- 1Key Laboratory of Digital Technology in Medical Diagnostics of Zhejiang Province, Dian Diagnostics Group Co., Ltd., Hangzhou, Zhejiang, China
- 2Department of Thyroid & Bariatric Metabolic Surgery, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Third Hospital of Shanxi Medical University, Taiyuan, Shanxi, China
- 3Clinical Genome Center, Dian Diagnostics Group Co., Ltd., Hangzhou, Zhejiang, China
NTRK fusions are validated oncogenic drivers of various adult and pediatric tumor types, including thyroid cancer, and serve as a therapeutic target. Recently, tropomyosin receptor kinase (TRK) inhibitors, such as entrectinib and larotrectinib, display promising therapeutic efficacy in NTRK-positive solid tumors. Although some NTRK fusion partners have been identified in thyroid cancer, the spectrum of NTRK fusion is not fully characterized. In this study, a dual NTRK3 fusion was identified by targeted RNA-Seq in a 47-year-old female patient with papillary thyroid carcinoma. The patient harbors a novel in-frame fusion between NTRK3 exon 13 and AJUBA exon 2, co-existing with a known in-frame fusion between ETV6 exon 4 and NTRK3 exon 14. The dual NTRK3 fusion was validated by Sanger sequencing and fluorescence in situ hybridization (FISH) but lack TRK protein expression as defined by pan-TRK immunohistochemistry (IHC). We supposed the pan-TRK IHC result to be falsely negative. In conclusion, we present the first case of a novel NTRK3-AJUBA fusion co-existing with a known ETV6-NTRK3 fusion in thyroid cancer. These findings extend the spectrum of translocation partners in NTRK3 fusion, and the effect of dual NTRK3 fusion on TRK inhibitor therapy and prognosis needs long-term follow-up.
Introduction
Thyroid cancer is one of the most common malignant tumors, with papillary thyroid carcinoma (PTC) as the predominant subtype. The worldwide incidence of thyroid cancer in adults has been increasing dramatically in the past three decades, especially for PTC (1). According to the latest epidemiological research, PTC was the main contributor to the rapid increase in thyroid cancer incidence, and was the only histological subtype that increased systematically in 25 studied countries (2). Neurotrophic tyrosine receptor kinase (NTRK) fusions are validated oncogenic drivers of various adult and pediatric tumor types, including NTRK1, NTRK2, and NTRK3, which encode the TRK proteins TRKA, TRKB, and TRKC, respectively (3). Since the initial discovery in colorectal carcinoma (4), NTRK fusions have been identified in 17 unique cancer types, including thyroid cancer (5). NTRK fusions are found at high frequencies(>90%) in rare cancer types (secretory carcinoma, secretory breast carcinoma, infantile fibrosarcoma, and cellular or mixed congenital mesoblastic nephroma), moderate frequencies (5%-25%) in some cancers (papillary thyroid cancer, spitzoid neoplasm, and gastrointestinal stromal tumor) and lower frequencies(<5%) in other common tumors (lung cancer, breast cancer, colorectal cancer, pancreatic cancers, melanoma, and other solid or hematologic cancers) (5).
For structurally persistent/recurrent locoregional or distant metastatic disease not amenable to radioactive iodine (RAI) therapy, the National Comprehensive Cancer Network (NCCN) Guidelines recommend genomic testing to identify actionable mutations (including NTRK fusions). In several small basket trials, entrectinib and larotrectinib display promising therapeutic efficacy with high and durable responses in NTRK fusion-positive pediatric and adult solid tumors (6, 7). Although several NTRK fusion partners have been identified in thyroid cancer (8), the spectrum of NTRK fusion is not fully characterized. In this case, we first report a novel NTRK3-AJUBA fusion co-existing with ETV6-NTRK3 fusion in PTC. We discuss the implications of this finding for targeted therapies and clinical outcomes.
