- Department of Gastroenterology, Lanzhou University Second Hospital, Lanzhou, Gansu, China
Discoidin domain receptors (DDRs) are receptor tyrosine kinases on the membrane surface that bind to extracellular collagens, but they are rarely expressed in normal liver tissues. Recent studies have demonstrated that DDRs participate in and influence the processes underlying premalignant and malignant liver diseases. A brief overview of the potential roles of DDR1 and DDR2 in premalignant and malignant liver diseases is presented. DDR1 has proinflammatory and profibrotic benefits and promotes the invasion, migration and liver metastasis of tumour cells. However, DDR2 may play a pathogenic role in early-stage liver injury (prefibrotic stage) and a different role in chronic liver fibrosis and in metastatic liver cancer. These views are critically significant and first described in detail in this review. The main purpose of this review was to describe how DDRs act in premalignant and malignant liver diseases and their potential mechanisms through an in-depth summary of preclinical in vitro and in vivo studies. Our work aims to provide new ideas for cancer treatment and accelerate translation from bench to bedside.
1 Background
Discoidin domain receptors (DDRs) are novel receptor tyrosine kinases (RTKs) discovered by Johnson et al. in 1993 (1). As a RTK family members, DDRs also include two subfamily members: discoidin domain receptor 1 (DDR1) and discoidin domain receptor 2 (DDR2) (2). Similar to other RTK family members, the structure of DDRs includes an extracellular region, a transmembrane (TM) domain, and an intracellular kinase region. Unlike other members of the RTK family, the extracellular regions of DDRs contain a globular discoidin (DS) domain of 155 amino acids, a discoidin-like (DS-like) domain and a long extracellular juxtamembrane (JM) region between the DS domain and the TM domain (3, 4). The JM segment is responsible for transmitting extracellular signals to the intracellular tyrosine kinase region (5). Both of these molecular structure features indicate that DDRs can bind to their ligands through an unusual transmembrane mechanism and play a vital role in cell biology. Based on the alternative splicing of intracellular kinase mRNA, there are five subtypes of DDR1: a, b, c, d, and e. However, only one single DDR2 subtype exists. There is kinase activity in the DDR1a, DDR1b and DDR1c coding sequences but not in the DDR1d or DDR1e coding sequences due to the deletions of exon 11 and exon 12, respectively (Figure 1) (6, 7). Notably, in addition to their role in collagen synthesis and degradation, DDRs promote tumour cell adhesion, migration, and differentiation and the release of inflammatory factors (8, 9).
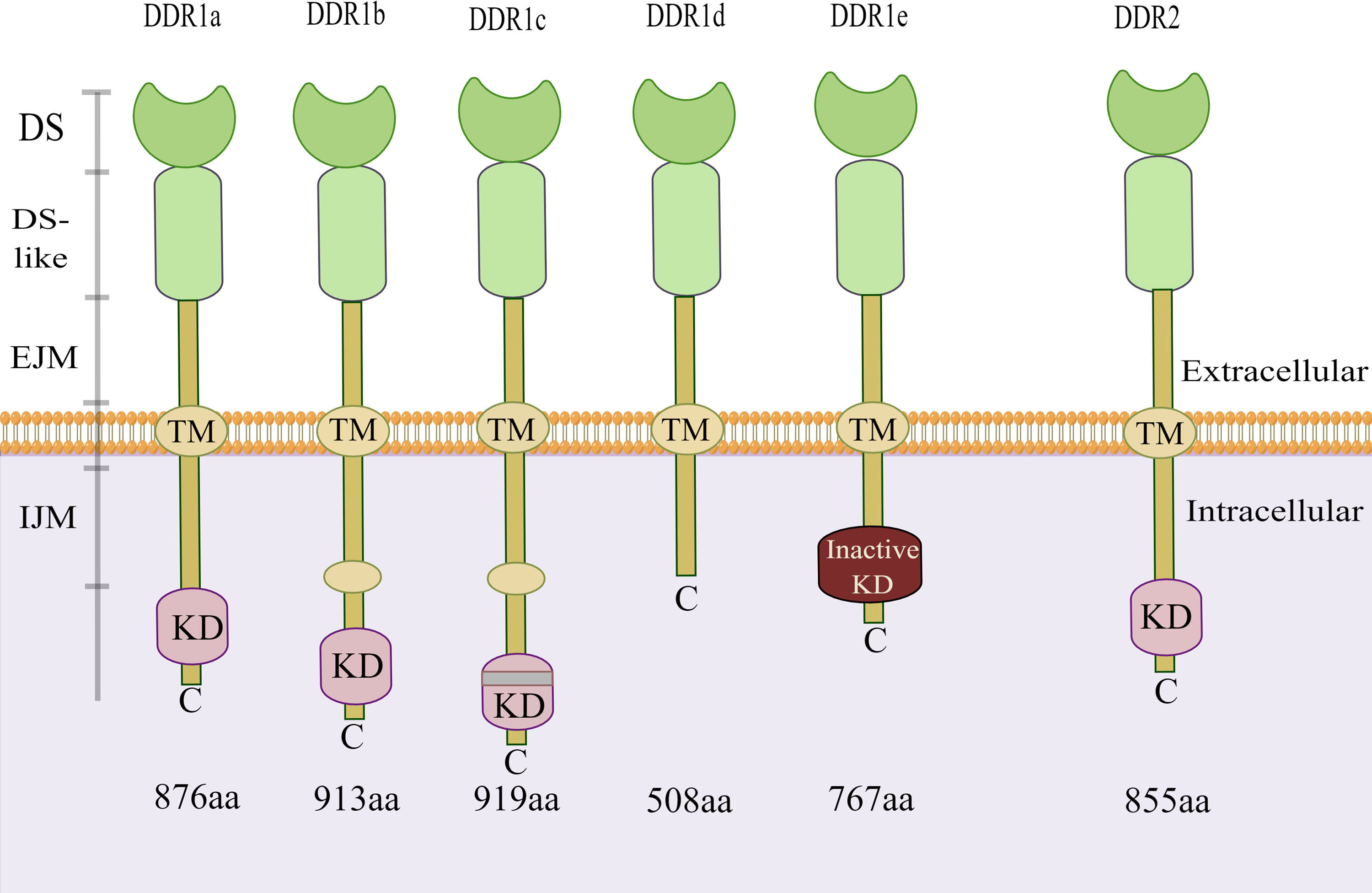
Figure 1 Structure of DDR1 and DDR2. There are five isoforms of DDR1. Among them, the kinase activity is present in the DDR1a, DDR1b and DDR1c coding sequences, but not in the DDR1d or DDR1e. However, only one single isoform of DDR2 exists. DDR, discoidin domain receptor; DS, discoidin domain; EJM, extracellular juxta-membrane; IJM, intracellular juxta-membrane; KD, kinase domain; TM, transmembrane domain. (The figure is drawn by Figdraw).
However, the underlying mechanisms of DDRs in premalignant and malignant liver disease remain obscure. In this review, the characteristics of the structure, activation and phosphotyrosine-based interactions of DDRs are briefly introduced, and the molecular mechanism of DDRs in the liver are systematically discussed, with the aim of promoting the development of DDR-targeted therapies and new cancer treatment methods.
2 Activation mechanism of DDRs
Most RTKs are activated by phosphorylated tyrosine residues within seconds of ligand binding, followed by a rapid decline in activity due to dephosphorylation or internalization and degradation of the receptor/ligand (10). Collagen, a key constituent of the extracellular matrix, is the specific ligand of DDRs, which are not activated by soluble peptide-like growth factors. The DS domain of DDRs binds with collagen (11). Unlike the signal transduction of other typical RTKs with acute and rapid responses, the binding of collagen to DDRs is slow and continuous (4). However, only collagen with a natural triple helical structure, but not heat-denatured collagen, can bind to DDRs (11, 12). The DS domain of DDR1 and DDR2 recognizes the specific amino acid sequence motif GVMGFO (O, hydroxyproline) on fibrillar collagen types I, II and III (13, 14). Collagen of the basement membrane (type IV) only activates DDR1, whereas collagens of types V and X only interact with DDR2 (15–17).
