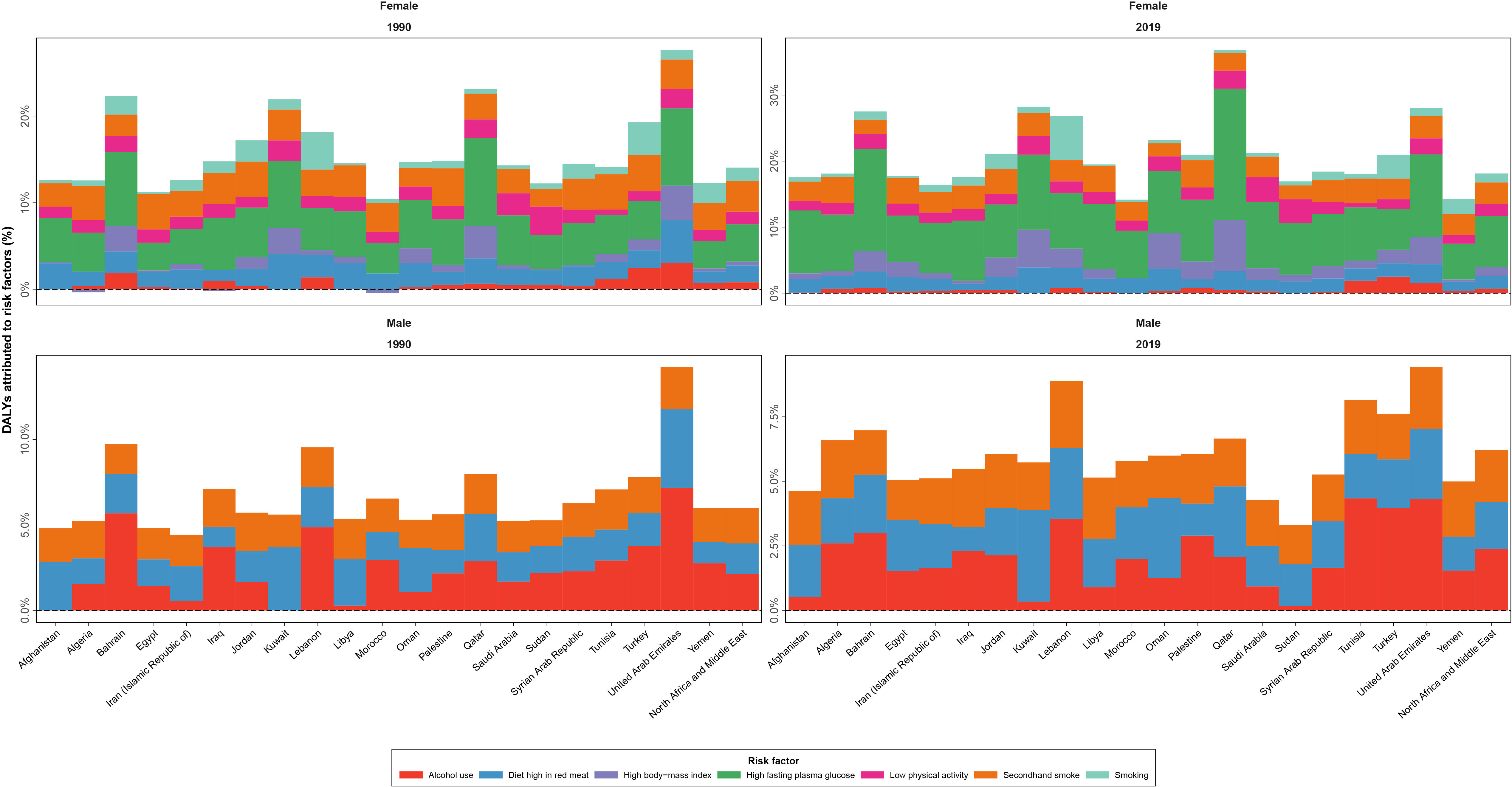
Figure 6 Disability-adjusted life years (DALYs) of breast cancer attributed to various risk factors contributing to this cancer by sex, in 1990 and 2019, in countries of the North Africa and Middle East region.
