
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 27 February 2023
Sec. Cancer Molecular Targets and Therapeutics
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1124039
Chimeric antigen receptor-T (CAR-T) cells and antibody-drug conjugates (ADCs) are promising therapeutic strategies in oncology. The carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5) is overexpressed in tumors including non-small cell lung cancer (NSCLC) and pancreatic ductal adenocarcinoma (PDAC), and is an attractive target for therapies based on CAR-T cell or/and ADCs. We previously developed a highly specific antibody-based CAR-T cells targeting CEACAM5 and the tumoricidal effect of CAR-T cells was proved against neuro-endocrine prostate cancer (NEPC) cells expressing CEACAM5. Here, we compare the anti-tumor efficacy of our CAR-T cells with that of an anti-CEACAM5 ADC being clinically evaluated against NSCLC. Our anti-CEACAM5 CAR-T cells showed cytotoxicity in a CEACAM5 surface concentration dependent manner and reduced tumor growth in both ADC-responsive and -non-responsive CEACAM5-expressing NSCLC cells in vitro and in vivo. In contrast, the ADC exhibited cytotoxicity independent on the CEACAM5 cell surface concentration. Even though clinical translation of CEACAM5 targeting CAR-T cell therapies is still in preclinical stage, our CAR-T cell approach could provide a potential therapeutic strategy for CEACAM5-positive cancer patients with resistance to ADCs.
Lung cancer is the prevalent cause of cancer related deaths worldwide (1). Recent advances in precision medicine have transformed lung cancer treatment from palliative chemotherapy to identification and targeting the genetic drivers of the disease (2, 3). Additionally, immunotherapy via the introduction of check point inhibitors has advanced further the methodologies for lung cancer treatment (4–6). The transformation of the way patients are treated has led to a significant prolongation of overall survival compared to traditional chemotherapy (5, 7) and to a continuous decrease in lung cancer-related deaths (8, 9). Non-small cell lung cancer (NSCLC) represents the most common and aggressive type of lung cancer (10) and development of drug resistance remains the main limitation for the treatment of NSCLC (5, 7, 11, 12). To address the challenge of drug resistance, new treatment approaches with a novel target are being sought. Recently, the human carcinoembryonic antigen-related cell adhesion molecule 5 (CEACAM5, CEA, or CD66e) has been identified as target for cancer immunotherapy (13).
CEACAM5 is a cell surface glycoprotein that is overexpressed in a variety of human tumors, including pancreatic cancers, breast cancers, lung cancer, and neuro-endocrine prostate cancer (NEPC) (14–16) and has been functionally associated with tumor differentiation, invasion, and metastasis (17–19). Currently, CEACAM5 has been targeted for developing immunotherapies such as bispecific T cell engagers (BiTEs), CAR-T cells, or ADCs (13, 16). The clinical trials using adoptive immunotherapies targeting CEACAM5 have shown limited success and have been complicated with adverse reactions. In contrast, the ADC, Tusamitamab Ravtansine (formerly SAR408701) has shown promising results and has been advanced to phase III trial (20, 21).
The anti-CEACAM5 ADC SAR408701, developed by Sanofi, is consisted of an anti-CEACAM5 antibody coupled to the maytansinoid agent DM4 with a cleavable linker, N-succinimidyl 4-(2-pyridyldithio) butyrate (SPDB) linker (16, 20). SAR408701 is administered intravenously as a conjugated antibody with an average drug-to-antibody ratio (DAR) of 3.8 (22). The binding of SAR408701 to CEACAM5 triggers antibody internalization, which eventually leads to release of the conjugated DM4 in a free form (23). The free DM4 binds to microtubules and suppresses their assembly (24), which leads to mitotic catastrophe (25). ADCs can potentially eliminate tumor cells by targeting tumor surface antigens acting as a membrane anchor. However, some ADCs show payload-induced toxicities, indicating limited therapeutic windows. In addition, oncogene mutations or resistance mechanisms can induce failure of ADCs (25–28). Possible resistance mechanisms of ADCs include: (1) less accessibility of ADC binding by reduced expression or mutation of target antigen (29), (2) high payload toxicity by up-regulation of drug efflux transporters (30), (3) changes in the intracellular routing or processing of ADCs (31), (4) payload drug resistance by tumor heterogeneity (32), and other mechanisms (25, 33). One approach for treating tumors exhibiting resistance to ADCs is to use CAR-T cells or BiTEs targeting the same antigen as the ADCs. CAR-T cell therapy is composed of T cells collected from autologous peripheral blood and engineered to express CARs specifically directing against the tumor surface antigen of interest (34). We previously identified a novel, fully human monoclonal antibody, 1G9, targeting membrane-proximal region of CEACAM5. CAR-T cells guided by the scFv 1G9 exhibited a potent cytotoxicity for NEPC in vitro and in vivo (35).
Here, we designed a model system using cell lines derived from NSCLC tumors, which are resistant to DM4 or an in-house developed analog of SAR408701, and our anti-CEACAM5 CAR-T cells showed CEACAM5-specific anti-tumor activities for DM4-resistant NSCLCs in vitro and in vivo. Our results suggest the potential of CAR-T cells-based approaches as a therapeutic strategy for ADCs-non-responsive patients.
H1975, A549, H1299, H2030, H2009, HPAC and HPAF-II cells were purchased from ATCC. H1975, A549, H1299, H2030, and H2009 cells were maintained in RPMI1640 (Gibco) supplemented with 10% v/v FBS (Gibco) and 1% penicillin-streptomycin (P/S, Gibco). HPAC cells were cultured in F12K (ATCC) with 10% FBS and 1% P/S. HPAF-II cells were maintained in EMEM (ATCC) with 10% FBS and 1% P/S. H1975-CEACAM5, H2009-CEACAM5, A549-CEACAM5, and H1299-CEACAM5, stably expressing CEACAM5, were generated by stable infection with lentivirus from the CEACAM5 lentiviral plasmid (Origene) using a commonly used protocol (36). Stably transfected cells were selected in RPMI1640 supplemented with 10% FBS, 1% P/S and 1 μg/ml (for A549-CEACAM5) or 2 μg/ml (for H1975-CEACAM5, H2009-CEACAM5, and H1299-CEACAM5) puromycin (Gibco). The cell surface CEACAM5 expression of the stably transfected cells was then assessed with PE-conjugated anti-CEACAM5 IgG1 (Miltenyi Biotec, 130-114-217) by flow cytometry. Anti-CEACAM5 CAR-T cells were generated as previously described (35) and expanded in the T cell media (RPMI1640 supplemented with extra 2mM glutamine, 10% human serum, and 1% P/S) in the presence of hIL-2 (fed every 2 days, 50 IU/ml, Miltenyi Biotec).