Results
Clinical and pathological features
A 47-year-old female was initially present with a thyroid nodule during a routine physical examination in 2021. Additional complaints include anemia, leukocytopenia, and complete right bundle branch block (CRBBB). The patient reported that she had three siblings and had no family history of cancer. Ultrasonography revealed diffuse echogenic changes in the parenchyma of the right lobe with multiple punctate calcifications (Thyroid Imaging Reporting and Data System (TIRADS) 5), multiple nodules with punctate calcification in the right lobe (TIRADS 4a), and multiple enlarged lymph nodes in the right II-IV levels and VI level. She was then diagnosed with malignancy and underwent extended radical thyroidectomy on 6 September 2021. Postoperative pathological examination identified papillary thyroid microcarcinoma (3-mm maximum diameter) in the left lobe, multifocal PTC (range 0.6-1.2cm in diameter) in the right lobe, lymph node metastasis (2/7) in left central, lymph node metastasis (13/17) in right central, lymph node metastases in right level II (3/5), and lymph node metastases in right level III-V (5/8). She was treated with iodine-131 once after surgery. This patient had been followed up for 19 months and remained free of recurrence to date, she was satisfied with the treatment.
Fusion description
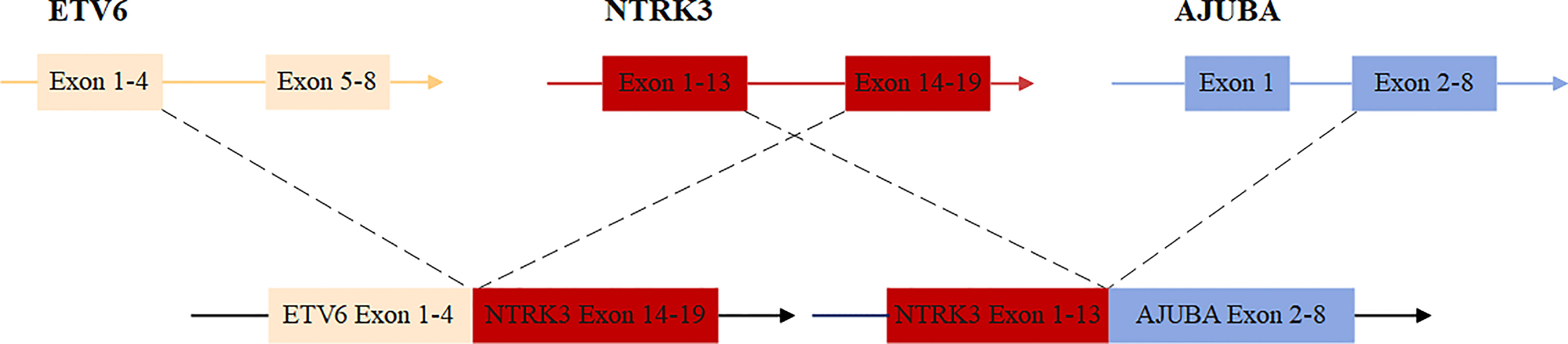
Freshly resected tumor tissue was used for gene fusion detection by targeted RNA sequencing, which was designed to target 22 genes frequently rearranged in thyroid carcinoma. Molecular testing revealed a novel fusion transcript between exon 13 of NTRK3 and exon 2 of AJUBA, co-existing with a known fusion transcript between exon 4 of ETV6 and exon 14 of NTRK3 (Figure 1). The bioinformatics analysis detected 5 unique reads spanning the NTRK3–AJUBA fusion breakpoint and 95 unique reads spanning the ETV6-NTRK3 fusion breakpoint. Meanwhile, we used Integrative Genome Viewer (IGV) to check and visualize the supporting reads that demonstrate the novel NTRK3–AJUBA fusion (Figure 2A) and ETV6-NTRK3 fusion (Figure 2B), respectively.
