
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 13 June 2023
Sec. Hematologic Malignancies
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1127112
 Peipei Yang†
Peipei Yang† Yali Tao†
Yali Tao† Ailin Zhao†
Ailin Zhao† Kai Shen
Kai Shen He Li
He Li Jinjin Wang
Jinjin Wang Hui Zhou
Hui Zhou Zhongwang Wang
Zhongwang Wang Mengyao Wang
Mengyao Wang Ying Qu
Ying Qu Li Zhang
Li Zhang Yuhuan Zheng
Yuhuan Zheng Ting Niu*
Ting Niu*Background: The overall survival of peripheral T-cell lymphoma (PTCL) is dismal. Histone deacetylase (HDAC) inhibitors have exhibited promising treatment outcomes for PTCL patients. Therefore, this work aims to systematically evaluate the treatment outcome and safety profile of HDAC inhibitor-based treatment for untreated and relapsed/refractory (R/R) PTCL patients.
Methods: The prospective clinical trials of HDAC inhibitors for the treatment of PTCL were searched on the Web of Science, PubMed, Embase, ClinicalTrials.gov, and Cochrane Library database. The pooled overall response rate, complete response (CR) rate, and partial response rate were measured. The risk of adverse events was evaluated. Moreover, the subgroup analysis was utilized to assess the efficacy among different HDAC inhibitors and efficacy in different PTCL subtypes.
Results: For untreated PTCL, 502 patients in seven studies were involved, and the pooled CR rate was 44% (95% CI, 39-48%). For R/R PTCL patients, there were 16 studies included, and the CR rate was 14% (95% CI, 11-16%). The HDAC inhibitor-based combination therapy exhibited better efficacy when compared with HDAC inhibitor monotherapy for R/R PTCL patients (P = 0.02). In addition, the pooled CR rate was 17% (95% CI, 13-22%), 10% (95% CI, 5-15%), and 10% (95% CI, 5-15%) in the romidepsin, belinostat, and chidamide monotherapy subgroups, respectively. In the R/R angioimmunoblastic T-cell lymphoma subgroup, the pooled ORR was 44% (95% CI, 35-53%), higher than other subtypes. A total of 18 studies were involved in the safety assessment of treatment-related adverse events. Thrombocytopenia and nausea were the most common hematological and non-hematological adverse events, respectively.
Conclusion: This meta-analysis demonstrated that HDAC inhibitors were effective treatment options for untreated and R/R PTCL patients. The combination of HDAC inhibitor and chemotherapy exhibited superior efficacy to HDAC inhibitor monotherapy in the R/R PTCL setting. Additionally, HDAC inhibitor-based therapy had higher efficacy in angioimmunoblastic T-cell lymphoma patients than that in other subtypes.
Peripheral T-cell lymphoma (PTCL) is a non-Hodgkin lymphoma that originates from post-thymic T-cells and presents with high heterogeneity. As stated by the latest classification of lymphoid neoplasms, PTCLs comprise various subtypes that exhibit different and complex clinicopathologic manifestations (1). Common subtypes include PTCL not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL), and anaplastic lymphoma kinase-negative anaplastic large-cell lymphoma (ALCL, ALK-neg). Most PTCLs exhibit an aggressive clinical course and inferior prognosis. A study that enrolled 166 untreated PTCL patients with a median follow-up of five years indicated that the rate of five-year overall survival (OS) of PTCL patients was 51% (2). In relapsed/refractory (R/R) PTCL patients, the median progression-free survival and median OS were 3.1 months and 5.5 months, respectively, as reported by a retrospective analysis (3). In a prospective study, the median OS of PTCL patients was only 5.8 months after relapse, demonstrating a dismal outcome (4).
The current frontline treatment of PTCL involves combination chemotherapy, typically including CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimen. Brentuximab vedotin has also been approved for the treatment of CD30-positive PTCL. Compared to CHOP, adding brentuximab vedotin to the CHP (cyclophosphamide, doxorubicin, prednisone) regimen has shown a meaningful improvement in the 5-year OS rate for CD30-positive PTCL patients, with a rate of 70.1% versus 61.0% (5). However, for subtypes such as PTCL-NOS and AITL, the prognosis remains unsatisfying. Therefore, there is a critical need for advanced therapeutic agents to improve the outcome.
Histone deacetylases (HDACs) are indispensable to controlling protein deacetylation in cells. They play a vital role in the epigenetic modulation of biological processes and regulate protein degradation (6, 7). However, the abnormal activity of HDACs is often related to disease progression (8), leading to increased attention to HDACs as therapeutic targets (9). Preclinical studies have demonstrated that HDAC inhibitors can inhibit tumor cell growth by apoptosis, cell cycle arrest, and cytokine regulation (10, 11). In addition, HDAC inhibitors exhibited antitumor efficacy in solid tumors and hematological malignancies (12, 13). Romidepsin is the first HDAC inhibitor approved for R/R PTCL across all subtypes. Romidepsin primarily inhibits class I HDACs and has a weak effect on class IIb HDACs (14). Another HDAC inhibitor, belinostat, can broadly inhibit all zinc-dependent HDAC enzymes and has been indicated for R/R PTCL (15). Chidamide, which can selectively target the catalytic pocket and inhibit class I and IIb HDACs, also showed promising efficacy in R/R PTCL treatment (16).
Clinical studies have been conducted to evaluate the safety and treatment outcomes of HDAC inhibitor monotherapy or HDAC inhibitor combined with conventional regimens for untreated or R/R PTCL patients in recent years. However, a comprehensive and quantitative meta-analysis of prospective trials evaluating the efficacy and safety of HDAC inhibitors is still lacking. This study aims to bridge the gap by providing a quantitative examination of the efficacy of HDAC inhibitor-based treatment for untreated or R/R PTCL patients, respectively. In the subgroup analysis, we compared the efficacy of monotherapy and combination therapy for R/R PTCL. The treatment outcome of different HDAC inhibitors has also been compared. Additionally, the treatment efficacy in different PTCL subtypes has been analyzed, in order to provide reliable evidence for the optimized application of HDAC inhibitors.
This study was reported in obedience to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. In order to identify studies evaluating HDAC inhibitor monotherapy and HDAC inhibitor-based combination therapy in PTCL patients, searches were conducted on various databases including Web of Science, PubMed, Embase, ClinicalTrials.gov, and Cochrane Library. The search strategy employed was related to “lymphoma, t-cell, peripheral” and “histone deacetylase inhibitor”, with detailed strategies outlined in the supplemental material. The retrieval time for the search was November 2022, and no language restrictions were imposed.
Two investigators (PY and YT) screened the search results and determined the eligibility independently. And disagreements were discussed and resolved. The studies which met these features were included (1): prospective clinical trials with large sample size (more than ten patients) (2); adult patients treated with HDAC inhibitors (3); the diagnoses including PTCL; and (4) sufficient data of efficacy and safety assessment. The exclusion criteria were as follows (1): a case report, letter, meeting abstract, or comment (2); studies without raw data on efficacy or safety.
Two investigators (PY and YT) independently collected the data, and resolved any discrepancies through discussion. For each included study, the following information and data were collected, including (1): basic study information, including the title, registration number, year of publication, first authors’ name, and study design (2); key characteristics, including the number of patients, disease state (untreated PTCL patients or R/R PTCL patients), median age and range, disease stage, treatment details, and PTCL subtypes (3); main outcomes, including overall response rate (ORR), complete response (CR) rate, and partial response (PR) rate (4); treatment-related adverse events (AEs) of all grades, and those of grade 3 or higher. For controlled trials, only the data from the HDAC inhibitor-based treatment arm were extracted.