Although their single DS domain contains sites for binding to collagen, DDRs require dimerization of the DS domain to bind collagen with high affinity (11). Strikingly, DDRs develop independent and stable dimers mediated by one leucine-based sequence motif in the transmembrane domains in the absence of ligand recognition, which distinguishes them from other RTKs (18). Furthermore, mutation of cysteine residues in the extracellular JM region in DDRs results in independent and covalent dimers, which occur during biosynthesis, according to the study authors (19). The collagen binding region of charged residues surrounded by the hydrophobic layer in the three surface-exposed loops of the DS domain is activated by forming lateral clusters after binding to collagen (Figure 2) (11, 20). In other words, the activation of DDRs and the lateral clusters between DDR proteins are reciprocal causations. The lateral clusters reinforce the binding of DDRs to collagen and induce the activation of DDRs, while the lateral clusters leading to trans-phosphorylation between dimers are the result of collagen binding (18, 21). DDRs regulate adhesion and traction force on collagen by binding to myosin IIA, which condenses collagen fibrils into a denser alignment (22, 23). Activation of the DDR kinase domain enhances the association of the kinase domain with myosin IIA filaments, optimizing myosin-dependent contractile force delivery to collagen fibrils (21). The magnitude of DDR activation is proportional to collagen contractile forces (21). Upon binding of DDRs to collagen, autophosphorylation of the intracellular JM region and tyrosine residues of the intracellular kinase region in DDRs occurs (10). This phosphorylation recruits intracellular signalling protein complexes, such as Src Homology-23 (SH2/3) and the phosphotyrosine binding (PTB) domain, for assembling and transmitting receptor signals that play a cellular signal transducing function (11). Moreover, there is a non-canonical but functional crosstalk between insulin/insulin-like growth factor system (IIGFS) and DDRs, which was recently discovered apart from canonical collagen-dependent DDR activation. Interestingly, DDR1 functionally interacts with IIGFS better than DDR2 (24). As a complex network, IIGFS is constituted of transmembrane receptors, corresponding ligands, and binding proteins (24). Insulin-like growth factor 1 receptor (IGF1R) and insulin receptor (IR)-A, which are members of transmembrane receptors of IIGFS, and their common ligands consisting of insulin-like growth factors (IGF1 and IGF2) and insulin, are involved in the crosstalk with DDR1 (24). Upon stimulation by cognate ligands, IGF1R or IR-A physically interacts with DDR1 to induce rapid and sustained DDR1 phosphorylation, independent of the binding capacity of DDR1 to collagen (25). IIGFS not only stimulates DDR1 phosphorylation but also up-regulates DDR1 protein level to some extent via the activation of phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) cascade and further inhibition of downstream miR-199a-5p, a negative regulator of DDR1 (26).
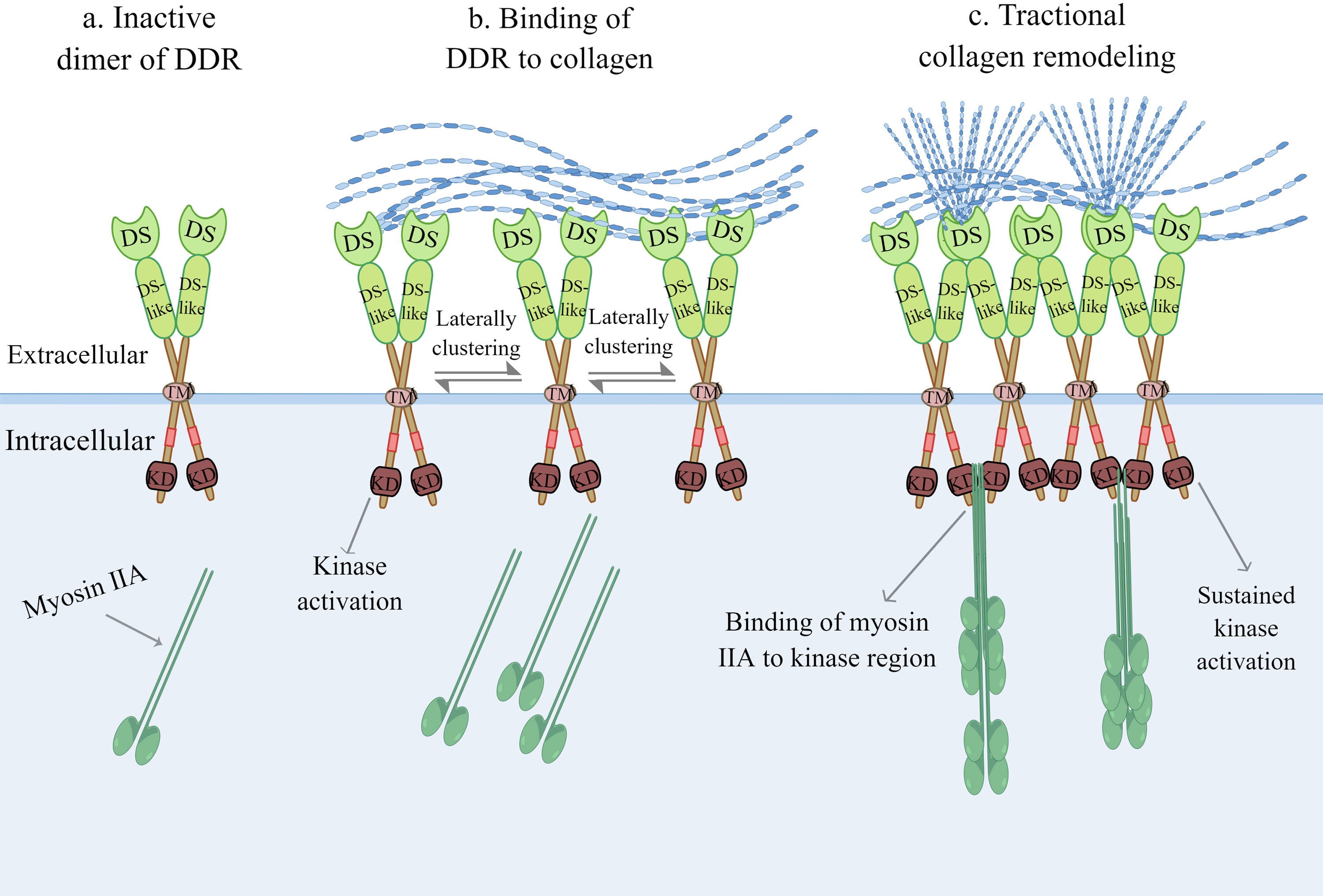
Figure 2 Collagen-induced DDR activation. (A) Inactive dimer of DDR without binding to collagen. (B) The activation of DDR and the lateral clusters between DDR proteins are a reciprocal causation. The lateral clusters reinforce the binding of DDR to collagen, meanwhile the increasing lateral clustering of dimers are the result of collagen binding. (C) Upon kinase domain activation, DDR regulates adhesion and traction force on collagen by binding to myosin IIA, which condenses collagen fibrils into more denser alignment. DDR, discoidin domain receptor; DS, discoidin domain; KD, kinase domain; TM, transmembrane domain. (The figure is drawn by Figdraw).
3 Phosphotyrosine-based interactions of DDRs
Upon ligand activation, distinct tyrosine residues in the intracellular JM and kinase regions are phosphorylated and act as docking sites for SH2/3 or PTB, which can serve as signal adaptors to assemble intracellular signalling molecules (Figure 3) (10). DDR1a has 13 tyrosine residues in the cytosolic JM and kinase regions, while both DDR1b and DDR1c have 15, and DDR2 has 14. It is beyond the scope of this paper to go into their tyrosine residues, given that both DDR1d and DDR1e, with an incomplete JM domain or the absence of an entire kinase domain, are nonfunctional truncated proteins (27). Interactors of DDRs localized on the phosphotyrosine residues of the receptor have been identified. Lemeer S et al. (28) outlined the tyrosine residue sites and corresponding interactors in the intracellular JM and kinase regions based on phosphopeptide affinity purifications in human placenta. For example, Crk2 (Tyr520), ShcA (Tyr513), C-terminal Src kinase (Csk) (Tyr520), STATs (Tyr543 and Tyr 547), Nck1/2 (Tyr569), phosphoinositide 3-kinase (PI3K) (Tyr703) and other phosphotyrosine interactors containing SH2/3 and PTB domains were detected to point to the docking sites of corresponding phosphotyrosine residues in the DDR1 receptor to transduce early signals from the receptor to the downstream cascade (28). Given that DDR1a lacks Tyr513 and Tyr520 docking sites compared with DDR1b/c, adaptor molecules, such as ShcA and Csk, associated with specific docking sites cannot bind to DDR1a (12). Regarding DDR2, Iwai LK et al. (29) identified two new docking sites in the DDR2 kinase domain, namely, Tyr684 and Tyr813. Interestingly, the docking site of Tyr481 in the JM region was shown to be constitutively phosphorylated, and Tyr471 was found to be a docking point for the adaptor ShcA (29, 30). Moreover, novel adaptor molecules, such as Grb2 and EphA2, were confirmed using a DDR1b immunoprecipitation assay (28). In addition to known adaptors with well-defined functions, other new adapters appear to involve RasGAP and VAV2/3. However, whether the new adaptor molecules bind to collagen-dependent activated receptors and generate signalling effects following binding remains to be seen.