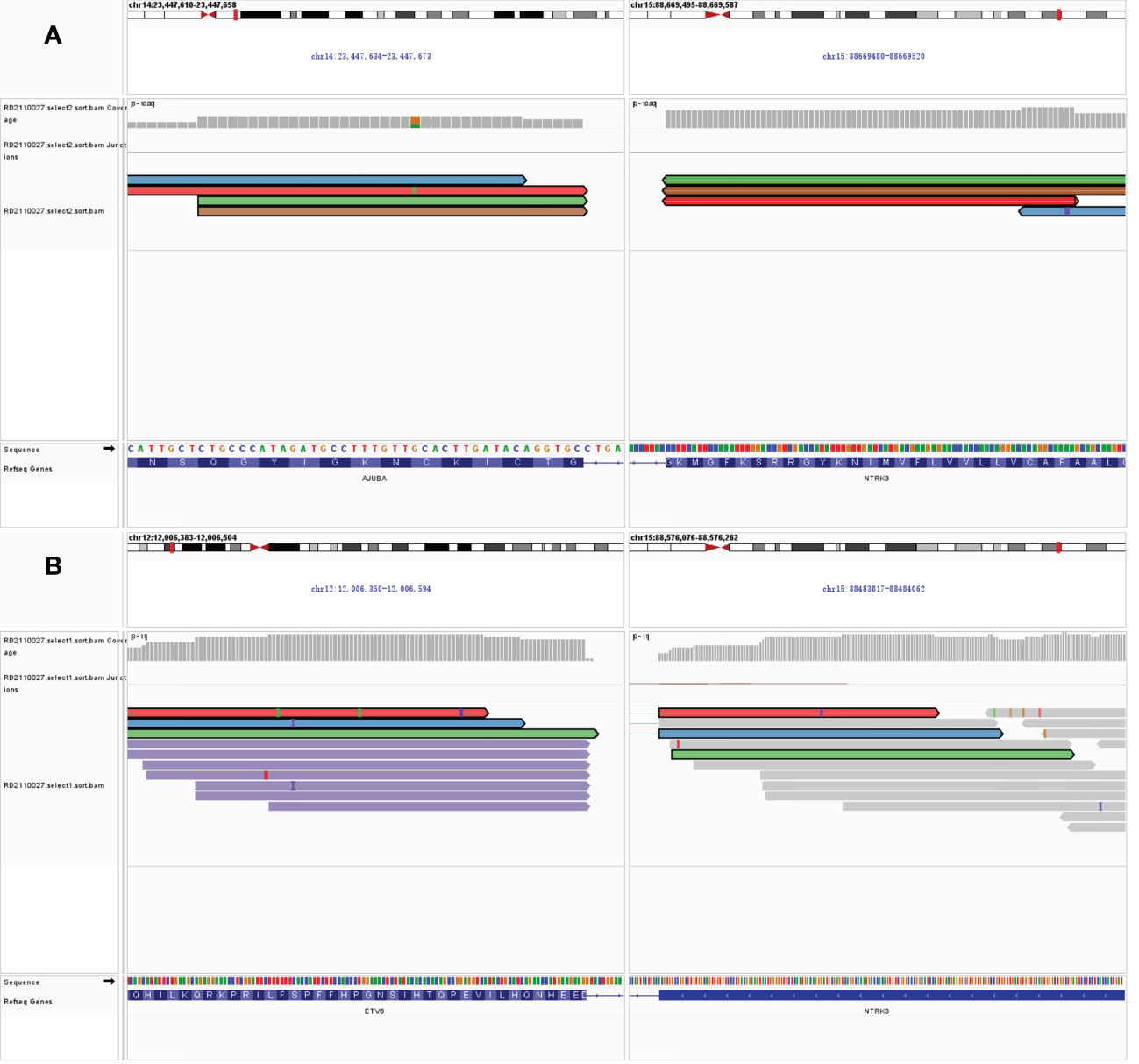
Figure 2 (A) Integrative Genome Viewer (IGV) snapshot of NTRK3–AJUBA fusion; (B) Integrative Genome Viewer (IGV) snapshot of ETV6-NTRK3 fusion.
We then used Sanger sequencing to validate the dual NTRK3 fusion. The results revealed that exon 13 of the NTRK3 gene on chromosome 15 is fused to exon 2 of the AJUBA gene on chromosome 14 (Figure 3A) and exon 4 of the ETV6 gene on chromosome 12 is fused to exon 14 of the NTRK3 gene on chromosome 15 (Figure 3B). Subsequently, fluorescence in situ hybridization (FISH) analysis, using a break-apart assay, confirmed NTRK3 fusion at the genomic level (Figure 4). In addition, Pan-TRK IHC was performed to validate the presence of NTRK fusion protein. However, this case showed negative cytoplasmic and nuclear staining for pan-TRK (Supplementary Figure 1), which was discordant with NGS and FISH results.