Human tumor and normal multiple frozen tissue arrays were purchased from Fisher scientific (50-180-886). 14 tumors and 14 correspondent normal tissues (brain, breast, colon, muscle, kidney, liver, lung, pancreas, prostate, skin, small intestine, stomach, ovary, and uterus) were mounted on a positively charged glass slide. For immunohistochemistry, the tissue slide was blocked with 10% normal horse serum for 1 h at 25°C and incubated with mouse anti-human CEACAM5 antibody (Novus biologicals, NB11058734, 1:100) for overnight at 4°C in humidified chamber. The tissue slide was washed with PBS, incubated with biotin-conjugated anti-mouse antibody (Invitrogen, B2763, 1:200) for 1 h at 25°C, and washed again with PBS. Slide was incubated with ImmunoCruz ABC staining (Santacruz, sc-516216) by the manufacturer instructions. Stains were then visualized using DAB peroxidase substrate (Santacruz, sc-249982). The positive pixel areas of CEACAM5 staining of the entire tissue were quantified using Image J software and the total CEACAM5 area of tumor tissue normalized to the total CEACAM5 pixel area of the corresponding normal tissue.
The cell killing activity of DM4 or the ADC SAR408701 analog was measured by CellTiter-Glo Luminescent cell viability assay kit (Promega, G7571). Cells (2.5×103 cells/well in white 96-well plate) were cultured for 12 h prior to treatment with the indicated doses of DM4 or ADC for 96 h at 37°C. Normalized % ATP values were calculated by normalizing luminescence values for buffer (DPBS or DMSO)-treated cells. The LDH-Glo cytotoxicity assay kit (Promega, J2381) was used to measure cell viability in treatment of anti-CEACAM5 CAR-T cells. Control T or CAR-T cells as effector cells were incubated with target cells (5×103 cells/well in 96-well plate) at the indicated E:T ratio for 24 h at 37°C. Controls conducted for the calculation of percent cytotoxicity were included according to the manufacturer’s instructions.
All studies were approved by the University of Pittsburgh institutional Animal Care and Use Committee. A549 cells (7×106/mice), A549-CEACAM5 cells (7×106/mice) or H1975-CEACAM5 cells (5×106/mice) resuspended in 200 μl of DPBS were subcutaneously injected into the right flank of female NSG mice (6-8 weeks old, The Jackson Laboratory). When the tumor volume reached approximately 150 mm3, mice were intravenously treated with ADC SAR408701 analog (10 mg/kg or 5 mg/kg), Control T (5x106/mice) or anti-CEACAM5 CAR-T cells (5×106 or 2×106/mice) every 4 days, two times, via tail vein. Tumor volume was measured by two-dimensional measurements with a caliper and calculated according to the formula V=0.5 × length × (width)2. Tumor growth inhibition (TGI) by anti-CEACAM5 ADC or CAR-T compared to that by vehicle or control T was determined on the last day of the study according to the formula: TGI (%) = [1-(-)/(-)] ×100, where Vf is the final mean tumor volume in the treated group (ADC or CAR-T cells), and Vi is the initial mean tumor volume in the control group (vehicle or control T cells). Animals were euthanized when the tumor volume reached >1-1.5 cm3.
Statistical analyses were performed using GraphPad Prism software (GraphPad, Inc.). Data are presented as the mean ±SD for representative data from three independent experiments. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used to evaluate the significance of differences. Survival curve was represented as Kaplan-Meier plots, with statistical significance determined by log-rank (Mantel-Cox) tests. P values less than 0.05 were considered statistically significant. P values less than 0.05, 0.01, 0.001, and 0.0001 are indicated as *, **, ***, and ****, in the respective figure.
To verify protein expression of CEACAM5 in human tissues, we performed IHC analysis with 14 different human tumor and 14 correspondent normal tissues (brain, breast, colon, muscle, kidney, liver, lung, pancreas, prostate, skin, small intestine, stomach, ovary, and uterus) on the tissue microarray. Lung tumor and pancreas tumor highly expressed CEACAM5 compared to normal lung and pancreas tissues (Figure 1A). In contrast, CEACAM5 expression was detected in normal tissue as well as tumor tissue from the colon, and no CEACAM5 was observed in normal and tumor of other tissues, as reported previously (16, 37). Recent data indicated that prostate cancer subtypes are differentiated as prostate adenocarcinoma (PrAd) and neuroendocrine prostate cancer (NEPC) (37). CEACAM5 is especially prominent as a therapeutic target in NEPC (35). In accordance with CEACAM5 overexpression in lung tumor tissues, the anti-CEACAM5 antibody drug-conjugate (ADC) SAR408701 is being evaluated in non-small cell lung cancer (NSCLC) with different interventions in clinical trials (16, 20).

Figure 1 CEACAM5 expressions in different normal/tumor tissues and DM4 sensitivity in NSCLCs and PDACs. (A) CEACAM5 immunohistochemistry (IHC) of 14 tumor tissues and 14 correspondent normal tissues in tissue microarray (left panel) and quantification values of IHC images (right panel). (B) DM4 response curves in NSCLCs (H1975, A549, H1299, H2030, and H2009 cells) and PDACs (HPAC and HPAF-II cells). Normalized % cell viability (ATP level) was calculated by normalizing luminescence values using buffer (DMSO)-treated respective cells. Results are shown as the mean ±SD for representative data from three independent experiments.
DM4 is a potent cytotoxic agent derived from maytansine that blocks tubulin polymerization and is used as a payload for ADC SAR408701. We determined the sensitivity of NSCLC cells (NSCLCs) and PDAC cells (PDACs) to DM4. Three NSCLCs, A549, H2030, and H1299 cells, responded to treatment with DM4, but two NSCLCs, H1975 and H2009 cells, showed resistance to DM4 (Figure 1B). The two tested PDACs, HPAC and HPAF-II cells, were sensitive to DM4 in vitro (Figure 1B). Vecchione, L. et al. reported that BRAF(V600E) is a predictive biomarker of the DM4 response in colon cancer PDX models (26). To identify whether the DM4 sensitivity would be predicted by oncogene mutation status, we examined oncogene mutations through Cancer Cell Line Encyclopedia (CCLE) database (38). No correlation was found between the observed DM4 sensitivity and oncogene(s) mutation status (Supplementary Table S1).