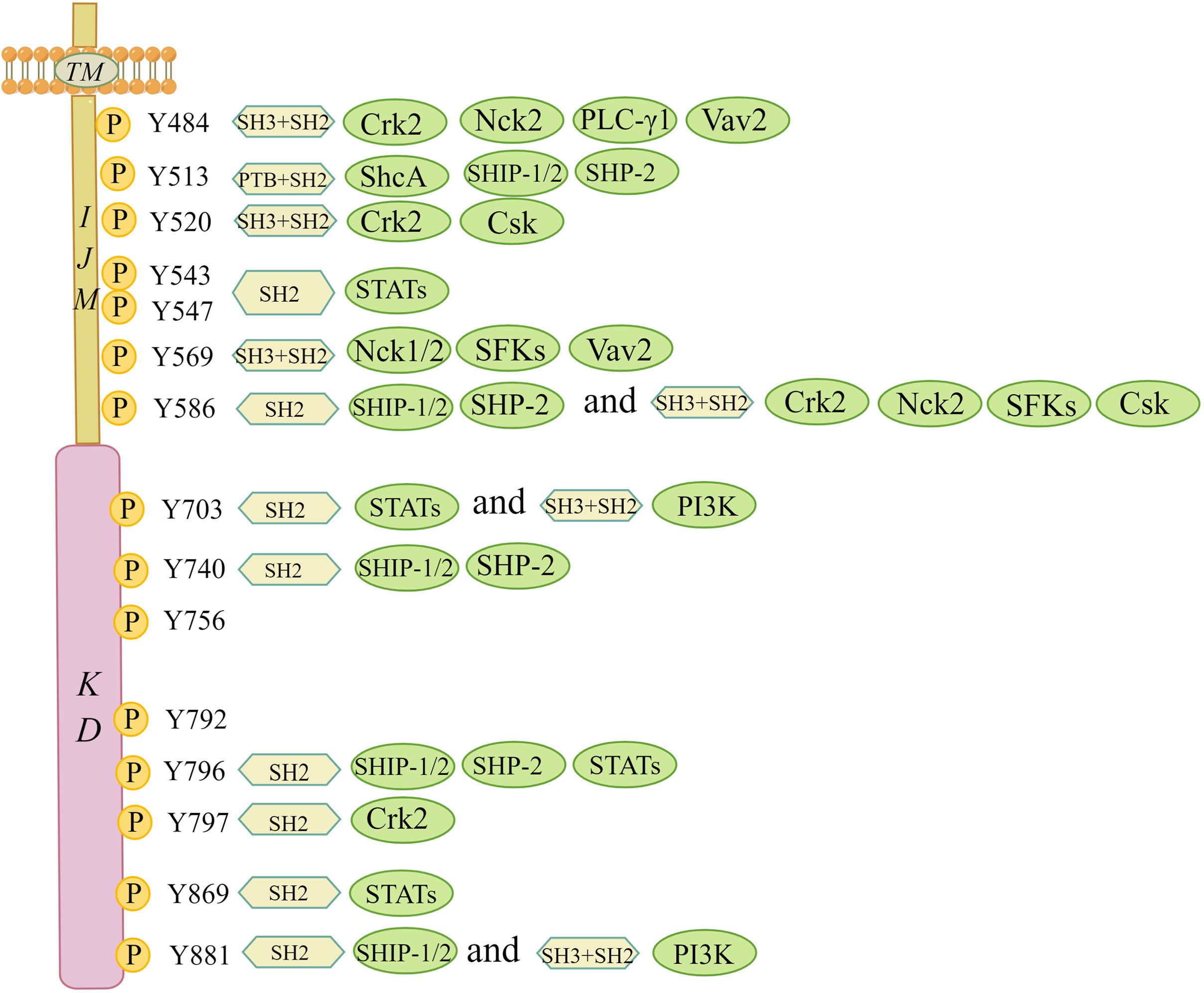
Figure 3 Interaction map of phosphotyrosine-based interactions of DDR1b isoform. This figure summarizes the tyrosine residue sites and corresponding interactors in the intracellular JM and kinase regions based on the study by Lemeer S et al. All phosphotyrosine interactors must rely on their SH2/3 and PTB domains in order to combine with the corresponding docking sites of tyrosine residues. Csk, C-terminal Src kinase; Crk2, adaptor protein Crk2; Nck1/2, adaptor protein Nck1/2; PI3K, phosphoinositide 3 kinase; PTB, phosphotyrosine-binding domain; PLC-γ1, phospholipase C γ1; SH2, Src homology 2 domain; SH3, Src homology 3 domain; ShcA, SH2-containing transforming protein A; SFKs, Src family tyrosine kinases; SHP-2, SH2 containing protein tyrosine phosphatase 2; SHIP-1/2, SH2-containing inositol polyphosphate 5- phosphatase 1/2; STATs, signal transducer and activator of transcriptions; TM, transmembrane domain; Vav2, guanine nucleotide exchange factor Vav2. (The figure is drawn by Figdraw).
4 DDRs as potential therapeutic targets in premalignant and malignant liver diseases
Several cell types express DDR1, mainly epithelial cells and smooth muscle cells, as well as other types of cells, such as the epithelium of the large intestine, lung and breast cells, adrenal cortical cells, pancreatic ducts, and macrophages (31, 32). DDR2 is mainly expressed in connective tissues of mesenchymal origin, including in cartilage and smooth muscle cells, fibroblasts and myofibroblasts, but also in skeletal muscle, kidney, myocardium, and lung cells (2, 23, 33, 34). Both DDR1 and DDR2 play important roles in the process of embryonic development. DDR1 is mainly involved in organ development and maturation, while DDR2 is involved in bone growth in humans (35). The physiological effects of DDRs do not persist into human adulthood, even though they play important physiological roles during embryonic development (27). Their physiological effects are not carefully elaborated here. The pathological functions of DDRs in premalignant and malignant liver diseases are described in the following sections (Table 1).
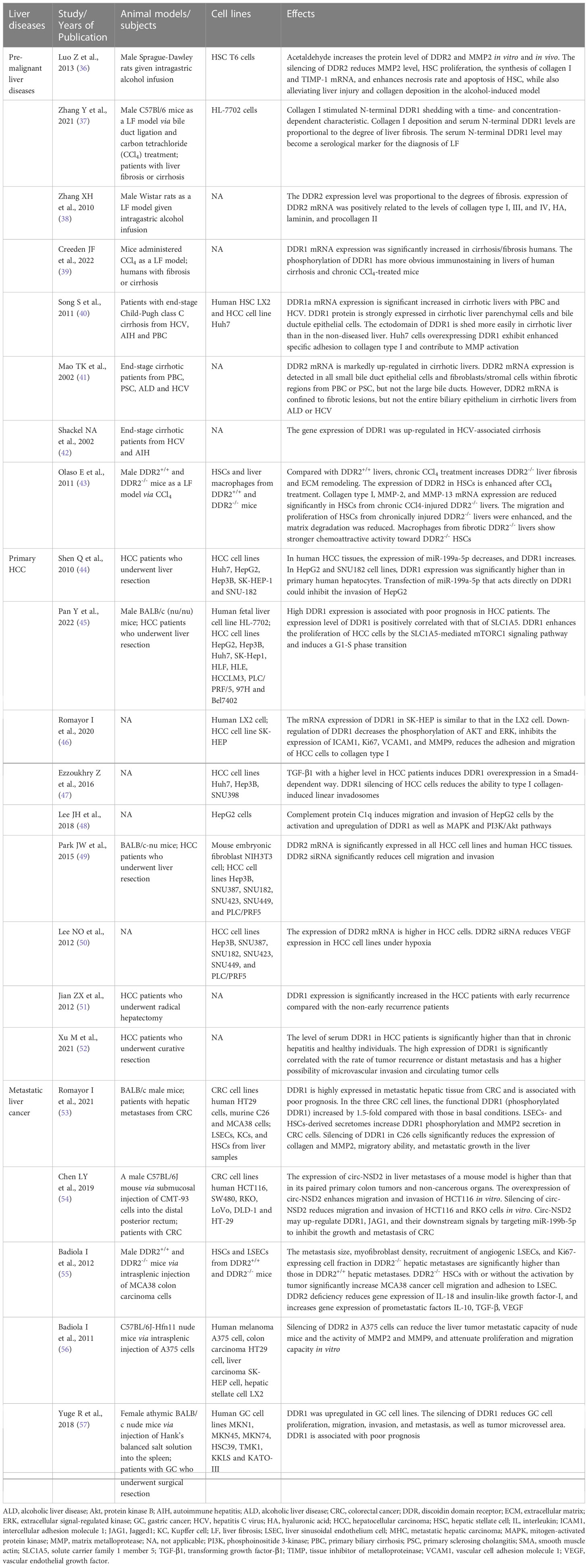
Table 1 The list of animal models/subjects, cell lines and effects of DDRs in premalignant and malignant liver diseases.
4.1 Premalignant liver diseases
The role of DDRs in the fibrosis stage of premalignant liver diseases appears to be complex, especially that of DDR2, which may exert diverse effects on the liver fibrosis stage. Overall, a positive correlation exists between the mRNA and protein expression levels of DDRs and the degree of liver fibrosis. Fibrotic livers, especially cirrhotic livers, express much higher levels of DDRs than nondiseased livers (37–42). The activation of cells that produce extracellular matrix (ECM) is an important step in tissue remodelling during liver fibrosis (58). During liver injury (prefibrotic stage), myofibroblasts derived from hepatic stellate cell (HSC) activation mainly produce collagen types I and III deposited into the nascent matrix (59). Lateral clusters of DDR proteins and enhanced binding of DDRs to collagen mediate tractional collagen remodelling, which is essential for mechanical compression and reorganization of collagen in the ECM during fibrosis (21). DDRs can also regulate cell adhesion, migration, differentiation and proliferation, depending on collagen quantity and configuration in the ECM. Song S et al. (40) showed that DDR1 overexpression enhanced hepatocyte adhesion to collagen type I and contributed to matrix metalloprotease (MMP) activation in vitro. Results from in vitro experiments suggested that DDR2 regulates the bioactivation of HSCs and the secretion of MMPs and promotes the development of fibrosis in early liver disease (36, 43). However, DDR2 may play a protective role in chronic and persistent liver fibrosis. Compared to DDR1-/- mice that were protected from chronic lung or kidney fibrosis, chronic liver fibrosis worsened in DDR2-/- mice (43, 60, 61). In disease progression, the balance between MMP2 and tissue inhibitor of metalloproteinase (TIMP) may be inclined toward MMP2. The activity of MMP2 is inhibited by TIMP in the early stage of liver injury, despite DDR2 promotion of MMP2 secretion. However, MMP2 performs limited collagen degradation, triggering proliferation, activation, and collagen production by more HSCs (62). In chronic liver fibrosis, DDR2-mediated MMP2 plays a more prominent role in the degradation of hepatic ECM than in the activation and proliferation of HSCs due to the decreased TIMP expression (62).