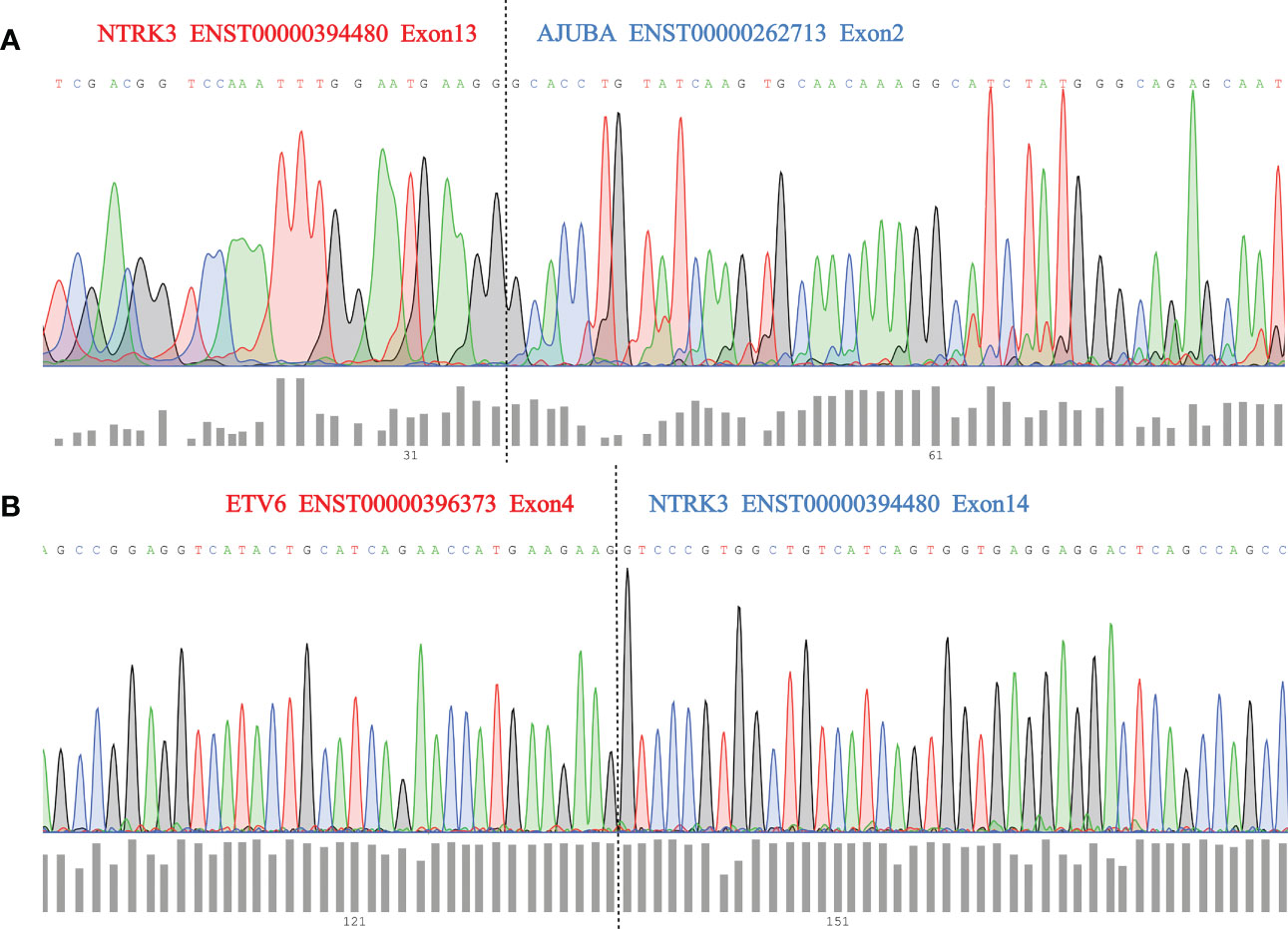
Figure 3 (A) Sanger sequencing validation of NTRK3–AJUBA fusion and dashed lines (black) indicate breakpoint position; (B) Sanger sequencing validation of ETV6-NTRK3 fusion and dashed lines (black) indicate breakpoint position.