To test in vitro cytotoxicity of CAR-T cells and ADC targeting CEACAM5, we successfully produced an analog of ADC SAR408701 derived from Sanofi (16). The ADC SAR408701 analog, produced by NJ Biopharmaceuticals, has a DAR of 3.7 as determined by reverse-phase (RP) chromatography coupled with mass spectrum (MS) analysis, and exhibits a homogenous folding as tested by size-exclusion chromatography (SEC) (Supplementary Figure S1). The CEACAM5 binding specificity and affinity of in-house developed ADC SAR408701 analog were also confirmed (data not shown). The CEACAM5 surface expression was first screened in 7 lung cancer cell lines, 4 pancreatic cancer cell lines, and 3 other cancer cell lines (Supplementary Figure S2). Unfortunately, total 7 lung cancer cell lines exhibited no CEACAM5 surface expression, so we generated CEACAM5-stably expressing DM4-sensitive (DM4S) NSCLCs, H1299-CEACAM5 and A549- CEACAM5. CEACAM5 expression was confirmed by flow cytometry (Figure 2A). ADC SAR408701 analog was tested in dose-response cell viability assays using four DM4S CEACAM5-positive cell lines (H1299-CEACAM5, A549-CEACAM5, HPAC, and HPAF-II) and two DM4S CEACAM5-negative cell lines (H1299 and A549). Treatment with ADC SAR408701 analog reduced cell viability in a dose-dependent manner with all CEACAM5-positive cells (Figures 2B, D). However, the ADC SAR408701 analog also showed a killing activity in DM4S CEACAM5-negative H1299 and A549 cell lines (Figure 2B) which may be attributed to the payload-induced toxicity independent of direct antigen-mediated internalization (39, 40). We next assessed the cytotoxicity of our anti-CEACAM5 CAR-T cells for DM4S NSCLCs and PDACs in vitro. Our anti-CEACAM5 CAR-T cells were previously generated and evaluated in prostate cancer cells in vitro and in vivo (35). Anti-CEACAM5 CAR-T cells showed a potent cytotoxicity against CEACAM5-positive NSCLCs (H1299-CEACAM5 and A549-CEACAM5) (Figure 2C) and PDACs (HPAC and HPAF-II) (Figure 2E). Contrary to the ADC SAR408701 analog, CAR-T cells did not exhibit non-specific toxicity in CEACAM5-negative NSCLCs (H1299 and A549) (Figure 2C).
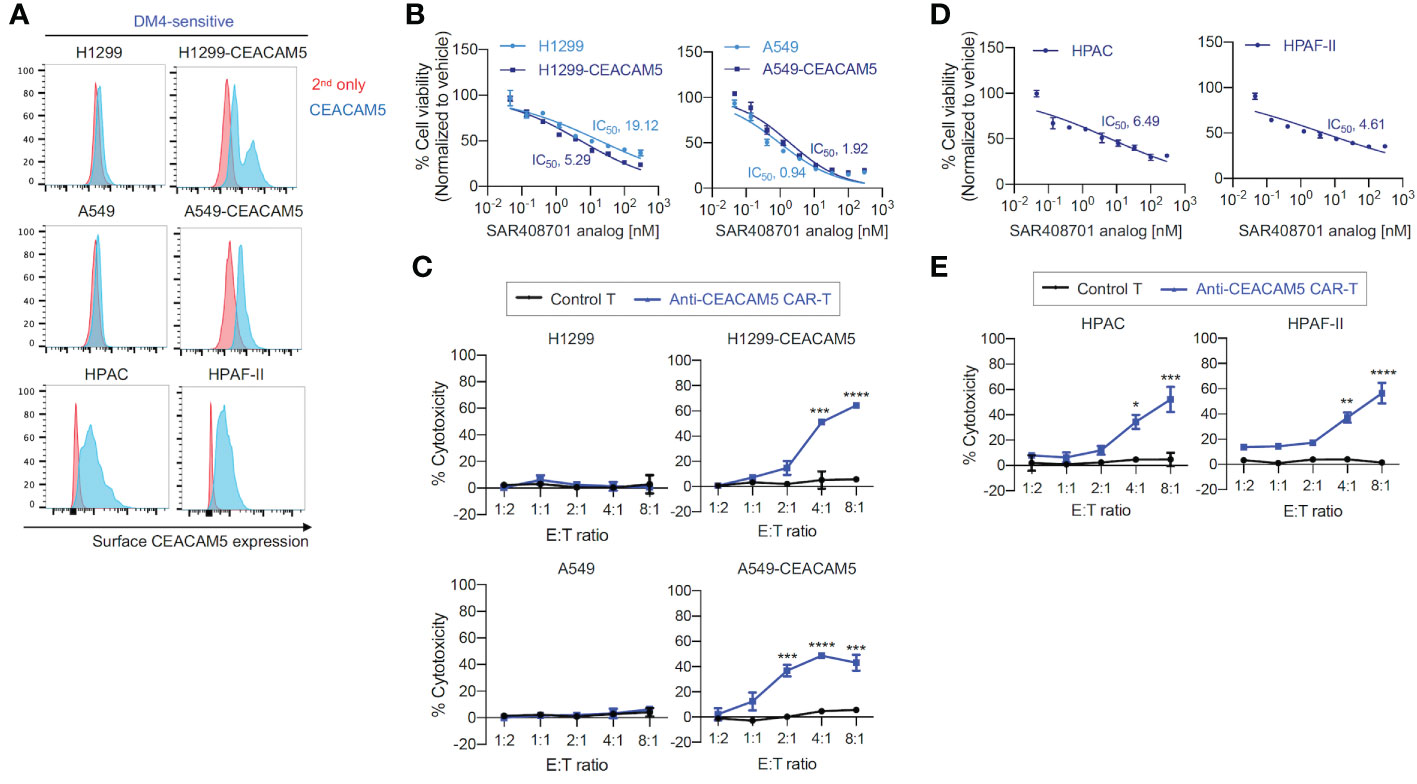
Figure 2 in vitro cell killing activities of the ADC SAR408701 analog and CAR-T cells targeting CEACAM5 in DM4S cells. (A) Cell surface CEACAM5 expression levels in DM4S NSCLCs (H1299, H1299-CEACAM5, A549, and A549-CEACAM5 cells) and PDACs (HPAC and HPAF-II cells). (B, D) Cell killing activities with ADC SAR408701 analog against DM4S NSCLCs (H1299, H1299-CEACAM5, and A549, A549-CEACAM5 cells) (B) and DM4S PDACs (HPAC, and HPAF-II cells) (D). Normalized % cell viability (ATP level) was calculated by normalizing luminescence values using vehicle (buffer)-treated respective cells. The IC50 were then determined by nonlinear regression plot of percent specific cytotoxicity versus Log10 concentration of ADC SAR408701 analog using GraphPad Prism software. (C, E) Cytotoxic activities (%) of anti-CEACAM5 CAR-T cells against DM4S NSCLCs (C) and PDACs (E). Significance was tested using one-way ANOVA, followed by the tukey’s multiple post hoc test. ****, P<0.0001; ***, P<0.001; **, P<0.01; *, P<0.05; versus control T at each E:T ratio. (B–E) Results are shown as the mean ±SD for representative data from three independent experiments.
