
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 February 2023
Sec. Molecular and Cellular Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1124949
This article is part of the Research Topic Molecular and Cellular Mechanisms for Cancer Therapy Resistance View all 7 articles
 Waleed Kian1
Waleed Kian1 Bilal Krayim1
Bilal Krayim1 Hadel Alsana2
Hadel Alsana2 Betsy Giles3
Betsy Giles3 Ofer Purim1
Ofer Purim1 Wafeek Alguayn4
Wafeek Alguayn4 Farouq Alguayn5
Farouq Alguayn5 Nir Peled1*
Nir Peled1* Laila C. Roisman1
Laila C. Roisman1Lung cancer is the most common cancer-related cause of death worldwide, most of which are non-small cell lung cancers (NSCLC). Epidermal growth factor receptor (EGFR) mutations are common drivers of NSCLC. Treatment plans for NSCLC, specifically adenocarcinomas, rely heavily on the presence or absence of specific actionable driver mutations. Liquid biopsy can guide the treatment protocol to detect the presence of various mechanisms of resistance to treatment. We report three NSCLC EGFR mutated cases, each treated with Osimertinib in a combination therapy regimen to combat resistance mechanisms. The first patient presented with EGFR L858R/L833V compound mutation with MET amplification alongside CEP85L-ROS1 fusion gene, the second with EGFR exon 19del and MKRN1-BRAF fusion, and the last EGFR L858R/V834L compound mutation with MET amplification. Each regimen utilized a tyrosine kinase inhibitor or monoclonal antibody in addition to osimertinib and allowed for a prompt and relatively durable treatment response.
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) have revolutionized the therapeutic paradigm for advanced non-small cell lung cancer (NSCLC) with EGFR mutations (1). The most prevalent EGFR mutations are the in-frame deletion of exon 19 del and the L858R point mutation in exon 21. L858R and del 19 are called “common mutations”; they account for up to 90% of EGFR mutations in NSCLC (2). The presence of a common mutation correlates with an improved overall response rate of EGFR-TKIs, specifically osimertinib in the aforementioned study (3). Osimertinib, an irreversible, third-generation EGFR-TKI, targets the Thr790Met resistance mutation as well as another common EGFR sensitizing mutations. In a phase III trial (AURA3), osimertinib demonstrated superior outcomes compared with platinum-based doublet chemotherapy (4, 5). In the phase III FLAURA trial, which compared osimertinib to first-generation EGFR-TKIs in advanced EGFR-mutant NSCLC, objective response rates (ORRs) were similar, but progression-free survival (PFS) and overall survival (OS) were significantly longer (18.9 vs 10.2 and 38.6 vs 31.8 months, respectively) (6, 7).
In recent years, widespread use of next-generation sequencing helped identify a variety of rare EGFR mutations. These uncommon mutations are a highly heterogenous group, including T790M, L833V, and other genomic alterations within exons 18-21 (3). In NSCLC, uncommon EGFR mutations can appear on their own or together with other EGFR mutations. Compound mutations are associated with more aggressive tumors (2). Data on the prevalence of EGFR compound mutations in NSCLC is affected by several factors, related to ethnicity, testing methods and reporting biases (8). A large amount of available evidence on testing is derived from studies conducted in Asian populations, with ~45–60% overall EGFR mutation rate (9, 10). According to Attili et al., the prevalence of EGFR compound mutations ranges from 4-6.7% to 26% of EGFR mutant cases (8). While three large studies in Caucasian populations have reported 5-7% compound EGFR mutations among EGFR-positive patients (11–13).
Although osimertinib represents a significant advancement in the field of targeted therapy, unfortunately, all tumors eventually develop acquired resistance; some mechanisms involve EGFR (C797X, G796X, L718Q, and exon 20 insertions), while others involve alternative pathways that lead to osimertinib inhibition bypass (MET amplification, HER2 alteration, RET fusion, BRAF alteration, KRAS mutation, PI3K alteration, and small cell transformation) (14).
We present three cases of NSCLC with EGFR mutations that were treated with osimertinib in a combination therapy regimen to combat resistance mechanisms. The first had an EGFR L858R/L833V compound mutation with MET amplification, as well as a CEP85L-ROS1 fusion gene, the second had an EGFR exon 19del and an MKRN1-BRAF fusion, and the last had an EGFR L858R/V834L compound mutation with MET amplification. In addition to osimertinib, each regimen included a tyrosine kinase inhibitor or monoclonal antibody. Despite their resistive mechanisms, osimertinib plus crizotinib or dabrafenib plus trametinib or amivantamab, respectively, allowed for a prompt treatment response.
A 73-year-old, non-smoking, Caucasian female with a history of hypertension presented to the hospital with dyspnea in March 2016. Soon after, she was diagnosed with a T3N2M0, Stage IIIB NSCLC adenocarcinoma, positive for the EGFR L858R mutation (Figure 1). In April 2016, platinum-based chemotherapy (carboplatin and pemetrexed) was initiated alongside 60 grays (Gy) of definitive radiation therapy.