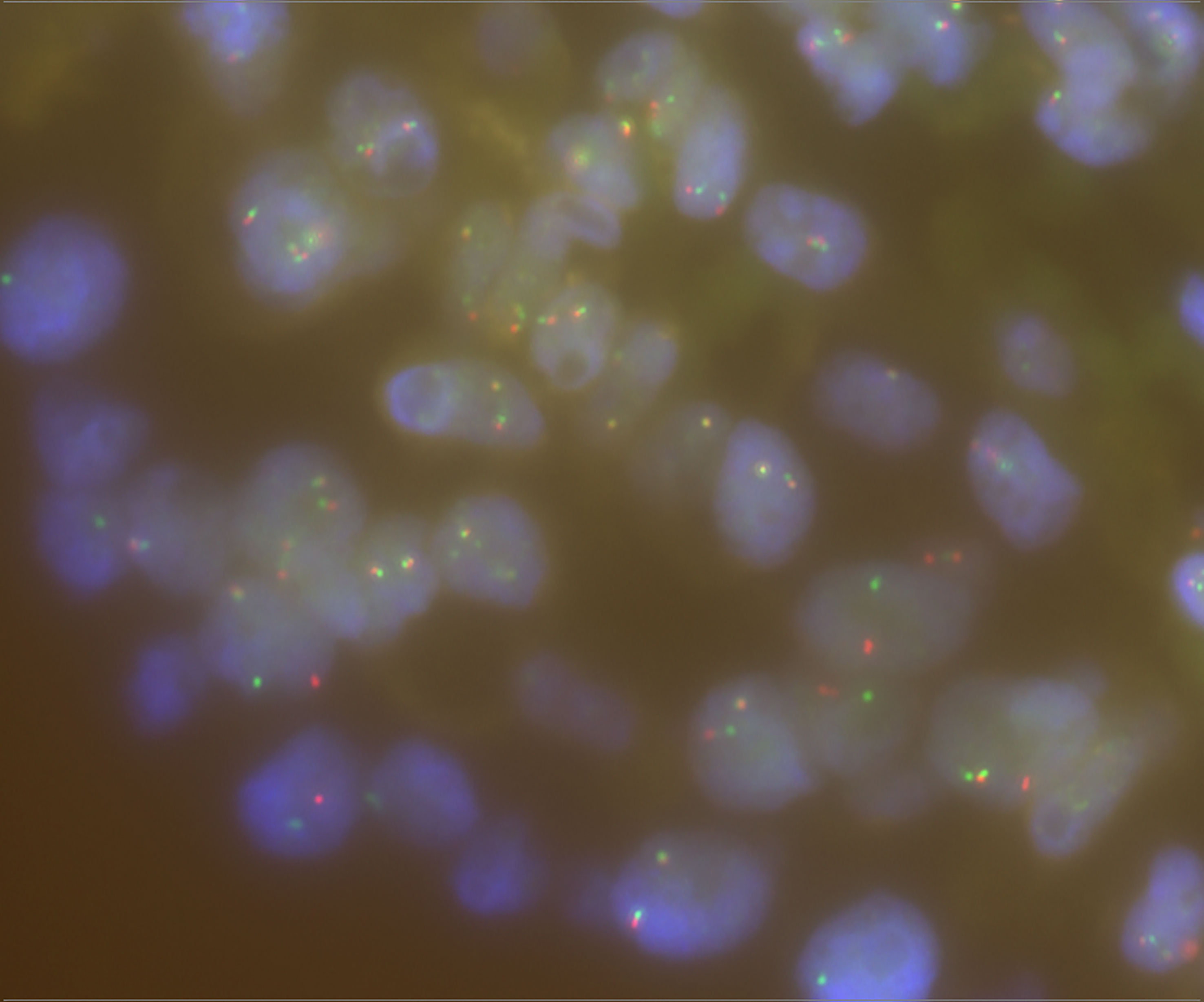
Figure 4 FISH break-apart assay for NTRK3 gene and separate red and green signals indicating rearrangement.
The NTRK3–AJUBA fusion protein is predicted to include LRRNT, LRR_8, LRRCT_2, Ig, and I-set domains encoded by NTRK3 and LIM domains encoded by AJUBA. The ETV6-NTRK3 fusion protein is predicted to include the PNT domain encoded by ETV6 and the whole protein tyrosine kinase domain encoded by NTRK3 (Supplementary Figure 2).
