
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 24 February 2023
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 13 - 2023 | https://doi.org/10.3389/fonc.2023.1123378
This article is part of the Research TopicAdvancing Precision Therapies in GlioblastomaView all 10 articles
 Shubin W. Shahab1,2*†
Shubin W. Shahab1,2*† Matthew Schniederjan3,4
Matthew Schniederjan3,4 Jose Velazquez Vega3,4
Jose Velazquez Vega3,4 Stephen Little5
Stephen Little5 Andrew Reisner2,4,6
Andrew Reisner2,4,6 Tobey MacDonald1,2,7
Tobey MacDonald1,2,7 Dolly Aguilera1,2
Dolly Aguilera1,2Introduction: Infant type hemispheric gliomas are a rare tumor with unique molecular characteristics. In many cases these harbor mutations in receptor tyrosine kinase pathways and respond to targeted therapy. Here we describe the case of an infant with this type of tumor with a novel ATIC-ALK fusion that has responded dramatically to the ALK inhibitor lorlatinib, despite being refractory to standard chemotherapy.
Case description: The infant was initially treated with standard chemotherapy and found to have an ATIC-ALK fusion. When surveillance imaging revealed progressive disease, the patient was switched to the ALK-inhibitor lorlatinib at 47 mg/m2/day. The patient demonstrated a significant clinical and radiographic response to the ALK inhibitor lorlatinib after just 3 months of treatment and a near complete response by 6 months of therapy.
Conclusion: The ALK inhibitor lorlatinib is an effective targeted therapy in infant type hemispheric glioma patients harboring ATIC-ALK fusion.
Infantile high-grade gliomas (HGG) are rare entities with unique molecular biology. These tumors are now codified in the World Health Organization (WHO) classification of Central Nervous System Tumors as infant-type hemispheric gliomas (herein referred to as ITHG) without a specific corresponding grade (1). In contrast to their adult counterpart, ITHGs exhibit better outcomes. Prior studies have demonstrated that patients under 4 years with HGG can have distinct molecular rearrangements and fusions and tend to have a more favorable prognosis compared to older patients (2, 3). One of the genes commonly altered in ITHGs is anaplastic lymphoma kinase (ALK), which encodes a receptor tyrosine kinase (RTK) that is also often rearranged, amplified or mutated in pediatric neoplasms, including anaplastic large cell lymphoma (ALCL), inflammatory myofibroblastic tumor (IMT), rhabdomyosarcoma, glioma and neuroblastoma (2). In a case series of infants with HGGs, a large number of cases demonstrated gene fusions that can be targeted with novel therapies. Among these, MET fusions were present in 4 samples, NTRK1/2/3 in 21 samples, ROS fusions in 9 samples and ALK fusions in 31 samples, the most common among which was the PPP1CB-ALK fusion (3). While the 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase (ATIC)-ALK fusion has been described in literature, it is a rare fusion event and has never been described in an ITHG. Here, we describe the case of a patient with an ITHG diagnosed at 3 months of age and exhibiting an ATIC-ALK fusion, who despite progression on standard chemotherapy, has had an extremely good radiographic and clinical response to the ALK inhibitor lorlatinib.