Second, we evaluated the effects of anti-CEACAM5 ADC SAR408701 analog and CAR-T cells on DM4-resistant (DM4R) NSCLCs (H1975 and H2009). CEACAM5-expressing NSCLCs - H1975-CEACAM5 and H2009-CEACAM5, were constructed (Figure 3A). The DM4R NSCLCs expressing or not expressing CEACAM5 showed a mild concentration-dependent response to treatment with the ADC SAR408701 analog (measured maximum of 20% cytotoxicity) (Figure 3B). In contrast, the anti-CEACAM5 CAR-T cells demonstrated a strong E:T ratio-dependent cytotoxicity for cells expressing CEACAM5 and no response to cells not expressing CEACAM5 (Figure 3C).
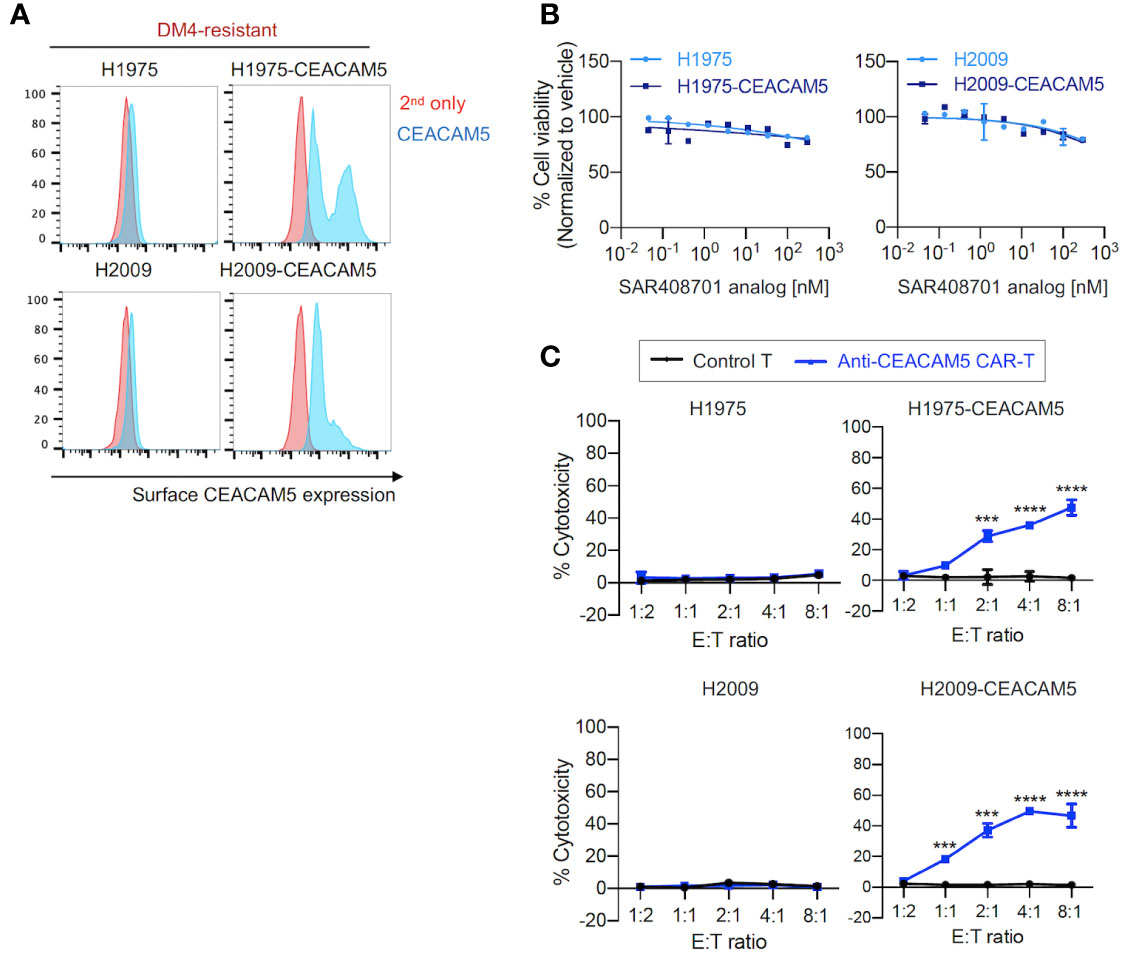
Figure 3 in vitro cell cytotoxicity assay of the ADC SAR408701 analog and CAR-T cells targeting CEACAM5 in DM4R cells. (A) Cell surface CEACAM5 expression levels in DM4R NSCLCs (H1975, H1975-CEACAM5, H2009, and H2009-CEACAM5 cells). (B) Cell killing activities with ADC SAR408701 analog against CEACAM5-positive DM4R cell lines (H1975-CEACAM5 and H2009-CEACAM5 cells) and CEACAM5-negative DM4R cell lines (H1975 and H2009 cells). Normalized % cell viability (ATP level) was calculated by normalizing luminescence values for vehicle (buffer)-treated respective cells. (C) Cytotoxic activities (%) of anti-CEACAM5 CAR-T cells against CEACAM5-positive and CEACAM5-negative DM4R NSCLC cells. Significance was tested using one-way ANOVA, followed by the tukey’s multiple post hoc test. ****, P<0.0001; ***, P<0.001; versus control T at each E:T ratio. (B, C) Results are shown as the mean ±SD for representative data from three independent experiments.