Discussion
In this report, we identified a dual NTRK3 fusion in a 47-year-old female patient with PTC by targeted RNA sequencing. To the best of our knowledge, this is the first report of a novel NTRK3-AJUBA fusion co-existing with ETV6-NTRK3 fusion in thyroid cancer or any other cancers. These findings extend the spectrum of translocation partners in NTRK3 fusions. Although this case showed negative cytoplasmic and nuclear staining for pan-TRK IHC, the dual NTRK3 fusion was detected by RNA-based NGS assay, and then validated by Sanger sequencing and FISH break-apart assay. Currently, there are no commercially available TRKC monoclonal antibodies (specific to NTRK3 fusions), and some cases with NTRK3 fusions showed negative results as defined by pan-TRK IHC (9, 10). Therefore, the Pan-TRK IHC result of this case may be falsely negative, and the RNA-based NGS assay was strongly recommended for NTRK3 fusion detection. These findings provide evidence for the selection of NTRK3 fusion detection methods and may contribute to precision diagnosis and treatment.
ETV6 encodes an ETS family transcription factor and is located on chromosome 12. NTRK3 encodes tropomyosin receptor kinase C (TRKC) and is located on chromosome 15. ETV6-NTRK3 chimeric was a common oncogene fusion in a variety of cancers, including infantile fibrosarcoma (11), acute myeloid leukemia (12), mammary analogue secretory carcinoma (13), congenital mesoblastic nephroma (14), secretory breast carcinoma (15) and radiation-related PTC (16). Here, we describe a patient with papillary thyroid carcinoma harboring an ETV6-NTRK3 fusion without radiation exposure and may benefit from TRK inhibitor therapy after possible tumor recurrence. AJUBA (Ajuba LIM Protein) gene functions as a scaffold participating in a variety of cellular processes, such as mitosis, motility, cell adhesion, gene transcription, cell differentiation, proliferation, and migration (17–20). Although numerous studies have demonstrated that AJUBA acts as an oncogene or tumor suppressor in different cancer types (19), the function of AJUBA in thyroid cancer remains unclear. Dysregulation of gene expression and gene mutations were the predominant genetic alteration in AJUBA, here we first reveal that AJUBA has undergone a gene fusion event, which is a new genomic alteration type in AJUBA.
This patient had been followed up for 19 months and remained free of recurrence or metastasis to date. The effect of nonreciprocal/reciprocal NTRK3 fusion on TRK inhibitor therapy and prognosis needs long-term follow-up. To the best of our knowledge, nonreciprocal/reciprocal NTRK3 fusion had not been reported before in thyroid cancer or any other cancers, possibly owing to the relatively low incidence of NTRK fusions in common tumors and the limitations of detection methods. Although the clinical significance of these nonreciprocal/reciprocal NTRK3 fusions is still unknown, nonreciprocal/reciprocal ALK fusions had previously been reported to be associated with brain metastases and worse progression-free survival (PFS) in patients with ALK-rearranged NSCLC who received first-line crizotinib (21), moreover, nonreciprocal/reciprocal ROS1 fusions may improve sensitivity to crizotinib and prolong PFS of patients with lung adenocarcinoma (22), indicating a potential role of the nonreciprocal/reciprocal NTRK3 fusion in targeted therapy and prognosis of thyroid carcinoma.
Materials and methods
Targeted RNA sequencing and identification of gene fusions
The fusion gene panel was designed to target 22 genes frequently rearranged in thyroid carcinoma, including ALK, BRAF, BRD4, DERL1, ERBB4, FGFR1, FGFR2, LTK, MAML2, MET, NTRK1, NTRK2, NTRK3, PAX8, PPARG, RAF1, RET, ROS1, SLC26A11, SLC5A11, THADA, and WNK1. Nucleic acid extraction, library preparation, hybrid Capture, and NGS (Illumina NextSeq 500, San Diego, CA) were carried out by the Key Laboratory of Digital Technology in Medical Diagnostics of Zhejiang Province. FASTQ sequencing files were aligned to the human reference genome (UCSC hg19; Feb 2009 release) with BWA software. STAR-Fusion and Arriba software was used to detect gene fusions. The candidate gene fusion transcripts were reviewed and visualized in Integrative Genome Viewer (IGV, Broad Institute, version 2.1.2).