A 3-month-old patient presented with poor head control, increased fatigue, right hemiparesis, feeding difficulties, decreased peripheral vision on the right side and progressively increasing head circumference. Emergency department evaluation showed bulging fontanelle, increased irritability and macrocephaly. A head CT revealed a large, left sided supratentorial mass with midline shift and hydrocephalus, requiring ventricular drain placement. MRI of the brain confirmed a large tumor centered in the posterior left cerebral hemisphere with associated severe mass effect, including subfalcine, uncal, and ascending transtentorial herniation. There was no evidence of metastatic disease in the brain or spine. The patient underwent a subtotal resection with significant blood loss intraoperatively requiring initiation of massive transfusion protocol. Pathology revealed a densely cellular HGG with focal pseudopalisading necrosis, gemistocytic morphology in a proportion of the tumor as well as elevated mitotic activity. A panel of immunohistochemistry supported the diagnosis of HGG (Figure 1). Furthermore, DNA methylation analysis classified the tumor as an ITHG. The sarcoma fusion panel (Archer FusionPlex) revealed fusion between ATIC (exon 7) and ALK (exon 20) (Figure 2). Additional (Ashion) testing through the precision medicine program at our center confirmed the ATIC-ALK fusion at both the DNA and RNA level. There was no evidence of germline mutations. Initial treatment with multiagent chemotherapy per the Baby POG protocol (5) induced a partial tumor response; however, after 7 cycles of this chemotherapy regimen, routine surveillance brain MRI demonstrated progressive disease. The patient underwent a second subtotal resection and resumed Baby POG chemotherapy for an additional 8 months. Due to further tumor progression in multiple areas of the resection cavity on brain MRI, and considering the result of tumor