The quality evaluation for the chosen studies was carried out using the Methodological Index for Nonrandomized Studies (MINORS). This index comprises eight items for non-randomized studies and four additional items for comparative studies (17). The scoring criteria were 0, 1, and 2 for not reported, reported but inadequate, and reported and adequate, respectively. Each included study was scored by two independent investigators (JW and HZ). Any discrepancies were resolved by consensus.
The statistical analysis was conducted by Revman 5.4 and STATA14 (Stata Corporation). The I-squared test (I2 test) was applied to the assessment of heterogeneity among the included studies. The I2 values of less than 25%, between 25% and 50%, and higher than 50% were considered as low heterogeneity, moderate heterogeneity, and significant heterogeneity, respectively. Additionally, the Galbraith plot was used to identify potential sources of heterogeneity. For studies with I2 higher than 50%, a random effects model was employed, whereas a fixed effects model was used for those with lower I2 values. To address any heterogeneity, subgroup analyses were performed for monotherapy versus combination therapy, different kinds of HDAC inhibitors, and three PTCL subtypes. Publication bias was assessed using the funnel plot. And the sensitivity analysis was performed to evaluate the robustness of the results.
We initially identified 1465 studies after the removal of 804 duplications. Then, the screen of the titles and abstracts of these studies was performed, and 87 potentially eligible studies were remained for full-text review and determination. Finally, 22 studies were included. The detailed procedure of study selection is shown in Figure 1.
The included studies were conducted from 2011 to 2022, and most of these were phase I or phase II single-arm studies, with only two randomized controlled trials. Among these studies, six enrolled untreated PTCL patients, 15 enrolled R/R PTCL patients, and one enrolled untreated and R/R PTCL patients. The subtypes of PTCL included in these studies were mainly PTCL-NOS, AITL, and ALCL, ALK-neg. In total, 502 untreated patients and 662 R/R patients were involved in our efficacy analysis. For untreated PTCL patients, all of the studies used HDAC inhibitor-based combination therapy, specifically the HDAC inhibitor plus CHOP regimen. Among these studies, three used romidepsin (18–20), one used belinostat (21), and three used chidamide (22–24). For R/R PTCL, nine studies reported HDAC inhibitor monotherapy. Of these, four studies used romidepsin (25–28), two studies used belinostat (29, 30), and three studies used chidamide (12, 31, 32). In addition, seven studies employed combination therapy, including six studies that used romidepsin (20, 33–37) and one study that used panobinostat (38). Table 1 shows the summarized overall characteristics of the 22 included studies.
All the included studies were evaluated by MINORS, and the detailed scores are shown in Figure S1. All of the studies reported the study purpose and successively enrolled patients, and the follow-up period was appropriate. However, most studies did not declare an unbiased endpoint assessment. According to the total scores, seven studies were of high quality (19, 23, 26, 28, 31–33), 11 studies were of moderate quality (12, 18, 21, 22, 24, 27, 29, 30, 35, 36, 38), and four studies were of low quality (20, 25, 34, 37).
Seven studies were analyzed to determine the efficacy of HDAC inhibitor-based combination therapy for untreated PTCL patients, and the studies reported ORR, CR rate, and PR rate. In total, the ORR of the included 502 patients was 72% (95% CI, 63-82%, random effect model). Of the seven studies, one study used belinostat-based treatment and enrolled 21 patients (21), resulting in an ORR of 86%. Three studies used chidamide-based treatment and totally included 224 patients (22–24), and the pooled ORR was 74% (95% CI, 57-92%). Three studies used romidepsin-based treatment and enrolled 257 patients (18–20), resulting in a pooled ORR of 64% (95% CI, 58-70%). The analysis revealed a significant difference in ORR among the three HDAC inhibitor-based combination therapies in untreated PTCL patients (P = 0.02) (Figure S2).
The pooled CR rate was 44% (95% CI, 39-48%, fixed effect model). Upon subgroup analysis, the CR rate for the belinostat-based treatment study was 67%. In the romidepsin-based treatment subgroup, the pooled CR was 43% (95% CI, 37-49%). For the chidamide-based therapy, the CR rate was 42% (95% CI, 36-49%). And the subgroup analysis indicated no statistical difference in CR rate among the different treatment options (P = 0.07) (Figure 2A).
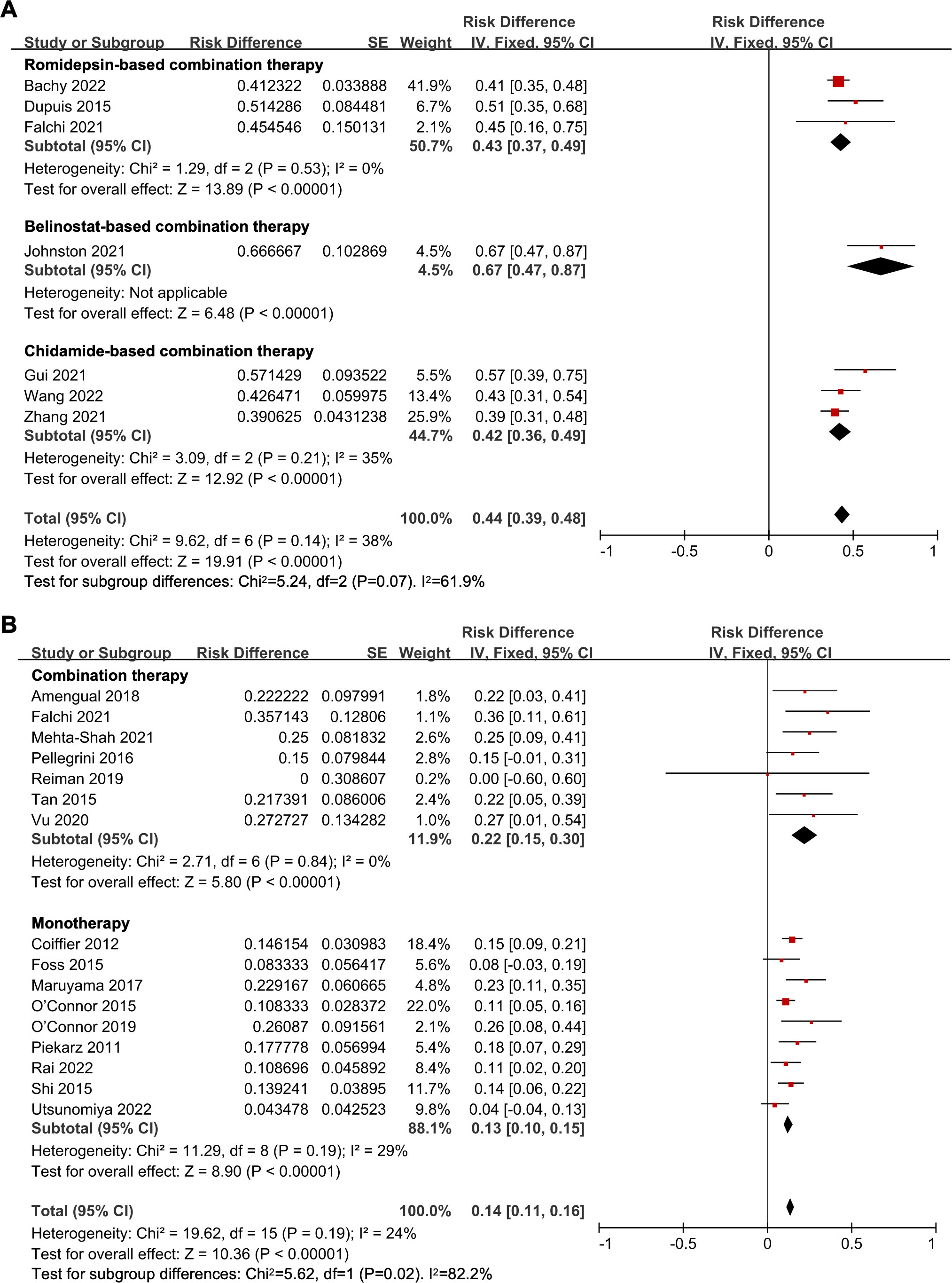
Figure 2 The forest plot of complete response rates in (A) untreated peripheral T-cell lymphoma patients and (B) relapsed/refractory peripheral T-cell lymphoma patients.
