
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 11 April 2023
Sec. Surgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1124014
This article is part of the Research Topic Video-Assisted Surgery in Oncology View all 13 articles
Background: Whether 4L lymph node dissection (LND) should be performed remains unclear and controversial. Prior studies have found that station 4L metastasis was not rare and that 4L LND may provide survival benefits. The objective of this study was to analyze the clinicopathological and survival outcomes of 4L LND from the perspective of histology.
Methods: This retrospective study included 74 patients with squamous cell carcinoma (SCC) and 84 patients diagnosed with lung adenocarcinoma (ADC) between January 2008 and October 2020. All patients underwent pulmonary resection with station 4L LND and were staged as T1-4N0-2M0. Clinicopathological features and survival outcomes were investigated based on histology. The study endpoints were disease-free survival (DFS) and overall survival (OS).
Results: The incidence rate of station 4L metastasis was 17.1% (27/158) in the entire cohort, with 8.1% in the SCC group, and 25.0% in the ADC group. No statistical differences in the 5-year DFS rates (67.1% vs. 61.7%, P=0.812) and 5-year OS rates (68.6% vs. 59.3%, P=0.100) were observed between the ADC group and the SCC group. Multivariate logistic analysis revealed that histology (SCC vs. ADC: OR, 0.185; 95% CI, 0.049–0.706; P=0.013) was independently associated with 4L metastasis. Multivariate survival analysis showed that the status of 4L metastasis was an independent factor for DFS (HR, 2.563; 95% CI, 1.282–5.123; P=0.008) but not for OS (HR, 1.597; 95% CI, 0.749–3.402; P=0.225).
Conclusion: Station 4L metastasis is not rare in left lung cancer. Patients with ADC have a greater predilection for station 4L metastasis and may benefit more from performing 4L LND.
Lung cancer is the leading cause of cancer-related mortality worldwide, with non-small-cell lung cancer (NSCLC) being the most frequent subtype (1). Lobectomy with systemic nodal dissection (SND) remains the fundamental approach for operable NSCLC patients because of its vital role in accurate staging and its therapeutic and prognostic implications (2). The National Comprehensive Cancer Network (NCCN) guidelines require the evaluation of a minimum of three N2 stations. For left-sided cancers, stations 4L, 5, 6, 7, 8, and 9 should be dissected (3). Often, station 4L is not routinely sampled or dissected during lung cancer resection out of concern for recurrent laryngeal nerve, thoracic duct, and aortic arch injury because of its anatomic location (4). Previous studies have shown that station 4L lymph nodes play a crucial role in the left bronchial-recurrent lymph node (LN) chain, an essential lymphatic pathway of the left lung (5, 6). Recently, some studies have assessed the clinical significance of 4L lymph node dissection (4L LND) in left lung cancer and found that station 4L metastasis was not rare and that 4L LND may provide survival benefits (7–12). However, whether 4L LND should be resected remains unclear and controversial because most guidelines have no detailed requirements. Furthermore, complete 4L LND is technically challenging and may cause postoperative complications. Thus, many surgeons choose not to perform 4L LND even in high-volume thoracic centers.
To the best of our knowledge, no study has been conducted to identify the clinicopathological features and survival outcomes of 4L LND in left lung cancer from a histological perspective. Therefore, the purpose of this study was to investigate the differences in clinicopathological features and survival outcomes between adenocarcinoma (ADC) and squamous cell carcinoma (SCC) after 4L LND.
We retrospectively reviewed the records of all patients who underwent left NSCLC surgery at our hospital between January 2008 and October 2020. The inclusion criteria were as follows: patients who underwent pulmonary resection (lobectomy or pneumonectomy) with SND or lymph node sampling, tumor pathology of ADC or SCC, and pathological stage of T1-4N0-2M0. The following patients were excluded: those with metastatic lung tumors or distant metastasis, those who underwent partial resection or segmentectomy, those who had no LN resection or unknown lymph node status (pNx), and those who received neoadjuvant therapy. Finally, 158 patients were enrolled in this study, and their clinicopathological characteristics were collected from the hospital electronic medical record system (Figure 1). All patients were staged according to the 8th edition of the American Joint Committee on Cancer (AJCC) lung cancer staging system (13). The study was approved by the Ethics Committee of Chinese People’s Liberation Army General Hospital. All patients signed the informed consent.