To further examine the therapeutic potentials of our CAR-T cells in vivo, we utilized mouse xenograft tumor models of CEACAM5-expressing DM4S (A549-CEACAM5), DM4R (H1975-CEACAM5) and CEACAM5-negative DM4S (A549) NSCLCs. Anti-CEACAM5 CAR-T cells and ADC SAR408701 were administered as Figure 4A. In mice bearing DM4S A549-CEACAM5, ADC SAR408701 analog treatment significantly reduced tumor growth and improved mouse survival in dose-dependent manner. CAR-T cells also showed a dose-dependent tumor growth inhibition (Figure 4B) and extended survival in CEACAM5-positive DM4S A549-CEACAM5 (Figure 4C). However, a different result was observed in the DM4R H1975-CEACAM5 tumors, where potent anti-tumor activity was observed for CAR-T cells-treated group, but not for the ADC SAR408701 analog treated group (Figures 4D, E). We sought further insights into the tumor growth inhibition activity of anti-CEACAM5 CAR-T and ADC SAR408701 analog by examining their activity in a CEACAM5-negative DM4S A549 tumor model, because the ADC SAR408701 analog showed a CEACAM5-independent killing activity in vitro cell-based system. The high dose (10 mg/kg) of ADC SAR408701 analog suppressed A549 tumor growth (51% TGI) even in the absence of expressed CEACAM5, but anti-CEACAM5 CAR-T displayed no tumor growth inhibition (Figures 4F, G). The mouse body weight, monitored as an indicator of drug toxicity, was similar compared with vehicle group (Supplementary Figure S3). These results indicate that our CAR-T cells therapy is effective and safe against NSCLCs and can be an alternative treatment strategy in ADC-non-responsive NSCLCs.
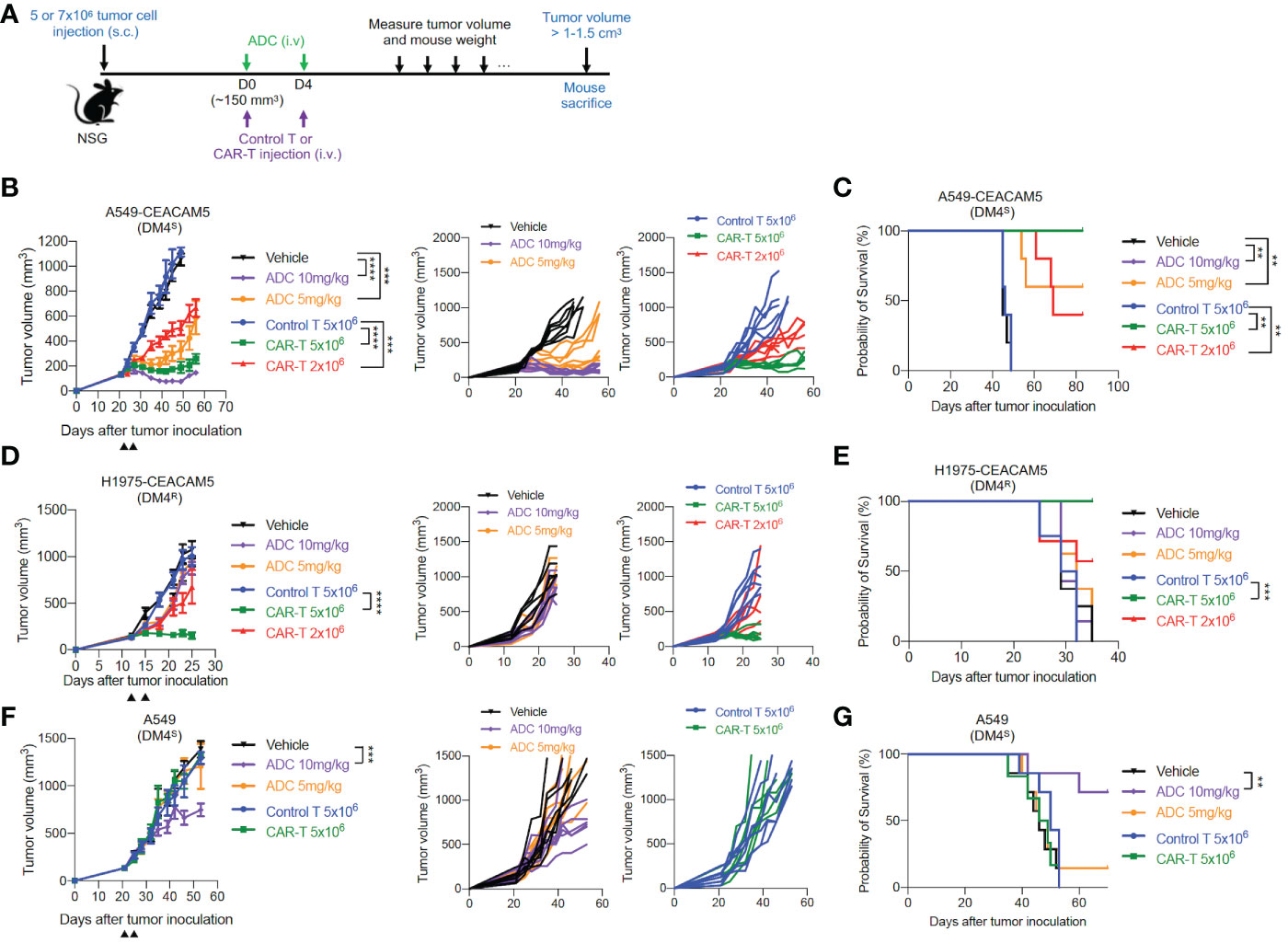
Figure 4 in vivo anti-tumor activities of the ADC SAR408701 analog and CAR-T cells targeting CEACAM5 in DM4S and DM4R NSCLC tumors. (A) Schematic representation of experimental design and treatment schedule for mice studies. (B, D, F) Tumor growth curve (left panels) and individual mice curves (right panels) of DM4S A549-CEACAM5 (B), DM4R H1975-CEACAM5 (D), and CEACAM5-negative DM4S A549 (F) tumors. Significance was analyzed by comparing the tumor volume (mm3) ±SD at endpoint of 1.0 cm3 (B) or end day (D, F) and determined using one-way ANOVA, followed by the tukey’s multiple post hoc test. ****, P<0.0001; ***, P<0.001. (C, E, G) Survival curve showing the efficacy of the ADC SAR408701 analog and CAR-T cells in NSG mice. Tumor volume (mm3) are shown as mean ±SD for n=6 or 7 per group. Survival was presented by Kaplan-Meier plot of percentage of mice with tumor volume ≥ 1-1.5 cm3. Significance was determined by log-rank (Mantel-Cox) test. ***, P<0.001; **, P<0.01.