Moreover, the pooled PR rate was 22% (95% CI, 19-26%, fixed effect model). For the subgroup analysis, the PR rate for belinostat-based treatment study was 19%. For romidepsin-based treatment, the estimated PR rate was 21% (95% CI, 16-26%). And for chidamide-based treatment group, the estimated PR rate was 24% (95% CI, 19-30%), rendering no significant difference in subgroup analysis (P = 0.62) (Figure S3).
The pooled efficacy analysis for R/R PTCL involved 16 studies. The ORR was 37% (95% CI, 31-42%, random effect model). In the HDAC inhibitor-based combination therapy subgroup, the ORR was higher at 45% (95% CI, 36-54%), whereas the HDAC inhibitor monotherapy subgroup showed a lower ORR at 33% (95% CI, 27-38%). The efficacy of HDAC inhibitor-based combination therapy was found superior to HDAC inhibitor monotherapy for R/R PTCL patients (P = 0.02) (Figure S4). Additionally, in the panobinostat-based combination treatment subgroup, the ORR was 43%. And the ORR of romidepsin-based combination treatment was 46% (95% CI, 36-55%), with no statistical difference between the two subgroups (P = 0.86) (Figure S5). Regarding monotherapy, subgroup analysis indicated that the pooled ORR was 32% (95% CI, 27-38%), 26% (95% CI, 19-33%), and 33% (95% CI, 26-41%) in the romidepsin, belinostat, and chidamide monotherapy subgroups, respectively. And the subgroup analysis revealed no significant difference (P = 0.28) (Figure S6).
The overall pooled CR rate was 14% (95% CI, 11-16%, fixed effect model). In the combination therapy group, the CR rate was 22% (95% CI, 15-30%). And in the HDAC inhibitor monotherapy subgroup, the CR rate was 13% (95% CI, 10-15%), which was significantly lower than that of the combination therapy (P = 0.02) (Figure 2B). The CR rate of panobinostat-based combination therapy was 22%, and the CR rate of romidepsin-based combination therapy was 23% (95% CI, 14-31%), with no statistical difference (P = 0.94) (Figure 3A). In addition, the pooled CR rate for monotherapy was 17% (95% CI, 13-22%), 10% (95% CI, 5-15%), and 10% (95% CI, 5-15%) in the romidepsin, belinostat, and chidamide monotherapy subgroups, respectively. No statistical discrepancy was observed in the CR rate among the three types of HDAC inhibitors (P = 0.06) (Figure 3B).
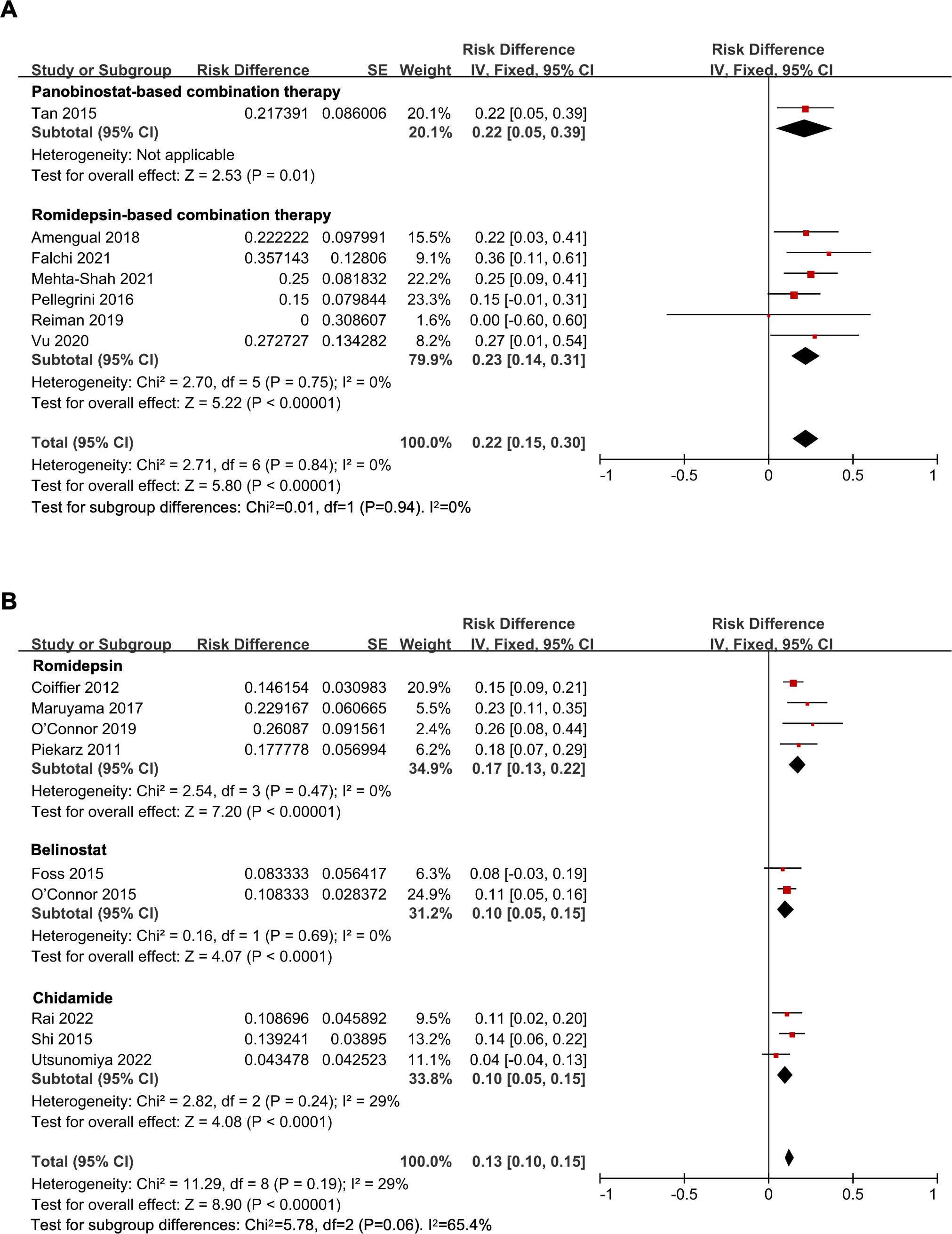
Figure 3 The forest plot of pooled complete response rate of (A) histone deacetylase inhibitor-based combination therapy and (B) histone deacetylase inhibitor monotherapy therapy in relapsed/refractory peripheral T-cell lymphoma patients.