Routine examinations after surgery were requested every 3 months for the first 2 years, and every 6 months thereafter for 5 years. After 5 years, the patients were assessed annually. The examinations included blood tumor marker testing, chest CT, ultrasound of the neck and abdomen, and head magnetic resonance imaging. Bone scans were performed in case of bone pain. Follow-up information was collected by official contact with patients or their relatives via telephone or from the hospital outpatient clinic records. The last follow-up was in May 2022. The primary endpoint was disease-free survival (DFS), which was defined as the time interval from the date of surgery to the first event (recurrence, metastasis, or NSCLC-related death) or last follow-up. Overall survival (OS) served as the secondary endpoint, defined as the time interval between the date of surgery and the date of death or the last follow-up. The DFS and OS were calculated in months.
All statistical analyses were performed using SPSS version 22 software (IBM Corporation, Armonk, NY, USA). Continuous variables are expressed as the mean with standard deviation as well as the median with a range of values. We used the Mann-Whitney U test to determine significant differences in continuous variables between the two groups. Every group with categorical variables is summarized with the frequency and percentage of the considered population, and statistical comparisons between the two groups were performed using the χ2 test. Survival curves were estimated using the Kaplan-Meier method, and the log-rank test was used to compare differences. The Cox proportional hazards model was applied in univariate analyses to determine the influence on patients’ risk of death. Predictive variables were selected based on univariate models (P-value <0.05). A two-sided P<0.05 was considered statistically significant.