4.2 Primary hepatocellular carcinoma
DDRs play important roles in various cancer types, such as lung, gastric, liver, breast, brain and other cancers (63). DDR1 regulates HCC invasion ability, likely through MMP2 and MMP9 in the ECM (64). A study by Shen Q et al. (44) indicated that miR-199a-5p targets the 3’-UTR of DDR1 mRNA to reduce the invasiveness of HCC, and high expression of DDR1 was found to be closely related to advanced HCC and a poor prognosis. Moreover, it is known that the amino acid transporter SLC1A5 and mTORC1 contribute to cellular glutamine transport and glutamine utilization by tumour cells, respectively (65, 66). The DDR1-STAT3 interaction could also be involved in HCC. DDR1 promotes the phosphorylation of STAT3, which in turn increases the DDR1 protein level. The positive feedback between DDR1 and STAT3 promotes HCC tumorigenesis and metastasis to the lung via promoting epithelial-mesenchymal transition (EMT) and glutamine metabolism (67). DDR1 also enhances the proliferation of HCC cells via the SLC1A5-mediated mTORC1 signalling pathway and induces G1-S phase transition (45). Romayor I et al. (46) demonstrated that inhibition of DDR1 expression or activity suppressed HCC cell adhesion and migration to the ECM in a manner dependent on intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) and inhibited HCC cell proliferation, MMP9-dependent degradation of the ECM and the phosphorylation of Akt and extracellular signal-regulated kinase (ERK), which are two pro-survival molecules for tumour cell growth. Transforming growth factor-β1 (TGF-β1) induces collagen crosslinking- and DDR1-mediated linear invadosome formation, which can be reversed by inhibiting DDR1 expression to attenuate the invasiveness of HCC cells (47). Moreover, as a novel ligand of DDR1, the complement protein C1q induces the migration and invasion of HCC cells by activating and upregulating DDR1 and the mitogen-activated protein kinase (MAPK) and PI3K/Akt pathways to increase the expressions of MMP2/9 and EMT-related proteins (48). Concerning DDR2, there is still prominent DDR2 protein expressed on hepatocytes, and inhibiting DDR2 expression can also inhibit the growth, invasion, and migration of HCC cells (49, 50). Furthermore, DDR2 siRNA in HCC cell lines reduces vascular endothelial growth factor (VEGF) expression, suggesting that VEGF may be a downstream target of the DDR2 gene (50).
Early recurrence of liver cancer following surgery is a major prognostic factor. The expression of DDR1 mRNA and protein is prominently higher in early recurrent tumour samples than in nonrecurrent tumour samples, suggesting that DDR1 may be a predictor of early recurrence after hepatectomy (51). Tumour microvascular invasion and circulating tumour cells, which are prognostic factors, are also closely associated with the overexpression of DDR1 (52). Moreover, the serum DDR1 level is significantly higher in HCC patients than in chronic hepatitis patients and healthy individuals (52). After long periods of DDR1 binding to collagen, the N-terminus (extracellular region) of DDR1 undergoes shedding under the induction of a membrane-anchored collagenase and MMPs (68–70). This phenomenon is more pronounced in liver cancer and likely explains the close association between DDR1 in serum and DDR1 in tumours. AFP is perhaps one of the most widely used biomarkers for the evaluation of HCC patient status (71). As more than 50% of HCC patients with low AFP levels or low recurrence risk still have postoperative recurrence and distant metastasis, the monitoring role of AFP faces stern challenges (72). DDR1 still has prognostic value, even in low-risk patients. Thus, the combination of serum DDR1 and tumour DDR1 levels with other monitoring methods, such as alpha fetoprotein (AFP), computed tomography (CT) or magnetic resonance imaging (MRI), might be helpful in forecasting recurrence. Moreover, in a phosphoproteomic screen, DDR1 was identified as a highly phosphorylated RTK in cholangiocarcinoma (73).
4.3 Metastatic liver cancer derived from colorectal cancer
Liver metastases and metastatic growth originating from human CRC predict poor prognosis. However, the potential mechanism of DDRs in metastatic liver cancer remains poorly understood. Romayor I et al. (53) showed that metastatic hepatic tissue from CRC expressed high levels of DDR1, which was associated with a poor prognosis. Silencing DDR1 significantly reduced tumour migratory ability and metastatic growth in the liver. The formation of a metastatic focus in the liver is highly dependent on the proliferation of primary tumour cells and the supportive microenvironment (74, 75). Given this information, the interaction between sinusoidal cells (SCs), composed of liver sinusoidal endothelial cells (LSECs), Kupffer cells (KCs) and HSCs, and CRC cells is essential for the metastasis, survival and proliferation of CRC cells in the liver (76). The secretomes of LSECs and HSCs induce increases in the levels of functional DDR1 (phosphorylated DDR1) and MMPs in CRC cells, which further promote CRC cell infiltration into the liver through matrix degradation and ECM remodelling (77). Furthermore, circ-NSD2, a circular noncoding RNA that is highly expressed in liver metastases, upregulates the expression of DDR1, JAG1 (Jagged 1) and their downstream signalling proteins to promote the migration, invasion and metastasis of CRC cells by targeting miR-199b-5p (54).
On the other hand, DDR2 might act as a liver metastasis suppressor through tumour-activated HSCs. We suggest that the most important reason lies in the interaction between DDR2-/- HSCs and cancer cells. Badiola I et al. (55) demonstrated that DDR2-/- HSCs have a higher myofibroblast transdifferentiation rate, release more endothelial cell adhesion molecules and migration-stimulating factors and overexpress key prometastatic factors, including interleukin (IL)-10, VEGF and TGF-β1, in response to CRC cells. However, inhibition of DDR2 signalling in HSCs reduces the expression of MMP2, which is involved in matrix degradation and metastasis promotion (62). Thus, the liver microenvironment produced by DDR2-/- HSC-derived myofibroblasts based on the stimulation of tumour tissue, rather than MMP2 released insufficiently by DDR2-/- HSCs, may be the major contributor to the development of liver metastases derived from colorectal cancer. Interestingly, DDR2 is mainly expressed in HSCs, although it is also seen in hepatocytes or cancer cells (49, 50). This DDR2 expression in different cell types shows functional divergence. This was demonstrated in another study by Badiola I et al. (56), which suggested that downregulation of DDR2 expression in human hepatoma and colon cancer cell lines attenuated their proliferation and migration capacity in vitro.
4.4 Metastatic liver cancer derived from other cancer types
DDR1 and DDR2 were found to be significantly overexpressed in gastric cancer (GC) cell lines compared to normal gastric mucosal cells, particularly in poorly differentiated GC cells (78, 79). As DDR1 and DDR2 expression increases in GC cells, early recurrence and peritoneal metastasis occur more frequently; thus, we speculate that DDR1 and DDR2 overexpression may also be closely related to further liver metastasis (80, 81). One recent study by Yuge R et al. (57) suggested that DDR1 expression in GC tissue was associated with poor prognosis. By silencing DDR1, GC cell lines were less prone to migration, invasion, and tube formation. DDR1 silencing also reduced GC tissue angiogenesis and lymphangiogenesis in a nude mouse model and almost completely blocked liver colonization and metastasis. Moreover, DDR2 overexpression promotes GC invasion, metastasis and EMT progression by activating mTORC2 and phosphorylating downstream Akt (78). If detected in the early stages of progression, DDR1 and DDR2 may serve as new and promising targets to prevent liver metastasis of GC. This effect of DDRs has also been shown in melanoma. Rather than being expressed in normal tissues, the expression of DDR2 in melanoma cells promotes malignant melanoma growth (82). After intrasplenic inoculation, the liver metastasis ability of DDR2-silenced melanoma cells is reduced compared with that of nonsilenced control cells. The mechanism may be that downregulation of DDR2 in melanoma cells decreases the activity of MMPs to suppress the proliferation, invasion and migration of the cells through inhibition of the ERK1/2 and nuclear factor kappa B (NF-κB) pathways (56).
5 DDR inhibitors
The slow development of liver fibrosis associated with a variety of chronic diseases usually takes years (83). There is a slow transition from normal tissue repair to profibrotic tissue and tissue remodelling resulting from a prolonged lesion (84). Early diagnosis and early treatment of liver fibrosis need urgent improvements, and antifibrotic treatment remains stagnant. In addition, the emerging evidence described in this review suggests that DDRs play a crucial role in tumour progression and metastasis. Therefore, this section explains how DDR kinase inhibitors and multikinase inhibitors are used in the treatment of fibrosis and cancer. The effects of multikinase inhibitors may be mediated in part through inhibiting DDRs.