The overall pooled PR rate of the included 16 trials was 17% (95% CI, 14-20%, fixed effect model). In the combination therapy, the PR rate was 24% (95% CI, 16-31%), while the HDAC inhibitor monotherapy subgroup, the PR rate was 16% (95% CI, 13-19%), with no statistical difference (P = 0.06) (Figure S7). The PR rate of the panobinostat-based combination therapy and the romidepsin-based combination therapy was 22% and 24% (95% CI, 16-33%), respectively. This subgroup analysis revealed no significant difference (P = 0.80) (Figure S8). In addition, subgroup analysis of monotherapy indicated that the pooled PR rate were 14% (95% CI, 10-18%), 15% (95% CI, 9-21%), and 20% (95% CI, 13-26%) in the romidepsin, belinostat, and chidamide monotherapy, respectively, without a statistical difference (P = 0.35) (Figure S9).
There were three studies involved in the subtype analysis in untreated PTCL patients (18, 23, 24). The ORR of all 196 patients was 68% (95% CI, 62-75%, fixed effect model). Of these, the pooled ORR in the PTCL-NOS, AITL, and ALCL, ALK-neg subgroups were 58% (95% CI, 45-71%), 71% (95% CI, 63-79%), and 76% (95% CI, 56-97%), respectively. However, no significant difference was observed (P = 0.17) (Figure 4).
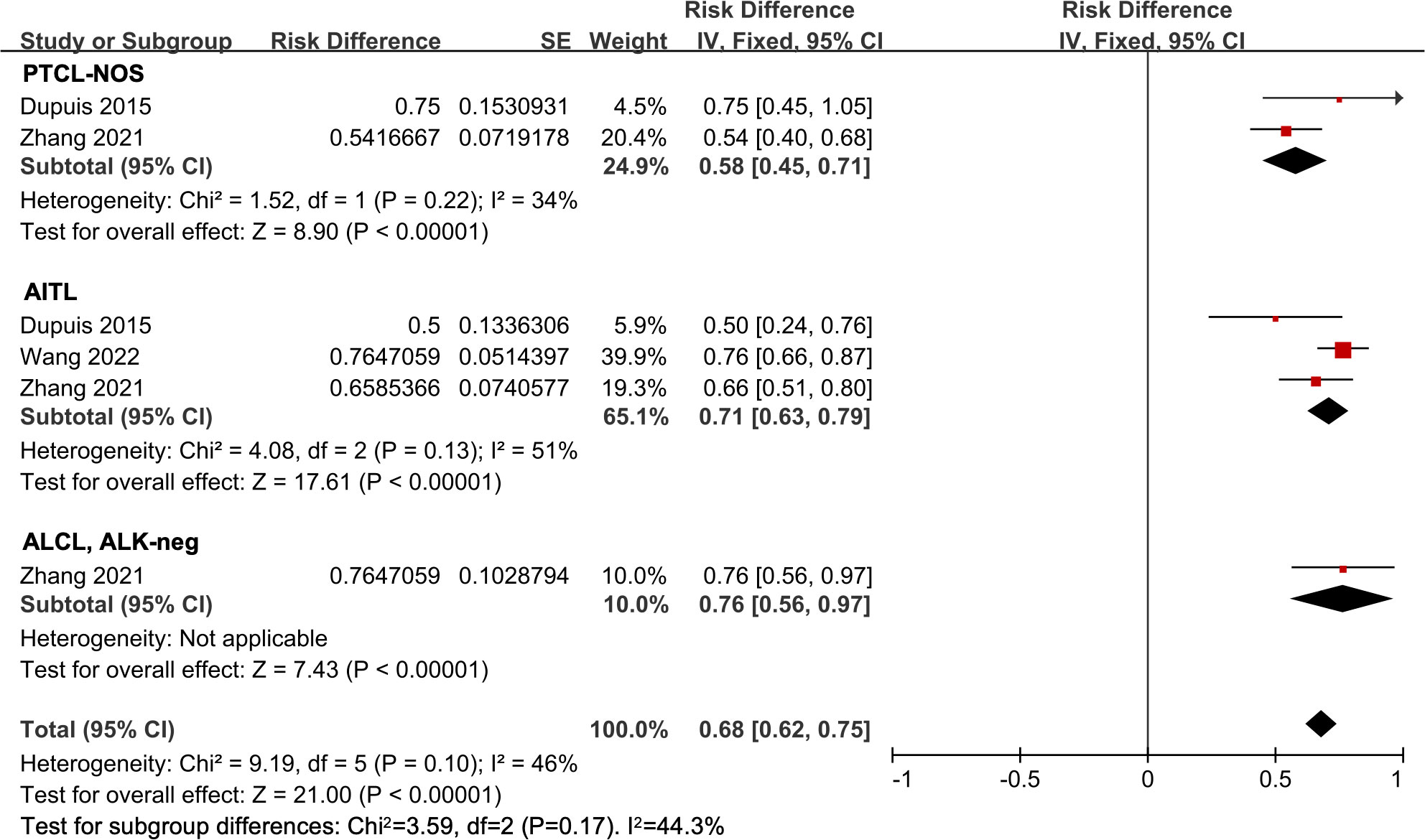
Figure 4 The forest plot of pooled overall response rate in different subtypes in untreated peripheral T-cell lymphoma patients. PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; AITL, angioimmunoblastic T-cell lymphoma; ALCL, ALK-neg, anaplastic lymphoma kinase-negative anaplastic large-cell lymphoma.
Eight studies were included in the histological subtype analysis in R/R PTCL patients (12, 25, 26, 29, 31, 33, 34, 37). The ORR of the all 418 patients was 32% (95% CI, 27-36%, fixed effect model). In the subgroup analysis, the pooled ORR was 29% (95% CI, 23-34%) in the PTCL-NOS patients. The pooled ORR in the ALCL, ALK-negative patients was 27% (95% CI, 16-38%). And in the AITL subgroup, the pooled ORR was 44% (95% CI, 35-53%), indicating a more efficient response compared to other subgroups (P = 0.01) (Figure 5).