The responsible risk factors for BC in male patients in GBD 2019 only included alcohol use, secondhand smoke, and diet high in red meat. The all-ages DALYs and deaths attributable to these risk factors were estimated to be approximately 1,359 (885 to 1871) and 48 (30 to 68) in 2019 in the NAME region. The attributable DALYs to alcohol use was the highest (573 [418 to 769]), followed by secondhand smoke (415 [95 to 742]), and diet high in red meat (401 [88 to 610]). The countries with the highest attributable DALY rates for BC in male patients’ risk factors in 2019 were Algeria (2.2 [1.3 to 3.5]), United Arab Emirates (1.6 [0.7 to 3.1]), and Oman (1.6 [0.8 to 2.4]), while Egypt (0.08 [0.04 to 0.14]), Syrian Arab Republic (0.1 [0.05 to 0.15]), and Saudi Arabia (0.2 [0.1 to 0.3]) had the lowest rates. However, regarding the change of attributable DALY rates change during the study period, Palestine (33.9% [−20.5 to 133.4]), Egypt (30.0% [−25.1 to 112.8]), and Iraq (19.8% [−30.6 to 110.8]) had the most immense increase, while Sudan (−49.3% [−75.3 to −1.1]), United Arab Emirates (−47.2% [−75.3 to 1.6]), and Qatar (−46.2% [−72.5 to 5.0]) had the highest decreases in this measure.
The major findings of this study on BC status in the female patients of the NAME region during 1990 to 2019 were the almost doubling of incidence and prevalence rates and the three times increased death and YLLs rates caused by BC. Increase in the age-specific incidence rates was the main contributor to the increasing incidence of BC rather than population growth and aging. Behavioral risk factors were recognized as the greatest burden of female BC among all risk factors. However, high FPG as a metabolic risk factor was found to have the largest impact in burden of BC among women inspecting each risk factor. We found BC epidemiology to be alarming in Lebanon, Qatar, and Bahrain in terms of incidence and in Afghanistan, Yemen, and Sudan in terms of higher lethality and worst care given to patients with BC.
This study showed increasing trends of incidence and deaths due to BC in the NAME region, in both female and male patients. Changes in incidence and deaths of BC in the past decades are attributed to various causes. The increasing trend of BC incidence is thought to be due to expanded life expectancies resulting from improvement in healthcare systems, increased age of first pregnancy, earlier menarche, decreasing prevalence of breast feeding as a protective factor for BC, and increased exposure to BC risk factors like increased smoking, alcohol use, physical inactivity, obesity, and unhealthy dietary habits (10, 41–44). Moreover, improvement in BC screening and diagnosis by implementation of cancer guidelines and better and widespread use of breast imaging techniques, added to the increased knowledge about BC and early screening programs, leads to greater diagnosis of this cancer (7).
The current study found higher incidence and prevalence of BC in countries with higher SDI and HAQ index and vice versa. This major finding was consistent with previous studies that showed that socio-demographic and development status of countries have a substantial impact on BC incidence and survival rates, as both reported to be higher in developed and high-income countries and regions (8, 23, 45, 46). Better primary care, higher care quality, better access to and higher prevalence of screening programs, and other independent factors lead to higher detection, incidence, and higher prevalence of BC due to better protection against mortality due to cancer in wealthier countries with better sociodemographic indicators (2, 47–49). Therefore, development and implementation of cost-effective screening and treatment interventions are necessary to effectively manage BC burden in lower SDI countries (50).
Results of this study revealed higher MIR and YLL/YLD ratio values for both female and male patients with BC in deprived countries of the region and those extremely affected by war and conflict in the past years including Afghanistan, Yemen, Sudan, Syrian Arab Republic, Iraq, and Libya. Interpreting raw epidemiologic measures is prone to underestimation due to lack of data from some countries with limited registries like those with poorly developed data gathering and reporting systems; thus, we used the two aforementioned indices to explore the BC status among the countries included in this study. The higher values of these two indices indicate poorer prognosis of BC in the countries mostly affected by war. Based on these findings, we hypothesize that in such countries, lack of proper screening programs and utilities, diagnosis of cancer in later and advanced stages with reduced curability, and altered cancer care lead to worse disease outcomes, and this is mainly highlighted in the NAME region (51–53). Regarding the overall decreasing trend of the two mentioned indices in the region and countries, it could be inferred that quality of care of BC had an improved status in the past decades in both female and male patients, which could be attributed to improvements in disease management and development of novel therapeutics, better access to treatment, and earlier detection of BC with improved screening and diagnostics (54–56).