Table 1 shows the clinical characteristics of the patients (n=158). A total of 74 (46.8%) and 84 (53.2%) patients were assigned to the SCC group and ADC group, respectively. Patients in the SCC group were older than those in the ADC group (P=0.001). Sixty-seven (90.5%) patients in the SCC group were male, whereas 38 (45.2%) male patients in the ADC group (P<0.001). The proportion of smoking history in the SCC group was significantly higher than that in the ADC group (P<0.001). Chest CT showed a central mass in 40 (54.1%) SCC cases and in 14 (16/7%) ADC patients (P<0.001). No differences were observed in terms of comorbidities, abnormality of tumor markers, tumor location, surgical procedure, surgical approach, lymph node dissection, postoperative complication, and adjuvant therapy.
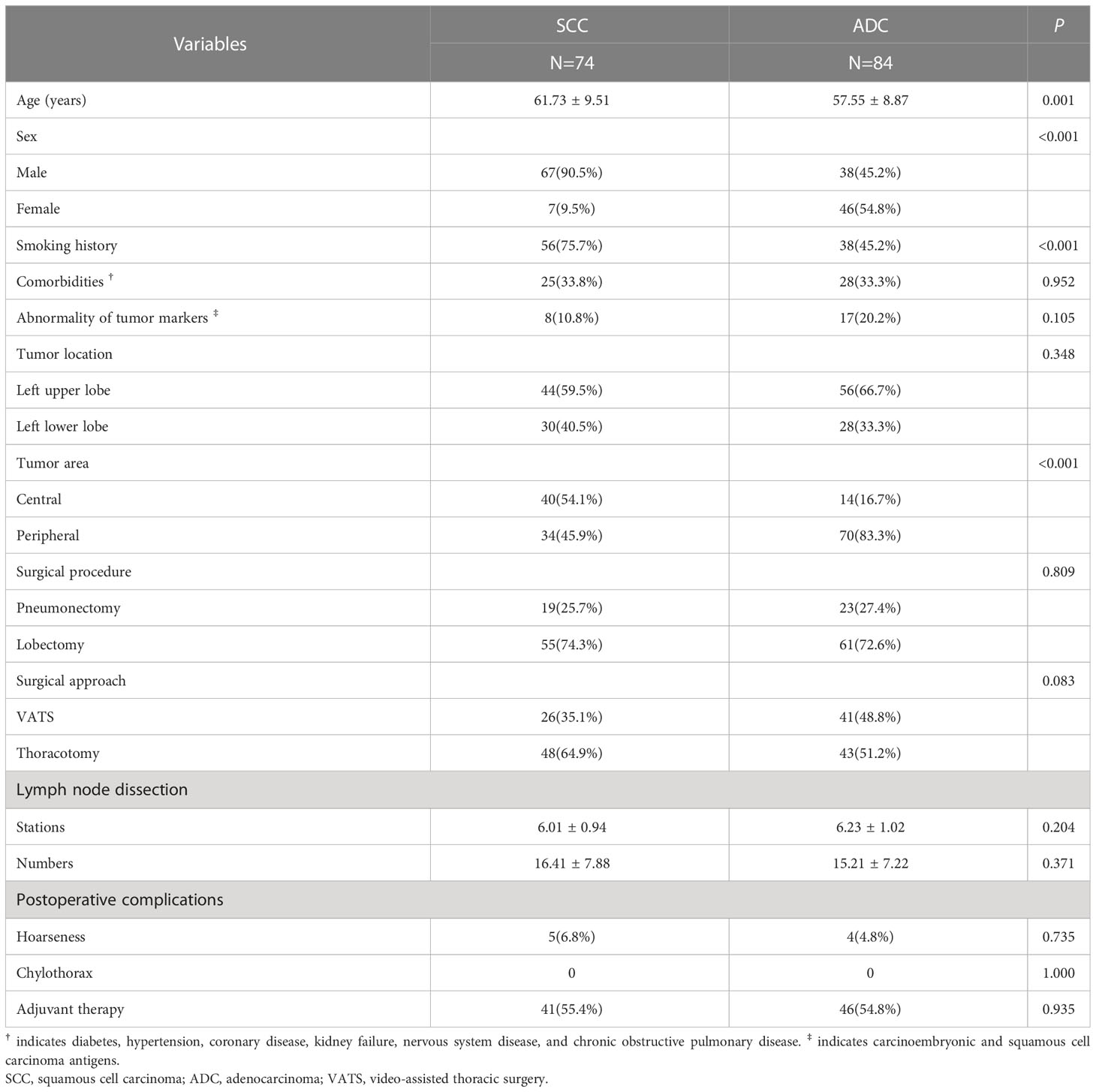
Table 1 Clinical characteristics of patients in the squamous cell carcinoma and adenocarcinoma groups.
Comparisons of the pathological findings between the SCC group and the ADC group are shown in Table 2. Differences were observed in terms of tumor diameter (P=0.002), visceral pleural (P=0.002), Ki-67 index (P=0.001), pT1 stage (P=0.032), and station 4L metastasis (P=0.005). The incidence of station 4L metastasis was 17.1% (27/158) in the entire cohort, with 8.1% in the SCC group and 25.0% in the ADC group. Two patients had skip station 4L metastasis in the ADC group and one patient had solitary station 4L metastasis in the SCC group. Four patients in the ADC group only had station 4L and 10 LN metastasis.