Most DDR inhibitors tend to be adenosine triphosphate (ATP)-competitive inhibitors, which can act on both DDR1 and DDR2 with poor kinase selectivity (85). The multikinase inhibitors dasatinib, imatinib, nilotinib, bafetinib, ponatinib, MGCD516 (sitravatinib) and LCB 03-0110 have been shown to inhibit both DDR1 and DDR2 kinase activity and are mainly applied in the treatment of chronic myeloid leukaemia (CML), lung adenocarcinoma, and colorectal cancer, among other cancer types (86–91). Among them, dasatinib can inhibit the proliferation of squamous cell carcinoma (SQCC) with DDR2 mutation, and ponatinib is prescribed to treat imatinib-resistant patients with CML (88, 92). Concerning DDR kinase inhibitors, the compound 7f is representative of a range of 2-amino-2,3-dihydro-1H-indene-5-carboxamide derivatives; compounds 2a, 4a, and 4b belong to a family of pyrazolourea-containing compounds; and DDR1-IN-1 and DDR1-IN-2 exhibit notable inhibitory effects on DDR1 and DDR2 kinase activity in cancer (93–95). WRG-28 has also been shown to inhibit tumour invasion and metastasis as a selective DDR2 small-molecule inhibitor (96). Moreover, Chen C et al. (97) reported that the novel DDR1 inhibitor AH-487/41940522 can inhibit collagen deposition in a rat model of idiopathic pulmonary fibrosis. Nilotinib has been shown to block the association of myosin IIA with the DDR1 kinase domain to reduce tractional collagen contraction in vitro (Figure 4) (21). Other potent lead compounds for inhibiting selectively DDRs include compounds 13-21 (98). Among them, compounds 13, 18, and 21 are representative of 1, 2, 3, 4-tetrahydroisoquinoline derivatives, pyrazole fused pyrimidine derivatives, and indazole derivatives against DDR1, respectively; compounds 14-16, compound 17, and compound 19 separately belong to di-amide derivatives, dasatinib analogs and indole analogs against DDR1 and DDR2; and the compound 20 derives from pyridine derivatives against DDR2 (98). Currently, several clinical trials have been conducted with drugs such as dasatinib, nilotinib, and sitravatinib for different cancers, including squamous cell lung carcinoma, advanced or refractory lymphoma, advanced solid malignancies, hematopoietic neoplasm, etc (99–104).
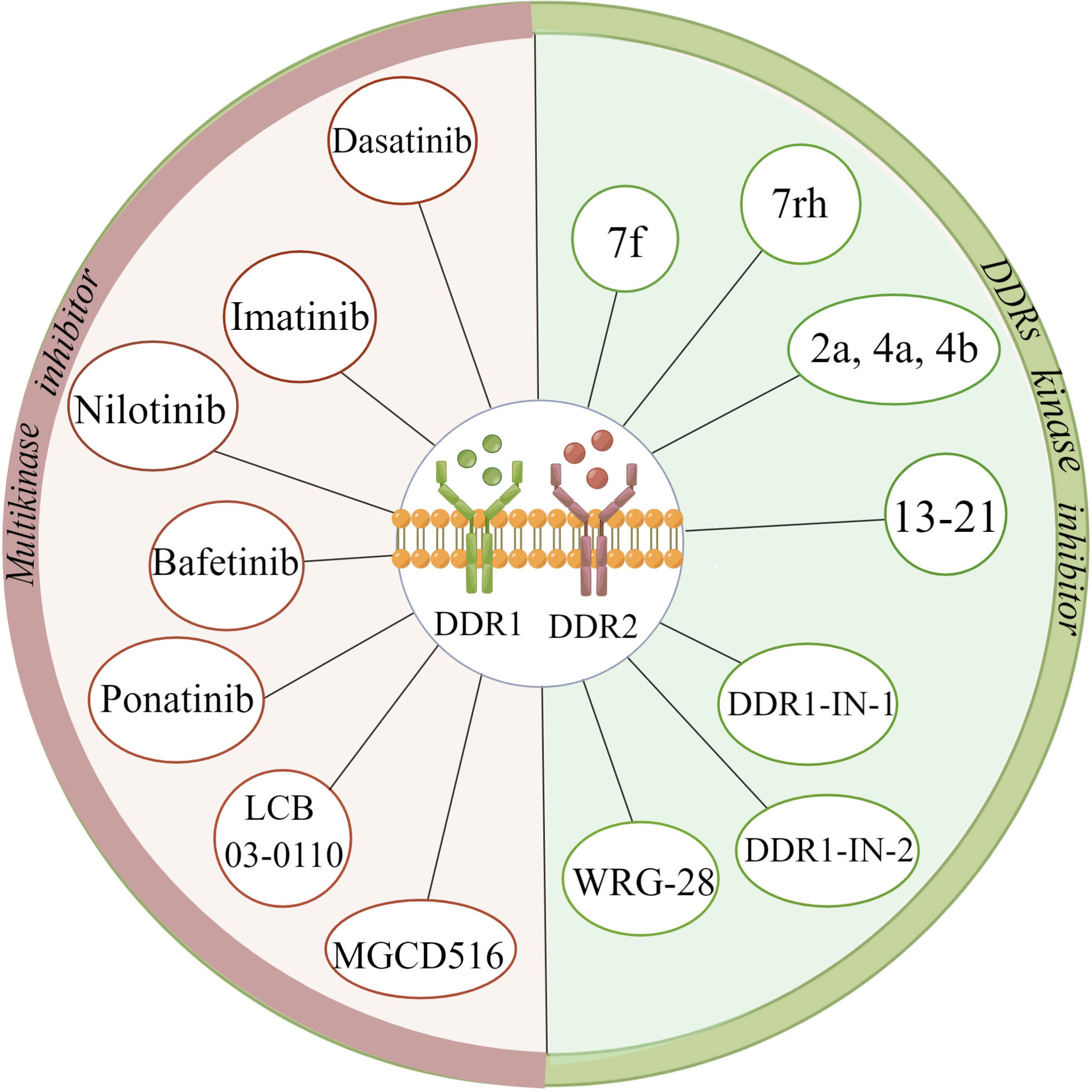
Figure 4 Summary of various discoidin domain receptors inhibitors. (The figure is drawn by Figdraw).
6 Drug resistance linked to DDRs
The mechanisms of drug resistance linked to DDRs are associated with DDR mutations. Part of DDR inhibitors loses their inhibitory activity on the background of DDR mutations. The aminopyrimidine head group of imatinib binds to the “gatekeeper” residue Thr701 in DDR1 via the hydrogen bond. In CML, the mutation of the “gatekeeper” Thr701 in DDR1 confers steric hindrance and causes resistance to imatinib (105). Ponatinib has been proven to circumvent the steric hindrance and may be selected for the treatment of imatinib-resistant CML patients (86). Considering that Ponatinib is much less selective than imatinib, DDR1-IN-1, therefore, is designed to have a similar pharmacophore model while conferring highly a selective pharmacological profile (94). Beauchamp et al. (106), found that the mutation of the “gatekeeper” T654I in DDR2 and loss of NF1 conferred acquired resistance to dasatinib in lung cancer cell lines via targeted exome sequencing. Although the phosphorylation of DDR2 in lung cancer cell lines was decreased by the administration of dasatinib, the acquired mutation in DDR2 T654I blocked this therapeutic effect. And, the loss of NF1 activated a bypass pathway that conferred the activation of the RAS signaling pathway. WRG-28 has been proven to inhibit the collagen-stimulated phosphorylation of DDR2 with T654I mutation via its allosteric action upon the extracellular domain (96). Interestingly, a case report by Pitini et al. (107), found a significant reduction in tumor size during administration of dasatinib in one patient with lung squamous SQCC accompanied by S768R mutation of DDR2 kinase gene. However, The high-frequency occurrence of dasatinib toxicity in clinical trials should be noted. Moreover, advanced melanoma with acquired BRAF (V600) mutations is resistant to initially effective BRAF/MEK inhibitors, which is the main reason restricting patient benefit. The key lies in the reactivation of alternate signaling networks, in which the DDR-mediated extracellular matrix is an important component of cancer cell adaptation and resistance to targeted therapy (108, 109). Thus, DDR-targeted therapy can enhance the efficacy of BRAF-targeted therapy to overcome drug resistance.
7 Conclusions
Here, we discussed the roles of DDR1 and DDR2 in premalignant liver diseases, primary HCC and metastatic liver cancer derived from several other cancer types. Briefly, the progression of liver fibrosis can be promoted when DDR1 is overexpressed in hepatocytes, and invasion, migration and liver metastasis can be stimulated when DDR1 is overexpressed in tumour cells. However, DDR2 signalling in HSCs can protect against chronic liver fibrosis progression but not early-stage liver injury (prefibrotic stage) and can inhibit liver metastasis of colorectal cancer. These perspectives are critically significant and are first described in detail in this review. Based on relevant original studies, DDR2 can also be expressed in HCC cells and plays a pathogenic role. Although the pathological functions of DDRs in premalignant and malignant liver diseases have been reported, to aid in the development of more targeted DDR inhibitors and facilitate their application in clinical practice, additional studies exploring the concrete molecular mechanisms of DDRs are needed.
Author contributions
HG: Writing-original draft. H-MX: Designing some contents of the manuscript. D-KZ: Writing, and revising the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 8216030085); and Talent Innovation and Entrepreneurship Project of Science and Technology Department of Lanzhou (grant number 2018-RC-76).