sequencing, the patient’s treatment was then switched to the ALK inhibitor lorlatinib at a dose of 47 mg/m2/day via gastrostomy tube. Subsequent brain MR imaging after 10 weeks of daily lorlatinib treatment revealed a partial response in the largest residual tumor lesion and complete response on the smaller tumor nodules (Figure 3) and 6 months after starting lorlatinib, near complete response on all the residual tumor nodules (Figure 3). At the time of this report, the patient continues on daily lorlatinib therapy (See timeline Figure 4). The only toxicities to date per common terminology criteria for adverse events (CTCAE, version 5.0) have been Grade 1 hypercholesterolemia, Grade 1 triglyceridemia and Grade 2 weight gain. No neurological toxicities have been observed. The clinical response has been remarkable with resolution of hemiparesis, improvement of gait, speech and language, and stabilization of visual deficits.
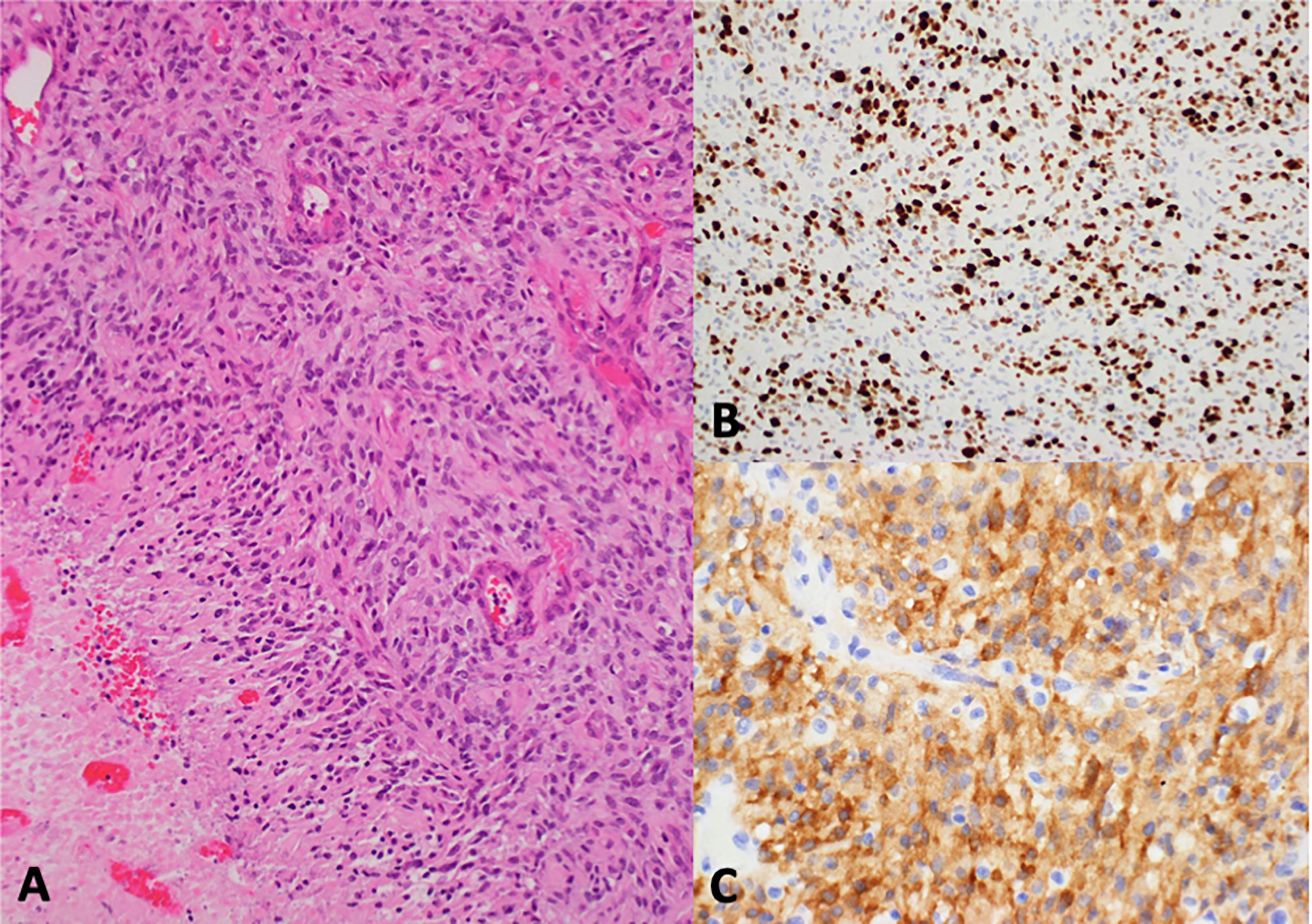
Figure 1 Histologic sections showed a highly cellular glial tumor with a solid growth pattern, brisk mitotic activity, microvascular proliferation, and palisading necrosis (A). Immunohistochemistry revealed high Ki-67 expression (B) and strong cytoplasmic ALK expression (C).
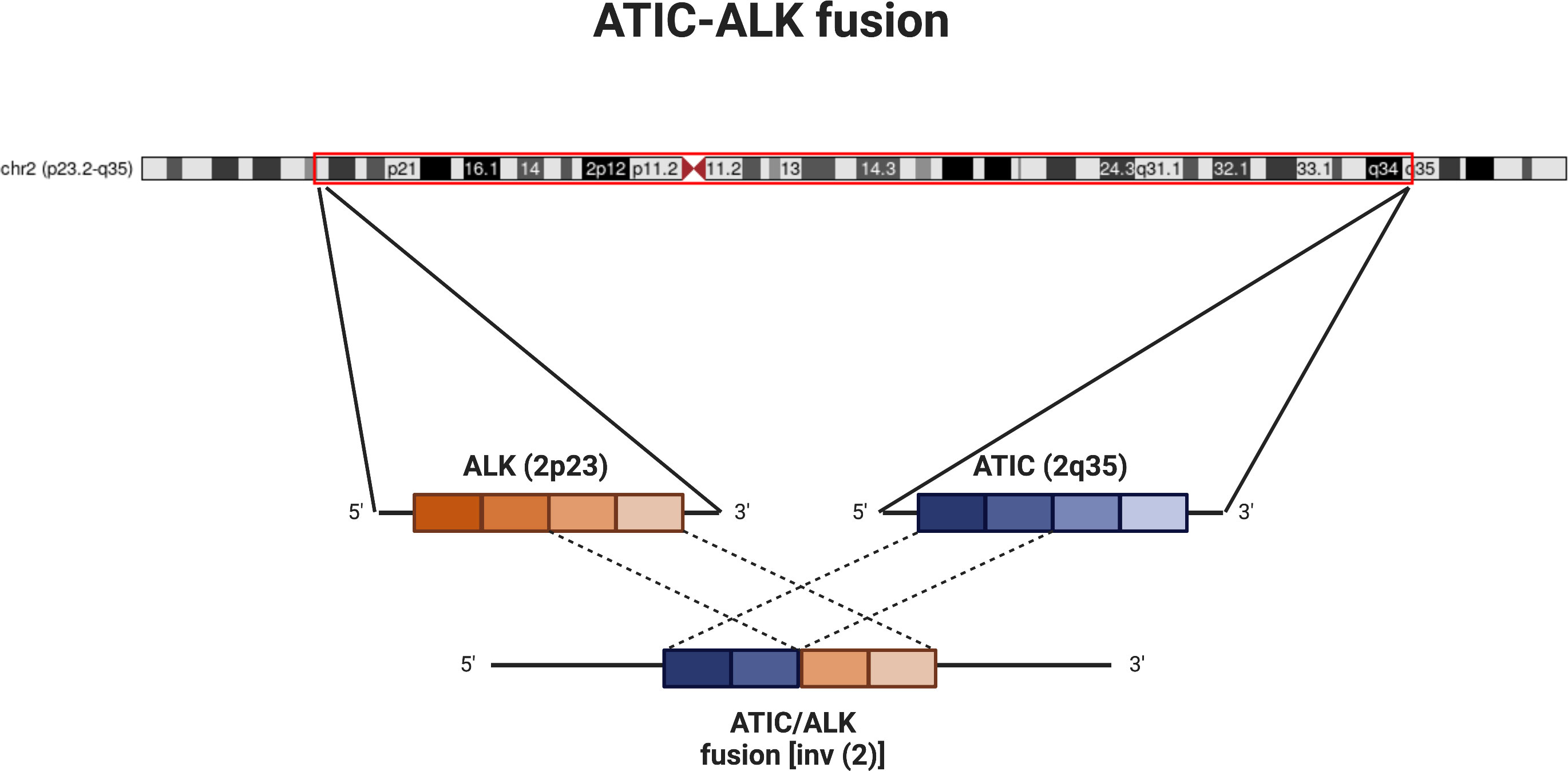
Figure 2 Schematic of ATIC-ALK fusion. Diagram showing chromosome 2 (courtesy of UCSC genome browser; http://genome.ucsc.edu (4); with approximate locations of ALK (2p23) and ATIC (2q35). The inversion and subsequent fusion between the 5’ end of ATIC to the 3’ end of ALK leads to activation of the ALK kinase. For our patient, the fusion is noted specifically between exon 7 of ATIC and exon 20 of ALK.

Figure 3 Pre (A, D, G), Post 1 (10 weeks) (B, E, H) and Post 2 (6 months) (C, F, I) ALK inhibitor administration. Pre-treatment images demonstrate nodular lesions near the splenium of the corpus callosum (arrows in D, G) and along the floor of the third ventricle (arrow in A). Post-treatment images (Post 1) demonstrate substantial decrease in size of both lesions (arrows in B, E, H). Continued decrease in lesion size is seen on Post 2 images (arrows in C, F, I).