As shown in Table 3, the 4L metastasis was significantly correlated with abnormalities in tumor markers (P=0.031), histology (P=0.005), visceral pleural invasion (P<0.001), lymphovascular invasion (P=0.001), moderate and poor cell differentiation (P=0.001 and P < 0.001, respectively), and other stations (station 5, P < 0.001; station 6, P=0.008; station 7, P=0.010; station 10, P<0.001). These statistically significant factors were further analyzed by multivariate logistic analysis, and the results revealed that histology (SCC vs. ADC: OR, 0.185; 95% CI, 0.049–0.706; P=0.013), station 5 metastasis (OR, 20.567; 95% CI, 5.520–76.6939; P<0.001), station 10 metastasis (OR, 10.607; 95% CI, 2.985–37.694; P<0.001), and poor cell differentiation (OR, 6.080; 95% CI, 1.655–22.340; P =0.007) were independently associated with 4L LN metastasis.
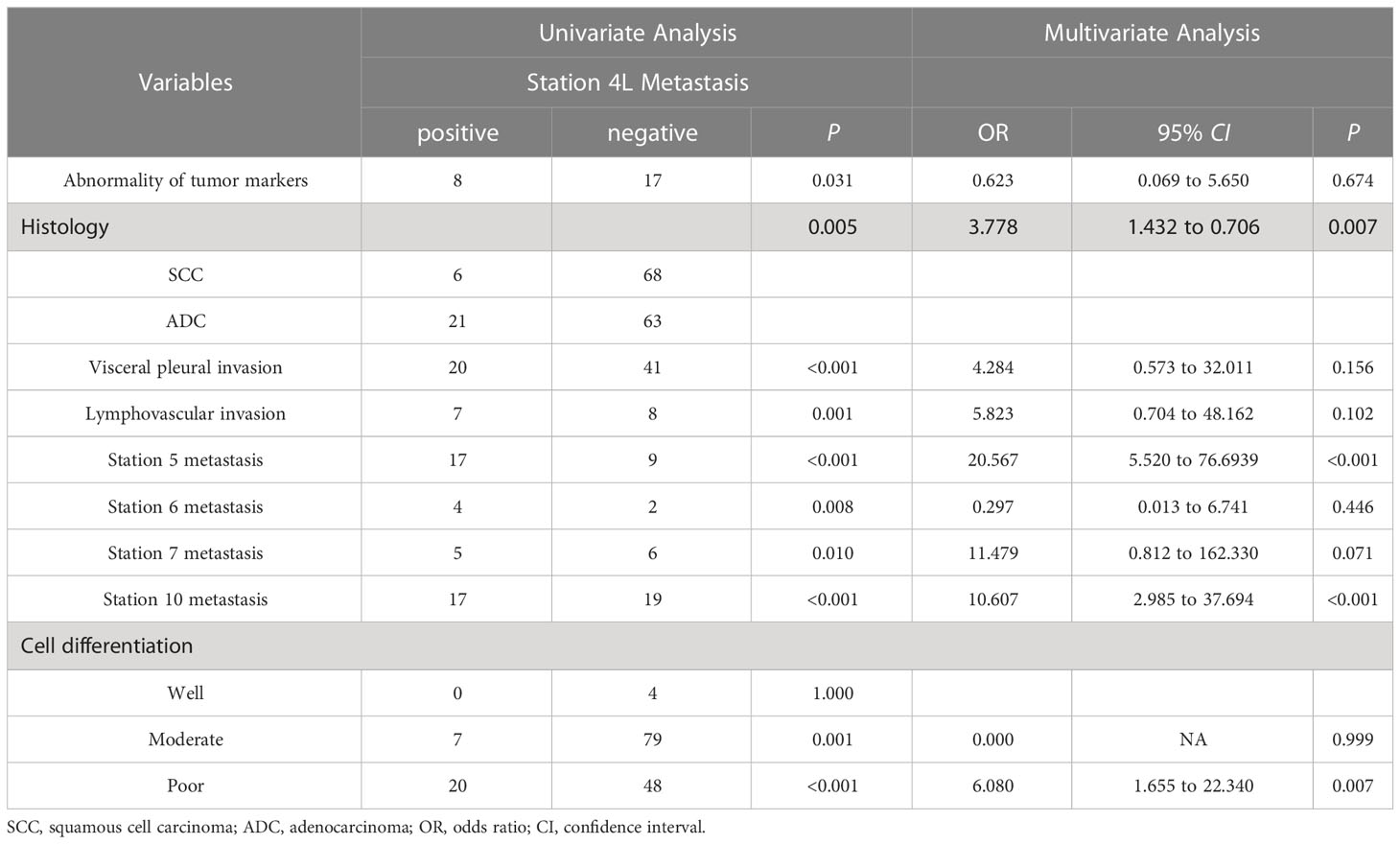
Table 3 Univariate and multivariate analysis of the correlation between clinicopathological factors and station 4L metastasis.
The median follow-up time was 36 months (range: 1–152 months). Fifty-seven patients died and 54 had recurrence or metastasis at the last follow-up. In the ADC group, 20 patients died and 20 patients had recurrence or metastasis. The rates of local recurrence and distant metastasis were 28.6% (24/84) and 23.8% (20/84), respectively. In the SCC group, 30 patients died and 34 patients had recurrence or metastasis. The rates of local recurrence and distant metastasis were 32.4% (24/74) and 16.2% (12/74), respectively. The 5-year DFS rates were 67.1% and 61.7% in the ADC group and SCC group, respectively. The 5-year OS rates in the two groups were 68.6% and 59.3%, respectively. The log-rank test showed no statistical differences in DFS (P =0.812; Figure 2A) and OS (P =0.100; Figure 2B) between the two groups.