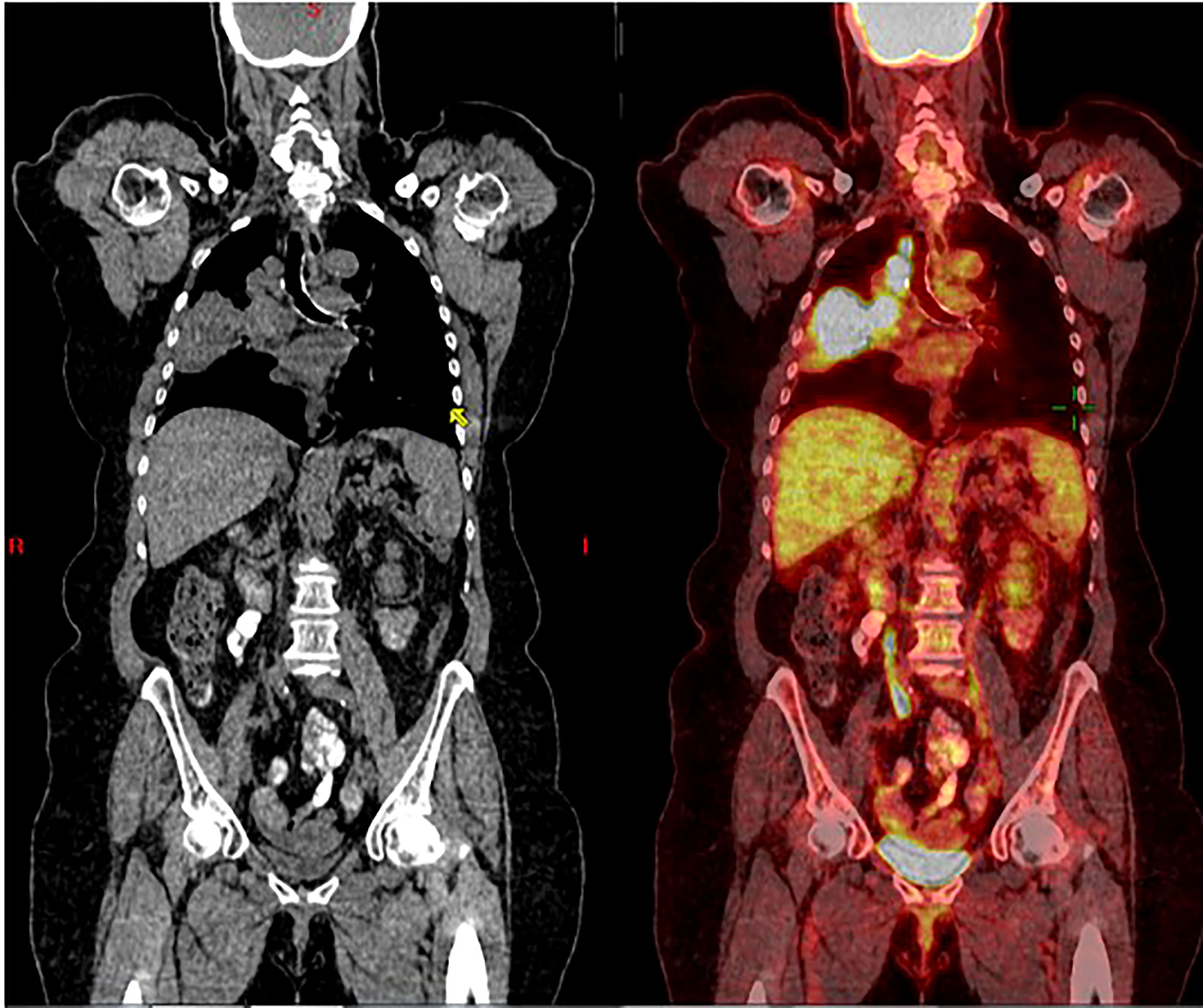
Figure 1 Coronal CT scan (left), and superimposed PET scan (right), from March 2016, show a large right lung mass (>5 cm) with increased uptake of FDG-18. There is also ipsilateral hilar and paratracheal lymphadenopathy with positive PET uptake, contributing to classification as unresectable, stage 3B NSCLC.
In May 2018, PET-CT showed a single liver metastasis. On liquid biopsy, circulating free DNA (cfDNA) included two mutations of EGFR: L858R (0.2%) and L833V (0.08%), as shown in Table 1. After identifying these mutations, erlotinib was initiated, and the patient demonstrated a partial response.
Disease progression was identified in January 2019 with a further enlargement of the single liver mass. Tests for T790M-mut were negative. The patient was then enrolled in a clinical trial where she received combined chemo-immunotherapy with platinum-based therapy and pembrolizumab. In November 2019, there was a size progression of the liver mass, prompting a liquid biopsy evaluation that identified progression of both L858R (8.2%) and L833V (7.2%) EGFR mutations, as illustrated in Table 1. The patient was then treated with liver stereotactic body radiation therapy (SBRT) of 50 Gy in 5 fractions. She continued maintenance therapy with oral vinorelbine until October 2020. Due to the continued liver mass progression, the patient underwent a left hepatectomy (Figure 2). Biopsy of the liver mass showed moderate to poorly differentiated adenocarcinoma, of lung origin, with Met-amplification (Table 1).