Acknowledgments
We appreciate the contributions of all the doctors, coworkers, and friends involved in this study and thank the editors and reviewers for their help with this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Johnson JD, Edman JC, Rutter WJ. A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin i-like domain. Proc Natl Acad Sci USA (1993) 90:5677–81. doi: 10.1073/pnas.90.12.5677
2. Vogel W. Discoidin domain receptors: Structural relations and functional implications. FASEB J (1999) 13 Suppl:S77–82. doi: 10.1096/fasebj.13.9001.s77
3. Noordeen NA, Carafoli F, Hohenester E, MA H, Leitinger B. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase ddr1. J Biol Chem (2006) 281:22744–51. doi: 10.1074/jbc.M603233200
4. Vogel WF, Abdulhussein R, Ford CE. Sensing extracellular matrix: An update on discoidin domain receptor function. Cell Signal (2006) 18:1108–16. doi: 10.1016/j.cellsig.2006.02.012
5. Carafoli F, Hohenester E. Collagen recognition and transmembrane signalling by discoidin domain receptors. Biochim Biophys Acta (2013) 1834:2187–94. doi: 10.1016/j.bbapap.2012.10.014
6. Carafoli F, MC M, Shiraishi K, Pecheva MA, Chan LY, Nan R, et al. Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory fab fragment reveals features important for signaling. Structure. (2012) 20:688–97. doi: 10.1016/j.str.2012.02.011
7. Carafoli F, Bihan D, Stathopoulos S, Konitsiotis AD, Kvansakul M, Farndale RW, et al. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure. (2009) 17:1573–81. doi: 10.1016/j.str.2009.10.012
8. Olaso E, HC L, Wang LH, Friedman SL. Impaired dermal wound healing in discoidin domain receptor 2-deficient mice associated with defective extracellular matrix remodeling. Fibrogenesis Tissue Repair (2011) 4:5. doi: 10.1186/1755-1536-4-5
9. Pozzi A, Yurchenco PD, Iozzo RV. The nature and biology of basement membranes. Matrix Biol (2017) 57-58:1–11. doi: 10.1016/j.matbio.2016.12.009
10. Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. (2010) 141:1117–34. doi: 10.1016/j.cell.2010.06.011
11. Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, ddr1 and ddr2. identification of collagen binding sites in ddr2. J Biol Chem (2003) 278:16761–9. doi: 10.1074/jbc.M301370200
12. Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell (1997) 1:13–23. doi: 10.1016/s1097-2765(00)80003-9
13. Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor ddr2 in collagen. J Biol Chem (2008) 283:6861–8. doi: 10.1074/jbc.M709290200
14. Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B. Collagen binding specificity of the discoidin domain receptors: Binding sites on collagens ii and iii and molecular determinants for collagen iv recognition by ddr1. Matrix Biol (2011) 30:16–26. doi: 10.1016/j.matbio.2010.10.004
15. Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, et al. Discoidin domain receptors: Unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem (2013) 288:7430–7. doi: 10.1074/jbc.R112.444158
16. Schlessinger J. Direct binding and activation of receptor tyrosine kinases by collagen. Cell. (1997) 91:869–72. doi: 10.1016/s0092-8674(00)80477-8
17. Leitinger B, Kwan AP. The discoidin domain receptor ddr2 is a receptor for type x collagen. Matrix Biol (2006) 25:355–64. doi: 10.1016/j.matbio.2006.05.006
18. Juskaite V, Corcoran DS, Leitinger B. Collagen induces activation of ddr1 through lateral dimer association and phosphorylation between dimers. Elife. (2017) 6:e25716. doi: 10.7554/eLife.25716
19. Xu H, Abe T, Liu JK, Zalivina I, Hohenester E, Leitinger B. Normal activation of discoidin domain receptor 1 mutants with disulfide cross-links, insertions, or deletions in the extracellular juxtamembrane region: Mechanistic implications. J Biol Chem (2014) 289:13565–74. doi: 10.1074/jbc.M113.536144
20. Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the ddr1 tyrosine kinase receptor. J Biol Chem (2004) 279:31462–70. doi: 10.1074/jbc.M400651200
21. Coelho NM, Arora PD, van Putten S, Boo S, Petrovic P, Lin AX, et al. Discoidin domain receptor 1 mediates myosin-dependent collagen contraction. Cell Rep (2017) 18:1774–90. doi: 10.1016/j.celrep.2017.01.061
22. Huang Y, Arora P, McCulloch CA, Vogel WF. The collagen receptor ddr1 regulates cell spreading and motility by associating with myosin iia. J Cell Sci (2009) 122:1637–46. doi: 10.1242/jcs.046219
23. Coelho NM, McCulloch CA. Contribution of collagen adhesion receptors to tissue fibrosis. Cell Tissue Res (2016) 365:521–38. doi: 10.1007/s00441-016-2440-8
24. Vella V, Malaguarnera R, Nicolosi ML, Morrione A, Belfiore A. Insulin/igf signaling and discoidin domain receptors: An emerging functional connection. Biochim Biophys Acta Mol Cell Res (2019) 1866:118522. doi: 10.1016/j.bbamcr.2019.118522
25. Malaguarnera R, Nicolosi ML, Sacco A, Morcavallo A, Vella V, Voci C, et al. Novel cross talk between igf-ir and ddr1 regulates igf-ir trafficking, signaling and biological responses. Oncotarget. (2015) 6:16084–105. doi: 10.18632/oncotarget.3177
26. Mata R, Palladino C, Nicolosi ML, Lo PA, Malaguarnera R, Ragusa M, et al. Igf-i induces upregulation of ddr1 collagen receptor in breast cancer cells by suppressing mir-199a-5p through the pi3k/akt pathway. Oncotarget. (2016) 7:7683–700. doi: 10.18632/oncotarget.6524
27. Leitinger B. Discoidin domain receptor functions in physiological and pathological conditions. Int Rev Cell Mol Biol (2014) 310:39–87. doi: 10.1016/B978-0-12-800180-6.00002-5
28. Lemeer S, Bluwstein A, Wu Z, Leberfinger J, Muller K, Kramer K, et al. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. J Proteomics (2012) 75:3465–77. doi: 10.1016/j.jprot.2011.10.007
29. Iwai LK, Payne LS, Luczynski MT, Chang F, Xu H, Clinton RW, et al. Phosphoproteomics of collagen receptor networks reveals shp-2 phosphorylation downstream of wild-type ddr2 and its lung cancer mutants. Biochem J (2013) 454:501–13. doi: 10.1042/BJ20121750
30. Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, et al. Discoidin domain receptor 2 interacts with src and shc following its activation by type i collagen. J Biol Chem (2002) 277:19206–12. doi: 10.1074/jbc.M201078200
31. Barker KT, Martindale JE, Mitchell PJ, Kamalati T, Page MJ, Phippard DJ, et al. Expression patterns of the novel receptor-like tyrosine kinase, ddr, in human breast tumours. Oncogene. (1995) 10:569–75.
32. Moll S, Desmouliere A, Moeller MJ, Pache JC, Badi L, Arcadu F, et al. Ddr1 role in fibrosis and its pharmacological targeting. Biochim Biophys Acta Mol Cell Res (2019) 1866:118474. doi: 10.1016/j.bbamcr.2019.04.004
33. Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin i subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. (1995) 10:609–18.