NSCLC is a primary type of lung cancer and one of the most common malignant tumors on a global scale (9, 41). CEACAM5, a glycosylated transmembrane protein, is often presented in lung cancer tumor tissues (42). CEACAM5-targeted therapies, including CAR-T cells (43) or ADCs (20), have been developed against lung cancer. Here, we investigated the efficacy of such therapeutic modalities targeting CEACAM5 in ADC-sensitive and -resistant NSCLC cell lines. CAR-T cells mediated MHC-unrestricted tumor cell killing by enabling T cells to bind target cell surface antigens (44). CAR-T cell therapies are being developed as potentially powerful immunotherapeutic tools. However, they remain unable successfully fight solid tumors in their current state (45, 46) due to heterogeneous tumor antigen expression (47), the immunosuppressive tumor microenvironment (48) and T cell exhaustion driven by chronic antigen exposure (49). Another targeted approach, ADC, is an evolving class of immunotherapeutics that consist of a cytotoxic agents linked covalently to an antibody. ADCs act through a series of processes including target cell binding, internalization, and release of cytotoxic payload (50). ADCs can potentially eliminate tumor cells by targeting tumor surface antigens. In some cases, however, neighboring cancer cells (bystander effects) or normal cells (toxicity) that do not express the tumor surface antigen can be abolished (51).
The preclinically validated anti-CEACAM5 ADC, SAR408701, was developed by Sanofi, and it is comprised of the antibody SAR408377 covalently linked to the cytotoxic agent maytansinoid DM4, a potent microtubule-destabilizing agent (16). SAR408701 is currently being evaluated in advanced colorectal, gastric, and non-small cell lung cancer patients. In an interim analysis of a first in-human study (NCT02187848) in patients with non-squamous NSCLC, SAR408701 showed an objective response rate (ORR) of only 23%, even in patients with ≥ 50% of CEACAM5-expressing tumor cells. One possibility for this significant difference could be the emergence of ADC resistant subclones caused by cell surface recycling of the targeted tumor antigen, altered internalization, or impaired release of the toxic payload into the cytosol (29, 52). In this study, we examined the response of NSCLCs or PDACs to single drug DM4, the payload of SAR408701, and identified that the ADC response of cell lines used in this study was determined by payload DM4 response. We compared the cytotoxic effects of in-house developed ADC SAR408701 analog and our anti-CEACAM5 CAR-T (35) to DM4S and DM4R NSCLCs and PDACs. Our anti-CEACAM5 CAR-T cells exhibited a potent cytotoxicity for both DM4S and DM4R CEACAM5-expressing NSCLCs or PDACs, both in vitro and in vivo. By contrast, SAR408701 analog only showed cytotoxicity to DM4S cells. Also, the ADC SAR408701 analog exhibited killing effects against DM4S NSCLCs irrespective of CEACAM5 expression. CEACAM5 was previously reported as a non-internalizing receptor or very slow internalization receptor (53). This property of CEACAM5 may contribute to the different efficacy of CAR-T cells and ADCs, but further experimentation to examine the detailed mechanism is needed.
In this study, two promising therapeutic strategies in oncology, CAR-T cell therapy and ADC, are compared in terms of efficacy and toxicity. Both strategies represent promising therapeutic modalities in spite of many safety issues in trials (54), lack of transparency in data sharing (55). Accordingly customized patient selection for each therapy is important (56, 57). In this regard, our anti-CEACAM5 CAR-T cells therapy can be a promising candidate for development as a potential treatment for ADC-non-responsive patients with CEACAM5-positive tumors.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by University of Pittsburgh institutional Animal Care and Use Committee.
Y-JK, JM, DD, and D-SB wrote the manuscript and analyzed the data. Y-JK and D-SB designed and performed the experiments. WL, DZ, and JM interpreted the data and edited the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by UPMC (United States) internal funding.
We would like to thank the members of the Center for Antibody Therapeutics in University of Pittsburgh for their helpful discussions.
Authors JM, DD and D-SB were employed by Abound Bio.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1124039/full#supplementary-material
1. Bray F, Ferlay J, Soerjomataram I, Sieg RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca-a Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Kalemkerian GP, Narula N, Kennedy EB. Molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement summary of the college of American Pathologists/International association for the study of lung Cancer/Association for molecular pathology clinical practice guideline update. J Oncol Pract (2018) 14(5):323–7. doi: 10.1200/JOP.18.00035
3. Kalemkerian GP, Narula N, Kennedy EB, Biermann WA, Donington J, Leighl NB, et al. Molecular testing guideline for the selection of patients with lung cancer for treatment with targeted tyrosine kinase inhibitors: American society of clinical oncology endorsement of the college of American Pathologists/International association for the study of lung Cancer/Association for molecular pathology clinical practice guideline update. J Clin Oncol (2018) 36(9):911–9. doi: 10.1200/JCO.2017.76.7293
4. Suraya R, Tachihara M, Nagano T, Nishimura Y, Kobayashi K. Immunotherapy in advanced non-small cell lung cancers: Current status and updates. Cancer Manag Res (2022) 14:2079–90. doi: 10.2147/CMAR.S366738
5. Cheng Y, Zhang T, Xu Q. Therapeutic advances in non-small cell lung cancer: Focus on clinical development of targeted therapy and immunotherapy. Medcomm (2021) 2(4):692–729. doi: 10.1002/mco2.105