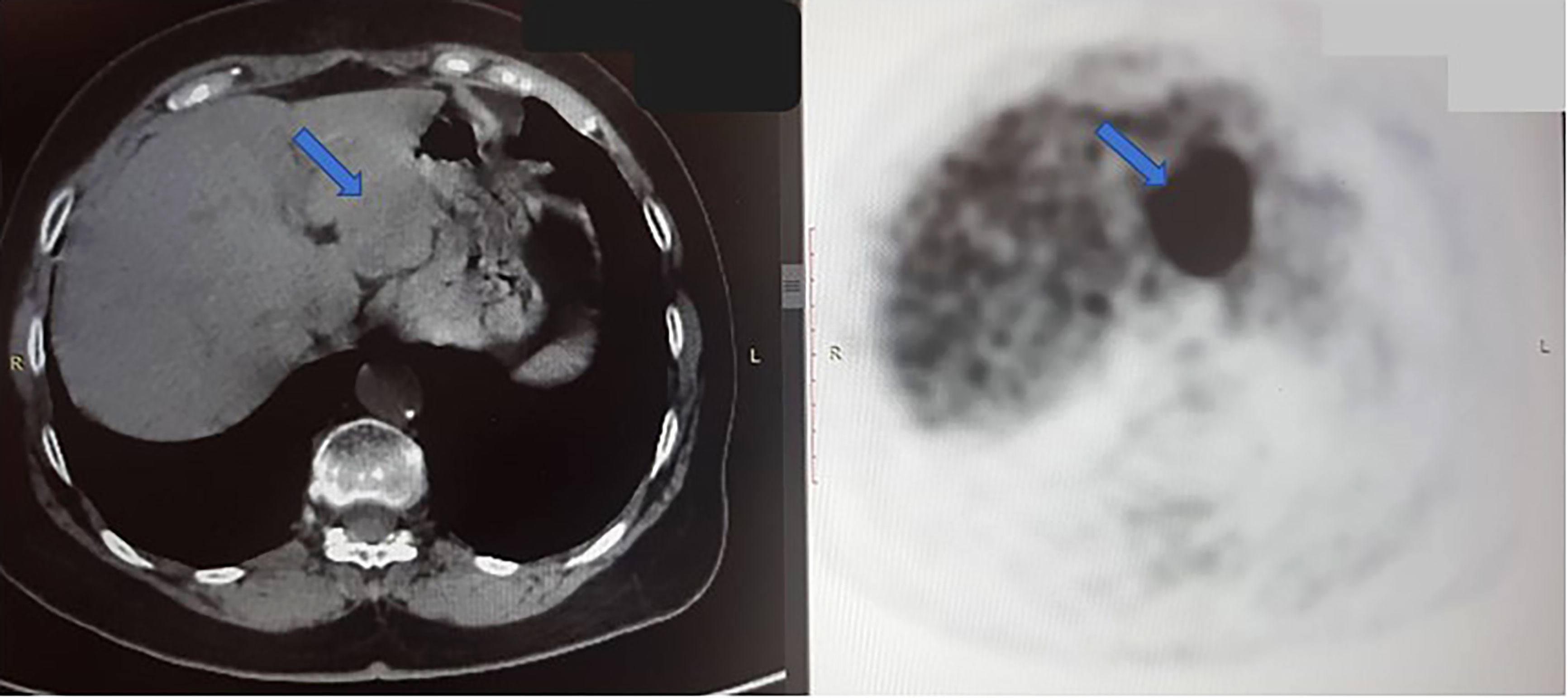
Figure 2 Axial CT scan (left) and PET scan (right) from October 2020, shows a large tumor in the left hepatic lobe (blue arrow). In PET scan, the dark mass shows an FDG-avid, metabolically active tumor. The right hepatic lobe is normal, without signs of malignancy at this time.
In December 2020, multiple liver metastases were identified on PET-CT (Figure 3A). At this time, another comprehensive genomic test (Tempus) was performed which identified further progression of EGFR- L858R (61.1%) and L833V mutations (63.4%), as well as the development of additional findings such as CEP85L-ROS1 fusion and MET amplification (6 copies), as shown in Table 1. Treatment with osimertinib (80mg daily) was then attempted, but the disease continued progressing (Figure 3B). At this time, crizotinib (250 mg bid) was added to the current regimen. Following 2-months of combination therapy, the liver mass substantially decreased in its size and numbers (Figure 3C). Dose reduction of crizotinib (250 mg once daily) was required following an ischemic stroke. In October 2021, the patient passed away due to further disease progression.
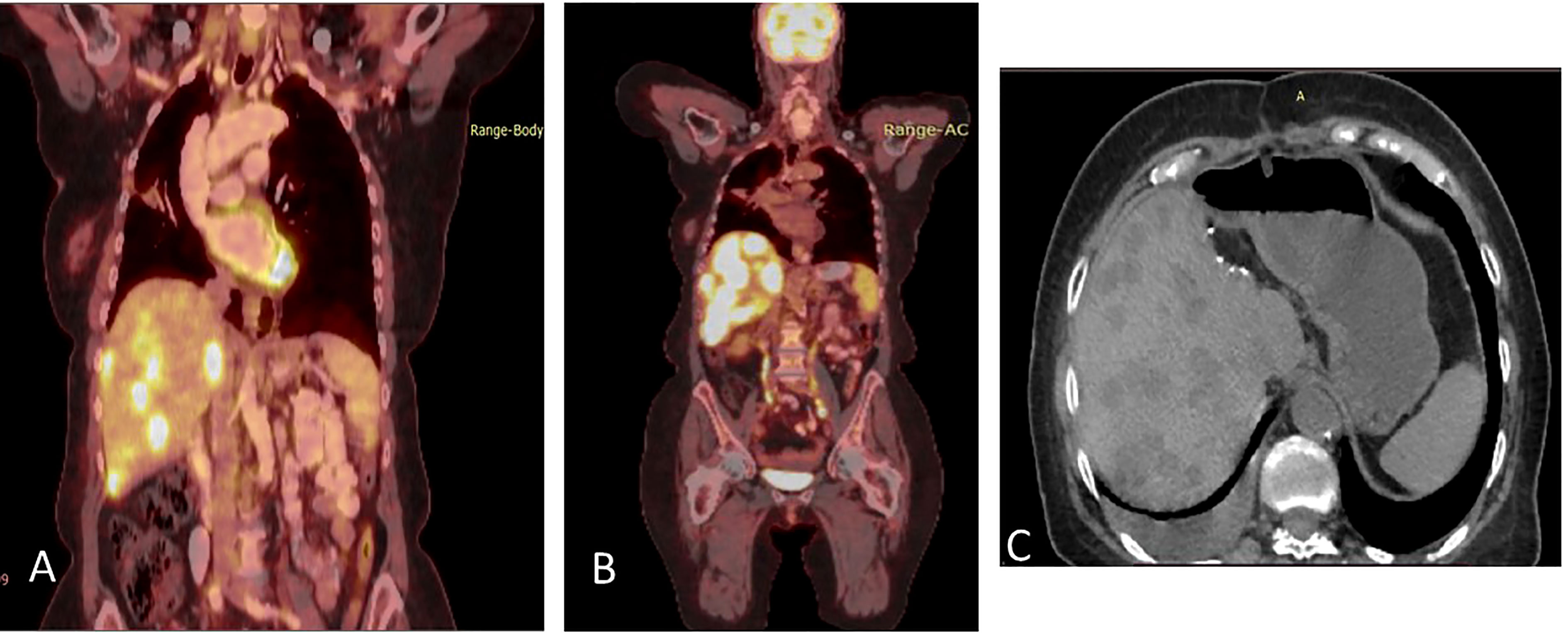
Figure 3 (A) Coronal section, superimposed PET-CT scan, December 2020. Four years after the first presentation of stage 3B NSCLC, the right lung shows a significant reduction in FDG-18 uptake and decreased tumor size, with only a scar, indicating the lung’s complete response to treatment. However, status-post left hepatectomy, there is increased FDG-18 uptake