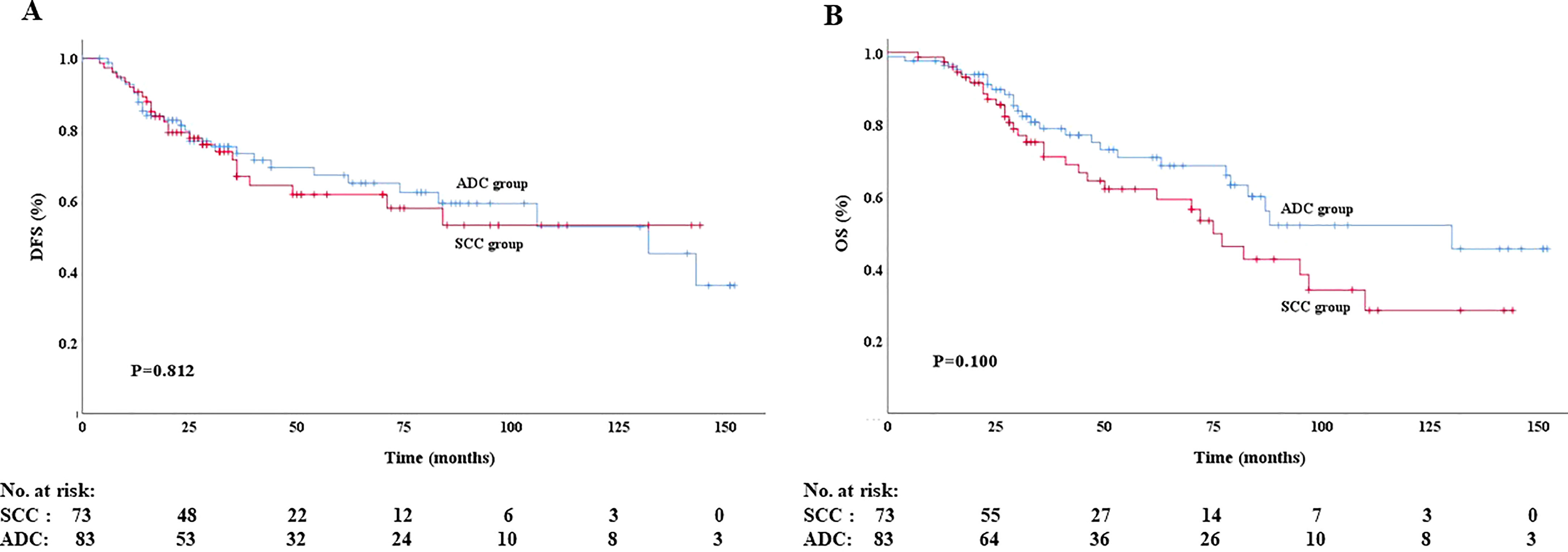
Figure 2 Disease-free survival (A) and overall survival (B) of patients in the ADC group and SCC group.
Some variables, such as sex, station 4L metastasis, station 7 metastasis, pT stage, and pTNM stage were significantly associated with DFS in the univariate analysis (P=0.042, P<0.001, P<0.001, P<0.001, and P<0.001, respectively). And the above five variables were also significantly associated with OS (P=0.003, P<0.001, P<0.001, P<0.001, and P<0.001, respectively). Additional multivariate analysis showed that status of 4L metastasis was an independent factor for DFS (HR, 2.563; 95% CI, 1.282–5.123; P=0.008), together with the status of station 7 LN metastasis, pT stage, and pTNM stage. Sex, status of station 7 LN metastasis, and pTNM stage were independent factors for OS (Table 4).
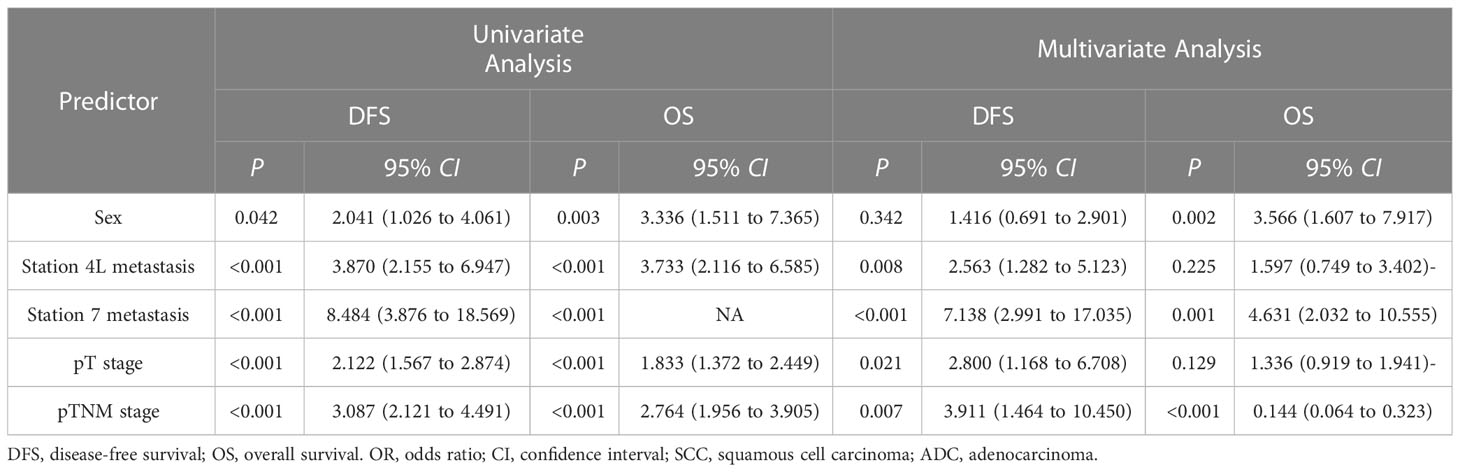
Table 4 Univariate and multivariate Cox regression analysis of prognostic factors in the SCC and ADC groups.