6. Batra U, Chufal KS, Nathany S, Ahmad I, Chowdhary RL, Sharma M, et al. Immunotherapy in advanced non-small-cell lung cancer (NSCLC) after progression on chemotherapy: Real-world results from a prospective institutional cohort. Immunotherapy (2022) 14(11):851–8. doi: 10.2217/imt-2021-0170
7. Tulpule A, Bivona TG. Acquired resistance in lung cancer. Annu Rev Cancer Biol (2020) 4(1):279–97. doi: 10.1146/annurev-cancerbio-030419-033502
8. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Ca-a Cancer J Clin (2018) 68(1):7–30. doi: 10.3322/caac.21442
9. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 72(1):7–33. doi: 10.3322/caac.21708
10. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc (2008) 83(5):584–94. doi: 10.1016/S0025-6196(11)60735-0
11. Chen P, Kuang P, Wang L, Li W, Chen B, Liu Y, et al. Mechanisms of drugs-resistance in small cell lung cancer: DNA-related, RNA-related, apoptosis-related, drug accumulation and metabolism procedure. Transl Lung Cancer Res (2020) 9(3):768–86. doi: 10.21037/tlcr-19-547
12. Frisone D, Friedlaender A, Addeo A, Tsantoulis P. The landscape of immunotherapy resistance in NSCLC. Front Oncol (2022) 12:817548. doi: 10.3389/fonc.2022.817548
13. Han ZW, Lyv ZW, Cui B, Wang YY, Cheng JT, Zhang Y, et al. The old CEACAMs find their new role in tumor immunotherapy. Invest New Drugs (2020) 38(6):1888–98. doi: 10.1007/s10637-020-00955-w
14. Zheng C, Feng J, Lu D, Wang P, Xing S, Coll JL, et al. A novel anti-CEACAM5 monoclonal antibody, CC4, suppresses colorectal tumor growth and enhances NK cells-mediated tumor immunity. PloS One (2011) 6(6):e21146. doi: 10.1371/journal.pone.0021146
15. DeLucia DC, Cardillo TM, Ang L, Labrecque MP, Zhang A, Hopkins JE, et al. Regulation of CEACAM5 and therapeutic efficacy of an anti-CEACAM5-SN38 antibody-drug conjugate in neuroendocrine prostate cancer. Clin Cancer Res (2021) 27(3):759–74. doi: 10.1158/1078-0432.CCR-20-3396
16. Decary S, Berne PF, Nicolazzi C, Lefebvre AM, Dabdoubi T, Cameron B, et al. Preclinical activity of SAR408701: A novel anti-CEACAM5-maytansinoid antibody-drug conjugate for the treatment of CEACAM5-positive epithelial tumors. Clin Cancer Res (2020) 26(24):6589–99. doi: 10.1158/1078-0432.CCR-19-4051
17. Blumenthal RD, Leon E, Hansen HJ, Goldenberg DM. Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer (2007) 7:2. doi: 10.1186/1471-2407-7-2
18. Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal (1991) 5(5):344–66. doi: 10.1002/jcla.1860050510
19. Powell E, Shao J, Picon HM, Bristow C, Ge Z, Peoples M, et al. A functional genomic screen in vivo identifies CEACAM5 as a clinically relevant driver of breast cancer metastasis. NPJ Breast Cancer (2018) 4:9. doi: 10.1038/s41523-018-0062-x
20. Gazzah A, Bedard PL, Hierro C, Kang YK, Razak AA, Ryu MH, et al. Safety, pharmacokinetics, and antitumor activity of the anti-CEACAM5-DM4 antibody-drug conjugate tusamitamab ravtansine (SAR408701) in patients with advanced solid tumors: first-in-human dose-escalation study. Ann Oncol (2022) 33(4):416–25. doi: 10.1016/j.annonc.2021.12.012
21. Pouzin C, Tod M, Chadjaa M, Fagniez N, Nguyen L. Covariate analysis of tusamitamab ravtansine, a DM4 anti-CEACAM5 antibody-drug conjugate, based on first-in-human study. CPT Pharmacometrics Syst Pharmacol (2022) 11(3):384–94. doi: 10.1002/psp4.12769
22. Pouzin C, Gibiansky L, Fagniez N, Chadjaa M, Tod M, Nguyen L. Integrated multiple analytes and semi-mechanistic population pharmacokinetic model of tusamitamab ravtansine, a DM4 anti-CEACAM5 antibody-drug conjugate. J Pharmacokinet Pharmacodyn (2022) 49(3):381–94. doi: 10.1007/s10928-021-09799-0
23. Chen H, Zongtao Lin Z, Arnst KE, Miller DD, Li W. Tubulin inhibitor-based antibody-drug conjugates for cancer therapy. Molecules (2017) 22(8):1281. doi: 10.3390/molecules22081281
24. Lopus M, Oroudjev E, Wilson L, Wilhelm S, Widdison W, Chari R, et al. Maytansine and cellular metabolites of antibody-maytansinoid conjugates strongly suppress microtubule dynamics by binding to microtubules. Mol Cancer Ther (2010) 9(10):2689–99. doi: 10.1158/1535-7163.MCT-10-0644
25. Díaz-Rodríguez E, Gandullo-Sánchez L, Ocaña A, Pandiella A. Novel ADCs and strategies to overcome resistance to anti-HER2 ADCs. Cancers (Basel) (2021) 14(1):154. doi: 10.3390/cancers14010154
26. Vecchione L, Gambino V, Raaijmakers J, Schlicker A, Fumagalli A, Russo M, et al. A vulnerability of a subset of colon cancers with potential clinical utility. Cell (2016) 165(2):317–30. doi: 10.1016/j.cell.2016.02.059
27. Prota AE, Bargsten K, Diaz JF, Steinmetz MO. A new tubulin-binding site and pharmacophore for microtubule-destabilizing anticancer drugs. Proc Natl Acad Sci USA. (2014) 111(38):13817–21. doi: 10.1073/pnas.1408124111
28. Hunter FW, Barker HR, Zipert B, Rothé F, Gebhart G, Martine J, et al. Mechanisms of resistance to trastuzumab emtansine (T-DM1) in HER2-positive breast cancer. Br J Cancer (2020) 122(5):603–12. doi: 10.1038/s41416-019-0635-y
29. Collins DM, Bossenmaier B, Kollmorgen G, Gerhard Niederfellner G. Acquired resistance to antibody-drug conjugates. Cancers (Basel) (2019) 11(3):394. doi: 10.3390/cancers11030394
30. Bugg BY, Danks MK, Beck WT, Suttle DP. Expression of a mutant DNA topoisomerase II in CCRF-CEM human leukemic cells selected for resistance to teniposide. Proc Natl Acad Sci USA (1991) 88(17):7654–8. doi: 10.1073/pnas.88.17.7654
31. Chalouni C, Doll S. Fate of antibody-drug conjugates in cancer cells. J Exp Clin Cancer Res (2018) 37(1):20. doi: 10.1186/s13046-017-0667-1
32. Yamazaki CM, Yamaguchi A, Anami Y, Xiong W, Otani Y, Lee J, et al. Antibody-drug conjugates with dual payloads for combating breast tumor heterogeneity and drug resistance. Nat Commun (2021) 12(1):3528. doi: 10.1038/s41467-021-23793-7
33. Marei HE, Cenciarelli C, Hasan A. Potential of antibody-drug conjugates (ADCs) for cancer therapy. Cancer Cell Int (2022) 22(1):255. doi: 10.1186/s12935-022-02679-8