in the right lobe of the liver, consistent with multifocal right hepatic metastatic disease. (B) Two months later, February 2021, there is further progression in size, number, and metabolic activity of metastases throughout the right lobe of the liver, indicating more aggressive disease. There remains no evidence of recurrent or residual disease in the lung. (C) Non-contrast, axial CT scan from April 2021 shows interval regression in size and number of liver masses. The white spots are sutures from the surgery to remove the left hepatic lobe (status-post left hepatectomy).
A 75-year-old, non-smoking, Caucasian female with a history of right thyroidectomy presented to the hospital with cough for several months with a left shoulder pain in August 2017. Soon after, she was diagnosed with a poorly differentiated NSCLC adenocarcinoma stage IIIB, with an EGFR exon 19 deletion. In September 2017, induction erlotinib was initiated with significant partial response to the primary lesion (Figures 4A, B). In January 2018, definitive radiation therapy 72 grays (Gy) were delivered followed by adjuvant erlotinib.
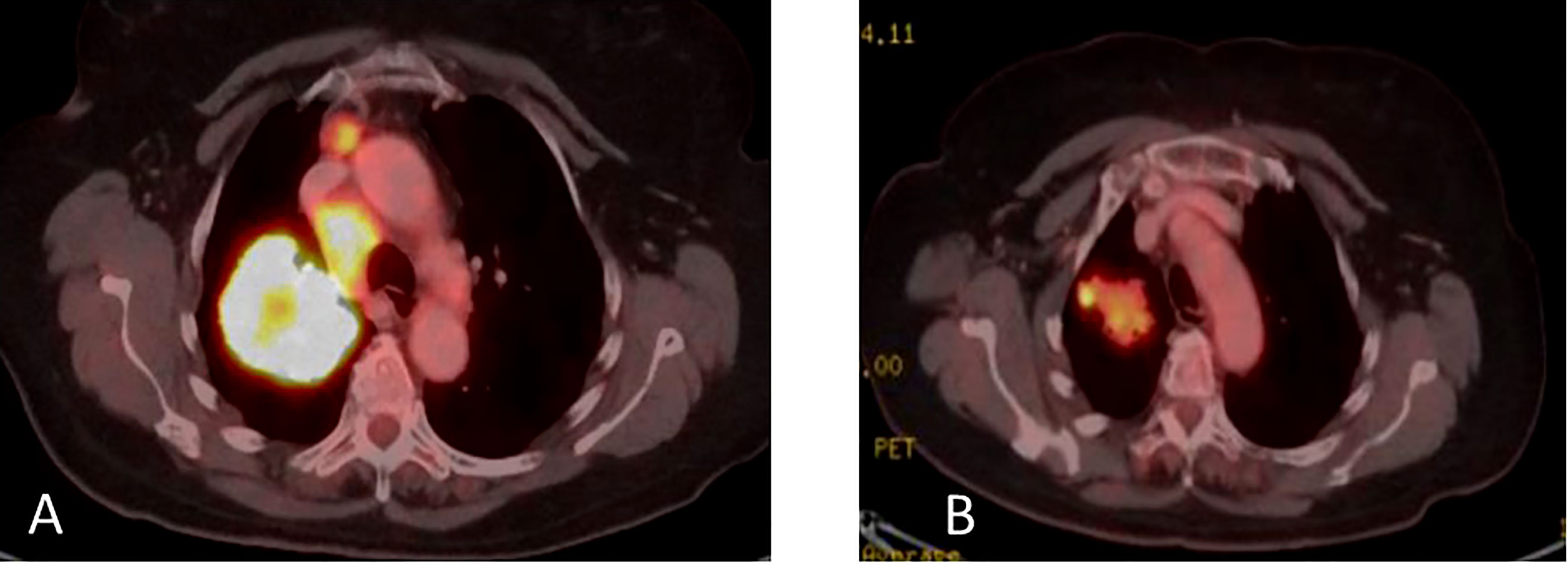
Figure 4 (A) Coronal PET-CT scan, from September 2017, show a large right lung mass with increased uptake of FDG-18. There is also ipsilateral hilar and mediastinal lymphadenopathy with positive PET uptake, contributing to classification as unresectable, stage 3B NSCLC. (B) January 2018, following induction erlotinib and sequential definitive radiation therapy 72 grays (Gy) indicating good partial response.