Sanger sequencing
Direct Sanger sequencing of PCR product was performed to validate the dual NTRK3 fusion. The NTRK3-AJUBA fusion transcripts were amplified using primer pairs as follows: primer forward: GTGTCCTGTTGGTGGTTCTCT and primer reverse: CAAAGCACTGGGTGTGGTAGA (product length 156bp). The ETV6-NTRK3 fusion transcripts were amplified using primer pairs as follows: primer forward: TGTAAAACGACGGCCAGTTCTTTCCAGGTGATGTGCTCT and primer reverse: CAGGAAACAGCTATGACCAAGCAGATTCAGACCCACAG (product length 559bp).
Fluorescence in situ hybridization
NTRK3 break-apart FISH was performed on FFPE samples. The FISH signals were scored by evaluating 100 tumor cell nuclei per case. Tumor cells showing split signals were concluded to have NTRK3 fusion. A threshold of 15% nuclei positive for a break-apart signal was considered positive for gene fusion.
Immunohistochemistry
IHC staining for pan-TRK expression was performed on the Benchmark Ultra platform (Ventana Medical Systems, Tucson, AZ) with Optiview DAB IHC Detection Kit, using a commercially available pan-TRK assay (rabbit monoclonal antibody, clone EPR17341, Assay, RTU, Roche, Ventana).
Data availability statement
The datasets presented in this article are not readily available because of ethical/privacy restrictions. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the ethics committee of Shanxi Bethune Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Conceptualization: Q-xY; validation: LQ; data curation: H-yW and LZ (4th author); writing—original draft preparation: Q-xY and W-jZ; writing—review and editing: LZ (6th author) and J-lH. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Shanxi Province “136 Revitalization Medical Project Construction Funds”.
Acknowledgments
We would like to thank the patient and her family for their contributions and support of our research.
Conflict of interest
Authors Q-xY, LQ, and LZ (6th author) were employed by Dian Diagnostics Group Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1123812/full#supplementary-material
References
1. Wild C, Weiderpass E, Stewart BW. World cancer report: cancer research for cancer prevention. IARC Press (2020). Available at: https://publications.iarc.fr/586
2. Miranda-Filho A, Lortet-Tieulent J, Bray F, Cao B, Franceschi S, Vaccarella S, et al. Thyroid cancer incidence trends by histology in 25 countries: a population-based study. Lancet Diabetes Endocrinol (2021) 9(4):225–34. doi: 10.1016/S2213-8587(21)00027-9
3. Tessarollo L, Tsoulfas P, Martin-Zanca D, Gilbert DJ, Jenkins NA, Copeland NG, et al. Trkc, a receptor for neurotrophin-3, is widely expressed in the developing nervous system and in non-neuronal tissues. Development (1993) 118(2):463–75. doi: 10.1242/dev.118.2.463
4. Martin-Zanca D, Hughes SH, Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. Nature (1986) 319(6056):743–8. doi: 10.1038/319743a0
5. Cocco E, Scaltriti M, Drilon A. Ntrk fusion-positive cancers and trk inhibitor therapy. Nat Rev Clin Oncol (2018) 15(12):731–47. doi: 10.1038/s41571-018-0113-0
6. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. New Engl J Med (2018) 378(8):731–9. doi: 10.1056/NEJMoa1714448
7. Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic ntrk fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol (2020) 21(2):271–82. doi: 10.1016/S1470-2045(19)30691-6
8. Park JC, Ashok A, Liu C, Kang H. Real-world experience of ntrk fusion-positive thyroid cancer. JCO Precis Oncol (2022) 6:e2100442. doi: 10.1200/PO.21.00442
9. Hechtman JF, Benayed R, Hyman DM, Drilon A, Zehir A, Frosina D, et al. Pan-trk immunohistochemistry is an efficient and reliable screen for the detection of ntrk fusions. Am J Surg Pathol (2017) 41(11):1547–51. doi: 10.1097/PAS.0000000000000911