34. Lee JE, Kang CS, Guan XY, Kim BT, Kim SH, Lee YM, et al. Discoidin domain receptor 2 is involved in the activation of bone marrow-derived dendritic cells caused by type i collagen. Biochem Biophys Res Commun (2007) 352:244–50. doi: 10.1016/j.bbrc.2006.11.010
35. Dorison A, Dussaule JC, Chatziantoniou C. The role of discoidin domain receptor 1 in inflammation, fibrosis and renal disease. Nephron. (2017) 137:212–20. doi: 10.1159/000479119
36. Luo Z, Liu H, Sun X, Guo R, Cui R, Ma X, et al. Rna interference against discoidin domain receptor 2 ameliorates alcoholic liver disease in rats. PloS One (2013) 8:e55860. doi: 10.1371/journal.pone.0055860
37. Zhang Y, Zhang Y, Liang H, Zhuo Z, Fan P, Chen Y, et al. Serum n-terminal ddr1: A novel diagnostic marker of liver fibrosis severity. J Clin Transl Hepatol (2021) 9:702–10. doi: 10.14218/JCTH.2021.00024
38. Zhang XH, Yan M, Liu L, Wu TJ, Ma LL, Wang LX. Expression of discoidin domain receptors (ddr2) in alcoholic liver fibrosis in rats. Arch Med Res (2010) 41:586–92. doi: 10.1016/j.arcmed.2010.10.010
39. Creeden JF, Kipp ZA, Xu M, Flight RM, Moseley H, Martinez GJ, et al. Hepatic kinome atlas: An in-depth identification of kinase pathways in liver fibrosis of humans and rodents. Hepatology (2022) 76:1376–1388. doi: 10.1002/hep.32467
40. Song S, Shackel NA, Wang XM, Ajami K, McCaughan GW, Gorrell MD. Discoidin domain receptor 1: Isoform expression and potential functions in cirrhotic human liver. Am J Pathol (2011) 178:1134–44. doi: 10.1016/j.ajpath.2010.11.068
41. Mao TK, Kimura Y, Kenny TP, Branchi A, Gishi RG, Van de Water J, et al. Elevated expression of tyrosine kinase ddr2 in primary biliary cirrhosis. Autoimmunity. (2002) 35:521–9. doi: 10.1080/0891693021000057784
42. Shackel NA, McGuinness PH, Abbott CA, Gorrell MD, McCaughan GW. Insights into the pathobiology of hepatitis c virus-associated cirrhosis: Analysis of intrahepatic differential gene expression. Am J Pathol (2002) 160:641–54. doi: 10.1016/S0002-9440(10)64884-5
43. Olaso E, Arteta B, Benedicto A, Crende O, Friedman SL. Loss of discoidin domain receptor 2 promotes hepatic fibrosis after chronic carbon tetrachloride through altered paracrine interactions between hepatic stellate cells and liver-associated macrophages. Am J Pathol (2011) 179:2894–904. doi: 10.1016/j.ajpath.2011.09.002
44. Shen Q, Cicinnati VR, Zhang X, Iacob S, Weber F, Sotiropoulos GC, et al. Role of microrna-199a-5p and discoidin domain receptor 1 in human hepatocellular carcinoma invasion. Mol Canc (2010) 9:227. doi: 10.1186/1476-4598-9-227
45. Pan Y, Han M, Zhang X, He Y, Yuan C, Xiong Y, et al. Discoidin domain receptor 1 promotes hepatocellular carcinoma progression through modulation of slc1a5 and the mtorc1 signaling pathway. Cell Oncol (Dordr) (2022) 45:163–78. doi: 10.1007/s13402-022-00659-8
46. Romayor I, Badiola I, Olaso E. Inhibition of ddr1 reduces invasive features of human a375 melanoma, ht29 colon carcinoma and sk-hep hepatoma cells. Cell Adh Migr (2020) 14:69–81. doi: 10.1080/19336918.2020.1733892
47. Ezzoukhry Z, Henriet E, Piquet L, Boye K, Bioulac-Sage P, Balabaud C, et al. Tgf-beta1 promotes linear invadosome formation in hepatocellular carcinoma cells, through ddr1 up-regulation and collagen i cross-linking. Eur J Cell Biol (2016) 95:503–12. doi: 10.1016/j.ejcb.2016.09.003
48. Lee JH, Poudel B, Ki HH, Nepali S, Lee YM, Shin JS, et al. Complement c1q stimulates the progression of hepatocellular tumor through the activation of discoidin domain receptor 1. Sci Rep (2018) 8:4908. doi: 10.1038/s41598-018-23240-6
49. Park JW, Lee YS, Kim JS, Lee SK, Kim BH, Lee JA, et al. Downregulation of discoidin domain receptor 2 decreases tumor growth of hepatocellular carcinoma. J Cancer Res Clin Oncol (2015) 141:1973–83. doi: 10.1007/s00432-015-1967-5
50. Lee NO, Park JW, Lee JA, Shim JH, Kong SY, Kim KT, et al. Dual action of a selective cyclooxygenase-2 inhibitor on vascular endothelial growth factor expression in human hepatocellular carcinoma cells: Novel involvement of discoidin domain receptor 2. J Cancer Res Clin Oncol (2012) 138:73–84. doi: 10.1007/s00432-011-1075-0
51. Jian ZX, Sun J, Chen W, Jin HS, Zheng JH, Wu YL. Involvement of discoidin domain 1 receptor in recurrence of hepatocellular carcinoma by genome-wide analysis. Med Oncol (2012) 29:3077–82. doi: 10.1007/s12032-012-0277-x
52. Xu M, Cui C. Discoidin domain receptor tyrosine kinase 1 (ddr1): A novel predictor for recurrence of hepatocellular carcinoma after curative resection. Med Sci Monit (2021) 27:e933109. doi: 10.12659/MSM.933109
53. Romayor I, Marquez J, Benedicto A, Herrero A, Arteta B, Olaso E. Tumor ddr1 deficiency reduces liver metastasis by colon carcinoma and impairs stromal reaction. Am J Physiol Gastrointest Liver Physiol (2021) 320:G1002–13. doi: 10.1152/ajpgi.00078.2021
54. Chen LY, Zhi Z, Wang L, Zhao YY, Deng M, Liu YH, et al. Nsd2 circular rna promotes metastasis of colorectal cancer by targeting mir-199b-5p-mediated ddr1 and jag1 signalling. J Pathol (2019) 248:103–15. doi: 10.1002/path.5238
55. Badiola I, Olaso E, Crende O, Friedman SL, Vidal-Vanaclocha F. Discoidin domain receptor 2 deficiency predisposes hepatic tissue to colon carcinoma metastasis. Gut. (2012) 61:1465–72. doi: 10.1136/gutjnl-2011-300810
56. Badiola I, Villace P, Basaldua I, Olaso E. Downregulation of discoidin domain receptor 2 in a375 human melanoma cells reduces its experimental liver metastasis ability. Oncol Rep (2011) 26:971–8. doi: 10.3892/or.2011.1356
57. Yuge R, Kitadai Y, Takigawa H, Naito T, Oue N, Yasui W, et al. Silencing of discoidin domain receptor-1 (ddr1) concurrently inhibits multiple steps of metastasis cascade in gastric cancer. Transl Oncol (2018) 11:575–84. doi: 10.1016/j.tranon.2018.02.003
58. Duscher D, Maan ZN, Wong VW, Rennert RC, Januszyk M, Rodrigues M, et al. Mechanotransduction and fibrosis. J Biomech (2014) 47:1997–2005. doi: 10.1016/j.jbiomech.2014.03.031
59. Wu K, Huang R, Wu H, Liu Y, Yang C, Cao S, et al. Collagen-binding vascular endothelial growth factor attenuates ccl4-induced liver fibrosis in mice. Mol Med Rep (2016) 14:4680–6. doi: 10.3892/mmr.2016.5826
60. Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med (2006) 174:420–7. doi: 10.1164/rccm.200603-333OC
61. Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, et al. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol (2011) 179:83–91. doi: 10.1016/j.ajpath.2011.03.023
62. Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, et al. Ddr2 receptor promotes mmp-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest (2001) 108:1369–78. doi: 10.1172/JCI12373
63. Mehta V, Chander H, Munshi A. Complex roles of discoidin domain receptor tyrosine kinases in cancer. Clin Transl Oncol (2021) 23:1497–510. doi: 10.1007/s12094-021-02552-6
64. Park HS, Kim KR, Lee HJ, Choi HN, Kim DK, Kim BT, et al. Overexpression of discoidin domain receptor 1 increases the migration and invasion of hepatocellular carcinoma cells in association with matrix metalloproteinase. Oncol Rep (2007) 18:1435–41. doi: 10.3892/or.18.6.1435
65. Jiang H, Zhang N, Tang T, Feng F, Sun H, Qu W. Target the human alanine/serine/cysteine transporter 2(asct2): Achievement and future for novel cancer therapy. Pharmacol Res (2020) 158:104844. doi: 10.1016/j.phrs.2020.104844
66. Kim SG, Hoffman GR, Poulogiannis G, Buel GR, Jang YJ, Lee KW, et al. Metabolic stress controls mtorc1 lysosomal localization and dimerization by regulating the ttt-ruvbl1/2 complex. Mol Cell (2013) 49:172–85. doi: 10.1016/j.molcel.2012.10.003
67. Lin Y, Jin H, Wu X, Jian Z, Zou X, Huang J, et al. The cross-talk between ddr1 and stat3 promotes the development of hepatocellular carcinoma. Aging (Albany NY) (2020) 12:14391–405. doi: 10.18632/aging.103482
68. Vogel WF. Ligand-induced shedding of discoidin domain receptor 1. FEBS Lett (2002) 514:175–80. doi: 10.1016/s0014-5793(02)02360-8
69. Shitomi Y, Thogersen IB, Ito N, Leitinger B, Enghild JJ, Itoh Y. Adam10 controls collagen signaling and cell migration on collagen by shedding the ectodomain of discoidin domain receptor 1 (ddr1). Mol Biol Cell (2015) 26:659–73. doi: 10.1091/mbc.E14-10-1463
70. Slack BE, Siniaia MS, Blusztajn JK. Collagen type i selectively activates ectodomain shedding of the discoidin domain receptor 1: Involvement of src tyrosine kinase. J Cell Biochem (2006) 98:672–84. doi: 10.1002/jcb.20812
71. Kim DJ, Cho EJ, Yu KS, Jang IJ, Yoon JH, Park T, et al. Comprehensive metabolomic search for biomarkers to differentiate early stage hepatocellular carcinoma from cirrhosis. Cancers (Basel) (2019) 11:1497. doi: 10.3390/cancers11101497
72. Yamao T, Yamashita YI, Imai K, Umezaki N, Tsukamoto M, Kitano Y, et al. Clinical significance of preoperative hepatocellular carcinoma with high lens culinaris agglutinin-reactive fraction of alpha-fetoprotein, but low alpha-fetoprotein. Anticancer Res (2019) 39:883–9. doi: 10.21873/anticanres.13189