In March 2019, on liquid biopsy, cfDNA included T790M (0.14%) and the EGFR exon 19 deletion (1.74%), as seen in Table 2. After identifying local progressive disease, erlotinib was continued alongside re-irradiation of stereotactic body radiation therapy.
Disease progression in the right pleura was identified in November 2019 with a right-sided pleural effusion. Osimertinib was initiated, demonstrating a partial response (Figures 5A, B).
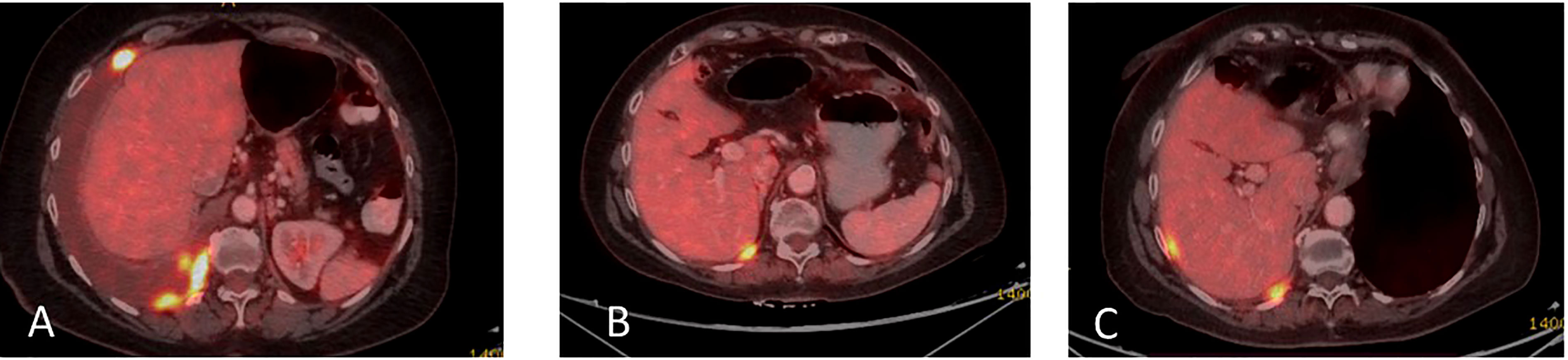
Figure 5 (A) Coronal PET-CT scan from November 2019 shows a pleural effusion and lesions with increased uptake of FDG-18, indicating disease progression. (B) Three months later, following Osimertinib demonstrated partial response. (C) February 2021, showing a new pleural lesion indicating disease progression on Osimertinib.
Upon mild local disease progression In February 2021, a lung biopsy, and a comprehensive genomic test (Tempus) were performed. Tissue biopsy demonstrated a poorly differentiated cancerous lesion with 50% Ki67 (neuroendocrine marker). At the same time a liquid biopsy resulted: EGFR exon 19 deletion (41%), EGFR T790M (11.9%), and a MKRN1-BRAF fusion gene, as shown in Table 2. In follow-up, the patient’s disease progression was mostly stable containing small nodules in the lungs (Figure 5C).
Osimertinib alone was continued until June 2021, at which time there was further disease progression into the pleura, liver, peritoneum, and bones (Figure 6A). To combat this, the patient underwent 3 cycles of carboplatin and pemetrexed, alongside Osimertinib with further disease progression (Figure 6B).

Figure 6 (A) Coronal PET-CT scan, from June 2021, showing new multiple lung and liver lesions with increased uptake of FDG-18, indicating further disease progression on osimertinib. (B) Two months later, August 2021, they demonstrated massive disease progression with lung, liver, and bone metastases following chemotherapy plus Osimertinib. (C) Three months later, November 2021, showed significantly decreased uptake of FDG-18, indicating partial response following adding dabrafenib plus trametinib to Osimertinib.
In August 2021, osimertinib was initiated alongside BRAF kinase inhibitor trametinib and dabrafenib, to combat the MKRN1-BRAF fusion gene acting as a mechanism of resistance. This resulted in clinical and RECIST partial response (Figure 6C). In February 2022, the patient passed away due to lung disease progression.
A 68-year-old, Caucasian female with a 10 pack-year history of smoking, in December 2019, was diagnosed with squamous NSCLC in the left upper lobe, stage T3N1M0.
In January 2020, a chemotherapy combination of carboplatin and paclitaxel were initiated alongside definitive radiation therapy (60 Gy) followed by maintenance durvalumab (6 cycles), demonstrating a partial response.
In September 2020, eight months after initial presentation, due to local progression, cfDNA liquid biopsy demonstrated EGFR L858R of 2.8% and V834L of 2.3%. Treatment with osimertinib (80mg daily) was initiated with significant partial response.
Upon disease progression in October of 2021, cfDNA liquid biopsy demonstrated EGFR L858R of 2.3%; V83L of 2.8%; with MET amplification. Brain MRI showed no metastases. Treatment with osimertinib (80mg daily) was continued. The decision was made to add 1050 mg of Amivantanab every two weeks to the patient’s treatment at this time. After four rounds of treatment, resulted in clinical and RECIST partial response (Figures 7A, B).