The mother of the child shared the following with the primary oncologist: “Our quality of life has improved significantly since switching to lorlatinib. Our child has had no admissions for fever or neutropenia, no need for transfusion of blood products, and has experienced no nausea or vomiting or additional hearing loss. Her appetite is back to normal, she no longer needs g-tube feeds, and is now eating all meals by mouth. She has also achieved more developmental milestones since starting lorlatinib.
Also, we have had fewer clinic visits and lab draws as she has needed less monitoring, going from weekly visits during standard chemotherapy to once every 6 weeks after starting lorlatinib. The central line has been removed. Our child is back at day care. “
ALK is a tyrosine kinase that can often be aberrantly expressed in cancers due to rearrangements or mutations and amplification events. In ALCL, where ALK oncogenic fusions were first described, the most frequent fusion partner is nucleolar protein nucleophosmin (NPM) and accounts for 40-60% of cases. Overall, ALK fusions occur in up to 90% of ALCLs in children, and up to 50% of adult cases (6). Other common fusion partners for ALK include TPM3, TPM4, CLTC and EML4, which are found across different tumor histologies including ALCL, IMT, papillary thyroid cancer, and renal cell carcinoma (2). The ATIC-ALK fusion is a rarer occurrence; The ATIC gene encodes a bifunctional enzyme involved in purine biosynthesis. During the rearrangement the portion of ATIC gene encoding the amino terminus is fused with the portion of ALK gene encoding its carboxy terminus, the product of which leads to a constitutively active ALK tyrosine kinase (7) (Figure 2).