Among the investigated BC risk factors in this study, high FPG and secondhand smoking were found to have the greatest burden. An established association between BC incidence and diabetes mellitus has been discovered before, as diabetes shares biologic pathways that can trigger BC, especially in older patients in postmenopausal stages and obese individuals (57–59). In this regard, more robust screening and preventive measures, and even pharmacological ones like metformin prescription interventions in a diabetic population, have shown promising results and are necessary to effectively prevent BC (60, 61). Also, the proven association between active smoking and BC, specifically smoking before first birth, is shown in literature (62–64). Interestingly, the current study found secondhand smoke to be a major risk factor of BC in both female and male patients. Similarly, recent studies also found the relation between passive smoking and risk of BC, and this highlights the importance of protecting women at a higher risk of BC due to exposure to secondhand smoke (42, 65).
A thorough investigation in the countries of the NAME region reveals practical points regarding the epidemiology of BC and the attributable risks of this condition. Many countries located in this region are developing, low- and middle-income countries (LMICs) coping with major obstacles to public health improvement, such as war and conflict and shortages in resources and infrastructures, leading to sub-optimal BC detection and care, including management and treatment (17, 66, 67). The poor prognosis of BC in these countries is mainly due to the detection of disease in advanced stages ending in poor outcomes and lower survival rates (68, 69). The 10-year survival rates of BC in this region vary between countries of the region and in comparison to developed countries (70). The frequent presence of the BRCA1 gene mutations in some countries of this region, which is a major risk factor of BC incidence, should be noted and various screening programs should be implemented to handle the burden of BC in NAME (71, 72). Multiple barriers to early access to screening and care for BC in different health system, health provider, and patient levels in the NAME region need to be addressed to improve BC status in this region (18). Psychological impact and socio-cultural issues are also major problems that influence the care of patients with BC in this region (73).
The importance of BC globally and regionally yielded many initiatives and preventive and surveillance programs to improve BC status. One of the most known ones is the National Comprehensive Cancer Network (NCCN), a union of leading cancer centers in the United States that provides cancer standard of care guidelines—named the NCCN Clinical Practice Guidelines in Oncology—for many cancers, including BC (74, 75). This guideline generally provides the most recent evidence-based information on the basics of BC, testing for BC, and treatment options of the condition (74). In order to implement the mentioned guidelines in the NAME region, the NCCN-MENA initiative first adopted the 2009 version of the guideline and modified it based on regional characteristics, especially risk factor-related items, since the profile varies among regions (10, 76). Development and update of such practical guidelines could successfully lead to a better care of BC in this region. Another example is the Breast Health Global Initiative (BHGI) that tries to control incidence and burden of BC in LMICs through behavior modification, reducing BC mortality by earlier detection of the condition, promotion of BC awareness, education, and self-examination, and modifying BC screening and treatment programs based on countries’ resources (17). A comprehensive framework developed for national BC control strategies mainly in countries located in Asia, Latin America, and the NAME region, proposed that four major actions are needed to successfully implement such framework, including building necessary infrastructures, expanding evidence, eliminating obstacles, and promoting patient support (21). Another promising progress in BC control in NAME was the implementation of a regional framework on cancer prevention and control in 2017, proposing public health approaches to regional policymakers to effectively control the most prevalent cancers of the region including BC, cervical cancer, and colorectal cancer (77). Also, the WHO’s regional guide on early detection of the most common cancers provides clear guidance on BC early detection approaches in line with local resources (78).
A more in-depth investigation of ongoing BC screening programs in the countries of this region using the WHO GHO database showed active programs in all countries in 2019 except for Afghanistan, Libya, Sudan, and Yemen. Also, this database revealed that except for Jordan, Libya, Syrian Arab Republic, and Yemen, all countries of this region had an operational policy/strategy/action plan for cancer in 2019. Additionally, a population-based cancer registry was available in all countries of the region except for Libya, Syrian Arab Republic, and Yemen in 2019 (35). Additional data on these indicators in different years are provided in Supplementary Tables 8–10. Measuring the estimated overall UHC index and the specific index on BC treatment showed the highest values for Kuwait, Qatar, and Lebanon and the lowest in Afghanistan, Yemen, and Sudan in 2019 (Supplementary Table 11) (36). The supplied material could help health authorities and policymakers in providing further actions to control the BC epidemic in the region.