10. Marchio C, Scaltriti M, Ladanyi M, Iafrate AJ, Bibeau F, Dietel M, et al. Esmo recommendations on the standard methods to detect ntrk fusions in daily practice and clinical research. Ann oncology: Off J Eur Soc Med Oncol (2019) 30(9):1417–27. doi: 10.1093/annonc/mdz204
11. Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel Etv6-Ntrk3 gene fusion in congenital fibrosarcoma. Nat Genet (1998) 18(2):184–7. doi: 10.1038/ng0298-184
12. Eguchi M, Eguchi-Ishimae M, Tojo A, Morishita K, Suzuki K, Sato Y, et al. Fusion of Etv6 to neurotrophin-3 receptor trkc in acute myeloid leukemia with T (12,15)(P13;Q25). Blood (1999) 93(4):1355–63. doi: 10.1182/blood.V93.4.1355
13. Drilon A, Li G, Dogan S, Gounder M, Shen R, Arcila M, et al. What hides behind the masc: clinical response and acquired resistance to entrectinib after Etv6-Ntrk3 identification in a mammary analogue secretory carcinoma (Masc). Ann oncology: Off J Eur Soc Med Oncol (2016) 27(5):920–6. doi: 10.1093/annonc/mdw042
14. Halalsheh H, McCarville MB, Neel M, Reynolds M, Cox MC, Pappo AS. Dramatic bone remodeling following larotrectinib administration for bone metastasis in a patient with trk fusion congenital mesoblastic nephroma. Pediatr Blood Cancer (2018) 65(10):e27271. doi: 10.1002/pbc.27271
15. Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the Etv6-Ntrk3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell (2002) 2(5):367–76. doi: 10.1016/s1535-6108(02)00180-0
16. Leeman-Neill RJ, Kelly LM, Liu P, Brenner AV, Little MP, Bogdanova TI, et al. Etv6-Ntrk3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer (2014) 120(6):799–807. doi: 10.1002/cncr.28484
17. Rauskolb C, Cervantes E, Madere F, Irvine KD. Organization and function of tension-dependent complexes at adherens junctions. J Cell Sci (2019) 132(7):jcs224063. doi: 10.1242/jcs.224063
18. Dommann N, Sanchez-Taltavull D, Eggs L, Birrer F, Brodie T, Salm L, et al. The lim protein ajuba augments tumor metastasis in colon cancer. Cancers (2020) 12(7):1913. doi: 10.3390/cancers12071913
19. Jia H, Peng H, Hou Z. Ajuba: an emerging signal transducer in oncogenesis. Pharmacol Res (2020) 151:104546. doi: 10.1016/j.phrs.2019.104546
20. Song N, Liu J, Zhang K, Yang J, Cui K, Miao Z, et al. The lim protein ajuba is a potential oncogenic target and prognostic marker in human cancer Via pan-cancer analysis. Front Cell Dev Biol (2022) 10:921897. doi: 10.3389/fcell.2022.921897
21. Zhang Y, Zeng L, Zhou C, Li Y, Wu L, Xia C, et al. Detection of Nonreciprocal/Reciprocal alk translocation as poor predictive marker in patients with first-line crizotinib-treated alk-rearranged nsclc. J Thorac Oncol (2020) 15(6):1027–36. doi: 10.1016/j.jtho.2020.02.007
Keywords: papillary thyroid carcinoma, NTRK3-AJUBA, ETV6-NTRK3, NGS, fusion
Citation: Yu Q-X, Zhao W-J, Wang H-Y, Zhang L, Qin L, Zhang L and Han J-l (2023) Case Report: Identification of a novel NTRK3-AJUBA fusion co-existing with ETV6-NTRK3 fusion in papillary thyroid carcinoma. Front. Oncol. 13:1123812. doi: 10.3389/fonc.2023.1123812
Received: 14 December 2022; Accepted: 07 April 2023;
Published: 28 April 2023.
Edited by:
Susanna Chiocca, European Institute of Oncology (IEO), ItalyReviewed by:
Mark Rosenzweig, Foundation Medicine Inc., United StatesAmandeep Kaur, NorthShore University HealthSystem, United States
Copyright © 2023 Yu, Zhao, Wang, Zhang, Qin, Zhang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emhhbmdsZWkzQGRhemQuY24=; Jian-li Han, MTI5MjIyNTkyMkBxcS5jb20=
†These authors have contributed equally to this work
 Qing-Xiang Yu
Qing-Xiang Yu Wen-Jun Zhao2†
Wen-Jun Zhao2†