The ATIC-ALK fusion has been reported in a variety of cancers including in IMT, ALCL, and NSCLC (2, 6–14); overall, it still accounts for only 0.1% of reported AACR Genie cases of ALK fusions (15). Thus, to our knowledge, this is the first report of a patient with an ITHG with the ATIC-ALK fusion treated with targeted therapy using an ALK inhibitor, even though other groups have reported the use of lorlatinib for ITHG with other ALK fusions (16).
ITHGs are unique in that most of these tumors harbor alterations in RTK oncogenes (up to 80% in one study (17)). It is possible that the presence of these RTK alterations may drive the biology behind the favorable prognosis seen in infant HGG (17) and may display maturation/differentiation following treatment.
ALK fusions in infant brain tumors have been reported (3, 18–21). In a large multi-institutional international cohort study (17) of infant gliomas, the reported ALK fusions were CCDC88A-ALK, EML4-ALK, PPP1CB-ALK and KTN1-ALK. In this study infants with ALK fusion positive HGGs had a worse outcome compared to ALK fusion positive low grade glioma (LGG), with only 57.1% alive at 3 years of median follow up. In another large study CLIP2-ALK, HIP1-ALK, MAD1L1-ALK, MAP2-ALK, MSI2-ALK, PRKAR2A-ALK, SPECC1L-ALK, SYNDIG1L-ALK and ZC3H7A-ALK fusions were reported (3). In one study, an infant with HGG and PPP1CB-ALK fusion had a complete resection and has not had progression of the tumor 3 years since diagnosis without additional therapy (22). On the other hand, more aggressive courses have been reported with ALK fusion positive infant HGG, and in some cases, response to targeted therapy with ALK inhibitors has been documented (16). ALK inhibitors have also demonstrated efficacy in patients with metastatic NSCLC (non-small cell lung cancer) to the brain, as well as in neuroblastoma and there are several clinical trials that are currently looking into the efficacy of ALK inhibitors in both CNS and non-CNS tumors (NCT03236675, NCT04774718, NCT02201992, NCT02568267, NCT03052608, NCT04589845, NCT02693535, NCT04541407, NCT04094610, NCT02650401).
Lorlatinib is a 3rd generation ALK inhibitor with improved CNS penetration compared to earlier generations, and may have some neurological toxicities including peripheral neuropathy, mental status and mood changes (23). In a clinical trial for adult patients with advanced ALK-positive lung cancer the 12-month event-free survival was 78% in the lorlatinib arm vs. 39% in the crizotinib arm. Additionally, the responses were significantly better with lorlatinib for subjects with intracranial metastatic disease, a subset in which 71% in the lorlatinib group achieved complete response in the brain (as opposed to 8% for crizotinib) (24).
At this time, the optimal pediatric dose, duration of treatment, or anticipated long term toxicities of using lorlatinib in children, let alone in infants, are not known. Our patient has so far experienced the most common toxicities of hypercholesterolemia, hypertriglyceridemia and weight gain, although some neurocognitive effects may be difficult to assess at this age. In a previously published case report of lorlatinib use in a child with HGG, the patient was treated with 95 mg/m2/day (twice the dose used in our patient) with similar toxicity profile (16). That patient received treatment for 8 months (with a 20% dose reduction due to weight gain) and then therapy was discontinued for a period before having to be resumed due to relapse. Another case of a young child with a recurrent infant-type hemispheric glioma with the ZNF397-ALK fusion has recently been published (25). In that study the patient received lorlatinib at 95 mg/m2/day as well. Significant complications were reported including weight gain greater than 130% from baseline. A dose reduction of 50% of lorlatinib was started, and while weight gain slowed, it did not stop. Eventually lorlatinib was discontinued after more than 1 year of treatment. Prior use of lorlatinib in children also included a phase I trial to evaluate doses on relapsed or recurrent neuroblastoma patients with ALK mutations/amplification who might have had prior exposure to other ALK inhibitors (26). In that study 5 dose levels (45, 60, 75, 95, 115 mg/m2/day) were evaluated in pediatric patients. Dose limiting toxicity (DLT) was the primary endpoint on the first 28 days and neurocognitive toxicity within 54 days of starting drug. Of note the youngest patient getting lorlatinib on this trial reported was 2 years old. No DLT was observed at the lowest 3 dose levels. At a dose of 95mg/m2/day 50% enrolled patients (5/10) had DLT’s. At 115/mg/m2/day 33% of enrolled patients (1/3; with expansion ongoing at the time of abstract publication) had a DLT of grade 3 diarrhea. Overall weight gain, hyperlipidemia, concentration/memory impairment, peripheral neuropathy, and peripheral edema were the most common adverse events reported. Interestingly, responses were seen early (median of 2 courses) and across dose levels.