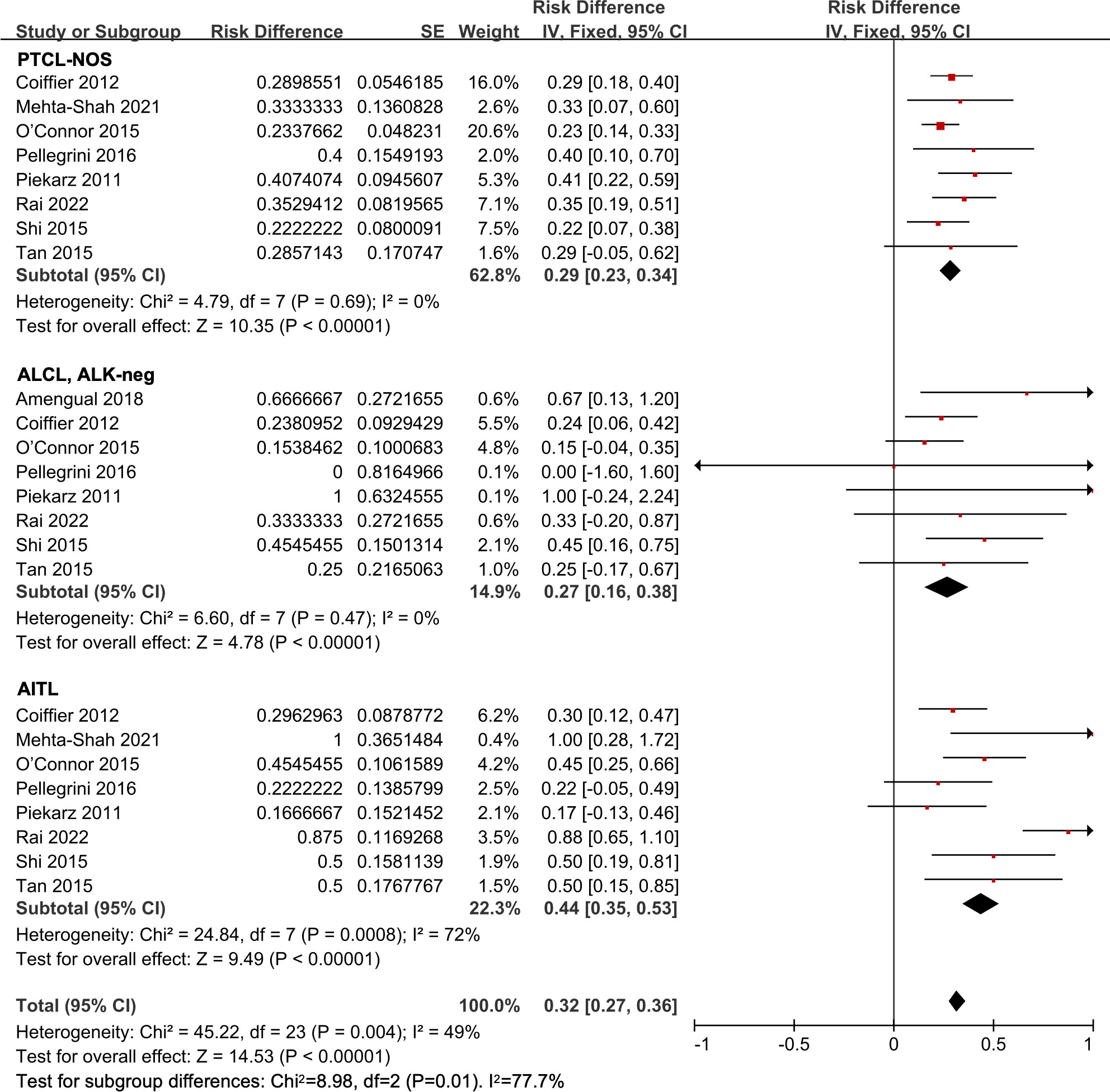
Figure 5 The forest plot of pooled overall response rate in different subtypes in relapsed/refractory peripheral T-cell lymphoma patients. PTCL-NOS, peripheral T-cell lymphoma not otherwise specified; AITL, angioimmunoblastic T-cell lymphoma; ALCL, ALK-neg, anaplastic lymphoma kinase-negative anaplastic large-cell lymphoma.
Eighteen studies were included for safety assessment (12, 18–33, 38). The AEs were graded in compliance with the National Cancer Institute Common Toxicity Criteria for AEs (CTCAE) (Version 3.0, 4.0, or 4.03). Thrombocytopenia was the most common, with a pooled incidence rate of 54% (95% CI, 32-77%) for all grades. Other common hematological AEs included neutropenia and leukopenia, and the incidence rates were 50% (95% CI, 32-67%) and 46% (95% CI, 24-69%), respectively. The most common grade 3 or higher hematological AE was neutropenia. Nausea was the most frequent non-hematological AE for all grades, with an incidence rate of 53% (95% CI, 36-70%). Moreover, nausea, fever/pyrexia, decreased appetite, and hypokalemia were common grade 3 or higher non-hematological AEs. The pooled incidence rate and the number of included studies in every AE are listed in Table 2.
The incidence rate of AEs of HDAC inhibitor monotherapy and HDAC inhibitor-based combination therapy of PTCL were compared. Subgroup analysis of AEs of all grades showed that combination therapy exhibited higher rates of anemia (55% vs. 24%, P = 0.001), nausea (78% vs. 41%, P = 0.0003), and hypokalemia (26% vs. 7%, P = 0.004), compared to monotherapy. Moreover, the subgroup analysis of grade 3 or higher AEs suggested that combination therapy showed higher occurrences of anemia (22% vs. 6%, P = 0.03), neutropenia (54% vs. 23%, P = 0.001), nausea (11% vs. 2%, P = 0.006), vomiting (7% vs. 2%, P = 0.004), and hypokalemia (8% vs. 2%, P = 0.03), but the lower occurrence of fatigue (1% vs. 10%, P = 0.02), when compared to HDAC inhibitor monotherapy.
Funnel plots were utilized to estimate the publication bias of the studies of R/R PTCL. Asymmetry was found in the results of ORR, CR rate, and PR rate, suggesting evidence of publication bias (Figure 6). The I2 test and Galbraith plots indicated that heterogeneity existed in the outcome of ORR of untreated PTCL, ORR and PR rate of R/R PTCL. And low risks of heterogeneity were found in the results of CR and PR rate of untreated PTCL, and CR rate of R/R PTCL (Figures S10-S15). The sensitivity analysis was conducted by omitting four low-quality studies (20, 25, 34, 37). For untreated PTCL patients, the pooled ORR was 73% (95% CI, 63-83%, random effect model) (Figure S16). And for R/R PTCL, the pooled ORR was 31% (95% CI, 27-35%, fixed effect model). Although combination therapy exhibited a higher ORR than monotherapy, no statistical discrepancy was found in the sensitivity analysis (P = 0.19) (Figure S17).
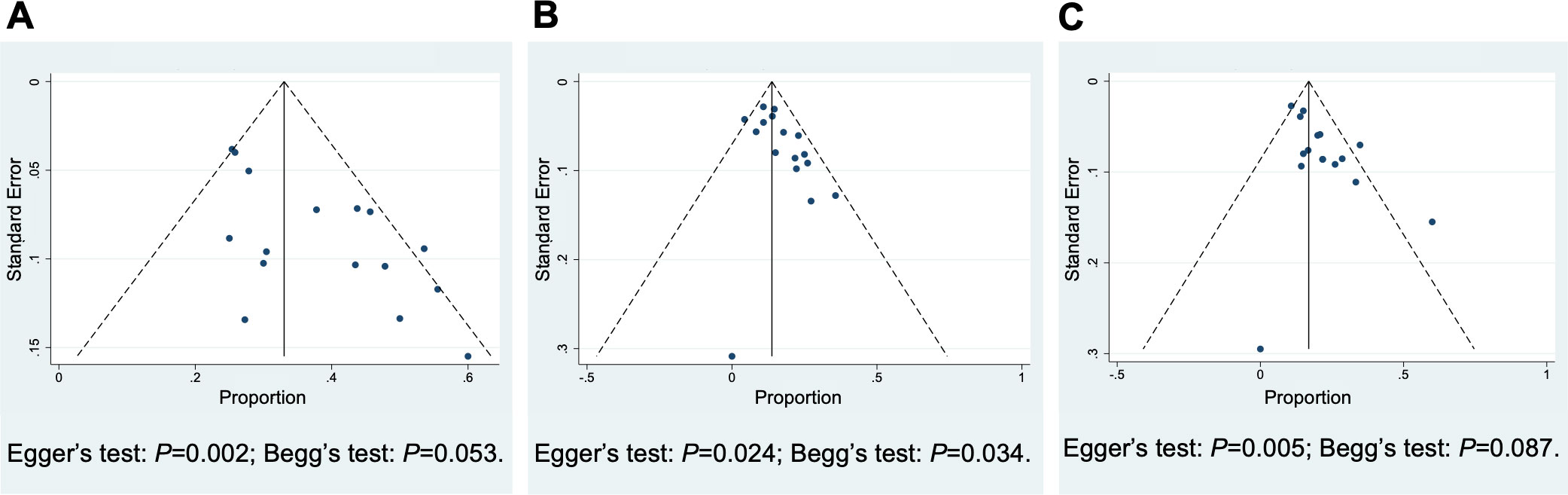
Figure 6 Funnel Plots of the studies included in relapsed/refractory peripheral T-cell lymphoma to estimate the publication bias. (A) Overall response rate, (B) complete response rate, (C) partial response rate.