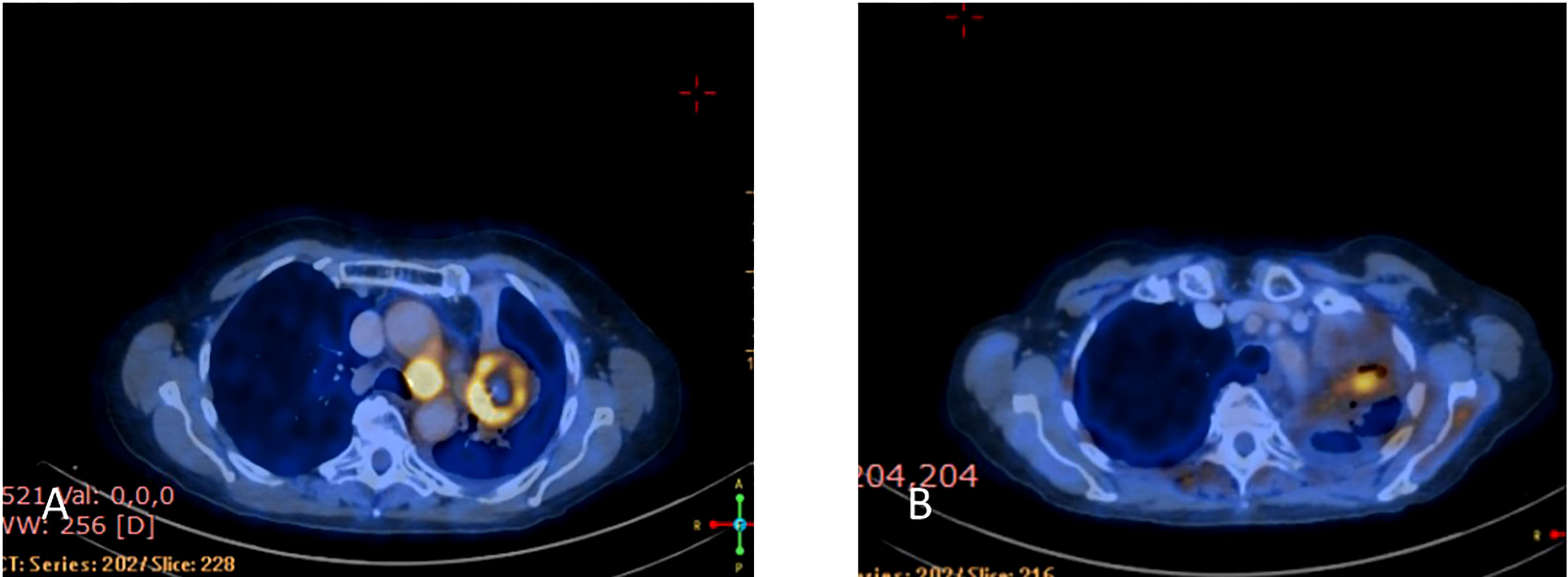
Figure 7 (A) Coronal PET-CT scan from October 2021 shows a left lung mass and ipsilateral pleural effusion with increased uptake of FDG-18, indicating disease progression on Osimertinib. (B) Three months later, January 2022, showed significantly decreased uptake of FDG-18, indicating partial response following adding Amivantamb to Osimertinib.
In March 2022, treatment was continued, with Amivantanab reduced from two-week intervals to three-week intervals due to grade 2 adverse events (diarrhea and paronychia). Liquid biopsy in May 2022 demonstrated total eradication of MET and EGFR from the cfDNA in the blood (Figure 8). Eight months later, the patient is still responding to the combination therapy.
Lung adenocarcinomas with common EGFR mutations are often responsive to EGFR TKIs, while uncommon and compound mutations react unpredictably. Approximately 40-45% of EGFR mutations possess an exon 21 L858R substitution (1, 3). L858R/L833V is a rare compound mutation found in 6% of lung adenocarcinoma EGFR mutation cases (2). Bonomi et al. reported a partial response to a 1st generation EGFR-TKI in patients harboring a compound L858R/L833V mutation (15). Bar et al. recently reported Osimertinib activity in uncommon EGFR mutated patients with 91% disease control and encouraging PFS and DOR (16).
Despite the fact that osimertinib is a significant advancement in the field of targeted therapy, sadly all tumors eventually develop acquired resistance; some mechanisms involve EGFR (C797X, G796X, L718Q, and exon 20 insertions), while others involve alternative pathways that lead to osimertinib inhibition bypass (MET amplification, HER2 alteration, RET fusion, BRAF alteration, KRAS mutation, PI3K alteration, and small cell transformation) (14).
Fuchs et al. discussed how mechanisms of resistance to osimertinib as a first-line therapy differ from those which develop in the second-line setting (17); MET amplification being a common mechanism of acquired resistance in the setting of first line osimertinib therapy. Interestingly the first patient developed further disease progression on osimertinib through MET amplification alongside the CEP85L-ROS1 fusion gene. As a result of this, crizotinib 250mg BID was added to osimertinib 80mg QD as this combination therapy had demonstrated effectiveness against this resistance mechanism. Crizotonib is a small molecule inhibitor of several receptor tyrosine kinases, including c-MET, ROS1 and ALK, which helps explain its efficacy in settings of MET amplification and rearrangements involving ROS1 (18).