Given that responses were seen at the lowest dose level, we selected this dose to start the treatment of our patient. Our patient has only experienced Grade 1 hypercholesterolemia, Grade 1 triglyceridemia and Grade 2 weight gain to date and as highlighted in the caregiver perspective has had significant improvement in quality of life. While targeted therapy clearly has a role in the relapsed/refractory setting, it remains to be seen whether this approach will be widely adopted as 1st line. In patients with ALK-rearranged non-small cell lung cancer targeted therapy appears to be superior than chemotherapy only regimens (27). Recently a meta-analysis investigated whether ALK-inhibitor therapy should be used first line in ALK-positive lung cancer patients who were also given chemotherapy (28). The authors noted that while there was an improvement in progression free survival, there was no significant improvement in overall survival. We recognize that ALK-positive lung cancer is a very different disease in a significantly older age group compared to ALK-positive infantile high-grade glioma and therefore, the outcomes may be very different. Furthermore, as with any case report, our observations may have been influenced by our subjective biases and may not be generalizable to other cases of high-grade glioma or even infants with IHTG. Thus, more studies are clearly needed. However, given the rarity of these fusions and the age of many of these patients, accruing enough patients for clinical trials may be difficult, although some data may be extrapolated from non-CNS studies or pathology agnostic trials in older patients.
This case highlights the importance of molecular information directing therapy in the clinical setting. We initially chose to treat our patient with standard chemotherapy per Baby POG after discussion within our group and with the parents. Specifically, the age of the child and the lack of data published in children less than 2 years of age were driving factors behind our decision. Additionally, the potential for development of resistance with prolonged exposure to this agent is also a consideration. Molecular profiling at the time of progression may help in our understanding of the pathways to resistance development and help find an alternative treatment. While there are no established combination treatment regimens with ALK inhibitors, several clinical trials are investigating rational strategies of combining ALK inhibitors with other agents.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethical review and approval were not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the minor(s)’ legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
SS, TM and DA contributed to the study conception and design. Sample collection was performed by AR. Imaging analysis and interpretation were performed by SL. Histopathological slide preparation and interpretation were performed by MS and JV. The first draft of the manuscript was written by SS and all authors commented on previous versions of the manuscript. All authors contributed to the article and approved the submitted version.
Sequencing costs were covered by the Aflac Precision Medicine Program, which receives generous support from the CURE Childhood Cancer Foundation.