34. Guedan S, Ruella M, June CH. Emerging cellular therapies for cancer. Annu Rev Immunol (2019) 37:145–71. doi: 10.1146/annurev-immunol-042718-041407
35. Baek DS, Kim YJ, Vergara S, Conard A, Adams C, Calero G, et al. A highly-specific fully-human antibody and CAR-T cells targeting CD66e/CEACAM5 are cytotoxic for CD66e-expressing cancer cells in vitro and in vivo. Cancer Lett (2022) 525:97–107. doi: 10.1016/j.canlet.2021.10.041
36. Elegheert J, Behiels E, Bishop B, Scott S, Woolley RE, Griffiths SC, et al. Lentiviral transduction of mammalian cells for fast, scalable and high-level production of soluble and membrane proteins. Nat Protoc (2018) 13(12):2991–3017. doi: 10.1038/s41596-018-0075-9
37. Lee JK, Bangayan NJ, Chai T, Witte ON. Systemic surfaceome profiling identifies target antigens for immune-based therapy in subtypes of advanced prostate cancer. Proc Natl Acad Sci USA (2018) 115(19):E4473–82. doi: 10.1073/pnas.1802354115
38. Bouhaddou M, DiStefano MS, Riesel EA, Carrasco E, Holzapfel HY, Jones DC, et al. Drug response consistency in CCLE and CGP. Nature (2016) 540(7631):E9–E10. doi: 10.1038/nature20580
39. Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer (2017) 117(12):1736–42. doi: 10.1038/bjc.2017.367
40. Dong Y, Liao H, Yu J, Fu H, Zhao D, Gong K, et al. Incorporation of drug efflux inhibitor and chemotherapeutic agent into an inorganic/organic platform for the effective treatment of multidrug resistant breast cancer. J Nanobiotechnol (2019) 17(1):125. doi: 10.1186/s12951-019-0559-y
41. Kim YJ, Baek DS, Lee S, Park D, Kang HN, Cho BC, et al. Dual-targeting of EGFR and neuropilin-1 attenuates resistance to EGFR-targeted antibody therapy in KRAS-mutant non-small cell lung cancer. Cancer Lett (2019) 466:23–34. doi: 10.1016/j.canlet.2019.09.005
42. Zhang X, Han X, Zuo P, Zhang X, Xu H. CEACAM5 stimulates the progression of non-small-cell lung cancer by promoting cell proliferation and migration. J Int Med Res (2020) 48(9):300060520959478. doi: 10.1177/0300060520959478
43. Liu Y, He Y. A narrative review of chimeric antigen receptor-T (CAR-T) cell therapy for lung cancer. Ann Transl Med (2021) 9(9):808. doi: 10.21037/atm-20-7626
44. Benmebarek MR, Karches CH, Cadilha BL, Lesch S, Endres S, Kobold S. Killing mechanisms of chimeric antigen receptor (CAR) T cells. Int J Mol Sci (2019) 20(6):1283. doi: 10.3390/ijms20061283
45. Kozani PS, Kozani PS, Najafabadi MA, Mirarefin SMJ, Rahbarizadeh F. Recent advances in solid tumor CAR-T cell therapy: Driving tumor cells from hero to zero? Front Immunol (2022) 13:795164. doi: 10.3389/fimmu.2022.795164
46. Sterner RC, Sterner RM. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J (2021) 11(4):69. doi: 10.1038/s41408-021-00459-7
47. Kailayangiri S, Altvater B, Wiebel M, Jamitzky S, Rossig C. Overcoming heterogeneity of antigen expression for effective CAR T cell targeting of cancers. Cancers (Basel) (2020) 12(5):1075. doi: 10.3390/cancers12051075
48. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene (2008) 27(45):5904–12. doi: 10.1038/onc.2008.271
49. Bettonville M, d'Aria S, Weatherly K, Porporato PE, Zhang J, Bousbata S, et al. Long-term antigen exposure irreversibly modifies metabolic requirements for T cell function. Elife (2018) 7:e30938. doi: 10.7554/eLife.30938
50. Fu Z, Li S, Han S, Shi C, Zhang Y. Antibody drug conjugate: the "biological missile" for targeted cancer therapy. Signal Transduct Target Ther (2022) 7(1):93. doi: 10.1038/s41392-022-00947-7
51. Boni V, Sharma MR, Patnaik A. The resurgence of antibody drug conjugates in cancer therapeutics: Novel targets and payloads. Am Soc Clin Oncol Educ Book (2020) 40:1–17. doi: 10.1200/EDBK_281107
52. Buongervino S, Lane MV, Garrigan E, Zhelev DV, Dimitrov DS, Bosse KR. Antibody-drug conjugate efficacy in neuroblastoma: Role of payload, resistance mechanisms, target density, and antibody internalization. Mol Cancer Ther (2021) 20(11):2228–39. doi: 10.1158/1535-7163.MCT-20-1034
53. Schmidt MM, Thurber GM, Wittrup KD. Kinetics of anti-carcinoembryonic antigen antibody internalization: effects of affinity, bivalency, and stability. Cancer Immunol Immunother (2008) 57(12):1879–90. doi: 10.1007/s00262-008-0518-1
54. Rasche L, Wäsch R, Munder M, Goldschmidt H, Raab MS. Novel immunotherapies in multiple myeloma - chances and challenges. Haematologica (2021) 106(10):2555–65. doi: 10.3324/haematol.2020.266858
55. Rosenthal M, Curry R, Reardon DA, Rasmussen E, Upreti VV, Damore MA, et al. Safety, tolerability, and pharmacokinetics of anti-EGFRvIII antibody-drug conjugate AMG 595 in patients with recurrent malignant glioma expressing EGFRvIII. Cancer Chemother Pharmacol (2019) 84(2):327–36. doi: 10.1007/s00280-019-03879-2
56. Dulan SO, Viers KL, Wagner JR, Clark MC, Chang B, Gorospe GL, et al. Developing and monitoring a standard-of-Care chimeric antigen receptor (CAR) T cell clinical quality and regulatory program. Biol Blood Marrow Transplant (2020) 26(8):1386–93. doi: 10.1016/j.bbmt.2020.03.021
Keywords: CEACAM5, NSCLCs, CAR-T, ADC, DM4 resistance, immunotherapy
Citation: Kim Y-J, Li W, Zhelev DV, Mellors JW, Dimitrov DS and Baek D-S (2023) Chimeric antigen receptor-T cells are effective against CEACAM5 expressing non-small cell lung cancer cells resistant to antibody-drug conjugates. Front. Oncol. 13:1124039. doi: 10.3389/fonc.2023.1124039
Received: 14 December 2022; Accepted: 08 February 2023;
Published: 27 February 2023.
Edited by:
Shiv K. Gupta, Mayo Clinic, United StatesReviewed by:
Sonia Jain, Mayo Clinic, United StatesCopyright © 2023 Kim, Li, Zhelev, Mellors, Dimitrov and Baek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimiter S. Dimitrov, bWl0NjY2NjY2QHBpdHQuZWR1; Du-San Baek, ZHViNUBwaXR0LmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.