Lymph node metastasis is a major metastatic pathway in NSCLC, which leads to poor prognosis. Thorough removal of lymph nodes is of great importance for precise stage assessment, prognosis prediction, and development of postoperative therapeutic strategies (14). The American College of Surgeons Oncology Group Z0030 trial suggested that all patients should undergo comprehensive lymph node evaluation (15). However, whether 4L LN should be resected remains unclear, as only the European Society of Thoracic Surgeons expert consensus guidelines and NCCN guidelines recommend 4L nodal evaluation for left-sided tumors, except in select circumstances (16) whereas others have no specific requirements (3, 17, 18). Wang et al. (7) raised the debate for a more comprehensive evaluation of the 4L station in patients with left-sided NSCLC.
In this study, we investigated the differences in clinicopathological features and survival outcomes between left-sided ADC and SCC after 4L LND. Our findings suggested that station 4L metastasis was not rare(17.1%), which is consistent with previous studies (7–12), and that patients with adenocarcinoma are more likely to have 4L lymph node metastasis. However, we found no difference in DFS and OS between the ADC and the SCC group. The status of station 4L metastasis was an independent factor for DFS, but not for OS.
A recent meta-analysis (12) and previous studies (7–11) had found that dissection of the 4L LN could significantly improve both the 5-year OS and DFS rates in patients with left-sided NSCLC. Specifically, Zhao et al. (9) found that patients with stage II, IIIA, and N2 disease in the 4L LND group had better survival outcomes than those without, whereas patients with stage I left-sided NSCLC had no survival benefit. Yang et al. (8) found that 4L LND only benefits patients with NSCLC in the left upper lobe, indicating that 4L LND may be unnecessary for left lower lobe tumors. From another perspective, our study found that patients with adenocarcinoma were more likely to have 4L lymph nodal metastasis than those with squamous cell carcinoma (25.0% vs. 8.1%; P=0.005), which may be the first report. We propose that it is of great necessity for left-sided adenocarcinoma to undergo 4L LND. To our knowledge, ADC develops and progresses quickly and has a poorer prognosis than SCC. However, no differences in DFS (P=0.812) and OS (P=0.100) between the ADC group and SCC group were observed in our cohort. We speculated that two main reasons may contribute to the contradictory results. First, dissection of 4L LN in the ADC group could yield more lymph nodes for examination and lead to more accurate node upstaging followed by adjuvant therapy, which might improve the DFS and OS. In our study, two patients (2.4%) had skip station 4L metastasis in the ADC group and one patient (1.4%) had single-station 4L metastasis in the SCC group, while four patients (4.8%) in the ADC group only had station 4L and 10 LN metastasis. This indicated that two patients were upstaged from N0 to N2 and four from N1 to N2 in the ADC group, and one patient was upstaged from N0 to N2 in the SCC group. Second, 4L LND could therapeutically clear lymph nodes with micrometastases, which might significantly reduce the risk of recurrence. Therefore, the poor prognosis in ADC patients may be compensated by the complete dissection of 4L LN and adjuvant treatment resulting from accurate node staging.
Fang et al. (11) demonstrated that cN2, stations 5 and 10 metastases were independent risk factors for station 4L metastasis, whereas Wang et al. (7) suggested that station 10 metastasis was independently associated with 4L metastasis. Our study revealed that histology, station 5 metastasis, station 10 metastasis, and poor cell differentiation were risk factors, implying that stations 5 and 10 metastases were common risk factors for station 4L metastasis. We speculate that this may be related to their transition zone (such as the aortopulmonary window and tracheobronchial angle) (19), which could explain the result that left upper lobe tumors had a greater preference for superior mediastinal LN metastasis than lower lobe tumors (20) and that 4L LND only benefits patients with NSCLC in the left upper lobe (8).
Interestingly, our study suggested that the status of station 4L metastasis is an independent factor for DFS, but not for OS, while the status of station 7 metastasis and pTNM stage were both independent predictors for DFS and OS. We speculate that the following reason may contribute to this finding. Station 4L LN involvement changed the pathological stage and remodeled the postoperative regimens, which temporarily influenced the DFS. However, overall survival still needs to be elevated in locally advanced and metastatic NSCLC, even with the rapid progress in immunotherapy and targeted therapy (21, 22).