The authors would like to acknowledge the American Association for Cancer Research and its financial and material support in the development of the AACR Project GENIE registry, as well as members of the consortium for their commitment to data sharing. Interpretations are the responsibility of study authors. We would also like to thank CURE childhood cancer foundation for supporting Aflac Precision Medicine Program. We are also forever grateful to the parents of our patient who agreed to share their story for the benefit of other children/families.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
2. Takita J. The role of anaplastic lymphoma kinase in pediatric cancers. Cancer Sci (2017) 108(10):1913–20. doi: 10.1111/cas.13333
3. Clarke M, Mackay A, Ismer B, Pickles JC, Tatevossian RG, Newman S, et al. Infant high-grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Cancer Discov (2020) 10(7):942–63. doi: 10.1158/2159-8290.CD-19-1030
4. Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, et al. The human genome browser at UCSC. Genome Res (2002) 12(6):996–1006. doi: 10.1101/gr.229102
5. Duffner PK, Horowitz ME, Krischer JP, Burger PC, Cohen ME, Sanford RA, et al. The treatment of malignant brain tumors in infants and very young children: an update of the pediatric oncology group experience. Neuro Oncol (1999) 1(2):152–61. doi: 10.1093/neuonc/1.2.152
6. Cao Z, Gao Q, Fu M, Ni N, Pei Y, Ou WB. Anaplastic lymphoma kinase fusions: Roles in cancer and therapeutic perspectives. Oncol Lett (2019) 17(2):2020–30. doi: 10.3892/ol.2018.9856
7. Colleoni GW, Bridge JA, Garicochea B, Liu J, Filippa DA, Ladanyi M. ATIC-ALK: A novel variant ALK gene fusion in anaplastic large cell lymphoma resulting from the recurrent cryptic chromosomal inversion, inv(2)(p23q35). Am J Pathol (2000) 156(3):781–9. doi: 10.1016/S0002-9440(10)64945-0
8. Trinei M, Lanfrancone L, Campo E, Pulford K, Mason DY, Pelicci PG, et al. A new variant anaplastic lymphoma kinase (ALK)-fusion protein (ATIC-ALK) in a case of ALK-positive anaplastic large cell lymphoma. Cancer Res (2000) 60(4):793–8. doi: 10.3892/or.2020.7767
9. Maes B, Vanhentenrijk V, Wlodarska I, Cools J, Peeters B, Marynen P, et al. The NPM-ALK and the ATIC-ALK fusion genes can be detected in non-neoplastic cells. Am J Pathol (2001) 158(6):2185–93. doi: 10.1016/S0002-9440(10)64690-1
10. Debiec-Rychter M, Marynen P, Hagemeijer A, Pauwels P. ALK-ATIC fusion in urinary bladder inflammatory myofibroblastic tumor. Genes Chromosomes Cancer (2003) 38(2):187–90. doi: 10.1002/gcc.10267
11. Matsubara K, Tanaka T, Taki T, Nakagawa A, Nigami H, Tamura A, et al. [ATIC-ALK-positive anaplastic large cell lymphoma: A case report and review of the literature]. Rinsho Ketsueki (2008) 49(5):325–30.
12. Damm-Welk C, Klapper W, Oschlies I, Gesk S, Rottgers S, Bradtke J, et al. Distribution of NPM1-ALK and X-ALK fusion transcripts in paediatric anaplastic large cell lymphoma: A molecular-histological correlation. Br J Haematol (2009) 146(3):306–9. doi: 10.1111/j.1365-2141.2009.07754.x
13. Tateishi Y, Okudela K, Kawai S, Suzuki T, Umeda S, Matsumura M, et al. Intraosseous inflammatory myofibroblastic tumor of the mandible with a novel ATIC-ALK fusion mutation: A case report. Diagn Pathol (2016) 11(1):132. doi: 10.1186/s13000-016-0586-z
14. Wu X, Zhou H, He Z, Zhang Z, Feng W, Zhao J, et al. Coexistence of a novel CCNY-ALK and ATIC-ALK double-fusion in one patient with ALK-positive NSCLC and response to crizotinib: a case report. Transl Lung Cancer Res (2020) 9(6):2494–9. doi: 10.21037/tlcr-20-1049