To address the poor outcome of PTCL, several agents have been evaluated in clinical trials, including monoclonal antibodies, hypomethylating agents, PI3K/Akt/mTOR pathway inhibitors, and HDAC inhibitors (39). And HDAC inhibitors have been extensively studied for the treatment of PTCL. Thus, in this paper, we conducted a systematic review of prospective clinical trials and a meta-analysis to evaluate the efficacy and safety profile of HDAC inhibitors for PTCL.
We analyzed the treatment efficacy in untreated PTCL patients by including seven studies, resulting in a pooled ORR of 72% and a pooled CR rate of 44%, which demonstrates high therapeutic efficacy. There was a significant difference in ORR among three HDAC inhibitor-based combination therapies (P = 0.02). The belinostat-based combination therapy demonstrated the best ORR. The chidamide-based combination therapy performed well, while the romidepsin-based combination therapy showed an inferior outcome to other HDAC inhibitors. These three HDAC inhibitors have different structures and various target specificities, as well as distinct pharmacokinetic and pharmacodynamic properties, which may lead to different outcomes (31). It’s noteworthy that only one study was included in the belinostat group, with a small sample size. Therefore, it is necessary to verify the efficacy of belinostat-based therapy in larger samples of untreated PTCL patients in the future. Five studies used HDAC inhibitor plus CHOP or CHOEP (CHOP plus etoposide) regimen. The CHOP or CHOP-like regimen is typically the frontline therapy for newly diagnosed PTCL patients. Our analysis included a controlled trial, which indicated that the ORR of CHOP therapy was 60%, and the ORR of the romidepsin plus CHOP therapy was 63%, with no significant difference observed (19). However, the duration of response in CR or PR patients was prolonged with the treatment of romidepsin plus CHOP. In a double-blind, randomized trial that enrolled previously untreated PTCL patients, the ORR was 72% with the CHOP therapy (40). In addition, a real-world study demonstrated that the ORR was 75% with CHOEP treatment and 65% with CHOP treatment (41). Moreover, in a retrospective analysis, untreated PTCL patients achieved an ORR of 82.6%, 76.1%, and 75.0% when treated with CHOP, CHOEP, and CHOEP alternating with a gemcitabine-based regimen, respectively (42). Although HDAC inhibitor-based combination therapies resulted in a high ORR in untreated PTCL, no additional benefits have been found with the treatment of HDAC inhibitor plus chemotherapy when indirectly compared with the CHOP or CHOP-like regimen. Thus, more controlled clinical trials are needed to identify promising therapies for untreated PTCL patients.
The clinical outcome is generally dismal in R/R PTCL patients. In our study, 16 studies enrolling 665 R/R PTCL patients were included, and most patients were at stage III or IV. Our findings suggested that the HDAC inhibitors have favorable efficacy in R/R PTCL patients, with an estimated ORR of 37%, a CR rate of 14%, and a PR rate of 17%. Furthermore, subgroup analysis indicated that HDAC inhibitor-based combination therapy showed significantly higher ORR and CR rate (45% and 23%, respectively) than HDAC inhibitor monotherapy. A meta-analysis was conducted to evaluate the treatment outcome and tolerability of novel drugs versus chemotherapy in R/R T-cell lymphoma. The ORR of single novel agents in R/R PTCL was 36%, which was lower than that of chemotherapy and the combination of chemotherapy and novel agents (43), which is partly consistent with our findings. No statistical differences were found in the subgroup analysis of the efficacy of different HDAC inhibitors, while in the monotherapy, romidepsin and chidamide exhibited slightly higher efficacy than belinostat. Chidamide, which was developed in China, showed promising therapeutic capacity. A meta-analysis involving prospective and retrospective studies of PTCL patients treated with romidepsin showed a CR rate of 20%. Controversially, the study found no statistical difference comparing romidepsin monotherapy with romidepsin-based combination therapy (44). The discrepancy may be attributed to the difference that our study included four kinds of HDAC inhibitor-related studies and conducted a more comprehensive analysis. Another study summarized the efficacy of belinostat for PTCL, and the result was consistent with our study (45).
The efficacy of HDAC inhibitors varies across different subtypes of PTCL. In untreated PTCL patients, the AITL and ALCL, ALK-neg subgroups showed higher ORR compared to the PTCL-NOS subgroup. In the R/R PTCL setting, HDAC inhibitors exhibited favorable efficacy in the AITL subgroup, while the outcome was still suboptimal in the PTCL-NOS subgroup. These results suggested that HDAC inhibitors were more effective in patients with AITL. Several genetic mutations have been found in AITL, including TET2, DNMT3A, and RHOA (46, 47). The mutations in epigenetic regulation genes may relate to the promising activity of epigenetic drugs (48). Identifying the genomic and molecular information, and exploring the relationship between therapeutic activity and disease subtypes can further enhance the precise management of the disease.
The analysis of treatment-related AEs indicated that hematological AEs frequently occurred, including thrombocytopenia, neutropenia, and leukopenia. Among these, thrombocytopenia was the most common AE, and a preclinical study reported that HDAC inhibitors decreased the release of platelets from megakaryocytes (49). Furthermore, the analysis of grade 3 or higher AEs indicated that hematological AEs occurred more frequently than non-hematological AEs. Most AEs were reversible, and supportive care was typically provided. Dose modification can be utilized to manage these AEs. Compared to HDAC inhibitor monotherapy, the HDAC inhibitor-based combination therapy showed a higher incidence of hematological AEs, including anemia and neutropenia. In a clinical trial, the romidepsin plus CHOP regimen resulted in severe AEs of hematological, gastrointestinal, and nutritional origin. Additionally, the addition of romidepsin led to frequent dose reduction, dose interruption, and low dose intensity of the CHOP regimen (19). Therefore, managing AEs and adjusting drug dosage are crucial when using combination therapy. Although some non-hematological AEs, such as nausea, vomiting, fatigue, and diarrhea, occurred frequently, they were primarily graded 1-2, with a low incidence of grade 3 or higher. The results are consistent with another meta-analysis that included romidepsin-related studies (44).
However, there are still inevitable limitations in this work. First, most of the included studies were single-arm trials and lacked comparability. Second, the studies used different versions of CTCAE, including version 3.0, 4.0, and 4.03. Additionally, four of the included studies had a MINORS score of nine, indicating low quality, which may affect the reliability of our findings.