The major limitations of this study include the nature of the main endeavor by IHME to estimate epidemiology and burden of BC and its risk factors. Availability and quality of the primary data and data processing and modeling complications are the main challenges in data estimation in GBD studies (33, 34). Despite these limitations, IHME tries to reassess and reevaluate its data processing method for each round of study, and the refinements lead to more stability of provided data. For the GBD 2019 study, for example, the clarification of the reference and alternative methods for measuring outcomes and the bias mapping from alternative to reference ones and enhancements in modeling by implementation of standard locations for estimating effects in models were conducted to produce the most precise estimations (33, 34). Regarding the BC epidemiologic measures, lack of data on various histopathologic subtypes of this cancer in the GBD study is the major shortcoming of this study. In GBD 2019 risk studies, reassessments of dose–response relationships and further investigations on the joint effects of risk factors were taken to make the risk estimations more robust (34). Regarding the included risk factors in this study, the major concern is the numerous risks of BC missed in the GBD study including genetic risk factors, BRCA, family history, radiation, hormone therapy, breastfeeding, early menstruation or late menopausal age, and number of children. Furthermore, another limitation of this study is the absence of estimation on some of the risk factors due to data scarcity, which complicates BC burden estimation in male population. Considering all these limitations, IHME tries to provide the most accurate epidemiologic data on diseases and risk factors through its meticulous methodologies. This study is a part of this project and explored BC epidemiology in the NAME region and provided data in the regional and national levels. Results of this study could successfully guide health authorities in multiple levels to adopt proper policies and needed actions to control the growing burden of BC in this region.
BC receives a special consideration when investigating the status of cancer in the NAME region. The increasing incidence and burden rates of BC in this region is remarkable, especially when considering limited resources and policies in the developing countries of this region, which make up a great proportion. Also, considering the presence of war and conflict in a number of countries in this region, the BC epidemic is prone to underestimation and mismanagement. Further investigations and careful resource allocations besides proper population-wide policies are needed to effectively mitigate the growing burden of this cancer in countries located in this region.
Publicly available datasets were analyzed in this study. This data can be found here: the Global Burden of Disease study data repository [https://vizhub.healthdata.org/gbd-results/].
The studies involving human participants were reviewed and approved by the institutional review board of the Endocrinology and Metabolism Research Institute at Tehran University of Medical Sciences (IR.TUMS.EMRI.REC.1400.031). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication. Please see the Supplementary Material (pages 44-45) for more detailed information about individual author contributions to the research, divided into the following categories: providing data or critical feedback on data sources; developing methods or computational machinery; providing critical feedback on methods or results; drafting the work or revising is critically for important intellectual content; and managing the estimation or publications process.
As a part of the Global Burden of Disease study, this study was funded by the Bill & Melinda Gates Foundation. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of this publication.