The CEP85L-ROS1 fusion pattern is one of many fusion genes that can be formed with the ROS1 proto-oncogene but has itself only been reported in a case of glioma (19, 20). Despite several years of lack of response to therapies and various treatment plans, this combination therapy demonstrated rapid disease response. To our knowledge, this is the second time that the presence of a CEP85L-ROS1 fusion gene has been identified. The present case is the first reported CEP85L-ROS1 fusion in adenocarcinoma of the lung (20). This patient’s rapid response to osimertinib-crizotinib combination treatment in the setting of metastatic NSCLC leads us to recommend its usage in future cases of CEP85L-ROS1 fusion.
For the second patient, the combination therapy regimen of osimertinib and dabrafenib plus trametinib, a BRAF and MEK1/2 kinase inhibitor, demonstrated a successful response to another EGFR exon 19 del with an MKRN1-BRAF fusion gene. Though this therapy has previously demonstrated a poorly tolerated patient response (21), this finding was not found in our presented patient. MKRN1-BRAF fusion was found in 22 head and neck carcinoma cases and 56 cases of colorectal carcinoma (22). Ross et al. reported a case report that trametinib treatment was effective in metastatic Spitzoid melanoma with ZKSCAN1-BRAF fusion (22). To our knowledge, MKRN1-BRAF fusion was not previously mentioned as acquired resistance to EGFR-TKIs. Despite being previously identified as poorly tolerated, the osimertinib and dabrafenib plus trametinib regimen have shown efficacy in response to BRAF fusion genes with no similarly reported side effects (21).
The last patient presented with metastatic NSCLC-squamous histology with EGFR L858R/V834L and progressed on osimertinib through MET amplification. The addition of amivantamab to osimertinib led to a rapid and sustained response with tolerable toxicities with total eradication of MET and EGFR from the cfDNA in the blood. According to a cohort analysis of the CHRYSALIS trial presented at the American Society of Clinical Oncology (ASCO) 2021 virtual annual meeting, dual EGFR targeting with amivantamab-vmjw (Rybrevant) plus lazertinib (Leclaza) led to durable responses in more than one-third of chemotherapy-naive patients with EGFR-positive non-small-cell lung cancer (NSCLC) whose disease had progressed on osimertinib (23). Amivantamab is a fully human bispecific antibody that targets EGFR and MET, while Lazertinib is a third-generation EGFR tyrosine kinase inhibitor. The updated results of the CHRYSALIS-2 study were presented in ASCO 2022, enrolled patients whose disease progressed on first/2nd-line osimertinib followed by platinum chemotherapy as the last line of therapy. The trial concluded that amivantamab and lazertinib showed encouraging antitumor activity with a manageable safety profile in an unselected population that has exhausted SOC osimertinib and chemotherapy (24). Squamous histology, on the other hand, was not included in this cohort.
In conclusion, this paper describes the treatment of three patients whose cancer has progressed while on osimertinib, demonstrating rare heterogeneity of resistance mechanisms that is potentially treatable. More research is needed to clearly define the best treatments for each resistance mechanism post-osimertinib progression.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conception and design: all authors. Manuscript writing: all authors. Final approval of manuscript: all authors.
NP declares Advisor & Honorarium from & Research with AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Foundation Medicine, Gaurdant360, Merk, MSD, Novartis, NovellusDx, Pfizer, Roche, Takeda. WK declares lecture fees from Bristol-Myers Squibb and MSD.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging treatment paradigms for EGFR-mutant lung cancers progressing on osimertinib: A review. J Clin Oncol (2020) 38(25):2926–36. doi: 10.1200/JCO.19.03123
2. Hayashi T, Kohsaka S, Takamochi K, Hara K, Kishikawa S, Sano K, et al. Clinicopathological characteristics of lung adenocarcinoma with compound EGFR mutations. Hum Pathol (2020) 103:42–51. doi: 10.1016/J.HUMPATH.2020.07.007
3. Astaras C, Bettini A, Betticher DC. Response to osimertinib in a patient with non-small cell lung cancer and two uncommon EGFR mutations. Heal TIMES Oncol Hematol (2020) 3(1):10–3. doi: 10.36000/HBT.OH.2020.03.01
4. Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami NK, Hye RH, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non–small-cell lung cancer: Data from a randomized phase III trial (Aura3). J Clin Oncol (2018) 36:2702–9. doi: 10.1200/JCO.2018.77.9363
5. Peled N, Kian W, Inbar E, Goldstein IM, Zemel M, Rotem O, et al. Osimertinib in advanced EGFR-mutant lung adenocarcinoma with asymptomatic brain metastases: an open-label, 3-arm, phase II pilot study. Neuro-oncology Adv (2021) 4(1). doi: 10.1093/NOAJNL/VDAB188
6. Reungwetwattana T, Nakagawa K, Cho BC, Cobo MC, Eun KB, Alessandro B, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol (2018) 36:3290–7. doi: 10.1200/JCO.2018.78.3118
7. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR -mutated advanced NSCLC. N Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