15. Consortium APG. AACR project GENIE: Powering precision medicine through an international consortium. Cancer Discov (2017) 7(8):818–31. doi: 10.1158/2159-8290.CD-17-0151
16. Bagchi A, Orr BA, Campagne O, Dhanda S, Nair S, Tran Q, et al. Lorlatinib in a child with ALK-Fusion-Positive high-grade glioma. N Engl J Med (2021) 385(8):761–3. doi: 10.1056/NEJMc2101264
17. Guerreiro Stucklin AS, Ryall S, Fukuoka K, Zapotocky M, Lassaletta A, Li C, et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun (2019) 10(1):4343. doi: 10.1038/s41467-019-12187-5
18. Gilani A, Donson A, Davies KD, Whiteway SL, Lake J, Desisto J, et al. Targetable molecular alterations in congenital glioblastoma. J Neurooncol (2020) 146(2):247–52. doi: 10.1007/s11060-019-03377-8
19. Mrowczynski OD, Payne R, Pu C, Greiner R, Rizk E. A unique case of a high-grade neuroepithelial tumor with EML4-ALK fusion in a five-Month-Old. Cureus (2020) 12(6):e8654. doi: 10.7759/cureus.8654
20. Zhong Y, Lin F, Xu F, Schubert J, Wu J, Wainwright L, et al. Genomic characterization of a PPP1CB-ALK fusion with fusion gene amplification in a congenital glioblastoma. Cancer Genet (2021) 252-253:37–42. doi: 10.1016/j.cancergen.2020.12.005
21. Roosen M, Ode Z, Bunt J, Kool M. The oncogenic fusion landscape in pediatric CNS neoplasms. Acta Neuropathol (2022) 143(4):427–51. doi: 10.1007/s00401-022-02405-8
22. Aghajan Y, Levy ML, Malicki DM, Crawford JR. Novel PPP1CB-ALK fusion protein in a high-grade glioma of infancy. BMJ Case Rep (2016) 2016. doi: 10.1136/bcr-2016-217189
23. Nagasaka M, Ge Y, Sukari A, Kukreja G, Ou SI. A user's guide to lorlatinib. Crit Rev Oncol Hematol (2020) 151:102969. doi: 10.1016/j.critrevonc.2020.102969
24. Shaw AT, Bauer TM, De Marinis F, Felip E, Goto Y, Liu G, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med (2020) 383(21):2018–29. doi: 10.1056/NEJMoa2027187
25. Greenwell AM, Baughan S, Altinok D, Marupudi NI, Kupsky W, Kumar-Sinha C, et al. Lorlatinib for the treatment of ALK fusion-positive infant-type hemispheric glioma: A case report. JCO Precis Oncol (2022) 6:e2200255. doi: 10.1200/PO.22.00255
26. Goldsmith KC, Kayser K, Groshen SG, Chioda M, Thurm HC, Chen J, et al. Phase I trial of lorlatinib in patients with ALK-driven refractory or relapsed neuroblastoma: A new approaches to neuroblastoma consortium study. J Clin Oncol (2020) 38(15_suppl):10504–4. doi: 10.1200/JCO.2020.38.15_suppl.10504
27. Kron A, Alidousty C, Scheffler M, Merkelbach-Bruse S, Seidel D, Riedel R, et al. Impact of TP53 mutation status on systemic treatment outcome in ALK-rearranged non-small-cell lung cancer. Ann Oncol (2018) 29(10):2068–75. doi: 10.1093/annonc/mdy333
Keywords: infant-type hemispheric glioma, high grade glioma, ALK fusion, lorlatinib, case report
Citation: Shahab SW, Schniederjan M, Vega JV, Little S, Reisner A, MacDonald T and Aguilera D (2023) Case report: ATIC-ALK fusion in infant-type hemispheric glioma and response to lorlatinib. Front. Oncol. 13:1123378. doi: 10.3389/fonc.2023.1123378
Received: 14 December 2022; Accepted: 09 February 2023;
Published: 24 February 2023.
Edited by:
Theophilos Tzaridis, Sanford Burnham Prebys Medical Discovery Institute, United StatesReviewed by:
Angela Mastronuzzi, Bambino Gesù Children’s Hospital (IRCCS), ItalyCopyright © 2023 Shahab, Schniederjan, Vega, Little, Reisner, MacDonald and Aguilera. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shubin W. Shahab, c3NoYWhhYkBlbW9yeS5lZHU=
†ORCID: Shubin W. Shahab, orcid.org/0000-0003-2323-5892
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.