With the technical development of video-assisted thoracic surgery, the safety and thoroughness of 4L LND have been demonstrated in several studies (23, 24). The rate of hoarseness (5.7%, 9/158) caused by left recurrent laryngeal nerve injury in our study was acceptable and consistent with that in previous studies (9). Therefore, concern regarding hoarseness as a complication is not an obstacle in 4L LND, and the survival benefit should be taken into consideration and consensus regarding 4L LND should be reached.
Our study has several limitations. First, this single-center retrospective study inevitably had the possibility of uncontrolled confounding or selection bias, and we could not use the propensity score matching method to reduce them because of the relatively small number of enrolled patients with 4L LND left-sided tumors. This could be overcome by using a larger sample size and conducting a multicenter randomized clinical trial. Second, we focused on patients who received 4L LND without comparing them to patients who did not undergo 4L LND, as in some other studies. These findings should not be overinterpreted.
In conclusion, station 4L metastasis is not rare in left lung cancer. Patients with adenocarcinoma have a greater predilection for station 4L metastasis and may benefit more from performing 4L LND.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Chinese People’s Liberation Army General Hospital. The ethics committee waived the requirement of written informed consent for participation.
LS: Conceptualization, Methodology, Data curation, Writing-Original draft preparation. JG: Data curation, Writing-Review and Editing. HC: Formal analysis. WZ: Conceptualization, Writing-Review and Editing. YL: Conceptualization, Supervision, Writing-Reviewing and Editing. All authors contributed to the article and approved the submitted version.
We thank Xueli Lv for her efforts in helping the statistical analysis for this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2022. CA Cancer J Clin (2022) 2:7–33. doi: 10.3322/caac.21708
2. Watanabe S, Asamura H. Lymph node dissection for lung cancer: Significance, strategy, and technique. J Thorac Oncol (2009) 4:652–7. doi: 10.1097/JTO.0b013e31819cce50
3. National Comprehensive Cancer Network. NCCN guideline for non-small cell lung cancer (2022). Available at: https://www.nccn.org/professionals/ (Accessed March 16, 2022).
4. Reinersman JM. Better survival after 4L lymph node dissection for early-stage, left-sided, non-small cell lung cancer: Are we debating a false duality? Ann Surg Oncol (2019) 26:1959–60. doi: 10.1245/s10434-019-07382-z
5. Riquet M. Bronchial arteries and lymphatics of the lung. Thorac Surg Clin (2007) 17:619–38. doi: 10.1016/j.thorsurg.2006.12.011
6. Shien K, Toyooka S, Soh J, Okami J, Higashiyama M, Kadota Y, et al. Clinicopathological characteristics and lymph node metastasis pathway of non-small-cell lung cancer located in the left lingular division. Interact Cardiovasc Thorac Surg (2015) 20:791–6. doi: 10.1093/icvts/ivv062
7. Wang YN, Yao S, Wang CL, Li MS, Sun LN, Yan QN, et al. Clinical significance of 4L lymph node dissection in left lung cancer. J Clin Oncol (2018) 36:2935–42. doi: 10.1200/JCO.2018.78.7101
8. Yang MZ, Hou X, Li JB, Cai JS, Yang J, Li S, et al. Impact of L4 lymph node dissection on long-term survival in left-side operable non-small-cell lung cancer: A propensity score matching study. Eur J Cardiothorac Surg (2020) 57:1181–8. doi: 10.1093/ejcts/ezaa008
9. Zhao KJ, Wei SY, Mei JD, Guo CL, Hai Y, Chen N, et al. Survival benefit of left lower paratracheal (4L) lymph node dissection for patients with left-sided non-small cell lung cancer: Once neglected but of great importance. Ann Surg Oncol (2019) 26:2044–52. doi: 10.1245/s10434-019-07368-x
10. Gryszko GM, Cackowski MM, Zbytniewski M, Woźnica K, Orłowski TM, Dziedzic DA. The impact of left lower paratracheal (4L) lymph node dissection on survival in patients with surgically treated left-sided NSCLC. Eur J Cardiothorac Surg (2021) 60:1201–9. doi: 10.1093/ejcts/ezab294