J M Acuna acknowledges academic support from Universidad Espiritu Santo College of Medicine. S Aljunid acknowledges the Department of Health Policy and Management, College of Public Health, Kuwait University and International Centre for Casemix and Clinical Coding, Faculty of Medicine, National University of Malaysia for the approval and support to participate in this research project. S Haque acknowledges the Deanship of Scientific Research, Jazan University, Saudi Arabia for providing the access of the Saudi Digital Library for this research work. Z Piracha and U Saeed acknowledge The International Center of Medical Sciences Research (ICMSR), Islamabad (44000) Pakistan. S Saeedi Moghaddam acknowledges the financial support by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2167-390884018. A M Samy acknowledges the support from the Egyptian Fulbright Mission program and Ain Shams University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1132816/full#supplementary-material
1. Global Burden of Disease 2019 Cancer Collaboration. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the global burden of disease study 2019. JAMA Oncol (2022) 8(3):420–44. doi: 10.1001/jamaoncol.2021.6987
2. Azadnajafabad S, Saeedi Moghaddam S, Keykhaei M, Shobeiri P, Rezaei N, Ghasemi E, et al. Expansion of the quality of care index on breast cancer and its risk factors using the Global Burden of Disease Study 2019. Cancer Med (2022) 12(2):1729–43. doi: 10.1002/cam4.4951
3. Azamjah N, Soltan-Zadeh Y, Zayeri F. Global trend of breast cancer mortality rate: A 25-year study. Asian Pacific J Cancer prevent: APJCP. (2019) 20(7):2015–20. doi: 10.31557/APJCP.2019.20.7.2015
4. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
5. GBD 2019 Adolescent Young Adult Cancer Collaborators. The global burden of adolescent and young adult cancer in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol (2022) 23(1):27–52. doi: 10.1016/s1470-2045(21)00581-7
6. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
7. Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res (2017) 50(1):33. doi: 10.1186/s40659-017-0140-9
8. Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pacific J Cancer prevent: APJCP. (2016) 17(S3):43–6. doi: 10.7314/APJCP.2016.17.S3.43
9. GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010-19: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London England) (2022) 400(10352):563–91. doi: 10.1016/S0140-6736(22)01438-6
10. Abulkhair O, Saghir N, Sedky L, Saadedin A, Elzahwary H, Siddiqui N, et al. Modification and implementation of NCCN guidelines on breast cancer in the Middle East and North Africa region. J Natl Compr Cancer Network: JNCCN. (2010) 8 Suppl 3:S8–S15. doi: 10.6004/jnccn.2010.0126
11. Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YC, et al. Breast cancer screening for women at average risk: 2015 guideline update from the american cancer society. Jama (2015) 314(15):1599–614. doi: 10.1001/jama.2015.12783
12. Tabár L, Dean PB, Chen TH, Yen AM, Chen SL, Fann JC, et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer (2019) 125(4):515–23. doi: 10.1002/cncr.31840
13. Fadhil I, Lyons G, Payne S. Barriers to, and opportunities for, palliative care development in the Eastern Mediterranean Region. Lancet Oncol (2017) 18(3):e176–e84. doi: 10.1016/S1470-2045(17)30101-8
14. Kulhanova I, Bray F, Fadhil I, Al-Zahrani AS, El-Basmy A, Anwar WA, et al. Profile of cancer in the Eastern Mediterranean region: The need for action. Cancer Epidemiol (2017) 47:125–32. doi: 10.1016/j.canep.2017.01.009
15. Pilleron S, Sarfati D, Janssen-Heijnen M, Vignat J, Ferlay J, Bray F, et al. Global cancer incidence in older adults, 2012 and 2035: A population-based study. Int J cancer. (2019) 144(1):49–58. doi: 10.1002/ijc.31664
16. Eastern Mediterranean Region Cancer Collaborators GBD. Burden of cancer in the Eastern Mediterranean Region, 2005-2015: findings from the Global Burden of Disease 2015 Study. Int J Public Health (2018) 63(Suppl 1):151–64. doi: 10.1007/s00038-017-0999-9
17. Anderson BO, Yip CH, Smith RA, Shyyan R, Sener SF, Eniu A, et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer (2008) 113(8 Suppl):2221–43. doi: 10.1002/cncr.23844
18. Bowser D, Marqusee H, El Koussa M, Atun R. Health system barriers and enablers to early access to breast cancer screening, detection, and diagnosis: a global analysis applied to the MENA region. Public Health (2017) 152:58–74. doi: 10.1016/j.puhe.2017.07.020
19. Mohammadi E, Aminorroaya A, Fattahi N, Azadnajafabad S, Rezaei N, Farzi Y, et al. Epidemiologic pattern of cancers in Iran; current knowledge and future perspective. J Diabetes Metab Disord (2021) 20(1):825–9. doi: 10.1007/s40200-020-00654-6
20. Rastad H, Khanjani N, Khandani BK. Causes of delay in seeking treatment in patients with breast cancer in Iran: a qualitative content analysis study. Asian Pacific J Cancer prevent: APJCP. (2012) 13(9):4511–5. doi: 10.7314/APJCP.2012.13.9.4511