In conclusion, our systematic review and meta-analysis demonstrates that HDAC inhibitors are effective treatment options for both untreated and R/R PTCL patients. The combination of HDAC inhibitor and chemotherapy exhibited superior efficacy to HDAC inhibitor monotherapy in the R/R PTCL setting. Moreover, HDAC inhibitor-based therapy was more effective in AITL patients than in other subtypes. Further research is needed, particularly randomized controlled trials, to robustly evaluate the treatment outcomes of HDAC inhibitors compared with other therapies. The potential for HDAC inhibitors combined with novel agents should also be explored.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
TN conceived the ideas of the manuscript and supervised the writing. PY and YT designed the study, searched the literature, extracted and analyzed the data, and drafted the initial manuscript. AZ designed the study and revised the manuscript. KS, HL, JW, and HZ assessed the quality of included studies and analyzed the data. ZW, MW, and YQ checked the collected data and revised the manuscript for intellectual content. LZ and YZ revised the manuscript and supervised the writing. All authors contributed to the article and approved the submitted version.
This work was supported by Incubation Program for Clinical Trials (No. 19HXFH030), Achievement Transformation Project (No. CGZH21001), 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (No. ZYJC21007), Translational Research Grant of NCRCH (No. 2021WWB03), Chengdu Science and Technology Program (No. 2022-YF05-01444-SN), Key Research and Development Program of Sichuan Province (No. 2023YFS0031), and National Key Research and Development Program of China (No. 2022YFC2502600, 2022YFC2502603).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1127112/full#supplementary-material
1. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the world health organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia (2022) 36(7):1720–48. doi: 10.1038/s41375-022-01620-2
2. d’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol (2012) 30(25):3093–9. doi: 10.1200/jco.2011.40.2719
3. Mak V, Hamm J, Chhanabhai M, Shenkier T, Klasa R, Sehn LH, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol (2013) 31(16):1970–6. doi: 10.1200/jco.2012.44.7524
4. Bellei M, Foss FM, Shustov AR, Horwitz SM, Marcheselli L, Kim WS, et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, international T-cell project. Haematologica (2018) 103(7):1191–7. doi: 10.3324/haematol.2017.186577
5. Horwitz S, O’Connor OA, Pro B, Trümper L, Iyer S, Advani R, et al. The ECHELON-2 trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann Oncol (2022) 33(3):288–98. doi: 10.1016/j.annonc.2021.12.002
6. Zhang J, Peng J, Huang Y, Meng L, Li Q, Xiong F, et al. Identification of histone deacetylase (HDAC)-associated proteins with DNA-programmed affinity labeling. Angew Chem Int Ed Engl (2020) 59(40):17525–32. doi: 10.1002/anie.202001205
7. Bahl S, Seto E. Regulation of histone deacetylase activities and functions by phosphorylation and its physiological relevance. Cell Mol Life Sci (2021) 78(2):427–45. doi: 10.1007/s00018-020-03599-4
8. Zhang H, Shang YP, Chen HY, Li J. Histone deacetylases function as novel potential therapeutic targets for cancer. Hepatol Res (2017) 47(2):149–59. doi: 10.1111/hepr.12757
9. Wang JC, Kafeel MI, Avezbakiyev B, Chen C, Sun Y, Rathnasabapathy C, et al. Histone deacetylase in chronic lymphocytic leukemia. Oncology (2011) 81(5-6):325–9. doi: 10.1159/000334577
10. Valdez BC, Brammer JE, Li Y, Murray D, Liu Y, Hosing C, et al. Romidepsin targets multiple survival signaling pathways in malignant T cells. Blood Cancer J (2015) 5(10):e357. doi: 10.1038/bcj.2015.83
11. Zhang Q, Wang S, Chen J, Yu Z. Histone deacetylases (HDACs) guided novel therapies for T-cell lymphomas. Int J Med Sci (2019) 16(3):424–42. doi: 10.7150/ijms.30154
12. Rai S, Kim WS, Ando K, Choi I, Izutsu K, Tsukamoto N, et al. Oral HDAC inhibitor tucidinostat in patients with relapsed or refractory peripheral T-cell lymphoma: phase IIb results. Haematologica (2022) 108(3):811–21. doi: 10.3324/haematol.2022.280996
13. Dong M, Ning ZQ, Xing PY, Xu JL, Cao HX, Dou GF, et al. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother Pharmacol (2012) 69(6):1413–22. doi: 10.1007/s00280-012-1847-5
14. Yazbeck VY, Grant S. Romidepsin for the treatment of non-hodgkin’s lymphoma. Expert Opin Investig Drugs (2015) 24(7):965–79. doi: 10.1517/13543784.2015.1041586
15. Campbell P, Thomas CM. Belinostat for the treatment of relapsed or refractory peripheral T-cell lymphoma. J Oncol Pharm Pract (2017) 23(2):143–7. doi: 10.1177/1078155216634178
16. Cao HY, Li L, Xue SL, Dai HP. Chidamide: targeting epigenetic regulation in the treatment of hematological malignancy. Hematol Oncol (2022) 1–9. doi: 10.1002/hon.3088
17. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg (2003) 73(9):712–6. doi: 10.1046/j.1445-2197.2003.02748.x
18. Dupuis J, Morschhauser F, Ghesquières H, Tilly H, Casasnovas O, Thieblemont C, et al. Combination of romidepsin with cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated patients with peripheral T-cell lymphoma: a non-randomised, phase 1b/2 study. Lancet Haematol (2015) 2(4):e160–5. doi: 10.1016/s2352-3026(15)00023-x
19. Bachy E, Camus V, Thieblemont C, Sibon D, Casasnovas RO, Ysebaert L, et al. Romidepsin plus CHOP versus CHOP in patients with previously untreated peripheral T-cell lymphoma: results of the ro-CHOP phase III study (Conducted by LYSA). J Clin Oncol (2022) 40(3):242–51. doi: 10.1200/jco.21.01815
20. Falchi L, Ma H, Klein S, Lue JK, Montanari F, Marchi E, et al. Combined oral 5-azacytidine and romidepsin are highly effective in patients with PTCL: a multicenter phase 2 study. Blood (2021) 137(16):2161–70. doi: 10.1182/blood.2020009004
21. Johnston PB, Cashen AF, Nikolinakos PG, Beaven AW, Barta SK, Bhat G, et al. Belinostat in combination with standard cyclophosphamide, doxorubicin, vincristine and prednisone as first-line treatment for patients with newly diagnosed peripheral T-cell lymphoma. Exp Hematol Oncol (2021) 10(1):15. doi: 10.1186/s40164-021-00203-8
22. Gui L, Cao J, Ji D, Zhang H, Fan Q, Zhu J, et al. Chidamide combined with cyclophosphamide, doxorubicin, vincristine and prednisone in previously untreated patients with peripheral T-cell lymphoma. Chin J Cancer Res (2021) 33(5):616–26. doi: 10.21147/j.issn.1000-9604.2021.05.08
23. Wang Y, Zhang M, Song W, Cai Q, Zhang L, Sun X, et al. Chidamide plus prednisone, etoposide, and thalidomide for untreated angioimmunoblastic T-cell lymphoma in a Chinese population: a multicenter phase II trial. Am J Hematol (2022) 97(5):623–9. doi: 10.1002/ajh.26499