73. Gu TL, Deng X, Huang F, Tucker M, Crosby K, Rimkunas V, et al. Survey of tyrosine kinase signaling reveals ros kinase fusions in human cholangiocarcinoma. PloS One (2011) 6:e15640. doi: 10.1371/journal.pone.0015640
74. Clark AM, Ma B, Taylor DL, Griffith L, Wells A. Liver metastases: Microenvironments and ex-vivo models. Exp Biol Med (Maywood) (2016) 241:1639–52. doi: 10.1177/1535370216658144
75. Van den Eynden GG, Majeed AW, Illemann M, Vermeulen PB, Bird NC, Hoyer-Hansen G, et al. The multifaceted role of the microenvironment in liver metastasis: Biology and clinical implications. Cancer Res (2013) 73:2031–43. doi: 10.1158/0008-5472.CAN-12-3931
76. Brodt P. Role of the microenvironment in liver metastasis: From pre- to prometastatic niches. Clin Cancer Res (2016) 22:5971–82. doi: 10.1158/1078-0432.CCR-16-0460
77. Eggert T, Greten TF. Tumor regulation of the tissue environment in the liver. Pharmacol Ther (2017) 173:47–57. doi: 10.1016/j.pharmthera.2017.02.005
78. Wang YG, Xu L, Jia RR, Wu Q, Wang T, Wei J, et al. Ddr2 induces gastric cancer cell activities via activating mtorc2 signaling and is associated with clinicopathological characteristics of gastric cancer. Dig Dis Sci (2016) 61:2272–83. doi: 10.1007/s10620-016-4116-3
79. Xie R, Wang X, Qi G, Wu Z, Wei R, Li P, et al. Ddr1 enhances invasion and metastasis of gastric cancer via epithelial-mesenchymal transition. Tumour Biol (2016) 37:12049–59. doi: 10.1007/s13277-016-5070-6
80. Jin H, Ham IH, Oh HJ, Bae CA, Lee D, Kim YB, et al. Inhibition of discoidin domain receptor 1 prevents stroma-induced peritoneal metastasis in gastric carcinoma. Mol Cancer Res (2018) 16:1590–600. doi: 10.1158/1541-7786.MCR-17-0710
81. Kurashige J, Hasegawa T, Niida A, Sugimachi K, Deng N, Mima K, et al. Integrated molecular profiling of human gastric cancer identifies ddr2 as a potential regulator of peritoneal dissemination. Sci Rep (2016) 6:22371. doi: 10.1038/srep22371
82. Zhang S, Bu X, Zhao H, Yu J, Wang Y, Li D, et al. A host deficiency of discoidin domain receptor 2 (ddr2) inhibits both tumour angiogenesis and metastasis. J Pathol (2014) 232:436–48. doi: 10.1002/path.4311
83. Sudjaritruk T, Bunupuradah T, Aurpibul L, Kosalaraksa P, Kurniati N, Sophonphan J, et al. Nonalcoholic fatty liver disease and hepatic fibrosis among perinatally hiv-monoinfected asian adolescents receiving antiretroviral therapy. PloS One (2019) 14:e226375. doi: 10.1371/journal.pone.0226375
84. Wynn TA, Ramalingam TR. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat Med (2012) 18:1028–40. doi: 10.1038/nm.2807
85. Rammal H, Saby C, Magnien K, Van-Gulick L, Garnotel R, Buache E, et al. Discoidin domain receptors: Potential actors and targets in cancer. Front Pharmacol (2016) 7:55. doi: 10.3389/fphar.2016.00055
86. Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, et al. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol (2008) 599:44–53. doi: 10.1016/j.ejphar.2008.10.014
87. Rix U, Remsing RL, Terker AS, Fernbach NV, Hantschel O, Planyavsky M, et al. A comprehensive target selectivity survey of the bcr-abl kinase inhibitor inno-406 by kinase profiling and chemical proteomics in chronic myeloid leukemia cells. Leukemia. (2010) 24:44–50. doi: 10.1038/leu.2009.228
88. Canning P, Tan L, Chu K, Lee SW, Gray NS, Bullock AN. Structural mechanisms determining inhibition of the collagen receptor ddr1 by selective and multi-targeted type ii kinase inhibitors. J Mol Biol (2014) 426:2457–70. doi: 10.1016/j.jmb.2014.04.014
89. Sun X, Phan TN, Jung SH, Kim SY, Cho JU, Lee H, et al. Lcb 03-0110, a novel pan-discoidin domain receptor/c-src family tyrosine kinase inhibitor, suppresses scar formation by inhibiting fibroblast and macrophage activation. J Pharmacol Exp Ther (2012) 340:510–9. doi: 10.1124/jpet.111.187328
90. Ambrogio C, Gomez-Lopez G, Falcone M, Vidal A, Nadal E, Crosetto N, et al. Combined inhibition of ddr1 and notch signaling is a therapeutic strategy for kras-driven lung adenocarcinoma. Nat Med (2016) 22:270–7. doi: 10.1038/nm.4041
91. Jeitany M, Leroy C, Tosti P, Lafitte M, Le Guet J, Simon V, et al. Inhibition of ddr1-bcr signalling by nilotinib as a new therapeutic strategy for metastatic colorectal cancer. EMBO Mol Med (2018) 10:e7918. doi: 10.15252/emmm.201707918
92. Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W, et al. Mutations in the ddr2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discovery (2011) 1:78–89. doi: 10.1158/2159-8274.CD-11-0005
93. Zhu D, Huang H, Pinkas DM, Luo J, Ganguly D, Fox AE, et al. 2-amino-2,3-dihydro-1h-indene-5-carboxamide-based discoidin domain receptor 1 (ddr1) inhibitors: Design, synthesis, and in vivo antipancreatic cancer efficacy. J Med Chem (2019) 62:7431–44. doi: 10.1021/acs.jmedchem.9b00365
94. Kim HG, Tan L, Weisberg EL, Liu F, Canning P, Choi HG, et al. Discovery of a potent and selective ddr1 receptor tyrosine kinase inhibitor. ACS Chem Biol (2013) 8:2145–50. doi: 10.1021/cb400430t
95. Richters A, Nguyen HD, Phan T, Simard JR, Grutter C, Engel J, et al. Identification of type ii and iii ddr2 inhibitors. J Med Chem (2014) 57:4252–62. doi: 10.1021/jm500167q
96. Grither WR, Longmore GD. Inhibition of tumor-microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of ddr2 extracellular domain. Proc Natl Acad Sci USA (2018) 115:E7786–94. doi: 10.1073/pnas.1805020115
97. Chen C, Deng J, Yu X, Wu F, Men K, Yang Q, et al. Identification of novel inhibitors of ddr1 against idiopathic pulmonary fibrosis by integrative transcriptome meta-analysis, computational and experimental screening. Mol Biosyst (2016) 12:1540–51. doi: 10.1039/c5mb00911a
98. Shenoy GP, Pal R, Purwarga MG, Singh E, Raghavendra NM, Dhiwar PS. Discoidin domain receptor inhibitors as anticancer agents: A systematic review on recent development of ddrs inhibitors, their resistance and structure activity relationship. Bioorg Chem (2023) 130:106215. doi: 10.1016/j.bioorg.2022.106215
99. Dasatinib in advanced squamous cell lung cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT01491633.
100. Testing dasatinib as a potential targeted treatment in cancers with DDR2 genetic changes . Available at: https://clinicaltrials.gov/ct2/show/NCT04439305.
101. Trial of dasatinib in patients with advanced cancers harboring DDR2 mutation or inactivating b-RAF mutation. Available at: https://www.clinicaltrials.gov/ct2/show/NCT01514864.
102. Bauer T, Cho BC, Heist R, Bazhenova L, Werner T, Goel S, et al. First-in-human phase 1/1b study to evaluate sitravatinib in patients with advanced solid tumors. Invest New Drugs (2022) 40:990–1000. doi: 10.1007/s10637-022-01274-y
103. Targeted therapy directed by genetic testing in treating patients with advanced refractory solid tumors, lymphomas, or multiple myeloma (The MATCH screening trial). Available at: https://clinicaltrials.gov/ct2/show/NCT02465060.
104. Adapting treatment to the tumor molecular alterations for patients with advanced solid tumors: MyOwnSpecificTreatment. Available at: https://clinicaltrials.gov/ct2/show/NCT02029001.
105. Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kuriyan J. Structural mechanism for sti-571 inhibition of abelson tyrosine kinase. Science. (2000) 289:1938–42. doi: 10.1126/science.289.5486.1938
106. Beauchamp EM, Woods BA, Dulak AM, Tan L, Xu C, Gray NS, et al. Acquired resistance to dasatinib in lung cancer cell lines conferred by ddr2 gatekeeper mutation and nf1 loss. Mol Cancer Ther (2014) 13:475–82. doi: 10.1158/1535-7163.MCT-13-0817
107. Pitini V, Arrigo C, Di Mirto C, Mondello P, Altavilla G. Response to dasatinib in a patient with sqcc of the lung harboring a discoid-receptor-2 and synchronous chronic myelogenous leukemia. Lung Canc (2013) 82:171–2. doi: 10.1016/j.lungcan.2013.07.004
108. Berestjuk I, Lecacheur M, Carminati A, Diazzi S, Rovera C, Prod'Homme V, et al. Targeting discoidin domain receptors ddr1 and ddr2 overcomes matrix-mediated tumor cell adaptation and tolerance to braf-targeted therapy in melanoma. EMBO Mol Med (2022) 14:e11814. doi: 10.15252/emmm.201911814
Keywords: discoidin domain receptors, liver, cancer, extracellular matrix, metastasis, therapy
Citation: Gong H, Xu H-M and Zhang D-K (2023) Focusing on discoidin domain receptors in premalignant and malignant liver diseases. Front. Oncol. 13:1123638. doi: 10.3389/fonc.2023.1123638
Received: 14 December 2022; Accepted: 03 March 2023;
Published: 15 March 2023.
Edited by:
Liang Qiao, Westmead Institute for Medical Research, AustraliaReviewed by:
Charles Saby, Université de Reims Champagne-Ardenne, FranceSylvie Brassart-Pasco, UMR7369 Matrice Extracellulaire et Dynamique Cellulaire (MEDyC), France
Copyright © 2023 Gong, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: De-Kui Zhang, emhhbmdkazg2MTZAMTI2LmNvbQ==
 Hang Gong
Hang Gong Hui-Mei Xu
Hui-Mei Xu De-Kui Zhang
De-Kui Zhang