8. Attili I, Passaro A, Pisapia P, Malapelle U, De Marinis F. Uncommon EGFR compound mutations in non-small cell lung cancer (NSCLC): A systematic review of available evidence. (2022). doi: 10.3390/curroncol29010024
9. Syahruddin E, Wulandari L, Muktiati NS, Rima A, Soeroso N, Ermayanti S, et al. Uncommon EGFR mutations in cytological specimens of 1,874 newly diagnosed Indonesian lung cancer patients. Lung Cancer Targets Ther (2018) 9:25. doi: 10.2147/LCTT.S154116
10. Zaini J, Syahruddin E, Yunus M, Andarini SL, Hudoyo A, Masykura N, et al. Evaluation of PCR-HRM, RFLP, and direct sequencing as simple and cost-effective methods to detect common EGFR mutations in plasma cell-free DNA of non-small cell lung cancer patients. Cancer Rep (Hoboken NJ) (2019) 2(4). doi: 10.1002/CNR2.1159
11. Sousa A, Silveira C, Janeiro A, Malveiro S, Oliveira AR, Felizardo M, et al. Detection of rare and novel EGFR mutations in NSCLC patients: Implications for treatment-decision. Lung Cancer (2020) 139:35–40. doi: 10.1016/J.LUNGCAN.2019.10.030
12. Martin J, Lehmann A, Klauschen F, Hummel M, Lenze D, Grohé C, et al. Clinical impact of rare and compound mutations of epidermal growth factor receptor in patients with non–Small-Cell lung cancer. Clin Lung Cancer (2019) 20(5):350–362.e4. doi: 10.1016/J.CLLC.2019.04.012
13. Evans M, O’Sullivan B, Smith M, Hughes F, Mullis T, Trim N, et al. Large-Scale EGFR mutation testing in clinical practice: Analysis of a series of 18,920 non-small cell lung cancer cases. Pathol Oncol Res (2019) 25(4):1401–9. doi: 10.1007/S12253-018-0460-2/METRICS
14. Ríos-Hoyo A, Moliner L, Arriola E. Acquired mechanisms of resistance to osimertinib–the next challenge. Cancers (Basel) (2022) 14(8). doi: 10.3390/CANCERS14081931
15. Bonomi M, Bonomi L, Sauta MG, Bettini AC, Squadroni M, Brena F, et al. Uncommon epidermal growth factor receptor (EGFR) mutations in non-small cell lung cancer and sensitivity to EGFR tyrosine kinase inhibitors, a two centers experience. Ann Oncol (2016) 27:iv9. doi: 10.1093/annonc/mdw332.20
16. Bar J, Peled N, Schokrpur S, Dudnik E, Wollner M, Girard N, et al. Uncommon EGFR mutations on osimertinib, real-life data (UNICORN study): Updated results, brain efficacy, and resistance mechanisms. J Clin Oncol (2022) 40(16_suppl):9109–9. doi: 10.1200/JCO.2022.40.16_SUPPL.9109
17. Fuchs V, Roisman L, Kian W, Daniel L, Dudnik J, Nechushtan H, et al. The impact of osimertinib’ line on clonal evolution in EGFRm NSCLC through NGS-based liquid biopsy and overcoming strategies for resistance. Lung Cancer (2021). doi: 10.1016/j.lungcan.2020.12.039
18. Rodig SJ, Shapiro IG. Crizotinib, a small-molecule dual inhibitor of the c-met and ALK receptor tyrosine kinases.
20. Davare MA, Henderson JJ, Agarwal A, Wagner JP, Iyer SR, Shah N, et al. Rare but recurrent ROS1 fusions resulting from chromosome 6q22 microdeletions are targetable oncogenes in glioma. Clin Cancer Res (2018) 24(24):6471–82. doi: 10.1158/1078-0432.CCR-18-1052
21. Dagogo-Jack I, Piotrowska Z, Cobb R, Banwait M, Lennerz JK, Hata AN, et al. Response to the combination of osimertinib and trametinib in a patient with EGFR-mutant NSCLC harboring an acquired BRAF fusion. J Thorac Oncol (2019) 14(10):e226–8. doi: 10.1016/J.JTHO.2019.05.046
22. Ross JS, Wang K, Chmielecki J, Gay L, Johnson A, Chudnovsky J, et al. The distribution of BRAF gene fusions in solid tumors and response to targeted therapy. Int J Cancer (2016) 138(4):881. doi: 10.1002/IJC.29825
23. Updated amivantamab and lazertinib combination data demonstrate durable responses and clinical activity for osimertinib-relapsed patients with EGFR-mutated non-small cell lung cancer. Available at: https://www.prnewswire.com/news-releases/updated-amivantamab-and-lazertinib-combination-data-demonstrate-durable-responses-and-clinical-activity-for-osimertinib-relapsed-patients-with-egfr-mutated-non-small-cell-lung-cancer-301295428.html (Accessed August 19, 2022).
24. Shu CA, Goto K, Ohe Y, Besse B, Lee SH, Wang Y, et al. Amivantamab and lazertinib in patients with EGFR-mutant non–small cell lung (NSCLC) after progression on osimertinib and platinum-based chemotherapy: Updated results from CHRYSALIS-2. J Clin Oncol (2022) 40(16_suppl):9006–6. doi: 10.1200/JCO.2022.40.16_SUPPL.9006
Keywords: EGFR, L833V, V834L, compound mutations, lung cancer, CEP85L-ROS1 fusion, MKRN1-BRAF, MET amplification
Citation: Kian W, Krayim B, Alsana H, Giles B, Purim O, Alguayn W, Alguayn F, Peled N and Roisman LC (2023) Overcoming CEP85L-ROS1, MKRN1-BRAF and MET amplification as rare, acquired resistance mutations to Osimertinib. Front. Oncol. 13:1124949. doi: 10.3389/fonc.2023.1124949
Received: 15 December 2022; Accepted: 30 January 2023;
Published: 27 February 2023.
Edited by:
Majid Momeny, University of California San Francisco, United StatesReviewed by:
Solmaz AghaAmiri, University of Texas Health Science Center at Houston, United StatesCopyright © 2023 Kian, Krayim, Alsana, Giles, Purim, Alguayn, Alguayn, Peled and Roisman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nir Peled, bmlycEBzem1jLm9yZy5pbA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.