24. Zhang W, Su L, Liu L, Gao Y, Wang Q, Su H, et al. The combination of chidamide with the CHOEP regimen in previously untreated patients with peripheral T-cell lymphoma: a prospective, multicenter, single arm, phase 1b/2 study. Cancer Biol Med (2021) 18(3):841–8. doi: 10.20892/j.issn.2095-3941.2020.0413
25. Piekarz RL, Frye R, Prince HM, Kirschbaum MH, Zain J, Allen SL, et al. Phase 2 trial of romidepsin in patients with peripheral T-cell lymphoma. Blood (2011) 117(22):5827–34. doi: 10.1182/blood-2010-10-312603
26. Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol (2012) 30(6):631–6. doi: 10.1200/jco.2011.37.4223
27. Maruyama D, Tobinai K, Ogura M, Uchida T, Hatake K, Taniwaki M, et al. Romidepsin in Japanese patients with relapsed or refractory peripheral T-cell lymphoma: a phase I/II and pharmacokinetics study. Int J Hematol (2017) 106(5):655–65. doi: 10.1007/s12185-017-2286-1
28. O’Connor OA, Özcan M, Jacobsen ED, Roncero JM, Trotman J, Demeter J, et al. Randomized phase III study of alisertib or investigator’s choice (Selected single agent) in patients with relapsed or refractory peripheral T-cell lymphoma. J Clin Oncol (2019) 37(8):613–23. doi: 10.1200/jco.18.00899
29. O’Connor OA, Horwitz S, Masszi T, Van Hoof A, Brown P, Doorduijn J, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol (2015) 33(23):2492–9. doi: 10.1200/jco.2014.59.2782
30. Foss F, Advani R, Duvic M, Hymes KB, Intragumtornchai T, Lekhakula A, et al. A phase II trial of belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol (2015) 168(6):811–9. doi: 10.1111/bjh.13222
31. Shi Y, Dong M, Hong X, Zhang W, Feng J, Zhu J, et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann Oncol (2015) 26(8):1766–71. doi: 10.1093/annonc/mdv237
32. Utsunomiya A, Izutsu K, Jo T, Yoshida S, Tsukasaki K, Ando K, et al. Oral histone deacetylase inhibitor tucidinostat (HBI-8000) in patients with relapsed or refractory adult T-cell leukemia/lymphoma: phase IIb results. Cancer Sci (2022) 113(8):2778–87. doi: 10.1111/cas.15431
33. Pellegrini C, Dodero A, Chiappella A, Monaco F, Degl’Innocenti D, Salvi F, et al. A phase II study on the role of gemcitabine plus romidepsin (GEMRO regimen) in the treatment of relapsed/refractory peripheral T-cell lymphoma patients. J Hematol Oncol (2016) 9:38. doi: 10.1186/s13045-016-0266-1
34. Amengual JE, Lichtenstein R, Lue J, Sawas A, Deng C, Lichtenstein E, et al. A phase 1 study of romidepsin and pralatrexate reveals marked activity in relapsed and refractory T-cell lymphoma. Blood (2018) 131(4):397–407. doi: 10.1182/blood-2017-09-806737
35. Reiman T, Savage KJ, Crump M, Cheung MC, MacDonald D, Buckstein R, et al. A phase I study of romidepsin, gemcitabine, dexamethasone and cisplatin combination therapy in the treatment of peripheral T-cell and diffuse large b-cell lymphoma; the Canadian cancer trials group LY.15 study†. Leuk Lymphoma (2019) 60(4):912–9. doi: 10.1080/10428194.2018.1515937
36. Vu K, Wu CH, Yang CY, Zhan A, Cavallone E, Berry W, et al. Romidepsin plus liposomal doxorubicin is safe and effective in patients with relapsed or refractory T-cell lymphoma: results of a phase I dose-escalation study. Clin Cancer Res (2020) 26(5):1000–8. doi: 10.1158/1078-0432.Ccr-19-2152
37. Mehta-Shah N, Lunning MA, Moskowitz AJ, Boruchov AM, Ruan J, Lynch P, et al. Romidepsin and lenalidomide-based regimens have efficacy in relapsed/refractory lymphoma: combined analysis of two phase I studies with expansion cohorts. Am J Hematol (2021) 96(10):1211–22. doi: 10.1002/ajh.26288
38. Tan D, Phipps C, Hwang WY, Tan SY, Yeap CH, Chan YH, et al. Panobinostat in combination with bortezomib in patients with relapsed or refractory peripheral T-cell lymphoma: an open-label, multicentre phase 2 trial. Lancet Haematol (2015) 2(8):e326–33. doi: 10.1016/s2352-3026(15)00097-6
39. Mina A, Pro B. T Time: emerging and new therapies for peripheral T-cell lymphoma. Blood Rev (2022) 52:100889. doi: 10.1016/j.blre.2021.100889
40. Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet (2019) 393(10168):229–40. doi: 10.1016/s0140-6736(18)32984-2
41. Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish lymphoma registry. Blood (2014) 124(10):1570–7. doi: 10.1182/blood-2014-04-573089
42. Liu X, Yang M, Wu M, Zheng W, Xie Y, Zhu J, et al. A retrospective study of the CHOP, CHOPE, and CHOPE/G regimens as the first-line treatment of peripheral T-cell lymphomas. Cancer Chemother Pharmacol (2019) 83(3):443–9. doi: 10.1007/s00280-018-3744-z
43. Shafagati N, Koh MJ, Boussi L, Park HJ, Stuver R, Bain P, et al. Comparative efficacy and tolerability of novel agents vs chemotherapy in relapsed and refractory T-cell lymphomas: a meta-analysis. Blood Adv (2022) 6(16):4740–62. doi: 10.1182/bloodadvances.2022007425
44. Du J, Han X, Lin S, Qiu C, Zhu L, Huang Z, et al. Efficacy and treatment-related adverse events of romidepsin in PTCL clinical studies: a systematic review and meta-analysis. Front Med (Lausanne) (2021) 8:732727. doi: 10.3389/fmed.2021.732727
45. Allen PB, Lechowicz MJ. Hematologic toxicity is rare in relapsed patients treated with belinostat: a systematic review of belinostat toxicity and safety in peripheral T-cell lymphomas. Cancer Manag Res (2018) 10:6731–42. doi: 10.2147/cmar.S149241
46. Odejide O, Weigert O, Lane AA, Toscano D, Lunning MA, Kopp N, et al. A targeted mutational landscape of angioimmunoblastic T-cell lymphoma. Blood (2014) 123(9):1293–6. doi: 10.1182/blood-2013-10-531509
47. Yao WQ, Wu F, Zhang W, Chuang SS, Thompson JS, Chen Z, et al. Angioimmunoblastic T-cell lymphoma contains multiple clonal T-cell populations derived from a common TET2 mutant progenitor cell. J Pathol (2020) 250(3):346–57. doi: 10.1002/path.5376
48. Zhang P, Zhang M. Epigenetic alterations and advancement of treatment in peripheral T-cell lymphoma. Clin Epigenet (2020) 12(1):169. doi: 10.1186/s13148-020-00962-x
Keywords: peripheral T-cell lymphoma, subtype, histone deacetylase inhibitor, efficacy, meta-analysis
Citation: Yang P, Tao Y, Zhao A, Shen K, Li H, Wang J, Zhou H, Wang Z, Wang M, Qu Y, Zhang L, Zheng Y and Niu T (2023) Efficacy and safety of histone deacetylase inhibitors in peripheral T-cell lymphoma: a systematic review and meta-analysis on prospective clinical trials. Front. Oncol. 13:1127112. doi: 10.3389/fonc.2023.1127112
Received: 19 December 2022; Accepted: 31 May 2023;
Published: 13 June 2023.
Edited by:
Lorenzo Falchi, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Locke Bryan, Augusta University, United StatesCopyright © 2023 Yang, Tao, Zhao, Shen, Li, Wang, Zhou, Wang, Wang, Qu, Zhang, Zheng and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Niu, bml1dGluZ0B3Y2hzY3UuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.