11. Fang LK, Wang LM, Wang YQ, Wu YH, Ye P, Lv W, et al. Predictors and survival impact of station 4L metastasis in left non-small cell lung cancer. J Cancer Res Cli Oncol (2019) 145:1313–9. doi: 10.1007/s00432-019-02880-9
12. Deng HY, Li DY, Qiu XM, Zhu DX, Tang X, Zhou Q. Dissection of 4L lymph node for left-sided non-small cell lung cancer: A meta-analysis. ANZ J Surg (2021) 91:E696–702. doi: 10.1111/ans.17131
13. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The eighth edition lung cancer stage classification. Chest (2017) 151:193–203. doi: 10.1016/j.chest.2016.10.010
14. Whitson BA, Groth SS, Maddaus MA. Surgical assessment and intraoperative management of mediastinal lymph nodes in non-small cell lung cancer. Ann Thorac Surg (2007) 84:1059–65. doi: 10.1016/j.athoracsur.2007.04.032
15. Darling GE, Allen MS, Decker PA, Ballman K, Malthaner RA, Inculet RI, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: Results of the American college of surgery oncology group Z0030 trial. J Thorac Cardiovasc Surg (2011) 141:662–70. doi: 10.1016/j.jtcvs.2010.11.008
16. Lardinois D, De Leyn P, Van Schil P, Porta RR, Waller D, Passlick B, et al. ESTS guidelines for intraoperative lymph node staging in non-small cell lung cancer. Eur J Cardio-thorac Surg (2006) 30:787–92. doi: 10.1016/j.ejcts.2006.08.008
17. Remon J, Soria JC, Peters S, on behalf of the ESMO Guidelines Committee. Early and locally advanced non-small-cell lung cancer: An update of the ESMO clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol (2021) 32:1637–42. doi: 10.1016/j.annonc.2021.08.1994
18. Detterbeck FC, Chansky K, Groome P, Bolejack V, Crowley J, Shemanski L, et al. The IASLC lung cancer staging project: Methodology and validation used in the development of proposals for revision of the stage classification of NSCLC in the forthcoming (eighth) edition of the TNM classification of lung cancer. J Thorac Oncol (2016) 11:1433–46. doi: 10.1016/j.jtho.2016.06.028
19. Shen LL, Yun TY, Guo JT, Liu Y, Liang CY. Clinicopathological characteristics and risk factors of station 4L lymph node metastasis of left non-small cell lung cancer clinicopathological characteristics and risk factors of station 4L lymph node metastasis of left non-small cell lung cancer. J South Med Univ (2020) 40:1793–8. doi: 10.12122/j.issn.1673-4254.2020.12.14
20. Shimada Y, Saji H, Kakihana M, Honda H, Usuda J, Kajiwara N, et al. Retrospective analysis of nodal spread patterns according to tumor location in pathological N2 non-small cell lung cancer. World J Surg (2012) 36:2865–71. doi: 10.1007/s00268-012-1743-5
21. Suraya R, Tachihara M, Nagano T, Nishimura Y, Kobayashi K. Immunotherapy in advanced non-small cell lung cancer: Current status and updates. Cancer Manag Res (2022) 14:2079–90. doi: 10.2147/CMAR.S366738
22. Hanna NH, Robinson AG, Temin S, Baker S Jr, Brahmer JR, Ellis PM, et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) joint guideline update. J Clin Oncol (2021) 39:1040–91. doi: 10.1200/JCO.20.03570
23. Nagashima T. Thoracoscopic left mediastinal lymph node dissection. Ann Transl Med (2016) 4:10. doi: 10.3978/j.issn.2305-5839.2015.12.58
24. Kim HJ, Kim YH, Choi SH, Kim HR, Kim DK, Park SI. Video-assisted mediastinoscopic lymphadenectomy combined with minimally invasive pulmonary resection for left-sided lung cancer: Feasibility and clinical impacts on surgical outcomes. Eur J Cardiothorac Surg (2016) 49:308–13. doi: 10.1093/ejcts/ezv077
Keywords: adenocarcinoma, squamous cell carcinoma, station 4L metastasis, prognosis, lymph node dissection
Citation: Shen L, Guo J, Zhang W, Liang C, Chen H and Liu Y (2023) Clinicopathological and survival outcomes of 4L lymph node dissection in left lung adenocarcinoma and squamous cell carcinoma. Front. Oncol. 13:1124014. doi: 10.3389/fonc.2023.1124014
Received: 14 December 2022; Accepted: 27 March 2023;
Published: 11 April 2023.
Edited by:
Long Jiang, First Affiliated Hospital of Guangzhou Medical University, ChinaReviewed by:
Elizabeth Gaughan, University of Virginia, United StatesCopyright © 2023 Shen, Guo, Zhang, Liang, Chen and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han Chen, aGFuY2hlbjE5ODZAaG90bWFpbC5jb20=; Yang Liu, c3VubnkzMDF4QHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.