21. Bridges JF, Anderson BO, Buzaid AC, Jazieh AR, Niessen LW, Blauvelt BM, et al. Identifying important breast cancer control strategies in Asia, Latin America and the Middle East/North Africa. BMC Health Serv Res (2011) 11:227. doi: 10.1186/1472-6963-11-227
22. GBD 2019 Iran Collaborators. Health system performance in Iran: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London England) (2022) 399(10335):1625–45. doi: 10.1016/S0140-6736(21)02751-3
23. Chen Z, Xu L, Shi W, Zeng F, Zhuo R, Hao X, et al. Trends of female and male breast cancer incidence at the global, regional, and national levels, 1990-2017. Breast Cancer Res Treat (2020) 180(2):481–90. doi: 10.1007/s10549-020-05561-1
24. Corbex M, Bouzbid S, Boffetta P. Features of breast cancer in developing countries, examples from North-Africa. Eur J Cancer (Oxford England: 1990). (2014) 50(10):1808–18. doi: 10.1016/j.ejca.2014.03.016
25. Gucalp A, Traina TA, Eisner JR, Parker JS, Selitsky SR, Park BH, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat (2019) 173(1):37–48. doi: 10.1007/s10549-018-4921-9
26. Abdelwahab Yousef AJ. Male breast cancer: epidemiology and risk factors. Semin Oncol (2017) 44(4):267–72. doi: 10.1053/j.seminoncol.2017.11.002
27. Ferzoco RM, Ruddy KJ. The epidemiology of male breast cancer. Curr Oncol Rep (2016) 18(1):1. doi: 10.1007/s11912-015-0487-4
28. Sarmiento S, McColl M, Musavi L, Gani F, Canner JK, Jacobs L, et al. Male breast cancer: a closer look at patient and tumor characteristics and factors that affect survival using the National Cancer Database. Breast Cancer Res Treat (2020) 180(2):471–9. doi: 10.1007/s10549-020-05556-y
29. Khan NAJ, Tirona M. An updated review of epidemiology, risk factors, and management of male breast cancer. Med Oncol (Northwood London England). (2021) 38(4):39. doi: 10.1007/s12032-021-01486-x
30. Bendardaf R, Saheb Sharif-Askari F, Saheb Sharif-Askari N, Yousuf Guraya S, AA S, Abusnana S. Incidence and clinicopathological features of breast cancer in the northern emirates: experience from sharjah breast care center. Int J Womens Health (2020) 12:893–9. doi: 10.2147/IJWH.S266335
31. Aryannejad A, Saeedi Moghaddam S, Mashinchi B, Tabary M, Rezaei N, Shahin S, et al. National and subnational burden of female and male breast cancer and risk factors in Iran from 1990 to 2019: results from the Global Burden of Disease study 2019. Breast Cancer Res (2023) 25(1):47. doi: 10.1186/s13058-023-01633-4
32. Global Burden of Disease Study 2019 (GBD 2019) Results [Internet]. Institute for Health Metrics and Evaluation (IHME) (2020). Available at: http://ghdx.healthdata.org/gbd-results-tool.
33. GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London England) (2020) 396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9
34. GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London England) (2020) 396(10258):1223–49. doi: 10.1016/S0140-6736(20)30752-2
35. THE GLOBAL HEALTH OBSERVATORY. World Health Organization (2021). Available at: https://www.who.int/data/gho.
36. GBD 2019 Universal Health Coverage Collaborators. Measuring universal health coverage based on an index of effective coverage of health services in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet (London England) (2020) 396(10258):1250–84. doi: 10.1016/S0140-6736(20)30750-9
37. Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet (London England). (2016) 388(10062):e19–23. doi: 10.1016/S0140-6736(16)30388-9
38. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol (2019) 5(12):1749–68. doi: 10.1001/jamaoncol.2019.2996
39. GBD 2016 Healthcare Access and Quality Collaborators. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet (London England) (2018) 391(10136):2236–71. doi: 10.1016/S0140-6736(18)30994-2
40. Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol (2017) 3(4):524–48. doi: 10.1001/jamaoncol.2016.5688
41. Boffetta P, Hashibe M, La Vecchia C, Zatonski W, Rehm J. The burden of cancer attributable to alcohol drinking. Int J cancer. (2006) 119(4):884–7. doi: 10.1002/ijc.21903
42. Carreras G, Lachi A, Boffi R, Clancy L, Gallus S, Fernandez E, et al. Burden of disease from breast cancer attributable to smoking and second-hand smoke exposure in Europe. Int J cancer. (2020) 147(9):2387–93. doi: 10.1002/ijc.33021
43. Chajes V, Romieu I. Nutrition and breast cancer. Maturitas (2014) 77(1):7–11. doi: 10.1016/j.maturitas.2013.10.004
44. Hashim MJ, Al-Shamsi FA, Al-Marzooqi NA, Al-Qasemi SS, Mokdad AH, Khan G. Burden of breast cancer in the arab world: findings from global burden of disease, 2016. J Epidemiol Global Health (2018) 8(1-2):54–8. doi: 10.2991/j.jegh.2018.09.003
45. Coleman MP, Quaresma M, Berrino F, Lutz JM, De Angelis R, Capocaccia R, et al. Cancer survival in five continents: a worldwide population-based study (CONCORD). Lancet Oncol (2008) 9(8):730–56. doi: 10.1016/S1470-2045(08)70179-7
46. Ji P, Gong Y, Jin ML, Hu X, Di GH, Shao ZM. The burden and trends of breast cancer from 1990 to 2017 at the global, regional, and national levels: results from the global burden of disease study 2017. Front Oncol (2020) 10:650. doi: 10.3389/fonc.2020.00650
47. Coccia M. The effect of country wealth on incidence of breast cancer. Breast Cancer Res Treat (2013) 141(2):225–9. doi: 10.1007/s10549-013-2683-y
48. Seneviratne S, Lawrenson R, Harvey V, Ramsaroop R, Elwood M, Scott N, et al. Stage of breast cancer at diagnosis in New Zealand: impacts of socio-demographic factors, breast cancer screening and biology. BMC cancer. (2016) 16:129. doi: 10.1186/s12885-016-2177-5
49. Azadnajafabad S, Saeedi Moghaddam S, Mohammadi E, Delazar S, Rashedi S, Baradaran HR, et al. Patterns of better breast cancer care in countries with higher human development index and healthcare expenditure: Insights from GLOBOCAN 2020. Front Public Health (2023) 11:1137286. doi: 10.3389/fpubh.2023.1137286
50. Hu K, Ding P, Wu Y, Tian W, Pan T, Zhang S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: an observational study based on the global burden of diseases. BMJ Open (2019) 9(10):e028461. doi: 10.1136/bmjopen-2018-028461
51. El Saghir NS, Soto Pérez de Celis E, Fares JE, Sullivan R. Cancer care for refugees and displaced populations: middle east conflicts and global natural disasters. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meeting. (2018) 38:433–40. doi: 10.1200/EDBK_201365
52. Mula-Hussain L, Alabedi H, Al-Alloosh F, Alharganee A. Cancer in war-torn countries: Iraq as an example. Laher I. (eds) In: Handbook of Healthcare in the Arab World (2021) Springer International Publishing, Cham. p. 481–94. doi: 10.1007/978-3-319-74365-3_152-1
53. Sahloul E, Salem R, Alrez W, Alkarim T, Sukari A, Maziak W, et al. Cancer care at times of crisis and war: the syrian example. J Global Oncol (2017) 3(4):338–45. doi: 10.1200/JGO.2016.006189
54. Ginsburg O, Yip CH, Brooks A, Cabanes A, Caleffi M, Dunstan Yataco JA, et al. Breast cancer early detection: A phased approach to implementation. Cancer (2020) 126 Suppl 10(Suppl 10):2379–93. doi: 10.1002/cncr.32887
55. Mutebi M, Anderson BO, Duggan C, Adebamowo C, Agarwal G, Ali Z, et al. Breast cancer treatment: A phased approach to implementation. Cancer (2020) 126 Suppl 10:2365–78. doi: 10.1002/cncr.32910
56. Rositch AF, Unger-Saldaña K, DeBoer RJ, Ng’ang’a A, Weiner BJ. The role of dissemination and implementation science in global breast cancer control programs: Frameworks, methods, and examples. Cancer (2020) 126 Suppl 10:2394–404. doi: 10.1002/cncr.32877
57. Boyle P, Boniol M, Koechlin A, Robertson C, Valentini F, Coppens K, et al. Diabetes and breast cancer risk: a meta-analysis. Br J cancer. (2012) 107(9):1608–17. doi: 10.1038/bjc.2012.414
58. Liao S, Li J, Wei W, Wang L, Zhang Y, Li J, et al. Association between diabetes mellitus and breast cancer risk: a meta-analysis of the literature. Asian Pacific J Cancer prevent: APJCP. (2011) 12(4):1061–5.
59. Wolf I, Sadetzki S, Catane R, Karasik A, Kaufman B. Diabetes mellitus and breast cancer. Lancet Oncol (2005) 6(2):103–11. doi: 10.1016/S1470-2045(05)01736-5
60. Bosco JL, Antonsen S, Sørensen HT, Pedersen L, Lash TL. Metformin and incident breast cancer among diabetic women: a population-based case-control study in Denmark. Cancer Epidemiol Biomarkers prevent: Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2011) 20(1):101–11. doi: 10.1158/1055-9965.EPI-10-0817
61. Hatoum D, McGowan EM. Recent advances in the use of metformin: can treating diabetes prevent breast cancer? BioMed Res Int (2015) 2015:548436. doi: 10.1155/2015/548436
62. Andersen ZJ, Jørgensen JT, Grøn R, Brauner EV, Lynge E. Active smoking and risk of breast cancer in a Danish nurse cohort study. BMC cancer. (2017) 17(1):556. doi: 10.1186/s12885-017-3546-4
63. Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Institute. (2013) 105(8):515–25. doi: 10.1093/jnci/djt023
64. Reynolds P. Smoking and breast cancer. J mammary gland Biol neoplasia. (2013) 18(1):15–23. doi: 10.1007/s10911-012-9269-x
65. Arafat HM, Omar J, Muhamad R, Al-Astani TAD, Shafii N, Al Laham NA, et al. Breast cancer risk from modifiable and non-modifiable risk factors among palestinian women: A systematic review and meta-analysis. Asian Pacific J Cancer prevent: APJCP. (2021) 22(7):1987–95. doi: 10.31557/APJCP.2021.22.7.1987
66. Fan L, Goss PE, Strasser-Weippl K. Current status and future projections of breast cancer in asia. Breast Care (Basel Switzerland). (2015) 10(6):372–8. doi: 10.1159/000441818
67. Kulhanova I, Znaor A, Shield KD, Arnold M, Vignat J, Charafeddine M, et al. Proportion of cancers attributable to major lifestyle and environmental risk factors in the Eastern Mediterranean region. Int J cancer. (2020) 146(3):646–56. doi: 10.1002/ijc.32284
68. Hisham AN, Yip CH. Spectrum of breast cancer in Malaysian women: overview. World J surgery. (2003) 27(8):921–3. doi: 10.1007/s00268-003-6976-x
69. Cecilio AP, Takakura ET, Jumes JJ, Dos Santos JW, Herrera AC, Victorino VJ, et al. Breast cancer in Brazil: epidemiology and treatment challenges. Breast Cancer (Dove Med Press). (2015) 7:43–9. doi: 10.2147/BCTT.S50361
70. Hassanipour S, Maghsoudi A, Rezaeian S, Arab-Zozani M, Mokhtari AM, Abdzadeh E, et al. Survival rate of breast cancer in eastern mediterranean region countries: A systematic review and meta-analysis. Ann Global Health (2019) 85(1):138. doi: 10.5334/aogh.2521
71. Laraqui A, Uhrhammer N, Rhaffouli HE, Sekhsokh Y, Lahlou-Amine I, Bajjou T, et al. BRCA genetic screening in Middle Eastern and North African: mutational spectrum and founder BRCA1 mutation (c. 798_799delTT) North African. Dis Markers (2015) 2015:194293. doi: 10.1155/2015/194293
72. Laitman Y, Friebel TM, Yannoukakos D, Fostira F, Konstantopoulou I, Figlioli G, et al. The spectrum of BRCA1 and BRCA2 pathogenic sequence variants in Middle Eastern, North African, and South European countries. Hum mutation. (2019) 40(11):e1–e23. doi: 10.1002/humu.23842
73. Salem H, Daher-Nashif S. Psychosocial aspects of female breast cancer in the middle east and north africa. Int J Environ Res Public Health (2020) 17(18):1–16. doi: 10.3390/ijerph17186802
74. Telli ML, Gradishar WJ, Ward JH. NCCN guidelines updates: breast cancer. J Natl Compr Cancer Network: JNCCN. (2019) 17(5.5):552–5. doi: 10.6004/jnccn.2019.5006
75. Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Network: JNCCN (2020) 18(4):452–78. doi: 10.6004/jnccn.2020.0016
76. Jazieh AR, Azim HA, McClure J, Jahanzeb M. The process of NCCN guidelines adaptation to the Middle East and North Africa region. J Natl Compr Cancer Network: JNCCN. (2010) 8 Suppl 3:S5–7. doi: 10.6004/jnccn.2010.0125
77. Pourghazian N, Sankaranarayanan R, Alhomoud S, Slama S. Strengthening the early detection of common cancers in the Eastern Mediterranean Region. Eastern Mediterr Health J = La Rev sante la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit. (2019) 25(11):767–8. doi: 10.26719/2019.25.11.767
78. World Health Organization. Early detection (2007). Available at: https://apps.who.int/iris/bitstream/handle/10665/43743/9241547338_eng.pdf.
Keywords: breast cancer, epidemiology, risk factor, Global Burden of Disease, Middle East, North Africa
Citation: Azadnajafabad S, Saeedi Moghaddam S, Mohammadi E, Rezaei N, Rashidi M-M, Rezaei N, Mokdad AH, Naghavi M, Murray CJL, Larijani B, Farzadfar F and GBD 2019 NAME Breast Cancer Collaborators (2023) Burden of breast cancer and attributable risk factors in the North Africa and Middle East region, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Front. Oncol. 13:1132816. doi: 10.3389/fonc.2023.1132816
Received: 28 December 2022; Accepted: 10 July 2023;
Published: 01 August 2023.
Edited by:
Maria Paula Curado, A.C.Camargo Cancer Center, BrazilReviewed by:
Gary Zirpoli, Boston University, United StatesCopyright © 2023 Azadnajafabad, Saeedi Moghaddam, Mohammadi, Rezaei, Rashidi, Rezaei, Mokdad, Naghavi, Murray, Larijani, Farzadfar and GBD 2019 NAME Breast Cancer Collaborators. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farshad Farzadfar, ZmFyemFkZmFyM0B5YWhvby5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.